Abstract
Background
The glandular odontogenic cyst (GOC) is a benign developmental cyst of the jaws that is characterized by a high recurrence rate.
Methods
A systematic review is presented of reported cases, case series, and retrospective studies of recurrent cases of glandular odontogenic cysts, to determine the overall and detailed demographic features with documentation of the specific histologic features of the initial presentation of each cyst. Searches of detailed databases were carried out to identify articles published in the English language from 1988 to 2023. The variables were demographics, patient symptoms, cyst location, radiographic features, histopathological findings, type of treatment, and minimum eight months of follow-up.
Results
Eighteen cases were identified: with an equal gender presentation of 50% females and 50% males. The average age was 44.7. The mean size was 3.5 cm. The most common location was in the anterior mandible in 50% (n = 9) of cases, followed by the posterior mandible 27.8% (n = 5). Most patients were asymptomatic 55.6% (n = 10). The most common histologic features at first diagnosis were mucous cells in 88.9% (n = 16), variable thickness with 83.3% (n = 15), eosinophilic cuboidal cells 88.9% (n = 16), microcysts 83.3% (n = 15), and clear cells 77.8% (n = 14) cases.
Conclusion
GOC has an aggressive behavior. Evidence was not conclusive to link any single or combination of histologic features to recurrence, and the strongest correlation for recurrence was the type of treatment. Since this is an uncommon cyst, more cases are needed. Follow-up should continue for at least five years, because recurrences were higher between years 3 and 5.
Keywords: Glandular odontogenic cyst, Histologic features, Recurrence, Treatment
Introduction
The glandular odontogenic cyst (GOC) is a benign developmental cyst of the jaws. It is suggested to arise from remnants of odontogenic epithelium [1]. In 1987, Padayachee and Van Wyk initially reported it as a sialodontogenic cyst, based on the possibility of salivary gland origin and then in 1988 Gardner et al. speculated an odontogenic origin and proposed the term GOC [2]. It accounts for 0.012–1.3% of all jaw cysts with a mean of 0.17% [3]. In 1992, the second edition of the World Health Organization’s (WHO) Histologic Typing of Odontogenic Tumors recognized it as a cyst arising in the tooth-bearing areas of the jaws, characterized histologically by an epithelial lining with cuboidal or columnar cells both at the surface and lining crypts or cyst-like spaces within the thickness of the epithelium [4]. The clinical significance of this cyst is its aggressive growth pattern and high recurrence rate [5].
GOCs can occur in all age groups; however, the peak incidence is in the 4th decade of life [6]. The anatomic prevalence favors the mandible (70%) rather than maxilla (30%) with the anterior mandible the most common of all the anatomic locations [7]. This cyst can expand buccolingually. Radiographically it presents as a unilocular or multilocular radiolucency with well-defined but in some cases ill-defined borders [6].
Histologically, GOCs have different glandular features according to Fowler et al.; microcysts, epithelial spheres, multiple compartments, and clear cells appear to be most helpful in distinguishing GOCs from GOC mimickers. Originally, it was reported that 10 histologic features could characterize GOC, but the definitive diagnosis can be rendered with confidence when 7 or more criteria are present [8]. An important distinction is that GOCs are sometimes multicompartmentalized, where these cases could be misinterpreted as central mucoepidermoid carcinoma (CMEC) of the jaws or as botryoid odontogenic cysts [5, 8]. In this regard, some authors consider that GOCs have an intimate relation with central mucoepidermoid carcinoma, and in some cases, a MEC can develop from the cystic epithelium of GOCs [9]. However, most of the evidence shows GOCs lack MAML2 rearrangements, the most common genetic fusion seen in MEC [10].
Different surgical procedures have been done for the management of GOCs, depending on the size and neighboring anatomic structures. Enucleation, curettage, and block resection have been used where enucleation and curettage have been more prone to recur [11]. In addition, the resection technique avoids in almost all cases the recurrence, but it is an invasive procedure with considerable surgical morbidity [12]. Several publications document a high recurrence for GOC ranging from 30 to 50% [8, 13].
Some of the factors contributing to recurrence may be the thickness of the cyst wall and the presence of microcysts which make complete removal of the cyst very difficult. According to Kaplan, recurrence was higher when the cyst was multilocular and when cortical integrity was compromised (71.4% vs. 47.1%) [14]. Cortical perforation may be associated with the presence of remnant cells/tissue in the periosteum and surrounding soft tissue [15]. Multilocular presentation has also been reported to develop more recurrence [14]. For that reason, some clinicopathological features may predict a high risk for recurrence. The aim of this systematic review is to identify recurrent cases of GOC from the literature, which document the clinical, radiologic, histopathological features, and treatment of the initial cyst that might allow better prediction of the recurrence.
Material and Methods
This systematic review followed the PRISMA (Preferred Reporting Items for Systematic Review and Meta-Analyses) guidelines [16].
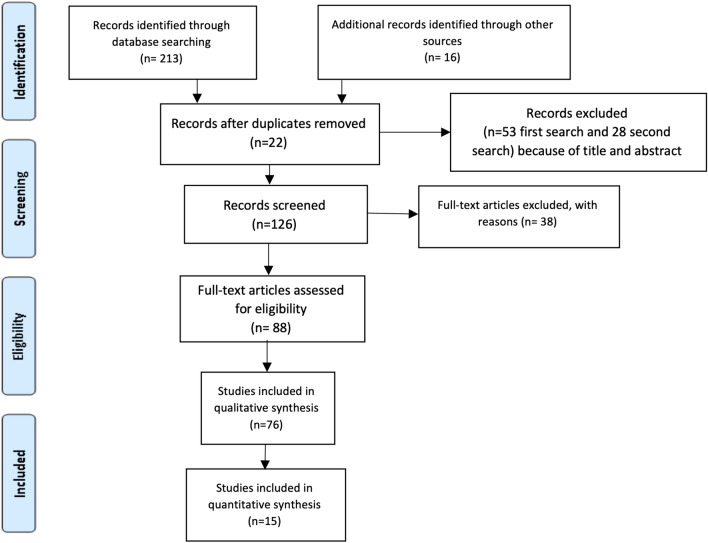
The research (Fig. 1 Flowchart) was based on the “PVO” framework for systematic reviews (P = patient/population, V = variable, O = outcome). The research question was “Which radiographic, histology presentation and type of surgery were commonly associated with high-rate recurrence of GOC.”
Fig. 1.
Flowchart
Protocol and Registration
The systematic review protocol was registered in the International Prospective Register of Systematic Review (PROSPERO)—Registration number: CRD42023484492.
Eligibility Criteria and Types of Studies
Inclusion criteria incorporated clinical and histopathologic studies on GOC reporting recurrences, with a minimal time of 8 months after the first diagnosis. The studies needed to have at least six histopathologic criteria by Fowler for a definite histologic diagnosis of GOC. Case reports [15, 17, 22–24, 26, 28, 30], case series [2, 13, 18, 25, 27, 29], and a retrospective study [11] were included. The studies needed to report on specific variables regarding clinical, radiologic, histologic features, and treatment of the first diagnosis associated with documented recurrent GOCs. Studies that lack important information, editorial letters, nonhuman research, or not English-documented studies were excluded. Two independent examiners (APL and AS) performed an electronic search in the PubMed/MEDLINE, Cochrane Library, and Scopus databases for articles published between 1988 and October 2023. Any discrepancies were resolved through debate with all authors for definitive accordance. The conducted search was conducted using the following medical subject headlines (MESH) terms: ‘Glandular odontogenic cyst’ OR ‘recurrence’ AND ‘follow-up.’ The reference lists of the included studies were also searched for possible additional articles.
Data Collection Process and Data Items
A data collection of each variable for the papers that met the inclusion criteria was performed. The following information was extracted from the included articles: Author, year of publication, type of study, demographics, radiographic presentation, histologic features, type of treatment, follow-up, and recurrence. Data were extracted individually by the reviewers (APL and AS) completing a data collection form in a standardized manner using Excel and Word (v.16/2020, Microsoft; Redmond, WA, USA). Correlation coefficients were calculated for all variables using Pearson’s correlation coefficients with significance set at α = 0.05.
Risk of Bias
The selected protocol was rigorously followed avoiding the risk of bias from differences in outcomes included from the evaluated primary studies reported and the variability of study designs. In this systematic review, the selected studies that met the criteria were graded according to the Oxford Center for Evidence-Based Medicine (CEBM) guidelines for level of evidence (Oxford Center for Evidence-Based Medicine, 2009) (Table 1). These guidelines analyze and rank studies from level 1 (highest) to 5 (lowest), being level 1 (randomized control trials), level 2 (prospective cohort clinical studies), level 3 (retrospective case-controlled studies), level 4 (case series studies), and level 5 (case-based reasoning and bench studies).
Table 1.
Oxford evidence level for recurrence of glandular odontogenic cysts articles included in the systematic review
| Author | Year | Type of study | # of patients | Recurrences | Treatment | Follow-up months | Evidence level |
|---|---|---|---|---|---|---|---|
| Martin-Chaves | 2021 | Case series | Three | One | Enucleation | 36 | 3b |
| Kaplan I | 2005 | Retrospective study | Seven | One | Enucleation + peripheral ostectomy | 12 | 3a |
| Gardner D | 1988 | Case series | Eight | Three (two cases well documented) | Enucleation | 44 | 3a |
| Enucleation | 39 | ||||||
| Oliveira JX | 2009 | Case report | One | One | Enucleation | 9 | 4 |
| de Campos | 2023 | Case report | One | One | Curettage and peripheric ostectomy | 8 after first dx and 15 after second surgery | 4 |
| Koppang HS | 1998 | Case series and review | Two | One | Enucleation | 14 | 3b |
| Momeni Roochi | 2015 | Case series | Four | Two | Enucleation peripheral osteotomy | 48 | 3a |
| Peripheral osteotomy | 144 | ||||||
| Motooka N | 2015 | Case report | One | One | Several enucleations | 24 | 4 |
| Hussain K | 1995 | Case series | Three | One | Curettage | 36 | 3b |
| Thor A | 2006 | Case report | One | One | First enucleation, then marginal resection and reconstruction | 120 | 3b |
| Savage N | 1996 | Case report | One | One | Enucleation | 12 | 4 |
| Jafarian A | 2015 | Case report | One | One | Enucleation and curettage | 36 | 3b |
| de Freitas S | 2017 | Case report | One | One | Curettage | 240 | 3b |
| Magnusson B | 1997 | Case series | Seven | Three (two after 8 months) | Enucleation | 36 | 3b |
| Enucleation | 36 | ||||||
| Turali S | 2012 | Case report | One | One | Enucleation and curettage | 72 | 3b |
Results
The results are summarized in Table 2.
Table 2.
Demographic of GOC recurrence, by gender, with the different variables
| Total sample size | 100% (n = 18) | |
|---|---|---|
| Age (years) | Mean ± SD (Min/Max) | [CI 95%] |
| 44.7 ± 14.7 (14/69) | [37.9–51.5] | |
| % (n) | [CI 95%] | |
| Sex | ||
| Female | 50% (n = 9) | [26.9–73.1] |
| Male | 50% (n = 9) | [26.9–73.1] |
| Clinical sign | ||
| Swelling | 88.9% (n = 16) | [74.4–100] |
| No Swelling | 5.6% (n = 1) | [− 5–16.2] |
| No Report | 5.6% (n = 1) | – |
| Symptoms | ||
| Asymptomatic | 55.6% (n = 10) | [32.6–78.6] |
| Pain | 5.6% (n = 1) | [− 5–16.2] |
| Pressure | 16.7% (n = 3) | [− 0.5–33.9] |
| Paresthesia | 5.6% (n = 1) | [− 5–16.2] |
| No report | 16.7% (n = 3) | – |
| Radiography | ||
| Unilocular | 61.1% (n = 11) | [38.6–83.6] |
| Multilocular | 38.9% (n = 7) | [16.4–61.4] |
| Well defined | 88.9% (n = 16) | [74.4–100] |
| Ill defined | 11.1% (n = 2) | [3.4–25.6] |
| Localization | ||
| Anterior mandible | 50% (n = 9) | [26.9–73.1] |
| Posterior mandible | 27.8% (n = 5) | [7.1–48.5] |
| Anterior maxilla | 16.7% (n = 3) | [− 0.5–33.9] |
| Anterior and posterior maxilla | 5.6% (n = 1) | [− 5–16.2] |
| Size of the lesion (mm) | 3.5 ± 1.2 (n = 13) | [2.8–4.2] |
| Tooth displacement | ||
| Yes | 27.8% (n = 5) | [7.1–48.5] |
| No | 66.7% (n = 12) | [44.9–88.5] |
| No report | 5.6% (n = 1) | – |
| Root resorption | ||
| Yes | 22.2% (n = 4) | [3–41.4] |
| No | 77.8% (n = 14) | [58.6–97] |
| Histology | ||
| Microcysts | ||
| Yes | 83.3% (n = 15) | [66.1–100] |
| No | 16.7% (n = 3) | [− 0.5–33.9] |
| Cilia | ||
| Yes | 44.4% (n = 8) | [21.4–67.4] |
| No | 55.6% (n = 10) | [32.6–78.6] |
| Mucous cells | ||
| Yes | 88.9% (n = 16) | [74.4–100] |
| No | 11.1% (n = 2) | [− 3.4–25.6] |
| Papillary tufting | ||
| Yes | 66.7% (n = 12) | [44.9–88.5] |
| No | 33.3% (n = 6) | [11.5–55.1] |
| Multiple compartments | ||
| Yes | 77.8% (n = 14) | [58.6–97] |
| No | 22.2% (n = 4) | [3–41.4] |
| Apocrine snouting | ||
| Yes | 33.3% (n = 6) | [16.3–47.2] |
| No | 66.6% (n = 12) | [53.6–87.6] |
| Epithelial spheres | ||
| Yes | 44.4% (n = 8) | [21.4 –67.4] |
| No | 55.6% (n = 10) | [32.6–78.6] |
| Variable thickness | ||
| Yes | 83.3% (n = 15) | [66.1–100] |
| No | 16.7% (n = 3) | [− 0.5–33.9] |
| Eosinophilic cuboidal cells | ||
| Yes | 88.9% (n = 16) | [74.4–100] |
| No | 11.1% (n = 2) | [3.4–25.6] |
| Clear cell | ||
| Yes | 77.8% (n = 14) | [58.6–97] |
| No | 22.2% (n = 4) | [3–41.4] |
| Treatment | ||
| Enucleation | 72.2% (n = 13) | [51.5–92.9] |
| Curettage and enucleation or peripheral osteotomy | 16.7% (n = 3) | [0.5–33.9] |
| Enucleation and peripheral osteotomy | 11.1% (n = 2) | [3.4–25.6] |
| Mean ± SD (n) | [CI 95%] | |
| Follow-up (months) | 56.4 ± 59.5 (n = 18) | [28.9–83.9] |
| Time of duration (months) | 10.8 ± 6.2 (n = 11) | [7.1–14.5] |
n number of cases, SD standard deviation CI confidence interval
Sex: For the 18 patients included in the review, 50% (n = 9) were female and 50% (n = 9) were male.
Age: The patients’ ages ranged from 14 to 69 years with an average of 44.7 years.
Clinical signs: Clinical information was available in 17 cases. Swelling was the most common presenting sign (88.9%, n = 16). Clinical signs were absent in one case, and one did not report any data.
Symptoms: Clinical symptoms were mentioned in 15 cases. Most patients reported being asymptomatic (55.6%, n = 10). Pain was described in 5.6% (n = 1) case and pressure was noted in 16.7% (n = 3) cases.
Time of duration before diagnosis: Time to diagnosis was reported in 11 cases with an average of 10.8 months.
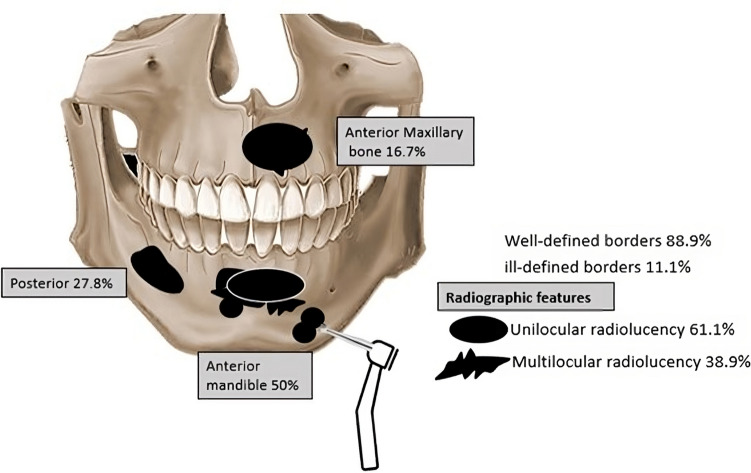
Radiographic pattern and border: Was recorded in all 18 cases. The most common radiographic finding was a unilocular radiolucency with 61.1% (n = 11) cases and a multilocular radiolucency with 38.9% (n = 7) cases. The radiographic borders were well defined in 88.9% (n = 16) of cases and ill-defined in 11.1% (n = 2) of cases (Fig. 2).
Fig. 2.
Graphic illustration with the different radiographic features and most common anatomic locations
Localization: The anterior mandible was affected in 50% (n = 9) of cases, followed by the posterior mandible 27.8% (n = 5). There were 16.7% (n = 3) cases in the anterior maxilla (Fig. 2).
Size of the lesion: Lesion size was documented in 13 cases and ranged from 2.8 to 5 cm, with a mean of 3.5 cm.
Tooth displacement: This variable was radiographically evident in 27.8% (n = 5) cases, and no evidence of tooth displacement was documented in 66.7% (n = 12) of cases, one case was not able to be evaluated.
Root resorption: Data was available for 18 cases. No root resorption was evident in 77.8% (n = 14) of cases whereas root resorption was present in 22.2% (n = 4) of cases.
Histopathological findings of the initial cyst: Features of microcyst presentation was evident in 72.5% (n = 13) cases, cilia in 44.4% (n = 8) cases, mucous cell in 88.9% (n = 16) of cases, papillary tufting in 38.9% (n = 7) of cases, multiple compartment was found in 33.3% (n = 6) cases, variable thickness in 83.3% (n = 15) cases, eosinophilic cuboidal cells in 83.3% (n = 15) cases, clear cells in 77.8% (n = 14) cases, apocrine snooting 33.3% (n = 6) cases, and epithelial spheres 27.5% (n = 5) cases.
Treatment: The variable was documented in 18 cases, with the most common therapeutic approach being enucleation 72.2% (n = 13) cases, followed by curettage and peripheral osteotomy with 16.7% (n = 3) cases.
Follow-up: Information was documented in all 18 cases. The follow-up time ranged from 8 to 240 months (mean of 56.4 months).
Discussion
Despite the extensive documented literature of case reports and reviews, some doubt remains regarding the recurrence of GOC. For this reason, the objective of this systematic review was to analyze documented GOCs recurrences, evaluating the initial clinical and histologic features with treatment modalities, trying to establish if any of those characteristics has more risk for recurrence.
Our study found equal gender presentation. Regarding the age, a mean of 44.7 years was found, with a range from 14 to 69 years [17, 18]. The youngest GOC patient found in the literature was an 11-year-old male documented by Faisal et al. [19], and we documented the youngest recurrence in a 14-year-old female by Savage [17]. Regarding age, GOCs are extremely rare in the second decade, and no reported cases were found in the first decade of life.
Chrcanovic and colleagues published a systematic review of GOC which included 169 cases [20], with bone expansion, (as swelling) in (73%, n = 103) patients and in our study swelling was the most common sign with (88.9%, n = 16). For symptoms our patients were (55.6% n = 10) asymptomatic [11, 13, 15, 17, 21–26] but we included pressure as a different variable for 16.7% [27, 28]. Adding those together will increase symptoms to 72.3%, compared with (75.7%, n = 106) in the Chrcanovic study [20].
The duration time before diagnosis was on average 10.8 months, mentioned in 11 cases with a range of 3 months [28] to 24 months [27].
Radiographically (Fig. 2) our study found a unilocular radiolucency in 61.1% of cases, [11, 15, 17, 21, 26–29] and multilocular in 38.9%, [13, 21–25, 29, 30] in concordance with the review by Chrcanovic with 61.5% for unilocular and 38.5% for multilocular, respectively [20]. Interestingly all the multilocular cases were found in the mandible, and multilocularity did not have as high an impact on the recurrence rate, p ≤ 0.05 as was mentioned by Kaplan et al. in 2005, who stated that the risk of a recurrence increases with size and multilocular appearance and possible difficulty for access and treatment [11]. On the other hand, the size was a factor associated with recurrence (p = 0.05).
The mandible was affected more frequently than the maxilla, with a 3 to 1 proportion, in concordance with the data by Macdonald showing 67% and Chrcanovic with 73.2% [6, 20]. The anterior mandible was affected in 50% in our study [13, 17, 22–24, 26, 28–30] and the posterior mandible with 27.8%, with a total of 77.8% affecting the mandible which was the most common anatomic area for recurrences. Our study found an average size of 3.5 cm which ranged from 2.8 cm [21] to 5 cm [23]. A larger size was found in the maxilla compared to the mandible with a difference of 1.2 cm [11, 15, 27, 32]. Even though the maxillary medullary spaces have less resistance, allowing the cyst to increase in size before evidence of signs or symptoms, no statistical difference was found for recurrence between the maxilla against the mandible. However, there was a statistically significant correlation between increased GOC size in males (p = 0.046) and a significant correlation between increased GOC size and a patient’s age (p = 0.05) in patients in the 4th decade (Figs. 3, 4, 5, 6).
Fig. 3.
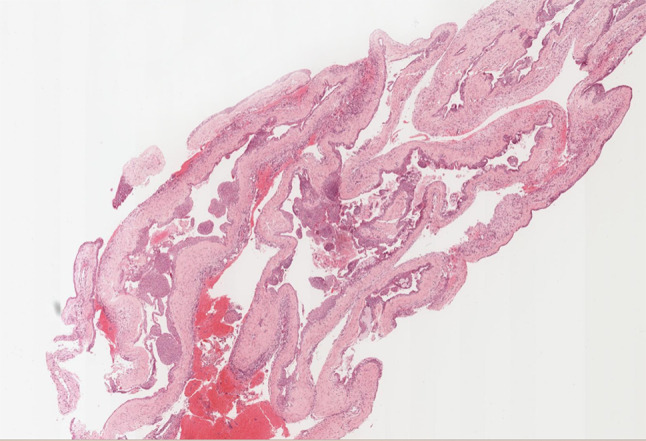
GOC Low power photomicrograph showing a GOC with multiple compartments, varying thickness of the epithelial lining, papillary ingrowth, and multiple spheres. H&E stain. Original magnification X 5
Fig. 4.
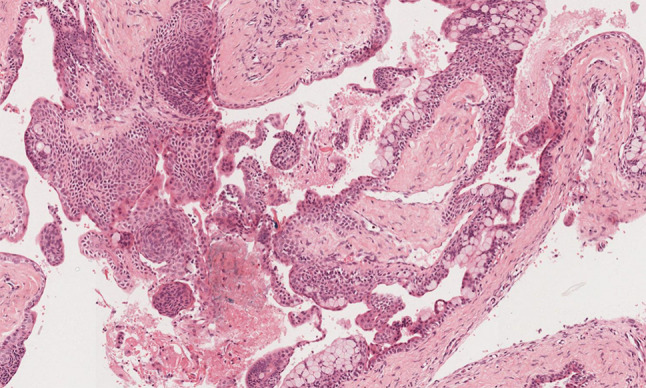
Midpower photomicrograph of GOC demonstrating papillary ingrowth, spheres, numerous mucous cells, and eosinophilic cells. H&E stain. Original magnification X 20
Fig. 5.
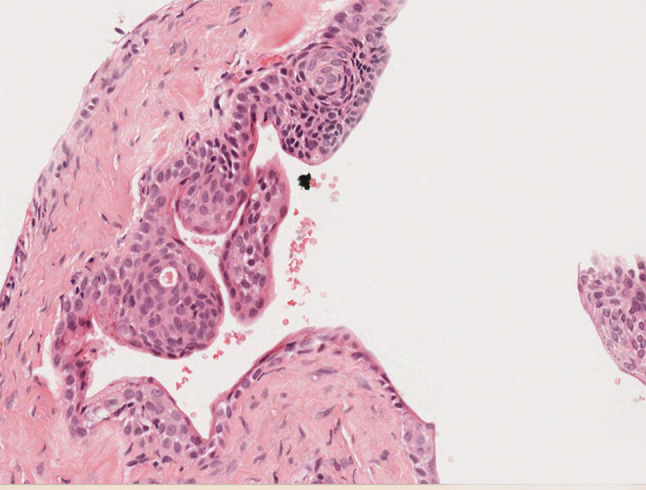
Higher power photomicrograph showing nodular spheres. H&E stain. Original magnification X 66
Fig. 6.

Higher power photomicrograph showing clear cells and surface hobnail cells with apocrine snouting. H&E stain. Original magnification X 132
Concerning tooth displacement at the initial diagnosis before recurrence, 27.8% [11, 22, 23, 26, 27] was found compared with 30.9% with the Chrcanovic general study [20]. Root resorption was reported in 22.2% of cases and were only found in the mandible [23–25, 27]. However, this data was not statistically significant for increased recurrence. According to Kaplan et al. in 2008 [3] the GOC has a high rate of cortical perforation with 61%; however, in our review exclusive for recurrence only one case in a male patient had cortical perforation reported by Kaplan in 2005 [11]. Additionally, in an extensive review by Chrcanovic in 2019, a low proportion of cases (26%) showed cortical perforation [20]. This suggests that cortical perforation is not a significant contributing factor for recurrence.
Histology
Histologic features of GOC can be seen in other pathologic lesions. The histologic differential diagnosis for GOC includes botryoid odontogenic cyst, a dentigerous cyst with mucous cell prosoplasia, nasopalatine duct cyst, and cystic mucoepidermoid carcinoma [48]. The botryoid odontogenic cyst does not characteristically have other features of GOC other than multiple compartments and nodular spheres [49]. Up to 10–23.8% of dentigerous cysts have mucous cells, but not other significant features of GOC and GOCs are not frequently pericoronal [50]. Nasopalatine duct cysts can have cilia and mucous cells in up to 24.4% of cases but typically not other features of GOC, and they occur only in the midline of the maxilla [51]. The most challenging differential is mucoepidermoid carcinoma (MEC) but rarely are MECs completely unicystic and they typically will not show the differentiation spectrum of GOCs [9, 10]. Most MECs are easily separated from GOC histologically. However, for some cases, demonstration of MAML2 can be a distinguishing feature [10].
The detailed histologic features of our recurrent GOCs are compared to those of GOCs in general in (Table 3). In general, the histologic features of recurrent GOCs paralleled those of Fowler and Chrcanovic [8, 20]. We did find epithelial spheres in only 44.8% of cases compared to 67.4% (Fowler) and 72.7% (Chrcanovic). But fewer epithelial spheres did not prove to be an independent predictor of recurrence. The presence of mucous cells, multiple compartments, and clear cells also did not show a higher risk for recurrence p ≥ 0.05. There were no histologic features that individually or in combination correlated with recurrence.
Table 3.
Comparison among the different histologic features of GOC, with previous studies
| Fowler et al. (2011) | Chrcanovic et al. (2018) | Peraza et al. (2023) |
|---|---|---|
| Parameter (n = 46) | Parameter (n = 161) cases | Parameter (n = 18) cases |
|
Eosinophilic cuboidal cells 100% (n = 46) |
Eosinophilic Cuboidal cells 95% (n = 153) |
Eosinophilic cuboidal cells 88.9% (n = 16) |
|
Intraepithelial microcysts 95.7%(n = 44) |
Intraepithelial microcysts 97.5% (n = 157) |
Intraepithelial microcysts 83.3% (n = 15) |
|
Apocrine snouting 91.3%(n = 42) |
Apocrine snouting 40.4% (n = 65) |
Apocrine snouting 33.3% (n = 6) |
|
Clear (vacuolated) cells 89.1%(n = 41) |
Clear cells 45.3% (n = 73) |
Clear cells 77.8% (n = 14) |
|
Variable thickness 89.1%(n = 41) |
Variable thickness 96.3% (n = 155) |
Variable thickness 83.3% (n = 15) |
|
Papillary projections 84.9%(n = 39) |
Papillary projections 77.6% (n = 125) |
Papillary projections 66.7% (n = 12) |
|
Mucous goblet cells 71.7%(n = 33) |
Mucous goblet cells 72% (n = 116) |
Mucous goblet cell 88.9% (n = 16) |
|
Epithelial spheres 67.4%(n = 31) |
Epithelial spheres 72.7% (n = 117) |
Epithelial spheres 44.4% (n = 8) |
|
Multiple compartments 63%(n = 29) |
Multiple compartment 70.2% (n = 113) |
Multiple compartment 77.8% (n = 14) |
|
Cilia 21.7%(n = 10) |
Cilia 61.5% (n = 99) |
Cilia 44.4% (n = 8) |
Previous criteria by Kaplan et al. in 2005 and 2008 [3, 11] mentioned 5 major diagnostic criteria for GOC and 4 minor but not obligatory criteria were required for the diagnosis. Fowler on the other hand reported 7 of ten criteria were more likely to definitively diagnosis GOC when compared to cases with 6 or less criteria. The recent WHO 2022 classification of odontogenic cysts did not require a specific number of criteria for the diagnosis but under essential criterion, there must be a lining epithelium with varying thickness, and hobnail cells [31, 32]. It is important to note that the most important differential diagnosis of GOC is intraosseous mucoepidermoid carcinoma, and MAML2 rearrangement was documented in approximately 75% of cases [10]. However, one GOC case was reported to have the MAML2 mutation [33]. In this regard, a study by Maruyama et al. found that CK13 was exclusively expressed in GOC lesions in comparison to central mucoepidermoid carcinoma [9], and on the contrary, the expression of epithelial membrane antigen and mammary serine protease inhibitor (MASPIN) can be helpful to distinguish intraosseous MEC, with almost no expression in GOC cases [40].
The recurrence rate reported by Fowler et al. was 50% with an average follow-up of almost 9 years [8]. Chrcanovic et al. reported a 21.6% recurrence rate for 33 cases in a 2-year follow-up of a total of 83 cases [20]. Kaplan et al. stated that recurrence occurred at a rate of 29.2%, with a mean of 2.9 years [11], and for this reason the recurrence data could be more accurate after at least 3 years of follow-up which would probably measure more accurately the recurrences. In this regard, from the total (100%) of recurrences in our study with an average of 8 months [26] and 240 months [28], we found a (61.1% n = 11) of recurrences during the first 39 months [11, 15, 17, 21, 24–27, 30, 32]. For this reason, long-term follow-up is advisable.
As it was documented in several studies, recurrence may be partly related to the thinness of the cyst wall that may detach or separate from the connective tissue, allowing microcysts and epithelial remains to stay [34, 35]. Many cysts are associated with tooth roots, and in an effort to save the teeth, surgery could leave cyst remnants that could lead to a higher risk for recurrence rate due to the incomplete removal of the lesion, similar to that of the odontogenic keratocyst behavior [36]. In our results, root resorption was not statistically significant for recurrence.
There is not an established treatment protocol for GOCs, where the types of treatment range from enucleation/curettage to block resection, with an almost null recurrence rate for resection, and with a conservative approach the relapses could surpass 50%. From our recurrence study, 8 cases were treated by enucleation only [17, 18, 21, 24, 25, 27, 30] and 13 cases were treated with enucleation and another adjunctive procedure, curettage, or peripheral ostectomy [11, 15, 17, 21–25, 27, 30, 32]. An additional two cases were treated with curettage only [13, 28] and one additional case was treated with curettage with peripheral ostectomy [26]. In this regard, the higher recurrence after treatment was for enucleation with (72.2% n = 13) and 16.7% (n = 3) in GOCs treated with curettage/enucleation and peripheral osteotomy. Regarding marsupialization, Kaplan in 2005 documented a single patient treated with marsupialization followed by enucleation when the cyst was close to vital structures, with a 6 years of follow-up without recurrence [11]. In 111 documented GOC cases in 2008 Kaplan et al. found that marsupialization was used in only 2.7% of the cases [3]. And in a well-documented GOC case by Cano et al. marsupialization was done and after 15-month surgery was performed without recurrence after a follow-up of 3 years [2].
Peripheral osteotomy after enucleation has been recommended as an adjunct approach broadly implemented in odontogenic keratocyst (OKC), using a rotary instrument to remove up to 5 mm of bone to ensure the elimination of any remaining epithelial lining, or remnants in the cyst wall [52]. However, according to Morgan et al. 11 patients with OKCs were treated with enucleation and peripheral ostectomy, with a recurrence rate of 18.18% [37], compared to the overall recurrence rate for OKC of 23.15% [38]. On the other hand, in an extensive study with 69 patients over a period of 13 years. Hresko et al. found a recurrence rate of 35% for primary ameloblastomas, without any statistically significant correlation between gender, age, type of ameloblastoma, location, radiologic, or histopathological features, but for incomplete surgical technique [39]. In this regard, in our study the type of surgery, with enucleation or combined with curettage, was statistically significant for the risk of recurrence p ≤ 0.05.
We suggest that GOCs treatment will need to involve adjunctive therapy in the first surgery after diagnosis, such as peripheral ostectomy with 5 mm with or without 5-FU, if the lesion is small. According to oral surgery techniques, if the bone cortex is compromised by GOCs, the treatment should be similar to the one for ameloblastoma with one centimeter margin. And with extra-osseous extension, surgery needs to be extended to the next biologic frontier, the resection should include the periosteal layer and if the periosteal layer is interrupted, then the covering muscle needs to be excised [41]. There is documentation of five GOC cases treated with Carnoy’s solution, before enucleation or curettage, by Bhat et al. [42], and three cases by Qin et al. 2004 [43]. However, the FDA banned the use of Carnoy’s in 2013 as it contained chloroform, known for carcinogenic properties [44]. A chloroform-free version can be used, but there is little evidence about the efficacy of this solution. Additionally, the 5-fluorouracil has been used in GOC as well, in a case report by Titinchi et al. who used it in the bony cavity after enucleation and peripheral ostectomy. This was subsequently removed 24 h post-operatively, with no recurrence after 14 months of follow-up [45]. Nonetheless, the study did not show a confirmation histology for GOC, and more time of follow-up is necessary. The 5-FU solution has shown less toxicity, in previous documented cases comparing both solutions in the management of OKC, where patients treated with Carnoy’s had an 18% of recurrence in a mean follow-up of 26.3 ± 1.8 months, and during that time no recurrences were observed in patients treated with 5-FU [46].
The basis of 5-FU is by inducing cytotoxicity promoting reduction of deoxythymidine monophosphate, which acts as an intracellular molecule for DNA repair and replication [47]. In this regard, a strong consideration should be given using 5-FU with more follow-up time. But with all surgically treated gnathic cysts/tumors, the published literature can only provide evidence for the minimal recurrence rate.
Limitation of the Study
During the review method, the histologic features of the first diagnosis of GOC, allowed comparison of recurrent GOCs to those reported in the literature whether they recurred or not. The histologic disparity with some features may be due to the number of cases examined that were exclusively based on recurrent lesions. We could hypothesize that the discrepancy between the previously reported histologic values with different features may be due to variations in the evaluations, and lack of information with the papers found. Besides the small number of well-documented cases, the Oxford system was used to reduce bias, additionally, the possibility that cases were missed cannot be completely excluded. Unpublished data were not identified even though we tried to contact some of the authors. A prospective controlled clinical trial would be more predictive of the recurrence, with emphases on histologic features as well as treatment, because the treatment of choice for GOCs are not definitive.
Conclusion
We were unable to find histologic features of a series of recurrent GOCs that singly, or in combination, were predictive of recurrence. Based on our study and review, it appears that conservative enucleation for GOC could be contraindicated as it is associated with recurrence rates as high as 50%. Although recurrence can be prevented with resection, because of its surgical morbidity, it appears the treatment of choice for GOCs is surgical removal followed by either 5-FU and/or peripheral ostectomy with about 5 mm of additional bone being removed from the lesion’s margins. Surgical resection should be reserved for larger and more anatomically complex cases.
Acknowledgements
Not applicable.
Author Contributions
APL Action performed Search and paper selection, Prospero writing, data analysis, Oxford codification, and discussion. AS Action performed paper selection, paper analyses, and excel writing. MNG Action performed discussion surgery writing part. NRG Action performed data analyses and statistic software analyses. MV Action performed excel data and Tables 1 and 2 and Fig. 2 design. MK Action performed Statistical software, comparing histopathologic features data. JPL Action performed references, Table 3, demographic, and Figure 1. JW Action performed writing results, Figs. 3–6, introduction, and discussion.
Funding
Not applicable.
Data Availability
Data analyses was performed with Microsoft Excel 2023 (Microsoft Corp, Redmond, Washington) and the Statistical Package for the Social Sciences (SPSS) software, version 20.0 Copyright IBM (SPSS Inc., Chicago, IL, USA). All information presented in the manuscript is available.
Code Availability
Statistical Package for the Social Sciences (SPSS) software, version 20.0 ©Copyright IBM (SPSS Inc., Chicago, IL, USA).
Declarations
Conflicts of interest
The authors declare no competing interests.
Ethical Approval
This study met criteria for nonhuman subject research and as a result board ethics approval was not required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gratzinger D, Salama ME, Poh CF, Rouse RV (2008) Ameloblastoma, calcifying epithelial odontogenic tumor, and glandular odontogenic cyst show a distinctive immunophenotype with some myoepithelial antigen expression. J Oral Pathol Med 37:177–184. 10.1111/j.1600-0714.2007.00613.x [DOI] [PubMed] [Google Scholar]
- 2.Cano J, Benito DM, Montáns J, Rodríguez-Vázquez JF, Campo J, Colmenero C (2012) Glandular odontogenic cyst: two high-risk cases treated with conservative approaches. J Craniomaxillofac Surg 40(5):e131–e136. 10.1016/j.jcms.2011.07.005 [DOI] [PubMed] [Google Scholar]
- 3.Kaplan I, Anavi Y, Hirshberg A (2008) Glandular odontogenic cyst: a challenge in diagnosis and treatment. Oral Dis 14(575):81 [DOI] [PubMed] [Google Scholar]
- 4.Kramer IR, Pindborg JJ, Shear M (1992) The WHO histological typing of odontogenic tumours. A commentary on the second edition. Cancer 70(12):2988–2994 [DOI] [PubMed] [Google Scholar]
- 5.Mervyn Shear PS (2007) Cysts of the oral and maxillofacial regions, 4th edn. Blackwell Publishing Ltd, Oxford [Google Scholar]
- 6.Macdonald-Jankowski DS (2010) Glandular odontogenic cyst: systematic review. Dentomaxillofac Radiol 39(3):127–139. 10.1259/dmfr/30943934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alaeddini M, Eshghyar N, Etemad-Moghadam S (2017) Expression of podoplanin and TGF-beta in glandular odontogenic cyst and its comparison with developmental and inflammatory odontogenic cystic lesions. J Oral Pathol Med 46:76–80 [DOI] [PubMed] [Google Scholar]
- 8.Fowler CB, Brannon RB, Kessler HP, Castle JT, Kahn MA (2011) Glandular odontogenic cyst: analysis of 46 cases with special emphasis on microscopic criteria for diagnosis. Head Neck Pathol 5(4):364–375. 10.1007/s12105-011-0298-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maruyama S, Mori T, Yamazaki M, Abé T, Ryo E, Kano H, Hasegawa G, Tanuma JI (2021) Central mucoepidermoid carcinoma arising directly from a glandular odontogenic cyst of the mandible: a case report. Diagn Pathol 16(1):61. 10.1186/s13000-021-01124-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bishop JA, Yonescu R, Batista D, Warnock GR, Westra WH (2014) Glandular odontogenic cysts (GOCs) lack MAML2 rearrangements: a finding to discredit the putative nature of GOC as a precursor to central mucoepidermoid carcinoma. Head Neck Pathol 8(3):287–290. 10.1007/s12105-014-0534-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaplan I, Gal G, Anavi Y, Manor R, Calderon S (2005) Glandular odontogenic cyst: treatment and recurrence. J Oral Maxillofac Surg 63(4):435–441. 10.1016/j.joms.2004.08.007 [DOI] [PubMed] [Google Scholar]
- 12.Gurler G, Al-Ghamian H, Aksakalli N, Delilbasi C (2017) Glandular odontogenic cyst: case series. Contemp Clin Dent 8(4):653–657. 10.4103/ccd.ccd_554_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hussain K, Edmondson HD, Browne RM (1995) Glandular odontogenic cysts. Diagnosis and treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 79(593):602 [DOI] [PubMed] [Google Scholar]
- 14.Kaplan I, Anavi Y, Manor R, Sulkes J, Calderon S (2005) The use of molecular markers as an aid in the diagnosis of glandular odontogenic cyst. Oral Oncol 41(895):902 [DOI] [PubMed] [Google Scholar]
- 15.Jafarian AH, Rahpeyma A, Khajehahmadi S (2015) Recurrent glandular odontogenic cyst of maxilla—a case report. Iran J Pathol 10(2):160–164 [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Savage NW, Joseph BK, Monsour PA, Young WG (1996) The glandular odontogenic jaw cyst: report of a case. Pathology 28(4):370–372. 10.1080/00313029600169384 [DOI] [PubMed] [Google Scholar]
- 18.Koppang HS, Johannessen S, Haugen LK, Haanaes HR, Solheim T, Donath K (1998) Glandular odontogenic cyst (sialo-odontogenic cyst): report of two cases and literature review of 45 previously reported cases. J Oral Pathol Med 27(9):455–462. 10.1111/j.1600-0714.1998.tb01984.x [DOI] [PubMed] [Google Scholar]
- 19.Faisal M, Ahmad SA, Ansari U (2015) Glandular odontogenic cyst—literature review and report of a paediatric case. J Oral Biol Craniofac Res 5(3):219–225. 10.1016/j.jobcr.2015.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chrcanovic BR, Gomez RS (2018) Glandular odontogenic cyst: an updated analysis of 169 cases reported in the literature. Oral Dis 24(5):717–724. 10.1111/odi.12719 [DOI] [PubMed] [Google Scholar]
- 21.Gardner DG, Kessler HP, Morency R, Schaffner DL (1988) The glandular odontogenic cyst: an apparent entity. J Oral Pathol 17(8):359–366. 10.1111/j.1600-0714.1988.tb01298.x [DOI] [PubMed] [Google Scholar]
- 22.Thor A, Warfvinge G, Fernandes R (2006) The course of a long-standing glandular odontogenic cyst: marginal resection and reconstruction with particulated bone graft, platelet-rich plasma, and additional vertical alveolar distraction. J Oral Maxillofac Surg 64(7):1121–1128. 10.1016/j.joms.2006.03.042 [DOI] [PubMed] [Google Scholar]
- 23.Turali S, Yazıcıoğlu D, Ergül KC, Telcioğlu NT, Karasu HA (2012) Recurrent glandular odontogenic cyst treatment. Kulak Burun Bogaz Ihtis Derg 22(3):176–180. 10.5606/kbbihtisas.2012.033 [DOI] [PubMed] [Google Scholar]
- 24.Motooka N, Ohba S, Uehara M, Fujita S, Asahina I (2015) A case of glandular odontogenic cyst in the mandible treated with the dredging method. Odontology 103(1):112–115. 10.1007/s10266-013-0143-0 [DOI] [PubMed] [Google Scholar]
- 25.Martins-Chaves RR, Granucci M, Gomez RS, Henriques de Castro W (2021) Glandular odontogenic cyst—a case series. J Oral Maxillofac Surg 79(5):1062–1068. 10.1016/j.joms.2020.10.030 [DOI] [PubMed] [Google Scholar]
- 26.de Campos WG, Araújo R, Martin V, Trierveiler M, Gomes P, Lemos CA (2023) Glandular odontogenic cyst in the anterior mandible: a case report of a conservative approach and a recurrence detection. Diagnostics 13(8):1452. 10.3390/diagnostics13081452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magnusson B, Göransson L, Odesjö B, Gröndahl K, Hirsch JM (1997) Glandular odontogenic cyst. Report on seven cases. Dentomaxillofac Radiol 26(1):26–31. 10.1038/sj.dmfr.4600205 [DOI] [PubMed] [Google Scholar]
- 28.de Freitas Silva BS, Yamamoto-Silva FP, Sena-Filho M, Silva Sant’Ana SS, Mariano-Júnior WJ, de Almeida OP, Estrela C (2017) 20-year follow-up of recurrent glandular odontogenic cyst mimicking a periapical lesion. J Endod 43(11):1915–1920. 10.1016/j.joen.2017.06.015 [DOI] [PubMed] [Google Scholar]
- 29.Momeni Roochi M, Tavakoli I, Ghazi FM, Tavakoli A (2015) Case series and review of glandular odontogenic cyst with emphasis on treatment modalities. J Craniomaxillofac Surg 43(6):746–750. 10.1016/j.jcms.2015.03.030 [DOI] [PubMed] [Google Scholar]
- 30.Oliveira JX, Santos KC, Nunes FD, Hiraki KR, Sales MA, Cavalcanti MG, Marcucci M (2009) Odontogenic glandular cyst: a case report. J Oral Sci 51(3):467–470. 10.2334/josnusd.51.467 [DOI] [PubMed] [Google Scholar]
- 31.El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ (2017) WHO classification of head and neck tumours. International Agency for Research on Cancer, Lyon [Google Scholar]
- 32.Soluk-Tekkesin M, Wright JM (2022) The World Health Organization classification of odontogenic lesions: a summary of the changes of the 2022 (5th) Edition. Turk Patoloji Derg 38(2):168–184. 10.5146/tjpath.2022.01573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greer RO, Eskendri J, Freedman P, Ahmadian M, Murakami-Walter A, Varella-Garcia M (2018) Assessment of biologically aggressive, recurrent glandular odontogenic cysts for mastermind-like 2 (MAML2) rearrangements: histopathologic and fluorescent in situ hybridization (FISH) findings in 11 cases. J Oral Pathol Med 47(2):192. 10.1111/jop.12658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osny FJ, Azevedo LR, Sant’Ana E, Lara VS (2004) Glandular odontogenic cyst: case report and review of the literature. Quintessence Int 35(5):385–389 [PubMed] [Google Scholar]
- 35.Krishnamurthy A, Sherlin HJ, Ramalingam K, Natesan A, Premkumar P, Ramani P, Chandrasekar T (2009) Glandular odontogenic cyst: report of two cases and review of literature. Head Neck Pathol 3:153–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naruse T, Yamashita K, Yanamoto S, Rokutanda S, Matsushita Y, Sakamoto Y, Sakamoto H, Ikeda H, Ikeda T, Asahina I, Umeda M (2017) Histopathological and immunohistochemical study in keratocystic odontogenic tumors: predictive factors of recurrence. Oncol Lett 13(5):3487–3493. 10.3892/ol.2017.5905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morgan TA, Burton CC, Qian F (2005) A retrospective review of treatment of the odontogenic keratocyst. J Oral Maxillofac Surg 63:635–639 [DOI] [PubMed] [Google Scholar]
- 38.Kaczmarzyk T, Mojsa I, Stypulkowska J (2012) A systematic review of the recurrence rate for keratocystic odontogenic tumour in relation to treatment modalities. Int J Oral Maxillofac Surg 41(6):756–767. 10.1016/j.ijom.2012.02.008 [DOI] [PubMed] [Google Scholar]
- 39.Hresko A, Palyvoda R, Burtyn O, Chepurnyi Y, Kopchak A, Helder M, Forouzanfar T (2022) Recurrent ameloblastoma: clinical manifestation and disease-free survival rate. J Oncol 2022:2148086. 10.1155/2022/2148086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vered M, Allon I, Buchner A, Dayan D (2010) Is maspin immunolocalization a tool to differentiate central low-grade mucoepidermoid carcinoma from glandular odontogenic cyst? Acta Histochem 112(2):161–168. 10.1016/j.acthis.2008.10.006 [DOI] [PubMed] [Google Scholar]
- 41.Hendra FN, Helder MN, Ruslin M, Van Cann EM, Forouzanfar T (2023) A network meta-analysis assessing the effectiveness of various radical and conservative surgical approaches regarding recurrence in treating solid/multicystic ameloblastomas. Sci Rep 13(1):8445. 10.1038/s41598-023-32190-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhat KK, Shetty P, Adyanthaya A, Adyanthaya S (2014) The glandular odontogenic cyst of the anterior maxilla: report of two cases. Malays Dent J 2014(36):15–19 [Google Scholar]
- 43.Qin XN, Li JR, Chen XM, Long X (2005) The glandular odontogenic cyst: clinicopathologic features and treatment of 14 cases. J Oral Maxillofac Surg 63(5):694–699. 10.1016/j.joms.2004.12.016 [DOI] [PubMed] [Google Scholar]
- 44.US Food and Drug Administration (1992) FDA compliance policy guides: section 460.200. Food and Drug Administration, Washington, DC. p 219
- 45.Titinchi F, Thompson J, Ranchod S (2023) Application of 5-fluorouracil in management of glandular odontogenic cyst. J Clin Exp Dent 15(6):e511–e513. 10.4317/jced.60092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ledderhof NJ, Caminiti MF, Bradley G, Lam DK (2017) Topical 5-fluorouracil is a novel targeted therapy for the keratocystic odontogenic tumor. J Oral Maxillofac Surg 75(3):514–524. 10.1016/j.joms.2016.09.039 [DOI] [PubMed] [Google Scholar]
- 47.Zhang N, Yin Y, Xu S, Chen W (2008) 5-Fluorouracil: mechanisms of resistance and reversal strategies. Molecules 13:1551–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chavez JA, Richter KJ (1999) Glandular odontogenic cyst of the mandible. J Oral Maxillofac Surg 57(4):461–464. 10.1016/s0278-2391(99)90291-4 [DOI] [PubMed] [Google Scholar]
- 49.Farina VH, Brandão AA, Almeida JD, Cabral LA (2010) Clinical and histologic features of botryoid odontogenic cyst: a case report. J Med Case Rep 4:260. 10.1186/1752-1947-4-260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takeda Y, Oikawa Y, Furuya I, Satoh M, Yamamoto H (2005) Mucous and ciliated cell metaplasia in epithelial lining of odontogenic inflammatory and developmental cysts. J Oral Sci 47:77–81 [DOI] [PubMed] [Google Scholar]
- 51.Cavalcante IL, Barros CC, Cunha JL, Cruz VM, Pedrosa GA, Santos AD et al (2021) Clinicopathologic features of nasopalatine duct cysts: a retrospective study in two Brazilian oral and maxillofacial pathology referral centers. Med Oral Patol Oral Cir Bucal 26(5):e676–e683. 10.4317/medoral.24718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scharffetter K, Balz-Herrmann C, Lagrange W, Koberg W, Mittermayer C (1989) Proliferation kinetics-study of the growth of keratocysts. Morpho-functional explanation for recurrences. J Craniomaxillofac Surg 17(5):226–233. 10.1016/s1010-5182(89)80074-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data analyses was performed with Microsoft Excel 2023 (Microsoft Corp, Redmond, Washington) and the Statistical Package for the Social Sciences (SPSS) software, version 20.0 Copyright IBM (SPSS Inc., Chicago, IL, USA). All information presented in the manuscript is available.
Statistical Package for the Social Sciences (SPSS) software, version 20.0 ©Copyright IBM (SPSS Inc., Chicago, IL, USA).