Abstract
Demons-Meigs syndrome is a very rare entity. It combines a benign ovarian “fibroma-like” tumor with ascites and hydrothorax. The notion of benignancy is the key point. CA-125 levels are most of the time normal, but high levels can be observed in rare cases which makes it difficult to have a diagnostic. We present here the case of a 43-year-old female patient who presented with abdominopelvic pain. Imaging discovered a 30 cm large intraabdominal mass with ascites and bilateral pleural effusion. Surgical resection of the tumor was performed, and pathology identified an ovarian fibroma. No postintervention complications were observed, with resorption of the ascites and hydrothorax.
Keywords: Demons-Meigs, Fibroma, CA-125, HE4, ROMA
Introduction
Demons-Meigs syndrome is a rare medical condition characterized by the triad of ovarian fibroma, ascites, and pleural effusion. Typically observed in women of reproductive age, Demons-Meigs syndrome often presents with symptoms such as abdominal distension, difficulty breathing, and discomfort. It's important for clinicians to differentiate Demons-Meigs syndrome from other conditions with similar presentations, such as ovarian malignancies. Staying vigilant for this rare syndrome in the clinical setting can contribute to timely and effective patient care.
Case report
A 43-year-old woman, gravida 4, para 4, consulted the Department of Obstetrics and Gynecology due to persistent abdominopelvic pain that had started 6 months prior. Her medical history did not reveal any existing medical conditions or previous surgical procedures. She denied experiencing chest pain, shortness of breath, or any deterioration in her general condition.
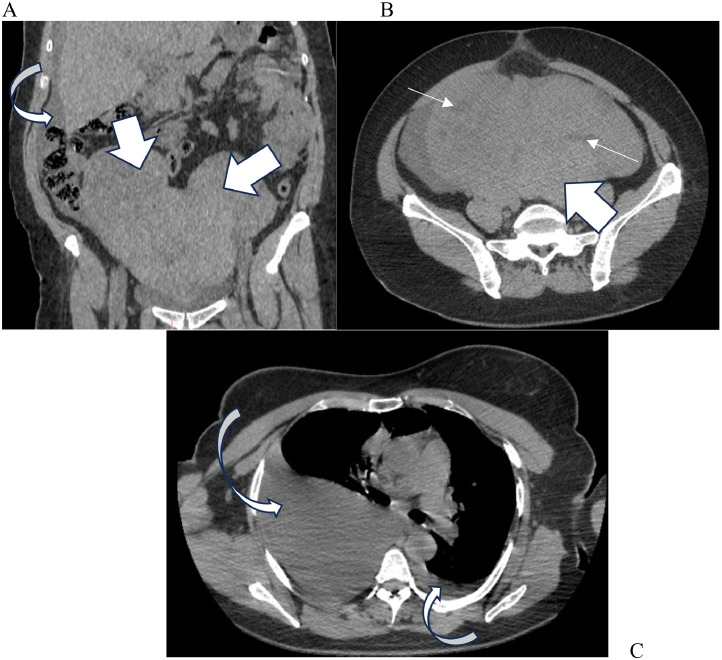
Upon examination, the patient was afebrile and stable at both hemodynamic and respiratory levels. The abdomen was distended and tender to palpation, revealing a mass reaching the level of the xiphoid appendix. A pleural effusion syndrome was observed on the right side. Computed tomography (CT) of the thorax, abdomen, and pelvis revealed a pelviabdominal mass measuring 30 × 26 × 13 cm, which appeared polylobed and was challenging to distinguish in terms of origin. Additionally, ascites and more severe bilateral pleurisy on the right side were evident (Figs. 1A-C).
Fig. 1.
(A) CT abdomen and pelvis identified a polylobed 30 × 26 × 13 cm pelvic mass (thick arrows) with ascites (bended arrows). (B) The mass is enhanced heterogeneously after contrast injection, and we note the presence of cystic components (thin arrows). (C) CT thorax demonstrated bilateral pleural effusion associated (bended arrows).
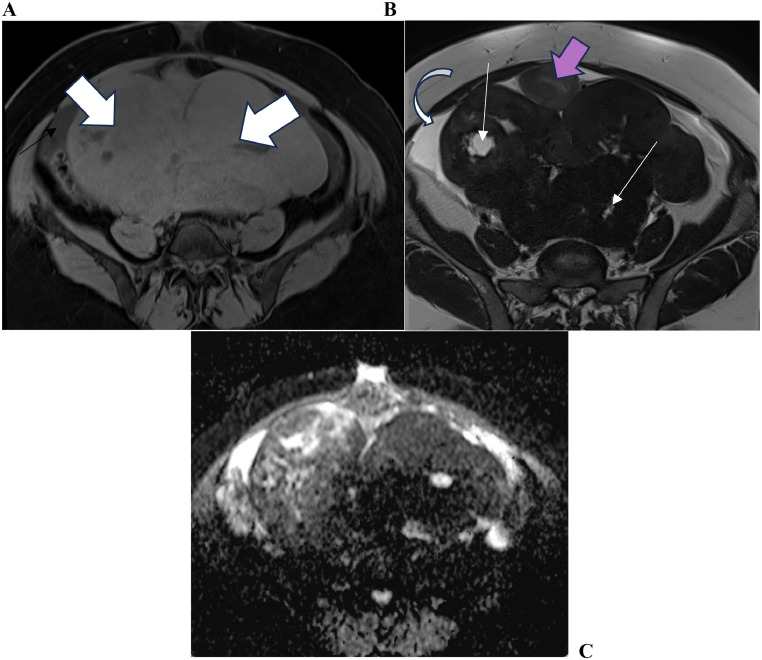
Subsequently, a pelvic MRI was prescribed as a complementary diagnostic tool to determine the origin of the masses. The MRI confirmed the presence of the pelvic mass, which seemed to originate from the left ovary. It exhibited areas of necrosis, exerting pressure on the uterus and bladder, causing them to descend (Figs. 2A-C).
Fig. 2.
(A) MRI T1 weighted image showing the well-limited mass (thick arrows), heterogeneously enhanced, with pelvic effusion (bended arrow). (B) T2 weighted image shows the mass in hyposignal with necrosis areas in hypersignal (thin arrows), pushing the uterus (pink arrow). (C) Diffusion weighted image showing a restriction of diffusion.
The patient had an elevated CA-125 level of 775 U/mL (0-35 U/mL) and a normal level of HE4 of 22,1 pmol/L (0-150 pmol/L). ROMA score was calculated at 1,45%, which indicates low risk of malignancy.
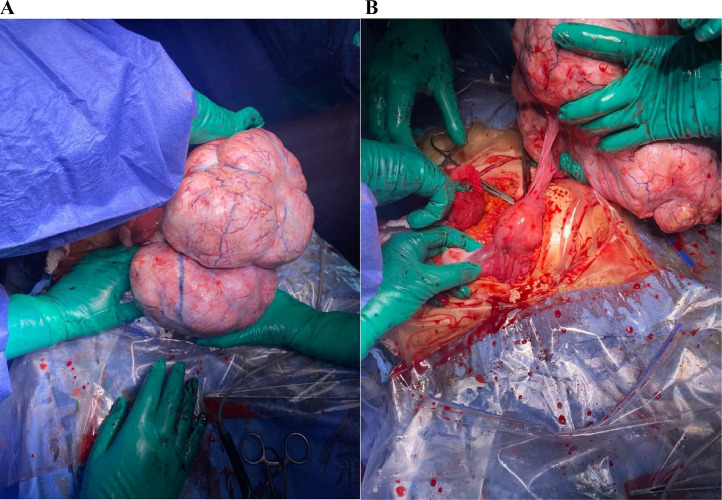
The patient underwent an exploratory laparotomy and a left adnexectomy in the operating room. A total of 750 mL of ascites were aspirated and sent for pathological examination. The surgical exploration revealed 3 uterine myomas and 1 giant, polylobed, and well-vascularized mass in the left ovary (Figs. 3A and B).
Fig. 3.
(A) Intraoperative view showing the giant pelvic mass and its polylobed aspect. (B) The mass originates from the left ovary.
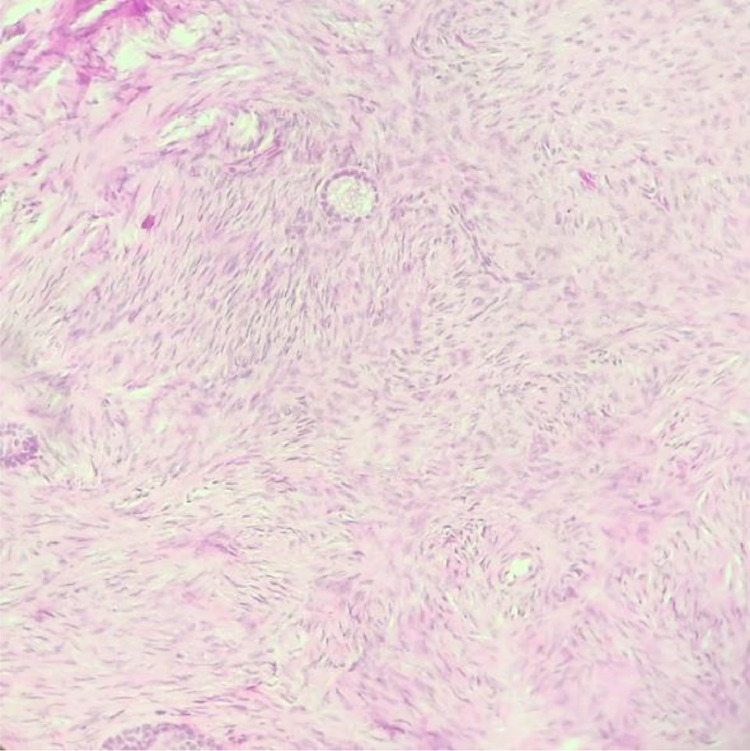
The tumor was completely resected and sent for pathology, along with a total left adnexectomy. No other intrabdominal lesions were identified, and the abdomen was closed. The patient did not experience any complications. The pathology report described a benign ovarian fibroma (Fig. 4), and no malignant cells were detected in the ascitic fluid.
Fig. 4.
Hematoxylin and eosin staining (x200): ovarian fibroma composed of cells showing spindled nuclei and scant eosinophilic.
Discussion
Ovarian fibromas and fibrothecomas are a rare entity, representing only 3.3% of ovarian tumors [1]. They typically occur in perimenopausal and menopausal patients, as seen in our report. While these tumors rarely occur before the age of 20, a few cases have been reported in the literature [2].
The clinical presentation is nonspecific and generally correlates with the mass's size, mainly manifesting as pelvic pain and abdominal distension, sometimes associated with metrorrhagias. In some exceptionally rare cases, fibrothecomas may lead to endocrine manifestations due to theca cells producing estrogens or androgens [3]. In our case, neither metrorrhagias nor endocrine manifestations were reported.
Imaging plays a crucial role in diagnosis, revealing the characteristic association of a pelvic mass with pleural and peritoneal effusion. MRI remains the preferred imaging modality for documenting pelvic pathologies. However, anatomopathology remains relevant to differentiate between Demons-Meigs and pseudo-Demons-Meigs syndromes.
The presence of an ovarian mass, with or without a decline in the general condition, naturally raises concerns about a malignant tumor, especially when CA-125 levels are elevated [4]. However, it is essential to consider the possibility of a benign etiology. Firstly, because the clinical presentation can mimic an advanced cancer state, with observed dyspnea caused by pleural effusion or potential abundant ascites. Without treatment, this can lead to the development of a cachectic state. Secondly, CA-125 lacks specificity, as elevated levels can occur in conditions such as endometriosis, menstruation, pregnancy, and benign ovarian, or uterine fibromas [5]. Higher CA-125 levels can also be associated with serious physiological disorders like heart failure, cirrhosis, coronary artery disease, and nonovarian malignancies, particularly lung and breast cancer [6].
For increased accuracy, human epididymis 4 (HE4) can be measured. It is a relatively new but promising biomarker used in diagnosing ovarian cancer, demonstrating greater sensitivity and specificity than CA-125 [7], [8], [9]. In our case, there was a contradiction between the 2 markers, with CA-125 levels suggesting malignancy, while HE4 results were reassuring. However, combining both markers is considered an efficient diagnostic tool, increasing their specificity [10]. These values are also used in the risk of ovarian malignancy algorithm (ROMA), developed by Moore in 2009 [10]. According to the meta-analysis conducted by Kaijser [11], ROMA has a sensitivity between 76% and 86% and a specificity between 74% and 95%. The cutoff level to classify patients in a high-risk group is 11.4% for premenopausal patients and 29.9% for menopausal patients. In our case, the score calculated was 1.45%, strongly indicating the benign nature of the mass.
The treatment for Demons-Meigs syndrome involves the surgical removal of the tumor. Our patient underwent a left adnexectomy, and no complications were observed. As Demons first stated, tumor removal is the most effective treatment for ascites and pleural effusion.
Differential diagnoses usually involve distinguishing malignant pelvic masses. In such cases, the clinical presentation may seem similar, but ascites and pleural effusion result from the tumor's extension.
Teaching point
Demons-Meigs syndrome is a well-known entity but remains rare. When a pelvic mass is associated with pleural and/or peritoneal effusion, the possibility of benignancy should be considered despite the alarming clinical presentation.
Patient consent
The written consent of the patient was obtained before submission.
CRediT authorship contribution statement
Each author contributed to imaging interpretation, surgery or pathology study of the ovarian mass.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Chechia A, Attia L, Temime RB, Makhlouf T, Koubaa A. Incidence, clinical analysis, and management of ovarian fibromas and fibrothecomas. Am J Obstet Gynecol. 2008;199(5):473.e1–473.e4. doi: 10.1016/j.ajog.2008.03.053. [DOI] [PubMed] [Google Scholar]
- 2.Laufer L, Barki Y, Mordechai Y, Maor E, Mares A. Ovarian fibroma in a prepubertal girl. Pediatr Radiol. 1996;26(1):40–42. doi: 10.1007/BF01403703. [DOI] [PubMed] [Google Scholar]
- 3.Siekierska-Hellmann M, Sworczak K, Babińska A, Wojtylak S. Ovarian thecoma with androgenic manifestations in a postmenopausal woman. Gynecol Endocrinol. 2006;22(7):405–408. doi: 10.1080/09513590600842539. [DOI] [PubMed] [Google Scholar]
- 4.Zhang M, Cheng S, Jin Y, Zhao Y, Wang Y. Roles of CA125 in diagnosis, prediction, and oncogenesis of ovarian cancer. Biochim Biophys Acta (BBA) 2021;1875(2) doi: 10.1016/j.bbcan.2021.188503. [DOI] [PubMed] [Google Scholar]
- 5.Yip HK, Huang LW, Lin YH, Hwang JL. Massive ascites caused by a large pedunculated subserosal uterine leiomyoma that has feeding arteries from peripheral tissues and exhibits elevated CA125: a case report of atypical Pseudo-Meigs’ syndrome. J Obstet Gynaecol. 2014;34(1):107–108. doi: 10.3109/01443615.2013.832736. [DOI] [PubMed] [Google Scholar]
- 6.Charkhchi P, Cybulski C, Gronwald J, Wong FO, Narod SA, Akbari MR. CA125 and ovarian cancer: a comprehensive review. Cancers. 2020;12(12):3730. doi: 10.3390/cancers12123730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore RG, McMeekin DS, Brown AK, DiSilvestro P, Miller MC, Allard WJ, et al. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol Oncol. 2009;112(1):40–46. doi: 10.1016/j.ygyno.2008.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scaletta G, Plotti F, Luvero D, Capriglione S, Montera R, Miranda A, et al. The role of novel biomarker HE4 in the diagnosis, prognosis and follow-up of ovarian cancer: a systematic review. Expert Rev Anticancer Ther. 2017;17(9):827–839. doi: 10.1080/14737140.2017.1360138. [DOI] [PubMed] [Google Scholar]
- 9.Urban N, McIntosh MW, Andersen M (Robyn), Karlan BY. Ovarian cancer screening. Hematol Oncol Clin North Am. 2003;17(4):989–1005. doi: 10.1016/s0889-8588(03)00063-7. [DOI] [PubMed] [Google Scholar]
- 10.Moore RG, Brown AK, Miller MC, Skates S, Allard WJ, Verch T, et al. The use of multiple novel tumor biomarkers for the detection of ovarian carcinoma in patients with a pelvic mass. Gynecol Oncol. 2008;108(2):402–408. doi: 10.1016/j.ygyno.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 11.Kaijser J, Belle VV, Gorp TV, Sayasneh A, Vergote I, Bourne T, et al. Prognostic value of serum HE4 levels and risk of ovarian malignancy algorithm scores at the time of ovarian cancer diagnosis. Int J Gynecol Cancer. 2014;24(7):1173–1180. doi: 10.1097/IGC.0000000000000181. [DOI] [PubMed] [Google Scholar]