Abstract
Rationale & Objective
Because of coronavirus disease 2019 (COVID-19), the US government issued emergency waivers in March 2020 that removed regulatory barriers around the use of telemedicine. For the first time, nephrologists were reimbursed for telemedicine care delivered during in-center hemodialysis. We examined the use of telemedicine for in-center hemodialysis during the first 16 months of the pandemic.
Study Design
We ascertained telemedicine modifiers on nephrologist claims. We used multivariable regression to examine time trends and patient, dialysis facility, and geographic correlates of telemedicine use. We also examined whether the estimated effects of predictors of telemedicine use changed over time.
Setting & Participants
US Medicare beneficiaries receiving in-center hemodialysis between March 1, 2020, and June 30, 2021.
Exposures
Patient, geographic, and dialysis facility characteristics.
Outcomes
The use of telehealth for in-center hemodialysis care.
Analytic Approach
Retrospective cohort analysis.
Results
Among 267,434 Medicare beneficiaries identified, the reported use of telemedicine peaked at 9% of patient-months in April 2020 and declined to 2% of patient-months by June 2021. Telemedicine use varied geographically and was more common in areas that were remote and socioeconomically disadvantaged. Patients were more likely to receive care by telemedicine in areas with higher incidence of COVID-19, although the predictive value of COVID-19 diminished later in the pandemic. Patients were more likely to receive care using telemedicine if they were at facilities with more staff, and the use of telemedicine varied by facility ownership type.
Limitations
Limited reporting of telemedicine on claims could lead to underestimation of its use. Reported telemedicine use was higher in an analysis designed to address this limitation by focusing on patients whose physicians used telemedicine at least once during the pandemic.
Conclusions
Some US nephrologists continued to use telemedicine for in-center hemodialysis throughout the pandemic, even as the association between COVID-19 incidence and telemedicine use diminished over time. These findings highlight unique challenges and opportunities to the future use of telemedicine in dialysis care.
Index Words: COVID-19 pandemic, hemodialysis, nephrologist, telemedicine, variation
Plain-Language Summary
Emergency waivers issued during the coronavirus disease 2019 pandemic enabled reimbursement to US nephrologists for telemedicine care delivered during in-center hemodialysis. Using modifiers from Medicare claims, we examined telemedicine use in the first 16 months of the pandemic. Reported telemedicine use peaked early in the pandemic and declined subsequently. Telemedicine use was more common in areas that were remote and socioeconomically disadvantaged and at facilities with more staff. Telemedicine use also varied by facility ownership type. Some nephrologists continued to use telemedicine for in-center hemodialysis throughout the pandemic, even as the association between coronavirus disease 2019 incidence and telemedicine use diminished over time. These findings highlight unique challenges and opportunities to the future use of telemedicine in dialysis care.
To maintain access to care while preventing the spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the Centers for Medicare and Medicaid Services (CMS) issued a series of emergency waivers in March 2020 that removed regulatory barriers to the use of telemedicine in the United States. For the 480,000 US patients receiving in-center hemodialysis, these waivers enabled their kidney care provider (nephrologist or affiliated advanced practice provider, hereafter referred to as “nephrologist”) to substitute face-to-face in-center hemodialysis visits with telemedicine encounters.1, 2, 3, 4 Nephrologists soon began conducting some of their in-center dialysis care by telemedicine, in which dialysis facility personnel relay a telemedicine device, such as a tablet or smart phone, to patients who then communicate with their nephrologist as they receive dialysis.5,6 This was the first time that telemedicine was widely used by nephrologists for in-center hemodialysis care.
Before the coronavirus disease 2019 (COVID-19) pandemic, interest in improving access to care and patient convenience fueled initiatives to promote the adoption of telemedicine in a variety of health care settings, including pilot interventions in chronic kidney disease and legislation permitting the use of telemedicine for home dialysis.7, 8, 9, 10, 11, 12, 13, 14 Concerns about infection risk during the COVID-19 pandemic created new incentives to use telemedicine. In hemodialysis, nephrologists could reduce the risk of contracting SARS-CoV-2 and spreading the virus to their patients by replacing face-to-face dialysis visits with telemedicine. Unlike the treatment of other chronic diseases, in which face-to-face outpatient visits occur in an office setting and in which patients incur the cost of travel to see their physician, nephrologists typically conduct in-person visits to patients receiving dialysis while patients are at their dialysis center receiving their treatment. These differences create a unique array of incentives and barriers to replacing in-person visits with telemedicine for in-center hemodialysis.
When telemedicine waivers were issued at the start of the COVID-19 pandemic, CMS instructed nephrologists to report the use of telemedicine on monthly dialysis billing claims. These instructions were disseminated widely to the nephrology community by professional societies.15 In this study, we use national Medicare claims data to examine patterns of reported telemedicine use for in-center hemodialysis care, including correlates of its use and how the use of telemedicine changed over time throughout the pandemic.
Methods
Study Population and Data Sources
In a retrospective cohort study, we used national Medicare claims data to identify all US patients receiving hemodialysis during the first 16 months of the COVID-19 pandemic from March 1, 2020, through June 30, 2021. We divided the 16-month period into 4 consecutive cohorts, in which each cohort included 4 months of data. Cohorts started on the following index dates: March 1, 2020; July 1, 2020; November 1, 2020; and March 1, 2021. We included adult in-center hemodialysis patients on each index date who had Medicare coverage and received dialysis for at least 6 months previously. We used 6 months of prior Medicare claims (inpatient hospitalization, outpatient institutional, and carrier) to identify patient comorbid conditions. We excluded patients with a kidney transplant in the 6 months before the index date.
We derived information about the use of telemedicine in each month from Medicare claims. We ascertained patient demographic information and Medicaid eligibility status from Medicare enrollment data. We used Dialysis Facility Reports to obtain facility-specific data at the start of each cohort.16 We used data from the 2020 US Census to obtain neighborhood-level socioeconomic data and population density in the area surrounding each dialysis facility.
We obtained information about the incidence (ie, case count) of COVID-19 in each county and month throughout the study from the USAFacts database.17 We selected this database because it was both comprehensive and involved efforts to clean and reconcile discrepancies in primary data.18 The USAFacts database has been used by other researchers to study COVID-19.19,20
Cohort Selection
We began our cohort selection by identifying all patients with an outpatient hemodialysis Medicare claim during the 16-month study period. We required that hemodialysis claims had a diagnosis of end-stage kidney failure and excluded claims with diagnoses of acute kidney injury. For each patient receiving dialysis during the study period, we created episodes of dialysis care from a set of consecutive dialysis claims. Dialysis care episodes started with the first outpatient hemodialysis claim and continued until loss of Medicare Parts A or B coverage or 60 days without a follow-up hemodialysis claim.
At each cohort index date, we identified all patients receiving in-center hemodialysis and followed these patients for the duration of the 4-month cohort period. We divided their dialysis episodes into calendar month, and only included the calendar months when a dialysis episode spanned the entire month and when the patients received at least one nephrologist visit based on a monthly capitation payment (MCP) claim in the month. We excluded months when patients received home dialysis for all or part of the month. Patients could appear in multiple months within a cohort and in multiple cohorts if they continued to receive in-center hemodialysis.
Outcomes
The primary study outcome was whether a patient was seen by their nephrologist during dialysis by telemedicine. Information about the use of telemedicine came from MCP claims, which nephrologists must submit each month to be reimbursed for dialysis care. Specifically, in March 2020, the CMS began requiring that nephrologists include a telemedicine modifier code in months when at least 1 visit was conducted by telemedicine. We collected data about telemedicine use for each patient-month included in the analysis.
Exposures and Model Covariates
We examined patient, dialysis facility, and geographic predictors of telemedicine use listed in Table 1.21 In the primary analysis, we summarized patient comorbid conditions using an Elixhauser mortality index (Item S1).22, 23, 24 Recognizing limitations in predicting illness acuity among patients with kidney failure from the Elixhauser Index, we conducted an additional analysis in which we examined each comorbid condition as an individual independent variable.25 For each census block, we used a previously published algorithm to calculate the Area Deprivation Index (ADI), which represents a measure of socioeconomic disadvantage in a geographic area.26,27 Quartiles of ADI scores and population density in the area of facilities were calculated using all of the 2020 census tracks in the contiguous 48 states. When examining dialysis facility ownership, we categorized facilities as nonprofit organization, for-profit independently owned, and owned by a large dialysis organization (LDO).
Table 1.
Baseline Characteristics by Cohort
| Baseline Characteristics | Cohort 1-4a (N = 267,434) | Cohort 1 (n = 217,813) | Cohort 2 (n = 213,620) | Cohort 3 (n = 210,743) | Cohort 4 (n = 170,457) |
|---|---|---|---|---|---|
| Patient factors | |||||
| Age (y) | |||||
| Mean (SD) | 63.9 (14.0) | 64.0 (13.9) | 64.0 (13.9) | 64.0 (13.9) | 64.5 (14.1) |
| Median (IQR) | 65.1 (54.8-73.8) | 65.1 (55.0-73.8) | 65.1 (55.0-73.8) | 65.2 (55.0-73.8) | 66.0 (55.4-74.6) |
| Female (n, %) | 116,273 (43.5) | 95,369 (43.8) | 93,428 (43.7) | 91,840 (43.6) | 74,483 (43.7) |
| Raceb (n, %) | |||||
| Non-Hispanic White | 97,034 (36.3) | 75,865 (34.8) | 74,775 (35.0) | 73,609 (34.9) | 63,292 (37.1) |
| African American | 99,172 (37.1) | 83,809 (38.5) | 81,625 (38.2) | 80,394 (38.1) | 61,096 (35.9) |
| Hispanic | 47,405 (17.7) | 39,060 (17.9) | 38,318 (17.9) | 37,827 (18.0) | 29,789 (17.5) |
| Asian or Pacific Islander | 12,494 (4.7) | 10,152 (4.7) | 10,012 (4.7) | 10,029 (4.8) | 8,577 (5.0) |
| North American Native | 4,711 (1.8) | 3,997 (1.8) | 3,834 (1.8) | 3,670 (1.7) | 3,130 (1.8) |
| Other | 6,618 (2.5) | 4,930 (2.3) | 5,056 (2.4) | 5,214 (2.5) | 4,573 (2.7) |
| Preindex home dialysis (n, %) | 1,534 (0.6) | 966 (0.4) | 797 (0.4) | 776 (0.4) | 749 (0.4) |
| Preindex Medicaid (n, %) | 141,757 (53.0) | 117,706 (54.0) | 114,294 (53.5) | 112,688 (53.5) | 89,445 (52.5) |
| Elixhauser mortality indexc | |||||
| Mean (SD) | 14.2 (15.6) | 14.6 (15.8) | 11.8 (13.5) | 12.2 (13.9) | 12.4 (14.0) |
| Median (IQR) | 8.0 (5.0-21.0) | 8.0 (5.0-22.0) | 6.0 (5.0-19.0) | 6.0 (5.0-19.0) | 6.0 (5.0-19.0) |
| Dementia (n, %) | 12,459 (4.7) | 10,732 (4.9) | 7,529 (3.5) | 7,648 (3.6) | 6,692 (3.9) |
| Obesity (n, %) | 45,375 (17.0) | 39,001 (17.9) | 23,287 (10.9) | 26,315 (12.5) | 20,638 (12.1) |
| Autoimmune disease or AIDS (n, %) | 13,238 (5.0) | 11,179 (5.1) | 8,165 (3.8) | 8,594 (4.1) | 6,849 (4.0) |
| Cardiovascular disease (n, %) | 119,737 (44.8) | 101,323 (46.5) | 70,370 (32.9) | 76,269 (36.2) | 60,700 (35.6) |
| Diabetes (n, %) | 155,043 (58.0) | 127,746 (58.6) | 105,912 (49.6) | 109,597 (52.0) | 86,704 (50.9) |
| Drug or alcohol abuse (n, %) | 8,697 (3.3) | 7,376 (3.4) | 4,866 (2.3) | 5,174 (2.5) | 3,958 (2.3) |
| Heart failure or valvular disease (n, %) | 95,285 (35.6) | 80,127 (36.8) | 56,047 (26.2) | 59,555 (28.3) | 47,901 (28.1) |
| Lung disease (n, %) | 56,594 (21.2) | 48,569 (22.3) | 32,354 (15.1) | 33,816 (16.0) | 26,793 (15.7) |
| Malignancy (n, %) | 17,998 (6.7) | 15,071 (6.9) | 10,455 (4.9) | 11,406 (5.4) | 9,501 (5.6) |
| Neurological disease or psychoses (n, %) | 49,724 (18.6) | 42,324 (19.4) | 29,569 (13.8) | 30,687 (14.6) | 25,732 (15.1) |
| Facility factorsd | |||||
| Nurses, per 100 patients | |||||
| Mean (SD) | 5.0 (2.7) | 5.0 (2.7) | 5.0 (2.7) | 5.0 (2.8) | 5.1 (3.3) |
| Median (IQR) | 4.6 (3.5-5.9) | 4.6 (3.5-5.9) | 4.6 (3.5-5.9) | 4.6 (3.5-5.9) | 4.6 (3.5-5.9) |
| N missing | 85 | 43 | 103 | 127 | 212 |
| Patient care technicians, per 100 patients | |||||
| Mean (SD) | 6.3 (2.4) | 6.3 (2.4) | 6.3 (2.4) | 6.3 (2.4) | 6.4 (3.3) |
| Median (IQR) | 6.3 (4.8-7.6) | 6.3 (4.9-7.6) | 6.3 (4.9-7.6) | 6.3 (4.9-7.6) | 6.3 (4.9-7.7) |
| N missing | 85 | 43 | 103 | 127 | 212 |
| Social workers, per 100 patients | |||||
| Mean (SD) | 0.9 (0.7) | 0.9 (0.7) | 0.9 (0.7) | 0.9 (0.7) | 0.9 (1.2) |
| Median (IQR) | 0.8 (0.6-1.0) | 0.8 (0.6-1.0) | 0.8 (0.6-1.0) | 0.8 (0.6-1.0) | 0.8 (0.6-1.0) |
| N missing | 85 | 43 | 103 | 127 | 212 |
| No. of patients | |||||
| Mean (SD) | 136.0 (74.4) | 136.7 (74.1) | 136.1 (74.0) | 136.0 (74.6) | 135.7 (75.5) |
| Median (IQR) | 122.0 (85.0-170.0) | 122.0 (86.0-171.0) | 122.0 (86.0-170.0) | 121.0 (85.0-170.0) | 121.0 (85.0-170.0) |
| N missing | 84 | 43 | 103 | 122 | 205 |
| % In-center dialysis patients | |||||
| Mean (SD) | 89.6 (14.6) | 89.7 (14.6) | 89.7 (14.5) | 89.8 (14.4) | 89.7 (14.4) |
| Median (IQR) | 99.1 (81.8-100.0) | 99.3 (82.1-100.0) | 99.2 (82.1-100.0) | 99.3 (82.1-100.0) | 99.1 (82.1-100.0) |
| N missing | 1,413 | 1,137 | 955 | 759 | 937 |
| Facility type (n, %) | |||||
| LDOe | 198,093 (74.1) | 161,747 (74.3) | 158,400 (74.2) | 156,012 (74.1) | 125,721 (73.9) |
| Non-LDO, profit | 41,807 (15.6) | 33,632 (15.4) | 33,144 (15.5) | 32,940 (15.6) | 26,228 (15.4) |
| Non-LDO, nonprofit | 27,444 (10.3) | 22,393 (10.3) | 21,975 (10.3) | 21,651 (10.3) | 18,257 (10.7) |
| N missing | 90 | 41 | 101 | 140 | 251 |
| Geographic factors | |||||
| Area Deprivation Index score | |||||
| Lowest quartile (n, %) | 49,752 (19.3) | 40,087 (19.1) | 39,214 (19.1) | 39,220 (19.4) | 33,478 (20.4) |
| 2nd quartile (n, %) | 63,749 (24.8) | 51,589 (24.6) | 50,715 (24.7) | 49,984 (24.7) | 41,645 (25.4) |
| 3rd quartile (n, %) | 71,970 (28.0) | 58,469 (27.9) | 57,377 (27.9) | 56,607 (27.9) | 45,640 (27.8) |
| Highest quartile (n, %) | 72,036 (28.0) | 59,525 (28.4) | 58,248 (28.3) | 56,757 (28.0) | 43,437 (26.5) |
| N missing | 9,927 | 8,143 | 8,066 | 8,175 | 6,257 |
| Population density | |||||
| Highest quartile (n, %) | 53,590 (20.5) | 43,960 (20.6) | 42,594 (20.4) | 42,226 (20.5) | 34,758 (20.9) |
| 3rd quartile (n, %) | 84,049 (32.1) | 68,430 (32.1) | 67,052 (32.1) | 66,149 (32.2) | 53,299 (32.0) |
| 2nd quartile (n, %) | 93,120 (35.6) | 75,510 (35.5) | 74,442 (35.7) | 73,306 (35.6) | 59,267 (35.6) |
| Lowest quartile (n, %) | 30,802 (11.8) | 25,109 (11.8) | 24,702 (11.8) | 24,094 (11.7) | 19,311 (11.6) |
| N missing | 5,873 | 4,804 | 4,830 | 4,968 | 3,822 |
Abbreviations: AIDS, acquired immune deficiency syndrome; LDO, large dialysis organization.
Characteristics of patients when they were enrolled into a cohort for the first time during the study period.
Based off of the Research Triangle Race code.
In a study of patients aged ≥66 years with Medicare who were hospitalized, the interquartile range of this Elixhauser comorbidity index was associated with a 30-day mortality rate range from ≈3% to ≈9%.21
Number of facility staff (patient care technician, or social worker) is defined as 100 ∗ (number of full-time staff + 0.5 ∗ number of part-time staff)/number of patients in a facility.
LDO is a large dialysis organization, which is defined as ownership by DaVita or Fresenius.
Although USAFacts publishes COVID-19 incidence for each county, many nephrologists see patients across multiple counties. When examining the risk of exposure to SARS-CoV-2, we considered how individual nephrologists were likely to change their practice patterns in response to the perceived risk of COVID-19 across the broader geographic area where they see patients.28 This exercise (described in Item S2) yielded a measure of COVID-19 risk around each dialysis facility most likely to influence nephrologist decisions about whether to see patients face-to-face or by telemedicine.
Statistical Model
We plotted trends over time in the use of telemedicine for in-center hemodialysis as well as geographic variation in its use. We described covariates of interest across all 4 cohorts. We used multivariable logistic regression models to examine independent associations among covariates of interest and the use of telemedicine. All models included separate categorical variables for each calendar month of the study period and used cluster-robust standard errors to account for repeated measures across patients.29
To examine whether the associations among correlates of interest and telemedicine use varied over the course of the pandemic, we created interaction terms representing the presence of each selected covariate and time on or after January 1, 2021. We selected the start of 2021 because this was the time when COVID-19 vaccination became available for health care providers and patients. Following vaccination, we expected nephrologists to become less sensitive to COVID-19 risk when making decisions about whether or not to use telemedicine. We focused this analysis of time interaction effects on COVID-19 incidence, facility, and geographic covariates. When we modeled continuous variables of interest as categorical variables (for ease of interpretation and presentation) in the primary analysis, we tested for interaction effects using the continuous form. To facilitate interpretation of the data, we present results from these analysis by displaying separate models stratified by each calendar year and P values representing the statistical significance of interaction terms in models that spanned both study years.
In a secondary analysis, we examined a restricted cohort that only followed up patients whose nephrologist had already reported the use of telemedicine on a claim during the pandemic for at least one of the nephrologist’s patients. This restriction focused the secondary analysis on nephrologists who had demonstrated their knowledge and ability to use the telemedicine modifiers during the pandemic. The study was approved by an institutional review board at Baylor College of Medicine.
Results
Baseline Characteristics and Unadjusted Outcomes
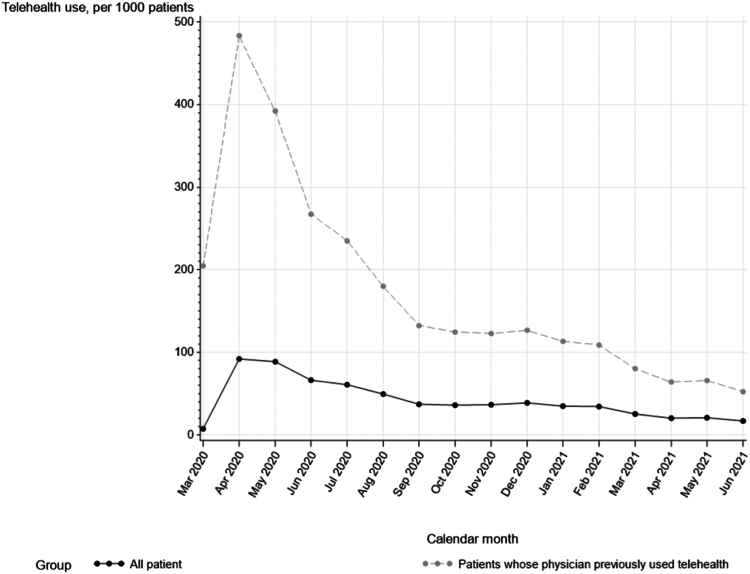
We identified 267,434 Medicare beneficiaries receiving in-center hemodialysis who were eligible for the study (Fig 1). The reported use of telemedicine varied over time, peaking at 9% of patients in April 2020 and declining to 2% of patients by June 2021. In a cohort restricted to patients whose nephrologists had already used telemedicine for at least 1 of their patients, telemedicine use was higher, peaking at 48% in April 2020 and declining to 5% in June 2021 (Fig 2).
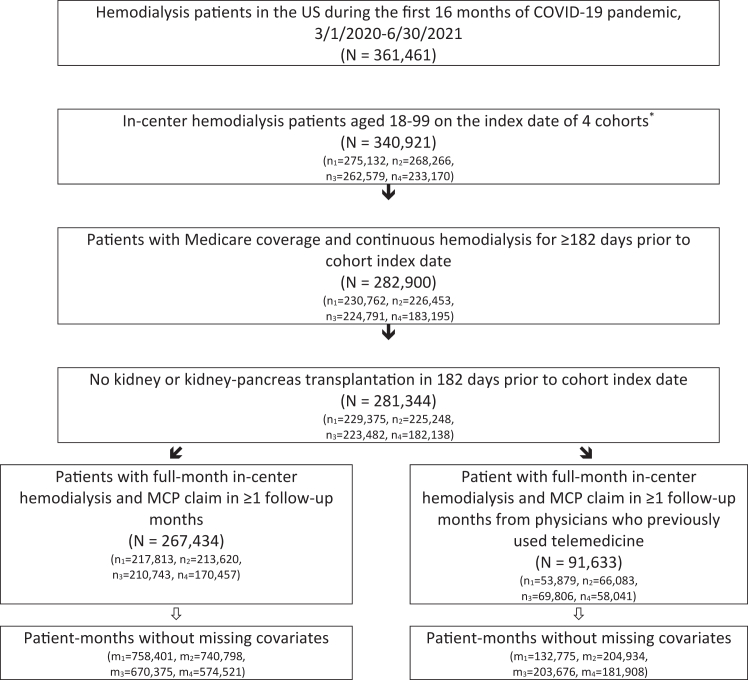
Figure 1.
Study selection flow diagram.
The index dates of the 4 cohorts were March 1, 2020; July 1, 2020; November 1, 2020; and March 1, 2021. Subscripts n1 through n4 refer to the number of patients in cohorts 1 through 4, respectively. Subscripts m1 through m4 refer to the number of patient-months in cohorts 1 through 4, respectively.
Figure 2.
Reported use of telemedicine over time.
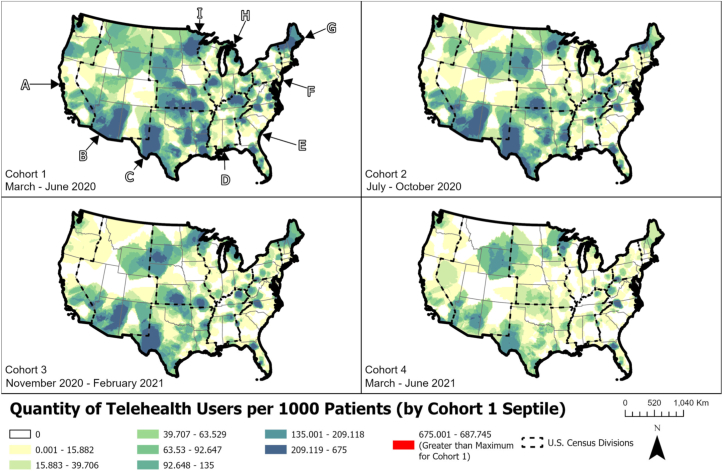
The use of telemedicine varied geographically. Early in the pandemic (March 2020-June 2020), it was used more frequently in areas of the Northeast, Midwest, Southwest, and Pacific Northwest. Later in the pandemic (March 2021-June 2021), it continued to be used at increased frequency in these same areas, except for parts of the Northeast and Pacific Northwest where its use declined (Fig 3).
Figure 3.
Geographic variation in telemedicine use over time.
A through I represent US Census Divisions. Abbreviations: A, Pacific; B, Mountain; C, West South Central; D, East South Central; E, South Atlantic; F, Middle Atlantic; G, New England; H, East North Central; I, West North Central.
The mean age of the study population was 64 years, and 44% were women. In total, 36% were non-Hispanic White race, 37% were African American race, 5% were Asian race, 2% were American Indian race, and 17% were of Hispanic ethnicity. In total, 53% of patients had dual Medicaid and Medicare coverage in the 6 months before each cohort index date. The mean Elixhauser mortality index was 14. The reported burden of comorbid conditions was highest in the first cohort and declined in later cohorts (Table 1).
Regression Results
In a multivariable regression model, the use of telemedicine peaked early in the pandemic and declined thereafter, similar to observed unadjusted trends. The incidence of COVID-19 in areas surrounding dialysis facilities was strongly associated with the use of telemedicine, with patients more likely to be seen by telemedicine if COVID-19 incidence was higher in the current or prior 2 months. Older patients were less likely to receive care by telemedicine, as were patients of African American race. Compared with patients of non-Hispanic White race, patients of North American Native race and of Hispanic ethnicity were more likely to receive nephrology care by telemedicine.
Dialysis facility characteristics were also related to the use telemedicine. Patients were more likely to receive nephrology care by telemedicine if they were at facilities with more social workers and more patient care technicians per patient and if they were at larger facilities (ie, those providing care to more patients). Patients were more likely to receive care by telemedicine if they were at an LDO-owned facility.
Patients were more likely to receive care by telemedicine if they lived in socioeconomically disadvantaged areas (represented by a higher ADI) or if they lived in more remote areas, with a step-wise increase in the use of telemedicine observed with higher ADI and decreased population density (Table 2).
Table 2.
Risk Factors for Telemedicine Use, by Calendar Year
| 2020-2021 |
By Calendar Year |
|||||
|---|---|---|---|---|---|---|
| 2020 |
2021 |
P Value for Interactionb | ||||
| OR (95% CI)a | P Valuea | OR (95% CI)a | OR (95% CI)a | |||
| Calendar month | Mar 2020 | 1.0 (referent) | 1.0 (referent) | — | ||
| Apr 2020 | 13.57 (12.88-14.30) | <0.0001 | 13.65 (12.95-14.38) | |||
| May 2020 | 13.13 (12.46-13.84) | <0.0001 | 13.23 (12.55-13.94) | |||
| Jun 2020 | 9.39 (8.90-9.91) | <0.0001 | 9.46 (8.97-9.98) | |||
| Jul 2020 | 7.91 (7.49-8.36) | <0.0001 | 8.07 (7.64-8.52) | |||
| Aug 2020 | 6.12 (5.79-6.47) | <0.0001 | 6.29 (5.95-6.65) | |||
| Sep 2020 | 4.59 (4.34-4.86) | <0.0001 | 4.65 (4.39-4.93) | |||
| Oct 2020 | 4.52 (4.27-4.79) | <0.0001 | 4.72 (4.45-5.00) | |||
| Nov 2020 | 3.95 (3.72-4.19) | <0.0001 | 4.12 (3.88-4.38) | |||
| Dec 2020 | 3.46 (3.26-3.68) | <0.0001 | 3.61 (3.39-3.85) | |||
| Jan 2021 | 2.73 (2.56-2.92) | <0.0001 | 1.0 (referent) | |||
| Feb 2021 | 3.02 (2.82-3.23) | <0.0001 | 0.96 (0.91-1.00) | |||
| Mar 2021 | 2.69 (2.51-2.88) | <0.0001 | 0.83 (0.78-0.87) | |||
| Apr 2021 | 2.49 (2.32-2.66) | <0.0001 | 0.70 (0.66-0.75) | |||
| May 2021 | 2.72 (2.55-2.90) | <0.0001 | 0.78 (0.73-0.83) | |||
| Jun 2021 | 2.20 (2.05-2.35) | <0.0001 | 0.64 (0.60-0.69) | |||
| COVID-19 incidence | ||||||
| Current month, per 1,000 people | 0-5 | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) | 0.0002 | |
| 6-10 | 1.07 (1.06-1.09) | <0.0001 | 1.05 (1.04-1.07) | 1.09 (1.06-1.13) | ||
| 10-20 | 1.27 (1.24-1.29) | <0.0001 | 1.25 (1.23-1.28) | 1.09 (1.04-1.14) | ||
| ≥21 | 1.34 (1.30-1.38) | <0.0001 | 1.34 (1.30-1.39) | 1.13 (1.06-1.21) | ||
| 1st month before current month, per 1,000 people | 0-5 | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) | 0.1338 | |
| 6-10 | 1.04 (1.02-1.05) | <0.0001 | 1.02 (1.01-1.04) | 1.02 (0.99-1.06) | ||
| 10-20 | 1.25 (1.23-1.28) | <0.0001 | 1.22 (1.19-1.25) | 1.17 (1.12-1.23) | ||
| ≥2 | 1.26 (1.23-1.30) | <0.0001 | 1.20 (1.15-1.26) | 1.32 (1.24, 1.41) | ||
| 2nd month before current month, per 1,000 people | 0-5 | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) | <0.0001 | |
| 6-10 | 1.06 (1.04-1.07) | <0.0001 | 1.04 (1.02-1.06) | 0.99 (0.96-1.03) | ||
| 10-20 | 1.17 (1.14-1.19) | <0.0001 | 1.21 (1.17-1.25) | 1.03 (0.98-1.08) | ||
| ≥21 | 1.20 (1.16-1.24) | <0.0001 | 1.69 (1.57-1.81) | 1.04 (0.99-1.10) | ||
| 3rd month before current month, per 1,000 people | 0-5 | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) | 0.0226 | |
| 6-10 | 1.01 (0.99-1.02) | 0.5349 | 0.96 (0.94-0.98) | 1.09 (1.05-1.13) | ||
| 10-20 | 0.98 (0.95-1.01) | 0.1415 | 0.93 (0.90-0.97) | 1.07 (1.02-1.12) | ||
| ≥21 | 1.01 (0.98-1.06) | 0.4740 | 1.12 (0.98-1.29) | 1.15 (1.09-1.22) | ||
| Patient factors | ||||||
| Age (y) | <50 | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) | — | |
| 50-64 | 1.00 (0.96-1.04) | 0.8444 | 0.99 (0.95-1.03) | 0.98 (0.92-1.05) | ||
| 65-79 | 0.95 (0.91-0.99) | 0.0097 | 0.94 (0.90-0.98) | 1.03 (0.96-1.11) | ||
| ≥80 | 0.90 (0.85-0.95) | 0.0001 | 0.89 (0.84-0.94) | 1.00 (0.91-1.10) | ||
| Sex | Male | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) | — | |
| Female | 1.05 (1.02-1.08) | 0.0017 | 1.05 (1.02-1.08) | 1.07 (1.02-1.12) | ||
| Race | Non-Hispanic White | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) | ||
| African American | 0.88 (0.85-0.92) | <0.0001 | 0.89 (0.86-0.93) | 0.92 (0.86-0.98) | ||
| Asian | 0.94 (0.86-1.02) | 0.1470 | 0.97 (0.89-1.06) | 1.18 (1.03-1.34) | ||
| Hispanic | 1.24 (1.19-1.30) | <0.0001 | 1.26 (1.21-1.31) | 1.60 (1.50-1.72) | ||
| North American Native | 2.58 (2.38-2.79) | <0.0001 | 2.49 (2.30-2.69) | 2.38 (2.12-2.66) | ||
| Other/unknown | 0.89 (0.80-0.99) | 0.0349 | 0.90 (0.81-1.00) | 1.04 (0.88-1.23) | ||
| Preindex home dialysis | No | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) | — | |
| Yes | 1.03 (0.89, 1.18) | 0.7214 | 1.06 (0.92-1.23) | 0.92 (0.66-1.30) | ||
| Preindex Medicaid | No | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) | — | |
| Yes | 0.90 (0.87-0.93) | <0.0001 | 0.89 (0.86-0.91) | 0.89 (0.85-0.94) | ||
| Elixhauser mortality index | 0.9998 (0.9992-1.0004) | 0.5585 | 0.9996 (0.9990-1.0003) | 0.9999 (0.9986-1.0012) | — | |
| Facility factors | ||||||
| Nurses, per 100 patients | ≤4 | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) | 0.0606 | |
| >4-6 | 1.03 (0.99-1.06) | 0.1409 | 1.02 (0.99-1.06) | 0.94 (0.89-1.00) | ||
| >6 | 0.92 (0.88-0.96) | <0.0001 | 0.91 (0.87-0.95) | 0.85 (0.79-0.91) | ||
| Patient care technicians, per 100 patients | ≤5 | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) | <0.0001 | |
| >5-8 | 1.18 (1.14-1.22) | <0.0001 | 1.21 (1.17-1.26) | 1.20 (1.13-1.28) | ||
| >8 | 1.28 (1.22-1.34) | <0.0001 | 1.34 (1.28-1.40) | 1.42 (1.32-1.53) | ||
| Social workers, per 100 patients | ≤0.5 | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) | 0.6404 | |
| >0.5-1 | 1.22 (1.16-1.28) | <0.0001 | 1.21 (1.15-1.27) | 1.45 (1.33-1.58) | ||
| >1 | 1.42 (1.33-1.51) | <0.0001 | 1.40 (1.32-1.49) | 1.66 (1.49-1.84) | ||
| No. of patients | <50 | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) | 0.2840 | |
| 50-99 | 1.14 (1.07-1.22) | <0.0001 | 1.18 (1.10-1.26) | 1.13 (1.01-1.26) | ||
| 100+ | 1.21 (1.12-1.30) | <0.0001 | 1.25 (1.16-1.35) | 1.36 (1.21-1.54) | ||
| In-center dialysis patients | <80% | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) | 0.0266 | |
| 80%-89% | 0.72 (0.68-0.76) | <0.0001 | 0.71 (0.68-0.75) | 0.71 (0.65-0.78) | ||
| 90%-100% | 1.01 (0.98-1.05) | 0.4901 | 1.02 (0.98-1.06) | 0.93 (0.87-0.99) | ||
| Facility type | LDO | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) | <0.0001 | |
| Non-LDO, profit | 0.67 (0.64-0.71) | <0.0001 | 0.67 (0.63-0.70) | 0.57 (0.52-0.62) | ||
| Non-LDO, nonprofit | 0.87 (0.82-0.92) | <0.0001 | 0.93 (0.88-0.98) | 1.24 (1.14-1.35) | ||
| Geographic factors | ||||||
| Area Deprivation Index score | Lowest | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) | 0.0028 | |
| 2nd quartile | 1.70 (1.61-1.80) | <0.0001 | 1.67 (1.58-1.76) | 1.34 (1.23-1.47) | ||
| 3rd quartile | 1.72 (1.63-1.82) | <0.0001 | 1.73 (1.64-1.83) | 1.68 (1.53-1.84) | ||
| 4th quartile | 2.19 (2.08-2.32) | <0.0001 | 2.19 (2.08-2.31) | 2.18 (2.00-2.39) | ||
| Population density | highest quartile | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) | <0.0001 | |
| 3rd quartile | 1.22 (1.16-1.28) | <0.0001 | 1.22 (1.16-1.27) | 0.86 (0.80-0.93) | ||
| 2nd quartile | 1.29 (1.23, 1.35) | <0.0001 | 1.28 (1.22-1.34) | 0.94 (0.87-1.02) | ||
| Lowest quartile | 1.34 (1.26-1.42) | <0.0001 | 1.33 (1.26-1.42) | 1.33 (1.22-1.46) | ||
Abbreviations: LDO, large dialysis organization.
From multiadjusted logistic regression model including calendar month, coronavirus disease 2019 (COVID-19) incidence rate, demographic characteristics, Elixhauser mortality index, facility and geographic factors. Generalized estimating equation used to address correlation among multiple patient observations.
Testing whether the dose-response relation between telemedicine use and COVID-19 incidence rate and facility factors is different between calendar years, ie, from a multiadjusted logistic regression model including calendar month, COVID-19 incidence rate category as continuous variable, demographic characteristics, Elixhauser mortality index, facility and geographic factors with ordinal variables as continuous ones, further including calendar year, interaction between calendar year and COVID-19 incidence rate, facility and geographic factors.
Patient comorbid conditions were not significantly associated with the use of telemedicine, whether measured using the Elixhauser Index in the primary model or when examined using separate comorbid conditions in a secondary analysis (Table S1). Predictors of telemedicine were similar in an analysis that was restricted to patients whose nephrologist had previously reported the use of telemedicine (Tables S2-3).
In an analysis of time interaction effects, variation in the incidence of COVID-19 in the current month became less predictive of telemedicine use later in the pandemic. The use of telemedicine increased significantly in nonprofit facilities in 2021 relative to LDO-owned facilities. Although patients in the lowest quartile of population density continued to receive telemedicine more frequently in 2021, the use of telemedicine in the second and third quartiles of population density moderated in 2021 such that its use in the second and third quartiles was no different from the highest population density quartile (Table 2).
Additional descriptive analyses are discussed in Item S3 and Tables S4-S5.30
Discussion
The use of telemedicine by US nephrologists providing in-center hemodialysis care during the COVID-19 pandemic peaked in the summer of 2020 and then declined during the first half of the following year. Telemedicine use in hemodialysis varied geographically. It was used more intensively in remote locations, in areas where the incidence of COVID was higher, and at facilities with more support staff. Although some patient demographic and socioeconomic characteristics were associated with the use of telemedicine, the burden of comorbid condition was not. These findings highlight similarities and differences between the use of telemedicine for in-center hemodialysis and its use in the treatment of other chronic diseases.
Before 2020, telemedicine visits during hemodialysis were generally not reimbursable through Medicare. Limited exceptions included a small number of patients enrolled in telemedicine pilot programs or receiving hemodialysis at rural, hospital-based, dialysis units located in designated professional shortage areas.31 On March 17, 2020, the CMS issued an emergency waiver that allowed in-center hemodialysis units to serve as the originating site for telemedicine encounters. The encounters could be conducted using everyday communications technologies that allowed interactive communication in real time, such as FaceTime or Skype.2 Subsequent guidance clarified that these encounters must be provided by platforms with combined audio and video formats and that telemedicine could be used for any MCP visit type, including the monthly comprehensive visit.15
Following the waivers, telemedicine use for in-center hemodialysis care increased sharply between April and June of 2020. This increase was in parallel with observations in the broader Medicare population, in which the use of telemedicine was found to have increased by more than 10-fold in the early months of the pandemic.32, 33, 34 Many of the reasons for increased use of telemedicine in other clinical settings, such as the easing of regulations related to internet and mobile device security, flexibility around the originating site for telemedicine visits, and efforts to reduce exposure to SARS-CoV-2, likely contributed to the adoption of telemedicine in hemodialysis.
After peaking in April 2020 at 9% of all patient-months, the reported use of telemedicine among patients receiving in-center hemodialysis began to decline. By June 2021, only 2% of patient-months included a telemedicine modifier claim. It is possible that this decline would have been steeper had there not been additional waves of COVID-19. In an analysis in which we examined the use of telemedicine among physicians who had previously billed the telemedicine modifier claim (indicating awareness of the billing code), telemedicine was used in 5% of patient-months by June 2021. This suggests that a small but sizable number of nephrologists continued to use telemedicine for in-center hemodialysis and might, therefore, be expected to continue using it if legislation permits continued reimbursement of telemedicine for in-center hemodialysis.
Declines in the use of telemedicine over time coincided with changes in the threat of COVID-19. We observed that the use of telemedicine for in-center hemodialysis at every point in time was closely associated with the incidence of COVID-19 in an area. When the incidence of COVID-19 was higher, nephrologists appeared more likely to replace face-to-face visits with telemedicine to avoid contracting and spreading SARS-CoV-2. Yet, an analysis of time interaction effects demonstrates that the magnitude of this association diminished over time. Access to effective vaccines, the evolution of less virulent strains of SARS-CoV-2, and increased comfort with screening procedures and contact precautions implemented in dialysis centers may have reduced nephrologists’ perceptions of the threat from COVID-19 spread.35, 36, 37 Telemedicine use in hemodialysis may have declined over time because nephrologists were less concerned about the spread of COVID-19 later in the pandemic.
Waning logistic support from dialysis facilities may have also contributed to declines over time in the use of telemedicine. In hemodialysis, a member of the dialysis facility staff, such as a nurse, patient care technician, or dietitian, typically relays the telemedicine device to patients and helps to establish a remote connection while the patient receives dialysis.5,6 Because this person is employed by the dialysis facility, and not by the nephrology practice, the use of telemedicine in dialysis requires an interest and willingness on the part of dialysis facilities to make staff available to facilitate the process. To deliver telemedicine effectively in the in-center hemodialysis setting, additional resources, generally in the form of staff time, need to be deployed so as to not compromise other patient-centered activities (eg, dietary counseling and vocational rehabilitation). We observed that patients were more likely to receive telemedicine care if they were at facilities with more patient care technicians and social workers, indicating the importance of facility staffing resources in making telemedicine possible. Patients receiving dialysis at facilities owned and operated by the 2 LDOs were also more likely to receive telemedicine from their dialysis care providers. The potential scope of telemedicine for future in-center hemodialysis care will depend on incentives and decisions involving dialysis facility administrators.
In some ways, population-level patterns of telemedicine use in in-center hemodialysis resembled other areas of health care. Older patients were less likely to receive care by telemedicine, similar to findings from a study of surgical patients during the pandemic.34 In this instance, technical assistance from dialysis facility staff may not have been sufficient to overcome barriers to the use of telemedicine among older patients. Patients living in socioeconomically disadvantaged areas were more likely to receive hemodialysis care by telemedicine, which is consistent with observed increases in the use of telemedicine among Medicare beneficiaries who are also eligible for Medicaid.32 In other clinical settings, associations among race, ethnicity, and telemedicine use in nondialysis populations vary.33,38, 39, 40 Among patients receiving in-center hemodialysis, African American race was associated with reduced likelihoods of telemedicine use, whereas Hispanic ethnicity was associated with an increased likelihood of use. It will be important to understand the ways in which telemedicine may affect the quality of care and access to care in underserved populations.
In nondialysis health care settings, patients living in urban areas were more likely to experience increases in the use of telemedicine in association with the COVID-19 pandemic.41 In contrast, patients receiving hemodialysis were more likely to receive care by telemedicine if they lived in less densely populated areas. This discrepancy may reflect differences in technological limitations across settings. In many chronic disease settings, patients receive telemedicine care from their homes. Technologic challenges, such as limited broadband internet access, may be more likely to impede the use of telemedicine from patient homes in rural locations.42 In contrast, patients receive telemedicine during hemodialysis from their dialysis center, where adequate internet connectivity is more likely to be available regardless of surrounding population density or socioeconomic status.
Increased use of telemedicine in remote hemodialysis settings also highlights the roles of convenience and cost reduction as key determinants of telemedicine use. One way that telemedicine can be more convenient than in-person visits is by eliminating the need for patients and clinicians to travel to the clinic. In most chronic disease settings, patients are the primary beneficiaries of this convenience because telemedicine enables them to see their physician from home rather than having to travel to the physician’s office. In contrast, in hemodialysis, it is the nephrologist who benefits mostly from the convenience of telemedicine. Patients must travel to their dialysis facility multiple times per week regardless of whether their nephrologist sees them in person or by telemedicine. Nephrologists may be using telemedicine more frequently in remote settings because travel distances (and therefore costs of face-to-face visits) are generally larger in remote settings. This has implications for the potential benefits of continued telemedicine use for in-center hemodialysis care. Rather than considering benefits in terms of convenience to patients, it may be more accurate to consider benefits in terms of reduced costs (ie, efficiency) on the part of clinicians.
Our study has several limitations. We rely on physicians to code telemedicine modifiers accurately. Physicians who were unaware of coding guidelines may have seen patients by telemedicine but not appropriately coded for the visit, leading to underestimation of the use of telemedicine. We attempted to address this limitation in an analysis in which we restricted the cohort to patients seen by physicians who had already used telemedicine. The telemedicine modifier code only indicated whether telemedicine was used for at least 1 of the monthly visits. Approximately 70% of patients are seen by their nephrology practitioner 4 or more times in a month.43,44 In months when telemedicine use was reported, we could not determine how many visits were conducted by telemedicine. Finally, measures of support staff were ascertained annually and did not reflect more granular staffing variations that might have occurred during peaks of the pandemic.
The role of telemedicine in in-center hemodialysis following elimination of the COVID-19 waivers remains uncertain. Emergency waivers that allowed telemedicine to be used for in-center hemodialysis in the United States expired on May 11, 2023, and, following a temporary extension period, telemedicine for in-center hemodialysis will no longer be reimbursed after December 31, 2024.45, 46, 47 Although several federal laws have been introduced to make aspects of telemedicine waivers permanent, none have explicitly addressed in-center hemodialysis.48, 49, 50 In other areas of health care, access to effective and efficient telemedicine care is associated with patient satisfaction and generally yields similar or improved clinical outcomes.51,52 Small pilot programs assessing the feasibility of using telemedicine for in-center hemodialysis care found that patient-reported and hard clinical outcomes were comparable to traditional in-person care.53, 54, 55, 56, 57, 58
As the threats related to COVID-19 wane, it will be important to consider how telemedicine could be used to improve the care of patients receiving in-center hemodialysis. Findings from this study suggest a potential role for telemedicine in the in-center hemodialysis setting going forward.
Article Information
Authors’ Full Names and Academic Degrees
Jingbo Niu, MD, DSc, Omar Rosales, MPH, Abiodun Oluyomi, PhD, Susie Q. Lew, MD, Wolfgang C. Winkelmayer MD, ScD, Glenn M. Chertow, MD, MPH, Kevin F. Erickson, MD, MS
Authors’ Contributions
Research idea and study design: KE, WW, GC, SL; data acquisition: KE, OR, AO; data analysis/interpretation: JN, OR, AO, WW, KE; statistical analysis: JN; WW; GC, KE; supervision or mentorship: KE, AO, WW, GC, SL. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
This work was funded by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (1R01DK128209-01).
Financial Disclosure
Kevin Erickson reports receiving personal fees from Acumen LLC, Outset Medical, and Boehringer Ingelheim and honoraria from Dialysis Clinics, Inc.
Peer Review
Received July 26, 2023, as a submission to the expedited consideration track with 1 external peer review. Direct editorial input from Statistical Editor and an Associate Editor, who served as Acting Editor-in-Chief. Accepted in revised form December 18, 2023. The involvement of an Acting Editor-in-Chief was to comply with Kidney Medicine’s procedures for potential conflicts of interest for editors, described in the Information for Authors & Journal Policies.
Footnotes
Complete author and article information provided before references.
Item S1: Calculating an Elixhauser Comorbity Index.
Item S2: COVID Data Collection and Preparation.
Item S3: Additional Descriptive Analyses.
Table S1: Risk Factors for Telemedicine Use, Adjusting for Individual Co-morbidities.
Table S2: Baseline Characteristics by Cohort, among Patient-Months from Physicians who Previously Used Telemedicine.
Table S3: Risk Factors for Telemedicine Use, among Patient Months from Physicians who Previously Used Telemedicine, by Calendar Year.
Table S4: Baseline Characteristics by Proportion of Patient-Months with Telemedicine Use.
Table S5: Facility and Geographic Characteristics by Region.
Supplementary Materials
Items S1-S3; Tables S1-S5.
References
- 1.Annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States. United States Renal Data System. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2021. [Google Scholar]
- 2.Medicare telemedicine healthcare provider fact sheet. Centers for Medicare and Medicaid Services. https://www.cms.gov/newsroom/fact-sheets/medicare-telemedicine-health-care-provider-fact-sheet Published March 17, 2020.
- 3.Additional background: sweeping regulatory changes to help U.S. healthcare system address COVID-19 patient surge. Centers for Medicare and Medicaid Services. https://www.cms.gov/newsroom/fact-sheets/additional-backgroundsweeping-regulatory-changes-help-us-healthcare-system-address-covid-19-patient Published March 30, 2020.
- 4.Neuman M. CMS expands Medicare coverage of telehealth services for in-center dialysis visits amid COVID-19. Nephrology News and Issues. March 20, 2020 [Google Scholar]
- 5.RPA Telehealth Survey Results Renal Physicians Association. https://cdn.ymaws.com/www.renalmd.org/resource/resmgr/legregscomp/public_policy/rev_rpa_th_results_summary_f.pdf Published June 2, 2020.
- 6.Lew S.Q., Kaur G., Sikka N., Erickson K.F. In-center hemodialysis unit patient experience with telehealth. Hemodial Int. 2023;27:193–196. doi: 10.1111/hdi.13065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehrotra A. The convenience revolution for treatment of low-acuity conditions. JAMA. 2013;310:35–36. doi: 10.1001/jama.2013.6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pearl R. Kaiser Permanente Northern California: current experiences with internet, mobile, and video technologies. Health Affairs (Project Hope) 2014;33:251–257. doi: 10.1377/hlthaff.2013.1005. [DOI] [PubMed] [Google Scholar]
- 9.Board on Health Care Services; Institute of Medicine . National Academies Press (US); Washington (DC): 2012. The Role of Telehealth in an Evolving Health Care Environment: Workshop Summary.https://www.ncbi.nlm.nih.gov/books/NBK207145/ [PubMed] [Google Scholar]
- 10.Telehealth in rural communities. Centers for Disease Control and Prevention. https://www.cdc.gov/chronicdisease/resources/publications/factsheets/telehealth-in-rural-communities.htm
- 11.Ishani A., Christopher J., Palmer D., et al. Telehealth by an interprofessional team in patients with CKD: a randomized controlled trial. Am J Kidney Dis. 2016;68:41–49. doi: 10.1053/j.ajkd.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 12.Ladino M.A., Wiley J., Schulman I.H., et al. Tele-nephrology: a feasible way to improve access to care for patients with kidney disease who reside in underserved areas. Telemed J E Health. 2016;22:650–654. doi: 10.1089/tmj.2015.0197. [DOI] [PubMed] [Google Scholar]
- 13.Narva A.S., Romancito G., Faber T., Steele M.E., Kempner K.M. Managing CKD by telemedicine: the Zuni Telenephrology Clinic. Adv Chronic Kidney Dis. 2017;24:6–11. doi: 10.1053/j.ackd.2016.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lew S.Q., Sikka N. Operationalizing telehealth for home dialysis patients in the United States. Am J Kidney Dis. 2019;74:95–100. doi: 10.1053/j.ajkd.2019.01.023. [DOI] [PubMed] [Google Scholar]
- 15.Guidance on billing and coding for remote nephrology services. Renal Physicians Association. https://cdn.ymaws.com/www.renalmd.org/resource/resmgr/covid_19/5-1_rpa_guidance_on_billing_.pdf
- 16.Dialysis Facility Report 2022. Centers for Medicare & Medicaid Services Data. https://data.cms.gov/sites/default/files/2022-02/FY2022_DFR_Sample_202107.pdf
- 17.US COVID-19 cases and deaths by state. USAFacts. https://usafacts.org/visualizations/coronavirus-covid-19-spread-map/
- 18.Detailed methodology and sources: COVID-19 Data. USAFacts. https://usafacts.org/articles/detailed-methodology-covid-19-data/
- 19.Neelon B., Mutiso F., Mueller N.T., Pearce J.L., Benjamin-Neelon S.E. Spatial and temporal trends in social vulnerability and COVID-19 incidence and death rates in the United States. PloS One. 2021;16 doi: 10.1371/journal.pone.0248702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kao S.Z., Sharpe J.D., Lane R.I., et al. Duration of behavioral policy interventions and incidence of COVID-19 by social vulnerability of US counties, April-December 2020. Public Health Rep. 2023;138:190–199. doi: 10.1177/00333549221125202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehta H.B., Li S., An H., Goodwin J.S., Alexander G.C., Segal J.B. Development and validation of the summary Elixhauser Comorbidity Score for use with ICD-10-CM-coded data among older adults. Annals of Internal Medicine. 2022;175:1423–1430. doi: 10.7326/M21-4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elixhauser comorbidity software for ICD-10-CM Healthcare Cost and Utilization Project AfHRaQ. https://hcup-us.ahrq.gov/toolssoftware/comorbidityicd10/CMR-User-Guide-v2023-1.pdf
- 23.Elixhauser A., Steiner C., Harris D.R., Coffey R.M. Comorbidity measures for use with administrative data. Medical Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Moore B.J., White S., Washington R., Coenen N., Elixhauser A. Identifying increased risk of readmission and in-hospital mortality using hospital administrative data: The AHRQ Elixhauser Comorbidity Index. Medical Care. 2017;55:698–705. doi: 10.1097/MLR.0000000000000735. [DOI] [PubMed] [Google Scholar]
- 25.McArthur E., Bota S.E., Sood M.M., et al. Comparing five comorbidity indices to predict mortality in chronic kidney disease: a retrospective cohort study. Can J Kidney Health Dis. 2018;5 doi: 10.1177/2054358118805418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh G.K. Area deprivation and widening inequalities in US mortality, 1969-1998. Am J Public Health. 2003;93:1137–1143. doi: 10.2105/ajph.93.7.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knighton A.J., Savitz L., Belnap T., Stephenson B., VanDerslice J. Introduction of an area deprivation index measuring patient socioeconomic status in an integrated health system: implications for population health. EGEMS (Wash DC) 2016;4:1238. doi: 10.13063/2327-9214.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Economic Research Service . Rural-Urban Commuting Area Codes (RUCA) United States Department of Agriculture; Washington DC: 2019. https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/documentation/ [Google Scholar]
- 29.Huber P.J. The behavior of maximum likelihood estimates under nonstandard conditions. Berkeley Symp on Math Statist and Prob. 1967;1:221–233. [Google Scholar]
- 30.Austin P.C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Statistics in Medicine. 2009;28:3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Centers for Medicare and Medicaid Services . Telehealth Services: Rural Health Series. US Department of Health and Human Services. Centers for Medicare and Medicaid Services; Baltimore, MD: 2018. https://www.hhs.gov/guidance/sites/default/files/hhs-guidance-documents/mln%20telehealth%20services%20booklet_42.pdf [Google Scholar]
- 32.Medicare telehealth, actions needed to strengthen oversight and help providers educate patients on privacy and security risks. US Government Acountability Office. https://www.gao.gov/products/gao-22-104454 Published September 26, 2022.
- 33.Samson L.W., Tarazi W., Turrini G., Sheingold S. Medicare beneficiaries’ use of telehealth in 2020: trends by beneficiary characteristics and location. US Department of Health and Human Services. Published December 3, 2021. https://connectwithcare.org/wp-content/uploads/2022/01/medicare-telehealth-report.pdf
- 34.Chao G.F., Li K.Y., Zhu Z., et al. Use of telehealth by surgical specialties during the COVID-19 pandemic. JAMA Surgery. 2021;156:620–626. doi: 10.1001/jamasurg.2021.0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verma A., Patel A.B., Tio M.C., Waikar S.S. Caring for dialysis patients in a time of COVID-19. Kidney Med. 2020 Oct 14;2(6):787–792. doi: 10.1016/j.xkme.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ikizler T.A., Kliger A.S. Minimizing the risk of COVID-19 among patients on dialysis. Nat Rev Nephrol. 2020;16(6):311–313. doi: 10.1038/s41581-020-0280-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kliger A.S., Silberzweig J. Mitigating risk of COVID-19 in dialysis facilities. CJASN. 2020;15:707–709. doi: 10.2215/CJN.03340320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stevens J.P., Mechanic O., Markson L., O'Donoghue A., Kimball A.B. Telehealth use by age and race at a single academic medical center during the COVID-19 pandemic: retrospective cohort study. J Med Internet Res. 2021;23 doi: 10.2196/23905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karimi M., Lee E.C., Couture S., et al. National survey trends in telehealth use in 2021: disparities in utilization and audio vs. video services. US Department of Health and Human Services, Office of Health Policy. https://aspe.hhs.gov/sites/default/files/documents/4e1853c0b4885112b2994680a58af9ed/telehealth-hps-ib.pdf Updated Feburary 1, 2022.
- 40.Cordasco K.M., Yuan A.H., Rollman J.E., et al. Veterans' use of telehealth for Veterans Health Administration Community Care Urgent Care during the early COVID-19 pandemic. Medical Care. 2022;60:860–867. doi: 10.1097/MLR.0000000000001777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chu C., Cram P., Pang A., Stamenova V., Tadrous M., Bhatia R.S. Rural telemedicine use before and during the COVID-19 pandemic: repeated cross-sectional study. J Med Internet Res. 2021;23 doi: 10.2196/26960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cortelyou-Ward K., Atkins D.N., Noblin A., Rotarius T., White P., Carey C. Navigating the digital divide: barriers to telehealth in rural areas. J Health Care Poor Underserved. 2020;31:1546–1556. doi: 10.1353/hpu.2020.0116. [DOI] [PubMed] [Google Scholar]
- 43.Erickson K.F., Tan K.B., Winkelmayer W.C., Chertow G.M., Bhattacharya J. Variation in nephrologist visits to patients on hemodialysis across dialysis facilities and geographic locations. CJASN. 2013;8:987–994. doi: 10.2215/CJN.10171012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brady B.M., Zhao B., Niu J., et al. Patient-reported experiences of dialysis care within a national pay-for-performance system. JAMA Internal Med. 2018;178:1358–1367. doi: 10.1001/jamainternmed.2018.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Executive Office of the President, Office of Management and Budget . Statement of Administration Policy. whitehouse.gov; Washington, DC: 2023. https://www.whitehouse.gov/wp-content/uploads/2023/01/SAP-H.R.-382-H.J.-Res.-7.pdf [Google Scholar]
- 46.Physicians and other clinicians: CMS flexibilities to fight COVID-19. Centers for Medicare and Medicaid Services. https://www.cms.gov/files/document/physicians-and-other-clinicians-cms-flexibilities-fight-covid-19.pdf [PubMed]
- 47.List of telehealth services for calendar year 2023. Centers for Medicare and Medicaid Services. https://www.cms.gov/medicare/coverage/telehealth/list-services Published February 13, 2023.
- 48.Payerchin R. House of Representatives reintroduces telehealth benefits bill. Medical Economics. February 6, 2023 [Google Scholar]
- 49.Congress H.R. 4040 - Advancing telehealth beyond COVID-19 act of 2021. US Congress. https://www.congress.gov/bill/117th-congress/house-bill/4040/text Published July 28, 2022.
- 50.Congress H.R. 2617 - Consolidated Appropriations Act, 2023. US Congress. https://www.congress.gov/bill/117th-congress/house-bill/2617 Published December 29, 2022.
- 51.Kruse C.S., Krowski N., Rodriguez B., Tran L., Vela J., Brooks M. Telehealth and patient satisfaction: a systematic review and narrative analysis. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2017-016242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Snoswell C.L., Chelberg G., De Guzman K.R., et al. The clinical effectiveness of telehealth: A systematic review of meta-analyses from 2010 to 2019. Journal of Telemedicine and Telecare. 2023;29:669–684. doi: 10.1177/1357633X211022907. [DOI] [PubMed] [Google Scholar]
- 53.Rumpsfeld M., Arild E., Norum J., Breivik E. Telemedicine in haemodialysis: a university department and two remote satellites linked together as one common workplace. Journal of Telemedicine and Telecare. 2005;11:251–255. doi: 10.1258/1357633054471885. [DOI] [PubMed] [Google Scholar]
- 54.Sicotte C., Moqadem K., Vasilevsky M., Desrochers J., St-Gelais M. Use of telemedicine for haemodialysis in very remote areas: the Canadian First Nations. Journal of Telemedicine and Telecare. 2011;17:146–149. doi: 10.1258/jtt.2010.100614. [DOI] [PubMed] [Google Scholar]
- 55.Whitten P., Buis L. Use of telemedicine for haemodialysis: perceptions of patients and health-care providers, and clinical effects. Journal of Telemedicine and Telecare. 2008;14:75–78. doi: 10.1258/jtt.2007.070411. [DOI] [PubMed] [Google Scholar]
- 56.Lunney M., Lee R., Tang K., et al. Impact of telehealth interventions on processes and quality of care for patients with ESRD. Am J Kidney Dis. 2018;72:592–600. doi: 10.1053/j.ajkd.2018.02.353. [DOI] [PubMed] [Google Scholar]
- 57.Bernstein K., Zacharias J., Blanchard J.F., Yu B.N., Shaw S.Y. Model for equitable care and outcomes for remote full care hemodialysis units. CJASN. 2010;5:645–651. doi: 10.2215/CJN.04550709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lunney M., Finlay J., Rabi D., Chandra T., Bello A., Tonelli M. eVisits in rural hemodialysis care: a qualitative study of stakeholder perspectives on design and potential impact to care. Am J Kidney Dis. 2020 Sep;76(3):441–444. doi: 10.1053/j.ajkd.2020.01.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Items S1-S3; Tables S1-S5.