Abstract
The reovirus ς1s protein is a 14-kDa nonstructural protein encoded by the S1 gene segment. The S1 gene has been linked to many properties of reovirus, including virulence and induction of apoptosis. Although the function of ς1s is not known, the ς1s open reading frame is conserved in all S1 gene sequences determined to date. In this study, we identified and characterized a variant of type 3 reovirus, T3C84-MA, which does not express ς1s. To facilitate these experiments, we generated two monoclonal antibodies (MAbs) that bind different epitopes of the ς1s protein. Using these MAbs in immunoblot and immunofluorescence assays, we found that L929 (L) cells infected with T3C84-MA do not contain ς1s. To determine whether ς1s is required for reovirus infection of cultured cells, we compared the growth of T3C84-MA and its parental strain, T3C84, in L cells and Madin-Darby canine kidney (MDCK) cells. After 48 h of growth, yields of T3C84-MA were equivalent to yields of T3C84 in L cells and were fivefold lower than yields of T3C84 in MDCK cells. After 7 days of growth following adsorption at a low multiplicity of infection, yields of T3C84-MA and T3C84 did not differ significantly in either L cells or MDCK cells. To determine whether ς1s is required for apoptosis induced by reovirus infection, T3C84-MA and T3C84 were tested for their capacity to induce apoptosis, using an acridine orange staining assay. In these experiments, the percentages of apoptotic cells following infection with T3C84-MA and T3C84 were equivalent. These findings indicate that nonstructural protein ς1s is not required for reovirus growth in cell culture and does not influence the capacity of reovirus to induce apoptosis. Therefore, reovirus replication does not require the full complement of virally encoded proteins.
Reoviruses contain a genome consisting of 10 discrete segments of double-stranded RNA (44). Each gene segment is monocistronic with the exception of the S1 gene, which encodes two proteins, viral attachment protein ς1 and nonstructural protein ς1s, in overlapping open reading frames (ORFs) (15, 21, 37). Studies using reassortant viruses to investigate mechanisms of reovirus pathogenesis indicate that the S1 gene segregates with strain-specific differences in reovirus growth in the intestine (4, 24), pathway of spread in the host (24, 25, 45), tropism for neural tissues (25, 50, 51), inhibition of DNA synthesis, (39, 46), and induction of apoptosis (35, 46, 47). In addition, mutations in the S1 gene are selected during persistent reovirus infections of cultured cells (23, 52, 55). For most properties linked to the S1 gene, a direct association with the ς1 protein has been deduced by the demonstration that a particular phenotype is determined by viral attachment (9, 31, 42, 51) or by the identification of mutations in the deduced amino acid sequence of ς1 without an attendant change in ς1s (2). For other S1-mediated properties, an association with ς1 is inferred from studies using UV-irradiated virions (39, 40, 47), which are incapable of expressing ς1s. Thus, previous studies of reovirus properties associated with the S1 gene have not provided insight into the function or importance of nonstructural protein ς1s.
The existence of the 14-kDa ς1s protein was first predicted upon the discovery that two discrete translation initiation sites on s1 mRNAs were protected by ribosomes in RNase protection assays (27). Polyclonal antisera raised to peptides corresponding to predicted antigenic regions of ς1s were used to demonstrate by both immunoprecipitation and immunofluorescence that ς1s is expressed in murine L929 (L) cells infected with type 3 reovirus and that ς1s has a cytoplasmic localization (8). Studies of the kinetics of ς1s expression indicate that ς1s appears in reovirus-infected cells 8 to 12 h postinfection (6, 21). Additional work suggests that ς1s also is capable of translocation into the nucleus (3).
The ς1s ORF is maintained in every S1 gene sequence determined to date and varies from 119 to 125 amino acids in length (1, 2, 7, 9, 12, 14, 29, 30, 32, 55). Alignments of ς1s-deduced amino acid sequences of prototype reovirus strains type 1 Lang (T1L), type 2 Jones, and type 3 Dearing (T3D) demonstrate that ς1s is highly divergent among strains of the three reovirus serotypes, sharing only 18 identical amino acid positions (14). Among 11 serotype 3 reovirus isolates, deduced amino acid sequences of ς1s share 59% sequence identity (12). The only region of ς1s conserved among all S1 gene sequences of the type 3 strains analyzed thus far is a highly basic region of approximately 8 to 12 amino acids near the amino terminus (7, 12, 30).
To characterize the role of ς1s in the reovirus life cycle, a variant of type 3 reovirus that does not express ς1s was identified and studied. Two hybridomas expressing anti-ς1s monoclonal antibodies (MAbs) were isolated, and these antibodies were used to confirm that ς1s is not expressed by the viral variant during infection of cultured cells. The requirement of ς1s for reovirus growth and cellular injury was determined in assays of viral yield and apoptosis induction. Results of these studies represent the first description of a viable reovirus null mutant.
MATERIALS AND METHODS
Cells and viruses.
Spinner-adapted murine L929 (L) cells were grown in either suspension or monolayer cultures in Joklik’s modified Eagle medium (Irvine Scientific, Santa Ana, Calif.) as previously described (9). Madin-Darby canine kidney (MDCK) cells were grown in modified Eagle’s medium (Gibco BRL, Gaithersburg, Md.) that was supplemented to contain 10% fetal bovine serum (Intergen, Purchase, N.Y.), 2 mM l-glutamine, 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 250 ng of amphotericin per ml (Irvine). Spodoptera frugiperda cells (Sf21 and High 5) (Clontech Laboratories, Palo Alto, Calif.) were grown in Grace’s insect cell medium (Gibco) supplemented to contain 10% fetal bovine serum, 2 mM l-glutamine, 50 U of penicillin per ml, and 50 μg of streptomycin per ml. Sp2/0-Ag14 myeloma cells (American Type Culture Collection, Rockville, Md.) and hybridoma cells were grown in Dulbecco’s modified Eagle medium (Gibco) supplemented to contain either 10% (DMEM-10) or 20% (DMEM-20) fetal bovine serum, 20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES) (Gibco), 1 mM sodium pyruvate (Gibco), 0.1 mM nonessential amino acids (Gibco), 2 mM l-glutamine, 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 250 ng of amphotericin per ml. Hybridoma cells were selected in DMEM-20 containing 0.1 mM hypoxanthine, 0.4 μM aminopterin, and 16 μM thymidine (HAT medium; Sigma, St. Louis, Mo.) and subcloned in DMEM-20 supplemented to contain 5% Hybridoma Cloning Factor (Igen, Gaithersburg, Md.).
Reovirus strain T3D is a laboratory stock. The reovirus field isolate strain type 3 clone 84 (T3C84) was isolated from a human host (12, 36). T3C84-MA was isolated by serial passage of T3C84 in murine erythroleukemia (MEL) cells as previously described (9). Purified virions were prepared using second-passage L-cell lysate stocks of twice-plaque-purified reovirus as previously described (18). Baculovirus vector strains were derived from Autographa californica nuclear polyhedrosis virus (Clontech).
Expression and purification of epitope-tagged ς1s.
A cDNA of the T3D reovirus S1 gene segment was generated by reverse transcriptase PCR amplification of purified reovirus double-stranded RNA, using primers specific for the noncoding regions of the S1 gene as previously described (9). The S1 cDNA was cloned into the pCR2.1 vector (Invitrogen, San Diego, Calif.), and this construct, termed pCR2.1-S1, was used as a template to amplify the ς1s ORF in subsequent PCRs. The octapeptide FLAG epitope tag (Kodak, New Haven, Conn.) was appended to the amino terminus of ς1s by PCR amplification of the ς1s ORF, using primers containing FLAG-encoding sequences. This PCR product was cloned into the pCR2.1 vector and then subcloned into the pBacPAK8 transfer vector (Clontech). Fidelity of the cDNA encoding the FLAG-ς1s fusion protein was confirmed by dideoxy chain-termination sequencing. Linearized BacPAK6 baculovirus genomic DNA (Clontech) and the recombinant pBacPAK8 transfer vector were cotransfected into Sf21 cells. Baculovirus recombinants arising from the cotransfection were plaque purified on Sf21 cell monolayers, and second-passage lysate stocks of recombinant baculovirus were generated by using Sf21 cells. The FLAG-ς1s fusion protein was expressed in High 5 cells infected with recombinant virus. Expressed ς1s protein in cell lysates was recovered using an affinity gel containing FLAG-specific MAb M2 (Kodak). After washing, fusion protein-containing affinity gel was heated at 100°C for 5 min in Laemmli sample buffer (28) and FLAG-ς1s protein was resolved using a preparative 14% polyacrylamide gel. Bands corresponding to FLAG-ς1s (∼15 kDa) were excised and electroeluted. The eluate was dialyzed against 150 mM Tris-HCl (pH 7.4) and concentrated using a Centricon filter (10,000 molecular weight cutoff) (Amicon, Beverly, Mass.).
Expression and purification of ς1s as a fusion with MBP.
The pCR2.1-S1 construct was used to amplify the ς1s ORF by PCR, and PCR products encoding full-length or truncated ς1s proteins were subcloned into the pMAL-c2 vector (New England Biolabs, Beverly, Mass.). Three maltose-binding protein (MBP)-ς1s constructs were generated: MBP plus full-length ς1s (MBP-ς1s/1–120), MBP plus ς1s amino acids 1 to 84 (MBP-ς1s/1–84), and MBP plus ς1s amino acids 1 to 42 (MBP-ς1s/1–42). The fidelity of cDNAs encoding the MBP-ς1s fusion proteins was confirmed by dideoxy chain-termination sequencing. Recombinant pMAL-c2 vectors were used to transform Escherichia coli DH5α, which was induced to express the fusion protein by the addition of 0.3 mM isopropyl-β-d-thiogalactopyranoside. After 3 to 4 h of growth, cells were pelleted by centrifugation and resuspended in column buffer (20 mM Tris-HCl [pH 7.4], 200 mM NaCl, 1 mM EDTA). Cells were lysed by sonication, and cellular debris was removed by centrifugation. Fusion proteins were purified by affinity chromatography using an amylose resin (New England Biolabs).
Indirect ELISA.
Detection of ς1s-specific antibodies was performed by enzyme-linked immunosorbent assay (ELISA), using FLAG-ς1s fusion protein as antigen. EIA/RIA plates (Costar, Cambridge, Mass.) were coated with 100 ng of FLAG-ς1s per ml and incubated with primary antibody (cell culture supernatants or serum). Horseradish peroxidase-conjugated sheep anti-mouse secondary antibody (Amersham, Arlington Heights, Ill.) was incubated with FLAG-ς1s and primary antibody, followed by the addition of 2,2′-azinobis(3-ethylbenzthiazoline)-sulfonic acid substrate (Sigma). Color reactions were quantitated in a Titertek Multiscan Plus ELISA plate reader (Flow Laboratories, McLean, Va.) at a wavelength of 405 nm.
Generation and characterization of anti-ς1s MAbs.
BALB/c mice were inoculated intraperitoneally with 50 μg of MBP-ς1s fusion protein combined with Ribi adjuvant (RIBI, Hamilton, Mont.). Booster inoculations were given every 3 weeks, and anti-ς1s antibody titers were monitored by indirect ELISA with FLAG-ς1s fusion protein as antigen. Once antibody titers exceeded 1:1,000 by ELISA, mice were boosted with MBP-ς1s in the absence of adjuvant and spleens were harvested 3 days later. Spleen cells were fused with Sp2/0-Ag14 myeloma cells using polyethylene glycol 4000 (Merck, Gibbstown, N.J.), and the products of each fusion were cultured on murine peritoneal macrophage feeder layers in HAT medium. When hybridomas were 10 to 20% confluent, supernatants from each colony were screened for anti-ς1s antibodies by indirect ELISA using FLAG-ς1s fusion protein as antigen. Cells from antibody-positive colonies were subcloned by limiting dilution. When subcloned colonies were 10 to 20% confluent, supernatants were again screened for anti-ς1s antibodies by indirect ELISA. Hybridoma cells secreting anti-ς1s antibodies were injected into the peritoneum of BALB/c mice, and ascitic fluid was harvested. MAbs were purified using Econo-Pac protein-A chromatography columns (Bio-Rad). Isotyping was performed using a capture ELISA hybridoma subtyping kit (Boehringer-Mannheim, Indianapolis, Ind.).
Immunoblot analysis of reovirus proteins.
L cells were adsorbed with reovirus strains at a multiplicity of infection (MOI) of 10 PFU per cell. After 0 to 28 h of incubation at 37°C, cytoplasmic extracts were prepared by washing cells in phosphate-buffered saline (PBS) followed by incubation in hypotonic lysis buffer (10 mM HEPES [pH 7.9], 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, and a protease inhibitor cocktail [5 μg of antipain per ml, 5 μg of aprotinin per ml, 5 μg of leupeptin per ml, 0.5 μg of pepstatin per ml, 7.5 μg of bestatin per ml, 4 μg of phosphoramidon per ml, and 5 μg of soybean trypsin inhibitor per ml]) at 4°C for 15 min. Nonidet P-40 was added to a final concentration of 0.65% (vol/vol), samples were vortexed, and cell membranes and nuclei were pelleted by centrifugation.
Protein extracts from L cells were electrophoresed in either 14% sodium dodecyl sulfate-polyacrylamide gels (28) (100 μg of total protein per lane) or 16.5% Tris-Tricine Ready Gels (Bio-Rad), transferred to a nitrocellulose membrane, and preincubated in a solution of Tris-buffered saline (TBS) containing 0.05% Tween-20 and 5% low-fat dry milk. The membrane was incubated with 5 μg of primary antibody per ml diluted in TBS plus Tween-20 and milk. After washing three times in TBS plus Tween-20, the membrane was incubated with horseradish peroxidase-conjugated sheep anti-mouse secondary antibody (Amersham) diluted 1:2,500 in TBS plus Tween-20 and milk. The membrane was washed three times in TBS plus Tween-20, incubated with enhanced chemiluminescent reagent (Amersham), and exposed to Biomax MR film (Kodak).
Immunofluorescence of reovirus-infected L cells.
L cells were grown on 12-mm glass coverslips (VWR Scientific, Atlanta, Ga.) for 2 days prior to infection. Cells were adsorbed with reovirus strains at an MOI of 10 PFU per cell at room temperature for 1 h. After 12 h of incubation at 37°C, cells were washed with PBS and fixed for 2 min in a 1:1 mixture of methanol and acetone. Cells were washed three times with PBS and incubated 10 min in PBS containing 1% Triton X-100 (PBS/Triton). Nonspecific binding of antibody to cells was blocked by incubation with 2% normal goat serum (NGS) diluted in PBS/Triton (PBS/Triton/NGS). Cells were then incubated for 45 min with anti-ς1s primary antibody (50 μg per ml diluted in PBS/Triton/NGS). After three washes in PBS/Triton/NGS, cells were incubated with biotinylated goat anti-mouse immunoglobulin G2a (IgG2a) (diluted 1:1,000 in PBS/Triton/NGS) (Amersham) for 45 min. Cells were washed three times in PBS/Triton/NGS and incubated with streptavidin-Cy2 conjugate (Amersham) (diluted 1:1,000 in PBS/Triton/NGS), TO-PRO-3 (Molecular Probes, Eugene, Ore.) (1:1,000), and anti-ςNS MAb 2H7 cross-linked to Cy3 (10 μg per ml) for 45 min. Cy2, Cy3, and TO-PRO-3 were visualized separately with excitation at 488, 543, and 643 nm, respectively, using a Zeiss LSM 410 confocal microscope equipped with a 63× Plan-Apochromat 1.4 NA oil-immersion objective lens. Images were processed using Adobe Photoshop 4.0.
Quantitation of reovirus growth in L cells and MDCK cells.
Cells (2 × 105 cells) grown in 24-well tissue-culture plates (Costar) were infected with reovirus strains at MOIs of 0.001 or 10 PFU per cell. Following viral adsorption for 1 h, the inoculum was removed, 1.0 ml of fresh medium was added, and cells were incubated at 37°C for defined intervals. Cells and culture media were frozen (−70°C) and thawed twice, and virus contained in cell lysates was titrated on L-cell monolayers by plaque assay (48). In experiments to determine viral titer in cell lysates and cell supernatants, cell culture medium was removed and replaced with an equal volume of PBS prior to freezing of cells.
Quantitation of apoptosis by AO staining.
L cells (2 × 105 cells) grown in 24-well tissue culture plates were infected with reovirus strains at an MOI of 100 PFU per cell. The percentage of apoptotic cells was determined by acridine orange (AO) staining as previously described (13, 35, 47). Briefly, cells were washed in PBS and incubated with trypsin-EDTA (Irvine). Cell culture medium, the PBS wash, and trypsinized cells were combined and centrifuged. The cell pellet was resuspended in approximately 25 μl of cell culture medium and stained with 2 μl of a solution containing 100 μg of AO (Sigma) per ml and 100 μg of ethidium bromide (Sigma) per ml. The percentage of cells exhibiting condensed chromatin was determined by epiillumination fluorescence microscopy using a fluorescein filter set (Nikon).
RESULTS
Identification of a ς1s-null reovirus variant.
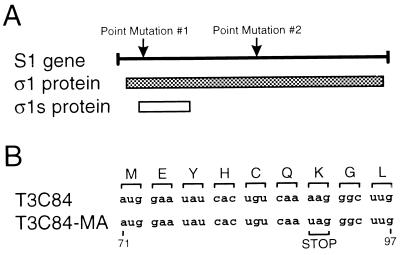
Reovirus strain T3C84, isolated from a human host in 1961 (12, 36), does not bind to or grow in MEL cells. Serial passage of T3C84 in MEL cells was used to select a viral variant that efficiently infects these cells (9). MEL-adapted viruses were plaque purified, and the S1 gene nucleotide sequence of a tenth-passage isolate (T3C84-MA) was determined. The S1 gene sequence of this strain was found to contain two point mutations (Fig. 1A). One of these is a U to C transition at nucleotide position 616, which results in a tryptophan to arginine substitution at amino acid position 202 in the ς1 protein (Fig. 1A, Point Mutation #1). The tryptophan to arginine mutation occurs in a region of ς1 important for its binding to the reovirus receptor on MEL cells, sialic acid (9, 11). The other point mutation in the T3C84-MA S1 gene is an A to U transversion at nucleotide position 89 (Fig. 1A, Point Mutation #2). This mutation results in a lysine to isoleucine substitution at amino acid position 26 in ς1 and the introduction of a stop codon following amino acid position 6 in ς1s (Fig. 1B).
FIG. 1.
(A) Linear depiction of the S1 gene and its protein products, ς1 and ς1s. Arrows indicate point mutations found in the S1 gene segment of T3C84-MA. (B) Nucleotide sequences of the first nine codons of the ς1s ORF of T3C84 and T3C84-MA. Amino acids in the single-letter code are shown above the corresponding nucleotide sequences in the S1 gene. A stop codon is shown at codon 7 in the T3C84-MA ς1s sequence.
Generation and characterization of anti-ς1s MAbs.
To determine whether ς1s is expressed in cells infected with T3C84-MA, MAbs were generated for use in immunoblot and immunofluorescence assays. Recombinant ς1s protein was expressed as a fusion protein with the FLAG epitope (FLAG-ς1s) in insect cells using a baculovirus expression system and with MBP (MBP-ς1s) in E. coli. Mice were immunized with MBP-ς1s and monitored by ELISA, using FLAG-ς1s as antigen, for production of antibodies against ς1s. Using this strategy, two ς1s-specific IgG2a MAbs, 2F4 and 3E2, were obtained that are capable of immunoblotting, immunostaining, and immunoprecipitating ς1s from cells infected with type 3 reovirus.
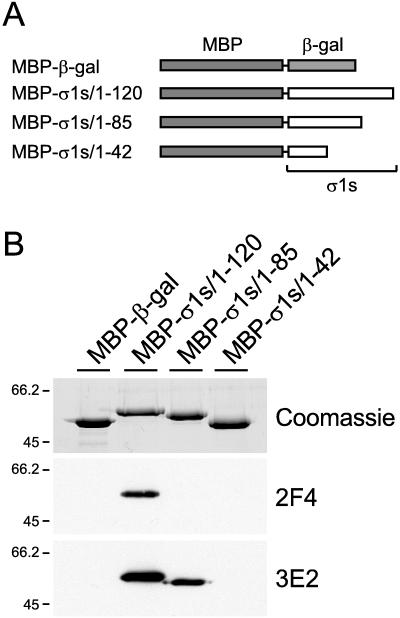
To determine whether these antibodies bind independent domains of the ς1s protein, full-length or truncated forms of ς1s were expressed as fusion proteins with MBP (Fig. 2A) and used as targets in immunoblot assays (Fig. 2B). MAb 2F4 recognized only the full-length ς1s fusion protein, MBP-ς1s/1–120, and did not bind either of the truncation mutants, MBP-ς1s/1–84 or MBP-ς1s/1–42. These data suggest that MAb 2F4 recognizes an epitope containing sequences in the carboxy-terminal one-third of ς1s (amino acids 85 to 120). MAb 3E2 recognized the full-length ς1s fusion protein and truncation mutant MBP-ς1s/1–84 but did not bind MBP-ς1s/1–42. These findings suggest that the epitope recognized by MAb 3E2 contains sequences from the middle portion of ς1s (amino acids 43 to 84) but not the carboxy-terminal one-third. Thus, ς1s-specific MAbs 2F4 and 3E2 bind discrete antigenic regions of the ς1s protein.
FIG. 2.
(A) Schematic of full-length and truncated forms of ς1s expressed as fusion proteins with MBP. Sequences of the T3D ς1s ORF were cloned into the pMAL-c2 vector. MBP fusion proteins containing β-galactosidase, full-length ς1s (MBP-ς1s/1–120), or truncations of ς1s (MBP-ς1s/1–84 and MBP-ς1s/1–42) were expressed in E. coli and purified by affinity chromatography using an amylose resin. (B) Immunoblot of MBP-ς1s fusion proteins using MAbs 2F4 and 3E2. The upper gel shows the MBP-β-galactosidase fusion protein as a control and the three MBP-ς1s fusion proteins after electrophoresis in a 10% polyacrylamide gel and staining with Coomassie blue (1 μg of protein per lane). The lower two gels are immunoblots of the same four proteins (20 ng of protein per lane) using MAbs 2F4 and 3E2 (5 μg per ml). Molecular size markers are given in kilodaltons.
To determine the capacity of the anti-ς1s MAbs to detect virally encoded ς1s and to define the kinetics of ς1s expression in reovirus-infected cells, L cells were infected with T3D, and immunoblotting with MAbs 2F4 and 3E2 was performed using cell lysates prepared at various intervals after infection (Fig. 3 and data not shown). The results demonstrate that ς1s expression is detectable in T3D-infected cells by 8 h postinfection, consistent with previously published findings (21). Thus, MAbs generated against an MBP-ς1s fusion protein bind native ς1s produced in reovirus-infected cells.
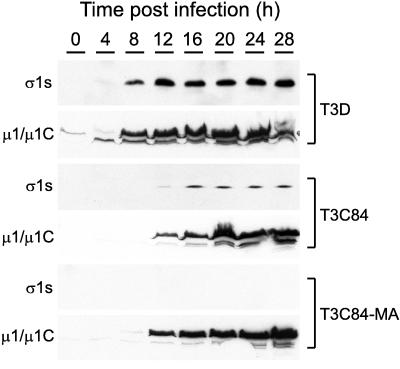
FIG. 3.
Time course of ς1s and μ1/μ1C expression during reovirus infection of L cells. Cells were infected with either T3D, T3C84, or T3C84-MA at an MOI of 10 PFU per cell and harvested at the time points indicated. Cytoplasmic extracts were prepared and electrophoresed in a 14% polyacrylamide gel (100 μg of protein per lane), transferred to nitrocellulose, and blotted with either anti-ς1s MAb 2F4 (5 μg per ml) or anti-μ1/μ1C MAb 8H6 (5 μg per ml).
Determination of ς1s expression in T3C84- and T3C84-MA-infected cells.
Sequence analysis of the T3C84-MA S1 gene suggested that ς1s would not be expressed by this strain due to the presence of a termination codon following amino acid position 6 in the ς1s ORF. To confirm that T3C84-MA is incapable of expressing ς1s, anti-ς1s MAb 2F4 was used to assess ς1s expression by immunoblot analysis (Fig. 3). Expression of ς1s was detected in T3D-infected and T3C84-infected L cells by 8 and 12 h postinfection, respectively; however, ς1s was not detected in T3C84-MA-infected L cells throughout the 28-h time course (Fig. 3). As a control for efficient infection of the cells, MAb 8H6 (49) was used to determine the levels of expression of structural protein μ1/μ1C (Fig. 3). The findings demonstrate that μ1/μ1C was efficiently expressed in cells infected with all three virus strains by 8 to 12 h postinfection. Identical results were obtained using ς1s-specific MAb 3E2 in experiments to assess ς1s expression in T3C84-MA-infected cells (data not shown). To exclude the possibility that downstream initiation products of ς1s are expressed in cells infected with T3C84-MA, lysates of T3D-, T3C84-, and T3C84-MA-infected L cells were resolved in a 16.5% Tris-Tricine gel and subjected to immunoblot analysis using MAb 2F4. No polypeptides smaller than full-length ς1s were detected in cells infected with the three virus strains (data not shown). These results demonstrate that neither full-length ς1s nor downstream initiation products of ς1s are expressed in cells infected with T3C84-MA.
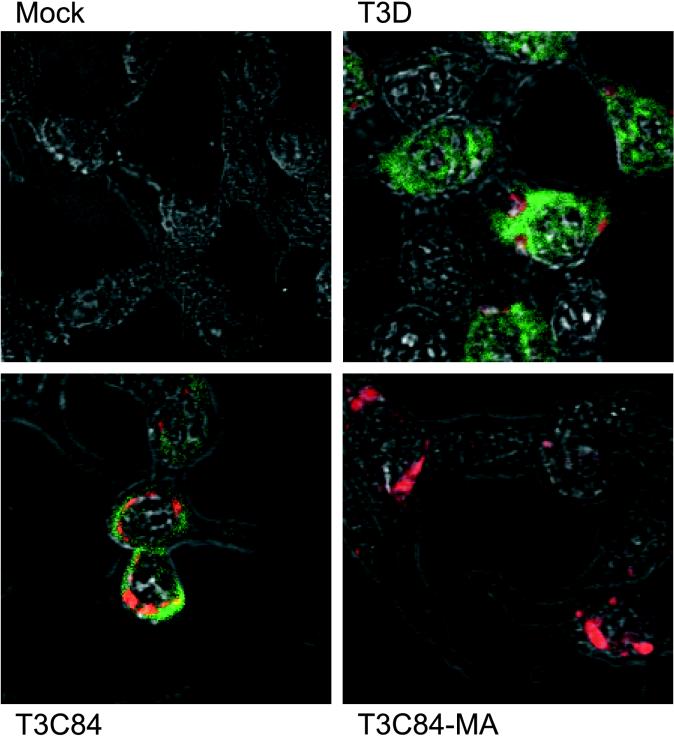
To confirm these results and to determine the subcellular localization of ς1s, immunofluorescence staining for ς1s in reovirus-infected cells was performed by using confocal microscopy. L cells were either mock infected or infected with T3D, T3C84, or T3C84-MA and stained with anti-ς1s MAb 2F4 at 12 h postinfection (Fig. 4). Cells also were stained with anti-ςNS MAb 2H7 (19) as a control for reovirus infection. Using this technique, ς1s and ςNS were detected in T3D-infected and T3C84-infected cells, but only ςNS was detected in T3C84-MA-infected cells. To determine whether ς1s is capable of translocation to the nucleus, reovirus-infected cells also were stained with TO-PRO-3, a nuclear dye (data not shown). In cells infected with either T3D or T3C84, ς1s was distributed throughout the cytoplasm and also was detected in the nucleus, overlapping in distribution with TO-PRO-3; ς1s was not concentrated at perinuclear sites of virus assembly (17, 34, 38). These data are consistent with immunoblot analyses of lysates obtained from reovirus-infected L cells and indicate that cells infected with T3C84-MA do not express ς1s.
FIG. 4.
Detection of ς1s and ςNS expression in reovirus-infected L cells by immunofluorescence staining. Cells grown on glass coverslips were mock-infected or infected with reovirus strains T3D, T3C84, or T3C84-MA at an MOI of 10 PFU per cell and fixed in methanol-acetone 12 h postinfection. Cells were incubated with anti-ς1s MAb 2F4 (50 μg per ml), followed by biotinylated goat anti-mouse IgG2a (1:1,000). Cells then were incubated with streptavidin-conjugated Cy2 and anti-ςNS MAb 2H7 cross-linked to Cy3 (10 μg per ml). Green fluorescence indicates ς1s; red fluorescence indicates ςNS. Immunofluorescence was visualized with a Zeiss LSM 410 confocal microscope.
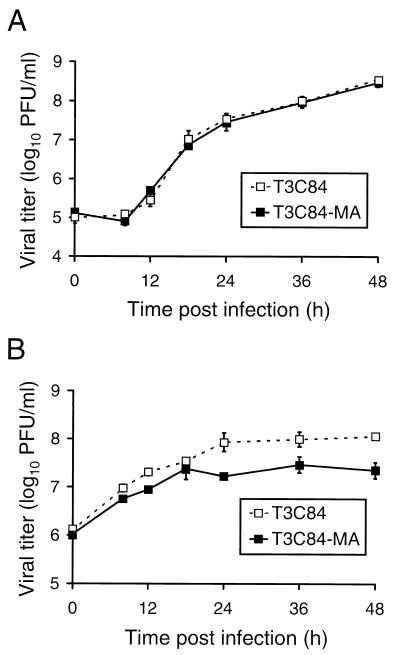
Growth of T3C84 and T3C84-MA in L cells and MDCK cells.
To assess the importance of ς1s in reovirus replication, strains T3C84 and T3C84-MA were used to infect L fibroblast cells and MDCK epithelial cells. Virus was adsorbed to both cell types at an MOI of 10 PFU per cell, and virus titers were determined in cell lysates at various times postadsorption (Fig. 5). After 48 h of growth in L cells, both T3C84 and T3C84-MA produced titers of approximately 5 × 108 PFU per ml, representing greater than a 1,000-fold increase in viral yield. Furthermore, T3C84 and T3C84-MA demonstrated identical growth kinetics in L cells during the assay period and displayed no significant differences in plaque morphology. In MDCK cells, T3C84 and T3C84-MA produced titers of 1.1 × 108 and 2.2 × 107 PFU per ml, respectively, after 48 h of growth. These findings suggest that the ς1s protein is not required for reovirus growth in cell culture but that expression of ς1s may provide a slight growth advantage in MDCK cells.
FIG. 5.
Growth of T3C84 and T3C84-MA in L cells (A) and MDCK cells (B). Cells (2 × 105) were infected with either T3C84 or T3C84-MA at an MOI of 10 PFU per cell. After adsorption for 1 h, the inoculum was removed and cells were incubated at 37°C for the times shown. Titers of virus in cell lysates were determined on L-cell monolayers by plaque assay. The results are presented as the mean viral titers for three independent experiments. Error bars indicate standard deviations of the means.
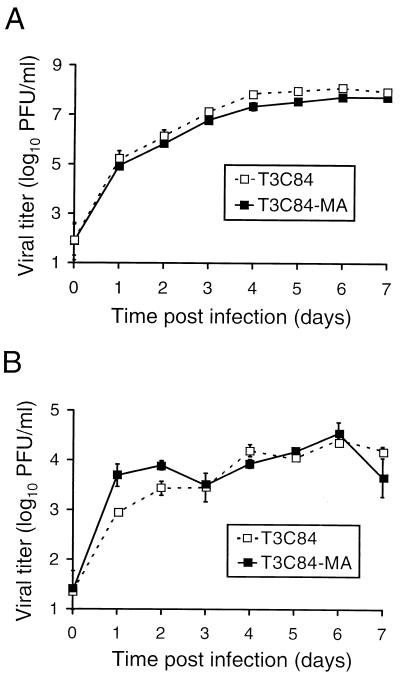
Growth of T3C84 and T3C84-MA in L cells and MDCK cells after viral adsorption at a low MOI.
To further assess whether ς1s confers any advantage to reovirus replication in L cells or MDCK cells, T3C84 and T3C84-MA were adsorbed to cells at an MOI of 0.001 PFU per cell and viral titers were determined at 24-h intervals for 7 days (Fig. 6). We reasoned that if ς1s were responsible for a small contribution to viral growth, then a difference in viral yield might be apparent in L cells after several cycles of infection, and the fivefold difference observed in MDCK cells would be enhanced. After 7 days of viral growth in L cells, T3C84 and T3C84-MA reached titers of 8.6 × 107 and 5.2 × 107 PFU per ml, respectively. In MDCK cells, T3C84 and T3C84-MA reached maximal titers of 2.7 × 104 and 3.5 × 104 PFU per ml, respectively, over a 7-day growth period. Therefore, after several cycles of viral replication, expression of the ς1s protein does not confer a discernible growth advantage in either L cells or MDCK cells.
FIG. 6.
Growth of T3C84 and T3C84-MA in L cells (A) and MDCK cells (B) after viral adsorption at a low MOI. Cells (2 × 105) were infected with either T3C84 or T3C84-MA at an MOI of 0.001 PFU per cell. After adsorption for 1 h, the inoculum was removed and cells were incubated at 37°C for the times shown. Titers of virus in cell lysates were determined on L-cell monolayers by plaque assay. The results are presented as the mean viral titers for three independent experiments. Error bars indicate standard deviations of the means.
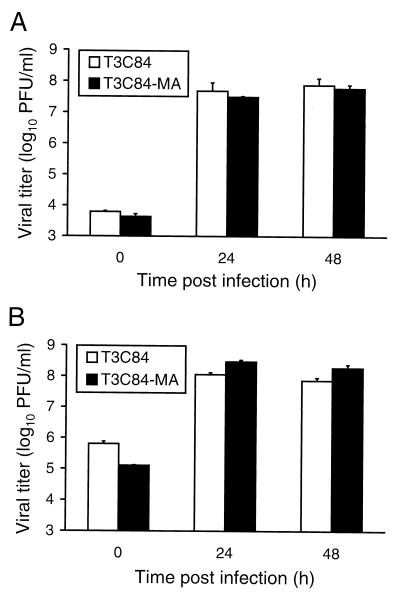
Yields of cell-free virus from cells infected with T3C84 and T3C84-MA.
In the experiments described above, viral yields were determined by titrating virus from lysates of infected cells. To determine whether ς1s plays a role in release of progeny virions from cells infected with reovirus, L cells were adsorbed with either T3C84 or T3C84-MA at an MOI of 10 PFU per cell and virus titers were determined for both culture supernatants and cell lysates at various times postadsorption (Fig. 7). After 48 h of growth, T3C84 and T3C84-MA produced titers of approximately 7.5 × 107 and 5.9 × 107 PFU per ml, respectively, in culture supernatants and 7.3 × 107 and 1.9 × 108 PFU per ml, respectively, in cell lysates. Moreover, T3C84 and T3C84-MA did not differ in the kinetics of viral release as judged by accumulation of viral titer in culture supernatants over time. Thus, the ς1s protein is not required for efficient release of progeny virions from reovirus-infected cells.
FIG. 7.
Yields of T3C84 and T3C84-MA in L-cell culture supernatants (A) and cell lysates (B). L cells (2 × 105) were infected with either T3C84 or T3C84-MA at an MOI of 10 PFU per cell. After adsorption for 1 h, the inoculum was removed and cells were incubated at 37°C for the times shown. Virus in culture supernatants and cell lysates was titrated on L-cell monolayers by plaque assay. The results are presented as the mean viral titers for three independent experiments. Error bars indicate standard deviations of the means.
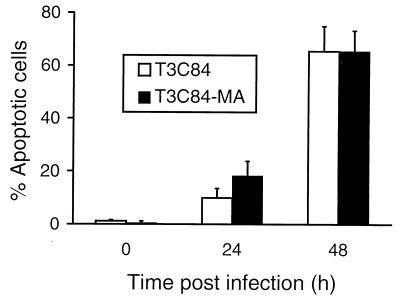
Apoptosis induction by T3C84 and T3C84-MA in L cells.
Differences in the capacity of reovirus strains to induce apoptosis in L cells (46, 47) and MDCK cells (35) have been mapped to the S1 gene segment, which encodes both viral attachment protein ς1 and nonstructural protein ς1s. To directly assess the role of ς1s in apoptosis, L cells were infected with reovirus strains T3C84 and T3C84-MA and apoptosis was quantitated using an AO staining assay (13, 47) (Fig. 8). AO is a fluorescent dye that allows cells undergoing apoptosis to be identified by the presence of condensed chromatin. T3C84 and T3C84-MA induced equivalent levels of apoptosis using this assay. This result indicates that the ς1s protein is not required for apoptosis induction by reovirus.
FIG. 8.
Apoptosis induction by T3C84 and T3C84-MA in L cells. Cells (2 × 105) were infected with either T3C84 or T3C84-MA at an MOI of 100 PFU per cell. After adsorption for 1 h, cells were incubated at 37°C for the indicated times and stained with AO. The results are expressed as the mean percentage of cells undergoing apoptosis for three independent experiments. Error bars indicate standard deviations of the means.
DISCUSSION
Reovirus nonstructural protein ς1s has no known function, although it is expressed at detectable levels during reovirus infection of cultured cells (6, 21) (Fig. 3 and 4). The fact that the ς1s ORF is conserved in every S1 gene sequence reported to date, representing 28 independent reovirus isolates (1, 2, 7, 9, 12, 14, 29, 30, 32, 55), suggests that ς1s confers some selective advantage to reovirus replication and plays an important biological role. Examination of the deduced amino acid sequences of nonstructural proteins of other members of the Reoviridae family does not reveal a homologue for the ς1s protein (10). Moreover, protein database searches (GenBank CDS translations, Brookhaven Protein Data Bank, SwissProt, and Protein Information Resource) do not identify proteins with significant primary sequence similarity to ς1s (10). Thus, the deduced amino acid sequence of ς1s does not lead to obvious inferences about its function.
The purpose of this study was to investigate the role of the ς1s protein in reovirus infection by characterizing a type 3 reovirus variant, T3C84-MA, that does not express ς1s. Reovirus strain T3C84-MA was isolated in a study of receptor-binding mutants of field isolate strains of type 3 reovirus. Nucleotide sequence analysis of the T3C84-MA S1 gene suggested that ς1s would not be expressed in cells infected with this variant (9). Consistent with this prediction, full-length or truncated forms of ς1s were not detected in T3C84-MA-infected L cells using immunoblot (Fig. 3 and data not shown) and immunofluorescence (Fig. 4) assays. These findings are in contrast to those obtained using either parental strain T3C84 or prototype strain T3D in which ς1s was found by immunofluorescence to be distributed throughout the cytoplasm and was detected at low levels in the nucleus. The finding that ς1s is capable of nuclear translocation in reovirus-infected cells confirms the results of a previous study that demonstrated nuclear localization of ς1s in COS cells transfected with ς1s (3).
Our findings clearly show that ς1s expression is not required for reovirus growth in cultured cells. Yields of T3C84 and T3C84-MA following adsorption at an MOI of 10 PFU per cell were equivalent after 48 h of growth in L cells (Fig. 5A) and differed minimally after 48 h of growth in MDCK cells (Fig. 5B). As a more stringent means to assess the possible contributions of ς1s to reovirus growth in cell culture, we compared the yields of T3C84 and T3C84-MA in L cells and MDCK cells after several cycles of viral replication, following infection at a low MOI. In these experiments, we again found no significant differences in yields of T3C84 and T3C84-MA in either cell type (Fig. 6). We also performed experiments to test whether ς1s contributes to the release of progeny virions from reovirus-infected cells. Similar to the results obtained in assays of viral growth, we found no difference between T3C84 and T3C84-MA in the kinetics or quantity of virus released into culture supernatants of infected cells (Fig. 7). Therefore, these results indicate that ς1s is dispensable for reovirus growth in cell culture and demonstrate that reovirus infection of cultured cells does not require the full complement of viral proteins.
Identification of T3C84-MA afforded the opportunity to directly test whether ς1s influences apoptosis induction by reovirus. In previous studies of reovirus-induced apoptosis of L cells and MDCK cells, differences in the capacity of type 1 and type 3 reovirus strains to induce apoptosis were linked by using reassortant viruses to the S1 gene (35, 46, 47). UV-irradiated reovirus virions, which are incapable of mediating viral protein synthesis (40), induce apoptosis efficiently (47), which suggests that ς1s is not involved in apoptosis induction. Concordantly, in the context of productive reovirus infection, T3C84-MA and its parent, T3C84, induced equivalent levels of apoptosis as determined by AO staining (Fig. 8). In a previous study, overexpression of T3D ς1s in murine C127 cells appeared to increase the cytopathicity of T1L and T3D (16), suggesting that the ς1s protein plays a role in reovirus-induced cell death. However, our findings indicate that apoptosis, which serves as an important mechanism of reovirus-induced cytopathicity in cell culture (35, 46, 47) and in vivo (33), is not affected by the absence of ς1s protein. These results support the hypothesis that ς1, and not ς1s, is the S1 gene product responsible for mediating differences in the capacity of reovirus strains to induce apoptosis.
Since ς1s is not required for reovirus infection of cultured cells or for the induction of apoptosis, strict retention of the ς1s ORF strongly suggests that the ς1s protein is important for viral growth or spread in vivo. There are ample precedents for viral proteins that are not required for growth in cultured cells yet play important roles in virus-host interactions (reviewed in reference 53). For example, the adenovirus E3 19-kDa protein is dispensable for viral replication in cultured cells (26) but modulates the host immune response by blocking cell surface expression of major histocompatibility complex (MHC) class I antigens (5). Similarly, the human cytomegalovirus US11 gene product, though not required for efficient growth in cell culture (22), down regulates MHC class I expression by targeting these molecules for proteosomal degradation (54). If ς1s plays an important role in reovirus replication in vivo, a ς1s-negative strain such as T3C84-MA would be expected to display altered virulence in comparison to wild-type reovirus. Because T3C84-MA and its parental strain, T3C84, also differ in the capacity to bind sialylated receptors (9), differences in the pathogenicity of these strains are not necessarily attributable to ς1s function. To address this issue, we currently are attempting to isolate a variant of T3C84 that binds sialic acid and retains the ς1s ORF.
Studies of other members of the Reoviridae family indicate that these viruses can tolerate genomic deletions and rearrangements yet retain the capacity to replicate in cell culture, albeit with less efficiency than wild-type viruses. For example, nondefective rotavirus strains have been described with deletions, truncations, or rearrangements of gene 5, which encodes NSP1 (20, 41, 43). In each of these cases, rotaviruses expressing truncated or rearranged NSP1 proteins exhibit reduced viral yields or decreased plaque size, suggesting that NSP1 affects the efficiency of viral growth. To our knowledge, reovirus strain T3C84-MA represents the first viable null mutant of mammalian reovirus and the first null mutant of the Reoviridae family without a detectable defect in viral growth.
Results presented here establish that reovirus nonstructural protein ς1s is not required for efficient viral growth in cell culture, making it unique among reovirus proteins. These results also suggest that the ς1s protein plays a role in virus-host interactions in vivo. Our future studies will focus on the delineation of ς1s-mediated effects on viral replication, cytopathology, and pathogenesis in mammalian hosts.
ACKNOWLEDGMENTS
We are grateful to Geoff Baer, Mehmet Goral, Larry Kerr, Aaron Shatkin, Paul Spearman, and Ken Tyler for expert advice. Guidance in the production of MAbs was provided by Randy Emmons. We thank Chris Aiken, Erik Barton, Patrick Green, and Denise Wetzel for careful reviews of the manuscript.
This work was supported by Public Health Service award T32 GM07347 from the National Institute of General Medical Studies for the Vanderbilt Medical Scientist Training Program (S.E.R.), Public Health Service award AI38296 from the National Institute of Allergy and Infectious Diseases, and the Elizabeth B. Lamb Center for Pediatric Research. Additional support was provided by Public Health Service awards P60 DK20593 for the Vanderbilt Diabetes Research and Training Center and CA68485 and DK20593 for the Vanderbilt Cell Imaging Resource.
REFERENCES
- 1.Bassel-Duby R, Jayasuriya A, Chatterjee D, Sonenberg N, Maizel J V, Jr, Fields B N. Sequence of reovirus haemagglutinin predicts a coiled-coil structure. Nature. 1985;315:421–423. doi: 10.1038/315421a0. [DOI] [PubMed] [Google Scholar]
- 2.Bassel-Duby R, Spriggs D R, Tyler K L, Fields B N. Identification of attenuating mutations on the reovirus type 3 S1 double-stranded RNA segment with a rapid sequencing technique. J Virol. 1986;60:64–67. doi: 10.1128/jvi.60.1.64-67.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belli B A, Samuel C E. Biosynthesis of reovirus-specified polypeptides: expression of reovirus S1 encoded sigma 1NS protein in transfected and infected cells as measured with serotype specific polyclonal antibody. Virology. 1991;185:698–709. doi: 10.1016/0042-6822(91)90541-i. [DOI] [PubMed] [Google Scholar]
- 4.Bodkin D K, Fields B N. Growth and survival of reovirus in intestinal tissue: role of the L2 and S1 genes. J Virol. 1989;63:1188–1193. doi: 10.1128/jvi.63.3.1188-1193.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burgert H, Maryanski J, Kvist S. “E3/19K” protein of adenovirus type 2 inhibits lysis of cytolytic T lymphocytes by blocking cell-surface expression of histocompatibility class I antigens. Proc Natl Acad Sci USA. 1987;84:1356–1360. doi: 10.1073/pnas.84.5.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cashdollar L W, Blair P, Van Dyne S. Identification of the ς1s protein in reovirus serotype 2-infected cells with antibody prepared against a bacterial fusion protein. Virology. 1989;168:183–186. doi: 10.1016/0042-6822(89)90420-0. [DOI] [PubMed] [Google Scholar]
- 7.Cashdollar L W, Chmelo R A, Wiener J R, Joklik W K. Sequences of the S1 genes of the three serotypes of reovirus. Proc Natl Acad Sci USA. 1985;82:24–28. doi: 10.1073/pnas.82.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ceruzzi M, Shatkin A J. Expression of reovirus p14 in bacteria and identification in the cytoplasm of infected mouse L cells. Virology. 1986;153:35–45. doi: 10.1016/0042-6822(86)90005-x. [DOI] [PubMed] [Google Scholar]
- 9.Chappell J D, Gunn V L, Wetzel J D, Baer G S, Dermody T S. Mutations in type 3 reovirus that determine binding to sialic acid are contained in the fibrous tail domain of viral attachment protein ς1. J Virol. 1997;71:1834–1841. doi: 10.1128/jvi.71.3.1834-1841.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connolly, J. L., and T. S. Dermody. Unpublished observation.
- 11.Dermody T S, Nibert M L, Bassel-Duby R, Fields B N. A ς1 region important for hemagglutination by serotype 3 reovirus strains. J Virol. 1990;64:5173–5176. doi: 10.1128/jvi.64.10.5173-5176.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dermody T S, Nibert M L, Bassel-Duby R, Fields B N. Sequence diversity in S1 genes and S1 translation products of 11 type 3 reovirus strains. J Virol. 1990;64:4842–4850. doi: 10.1128/jvi.64.10.4842-4850.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duke R C, Cohen J J. Morphological and biochemical assays of apoptosis. In: Coligan J E, editor. Current protocols in immunology. New York, N.Y: Wiley & Sons; 1992. pp. 17.1–17.16. [Google Scholar]
- 14.Duncan R, Horne D, Cashdollar L W, Joklik W K, Lee P W. Identification of conserved domains in the cell attachment proteins of the three serotypes of reovirus. Virology. 1990;174:399–409. doi: 10.1016/0042-6822(90)90093-7. [DOI] [PubMed] [Google Scholar]
- 15.Ernst H, Shatkin A J. Reovirus hemagglutinin mRNA codes for two polypeptides in overlapping reading frames. Proc Natl Acad Sci USA. 1985;82:48–52. doi: 10.1073/pnas.82.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fajardo K E, Shatkin A J. Expression of the two reovirus S1 gene products in transfected mammalian cells. Virology. 1990;178:223–231. doi: 10.1016/0042-6822(90)90397-a. [DOI] [PubMed] [Google Scholar]
- 17.Fields B N, Raine C S, Baum S G. Temperature-sensitive mutants of reovirus type 3: defects in viral maturation as studied by immunofluorescence and electron microscopy. Virology. 1971;43:569–578. doi: 10.1016/0042-6822(71)90282-0. [DOI] [PubMed] [Google Scholar]
- 18.Furlong D B, Nibert M L, Fields B N. Sigma 1 protein of mammalian reoviruses extends from the surfaces of viral particles. J Virol. 1988;62:246–256. doi: 10.1128/jvi.62.1.246-256.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goral, M. I., G. S. Baer, P. R. Hazelton, S. E. Rodgers, K. M. Coombs, and T. S. Dermody. Reovirus non-structural protein ςNS localizes to viral inclusions and is required for viral assembly. Submitted for publication.
- 20.Hua J, Patton J T. The carboxyl-half of the rotavirus nonstructural protein NS53 (NSP1) is not required for virus replication. Virology. 1994;198:567–576. doi: 10.1006/viro.1994.1068. [DOI] [PubMed] [Google Scholar]
- 21.Jacobs B L, Samuel C E. Biosynthesis of reovirus-specified polypeptides: the reovirus S1 mRNA encodes two primary translation products. Virology. 1985;143:63–74. doi: 10.1016/0042-6822(85)90097-2. [DOI] [PubMed] [Google Scholar]
- 22.Jones T, Muzithras V, Gluzman Y. Replacement mutagenesis of the human cytomegalovirus genome: US10 and US11 gene products are nonessential. J Virol. 1991;65:5860–5872. doi: 10.1128/jvi.65.11.5860-5872.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kauffman R S, Ahmed R, Fields B N. Selection of a mutant S1 gene during reovirus persistent infection of L cells: role in maintenance of the persistent state. Virology. 1983;131:79–87. doi: 10.1016/0042-6822(83)90535-4. [DOI] [PubMed] [Google Scholar]
- 24.Kauffman R S, Wolf J L, Finberg R, Trier J S, Fields B N. The sigma 1 protein determines the extent of spread of reovirus from the gastrointestinal tract of mice. Virology. 1983;124:403–410. doi: 10.1016/0042-6822(83)90356-2. [DOI] [PubMed] [Google Scholar]
- 25.Kaye K M, Spriggs D R, Bassel-Duby R, Fields B N, Tyler K L. Genetic basis for altered pathogenesis of an immune-selected antigenic variant of reovirus type 3 Dearing. J Virol. 1986;59:90–97. doi: 10.1128/jvi.59.1.90-97.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelly T, Lewis A. Use of nondefective adenovirus-simian virus 40 hybrids for mapping the simian virus 40 genome. J Virol. 1973;12:643–652. doi: 10.1128/jvi.12.3.643-652.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kozak M. Analysis of ribosome binding sites from the S1 message of reovirus. Initiation at the first and second AUG codons. J Mol Biol. 1982;156:807–820. doi: 10.1016/0022-2836(82)90143-7. [DOI] [PubMed] [Google Scholar]
- 28.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 29.Munemitsu S M, Atwater J A, Samuel C E. Biosynthesis of reovirus-specified polypeptides. Molecular cDNA cloning and nucleotide sequence of the reovirus serotype 1 Lang strain bicistronic s1 mRNA which encodes the minor capsid polypeptide sigma 1a and the nonstructural polypeptide sigma 1bNS. Biochem Biophys Res Commun. 1986;140:508–514. doi: 10.1016/0006-291x(86)90761-8. [DOI] [PubMed] [Google Scholar]
- 30.Nagata L, Masri S A, Mah D C, Lee P W K. Molecular cloning and sequencing of the reovirus (serotype 3) S1 gene which encodes the viral cell attachment protein 1. Nucleic Acids Res. 1984;12:8699–8710. doi: 10.1093/nar/12.22.8699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nibert M L, Chappell J D, Dermody T S. Infectious subvirion particles of reovirus type 3 Dearing exhibit a loss in infectivity and contain a cleaved ς1 protein. J Virol. 1995;69:5057–5067. doi: 10.1128/jvi.69.8.5057-5067.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nibert M L, Dermody T S, Fields B N. Structure of the reovirus cell-attachment protein: a model for the domain organization of ς1. J Virol. 1990;64:2976–2989. doi: 10.1128/jvi.64.6.2976-2989.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oberhaus S M, Smith R L, Clayton G H, Dermody T S, Tyler K L. Reovirus infection and tissue injury in the mouse central nervous system are associated with apoptosis. J Virol. 1997;71:2100–2106. doi: 10.1128/jvi.71.3.2100-2106.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rhim J S, Jordan L E, Mayor H D. Cytochemical, fluorescent-antibody and electron microscopic studies on the growth of reovirus (ECHO 10) in tissue culture. Virology. 1962;17:342–355. doi: 10.1016/0042-6822(62)90125-3. [DOI] [PubMed] [Google Scholar]
- 35.Rodgers S E, Barton E S, Oberhaus S M, Pike B, Gibson C A, Tyler K L, Dermody T S. Reovirus-induced apoptosis of MDCK cells is not linked to viral yield and is blocked by Bcl-2. J Virol. 1997;71:2540–2546. doi: 10.1128/jvi.71.3.2540-2546.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosen L, Hovis J F, Mastrota F M, Bell J A, Huebner R J. Observations on a newly recognized virus (Abney) of the reovirus family. Am J Hyg. 1960;71:258–265. doi: 10.1093/oxfordjournals.aje.a120109. [DOI] [PubMed] [Google Scholar]
- 37.Sarkar G, Pelletier J, Bassel-Duby R, Jayasuriya A, Fields B N, Sonenberg N. Identification of a new polypeptide coded by reovirus gene S1. J Virol. 1985;54:720–725. doi: 10.1128/jvi.54.3.720-725.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharpe A H, Chen L B, Fields B N. The interaction of mammalian reoviruses with the cytoskeleton of monkey kidney CV-1 cells. Virology. 1982;120:399–411. doi: 10.1016/0042-6822(82)90040-x. [DOI] [PubMed] [Google Scholar]
- 39.Sharpe A H, Fields B N. Reovirus inhibition of cellular DNA synthesis: role of the S1 gene. J Virol. 1981;38:389–392. doi: 10.1128/jvi.38.1.389-392.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shaw J E, Cox D C. Early inhibition of cellular DNA synthesis by high multiplicities of infectious and UV-irradiated reovirus. J Virol. 1973;12:704–710. doi: 10.1128/jvi.12.4.704-710.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taniguchi K, Kojima K, Urasawa S. Nondefective rotavirus mutants with an NSP1 gene which has a deletion of 500 nucleotides, including a cysteine-rich zinc finger motif-encoding region (nucleotides 156 to 248), or which has a nonsense codon at nucleotides 153 to 155. J Virol. 1996;70:4125–4130. doi: 10.1128/jvi.70.6.4125-4130.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tardieu M, Powers M L, Weiner H L. Age-dependent susceptibility to reovirus type 3 encephalitis: role of viral and host factors. Ann Neurol. 1983;13:602–607. doi: 10.1002/ana.410130604. [DOI] [PubMed] [Google Scholar]
- 43.Tian Y, Tarlow O, Ballard A, Desselberger U, McCrae M A. Genomic concatemerization/deletion in rotaviruses: a new mechanism for generating rapid genetic change of potential epidemiological importance. J Virol. 1993;67:6625–6632. doi: 10.1128/jvi.67.11.6625-6632.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tyler K L, Fields B N. Reoviruses. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1597–1623. [Google Scholar]
- 45.Tyler K L, McPhee D A, Fields B N. Distinct pathways of viral spread in the host determined by reovirus S1 gene segment. Science. 1986;233:770–774. doi: 10.1126/science.3016895. [DOI] [PubMed] [Google Scholar]
- 46.Tyler K L, Squier M K T, Brown A L, Pike B, Willis D, Oberhaus S M, Dermody T S, Cohen J J. Linkage between reovirus-induced apoptosis and inhibition of cellular DNA synthesis: role of the S1 and M2 genes. J Virol. 1996;70:7984–7991. doi: 10.1128/jvi.70.11.7984-7991.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tyler K L, Squier M K T, Rodgers S E, Schneider B E, Oberhaus S M, Grdina T A, Cohen J J, Dermody T S. Differences in the capacity of reovirus strains to induce apoptosis are determined by viral attachment protein ς1. J Virol. 1995;69:6972–6979. doi: 10.1128/jvi.69.11.6972-6979.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Virgin H W, IV, Bassel-Duby R, Fields B N, Tyler K L. Antibody protects against lethal infection with the neurally spreading reovirus type 3 (Dearing) J Virol. 1988;62:4594–4604. doi: 10.1128/jvi.62.12.4594-4604.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Virgin H W, IV, Mann M A, Fields B N, Tyler K L. Monoclonal antibodies to reovirus reveal structure/function relationships between capsid proteins and genetics of susceptibility to antibody action. J Virol. 1991;65:6772–6781. doi: 10.1128/jvi.65.12.6772-6781.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weiner H L, Drayna D, Averill D R, Jr, Fields B N. Molecular basis of reovirus virulence: role of the S1 gene. Proc Natl Acad Sci USA. 1977;74:5744–5748. doi: 10.1073/pnas.74.12.5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weiner H L, Powers M L, Fields B N. Absolute linkage of virulence and central nervous system tropism of reoviruses to viral hemagglutinin. J Infect Dis. 1980;141:609–616. doi: 10.1093/infdis/141.5.609. [DOI] [PubMed] [Google Scholar]
- 52.Wetzel J D, Wilson G J, Baer G S, Dunnigan L R, Wright J P, Tang D S H, Dermody T S. Reovirus variants selected during persistent infections of L cells contain mutations in the viral S1 and S4 genes and are altered in viral disassembly. J Virol. 1997;71:1362–1369. doi: 10.1128/jvi.71.2.1362-1369.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Whitton J L, Oldstone M B A. Immune responses to viruses. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 345–374. [Google Scholar]
- 54.Wiertz E, Jones T, Sun L, Bogyo M, Geuze H, Ploegh H. The human cytomegalovirus US11 gene product dislocates MHC class I heavy chains from the endoplasmic reticulum to the cytosol. Cell. 1996;84:769–779. doi: 10.1016/s0092-8674(00)81054-5. [DOI] [PubMed] [Google Scholar]
- 55.Wilson G J, Wetzel J D, Puryear W, Bassel-Duby R, Dermody T S. Persistent reovirus infections of L cells select mutations in viral attachment protein ς1 that alter oligomer stability. J Virol. 1996;70:6598–6606. doi: 10.1128/jvi.70.10.6598-6606.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]