Abstract
Schwannomas localized in the sacrum are relatively infrequent, accounting for 1–5% of all spinal axis schwannomas; they present with vague symptoms or are symptomless, so often grow to a considerable size before detection. Sacral schwannomas occasionally present with enormous dimensions, and these tumors are termed giant sacral schwannomas. However, their surgical removal is challenging owing to an abundant vascularity. The present study retrospectively analyzed the clinical and follow-up data of a patient with a giant sacral schwannoma. The patient experienced numbness in the left buttock and lower extremity, with radiating pain in the sole of the foot that had persisted for 3 years. A presacral mass was found by computed tomography examination 6 months after the stool had become thin. A tumor resection was performed using the anterior abdominal approach. A schwannoma was diagnosed by postoperative pathology. The postoperative course was uneventful, with the complete resolution of symptoms during the 21-month clinical follow-up. Overall, the present study reports the case of a giant sacral schwannoma with pelvic pain that was resected without complications and also discusses its successful management. Additionally, the study presents a systematic review of the literature. We consider that the surgical treatment of giant sacral schwannomas with piecemeal subtotal excision can achieve good outcomes, avoiding unnecessary neurological deficits.
Keywords: sacral schwannoma, pre-sacral, operation
Introduction
Sacral schwannomas are infrequent tumors accounting for 1–5% of all spinal schwannomas (1–3). The tumors occur most commonly at 50–60 years of age (median, 56 years) (4). In 1932, Masson revealed that Schwann cells were derived from the nerve sheaths of myelinated nerves (5). Schwannomas can occur in any Schwann cell-containing body part, such as the head, neck and extremities, but retroperitoneal schwannomas are rare. Retroperitoneal schwannomas are solid or cystic benign tumors encapsulated within a membrane; they are often located in the paraspinal and presacral areas. Latent symptomatic retroperitoneal schwannomas account for 47.7–66.6% of all schwannomas (6). In addition, 5–8% of schwannomas are accompanied by neurofibromatosis (7). Sacral schwannomas have a relatively slow growth rate, along with a large retroperitoneal space, excellent ductility and lack of pain transmission. Consequently, when detected, sacral schwannomas have a large volume and present with numerous degenerative pathological changes, such as cystic degeneration, calcification and hemorrhage, with cystic degeneration and calcification occurring in 60 and 23% of individuals, respectively (8). Computed tomography (CT) or magnetic resonance imaging (MRI) results exhibiting these features allow clinicians to consider the possibility of sacral schwannomas.
The incidence of malignant schwannomas, in which the nerve sheath membrane tends to undergo malignant differentiation, is 0.001% and accounts for 3–10% of soft-tissue sarcomas (9). Malignant schwannomas are considered high-grade sarcomas that are highly malignant, strongly invasive, and prone to recurrence and metastasis, as well as having a low long-term survival rate; therefore, an early diagnosis is particularly critical. According to the literature, 15–70% of patients with malignant schwannomas have combined neurofibromatosis type 1, which may be associated with the malignancy development of peripheral neurofibromas (10,11). The World Health Organization (WHO) has used the terms ‘malignant Schwann cell tumor’, ‘neurofibrosarcoma’ and ‘malignant schwannoma’ to indicate the origin and malignant behavior of schwannomas. In 2000, schwannomas were formally defined by the WHO as any malignant tumor originating in the peripheral nerves or showing nerve sheath differentiation, except for tumors originating in the nerve periphery or peripheral neurovascular system. Malignant schwannomas, including two specific subtypes, epithelioid malignant schwannomas and malignant salamander tumors, were classified as soft-tissue tumors in 2013 (12).
As sacral schwannomas usually exhibit slow growth and unspecific symptoms, diagnosis and treatment are often delayed until the tumor enlarges (1). Most patients do not show specific clinical manifestations and characteristic imaging features; thus, the preoperative diagnosis of sacral schwannomas is usually difficult (13). Benign schwannomas are differentiated from malignant ones based on the duration of the disease, clinical neuroerosive corrosive symptoms, capsule integrity on CT or MRI, and pathological examination results. The present study describes the surgical management of a giant sacral schwannoma and reviews the relevant literature, particularly associated case reports.
Case report
A 54-year-old man complaining of paraesthesia and radiating pain in the lower left extremity visited Jiaozhou Branch of Shanghai East Hospital, Tongji University (Qingdao, China) in February 2022. Radiating pain in the sole of the foot had increased in intensity gradually during the last 3 years, and the stool had become thin in size over the last 6 months. The patient also had a history of mild back pain and sciatica over the last 3 years and had been diagnosed previously as having L3,4, L4,5, L5 and S1 lumbar disc herniation on the basis of CT images. Left foot CT and MRI revealed osteochondral injury with degenerative cysts in the inner side of the trochlea in the left talus and a small effusion in the left ankle. The patient had undergone some massage therapy and physiotherapy in a number of the larger hospitals in Qingdao. The diagnosis was delayed, as an abdominal CT had not been performed. A history of trauma was not observed, and the patient was otherwise healthy. The physical examination revealed mild limitation in the movement of the left lower extremity, atrophy in the left buttock, thigh and calf muscles, and skin hypoesthesia. The circumference of the left lower extremity was 34 cm at 10 cm above the knee, 30 cm at 10 cm below the knee and 19.4 cm at 5 cm above the ankle. The circumference of the right lower extremity was 35.5 cm at 10 cm above the knee, 31.8 cm at 10 cm below the knee and 20.3 cm at 5 cm above the ankle. Contrast-enhanced CT of the pelvis demonstrated a cystic-solid mass on the left side of the pelvis. Three-dimensional CT reconstruction technology revealed that the feeding artery that originated from the internal iliac artery flowed into the tumor. There were more branches of the left internal iliac artery than usual, and tortuous branches were observed along the tumor margin (Fig. 1A-C). The diagnosis at the time of hospital admission was of a pelvic tumor with nerve compression syndrome in the left lower extremity. After the patient underwent blood routine, coagulation function, liver and kidney function, blood glucose, blood fat, electrolyte, carcinoembryonic antigen, α-fetoprotein, cancer antigen (CA)19-9, CA125 and CA153 tests, abdominal ultrasound and lower extremity electromyogram examinations, and was administered preoperative multi-disciplinary treatment, the pelvic tumor was resected using the transabdominal anterior approach in March 2022. After opening the abdomen, a large encapsulated retroperitoneal mass of ~5 cm in diameter was detected by the anterior approach in the left side of the pelvis region, displacing the urinary bladder upwards and rectosigmoid colon to the right side. The anterior approach has the advantage of not causing spinal instability. The lesion was immobile, without evidence of local invasion. The excision of the mass was challenging. Due to its size, the posterior peritoneum was opened over the mass. The sigmoid colon was mobilized from the presacral space to expose the tumor mass. The ureters were identified bilaterally and protected. A plane was then established between the tumor mass and the presacral alar tissue. As the tumor's blood supply originated from the left internal iliac artery, and thick, tortuous veins were observed on the tumor surface, the left internal iliac artery was first ligated and severed. The tumor was firmly attached by fibrous tissue to the presacral fascia and left iliac vein. A tissue block was collected from the tumor surface and subjected to rapid frozen pathology. The pathology revealed a benign schwannoma. Using an ultrasonic knife, in circumferential fashion, the tumor was completely mobilized from the sigmoid colon and upper rectum, the left iliac vein and the other surrounding anatomical structures with great care for exact hemostasis. The tumor envelope was then incised along the longitudinal axis of the nerve, and using sharp dissection. Finally, the remaining attachment of the tumor tissue to the presacral nerve root(s) was identified and carefully dissected free with ligasure device. The tumor was then completely resected by an intralesional resection (the indications of intralesional resection included the large size of the tumor and the clinical manifestations of nerve bundle erosion). For giant schwannomas, we believe it is not possible to lift the peritoneal sac as the tumor mass is so large. In addition, this mass had to be broken up to safely dissect the iliac vessels and ureters, Moreover, it was a multidisciplinary event, especially for the digestive surgeon, who had a perfect knowledge about this type of surgical approach while being an expert in laparoscopic surgery. The digestive surgeon performed the first operating phase with the surgical approach and the identification of vessels and ureters, and the neurosurgeon, by virtue of having knowledge of the lumbosacral plexus, helped with the identification of the sacral roots and the excision of the lesion. This allowed the association of two complementary specialists and reduced both operating time and surgical risks.
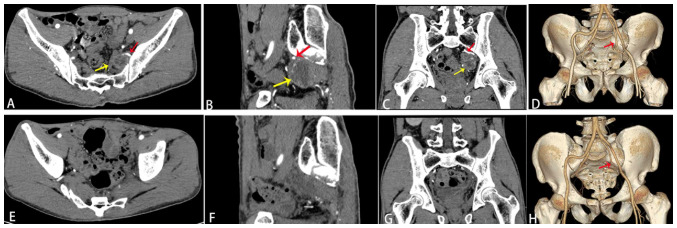
Figure 1.
Images of sacral schwannoma. (A) Horizontal view of enhanced CT on the left side of the pelvic region showing a round, 5.1×4.3-cm cystic-solid mass. (B) Sagittal view of enhanced CT on the left side of the pelvis region showing the tumor partially adjacent to the sacrum and had abundant blood vessels. (C) Coronal enhanced CT on the left side of the pelvic region showing delayed slight enhancement of the tumor capsule wall. (D) Three-dimensional CT reconstruction showing that the feeding artery originating from the internal iliac artery cranially flows into the tumor, the left internal iliac artery branches are increased in number, and tortuous branches are present along the tumor margin. (E-G) Postoperative enhanced CT showing that the tumor was completely resected, and the blood vessels were significantly reduced in the surgical area. (E) Horizontal, (F) sagittal and (G) coronal enhanced CT views, and (H) three-dimensional CT reconstruction showing the severed left internal iliac artery and the remaining stump of the artery. The red arrow indicates the internal iliac artery and the yellow arrow indicates the tumor. CT, computed tomography.
The central region of the tumor was cystic in appearance and the tumor measured 5×4×4 cm3 (Fig. 2A and B). A schwannoma was diagnosed on the basis of the postoperative pathology. Specimens were fixed in 10% neutral formalin solution at room temperature for 24 h, dehydrated, embedded and serially sliced into 4-µm sections. One slice of the sample was used for H&E staining. The sections were baked at 60°C for 30 min, and the sections were viewed under an optical microscope. For the immunohistochemical analysis, specimens were fixed in 10% neutral formalin solution at room temperature for 24 h, dehydrated, embedded and serially sliced into 4-µm sections. Antigen retrieval was performed using endogenous peroxidase blocker at room temperature for 10 min, and the non-specific antigens were blocked. The immunohistochemical SP method was performed. Sections were then incubated overnight at 4°C with primary monoclonal antibodies (ready-to-use; rabbit anti-human S-100, cat. no. ZM-0224; Vimentin, cat. no. ZM-0260; and Ki-67, cat. no. ZM-0224; OriGene Technologies, Inc.), followed by incubation at room temperature for 30 min with enzyme-labeled sheep anti-mouse/rabbit IgG polymer secondary antibody (ready-to-use; cat. nos. PV-6000D and IB000084; OriGene Technologies, Inc.) with diaminobenzidine (DAB) substrate buffer solution and DAB concentrated colour reagent, and counterstained with hematoxylin at room temperature for 5 min. The negative control group was studied using the same steps, but phosphate-buffered saline was used instead of primary antibody. The sections were viewed under a BX51 optical microscope (Olympus Corporation) at ×200 and ×400 magnification.
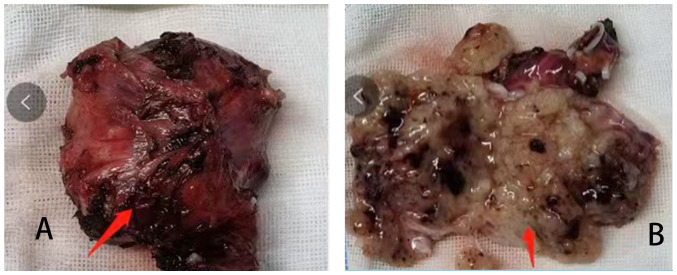
Figure 2.
Resected sacral schwannoma. (A) The tumor was quasi-circular and cyst-solid, with thick veins on the surface. (B) The dissected tumor showed cystic changes and intracapsular bleeding.
Immunohistochemical observations were as follows: S-100-positive, vimentin-positive and Ki-67-positive diffuse tumor cells were observed, and the Ki-67 index of the original tumor was 1% (Fig. 3A-D). Postoperative three-dimensional CT reconstruction technology revealed that the tumor was completely resected, with a significant reduction in blood vessels in the surgical area. The left internal iliac artery was severed and only a stump of the artery remained (Fig. 1D-F). Follow-up of the patient was performed every 6 months, including EMG of the left lower extremity, enhanced CT and MRI. No recurrence was observed during the 21-month follow-up, and the patient did not receive any further treatment.
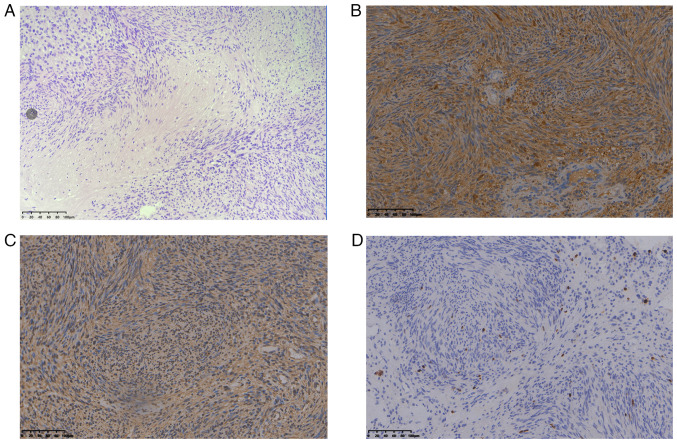
Figure 3.
Pathology of sacral schwannoma. (A) Microscopically, a large number of densely spindle cells can be seen in the Antoni A area and sparse tumor cells rich in mucous matrix in the Antoni B area (hematoxylin and esoin; magnification, ×200). (B) S-100-positive expression (magnification, ×200). (C) Vimentin-positive expression (magnification, ×200). (D) Ki-67-positive expression (magnification, ×200).
Discussion
Although the pathogenesis of schwannomas is unclear, their occurrence may be related to relevant gene mutations. Peripheral nerve schwannomas are well demarcated, encapsulated tumors, typically round and attached to the nerve; they arise from the Schwann cells and are eccentric in location, involving one or two fascicles, and sparing and displacing the other neural fascicles of the involved nerve. The tumors occur within the endoneurium and are surrounded by perineurium and fibrous epineurium that encapsulate the tumor (14). In retroperitoneal schwannomas, the mean tumor growth rate is 1.9 mm/year. The majority of peripheral tumors are solitary (96%), while only 4% are plexiform (15). According to their anatomical locations, schwannomas are divided into intraosseous, dumbbell and retroperitoneal types. The intraosseous type refers to tumors located in or invading the spinal canal. Dumbbell-type schwannomas are tumors extending outside the spinal canal through the intervertebral foramen. Retroperitoneal-type schwannoma refers to a tumor located in the posterior peritoneum outside the spinal canal (16). The tumor size is generally based on length, but the size of the dumbbell type is based on the largest part of the tumor. Sacral schwannomas have a slow growth rate. Moreover, schwannomas display strong concealment abilities due to the large pelvic space. Various clinical symptoms, including pelvic organ or nerve tissue compression, bone destruction, sciatica, lower back pain, bladder and rectum compression due to difficultyin urination and defecation, and weakness in lower limbs, are associated with sacral schwannomas (17–19). Feldenzer et al (20) found that 67% of patients with sacral schwannomas exhibited rectal irritation symptoms due to tumor compression. Therefore, a delayed diagnosis is a clinical feature of sacral schwannomas. In the present case, the patient had suffered from numbness in the left buttock and lower extremity, with persistent radiating pain in the foot sole for 3 years. The patient had visited several hospitals in Qingdao previously, but only the presence of lumbar and foot lesions was determined by the treating physicians, who ignored the results of the abdominal CT examination. The tumor remained undetected until the patient developed rectal compression symptoms and underwent an abdominal CT, which is an informative lesson for clinicians. Clinically, the numbness of the skin of the lower limbs on the affected side and the presence of muscle atrophy suggested that the tumor had eroded nerve bundles. According to one previous study, the mean time for a sacral schwannoma diagnosis was 5.2 years, and the longest time was 11 years (21). In general, sacral malignant tumors are common in chordoma and chondrosarcoma, and are metastatic, whereas sacral benign tumors are common in giant osteocytoma, aneurysmal bone cysts and osteoblastoma (22). However, sacral schwannomas are rare and need to be differentiated from fibrosarcomas, liposarcomas, astrocytomas, hydatid cysts, hematomas and connective tissue diseases (23).
On enhanced CT scans, the cystic wall presents with delayed slight enhancement, and parenchymal components exhibit plaque-like progressive but irregular enhancement. MRI T2-weighted imaging (T2WI) sequencing displays the following pathological characteristics of the internal tumor: The Antoni A area with dense cells showing an equal or slightly high signal, and the Antoni B area rich in stroma and mucus showing a higher signal (24). Verstraete et al (13) and Cuesta et al (4) summarized the imaging data of schwannomas and found tumors with low-density changes on enhanced CT, whereas unique changes were present in the schwannomas on MRI (a low or equal signal on T1WI and a high or heterogeneous signal on T2WI). Pan et al (25) reported the following diversified CT and MRI changes: Hemorrhage, degeneration, necrosis and liquefaction. On MRI, the low- and high-density signals on T1WI and T2WI, respectively, helped surgeons to provide a reference for a schwannoma diagnosis before the operation. Makni et al (26) suggested that the CT density helped to evaluate fluid and adipose tissues, determine how tumors and the sacrum or the coccyx and rectum are anatomically related, and determine the presence or absence of calcification (excluding chordoma). MRI can help estimate the tumor size, exact location and relationship of the tumor with adjacent nerves. Preoperative CT-based 3D reconstruction technology can more clearly reveal the relationship between tumors and adjacent blood vessels and nerves (27). Large volumes are characteristic of retroperitoneal schwannomas. In a previous study, CT revealed that degeneration and hemorrhage often occur in the tumor body, and calcification was observed (28). Additionally, a single benign schwannoma can be detected using fluorodeoxyglucose positron emission tomography/CT (29). The standard agreed diagnostic criterion for giant schwannomas is a tumor size of ≥5 cm on CT or MRI (1,30). In the present case, enhanced CT and three-dimensional reconstruction of the pelvis unveiled that the tumor was round, cystic-solid, with a thin cystic wall and smooth inner and outer walls. The tumor exhibited uniform enhancement, predominantly hypointense, with delayed and mild enhancement of the cystic wall. The tumor was partially adjacent to the sacrum, and the branches of the left internal iliac artery were increased in number and traveled along the tumor margin. The venous phase exhibited further mild progressive enhancement, with cloudy flocculent. The demarcation between high and low densities was more clearly defined. The aforementioned imaging changes were consistent with the imaging features of schwannomas. Enhanced CT and three-dimensional reconstruction of the pelvis revealed that the tumor was completely resected, and the blood vessels in the surgical area were reduced in number. The left internal iliac artery was severed and only a stump of the artery remained. With sacral schwannoma, accurate preoperative imaging evaluation is important for choosing a rational surgical approach (both to completely resect the tumor and to avoid nerve trunk damage). In the case, preoperative three-dimensional CT reconstruction revealed that the tumor originated from the left internal iliac artery, suggesting that surgeons should make clear the relationship between tumor and blood vessels, and choose a reasonable surgical approach, so as to avoid accidental massive haemorrhage during the operation. Moreover, a MRI view that shows the continuity to a nerve root may help the diagnosis. In the present case, MRI was not performed before the operation, which is a limitation of the study.
In the present study, based on the gross pathological examination, the schwannoma was white/yellow with a hard texture. Under the microscope, several densely arranged spindle cells were observed in the Antoni A area, and fewer tumor cells with the mucinous matrix were observed in the Antoni B area. Xu et al (23) and Yu et al (29) reported that schwannoma pathology is based on degenerative changes and nuclear atypia. Immunohistochemical analyses, such as analysis of levels of S-100, neuron-specific enolase or vimentin, are crucial for the pathological diagnosis. Ki-67 refers to the nuclear proliferation index. This index reflects cell proliferation and simultaneously indicates the probability of tumor recurrence after operation. Ogose et al (30) reported that the Ki-67 index of presacral schwannomas was generally <1%. In the present case, the gross specimen view revealed that the tumor was quasi-circular, cystic-solid, and thick veins were observed on the surface. On dissecting the tumor, cystic degeneration and intracapsular hemorrhagic changes were found. Under the microscope, numerous densely arranged spindle cells were noted in the Antoni A area, and sparse tumor cells rich in mucus stroma were observed in the Antoni B area. Positive expression of S-100, vimentin and Ki-67 was also observed. Schwannoma is a tumor originating from the mesenchymal tissues. The positive expression of S-100, a neuroendocrine marker, indicates that the tumor originates from neural tissues (31,32). Vimentin is a fibroblast component that belongs to the cytoskeleton; it is mainly distributed among cytoskeletal elements, as well as in tumors originating from mesenchymal cells, such as schwannomas or neurofibromas. Vimentin-positive expression also suggests a tumor originating from neural tissues (33). Ki-67 is a nuclear antigen that reflects cell proliferative activity (34,35). In the present case, the Ki-67 index of the original tumor was 1%, which indicated a benign tumor, and there was a low recurrence rate. Ozdemir et al (36) found that 50% of patients with sacral schwannomas exhibited nerve erosion, and emphasized that invasion and erosion are two different concepts. Invasion is tissue destruction by the tumor, whereas erosion is tissue replacement by the tumor. These concepts suggest that surgeons should consider both the thoroughness of tumor resection and the preservation of the nerve function when opting for surgical procedures. Considering the pathological characteristics of giant sacral schwannomas, surgeons must perform a reasonable operation rather than a perfect operation.
The envelopes of schwannomas are normal nerve sheath tissues that are not a part of the tumor; thus, the encapsulated tumor should be completely removed. A potential risk of nerve injury may arise when approaches such as extracapsular tumor resection are used to deliberately reduce the recurrence rate. Therefore, the complete resection of the enveloped tumor is sufficient for preventing its recurrence, protecting nerve functions, reducing nerve damage and reducing the probability of surgical complications. Studies have highlighted the use of 3D imaging technology-based preoperative needle biopsy for designing surgical protocols, as well as the need for multidisciplinary team collaboration and careful discussion, to avoid any possible complications (3,37). The use of a preoperative needle biopsy remains controversial. The advocators of this technique believe that a biopsy can help in making a clear diagnosis of the tumor, excluding lymphoma, and in the selection of reasonable surgical procedures. The opponents argue that a risk of infection and bleeding exists; nonetheless, most scholars have advocated for a preoperative hollow-needle biopsy (2,22,38). No consensus exists on the surgical management of sacral schwannomas. Surgical procedures may include a total or subtotal resection or a partial resection of the schwannoma. In the so-called subtotal resection, the tumor is resected to ≥90%, while <90% of the tumor is resected in a partial resection (1,18). Handa et al (16) reported that giant sacral schwannomas were surgically removed in 11 patients who were followed up for a mean time of 50 months, and that no recurrence was observed in 8 patients who underwent a total or partial resection, whereas recurrence was observed in 2 out of the 3 patients who underwent a partial resection. Using curettage and radiotherapy approaches for giant sacral schwannomas in 4 patients maximally protect the sacral nerve bundle in a study by Chandhanayingyong et al (39); however, the recurrence rate was 54%. Some scholars have proposed that nerve monitoring should be performed during the removal of giant sacral schwannomas to achieve maximal tumor resection while avoiding nerve injury and reducing tumor recurrence (19,40). The diagnosis must be reliable before surgical treatment for giant sacral schwannoma, avoiding the intralesional resection of retroperitoneal malignancies (41). In this present case, the abdomen was explored using the anterior approach, and the posterior peritoneum was opened. The tumor's blood supply originated from the left internal iliac artery, and thick, tortuous veins were observed on the tumor surface. The left internal iliac artery was first ligated and severed, and the nerve trunk was completely exposed at the tumor's base. Using an ultrasonic knife, the tumor envelope was separated, and the nerve trunk at the tumor's base was completely exposed to determine the tumor-nerve association. A tissue block was collected from the tumor surface and subjected to rapid frozen pathology. The pathology revealed a benign schwannoma. The tumor envelope was then incised along the longitudinal axis of the nerve, and using sharp dissection, the tumor was completely resected (intralesional resection indications include the large size of the tumor and the clinical manifestations of nerve bundle erosion). While resecting the schwannoma, nerve bundle damage was avoided as much as possible. Most studies have recommended careful intracapsular excision of the tumor to minimize postoperative neural deficits, since most schwannomas are encapsulated. If nerve fibers surround the tumor surface, intracapsular enucleation can be carried out while preserving nerve fibers by making a small longitudinal incision in the capsule (14,23,42,43). A number of studies have used laparoscopy or robotic assistance to clearly observe and enlarge the characteristics of tissue structures in a narrow space, remove tumors completely and protect nerve structure integrity, in alignment with the concept of precision and minimally invasive surgeries (2,44).
Adjuvant therapies for sacral schwannomas are embolization, chemotherapy, hyperthermia, Gamma Knife® therapy and cryosurgery (30,45). Different postoperative complications and prognoses of sacral schwannomas have been reported. Based on a review of the literature, Paulo et al (21) reported that of the 68 patients with sacral schwannomas, 10% had movement disorders, 6% had incontinence, 18% developed recurrence and 9% underwent reoperation after the initial surgery. Mualem et al (46) reported that, in 27 patients in whom sacral schwannomas were resected, 59.3% experienced a complete recovery from symptoms, 33.3% displayed a partial recovery from symptoms and 11.1% showed no symptom recovery. Yu et al (29) reported 12 cases of sacral schwannoma, 6 of which exhibited Ki-67 levels of >2% and 4 of which displayed postoperative recurrence. Thus, the postoperative recurrence of schwannomas is associated with the surgical procedure and biological tumor characteristics. The recurrence of schwannoma is affected by tumor size and the degree of resection. Therefore, laparoscopic or robotic surgery is recommended for giant sacral schwannomas, as the tumor location is deep and microscopic surgery cannot be performed. The magnification and clarity of image technology can facilitate minimally invasive and complete removal of the tumor, and prevent nerve damage while reducing the recurrence rate. In the present case, no recurrence was observed during the 21-month follow-up period and the patient was asymptomatic.
In conclusion, sacral schwannomas are rare, and their clinical diagnosis is frequently delayed. Furthermore, surgical procedures for removing sacral schwannomas are diverse. Problems such as postoperative nerve injuries and tumor recurrence may be a challenge for surgeons when choosing appropriate treatment strategies for sacral schwannomas. Therefore, future studies should concentrate on measures for avoiding nerve injuries and reducing the postoperative recurrence rate.
Acknowledgements
The authors would like to thank Professor Yang Qing (Department of Pathology, Jiaozhou Branch of Shanghai East Hospital, Tongji University, Qingdao, Shandong, China) for guidance and help with the pathology in this study.
Funding Statement
This study was funded by the Military Logistic Project of China (grant no. AWS17J008).
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
SZ and SW conceived the report and participated in data acquisition and the drafting of the manuscript. HC and YZ participated in the conception and design of the report, and critically revised the manuscript. LL and WD collected imaging data and performed study revisions. XM was responsible for the pathological analysis. SZ and HC confirm the authenticity of all the raw data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The reporting of this study conforms to the CARE guidelines. This study was exempt from ethical approval by the ethics committee of the Jiaozhou Branch of Shanghai East Hospital, Tongji University.
Patient consent for publication
Written informed consent was obtained from the patient for publication of this case report and the accompanying images.
Competing interests
The authors declare that they have no competing interests.
Authors' information
ORCID: Professor Haibo Chu, https://orcid.org/0000-0002-3568-6431.
References
- 1.Macciò A, Kotsonis P, Aste L, Voicu MA, Madeddu C, Conti C, Camparini S. An interdisciplinary approach for laparoscopic removal of a large retroperitoneal pelvic schwannoma attached to vital vessels: A case report. Medicine (Baltimore) 2019;98:e181492020. doi: 10.1097/MD.0000000000018149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohsawa M, Miguchi M, Yoshimitsu M, Oishi K, Kohashi T, Hihara J, Mukaida H, Kaneko M, Egi H, Ohdan H, Hirabayashi N. Laparoscopic excision of a retroperitoneal schwannoma: A case report. Asian J Endosc Surg. 2019;12:192–196. doi: 10.1111/ases.12607. [DOI] [PubMed] [Google Scholar]
- 3.Korduke O, Omar A, Viner W, Kodeda K. Pre-sacral schwannoma. ANZ J Surg. 2020;90:1805–1807. doi: 10.1111/ans.15707. [DOI] [PubMed] [Google Scholar]
- 4.Cuesta JP, Rodríguez LC, Bastidas N, Hernández M. Sacral schwannoma a case report and review of the literature. Neuroradiol J. 2023;36:371–374. doi: 10.1177/19714009221140486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masson P. Experimantal and spontaneous schwannomas (peripheral glinomas): IL spontaneous schwannomas. Am J Pathal. 1932;8:389–416. [PMC free article] [PubMed] [Google Scholar]
- 6.Brian KPG, Tan YM, Chuang YFA, Pierce KHC, London LPJO, Wong WK. Retroperitoneal schwannoma. Am J Surg. 2006;192:14–18. doi: 10.1016/j.amjsurg.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 7.Liu QY, Lin XF, Zhang WD, Li HG, Gao M. Retroperitoneal schwannomas in the anterior pararenal space: Dynamic enhanced multi-slice CT and MR findings. Abdom Imaging. 2013;38:201–210. doi: 10.1007/s00261-012-9882-6. [DOI] [PubMed] [Google Scholar]
- 8.Kudo T, Kawakami H, Kuwatani M, Ehira N, Yamato H, Eto K, Kubota K, Asaka M. Three cases of retroperitoneal schwannoma diagnosed by EUS-FNA. World J Gastroenterol. 2011;17:3459–3464. doi: 10.3748/wjg.v17.i29.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yun JS, Lee MH, Lee SM, Lee JS, Kim HJ, Lee SJ, Chung HW, Lee SH, Shin MJ. Peripheral nerve sheath tumor: differentiation of malignant from benign tumors with conventional and diffusion-weighted MRI. Eur Radio. 2020;31:1548–57. doi: 10.1007/s00330-020-07234-5. [DOI] [PubMed] [Google Scholar]
- 10.Stramare R, Beltrame V, Gazzola M, Gerardi M, Scattolin G, Coran A, Faccinetto A, Rastrelli M, Rossi CR. Imaging of soft-tissue tumors. J Magn Reson Imaging. 2013;37:791–804. doi: 10.1002/jmri.23791. [DOI] [PubMed] [Google Scholar]
- 11.Baheti AD, O'Malley RB, Kim S, Keraliya AR, Tirumani SH, Ramaiya NH, Wang CL. Soft-tissue sarcomas: An update for radiologists based on the revised 2013 world health organization classification. AJR. 2016;206:924–932. doi: 10.2214/AJR.15.15498. [DOI] [PubMed] [Google Scholar]
- 12.Doyle LA. Sarcoma classification: an update based on the 2013 world health organization classification of tumors of soft tissue and bone. Cancer. 2014;120:1763–1774. doi: 10.1002/cncr.28657. [DOI] [PubMed] [Google Scholar]
- 13.Verstraete KL, Achten E, De Schepper A, Ramon F, Parizel P, Degryse H, Dierick AM. Nerve sheath tumors: Evaluation with CT and MR imaging. J Belge Radiol. 1992;75:311–320. [PubMed] [Google Scholar]
- 14.Suárez C, López F, Rodrigo JP, Mendenhall WM, de Bree R, Mäkitie AA, Vander Poorten V, Takes RP, Bondi S, Kowalski LP, et al. Benign peripheral non-cranial nerve sheath tumors of the neck. Adv Ther. 2022;39:3449–3471. doi: 10.1007/s12325-022-02191-5. [DOI] [PubMed] [Google Scholar]
- 15.El Sayed L, Masmejean EH, Parfait B, Kalamarides M, Biau D, Peyre M. Natural history of peripheral nerve schwannomas. Acta Neurochir (Wien) 2020;162:1883–1889. doi: 10.1007/s00701-020-04430-6. [DOI] [PubMed] [Google Scholar]
- 16.Handa K, Ozawa H, Aizawa T, Hashimoto K, Kanno H, Tateda S, Itoi E. Surgical management of giant sacral schwannoma: A case series and literature review. World Neurosurg. 2019;129:e216–e223. doi: 10.1016/j.wneu.2019.05.113. [DOI] [PubMed] [Google Scholar]
- 17.Khan UA, Ismayl G, Malik I. Giant sacral schwannoma treated with a 360 approach: A rare case and systematic review of the literature. World Neurosurg. 2018;115:65–72. doi: 10.1016/j.wneu.2018.03.203. [DOI] [PubMed] [Google Scholar]
- 18.Tahta A, Dinc C, Ozdenkaya Y, Cakir A. Giant sacral schwannoma causing bilateral hydronephrosis: Case report and review of the literature. World Neurosurg. 2020;142:184–187. doi: 10.1016/j.wneu.2020.06.213. [DOI] [PubMed] [Google Scholar]
- 19.Ragurajaprakash K, Hanakita J, Takahashi T, Ueno M, Minami M, Tomita Y, Tsujimoto Y, Kanematsu R. Giant invasive sacral schwannoma with aortic bifurcation compressionand hydronephrosis. World Neurosurg. 2020;135:267–272. doi: 10.1016/j.wneu.2019.12.088. [DOI] [PubMed] [Google Scholar]
- 20.Feldenzer JA, McGauley JL, McGillicuddy JE. Sacraland presacral tumors: Problems in diagnosis and management. Neurosurgery. 1989;25:884–891. doi: 10.1097/00006123-198912000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Paulo D, Semonche A, Tyagi R. Surgical management of lumbosacral giant invasivespinal schwannoma: Acase report and literature review. World Neurosurg. 2018;114:13–21. doi: 10.1016/j.wneu.2018.02.146. [DOI] [PubMed] [Google Scholar]
- 22.Turner ML, Mulhern CB, Dalinka MK. Lesions of the sacrum differential diagnosis and radiological evaluation. JAMA. 1981;245:275–277. doi: 10.1001/jama.1981.03310280047030. [DOI] [PubMed] [Google Scholar]
- 23.Xu H, Sha N, Li HW, Bai M, Chen X, Hu HL, Wu CL. A giant pelvic malignant schwannoma: A case report andliterature review. Int J Clin Exp Pathol. 2015;8:15363–15368. [PMC free article] [PubMed] [Google Scholar]
- 24.Leclerc A, Lebreton G, Huet A, Alves A, Emery E. Management of giant presacral schwannoma. Clinical series and literature review. Clin Neurol Neurosurg. 2021;200:106409. doi: 10.1016/j.clineuro.2020.106409. [DOI] [PubMed] [Google Scholar]
- 25.Pan W, Wang Z, Lin N, Huang X, Liu M, Yan X, Ye Z. Clinical features and surgical treatment of sacral schwannomas. Oncotarget. 2017;8:38061–38068. doi: 10.18632/oncotarget.16968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Makni A, Fetirich F, Mbarek M, Ben Safta Z. Presacral schwannoma. J Visc Surg. 2012;149:426–427. doi: 10.1016/j.jviscsurg.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Kinoshita T, Naganuma H, Ishii K, Itoh H. CT features of retroperitoneal neurilemmoma. Eur J Radiol. 2015;27:67–71. doi: 10.1016/S0720-048X(97)00032-6. [DOI] [PubMed] [Google Scholar]
- 28.Bai X, Wang XM. Solitary benign schwannoma mimics residual malignancy on FDG PET/CT. Clin Nucl Med. 2018;43:782–784. doi: 10.1097/RLU.0000000000002245. [DOI] [PubMed] [Google Scholar]
- 29.Yu NH, Lee SE, Jahng TA, Chung CK. Giant invasive spinal schwannoma: Its clinical features and surgical management. Neurosurgery. 2012;71:58–66. doi: 10.1227/NEU.0b013e31824f4f96. [DOI] [PubMed] [Google Scholar]
- 30.Ogose A, Hotta T, Hatano H, Kawashima H, Umezu H, Higuchi T, Endo N. Presacral multiple cellular schwannomas. Spine (Phila Pa 1976) 2003;28:E426–E429. doi: 10.1097/01.BRS.0000092344.14376.D9. [DOI] [PubMed] [Google Scholar]
- 31.Mokhtari M, Iranpour P, Golbahar Haghighi A, Ghahramani L. Schwannoma of the rectosigmoid colon. Adv Biomed Res. 2022;11:5. doi: 10.4103/abr.abr_91_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doan L, Cassarino DS. A rare case of cutaneous pseudoglandular schwannoma. J Cutan Pathol. 2023;50:798–800. doi: 10.1111/cup.14477. [DOI] [PubMed] [Google Scholar]
- 33.Bohlok A, El Khoury M, Bormans A, Galdon MG, Vouche M, El Nakadi I, Donckier V, Liberale G. Schwannoma of the colon and rectum: A systematic literature review. World J Surg Oncol. 2018;16:125. doi: 10.1186/s12957-018-1427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prueter J, Norvell D, Backous D. Ki-67 index as a predictor of vestibular schwannoma regrowth or recurrence. J Laryngol Otol. 2019;133:205–207. doi: 10.1017/S0022215119000549. [DOI] [PubMed] [Google Scholar]
- 35.Singh A, Aggarwal M, Chadalavada P, Siddiqui MT, Garg R, Lai K, Chahal P. Natural history of gastrointestinal schwannomas. Endosc Int Open. 2022;10:E801–E808. doi: 10.1055/a-1784-0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ozdemir N, Bezircioğlu H, Akar O. Giant erosive spinal schwannomas: Surgical management. Br J Neurosurg. 2010;24:526–531. doi: 10.3109/02688697.2010.487129. [DOI] [PubMed] [Google Scholar]
- 37.Lin CL, Fang JJ, Lin RM. Resection of giant invasive sacral schwannoma using image-based customized osteotomy tools. Eur Spine J. 2016;25:4103–4107. doi: 10.1007/s00586-016-4782-z. [DOI] [PubMed] [Google Scholar]
- 38.Çağlı S, Işık HS, Yıldırım U, Akıntürk N, Zileli M. Giant sacral schwannomas. J Neurooncol. 2012;110:105–110. doi: 10.1007/s11060-012-0941-1. [DOI] [PubMed] [Google Scholar]
- 39.Chandhanayingyong C, Asavamongkolkul A, Lektrakul N, Muangsomboon S. The management of sacral schwannoma: Report of four cases and review of literature. Sarcoma. 2008;2008:845132. doi: 10.1155/2008/845132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Colonna MR, Costa AL, Mastrojeni C, Rizzo V, Nirta G, Angileri FF, Ieni A, Milone E, Macrì A. Giant sacral schwannoma excised under intraoperative neuromonitoring in an elderly patient: case report. J Surg Case Rep. 2021;2021:rjab460. doi: 10.1093/jscr/rjab460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berner EA, Hung YP, Nielsen GP, Lozano-Calderón SA. Malignant peripheral nerve sheath tumors arising from schwannomas: Case series and literature review. APMIS. 2021;129:524–532. doi: 10.1111/apm.13139. [DOI] [PubMed] [Google Scholar]
- 42.Ram S, Vivek V, Shekhar R, Gabbita AC, Ganesh K. Giant Cervicodorsal Schwannoma. J Exp Ther Oncol. 2019;13:155–158. [PubMed] [Google Scholar]
- 43.Ozturk C, Mirzanli C, Karatoprak O, Tezer M, Aydogan M, Hamzaoglu A. Giant sacral schwannoma: A case report and review of the literature. Acta Orthop Belg. 2009;75:705–710. [PubMed] [Google Scholar]
- 44.Yin J, Wu H, Tu J, Zou C, Huang G, Xie X, He Y, Shen J. Robot-assisted sacral tumor resection: A preliminary study. BMC Musculoskelet Disord. 2018;19:186. doi: 10.1186/s12891-018-2084-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang CC, Chen HC, Chen CM. Endoscopicresection of a presacral schwannoma: Case report. J Neurosurg Spine. 2007;7:86–89. doi: 10.3171/SPI-07/07/086. [DOI] [PubMed] [Google Scholar]
- 46.Mualem W, Ghaith AK, Rush D, Jarrah R, Alexander Y, Zamanian C, Atkinson JLD, Yaszemski MJ, Krauss WE, Spinner RJ, Bydon M. Surgical management of sacral schwannomas: A 21-year mayo clinic experience and comparative literature analysis. J Neurooncol. 2022;159:1–14. doi: 10.1007/s11060-022-03986-w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated in the present study may be requested from the corresponding author.