Abstract
We describe a novel epididymis-specific cDNA named Glb1l4, which was isolated from rat epididymis by differential display of mRNAs. Glb1l4 cDNA contains 2607 nucleotides and encodes a 637-amino acid protein with 50% similarity to mouse beta-galactosidase. The gene is located on chromosome 8q13, spanning 21 exons. Northern blot analysis reveals that Glb1l4 is specifically expressed in the caput region of epididymis and upregulated by androgen. A specific polyclonal antiserum against the N-terminal peptide of GLB1L4 has been produced. Western blot analysis and immunohistochemistry assay reveal that GLB1L4 is specifically expressed in the principal cells of the caput epididymis. Interestingly, its expression peaks at Postnatal Day 45 in mRNA level and at Postnatal Day 60 in protein level while the epididymis column cells undergo differentiation. Moreover, within this very period this secretory protein is confined inside the cell with a change of subcellular distribution pattern, which implies its important roles in the cell differentiation process. Only after the epididymal epithelium differentiation is completed and the spermatozoa enter the epididymal lumen is the GLB1L4 secreted into the luminal fluid and bound on the sperm head. Our results suggest that GLB1L4 may play various roles in principal cell differentiation and sperm maturation.
Keywords: developmental biology, epididymal development, epididymis, gene regulation, Glb1l4, male reproductive tract, novel gene, principal cell differentiation, sperm, sperm maturation
Introduction
The mammalian epididymis is a highly coiled duct that links the efferent ducts to the vas deferens. Overwhelming evidence has indicated the important roles of the epididymis for the maturation, concentration, protection, and storage of sperm [1]. Based on the distinct morphological changes and gradients in gene expression and secretion in the tubule, the epididymis is mainly divided into four major regions: the initial segment, caput, corpus, and cauda [2–4]. It has been demonstrated that the regional specificity of gene expression in epithelial cells is crucial to establish the luminal fluid microenvironment in the epididymis [5]. The spatial and temporal alterations of gene expression in the epididymis are critical to its development and function maintenance, and some stringent regulation must be involved in this series of complex events [6]. Therefore, searching for the regional specifically expressed genes in the epididymis has been a crucial step toward understanding the epididymal development and function.
It has been known that the changes in morphology and function of the developing epididymis are accompanied by the expression of genes that code for such proteins as AR and ER, and junctional proteins, including OCLN and CDH1 [1]. Among epididymis-specific genes, the NPC2 (HE1) mRNA is upregulated markedly at puberty until adulthood, whereas the NPC2 protein appears maximally in the cauda epididymis of the 3- to 4-wk-old pig and in the corpus/caput epididymis of the adult animal. In contrast to NPC2, the top expression of WFDC2 (HE4) protein occurs in the corpus/caput epididymis of the juvenile animal and in the cauda epididymis of the adult [7]. However, no gene maximally expressed at puberty and involved in epididymal cell differentiation as well as regional development has been reported. Consequently, the knowledge of developmental regulation of the epididymis is very limited at present. Thus, identification of the genes that are involved in epididymal development is crucial for advancing our understanding of the epididymal function.
Beta-d-galactosidase (EC 3.2.1.23) is an exoglycosidase that cleaves β-linked terminal galactosyl residues from a variety of natural and artificial substrates [8]. It belongs to glycoside hydrolase family 35. The enzyme is expressed in all tissues examined so far, including male reproductive tissues, such as testis and epididymis [9], and acts as digestion of specific terminal glycosyl residues from glycoproteins and glycolipids. Dutta and Majumder [10] partially purified an approximately 50-kDa β-d-galactosidase from rat epididymis, in which the enzyme activity is increased markedly (4-fold) in adult rats compared with that of 24-day-old rats. Another β-d-galactosidase with two molecular forms (97 and 84 kDa) has also been isolated and purified from rat epididymis luminal fluid which possesses a significant similarity (63% residue identity) with mouse β-d-galactosidase precursor in its 11 N-terminal amino acids, as demonstrated by N-terminal amino acid analysis [11]. It has been reported that 86%–90% of total β-galactosidase activity in rat epididymis exists in luminal fluid of the epididymis, and only 10%–14% of that originated from sperm [12]. All the data above indicate that different members of β-galactosidase family exist in rat epididymis.
In this article, we describe the cloning of a β-galactosidase-like gene named Glb1l4 in the rat epididymis and the characteristics of this gene at both the mRNA and protein levels. The results suggest that this protein may play important roles in the epididymis development and sperm maturation.
Materials and Methods
Animals
Healthy male Sprague-Dawley rats and male New Zealand white rabbits, supplied by The Animal Center of the Chinese Academy of Sciences (Shanghai, China) and housed under standard laboratory conditions, were used in the present study. Experiments were conducted according to a protocol approved by the Institute Animal Care Committee. The protocol conformed to internationally accepted guidelines for the humane care and use of laboratory animals.
Differential Display RT-PCR
The methods for differential display reverse transcription-polymerase chain reaction (DD-RT-PCR) were used as described previously [13]. Briefly, total RNA (30 μg) was isolated from the caput, corpus, and cauda regions of the adult rat epididymis and then digested with 10 units of RNase-free DNase I (Gibco BRL) at 37°C for 15 min. Two micrograms of digested RNA was reverse transcribed using 2.5 μM lower primer T11CA (5′-TTTTTTTTTTTCA-3′) with 400 units of M-MLV reverse transcriptase (Gibco BRL) at 37°C for 60 min. One twentieth of reverse transcription products were amplified using 2.5 μM of the upper primer 503 (5′-CTTTCTACCC-3′) and 0.5 μM of the lower primer T11CA in a 20-μl volume containing 10 mM Tris-HCl (pH 9.0), 1.5 mM MgCl2, 50 mM KCl, 0.1% Triton X-100, 4 μM each dinucleotide triphosphate (dNTP), 1 μCi α-32P-dATP, and 3 units of Taq polymerase. Polymerase chain reaction was carried out as follows: 94°C for 5 min, then 40 cycles of 94°C for 30 sec, 40°C for 60 sec, and 72°C for 50 sec, with a final elongation of 72°C for 10 min. Polymerase chain reaction products were precipitated with 2 volumes of ethanol and then fractionated by 0.2 mm of 6% sequencing gel. One band named the gene bridging integrator 2, which is differentially displayed in sequencing gel, was cut out and ethanol precipitated. The gene bridging integrator 2 was reamplified in the same conditions described above, but with 40 μM instead of 4 μM each dNTP, and no isotope was used. This DNA fragment mixture was cloned into pBluescript SK−. The clones were screened by reverse Northern blot analysis with 32P-labeled total RNA derived from each region. The positive clone was radiolabeled using Prime-a-Gene System (Promega) as the bridging integrator 2 probe. Two bands, about 2.6 kb and 0.6 kb, were specifically hybridized in the caput region of rat epididymis by the bridging integrator 2 probe. Clone that hybridized to the 2.6-kb band was screened out and named Glb1l4. Another one had not been identified yet.
5′-RACE Analysis
The 5′-RACE analysis was performed according to the instruction manual of the 5′-RACE System for Rapid Amplification of cDNA Ends, Version 2.0 (Gibco BRL) with a minor modification. A total of 500 ng of random hexamer was used instead of Glb1l4 gene-specific primer (Gsp) in reverse transcription reaction, and the reverse transcription products were used as templates in 5′-RACE. The 2.6-kb full-length cDNA of Glb1l4 was obtained by successive 5′-RACE reactions based on Glb1l4 DD-RT-PCR fragment. In the first round, a 566-bp fragment was amplified by Gsp1 (5′-ACAAAGAAAGTGAGTGGG TAAAGCC-3′) and Gsp2 (5′-AAGCCTATTTCGTTGTGAGCATC-3′). A 722-bp fragment was amplified by Gsp3 (5′-GGAGGATCTCCAGCTTT CAAGTGACT-3′) in the second round, and a 1345-bp fragment was amplified by Gsp4 (5′-TGAAATTCCTGGTACTCGGGTGTGT-3′) in the third round.
RNA Isolation and Northern Blot Analysis
RNA isolation and Northern blot analysis were performed according to the procedure described previously [14]. Twelve micrograms of total RNAs from each sample was loaded in each lane. The probe was a 32P-labeled Glb1l4 cDNA fragment (from 2279 to 2615 bp in cDNA; total of 337 bp). An 18s r-RNA hybridization signal was used as a loading control. Autoradiographs with pronounced differences in expression were analyzed by densitometry.
RT-PCR
Total RNA isolated from rat caput epididymis was reverse transcribed by M-MLV Reverse Transcriptase (Promega, Madison, WI) according to the manufacturer's recommendations. The rat Glb1l4 full-length cDNA was amplified by PCR with the P1 primer (5′-CTGACAGTGACTAGCTTCGAG-3′) and P2 primer (5′-GCCTATTTCGTTGTGAGCATC-3′) at denaturing, annealing, and extension temperatures of 95°C for 30 sec, 55°C for 50 sec, and 72°C for 2 min, respectively, with Ex-Taq (TaKaRa). To check the ratio of two transcripts, primer P7 (5′-CTTGACTTTCAGACCTACCCT-3′) was designed, and a 337-bp cDNA product was amplified by PCR with primer P1 and P7 at denaturing, annealing, and extension temperatures of 95°C for 30 sec, 58°C for 30 sec, and 72°C for 30 sec, respectively, with Ex-Taq (TaKaRa).
Ethane-1,2-Dimethyl Sulphonate Treatment of Rats
Adult rats (250–350 g weight) received a single intraperitoneal injection of ethane-1,2,dimethyl sulphonate (EDS; 75 mg/kg body weight) in dimethyl sulfoxide (DMSO)/H2O (1:3 v/v). Animals (n = 5 for each time point) were killed at 1, 3, 7, 14, 21, 28, and 42 days after EDS injection. The additional animals injected with DMSO/H2O (1:3 v/v) only were allowed to survive for 7 (n = 4) and 14 (n = 4) days after injection and were used as vehicle controls. Other animals (n = 3) were used as normal controls. Before killing, 5 ml of blood was drawn from each animal for testosterone evaluation [15]. Total RNA was extracted in the caput epididymis of these rats, and Northern blot assay was done as described as above.
Antibody
The cDNA fragments coding for N-terminal peptide (1–200 amino acids) were amplified by PCR using the following primers. The forward primer was 5′-CATATGAGCATGTGGACC-3′, and the reverse primer was 5′-CTCGAGTTACACAGCAATGATGGG-3′. The fragment with an NdeI site on its 5′ end and a HindIII site on its 3′ end was inserted to the pET28 (a) vector (Novagen). The expression vector was constructed according to the standard protocol in pET expression manual. The recombinant protein in the inclusion bodies was induced by IPTG in the strain Escherichia coli BL21 (Novagen). The purification of the recombinant protein from inclusion bodies and the rabbit polyclonal antiserum against GLB1L4 recombinant protein were performed as described previously [16]. Affinity-purified polyclonal antibody (immunoglobulin G [IgG] fraction) was prepared as described previously [17]. The eluted IgG fraction was dialyzed extensively in 1 M Tris-Cl (pH 7.5). The purified antibody was aliquoted and stored at −80°C until use.
Protein Extract and Western Blot Assay
Total protein extracts of rat tissues were prepared as described previously [18] except for the addition of a protease inhibitor cocktail (Pierce) in the homogenizing buffer instead of individual protease inhibitors. Protein extracts for two-dimensional gel were prepared as described [19].
Total protein extracts (30 μg) for each sample were electrophoresed on 12% polyacrylamide gels (SDS-PAGE), electroblotted onto Hybond-P membranes (Amersham Pharmacia Biotech), immunoblotted, and visualized by the ECL analysis system (Amersham Pharmacia Biotech). Anti-GLB1L4 antibody (dilution 1:10 000) was used as primary antibody. The second antibody (dilution 1:15 000) was the goat horseradish peroxidase-conjugated anti-rabbit IgG (Calbiochem). The Western blot analysis of two-dimensional gel electrophoresis was performed as described previously [19], except that the second antibody was diluted 1:10 000.
Immunohistochemistry and Immunofluorescence Staining
Tissue section preparation and immunohistochemical staining were performed as described previously [18]. Primary and secondary antibodies were diluted in PBS containing 10% (v/v) normal goat serum. The 1:200 diluted anti-GLB1L4 antiserum was applied overnight at 4°C and 1:200 diluted horseradish peroxidase-conjugated goat-anti-rabbit IgG was incubated for 1 h at room temperature. As a negative control, serial sections were subjected to the same procedure, with normal rabbit serum replacing the primary antibody.
For immunofluorescence staining, the method was used as described previously [20]. The primary antibodies were rabbit anti-GLB1L4 antibody (1:200) and chicken anti-ATP6E IgY (1:200; Genway Biotech Inc., San Diego, CA). The secondary antibodies were fluorescein isothiocyanate (FITC)-labeled anti-rabbit IgG (1:500; Sigma) and rhodamine-conjugated bovine anti-chicken IgY (1:200; Santa Cruz Biotechnology). The slices were mounted with 80% glycerol and examined on a confocal microscope (Lecia TCS SP2 AOBS) or an Olympus BX-52 microscope.
Indirect Immunofluorescence Detection of Protein Associated with Spermatozoa
The method of indirect immunofluorescence detection of protein associated with spermatozoa was used as described previously [21]. The primary antibody that was applied to the spermatozoa was the polyclonal anti-GLB1L4 serum (diluted 1:200 in PBS containing 10% goat serum) with incubation overnight at 4°C, and preimmune rabbit serum was used as the control.
Results
Cloning of the Rat Glb1l4 cDNA Sequence
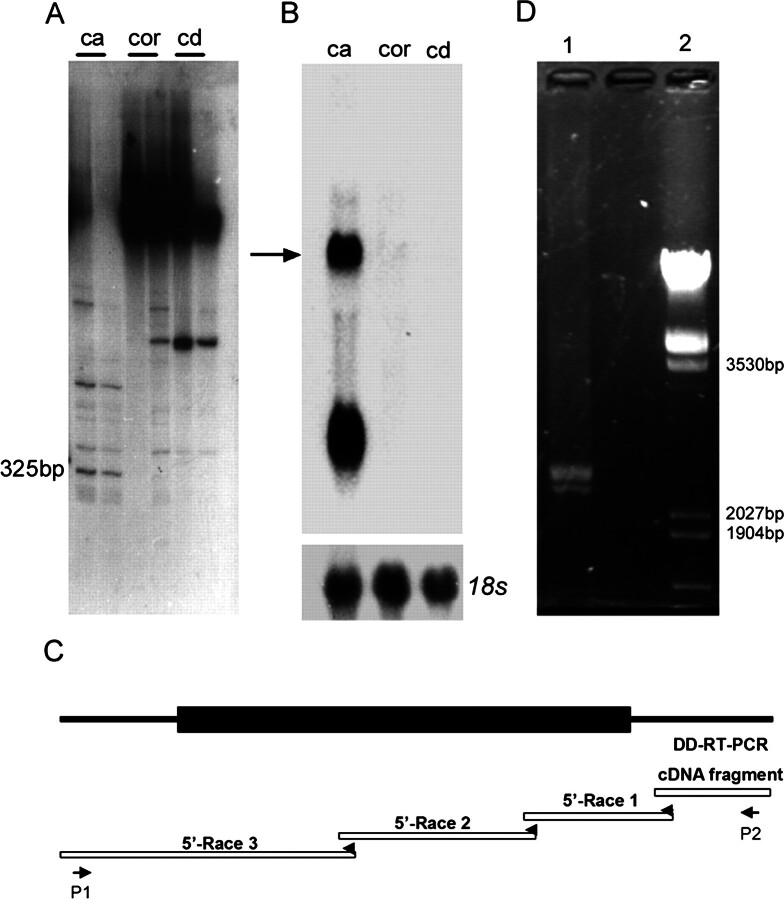
Caput, corpus, and cauda epididymis of adult Sprague-Dawley rats were screened for region-specific gene expression by differential display of mRNA. One 325-bp cDNA fragment mixture that specially expressed in the caput region was obtained using upper primer 503 and lower primer T11CA (Fig. 1A). Two bands in rat caput epididymis were detected by Northern blot analysis using this 3′-end cDNA clone fragment as a probe (Fig. 1B). The gene coding for the larger mRNA was named Glb1l4. Based on the sequence of this 325-bp DD-RT-PCR fragment, gene-specific primers were designed, and the sequence toward the 5′ end was extended with 456, 593, and 1232 bp, respectively, through three consecutive 5′-RACE reactions (Fig. 1C). The full-length cDNA of Glb1l4 was 2607 bp (excluding the poly(A) tail). A polyadenylation signal (AATAAA) was located at 2530–2535 bp, 72 bp upstream of the poly(A) tail. Two full-length Glb1l4 cDNAs (2607 and 2401 bp) were amplified by RT-PCR with rat epididymal total RNAs using P1 (5′-CTGACAGTGACTAGCTTCGAG-3′) and P2 (5′-GCCTATTTCGTTGTGAGCATC-3′) as primers (Fig. 1D). Sequencing analysis verified that Glb1l4 had two alternative spliced transcripts. The short one contained a 146-bp deletion between 114 and 259 bp in the 5′ untranslated region. The ratio of the two transcripts was 3:1. Its sequence was further confirmed by sequence analysis from four different healthy male Sprague-Dawley rats and submitted into the GenBank database under the accession number AY040870.
Fig. 1.
Cloning of the full length of rat Glb1l4. A) A DD-RT-PCR analysis of differentially expressed mRNAs in the epididymides of rats. Shown are differentially expressed transcripts obtained with combinations of upper primer 503 (CTTTCTACCC) and lower primer (T11CA) in three regions of the rat epididymis: caput (ca), corpus (cor), and cauda (cd). A 325-bp fragment is principally expressed in the caput region. B) The fragment obtained from A was used as a probe in three regions of the rat epididymis to identify mRNAs hybridized by Northern blot analysis. The Glb1l4 mRNA is indicated by an arrow. C) Diagram of cloning for Glb1l4 cDNA by 5′-RACE. Based on the 325-bp sequence of the DD-RT-PCR fragment, gene-specific primers were designed and the sequence toward the 5′ end was extended with 456, 593, and 1232, respectively, through three consecutive 5′-RACE reactions. D) Amplification of full-length Glb1l4 cDNA by RT-PCR. Two bands were amplified by the specific primers P1 and P2. The products of RT-PCR were loaded in lane 1, and DNA size markers were loaded in lane 2.
As shown in Figure 2, Glb1l4 contained an open reading frame coding for a protein with 637 amino acids. The N-terminal 24 amino acids probably form a signal peptide, as predicted with the SignalP 3.0 Server (http://www.cbs.dtu.dk/services/SignalP/). Cleavage of this peptide would lead to a mature protein of 613 amino acids with an estimated molecular mass of 70.2 kDa and isoelectric point (pI) of 9.44. One potential N-glycosylation site at N35 was predicted. Other potential sites of posttranslational modification are listed as follows: 12 protein kinase C phosphorylation sites located at T46RR, S85LR, T167YK, S240LR, T252VR, T408TK, S411SR, T446SR, S470NK, T481KK, T486LR, and T630SR; nine N-myristoylation sites located at G150GLPSW, G322TNFGL, G326LIGGA, G329GAQSA, G330AQSAE, G427QSFGY, G442GLLTS, G443LLTSR, and G500QDMNK; seven casein kinase II phosphorylation sites located at T234ADD, T262YED, T342SYD, S369VTD, T397LWE, S470NKE, and S499GQD; and one tyrosine kinase phosphorylation site located at R469SNKELLPY (Fig. 2).
Fig. 2.

The cDNA and deduced amino acid sequences of Glb1l4. The initial and terminal codons are boxed. The polyadenylation signal (AATAAA) is underlined. The sequences for primers P1 and P2 are underlined with arrows. The 146-bp sequence deleted in the short form is shadowed gray. The N-glycosylation (N35) site is boxed; 11 protein kinase C phosphorylation sites are underlined; six casein kinase II phosphorylation sites are double underlined; S470, which may be both a protein C kinase phosphorylation site and a casein kinase II phosphotylation site, is triple underlined; nine N-myristoylation sites are bracketed; and one tyrosine kinase phosphorylation site is circled. Numbers on the left and on the right correspond to nucleotide and amino acid positions, respectively.
A BLAST search in the GenBank protein database shows that rat GLB1L4 protein shares 51% similarity to mouse galactosidase beta 1 (accession number NP_033882) and about 50% similarity to human, dog, and cattle β-galactosidases, but no rat β-galactosidase sequence has been reported previously in the GenBank protein database. Rat GLB1L4 has the conserved catalytic proton donor residue Glu205 but lacks the catalytic nucleophile residue (Val279 instead of Glu). Furthermore, although the GLB1L4 is highly similar to β-galactosidase, the homologies are not clustered at the most highly conserved regions (A–H) but, rather, disperse in the full peptide evenly. Additional amino acids are inserted at the F region (Fig. 3). Our data suggest that rat GLB1L4 is a β-galactosidase-like protein, but it may not be a classical β-galactosidase.
Fig. 3.
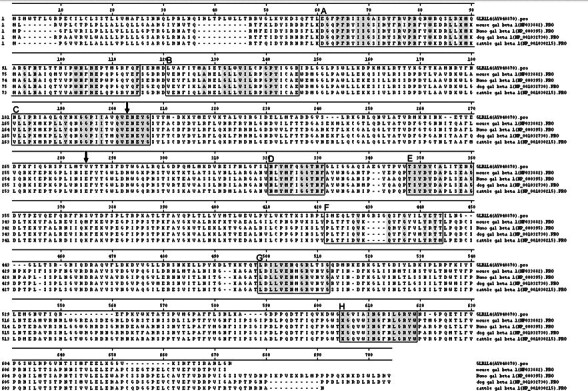
Amino acid sequence alignment of rat GLB1L4 and members of the beta-galactosidase family from mice, humans, dogs, and cattle. The boxes show the conserved regions of beta-galactosidase (Glyco_hydro_35 block number IPB001944A–IPB001944H). The amino acid sequences in eight conserved regions of five proteins are shadowed in gray. The active sites of catalytic proton donor E205 and catalytic nucleophile E279 are indicated by arrows. The catalytic nucleophile site E279 is replaced by V279 in rat GLB1L4 protein.
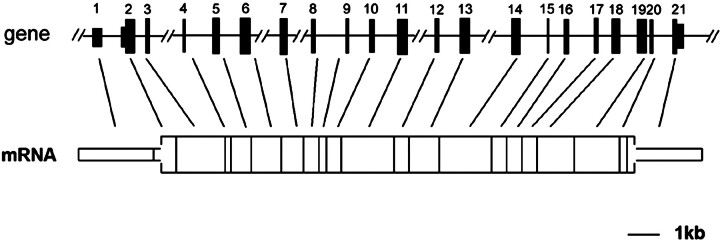
The Glb1l4 gene locus was mapped to rat chromosomes 8q13. It spanned about 41 kb of genomic DNA consisting of 20 introns and 21 exons (Fig. 4 and Table 1).
Fig. 4.
The genomic structure of the rat Glb1l4 gene. The Glb1l4 gene diagram shows the relative lengths of exons 1–21 and intervening introns. Line breaks indicate that the introns have lengths greater than 2 kb and are not drawn to scale. The open box represents the ORF of Glb1l4 mRNA. The narrow bar represents the 5′ and 3′ untranslated regions of the Glb1l4 mRNA.
Table 1.
Exon-intron boundaries in rat Glb1l4 gene.
| Exon no. (bp) | Exon size | Sequence at exon-intron junctiona | ||
|---|---|---|---|---|
| 5′ Splice donor | Intron size (kb) | 3′ Splice acceptor | ||
| 11 | 256 | GGATGAG gtgatgc | 1.4 | catttag CATGTGG |
| 12b | 93 | TACCCAG gtttgtt | 0.6 | cttgcag GCTTAAT |
| 13 | 198 | TTACCAC gtgagtc | 4.5 | atgatag GCATATT |
| 14 | 69 | ACCTCGT gtaggta | 0.9 | attccag AGCTTTT |
| 15 | 96 | TGCCCAG gtaagtt | 0.8 | atcatag CTGGTTG |
| 16 | 109 | ACTCCAG gtaaaac | 2.8 | ccctcag TATAACA |
| 17 | 92 | GAAAACG gtaagtg | 3 | tccttag GCTTTAG |
| 18 | 83 | CAAAACG gtatggt | 1 | cccacag TACTGGC |
| 19 | 65 | CATTCAG gtaaggg | 0.7 | ccctcag GGTAGAA |
| 10 | 85 | CCACAGA gtgagtc | 0.8 | tctgcag ATGTTGA |
| 11 | 138 | AGCTATG gtgagta | 2.7 | tctttag ACTACTG |
| 12 | 81 | GTTACAG gtactca | 0.6 | attgcag ATTTTAG |
| 13 | 107 | GGTCAAG gtgcgtg | 11.5 | gccacag ACAACCA |
| 14 | 147 | GGCCCAG gtaggga | 2.2 | ctggcag GTGTTTT |
| 15 | 68 | CAAAGAT gtatgta | 0.5 | tgaatag CTCACCA |
| 16 | 88 | AGAAAAG gtgggct | 0.8 | cctatag GTTTAAC |
| 17 | 88 | TACAAAG gtgggtg | 0.5 | ctcttag GGAATTT |
| 18 | 112 | AGTAAAG gttagtg | 0.7 | ttttcag GACTGGG |
| 19 | 117 | TAATACG gtgaatt | 0.1 | tgtgcag ATCATAA |
| 20 | 64 | CATCTAG gtatgcc | 0.7 | ttttcag GACATTG |
| 21 | 447 | AATGGTG | ||
Uppercase and lowercase letters indicate exon and intron sequences respectively; conserved splice donor and acceptor and acceptor sites (gt … ag) are bolded.
The open reading frame starts from this exon.
Epididymis-Specific and Androgen-Upregulated Expression of Glb1l4 mRNA
To examine whether Glb1l4 is specifically expressed in the epididymis, total RNAs isolated from 18 different tissues of adult male rat were analyzed by Northern blot analysis. As shown in Figure 5A, rat Glb1l4 mRNA was only expressed in the caput region of epididymis but not in other 17 different tissues.
Fig. 5.
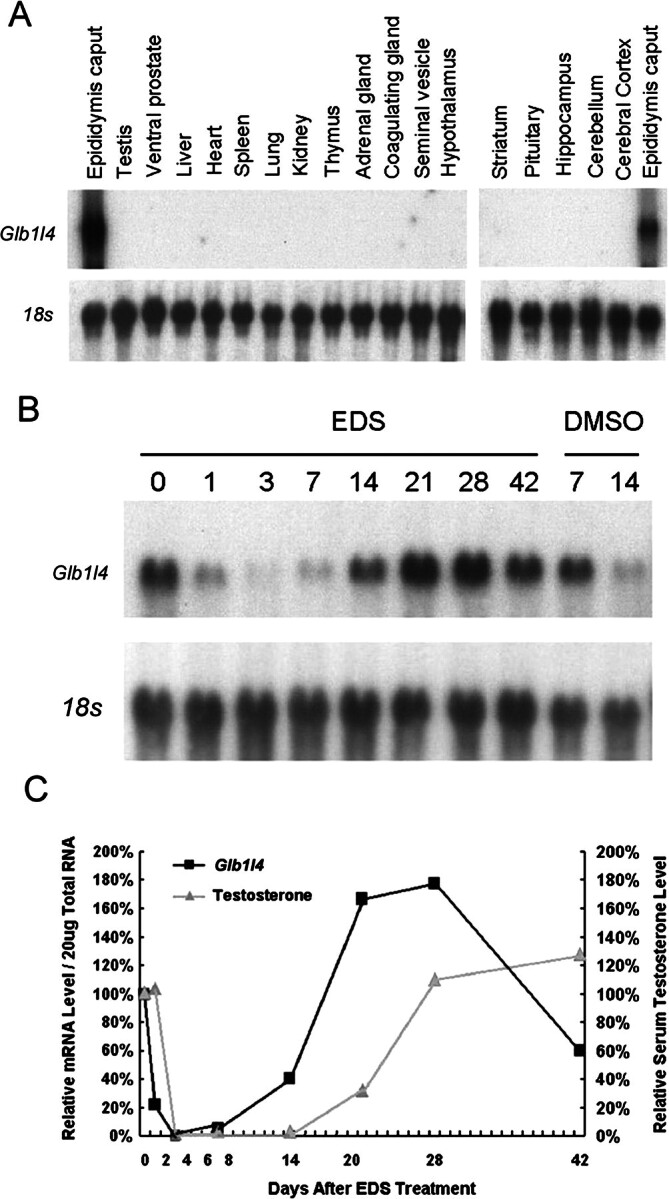
Tissue distribution and androgen manipulation of rat Glb1l4 mRNA analysis by Northern blot analysis. A) Total RNAs (20 μg/lane) from various tissues were used for Northern blot analysis. A specific band was detected in the caput epididymis only. The 18s rRNA was used as internal control. B) Testosterone increases Glb1l4 expression in the epididymis. Northern blot analyses were performed with total rat epididymis RNA (20 μg/lane) from pooled tissues (n = 5 for each time point, expressed in days) following EDS or DMSO (vehicle control) treatment and were hybridized with a Glb1l4 probe. The blot was reprobed for 18s ribosomal RNA to verify RNA integrity and equal loading. C) Quantitative analysis of Glb1l4 mRNA and serum testosterone following EDS treatment confirmed that Glb1l4 mRNA levels were correlated with changes in serum testosterone. Results are means from two independent experiments. Values were normalized to levels in control animals prior to EDS treatment (Day 0) and then were plotted as relative fold increases.
Because most epididymis-specific genes have been known to be regulated by androgens, the effect of androgen manipulation on Glb1l4 mRNA expression was investigated in the rat model treated with EDS, which is able to selectively and reversibly destroy the androgen-generated Leydig cells in rat testis. As shown in Figure 5, B and C, Glb1l4 mRNA in the epididymis was dramatically and concomitantly decreased in abundance as rat serum testosterone level drastically decreased 3 days after EDS administration. During the 4 wk after EDS treatment, Glb1l4 mRNA gradually returned to normal levels in parallel with serum testosterone restoration. The results suggest that transcription of Glb1l4 mRNA is upregulated by androgen in vivo. This conclusion also was supported by castration and testosterone replacement rat models (data not shown).
The Native Status of GLB1L4 Protein in Rat Epididymis
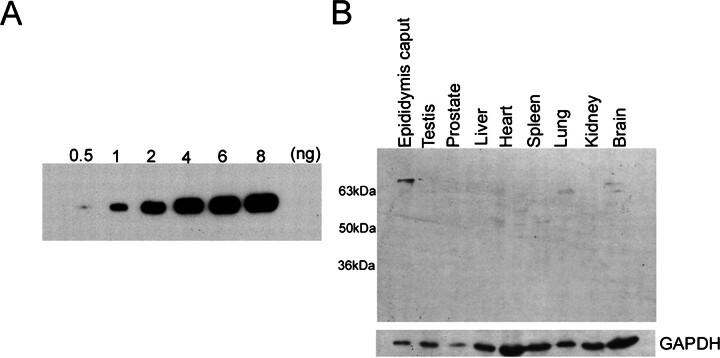
To investigate the native status and localization of the protein in the epididymis, the rabbit polyclonal antisera against the recombinant N-terminal peptide of GLB1L4 was raised, and the sensitivity and specificity of the antisera were verified by Western blot analysis. As shown in Figure 6A, 1-ng N-terminal antigen can be readily detected by the antisera with a 1:10 000 dilution. A distinct band of 70 kDa was detected in the protein extract from the epididymis but not in the testis, prostate, liver, heart, spleen, lung, and kidney (Fig. 6B), which were consistent with the tissue distribution pattern of the Glb1l4 mRNA demonstrated by Northern blot analysis.
Fig. 6.
The sensitivity and specificity of the two polyclonal antisera against the N-terminal peptide of GLB1L4 were analyzed by Western blot analysis. In A, 0.5, 1, 2, 4, 6, and 8 ng of N-terminal antigen peptide were loaded. The antiserum can detect as low as 0.5 ng of antigen by Western blot analysis. B) Protein extracts (40 μg/lane) from various tissues were analyzed by Western blot analysis. GAPDH was used as an internal control.
Region- and Cell-Specific Expression of Rat GLB1L4 in the Epididymis
The expression of GLB1L4 in epididymis was characterized by immunohistochemistry assay in the 90-day-old male rat. As shown in Figure 7A, a gradually decreasing immunoreactivity was detected from the caput region to the cauda region. The magnified images in Figure 7B clearly showed that no signal was found in the initial segment (Fig. 7Ba), and the immunoreactive signal was strong in the caput region (Fig. 7B, b and c), moderate in the corpus region (Fig. 7B, d and e), and faint in the cauda region (Fig. 7Bf).
Fig. 7.
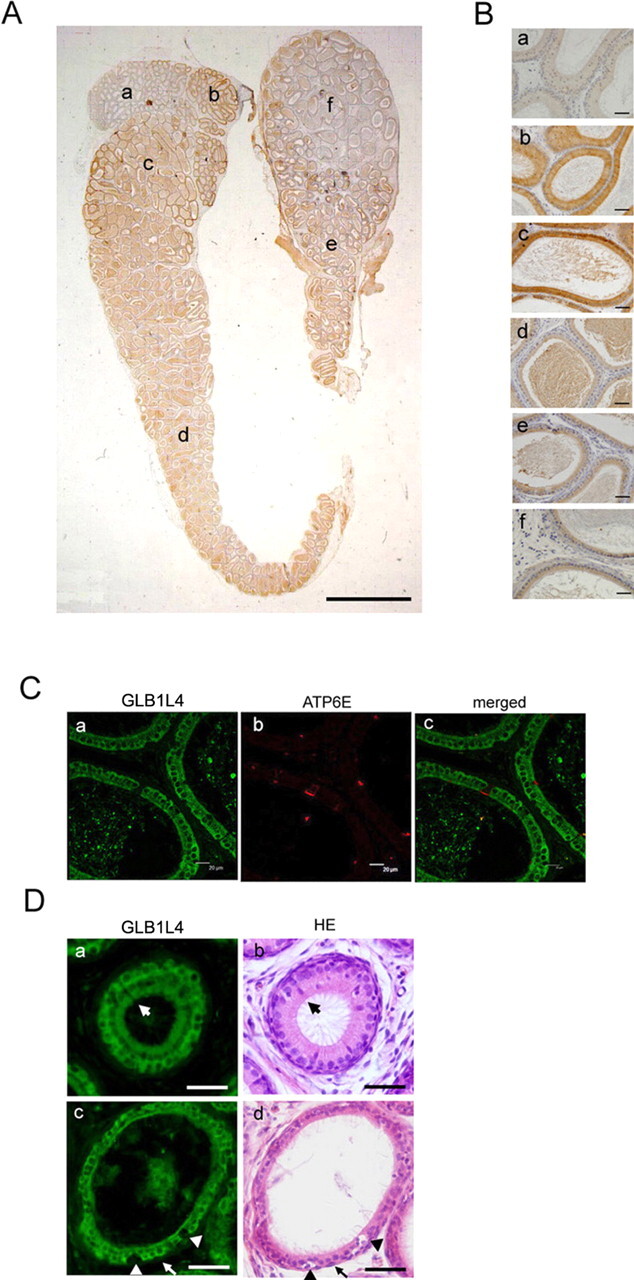
The region-specific and cell-specific localization of rat GLB1L4 in the rat epididymis. A) The regional expression pattern of GLB1L4 in the whole epididymis of a 120-day-old rat. Bar = 2 mm. B) Magnified photographs for individual fields of A: the initial segment (a), the proximal caput (b), the caput (c), the corpus (d), the distal corpus (e), and the cauda (f). Bars = 50 μm. C) Immunofluorescence detection of GLB1L4 (a, FITC labeled), ATP6E (b, rhodamine labeled for clear cells), and their colocalization (c). Bars = 20 μm. D) Immunofluorescence detection of GLB1L4 (a and c) and HE staining (b and d) of the same section, showing that the narrow cell (large arrows), halo cell (arrowheads), and basal cell (small arrows) are not reactive. Bars = 50 μm.
To further identify the localization of this protein in the epididymis, the cell-specific marker and hematoxylin-eosin (HE) staining were used to distinguish the different cell populations. As shown in Figure 7C, by double immunofluorescent staining of the same section, the GLB1L4 with green signal presented only in the principal cells but not in the clear cells, which displayed red signal using anti-ATP6E IgY as the clear cell marker. The immunofluorescence and HE staining of the same sections showed that no positive GLB1L4 fluorescence signal was detected in the narrow cells, halo cells, and basal cells (Fig. 7D). Collectively, the results demonstrated that GLB1L4 was only expressed in the principal cells of the epididymis.
Expression of Glb1l4 During Epididymal Postnatal Development
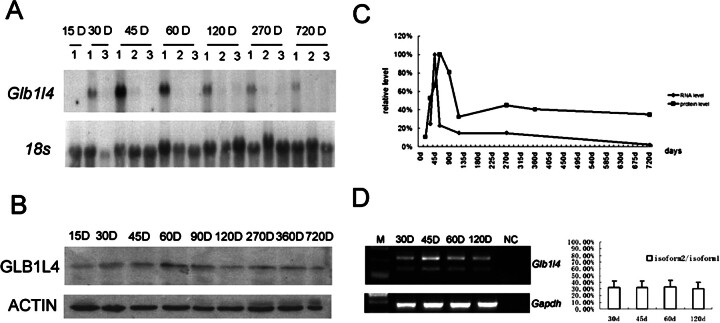
The expression of rat Glb1l4 in the epididymis was further characterized at different developmental stages. The onset of Glb1l4 mRNA expressed in the epididymis was around 15 days after birth and reached to a peak (4-fold increase) at the age of 45 days. After Postnatal Day 60, the expression of Glb1l4 mRNA was decreased sharply but remained at a low level until the rats aged (Fig. 8A). At the protein level, the peak of GLB1L4 was at Postnatal Day 60, implying a translation delay (Fig. 8B). The expression of rat Glb1l4 peaks in a very narrow time window at adolescence in both RNA and protein levels (Fig. 8C), implying that Glb1l4 plays an important role at puberty. The two alternatively spliced transcripts of Glb1l4 also were examined by RT-PCR during development, showing that the 3:1 ratio of long transcript and short transcript was not changed during the epididymal development (Fig. 8D).
Fig. 8.
Expression of Glb1l4 in rat epididymis during the entire life span in days (D). A) Northern blot analysis for Glb1l4 mRNA and 18s rRNA from the caput region (1), the corpus region (2), and the cauda region (3) during development. B) Western blot analysis of GLB1L4 and ACTIN proteins during development. D, days. C) Quantitative analysis of the relative mRNA and protein expression levels in rat epididymides at different ages. RNA and protein were pooled from three animals per group. D) Left: RT-PCR of two isoforms of Glb1l4 mRNA expression in rat caput epididymis from 30, 45, 60, and 120 days (D) after birth. Gapdh was used as internal control. NC, negative control. Right: semiquantitative analysis of the ratio of isoform2:isoform1 in rat epididymis at different ages. d, days.
For further understanding the role of GLB1L4 protein in epididymal cell differentiation during development, immunohistochemistry analysis was performed. Positive GLB1L4 signal was detected as early as Postnatal Day 14 (Fig. 9A, a–d). At Postnatal Day 21 (Fig. 9A, e–h), the signal was increased mainly in the epithelial cells of the initial segment and the caput region, a weak signal with a checkerboardlike pattern was seen in the corpus region, and almost no staining was detected in the cauda region. At Postnatal Day 69, the signal was maintained at a high level in the caput region but faded in the initial segment (Fig. 9Aq). From Day 69 (Fig. 9A, q–t) to Day 120 (Fig. 9A, u–x), the signal was still observed in the caput region, but it was very weak in the other regions of the epididymis. Interestingly, the subcellular localization of GLB1L4 in cytoplasm also changed during development. At Postnatal Day 21, it was noticed that the staining was located in the low columnar cells and scattered uniformly over the entire cytoplasm (Fig. 9Bb). By Postnatal Day 49, the distribution of GLB1L4 in the epithelium cell appeared to be polarized and located mainly in the apical and basal sides of the principal cells (Fig. 9Bd). At Postnatal Day 69, the protein distribution was resumed to the entire cytoplasm (Fig. 9Be). As a negative control, no staining was observed using normal rabbit serum (Fig. 9Ay).
Fig. 9.
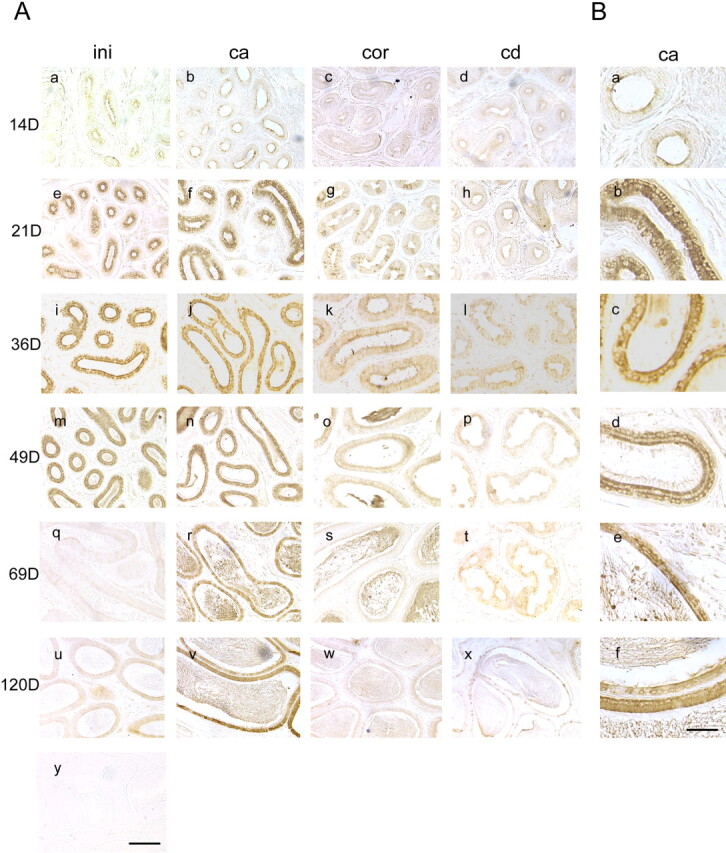
The spatial and temporal expressions of rat GLB1L4 in rat epididymis. The GLB1L4 protein localization in different regions of the rat epididymis at different ages was determined by immunohistochemistry. The ages in days (D) are shown on the left, and the regions of the epididymis are shown at the top. Panel y, as detected by preimmune serum, served as negative control. ini, the initial segment; ca, the caput region; cor, the corpus region; cd, the cauda region. Bar = 100 μm. B) Each panel is a highly magnified image of the caput epididymis as shown in A (i.e., Ab, Af, Aj, An, Ar, and Av). Bar = 25 μm.
GLB1L4 Is Secreted into the Lumen after Epididymis Development
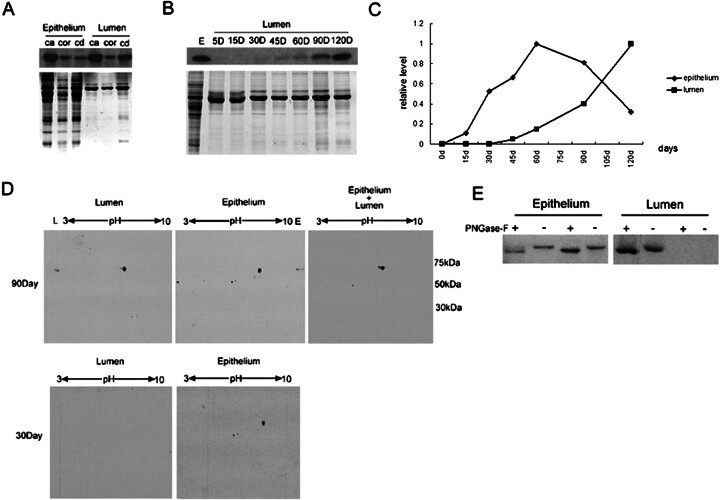
Curiously, we noticed that GLB1L4, as a secretory protein, could be found in the lumen fluid of an adult but not a pubertal rat (Fig. 9). There could be two possibilities: 1) the positive signal in the lumen is caused by an unknown protein, cross-reactive, with GLB1L4 antisera, and 2) GLB1L4 revealed different secretory patterns before and after the epididymal development. To confirm whether the staining in the epididymal lumen after Postnatal Day 49 was caused by GLB1L4 secreted into the lumen, protein extracts of the epididymal epithelial cells and lumen fluid from the caput region at Postnatal Day 90 were analyzed by Western blot analysis. The same size band was detected in the protein extracts of ether rat epididymal epithelium or the total lumen fluid (Fig. 10A), indicating that GLB1L4 protein was secreted from epithelial cells into the lumen.
Fig. 10.
Comparison of GLB1L4 in the rat epididymal epithelium and lumen by Western blot analysis. A) Western blot analysis of GLB1L4 from rat epididymal epithelium and lumen protein extracts of the caput (ca), corpus (cor), and cauda (cd) regions. Protein (30 μg) was loaded in each lane. The same volume of protein was separated by electrophoresis and stained by Coomassie blue to show the equal loading. B) Western blot analysis of GLB1L4 from epididymal lumen protein during development. Protein (20 μg) was loaded in each lane. The same volume of protein was separated by electrophoresis and stained by Coomassie blue to show the equal loading. E, epididymis; D, days. C) Quantitative analysis of the relative protein expression levels in epididymal epithelium and lumen fluid at different ages in days (d). Protein extracts were pooled from three animals per group. D) Lumen (100 μg), epididymal epithelium (100 μg), and epithelium plus lumen (200 μg epithelium and 100 μg lumen) proteins were separated by 2D gel electrophoresis and detected using anti-GLB1L4 antisera. Molecular mass is indicated on the right (kDa), and pI values are shown on the top. E) The change in molecular mass of mature (90-day-old; left) and adolescent (30-day-old; right) rat GLB1L4 before (−) and after (+) deglycosylation by PNGase-F. Epithelium, epididymal epithelium protein; Lumen, lumen fluid protein.
The expression of GLB1L4 was further analyzed in proteins of luminal fluid at different ages. Figure 10B shows that no GLB1L4 protein was in the lumen fluid before Postnatal Day 45. Thereafter, it began to be secreted, and it gradually increased in abundance in the lumen. The temporal expression pattern of GLB1L4 in the lumen fluid was opposite that in the epididymal epithelium (Fig. 10C). It is therefore plausible that GLB1L4 would be secreted into the lumen from the epididymal epithelium cell during puberty.
To investigate whether the secretion of the protein was caused by protein modification, two-dimensional (2D) gel electrophoresis followed by Western blot analysis was performed. Figure 10D shows that one spot at 70 kDa, pI 9.0 was detected in the epididymal epithelium protein extracts from the caput region of 90-day and 30-day rats. In the total luminal protein, however, two spots with the same molecular mass but slightly different charges were detected (Fig. 10D, left). The spot of acidic isoform was smaller than that of the basic isoform. To investigate how GLB1L4 was changed in charge in epididymal lumen fluid, the protein extracts from epididymal epithelium and lumen fluid protein were pooled together and analyzed by 2D gel electrophoresis and Western blot analysis. Two spots detected from pooled proteins were the same molecular mass and charge as the protein from the lumen fluid only (Fig. 10D, right). These data suggested that a small percentage of GLB1L4 became more acidic after being secreted into the epididymal lumen.
To determine what caused GLB1L4 to be secreted from the epithelium into lumen fluid, the deglycosylation and phosphorylation assays were performed based on the presence of many potential N-glycosylation and phosphorylation sites in the sequence (Fig. 2). The epithelium and lumen proteins of mature (90-day-old) and adolescent (30-day-old) rats were incubated in the absence and presence of N-glycanase and were subsequently analyzed by Western blot analysis. After deglycosylation, a slightly decreased molecular mass of rat GLB1L4 (Fig. 10E) was detected, suggesting that GLB1L4 from epididymal epithelium and lumen fluid at different ages was uniformly glycosylated. We also investigated whether the more acidic form of GLB1L4 is due to phosphorylation of tyrosine and threonine residues. The results showed that rat GLB1L4 from epithelium and lumen fluid is not phosphorylated at tyrosine and threonine residues (data not shown).
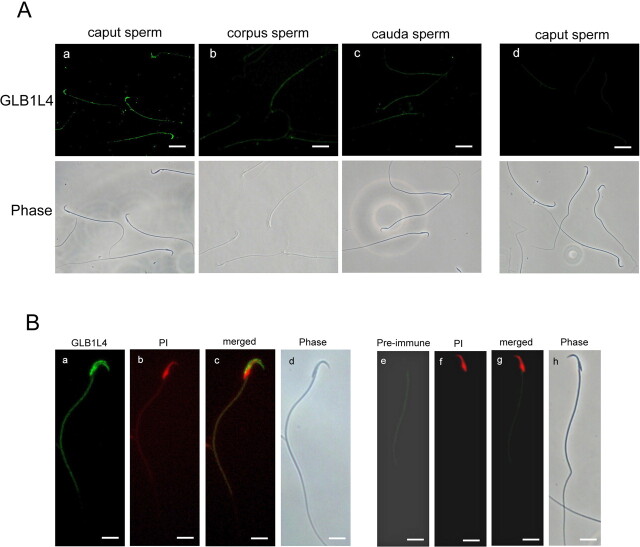
Immunolocalization of Rat GLB1L4 on Sperm
The presence of rat GLB1L4 in the epididymal lumen indicated that it may interact with spermatozoa. Indirect immunofluorescence staining showed that some caput spermatozoa can bind rat GLB1L4, whereas the corpus and cauda spermatozoa did not show fluorescent staining (Fig. 11A), suggesting a loss of rat GLB1L4 during epididymal transit. It was noticed that GLB1L4 was concentrated over the acrosomal region of spermatozoa (Fig. 11B, a–d). The negative control (preimmune serum) had no signal at all (Fig. 11, Ad and B, e–h).
Fig. 11.
Rat GLB1L4 localization on spermatozoa derived from the caput, corpus, and cauda epididymal regions by immunofluorescence. A) Immunolocalization of rat GLB1L4 (FITC labeled) on spermatozoa isolated from different epididymal regions: (a) caput spermatozoa, (b) corpus spermatozoa, (c) cauda spermatozoa, and (d) caput spermatozoa as detected by preimmune serum as negative control. Bottom row of photographs are phase-contrast images of the spermatozoa shown in the photographs in the upper row. Bars = 10 μm. B) Highly magnified images of the GLB1L4 protein localization on caput spermatozoa: (a) the immunofluorescence of rat GLB1L4 (FITC labeled); (b) spermatozoa nuclear detected by propidium iodide (PI; rhodanmine labeled); (c) the merged photographs of a and b; (d) phase-contrast view of sperm in a. e) Caput spermatozoa detected by preimmune serum as negative control; (f) spermatozoa nuclear detected by PI; (g) the merged photographs of e and f; and (h) phase-contrast view of sperm in e. Bars = 5 μm.
Discussion
In the present study, we isolated and characterized of a novel rat epididymis-specific, caput region, principal cell-exclusive, β-galactosidase-like gene Glb1l4 at both mRNA and protein levels. Certain rat epididymal β-galactosidases have been reported previously in the literature. For examples, Sosa et al. [22] purified a 64-kDa β-galactosidase in rat epididymal fluid, and Dutta et al. [10] reported a 50-kDa β-galactosidase whose enzyme activity increased markedly (4-fold) in the epididymis of adult rats compared with that of 24-day-old rats. Although cDNAs of these enzymes have not been cloned, their molecular masses are apparently smaller than that of GLB1L4. Tulsiani et al. [11] isolated and purified two forms (97 kDa and 84 kDa) of β-d-galactosidase in the rat epididymal lumen fluid. After removal of N-linked glycans by hydrolysis with N-glycanase or endoglycosidase F, both forms of the β-d-galactosidase released a 70-kDa polypeptide with the same N-terminal amino acid sequence which had 63% residue identity to that of the mouse β-d-galactosidase precursor. They thought that the two forms of the enzyme originated from a same or similar 70-kDa polypeptide with different N-linked glycans [11, 23]. Nonetheless, both their molecular mass and N-terminal amino acid sequence were different from that of GLB1L4 protein. In addition, these proteins were reported to have maximum enzymatic activity under acid conditions [11]. In contrast, recombinant GLB1L4 protein expressed in eukaryote cells did not show any galactosidase activity at pH 4–7 (data not shown). It is not surprising that GLB1L4 possessed some sequence similarities to β-d-galactosidase, but those amino acids are not located at those important structure domains; rather, they are dispersed evenly over the whole peptide. Moreover, GLB1L4 lacks an active nucleophile site. Thus, this newly discovered gene Glb1l4 may be a unique member of the β-galactosidase family and play important roles other than the general enzyme activity of β-galactosidase in the epididymis.
The molecular mechanism underlying epididymal development and caput region-specific differentiation is still unknown. Because of the temporal and special expression characteristics of GLB1L4 in the epididymis, we are encouraged to suppose that this protein may play some roles in two processes (i.e., epididymal development and sperm maturation). Our findings include that 1) Glb1l4 is the first reported epididymis-specific gene that is maximally expressed at puberty; 2) GLB1L4 protein is specifically expressed in the principal cells of the caput epididymis; and 3) GLB1L4 protein distribution in the principal cells is changed in the developing process of the epididymis. All the data strongly suggest a notion that GLB1L4 may act as a regulator of columnar cell differentiation. It also was reported that during the epididymal differentiation period, a dramatic cell shape change from a low columnar cell to a tall and thin principal cell occurred [1]. For example, in 21-day-old animals, the principal cells are low columnar. After Postnatal Day 35, a dramatic change in size occurs, and a tall, columnar cell type was observed [24]. We also noted in this study that the changes in the distribution of GLB1L4 protein in the cells occur in a period of time when the prolongated principal cell appears. During the principal cell elongation, the GLB1L4 protein is scattered throughout the cytoplasm first, and it is later confined to the apical and basal sides of the cell. When differentiation of principal cell is completed, GLB1L4 protein is redistributed back to the entire cytoplasm. A very nice coincidence of the spatial and temporal expression of GLB1L4 and the developing process of the epididymis support the assumption that GLB1L4 might participate in the differentiation of principal cell in the epididymis. Generation of conditional knockout mice to verify this hypothesis is on the way. Besides, the caput region-specific differentiation control also made us feel puzzled. We do not know at present whether there are any different subtypes of principal cells in different epididymal regions and whether the formation of these subtypes is controlled by different ways. Anyhow, the studies using the phenotype of the GLB1L4 conditional knockout mice would be able to answer these questions.
As shown in Figure 10C, the temporal expression pattern of GLB1L4 in the luminal fluid is different from that in the epididymal epithelium. It appears that GLB1L4 stays in the cytoplasm of epididymal epithelial cells during puberty, then moves into lumen and binds to sperm during adulthood. This raises the issue of how the secretion of GLB1L4 is temporally controlled. Interestingly, the size of this protein remains unchanged both in adulthood and in puberty, suggesting that the signal peptide is not removed after secretion. Another interesting observation is that only a small percentage of GLB1L4 is modified in the lumen (Fig. 10D). Two possible mechanisms for the regulation of GLB1L4 expression would need to be further investigated. 1) Posttranslational modifications, such as phosphorylation and N-myristoylation, might regulate its secretory ability, and 2) GLB1L4-binding protein(s) in principal cell cytoplasm might control its secretion. In fact, our results (Fig. 10F) have ruled out the first possibility. Identification of the GLB1L4-binding proteins in principal cells during puberty and adulthood might be able to address the second possibility.
In summary, findings from our current studies raise several important issues. 1) What is the molecular mechanism of epididymis development, especially how the caput region principal cells are developed from the columnar cells? 2) How is the secretion of GLB1L4 protein controlled in a timely manner? 3) What role does GLB1L4 play in sperm maturation after binding to sperm? The answers to these questions will further advance our knowledge of male reproductive functions and the β-galactosidase family functions.
Contributor Information
Wei Zhen, Shanghai Key Laboratory for Molecular Andrology, State Key Laboratory of Molecular Biology, Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, Shanghai, China; The Graduate School of the Chinese Academy of Sciences, Shanghai, China.
Peng Li, Shanghai Key Laboratory for Molecular Andrology, State Key Laboratory of Molecular Biology, Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, Shanghai, China.
Bin He, Shanghai Key Laboratory for Molecular Andrology, State Key Laboratory of Molecular Biology, Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, Shanghai, China.
Juyuan Guo, Shanghai Key Laboratory for Molecular Andrology, State Key Laboratory of Molecular Biology, Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, Shanghai, China.
Yong-Lian Zhang, Shanghai Key Laboratory for Molecular Andrology, State Key Laboratory of Molecular Biology, Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, Shanghai, China; Shanghai Institute of Planned Parenthood Research, Shanghai, China.
References
- 1. Rodriguez CM, Kirby JL, Hinton BT. The development of the epididymis.In:Robaire B, Hinton BT. The Epididymis: From Molecules to Clinical Practice. New York: Kluwer Academic/Plenum Publishers; 2002:251–267. [Google Scholar]
- 2. Connwall GA, Hann SR. Specialized gene expression in the epididymis. J Androl 1995; 16:379–383. [PubMed] [Google Scholar]
- 3. Hamilton DW. Structure and function of the epithelium lining the ductuli efferents, ducts epididymis and ductus deferens in the rat.In:Greep RO, Astwood EB. Handbook of Physiology, vol. 5. Baltimore: Waverly Press; 1975:259–383. [Google Scholar]
- 4. Miler RJ, Killian GJ. Morphometric analysis of the epididymis from normal and vasectomized rats. J Androl 1987; 8:279–291. [DOI] [PubMed] [Google Scholar]
- 5. Wong PYD, Au CL, Ngai HK. Electrolyte and water transport in rat epididymis: its possible role in sperm maturation. Int J Androl 1978; 1:600–628. [Google Scholar]
- 6. Rodriguez CM, Kirby JL, Hinton BT. Regulation of gene transcription in the epididymis. Reproduction 2001; 122:41–48. [DOI] [PubMed] [Google Scholar]
- 7. Uhlenbruck F, Sinowatz F, Amselgruber W, Kichhoff C, Ivell R. Tissue-specific gene expression as an indicator of epididymis-specific functional status in the boar, bull and stallion. Int J Androl 1993; 16:53–61. [DOI] [PubMed] [Google Scholar]
- 8. Conzelmann E, Sandhoff K. Glycolipid and glycoprotein degradation. Adv Enzymol Relat Areas Mol Biol 1987; 60:89–216. [DOI] [PubMed] [Google Scholar]
- 9. Conchie J, Findlay J, Levvy GA. Mammalian glycosidases; distribution in the body. Biochem J 1959; 71:318–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dutta P, Majumder GC. Purification and characterization of rat epididymal neutral beta- galactosidase and its changes during in vivo development. Biochem Cell Biol 1993; 71:22–26. [DOI] [PubMed] [Google Scholar]
- 11. Tulsiani DRP, Skudlarek MD, Araki YA, Orgebin-Crist MC. Purification and characterization of two forms of β-D-galactosidase from rat epididymal luminal fluid: evidence for their role in the modification of sperm plasma membrane glycoproteins. Biochem J 1995; 305:41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fornes WM, Sosa MA, Bertini F, Burgos MH. Vesicles in rat epididymal fluid. Existence of two populations differing in ultrastructure and enzymatic composition. Andrologia 1995; 27:233–237. [DOI] [PubMed] [Google Scholar]
- 13. Liang P, Pardee AB. Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction [see comments]. Science 1992; 257:967–971. [DOI] [PubMed] [Google Scholar]
- 14. Li P, Chan HC, He B, So SC, Chung YW, Shang Q, Zhang YD, Zhang YL. An antimicrobial peptide gene found in the male reproductive system of rats. Science 2001; 291:1783–1785. [DOI] [PubMed] [Google Scholar]
- 15. Morris ID, Phillips DM, Bardin CW. Ethylene dimethanesulfonate destroys Leydig cells in the rat testis. Endocrinology 1986; 118:709–719. [DOI] [PubMed] [Google Scholar]
- 16. Hu YX, Guo JY, Shen L, Zhang ZC, Zhang YL. Get effective polyclonal antisera in one month. Cell Res 2002; 12:157–160. [DOI] [PubMed] [Google Scholar]
- 17. Gu J, Stephenson CG, Iadarola MJ. Recombinant proteins attached to a nickel-NTA column: sue in affinity purification of antibodies. Biotechniques 1994; 17:257, 260,, 262. [PubMed] [Google Scholar]
- 18. Xiao LQ, Liu AH, Zhang YL. An effective method for raising antisera against beta-defensins: double-copy protein expression of mBin1b in E. coli. Acta Biochim Biophys Sin (Shanghai)2004; 36:571–576. [DOI] [PubMed] [Google Scholar]
- 19. Yuan H, Liu A, Zhang L, Zhou H, Wang Y, Zhang H, Wang G, Zeng R, Zhang YL, Chen Z. Proteomic profiling of regionalized proteins in rat epididymis indicates consistency between specialized distribution and protein functions. J Proteome Res 2006; 5:299–307. [DOI] [PubMed] [Google Scholar]
- 20. Hu YX, Zhou ZY, Xu C, Shang Q, Zhang YD, Zhang YL. Androgen down-regulated and region-specific expression of germ cell nuclear factor in mouse epididymis. Endocrinology 2003; 144:1612–1619. [DOI] [PubMed] [Google Scholar]
- 21. Zhu C, Liu Q, Zhang L, Yuan HX, Zhen W, Zhang J, Chen Z, Hall SH, French FS, Zhang Y. RNase9, an androgen-dependent member of the RNase A family, is specifically expressed in the rat epididymis. Biol Reprod 2007; 76:63–73. [DOI] [PubMed] [Google Scholar]
- 22. Sosa MA, Barbieri AM, Bertini F. Purification and characterization of beta-galactosidase from rat epididymal fluid. Andrologia 1996; 28:217–221. [DOI] [PubMed] [Google Scholar]
- 23. Skudlarek MD, Tulsiani DR, Orgebin-Crist MC. Rat epididymal luminal fluid acid beta-D-galactosidase optimally hydrolyses glycoprotein substrate at neutral pH. Biochem J 1992; 286:907–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hermo L, Barin K, Robaire B. Structural differentiation of the epithelial cells of the testicular excurrent duct system of rats during postnatal development. Anat Rec 1992; 233:205–228. [DOI] [PubMed] [Google Scholar]