Abstract
Objectives
To describe the feasibility and evaluate the performance of multiphasic photon-counting detector (PCD) CT for detecting breast cancer and nodal metastases with correlative dynamic breast MRI and digital mammography as the reference standard.
Methods
Adult females with biopsy-proven breast cancer undergoing staging breast MRI were prospectively recruited to undergo a multiphasic PCD-CT using a 3-phase protocol: a non-contrast ultra-high-resolution (UHR) scan and 2 intravenous contrast-enhanced scans with 50 and 180 s delay. Three breast radiologists compared CT characteristics of the index malignancy, regional lymphadenopathy, and extramammary findings to MRI.
Results
Thirteen patients underwent both an MRI and PCD-CT (mean age: 53 years, range: 36-75 years). Eleven of thirteen cases demonstrated suspicious mass or non-mass enhancement on PCD-CT when compared to MRI. All cases with metastatic lymphadenopathy (3/3 cases) demonstrated early avid enhancement similar to the index malignancy. All cases with multifocal or multicentric disease on MRI were also identified on PCD-CT (3/3 cases), including a 4 mm suspicious satellite lesion. Four of five patients with residual suspicious post-biopsy calcifications on mammograms were detected on the UHR PCD-CT scan. Owing to increased field-of-view at PCD-CT, a 5 mm thoracic vertebral metastasis was identified at PCD-CT and not with the breast MRI.
Conclusions
A 3-phase PCD-CT scan protocol shows initial promising results in characterizing breast cancer and regional lymphadenopathy similar to MRI and detects microcalcifications in 80% of cases.
Advances in knowledge
UHR and spectral capabilities of PCD-CT may allow for comprehensive characterization of breast cancer and may represent an alternative to breast MRI in select cases.
Keywords: photon-counting CT, breast imaging, ultrahigh-resolution CT, multi-energy CT
Introduction
Of the available imaging modalities for detecting and staging breast cancer, magnetic resonance imaging (MRI) has the highest sensitivity for breast cancer detection and an expanding set of indications for use in preoperative locoregional staging. When compared to mammography and US, breast MRI has shown superiority in primary tumor size estimation, as well as detection of multifocal and multicentric small foci of disease.1,2 However, breast MRI cannot detect breast microcalcifications. An additional drawback to MRI is that some patients cannot undergo the study due to implanted devices, claustrophobia, limited access, or cost. Finally, breast MRI is a longer exam, with most protocols ranging between 45 and 30 min.
Conventional whole-body CT is not a modality routinely utilized for diagnostic breast imaging due to its limited spatial resolution (300-500 µm) compared with mammography (100 µm), higher glandular radiation dose, and inferior soft-tissue contrast compared with MRI. Photon-counting detector (PCD) CT is a novel technology that has the advantages of improved isotropic spatial resolution (<150 µm), permitted by the smaller detector pixel sizes and the omission of reflective septae in the PCD CT detector. With PCDs, the electrical signal generated by each X-ray photon is proportional to the energy of the individual photon. This energy discriminating capability provides for improved quality of multienergy material-specific imaging (eg, iodine distribution maps) without the loss of temporal resolution, now available with every acquisition. PCD-CT specific kernels, with appropriate noise reduction methods such as iterative reconstruction, translate into image sharpness, contributing to improved demarcation and characterization of low contrast soft tissue breast masses. And all at a reduced radiation dose compared to conventional clinical CT systems.3–5 These features may allow for a comprehensive evaluation of the breast on CT.6
Our aim is to demonstrate the feasibility of a 3-phase contrast enhanced breast PCD-CT protocol to detect and fully characterize breast cancer and adenopathy using correlative dynamic breast MRI and digital mammography as the reference standard.
Methods
In this prospective, Institutional-Review-Board-approved study, adult female patients with biopsy proven breast cancer scheduled for a staging breast MRI were recruited to undergo a PCD-CT. Patients were included only if they underwent the clinical contrast-enhanced breast MRI. Patients were excluded if they were <18 years of age; pregnant; had poor renal function (estimated glomerular filtration rate ≤ 60 mL/min); or had a history of prior moderate or severe reaction to iodinated contrast material.
Breast MRIs were performed with a 1.5 T MRI system (Optima 450, GE Healthcare, Milwaukee, WI), with patients placed prone in an 8-channel breast coil (USA Instruments, GE Healthcare, Aurora, OH). Multiplanar multisequence imaging was performed with a dynamic contrast-enhanced protocol (Table 1).
Table 1.
Dynamic breast MRI acquisition parameters.
| Imaging parameter | Axial T2 IDEAL | Axial vibrant pre- and post-contrast |
|---|---|---|
| Sequence | 2D FRFSE-XL | 3D vibrant |
| TE | 102 | Min full |
| TR | 4500 | 6.9 |
| Flip angle (degree) | 160 | 10 |
| Section thickness (mm) | 4 | <2 (1.8) |
| FOV (cm) | 26 or more to fit | 26 or more to fit |
| Frequency direction | A/P | A/P |
All axial imaging is bilateral.
Abbreviations: A/P = anteroposterior; S/I = superior/inferior; STIR = short inversion-recovery; TE = echo time; TR = repetition time; 2D = 2 dimensional; 3D = 3 dimensional.
PCD-CT scans were performed on a dual-source system (NAEOTOM Alpha, Siemens Healthineers, Forchheim, Germany) at 120 kV.7 Patients were scanned prone, with the breasts in a pendant geometry using a custom-made positioning aid, simulating positioning at MRI.8 Image acquisition utilized a 3-phase breast imaging protocol (with a chest scan field of view): first, non-contrast scan acquired with ultra-high-resolution (UHR) (<150 µm in plane spatial resolution) mode with attention to selecting the smallest focal spot; followed by 2 multi-energy scans with 50- and 180-s delay post IV contrast injection, acquired with a standard- and a low-dose spectral mode, respectively. Additional scanning parameters are summarized in (Table 2). The IV contrast material (Iohexol, Omnipaque 350, GE Healthcare, Chicago, IL, United States) was injected at a rate of 3-5 mL/s applying a weight-based dose selection, not to exceed 150 mL for each case. The contrast enhanced scans were reconstructed with commercially available material decomposition software to generate quantitative iodine maps, which increased conspicuity of enhancing lesions and iodine uptake quantification.9,10
Table 2.
PCD-CT 3 phase breast protocol acquisition and reconstruction parameters.
| SIEMENS NAEOTOM Alpha | |
|---|---|
| Scan mode | RoutineSpiralAdultThoraxHighresultraQuantumplus |
| Base protocol | Thorax |
| Acquisition # | #1 non-contrast |
| Eff. mAs | 125 |
| Tube potential, kV | 120 |
| Collimation (mm) | 120 × 0.2 |
| Pitch | 0.8 |
| Rotation time (s) | 1 |
| Delay (s) | 0 |
| CTDI (mGy) | 10 |
| Slice thickness (mm) | 0.2 |
| Increment (mm) | 0.15 |
| Kernel | Br84 |
| IR/IR strength | ON/3 |
| Matrix size | 1024 |
| SIEMENS NAEOTOM Alpha | ||
|---|---|---|
| Scan mode | RoutineSpiralAdultThoraxQuantumplus | |
| Base protocol | Thorax | |
| Acquisition # | #2 CE 50 s | #3 CE 180 s |
| CARE keV IQ level | 180 | 90 |
| Eff. mAs | 128 | 64 |
| Tube potential, kV | 120 | |
| Collimation (mm) | 144 × 0.4 | |
| Pitch | 0.6 | |
| Rotation time (s) | 0.5 | |
| Delay (s) | 50 | 180 |
| CTDI (mGy) | 10 | 5 |
| Slice thickness (mm) | 0.6 | |
| Increment (mm) | 0.5 | |
| Kernel | Br56 | |
| IR/IR strength | ON/2 | |
| Matrix size | 1024 | |
Abbreviations: CE = contrast-enhanced; CTDIvol = volume computed tomography dose index; IR = iterative reconstruction.
Working in consensus, 3 unblinded breast radiologists characterized the breast lesions based on tumor size, enhancement pattern, presence of microcalcification, regional nodal metastatic disease, and extramammary findings. These were compared with correlative breast MRI and mammograms as the reference imaging standard.
Results
To date, 13 patients have been scanned in this study, with a mean age of 53 years (range 36-75 years). In 11/13 cases, a suspicious mass or non-mass enhancement were detected on PCD-CT compared to MRI, as illustrated in (Figure 1). Enhancement on CT was similar to MRI in 5 cases, less than MRI in 5 cases, and slightly more than MRI in a single case. Range of enhancement size identified on CT was from 0.4 cm to 11.9 cm. Subjectively, enhancement was more avid on the 180 second delay scan in 7 cases, more avid on the 50 second delay scan in 3 cases, and similar or minimally enhancing on both delays in 1 case. The histologic cancer types identified were Invasive Ductal Carcinoma (n = 10) and Ductal Carcinoma in Situ (DCIS) (n = 1). Of the 2 cases that did not demonstrate enhancement with PCD-CT, one was a biopsy proven Paget’s disease of the nipple with asymmetric nipple enhancement better seen on MRI. In the other case, a biopsy performed at the interval between the MRI and CT exams removed the group of suspicious microcalcifications where the enhancement had been seen at MR, with no residual calcifications post biopsy. Four of five patients with residual post-biopsy microcalcifications detected with mammography were identified on the UHR PCD-CT scan. Multifocal or multicentric disease in all 3 cases were visualized comparably with PCD-CT and MRI. In one of these cases, CT accurately identified small foci of enhancement comparable to that seen on MRI (Figure 2). Metastatic lymphadenopathy, detected in 3/3 cases with both modalities, showed early avid enhancement similar to the primary tumor emphasized on PCD-CT (Figure 3). Incidental findings noted with PCD-CT involved pulmonary and bony lesions, including a lytic sub-centimeter thoracic vertebral metastasis that was not seen on MRI. The estimated organ dose to the breast and the effective dose for our triple phase protocol are 26 mGy, and 12 mSv, respectively. Study results are summarized in (Table 3).
Figure 1.
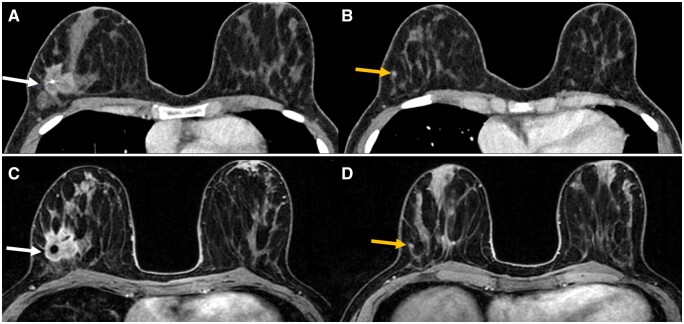
A 35-year-old patient with an index malignancy and a satellite lesion in the right breast detected on PCD-CT and MRI. Contrast enhanced PCD-CT with a 3.2 cm irregular spiculated mass containing biopsy clip in the right upper outer far posterior depth breast (A, white arrow). A tiny 4 mm suspicious focus inferior to the index malignancy is visualized on contrast enhanced PCD-CT (B, yellow arrow). MRI T1 with contrast demonstrates an enhancing spiculated irregular mass in the right upper outer, far posterior depth breast (C, white arrow). MRI T1 with contrast also demonstrating an enhancing 4 mm focus inferior to the breast mass (D, yellow arrow). Abbreviation: PCD = photon-counting detector.
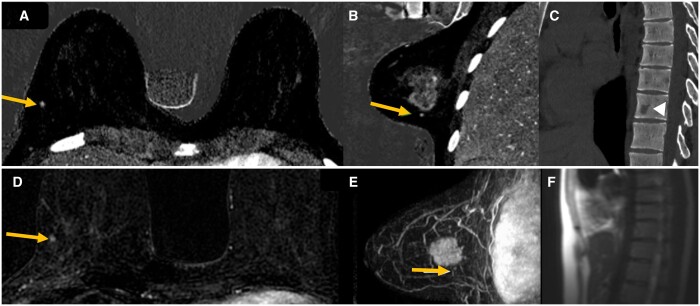
Figure 2.
The patient shown on the previous image with the primary breast mass, small satellite focus and a vertebral metastasis. The 4 mm enhancing focus, located 6 mm inferior to the known index malignancy in the right breast is nicely depicted on contrast enhanced PCD-CT iodine axial and sagittal images inferior to the index malignancy (A and B, yellow arrow). Small subcentimeter lucent lesion in the T10 vertebral body only seen on contrast enhanced PCD-CT (C, open arrow). This thoracic lesion was later biopsied positive for breast metastasis. Comparison MRI post contrast subtraction images again demonstrate this small enhancing right breast focus (D, yellow arrow). MRI MIP sagittal image with small enhancing focus inferior to the right breast mass (E, yellow arrow). Retrospectively the thoracic vertebral lesion is not visualized on MRI scout images and was not included within the field of view on axial MRI images (F). Abbreviation: PCD = photon-counting detector.
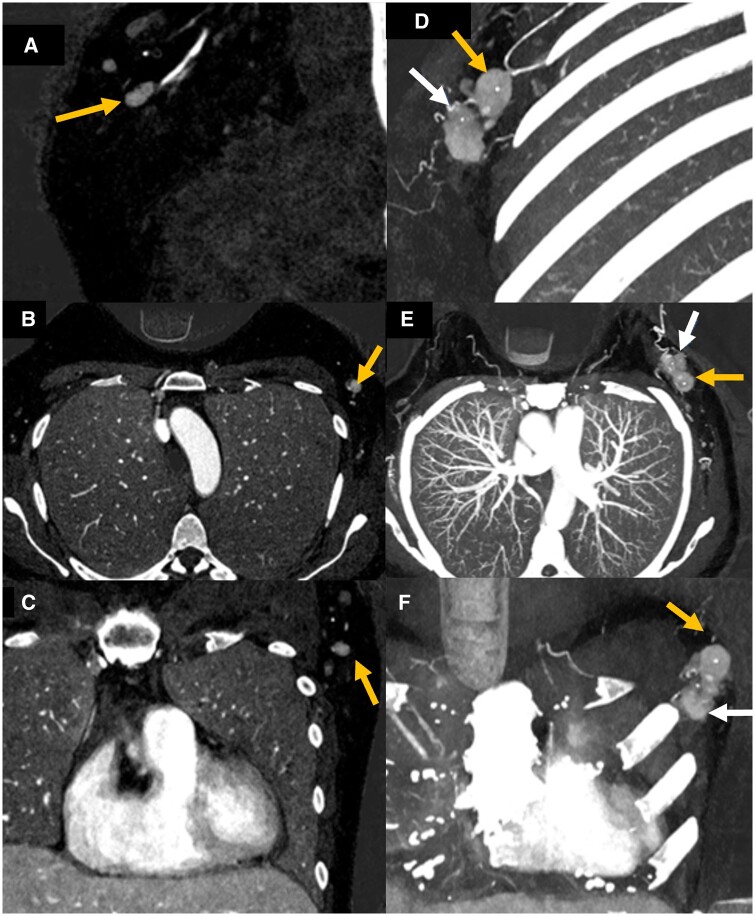
Figure 3.
A 55-year-old patient with metastatic lymphadenopathy. Contrast enhanced PCD-CT with iodine axial, sagittal, and coronal images showing an irregular left level 1 axillary node enhancing more avidly when compared to adjacent lymph nodes (A–C, yellow arrow). Contrast-enhanced PCD-CT with iodine map MIP images in axial, sagittal, and coronal planes demonstrating both the known left breast malignancy (D–F, white arrow) and adjacent enhancing biopsy proven left axillary metastasis (yellow arrow) which enhances significantly more than the adjacent normal nodes. Abbreviation: PCD = photon-counting detector.
Table 3.
Study results summary.
| Characteristic | Total | PCD | MRI |
|---|---|---|---|
| Mass/NME | 13 | 11 | 13 |
| Microcalcifications | 5 | 4 | NA |
| Multifocal/centric disease | 3 | 3 | 3 |
| Metastatic lymphadenopathy | 3 | 3 | 3 |
| Incidentals | 2 | 2 | 0 |
Abbreviation: NME = non-mass enhancement.
Discussion
We report on a triple phase photon-counting CT protocol for the staging work-up of breast cancer. In 11 patients with biopsy proven breast cancer, suspicious enhancing masses and non-mass enhancement, regional adenopathy, and 80% of cases with microcalcifications were detected on CT, with dynamic breast MRI and digital mammography as reference standards. These initial results are promising. Incidental pulmonary and bony findings were detected in 3 patients, with one case significantly impacting patient management secondary to a bony metastasis.
The potential role of breast CT was previously demonstrated in the evaluation of breast implant integrity.8 A non-contrast dual-energy CT scan, providing material specific information regarding the presence of extracapsular silicone, can be utilized in patients for whom an MRI study is not suitable. Breast CT for the evaluation of breast malignancy is less extensively studied due to the limited spatial resolution of conventional CT systems, relatively high glandular radiation dose, and high sensitivity and lack of radiation in breast MR. PCD-CT has technical advantages that provide very rich image contrast information, due to its ability to capture the energy of each individual X-ray photon, which is critical for a task involving delicate differences in soft tissue attenuation, at a reduced radiation dose.11 Rajendran et al7 demonstrated the superior technical capabilities (high spatial and temporal resolution with up to 30% radiation dose reduction) of the first clinical PCD-CT system in human subjects across a variety of imaging tasks.
In our limited number of cases, we detected increased enhancement on CT versus MRI in 1 case, enhancement similar to MRI in 5 cases, and enhancement less than MRI in 5 cases. Given these results, we will aim to adjust the post contrast phase of imaging delay after contrast injection for optimal enhancement. Currently, we utilize a triple phase protocol to investigate tumor contrast uptake and conspicuity, including a UHR scan for the detection of microcalcifications. The average breast radiation dose in our protocol was about 26 mGy, which is higher compared to a maximum dose of about 18 mGy, and 10 mGy at 2 phase contrast enhanced cone beam breast CT and a digital mammography, respectively,12,13 although detailed Monte-Carlo based dosimetry of our scanning geometry has not been performed. We are aiming to reduce the average radiation dose in the next phase of this study, by eventually eliminating one of the two post-contrast phases. Of note, the patients in our study were those with known malignancy, many of which will receive radiation therapy. In this study, we explored utilization of an in-house convolutional neural network based denoising algorithm for the detection of microcalcifications14; however, further work is needed to better characterize the calcifications under PCD-CT.15 Limitations of this study include its feasibility study design and small number of subjects. The clinical actual value of PCD-CT breast CT is yet to be determined with prospective studies.
Conclusion
Novel PCD-CT technology using a dedicated 3-phase breast imaging protocol shows promising initial results for detecting the primary tumor and lymph node staging when compared to MRI. Contrast enhanced breast CT could potentially be utilized as a lower cost, faster alternative in a subset of patients unable to undergo breast MRI.
Acknowledgements
The authors thank Yong Lee and Ryan Jacobson for patient recruitment and Kevin Kimlinger for manuscript preparation.
Contributor Information
Mariana Yalon, Department of Radiology, Mayo Clinic, Rochester, MN, 55905, United States.
Tiffany Sae-Kho, Department of Radiology, Mayo Clinic, Rochester, MN, 55905, United States.
Akriti Khanna, Department of Radiology, Mayo Clinic, Rochester, MN, 55905, United States.
Shaojie Chang, Department of Radiology, Mayo Clinic, Rochester, MN, 55905, United States.
Boleyn R Andrist, Department of Radiology, Mayo Clinic, Rochester, MN, 55905, United States.
Nikkole M Weber, Department of Radiology, Mayo Clinic, Rochester, MN, 55905, United States.
Safa Hoodeshenas, Department of Radiology, Mayo Clinic, Rochester, MN, 55905, United States.
Andrea Ferrero, Department of Radiology, Mayo Clinic, Rochester, MN, 55905, United States.
Katrina N Glazebrook, Department of Radiology, Mayo Clinic, Rochester, MN, 55905, United States.
Cynthia H McCollough, Department of Radiology, Mayo Clinic, Rochester, MN, 55905, United States.
Francis I Baffour, Department of Radiology, Mayo Clinic, Rochester, MN, 55905, United States.
Funding
Research support for this work was provided, in part, to Mayo Clinic from Siemens Healthineers, who provided the scanner used in this work.
Conflicts of interest
C.H.M. is the recipient of a research grant to the institution from Siemens Healthineers. For the remaining authors, no conflicts were declared.
References
- 1. Plana MN, Carreira C, Muriel A, et al. Magnetic resonance imaging in the preoperative assessment of patients with primary breast cancer: systematic review of diagnostic accuracy and meta-analysis. Eur Radiol. 2012;22(1):26-38. [DOI] [PubMed] [Google Scholar]
- 2. Mann RM, Cho N, Moy L.. Breast MRI: state of the art. Radiology. 2019;292(3):520-536. [DOI] [PubMed] [Google Scholar]
- 3. Leng S, Yu Z, Halaweish A, et al. Dose-efficient ultrahigh-resolution scan mode using a photon counting detector computed tomography system. J Med Imaging (Bellingham). 2016;3(4):043504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rajendran K, Petersilka M, Henning A, et al. Full field-of-view, high-resolution, photon-counting detector CT: technical assessment and initial patient experience. Phys Med Biol. 2021;66(20): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Leng S, Bruesewitz M, Tao S, et al. Photon-counting detector CT: system design and clinical applications of an emerging technology. Radiographics. 2019;39(3):729-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Berger N, Marcon M, Saltybaeva N, et al. Dedicated breast computed tomography with a photon-counting detector: initial results of clinical in vivo imaging. Invest Radiol. 2019;54(7):409-418. [DOI] [PubMed] [Google Scholar]
- 7. Rajendran K, Petersilka M, Henning A, et al. First clinical photon-counting detector CT system: technical evaluation. Radiology. 2022;303(1):130-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Glazebrook KN, Doerge S, Leng S, et al. Ability of dual-energy CT to detect silicone gel breast implant rupture and nodal silicone spread. AJR Am J Roentgenol. 2019;212(4):933-942. [DOI] [PubMed] [Google Scholar]
- 9. Fulwadhva UP, Wortman JR, Sodickson AD.. Use of dual-energy CT and iodine maps in evaluation of bowel disease. Radiographics. 2016;36(2):393-406. [DOI] [PubMed] [Google Scholar]
- 10. McCollough CH, Leng S, Yu L, Fletcher JG.. Dual- and multi-energy CT: principles, technical approaches, and clinical applications. Radiology. 2015;276(3):637-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nehra AK, Rajendran K, Baffour FI, et al. Seeing more with less: clinical benefits of photon-counting detector CT. Radiographics. 2023;43(5):e220158. [DOI] [PubMed] [Google Scholar]
- 12. Ma Y, Liu A, O'Connell AM, et al. Contrast-enhanced cone beam breast CT features of breast cancers: correlation with immunohistochemical receptors and molecular subtypes. Eur Radiol. 2021;31(4):2580-2589. [DOI] [PubMed] [Google Scholar]
- 13. Wienbeck S, Lotz J, Fischer U.. Review of clinical studies and first clinical experiences with a commercially available cone-beam breast CT in Europe. Clin Imaging. 2017;42:50-59. [DOI] [PubMed] [Google Scholar]
- 14. Huber NR, Ferrero A, Rajendran K, et al. Dedicated convolutional neural network for noise reduction in ultra-high-resolution photon-counting detector computed tomography. Phys Med Biol. 2022;67(17): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McCollough CH, Rajendran K, Baffour FI, et al. Clinical applications of photon counting detector CT. Eur Radiol. 2023;33(8):5309-5320. [DOI] [PMC free article] [PubMed] [Google Scholar]