Summary
The gut microbiome functions like an endocrine organ, generating bioactive metabolites, enzymes or small molecules that can impact host physiology. Gut dysbacteriosis is associated with many intestinal diseases including (but not limited to) inflammatory bowel disease, primary sclerosing cholangitis-IBD, irritable bowel syndrome, chronic constipation, osmotic diarrhoea and colorectal cancer. The potential pathogenic mechanism of gut dysbacteriosis associated with intestinal diseases includes the alteration of composition of gut microbiota as well as the gut microbiota–derived signalling molecules. The many correlations between the latter and the susceptibility for intestinal diseases has placed a spotlight on the gut microbiome as a potential novel target for therapeutics. Currently, faecal microbial transplantation, dietary interventions, use of probiotics, prebiotics and drugs are the major therapeutic tools utilized to impact dysbacteriosis and associated intestinal diseases. In this review, we systematically summarized the role of intestinal microbiome in the occurrence and development of intestinal diseases. The potential mechanism of the complex interplay between gut dysbacteriosis and intestinal diseases, and the treatment methods are also highlighted.
Keywords: gut microbiome, immune response, intestinal diseases, prebiotics, probiotics
Introduction
Gut microbiota is an assortment of micro-organisms inhabiting the length and width of the mammalian gastrointestinal tract (GIT). The composition of this microbial community is host specific, evolving throughout an individual’s lifetime and susceptible to both exogenous and endogenous modifications. The intestinal microbiota is a complex ecosystem and over 1000 human species have been described, mainly including Firmicutes, Bacteroidetes, Actinomycetes, Proteophylum and Verrucomicrobia (Chai et al. 2018). Once the intestinal microbiota is maladjusted, the development is facilitated of a variety of intestinal diseases, such as inflammatory bowel disease (IBD), primary sclerosing cholangitis (PSC)-IBD, irritable bowel syndrome (IBS), chronic constipation (CC), osmotic diarrhoea and colorectal cancer. On the other hand, recent studies revealed that intestinal diseases could elicit a variety of effects on the host gut microbiota. Indeed, the mechanism and interaction of gut microbiome functions and intestinal diseases remains largely undefined, but include the activity of signalling molecules and recognition of bacterial epitopes by both intestinal epithelial and mucosal immune cells.
The composition of gut microbiota and the gut microbiota–derived signalling molecules can be central and contributing factors affecting human health and disease. So, they are the main target in the mechanistic investigations of gut dysbacteriosis and intestinal disease. Gut microbes help transmit hormonal signals, regulate the immune system, produce metabolic end-products, such vitamins and short chain fatty acids (SCFAs), including butyrate, propionate and acetate. Butyrate and propionate have regulatory effects on intestinal physiology and immune function and protective effect in the colon. Acetate is the main substrate for lipogenesis and gluconeogenesis. These SCFAs play important roles in many aspects, such as regulating inflammation and peripheral immune function of adipose tissue, mediating appropriate immune responses, oral tolerance. Intestinal microbiota can also maintain health and ward off disease by secreting proteases of their own. Of course, not all gut microbiota is good for host survival. Bacteria such as Escherichia coli, Fusobacterium nucleatum and Helicobacter pylori can cause pathogenic reactions in the host. Studies have shown that biofilms protect pathogenic bacteria against host immune response in the respiratory tract and such biofilms have also been observed in the compromised intestinal tract (Wang et al. 2016). Secondary fatty acids and toxic metabolites can cause intestinal damage in different ways. Compounds such as phenol, ammonia, para-cresol, hydrogen sulphide and amine induce on inflammation, DNA damage, intestinal leakage and cancer. These processes could be inhibited by dietary fibre or plant-based food consumption, suggesting that intestinal microbiota also play an important role in the fermentation of carbohydrates.
The many links between the altered gut microbial community, metabolites and susceptibility for CVD and metabolic diseases has placed a spotlight on the gut microbiome as a potential novel target for therapeutics. Currently, faecal microbial transplantation (FMT), diet interventions and drugs are the major therapeutic tool utilized to impact dysbacteriosis and intestinal diseases. Additional approaches include the consumption of prebiotics, probiotics and postbiotics, whereas small-molecule inhibitors of defined microbial enzyme pathways are also promising.
Intestinal diseases
The GIT is a complex combination of micro-organisms, epithelial cells (EC), immune cells and food antigens. Changes in the functional composition of intestinal microbiota, which cause metabolic disorders, have been linked to certain human diseases, particularly with IBD (inflammatory bowel disease—Crohn’s disease, ulcerative colitis (UC)), IBS, CC, osmotic diarrhoea and colorectal cancer (Table 1).
Table 1.
Summary of studies investigated links between gut microbiota and intestinal diseases
| Increase | Decrease | Pathological features | |
|---|---|---|---|
| IBD | Proteobacteria | Firmicutes | Disruption of intestinal barrier |
| Intestinal mucosal inflammation | |||
| Bacterial antigen translocation increased | |||
| PI-IBD | Escherichia, Veillonella, Megasphera and Lachnospiraceae | Prevotella | Bacterial adhesion |
| Roseburia bacteroidetes Lactobacillus | Inflammatory autoimmune disease | ||
| IBS | Methane bacteria | Lactobacillus | Gastrointestinal infections |
| Bifidobacterium | Intestinal bacterial overgrowth (SIBO) | ||
| Coliform and pathogen translocation increased | |||
| Chronic constipation | Bacteroides | Firmicutes, Clostridium, Lactobacillus, Desulfovibrio, and Methylobacterium | Intestinal SERT |
| Akkermansia muciniphila Bacteroides | Decrease the level of 5-HT | ||
| Osmotic diarrhea | Bacteroides | S24-7 bacteria | Changes in intestinal osmotic pressure |
| Y deformation bacteria | |||
| CRC | Fusobacterium nucleatum, P. anaerobius, Peptostreptococcus stomatis, Solobacterium moorei, Gemella morbillorum and Parvimonas micra | Transform the microbiome | |
| Create a tumour environment | |||
| Tumour cell proliferation | |||
| The inflammatory response |
IBD
In recent years, many studies have found that the gut microbiota is likely to be the most critical environmental factor in the pathogenesis of IBD. Specific members of the intestinal microbiota have found to be dramatically affected in IBD in human. A recent study focused on the microbial contribution to IBD and attempted to identify members of the human gut microbiota that may preferentially impact IBD susceptibility and/or severity (Palm et al. 2014). In normal conditions, the intestine produces a highly effective antimicrobial molecule (IgA) against disease-causing bacteria. Mice studies showed that there are four groups of intestinal microbiota that bind IgA in the host: Bacteroidales, Lactobacillus, unclassified Erysipe-lotrichaceae and Segmented Filamentous Bacteria (SFB) (Goto 2019). These were significantly enriched, especially SFB. Using specialized IgA binding technique, colitis causing bacteria, Prevotellaceae, Helicobacter sp. flexispira, SFB were also identified in the intestinal tract of mice models. SFB is a commensal organism that can be attached to the intestinal wall of a variety of animals. It was found that the distribution of SFB in the intestinal mucosa was uneven, its adhesion mainly occurred at the end of the ileum, which was related to the increase of SFB-specific Th17 cells, by studying the relationship between SFB colonization and Th17 cells induction kinetics with quantifying SFB and adherent SFB in intestinal mucosa, and CD4 T-cell-specific SFB in lamellar laminae of mice (Farkas et al. 2015). While the presence of SFB in children has recently been reported, its levels are very low (Chen et al. 2018). It was found by RT-qPCR that the expression of Th17 pathway gene, T and B cell receptor signalling markers in SFB positive samples were up-regulated. When reproduced in large cohorts, it can be speculated that SFBs or related Gram-positive anaerobes play a role in human intestinal immunity.
In patients with IBD, the abundance of intestinal symbiotic microbiota decreased, and that deficiency led to decreased secretion of antimicrobial peptides (α-defensins) from Paneth and Goblet cells and increased REGIIIγ (Schultz et al. 2017). Notably, IBD patients showed a reduction of some Firmicutes (Faecalibacterium prausnitzii; a butyrate-producer), Verrucomicrobia (Akkermansia muciniphila; a propionate-producer) and an increase of Proteobacteria (Mahalhal et al. 2018). The increased level of Proteobacterias led to the reduction of SCFA that induces excessive production of IgG to target the symbiotic microbiota. Activated macrophages produce pro-inflammatory cytokines that overstimulate Th1 or Th17 cells, to induce inflammation (Schultz et al. 2017). The loss of intestinal barrier integrity leads to increased bacterial antigen translocation and stimulation of the inflammatory response of intestinal mucosa, which is the core pathological feature of IBD. Intestinal mucus and cells tight junctions (TJs) play a major role in maintaining the integrity of the intestinal barrier. Previous studies also have shown that loss or imbalance in the production of mucus or TJs can induce colitis, for example, mice lacking claudin 7, Hnf4a and Muc2 spontaneously caused colitis. Moreover, mucus barrier and TJs are interdependent. This interdependence may also contribute to feedback in the inflammatory response, making the IBD permanent and perpetuate.
Primary sclerosing cholangitis-IBD is a hepatic-biliary-intestinal axis-associated inflammatory autoimmune disease. The intestinal microbiota may affect the onset. Studies have shown that the intestinal microbiota of PSC-IBD patients has significant increase in adhesion compared to patients with IBD. Escherichia, Lachnospiraceae, Veillonella and Megasphera were significantly elevated in patients with PSC-IBD compared to controls (Kummen et al. 2017). The genes of these bacteria encode an amine oxidase that acts as a VAP1 substrate. VAP1 plays an important role in the recruitment of effector cells to the liver, both as an adhesion molecule and as a semi-carbazide-sensitive amine oxidase. The abundance of Prevotella and Roseburia (that includes butyrate producers) in PSC patients was reduced, and there were almost no Bacteroides sp.
Salmonella-infected disease
Studies also have reported the impact of Salmonella infection on IBD patients. Salmonellae typhimurium enter into the neutrophils in the intestinal lumen, where ROS (reactive oxygen species) are produced. The ROS act as the electron acceptor of Salmonella electron transport chain, thus making abundant growth of Salmonellae typhimurium, accounting for a major microbial population. An abundant Salmonella population can reach the Peyer’s patches by invading dendritic cells (DCs) in the epithelial barrier or M cells in the lumen and can be identified by the presence of their glycoprotein-2. Salmonella can also enter ECs to form membrane folds or interrupt the TJs by itself, thereby inducing inflammation of the intestinal epithelium in patients with IBD. On the basolateral side, TLR5 can be stimulated to produce NF-kB. Once Salmonella enters ECs, it blocks the autophagosome pathway preventing self-degradation (Lodes et al. 2004). At the same time, changes in the fungal microbiota were also detected in the IBD patients, such as an increase in the ratio of Basidiomycetes to the Ascomycetes. Compared to healthy people, IBD patients have lower proportion of S. cerevisiae, and higher proportion of Candida albicans (Sokol et al. 2017). Saccharomyces boulardii is marketed as a probiotic and this and S. cerevisiae have a beneficial effect on IBD in a mouse model (Khatri et al. 2017). Candida albicans is the most common fungus in human intestinal microbiota and has an improved colonization ability in an inflammatory environment, and aggravates the inflammatory response of colitis mice (Richard et al. 2015).
IBS
Irritable bowel syndrome is a gastrointestinal dysfunction disease, often manifested by abdominal pain, intestinal distortion and excretion. There are three types of IBS (Chang 2018): constipation-predominant (IBS-c), diarrhoea-predominant IBS (IBS-d) and mixed IBS (IBS-m). IBS-c is a disease characterized by CC with abdominal pain, with the primary symptom being acute pain and the secondary symptom being constipation (Chandar 2017). According to Rome IV criteria, IBS-c is specifically defined as: pain related constipation occurs more than 3 days a month; symptoms persist for three consecutive months; more than 25% of stools can be described as hard, and <25% of stools can be described as soft (Cash 2018). The common symptoms of IBS-d patients are stomach pain, stomach spasm, frequent diarrhoea, flatulence, urgency and diarrhoea. The number and proportion of normal healthy bacteria in the digestive system of IBS-d patients are out of balance (Rosa et al. 2016). IBS-m is specifically defined as: at least 25% of the stool can be described as hard, at least 25% of the stool can be described as soft or even liquid with unclear edges or no shape. The abnormal peristalsis of IBS-m patients are manifested in constipation and diarrhoea (Pimentel 2018). Gastrointestinal infections and psychological disorders are two risk factors for the development of IBS. It was found that patients with high stress and mental disorders had a high risk of contracting IBS (Donnachie et al. 2018). Since the first description of microbiota comparison of a large IBS cohort with healthy controls (Rajilić-Stojanović et al. 2011), a large number of studies have revealed specific microbiota signatures in IBS patients but its causality has not yet been established (Barbara et al. 2019). Of interest, in IBS patients, E. coli and Salmonella were found to pass through the epithelium only, significantly increasing the rate of epithelial passage. Furthermore, vasoactive intestinal peptide levels, tryptase levels, mast cell counts and mast cells expressing VPAC1 were elevated in the plasma of IBS patients. In addition, the translocation of commensals and pathogenic bacteria in colonic epithelial tissue are increased in IBS patients. Significant changes in gene expression were found in the intestinal lactic acid bacteria, Bifidobacteria, and Clostridium natto in patients with IBS (Liu et al. 2017a). The lactic acid bacteria and Bifidobacteria were significantly reduced in IBS-d patients, and it was hypothesized that probiotics could improve IBS-d, and a compelling case has been made for the probiotic activity of a Bifidobacterium strain (O’Mahoney 2005). IBS-c patients do not have these two bacteria, but the content of methanogens is high, so it is suspected that the pathogenesis of IBS-c may also be related to methanogen bacteria (Liu et al. 2017a).
Chronic constipation
Chronic constipation is a functional gastrointestinal disorder that causes intestinal disorders. The difference between IBS-c and CC is that CC patients have mainly difficulties in defecation and have hard stool (Vazquez Roque and Bouras 2015). Moreover, defecation in CC patients is associated with pain that disappears when the stool is produced (Rao et al. 2016). The relationship between CC and intestinal microbiota has been studied, with specific attention for the role of the serotonin transporter (SERT), a transmembrane transporter that is involved in the re-uptakes of excess serotonin (5-HT) from specific sites and participates in the regulation of gastrointestinal motility (Cao et al. 2017). Mice that had received microbiota from CC patients showed constipation symptoms and reduced bowel movements per unit time. In intestinal tissue of these mice, mRNA and protein levels of the serotonin transporter SERT were significantly elevated while serotonin levels were significantly reduced, that is inversely proportional to intestinal transit time. At the same time, the intestinal microbiota of FMT mice is dysregulated (in which the thick-walled bacteria, Clostridium, Lactobacillus, Desulfovibrio and Methylobacterium are reduced, the Bacteroides and Akkermansia are increased) and the intestinal barrier function is impaired. As the expression of SERT in the intestine is increased, the level of 5-HT is decreased, thereby inhibiting intestinal movement and promoting CC (Cao et al. 2017).
Osmotic diarrhoea
Osmotic diarrhoea is the consequence of different diseases including medication with different laxatives. It occurs when an orally ingested food is not fully absorbed in the small intestine, thus exerting an osmotic force which draws fluid into the intestine (Tropini et al. 2018). Lactose intolerance is a disorder of intestinal digestion, which is related to the decrease or complete loss of lactase chlorizin hydrolase (LPH) activity. Congenital lactase deficiency represents one type of lactose intolerance (Amiri et al. 2015). It is characterized by decreased or complete loss of lactase activity at birth. Due to the lack of this enzyme, the disaccharide lactose cannot be digested into glucose and galatose, leading to poor carbohydrate absorption and diarrhoea. Moreover, there will be osmotic diarrhoea when nutrients (especially small-sized nutrients) produce osmotic force, which causes water to enter the lumen (Thiagarajah et al. 2018). Celiac disease (CD) is an allergic enteropathy, which is also accompanied by osmotic diarrhoea. Malabsorption and chronic pancreatitis are also reported to cause osmotic diarrhoea (Tropini et al. 2018; Kempeneers et al. 2020). In addition, the use of laxatives containing phosphate or magnesium sulphate, and the overuse of drugs such as sorbitol usually resulted in osmotic diarrhoea (Liauw and Saibil 2019). There are also research findings indicating that the microbiota of stool and mucosa in patients with osmotic diarrhoea were significantly different (Gorkiewicz et al. 2013), for example, Firmicutes was the main bacteria in the mucous membrane, and Bacteroidetes was the main bacteria in the stool. Osmotic diarrhoea decreased the richness of phylogeny and showed a strong tendency to balance individualized microbiota on the mucosa (Gorkiewicz et al. 2013). In addition, diarrhoea caused significant relative changes in phyla Bacteroidetes and Firmicutes, and the number of Proteobacteria on the mucosa increased relatively.
Colorectal cancer
Colorectal cancer (CRC) patients have found to be enriched in colonic F. nucleatum, P. anaerobius, Peptostreptococcus stomatis, Solobacterium moorei, Gemella morbillorum and Parvimonas micra work together to form a network of bacteria in CRC (Yu et al. 2017). CRC appears to be affected by pathogens that play a major role in compare to infectious disease. Although the abundance of these key pathogens is relatively low, they can combine with other species to transform the microbiome and create a tumour-producing environment. When ordinary and germ-free mice are fed with faeces from CRC patients or healthy donors, the CRC mice developed highly atypical hyperplasia and a significant increase in the proportion of naked eye polyps. They also exhibited germ cell proliferation, had reduced faecal bacteria abundance and altered microbiota composition (Yu et al. 2017).
The CRC microbiome in mice also induced multiple inflammations and activate the carcinogenic pathways. The expression of cytokines regulating inflammation in the small intestine of mice was increased including CXcr1/2, IL-17a, IL-22, and IL-23a, IL22, IL23. These mice also showed an increased incidence of tumours in the colon, while inhibiting IL17A controlled tumours growth with the increase in the proportion of Th1 and Th17 cells (Wong et al. 2017). Interestingly, IL-22 is also an important extracellular regulator of DNA damage response in colon stem cells, which plays a role in preventing the accumulation of potentially dangerous mutations. It has been pointed out that the intestinal epithelial stem cells of IL-22 damaged mice accumulated oncogene mutations, which accelerated the development of cancer cells, and easily caused colon cancer (Gronke et al. 2019). In addition, CRC microbiome may affect the expression of other cytokines, for example, the upregulation of membrane receptor genes such as CXCR1 and CXCR2 confirms the chemotaxis of immune cells. At the same time, it was also found that the expression of several cell proliferation genes was increased, such as MKI67 and MCM2, AURUKA and CDC20 (Wong et al. 2017).
The mechanism of action of gut bacteria in intestinal disease development
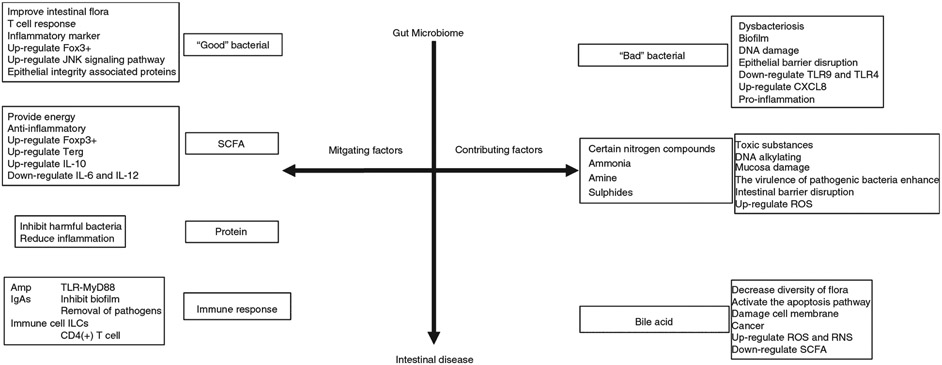
Most studies have shown that intestinal bacteria are associated with the development of various diseases, but few have systematically reported the molecular mechanisms by which intestinal bacteria affects diseases. Most bacteria in intestine form complex networks, including the beneficial bacteria and harmful bacteria (Fig. 1). Under normal circumstances, they exist optimally and are beneficial to the host health, but when imbalanced, it increases the risk for diseases. The secretion of bacterial biofilm is also one of the factors that lead to development of human diseases. The metabolites, such as the SCFA, toxic products, bile acid, etc. of intestinal microbiota can also affect the health of the host. In contrast, they can protect the host from pathogen invasion by activating immune defense.
Figure 1.
Gut microbiota and possible molecular pathways linked to intestinal diseases.
‘Good’ bacteria and ‘Bad’ bacteria
There is a considerable body of evidence to support the hypothesis that the endogenous intestinal microbiota plays a crucial role in the pathogenesis of intestinal disease such as IBD and its variants and related disorders (Weingarden and Vaughn 2017). In recent years, the prevalence of these diseases has increased significantly, which may be related to changes in the environment and lifestyle. The change of life style may have altered the initial development or stable maintenance of microbiota, thus changes the ‘normal’ or healthy composition of microbiota, namely dysbiosis (Round and Mazmanian 2009). Clinical, epidemiological and immunological studies indicate that a change of intestinal microbiota may be an important factor in the occurrence of many inflammatory diseases (Levy et al. 2017). Intestinal bacteria are an important part of immune system development and function. Therefore, the lack of beneficial micro-organisms to promote appropriate immune development (due to ecological imbalance) may lead to inflammatory response, which is the basis of various human immune diseases.
The intestinal bacterial population in healthy adults is rich and has a high diversity. The composition of microbial community is significantly different in different niche of GIT. In human body, Proteobacteria and Clostridium species are mainly located in the small intestine; Bacteroidae and Clostridiaceae families are mainly located in colon and cecum, and Lactobacilli, Streptococci and Enterococcus are mainly located in jejunum and ileum. In mice, Ruminococccae, Rikenellaceae and Lachnospiraceae are mainly located in cecum, Bacteroidaceae, Rikenellaceae and Prevotellaceae are mainly located in colon; Bacteroides and Firmicutes are mainly located in intestinal cavity, and SFB and H. pylori are mainly located in mucous layer of the small intestine (Donaldson et al. 2016).
Each type of bacteria plays a role in maintaining their dynamic balance. Beneficial bacteria in the gut are called probiotics and harmful bacteria are pathogenic microbes. ‘Good’ bacteria in gut alleviate many intestinal diseases and play a positive role in human health. Lactobacillus rhamnose GG secretion (LSM, Lactobacillus soluble medium) were recovered from the PBMC culture (Ludwig et al. 2018), and the LSM regulate the function of DC by strengthening the ability of the T cell response, evidenced by increase IL-2 and IFN-γ producing T cells. Enhanced Foxp3+ expression is also observed and this study reveals that LSM can modulate DC function suggesting that LSM can provide alternative approach to improve adaptive immune defense system when live bacteria transplantation is not feasible. The expression of inflammatory markers (IL-1β, IL-12) and epithelial-integrity related proteins (TGFβ, ICAM1) in the colon alters the expression of microRNA, changes in the ratio of Firmicutes to Bacteroides induced by DSS (Rodriguez-Nogales et al. 2018). Bacillus coagulans MTCC 5856 can improve depression and gastrointestinal symptoms in patients with severe IBS (Majeed et al. 2018), and Bifidobacterium longum NCC3001 also shows similar effect in IBS patients (Pinto-Sanchez et al. 2017). In cancer, through mass spectrometric analysis, it is observed that Lactobacillus casei ATCC 334 can produce iron pigment, which plays a role in inhibiting tumour progression by activating JNK signalling pathway (Konishi et al. 2016). A randomized clinical trial showed that a combination of probiotic supplements containing five strains of Lactobacillus and Bifidobacterium could improve the symptoms of CD in patients with IBS (Francavilla et al. 2019). It was also observed that patients had increased intestinal Lactobacillus, Staphylococcus and Bifidobacterium, so their effects were related to the changes of intestinal microbiota. The Bifidobacterium adolescentis can treat constipation (Wang et al. 2017).
‘Bad’ bacteria in gut on the other hand promote many intestinal diseases and play a negative role in human health. Bacteria can produce multilayered agglomerations, called biofilms, which protect them from physical stress (fluid flow, scraping, epithelial turnover) in intestine lumen, and help spread resistance genes (Balcazar et al. 2015). By destroying mucus and depleting goblet cells, the intestinal pathogenic bacteria create the microbial biofilm that contact the epithelium, and the plankton can spontaneously migrate out of the damaged biofilm. Enteropathogenic bacteria can also increase the virulence of phytoplankton symbionts, induce their adhesion and infiltration, weaken the epithelial barrier and promote inflammatory response. The role of biofilms in disease-related micro-organisms in IBD, CRC and intestinal damage has been confirmed. Microscopic results showed that patients with IBD had dense Bacteroides fragilis biofilms (Swidsinski et al. 2005). In addition, the mean value of mucosal biofilm density with IBD was significantly different, about 100 times (Swidsinski et al. 2005), compared to IBS patients and healthy subjects. Intestinal diseases such as IBD are associated with imbalance of microbiota and destruction of mucosal epithelium, thus promoting species migration. Biofilms help pathogens escape their hosts’ defenses, which can lead to disease progression (Hoarau et al. 2016).
Many anaerobic and aerobic micro-organisms, such as Klebsiella pneumoniae, E. coli and Fusobacillus nuclei, settle in the intestines and wounds all over the body (Bertesteanu et al. 2014). When bacteria colonized at the wound forms biofilms that slows or prevent wound healing. Biofilms containing enterotoxigenic B. fragilis (ETBF) and F. nucleatum were detected in tumour tissues (Kim et al. 2019). Biofilms are also observed in the nontumour mucosa tissue. Therefore, mature biofilms, when present in healthy tissues near CRC or IBD infected tissues, serve as an early warning signal for the critical transformation of the intestinal environment to imbalance, injury and pathogen infection. Bacteria that form pathogenic biofilms and their secretions can be used as signals to detect intestinal diseases. Such as Intestinal markers: (i) CRC: nucleatum, enterotoxigenic B. fragilis (ETBF, pks+E. coli, N1N12diacetyl-spermine); (ii) Gut wounds: F. nucleatum, E. coli, K. pneumoniae; (iii) IBD: B. fragilis. Intestinal contact with enteropathogenic bacteria, especially Giardia sp. and Campylobacter sp. results to pathogenic imbalance of mucosal microbiota bacteria. Campylobacter sp. and Giardia sp. are also a typical risk factor for the occurrence of post infectious IBS (PI-IBS) (Svendsen et al. 2019). Also, Citrobacter rodentium colitis infections causes intestinal allergy in mice. Infectious gastroenteritis worsens IBD symptoms, with Campylobacter jejuni, Salmonella sp. or E. coli. The pathogenesis of IBD is mainly disruption of balance between gut microbes and host immunity.
The expression of Firmicutes in patients with IBD was decreased whereas the number of pathogenic deformation bacteria was increased (Kolho et al. 2015). The decrease of Prevotella sp., Clostridium leptum and microbial diversity leads to increase of faecal calprotectin, which indicates inflammation. As a result, it appears that IBD is not only affected by a single microbe but by multiple microorganisms. However, the mechanism and role of underlying pathogens in causing IBD is not yet clear, and these problems need to be further addressed. In IBD, abdomen often have clinical complications, such as gastroenteritis, and it has been shown that C. jejuni plays a role in the development of complications (Rostami et al. 2015).
Escherichia coli was isolated from the intestines of mice with colitis, concurrently DNA damage and cancer were also observed (Arthur et al. 2014). Campylobacter jejuni also damage the polarity of TLR9, which in turn destroys the TLR9 induced epithelial barrier and increases CXCL8 production indicating that the infection of C. jejuni could induce the inflammatory response of experimental colitis in mice (Reti et al. 2015). Giardia sp. and C. jejuni can advance the release of plankton micro-organisms, and C. jejuni can activate the potential toxic genes of E. coli to promote adhesion of E. coli to human intestinal cells. This process is aided by the up-regulation of pro-inflammatory interleukin 8 (CXCL8) expression and down-regulation of toll-like receptor 4 (TLR4) expression. These findings may shed light on how intestinal pathogens can turn symbiotic bacteria into pathogens during the acute phase of infection.
Metabolites
Fatty acids
It is reported in the literatures that fatty acids including SCFAs, medium-chain fatty acids (MCFAs), long-chain fatty acids (LCFAs) could influence the intestinal microbiota (Bichkaeva et al. 2018). Anaerobic micro-organisms ferment undigested food and other host metabolites in the large intestine to produce beneficial SCFAs, including formic acid (C1), acetic acid (C2), propionic acid (C3), butyric acid (C4), isobutyric acid (C4), isovaleric acid (C5), hexanoic acid (C6). SCFA are absorbed from the intestinal cavity, but their subsequent distribution and metabolism within the host cells are different. Butyrate mainly provides energy for ECs, propionate is mainly responsible for metabolism in the liver and acetate can exist in a higher concentration in peripheral blood. Recent studies have shown that SCFAs have some anti-inflammatory effects and can modulate regulatory T cells in the colon of mice (Yu et al. 2019). Also, the anti-inflammatory effects of SCFAs have important effects on host cells and intestinal microbiota. SCFAs, produced by the intestinal bacteria through fermentation can increase the number of intestinal TReg cells and activate to secrete IL-10 in specific pathogen free mice (Smith et al. 2013). Extracellular SCFAs interact with host cell surface receptors. All host cells expressed GPR41 (FFA1), GPR43 (FFA2) and GPR109A. GPR43 interacts with propionate, butyrate and acetate, GPR41 interact with propionic acid in strongest saline condition but GPR109A only interact with butyrate (Feng et al. 2018; Li et al. 2018). The interaction of butyrate with GPR109A may induce anti-inflammatory effect. Butyrate can also play an anticancer role by restraining the proliferation and selectively promoting apoptosis of CRC cells (Fung et al. 2012).
Medium-chain fatty acids, including octanoic acid (C8), capric acid (C10), lauric acid (12), must be ingested and not produced by gastrointestinal micro-organisms. MCFA isolated from skin lipids and milk with high concentrations has direct antibacterial effects on a variety of pathogenic Gram-positive and Gram-negative micro-organisms (Fischer et al. 2012), including Clostridium perfringens, Listeria monocytogenes and C. jejuni. However, studies in broiler chickens and piglets have shown that supplementation of MCFA capric acid, lauric acid and caprylic acid can enrich some pathogenic Enterobacteriaceae populations, such as Citrobacter sp. and E. coli, while inhibiting symbiotic species belonging to Enterobacteriaceae, such as Lactobacillus sp. (van der Hoeven-Hangoor et al. 2013). The mechanism of these changes in intestinal microbiota caused by MCFA on host physiology and immunity is not clear. In addition, MCFAs seems to be able to enrich and inhibit ‘symbiotic’ microbiota and pathogenic micro-organisms. The contradictory role of MCFAs in shaping intestinal microbiota may be due to the internal differences of intestinal microbiota among animals used in vivo models, the types of MCFA supplementation and the location of 16S rRNA sequencing and analysis of intestinal microbiota collected from animals.
Like MCFA, LCFAs, including myristic acid (C14), palmitic acid (C16), must be ingested. Among them, omega-6 fatty acids—derived lipid metabolites are produced by symbiotic bacteria, especially Lactobacillus (Hirata and Kunisawa 2017). It was also found that high saturated long-chain fatty (LCFA) was associated with increased intestinal motility in NMS rats. The increased saturated long-chain fatty was positively correlated with the increased abundance of Prevotella, Lactobacillus and Alistipes (Zhao et al. 2018). Most studies on LCFAs are focused on macrophages, however, the function of most immune cells may be interfered by the number and type of LCFA in the environment. Omega-3 LCFAs plays an anti-inflammatory role by acting at various levels. GPR40 and GPR120 are two known GPCRs that are sensitive to the metabolites of LCFA, especially omega-3 LCFA. In macrophages, activation of GPR40 and GPR120 has been shown to inhibit the stimulation of NLRP3 inflammatory bodies, and GPR120 can additionally inhibit NF-κB signalling (Zhang and Qiu 2019). These anti-inflammatory effects involve the recruitment of β-arrestin 2 and insulin sensitization. A high-fat diet stimulates inflammatory signalling pathways and is also associated with increased intestinal permeability by affecting intestinal microbiota (Xie et al. 2020).
Protein and toxic compounds
It’s not just the microbes in the gut that can affect the health, but the proteases that are secreted by these microbes are also very important in developing diseases, like enteritis. AimA is an immuno-regulatory protein that is secreted by Aeromonas (Rolig et al. 2018). The symbiotic bacteria inhibit the harmful bacteria and intestinal inflammation in the host. In zebrafish model, this bacterium reduces inflammation and in chemical model, AimA prevent the excessive buildup of neutrophils, and prevent septic shock. Some bacteria also secrete amino acid-derived antibiotics to fight disease. Gut bacteria, Clostridium scindens and Clostridium sordellii, secrete tryptophan derived antibiotics 1-acetyl-β-carotine and turbomycin A, respectively (Kang et al. 2019). These two antibiotics inhibit the growth of Clostridium difficile and other intestinal bacteria by inhibiting the formation of the diaphragm during bacteria’s split phase. Olsenella scatoligenes (Liu et al. 2018) can secrete indoleacetic decarboxylase in the intestinal tract, which plays a key role in the process of tryptophan fermentation to form faecosine.
Bacterial fermentation of aromatic amino acids produces a range of metabolites, some of which are toxic, including certain nitrogen compounds, ammonia, amine and sulphides, etc. Some nitrogenous compounds, especially nitrites, increase the risk of cancer through DNA alkylation (Li et al. 2017). Ammonia is also a carcinogen at low concentrations and has been shown to be associated with mucosal damage and colorectal adenocarcinoma in animal models (Windey et al. 2012). Polyamine synthesis has been found in intestinal bacteria. High concentration of polyamine is toxic, which is involved in various diseases such as oxidative stress and cancer, and many studies suggest that oxidative stress caused by polyamine catabolism is the mechanism of toxicity (Olin-Sandoval et al. 2019). Pathogens, such as Shigella flexneri, Priceenterica subsp., Streptococcus pneumoniae, H. pylori and Enterica serovar Typhimurium employ polyamines to enhance their virulence (Di Martino et al. 2013). A small amount of sulphate reducing bacteria (such as Desulfovibrio) has been detected in most individuals that use lactate salts as a common substrate for growth and sulphide formation. Sulphide is not only toxic to colon cells but also inhibits the oxidation of butyrate and thereby destroys the integrity of the colon cell barrier (Blachier et al. 2017). This study observed that at a very low concentration (0·25–2 mmol l−1), sulphide produces ROS that is known to induce DNA damage resulting in cancer development.
Bile acid
Bile acid is a natural surfactant derived from cholesterol. They are important for lipid digestion, antimicrobial defense and glucose metabolism (Shapiro et al. 2018). Some bile acids destroy cell membrane because of their hydrophobicity, while others protect intestinal epithelium and are resistant to pathogens such as C. difficile (Buffie et al. 2015). Bile acids also have effects on gut related inflammation, which may be realized by regulating intestinal mucosal immune cells. The immunoregulation of bile acids is mostly studied in the background of innate immunity. Bile acids play an anti-inflammatory role in the innate immune system by inhibiting NF-κB-dependent signalling pathways and NLRP3-dependent inflammatory activities, but it is not clear whether they will affect adaptive immune cells, such as express IL-17a (TH17 cells) or regulatory T cells (T reg cells). Two lithocholic acid (LCA) metabolites found in humans and rodents directly affect CD4 (+) T cells (Kakiyama et al. 2014): the 3-OxoLCA can inhibit the differentiation of TH17 cells by directly binding with the key transcription factor retinoid-related orphan receptor-γt (RORγt); the isoalloLCA promotes the differentiation of T reg cells by producing mitochondrial ROS (mitoROS), which leads to the increase of Foxp3 expression (Hang et al. 2019). This suggests that bile acid metabolites control the host immune response by directly regulating the balance of TH17 and T reg cells.
Bile acids have strong antibacterial activity and may affect the composition of intestinal microbiota. The intestinal microbiome cannot transform primary bile acids, and when part of the residual bile acid passes through large intestine, the microbiome population in the large intestine changes significantly. The primary bile acid transforms into several different secondary bile acids, mainly deoxycholic acid and licholic acid. Mice when fed a diet with deoxycholic acid showed reduced generation of SCFAs and altered microbiota diversity, such as reduction of bacteroides, relative increase of Gammaproteobacteria and certain Firmicutes (Zeng et al. 2019). Bile acids activates reactive oxygen (ROS) and reactive nitrogen (RNS) species production during metabolism, and they can damage DNA, so they have a carcinogenic effect (Li and Cao 2016). The mechanism for controlling the toxicity of bile acid in cells is complex. Secondary bile acids are more capable of destroying cell membranes, and destroy membrane-related proteins (NAD(P)H oxidases and phospholipase (A2)) that cause ROS production, but may also do so by other mechanisms. For example, bile acids interact with nuclear receptors to activate signalling pathways that induce apoptosis. However, some bile acids also act as detoxification. Ursodeoxycholic acid seems to inhibit ROS and protect cells from deoxycholic acid. Song et al. showed that the primary bile acids (such as ursodeoxycholic acid) and some effective secondary bile acids (such as cholelithic acid) in the gut regulate RORγt+ Treg cells through BAR VDR (bile acid receptor vitamin D receptor) (Song et al. 2020). One of the causes of human IBD and colorectal cancer is the disorder of intestinal bile acid (Postler and Ghosh 2017). Therefore, human VDR gene variation associated with IBD may affect the susceptibility of the disease through the wrong control of intestinal Treg cell pool.
The immune response
The immune defense system of the intestinal tract can control the imbalance of intestinal environment caused by bacterial microbiota and pathogenic bacteria. The first step in gut defense uses antimicrobial peptides secreted by gut ECs. Antimicrobial peptides (Amp), respond to colonization of the gut microbiome. Epithelial cells secrete antimicrobial peptides against immune intolerant pathogens or symbionts within mucosal layers. Paneth cell and ECs identify signature of microbe associated molecular patterns (MAMPs), through receptors such as MAMPs, NLRs, TLRs 2,4,5,9 and other RIG molecules. When mice treated with antibiotics after supplementation with probiotics (e.g., Lactobacillus), can trigger intracellular TLR-MyD88 pathway and restore certain intestinal defense capacity.
At birth, IgAs is produced by the induction of intestinal microbial factors by AHR ligands, such as indoles (Culbreath et al. 2015). The less the immunoglobulin secreted, the lesser the intestinal barrier integrity preserves. Breast milk can provide IgAs and play a protective role before the complete establishment of the intestinal immune defense system (Rogier et al. 2014). Immunoglobulin IgAs bind to the surface of bacteria and produce effective signals to gut immune cells to identify the bacteria and perform phagocytosis. So, IgAs can act as a second line of defense for gut immunity. Studies have shown that the gut immune system not only protects host cells, but also assists in the growth of certain symbiotic bacteria (Dishaw et al. 2016). IgA cross-linked bacteria can effectively block epithelial barrier translocation and thus inhibit the formation of biofilms. Many studies showed that IgA also exhibit a host–microbial symbiosis system, in addition to removing pathogens (Donaldson et al. 2018). IgA also help bacteria to grow and interact with the environment (Moor et al. 2017). Innate and adaptive immune cells act as a third line of defense.
During inflammatory response in the bacteria–host barrier surface, lymphoid cells (ILCs) serve as the first line of defense for lymphatic defense (especially the intestinal epithelium). When subjected to irritation, they do not exhibit specific T cell receptor (TCRs) to precise antigens, nor do they undergo clonal selection and expansion, but, a rapid response can be made in case of bacterial invasion and tissue damage by activating AMP production and triggering local immune responses. This mechanism is associated with the production of many cytokines. The nuclear hormone receptor, retinoid-related orphan receptor and ɤ-T trigger inflammatory responses in lymphocytes differentiate ILC1, ILC2 and ILC3. ILC3 secrete IL22 and IL17 that can act on ECs, improve the secretion of antimicrobial peptides (Amp) and nonspecifically protect commensals. The function of ILCs is to regulate adaptive immune CD4 (+) T cells. Targeting ILCs in mice through genetic or antibody mediated depletion strategies could be beneficial to maintain proper adaptive immune system (Eberl et al. 2015). ILCs interact with CD4 (+) T cells via MHCII dependence, which limits the response of histopathological adaptive immune cells to symbiotic bacteria, thus maintaining intestinal homeostasis (Hepworth et al. 2013). TRegs lymphocytes that secrete IL-10 play an anti-inflammatory role. Intestinal TRegs is controlled by GM-CSF derived from ILCs in the gut. The ability of ILC to produce GM-CSF without macrophages induces microbial signals and the production of interleukin-1β (Mortha et al. 2014).
It is observed that symbiotic microbes enhance communication between lymphocytes and innate myeloid cells and maintain immune homeostasis in the gut. Mice lacking GM-CSF regulate the effector function of mononuclear phagocytes and reduce the number of TRegs, and their role in bacterial translocation into tissue may be affected. When different subunits of typical DCs interact with ILCs and T cells, they also promote the ILC1/Th1/ CTL- or ILC3/ Th17 response (Murphy et al. 2016). Therefore, DCs can also control ILCs. Dendritic cells further activate T lymphocytes and promote the secretion of epithelial AMP by regulating ILCs. Other ILCs thought to be mucosa-associated invariant T cells (MAIT) that are mainly found in the intestinal epithelium, which also incorporate a rapid response to the microbiota, which can trigger inflammation, especially when identifying microbial derivatives (such as riboflavin derivatives) (Powell and MacDonald 2017). However, more data are needed on these cells for further conclusion.
Treatment and adjustment
Imbalance of intestinal microbiota affects human intestinal health. Therefore, restoring the balance of the intestinal environment could be a potential therapeutic target. Recent studies have shown that faecal microbiota transplantation can effectively achieve the microbiota reconstruction, which play the role of prevention, mitigation and treatment to health. In addition, prebiotics, antibiotics, traditional Chinese herbs can also be used to regulate intestinal microbiota to treat the intestinal diseases.
Faecal microbiota transplantation
Faecal microbiota transplantation is a method to treat gastrointestinal diseases by transplanting the faecal filtrate of healthy donors to the GIT of the recipients and restoring the diversity of microbiota. In modern medicine, various experiments have shown that FMT has achieved remarkable results in the treatment of various types of diseases. At present, thousands of cases of C. difficile infections (Mills et al. 2018) and IBD (Paramsothy et al. 2017) have been successfully treated. Some clinical data show that patients with recurrent C. difficile infection (CDI) cease its recurrence by FMT, and their urethral infections are relieved or cured. In a similar case, a 19-year-old woman with Crohn’s disease developed C. difficile infection, and traditional therapies such as metronidazole had no effect, after two faecal bacterial transplants from the mother, C. difficile infection was relieved and lasted for 12 months (Oppfeldt et al. 2016). Similarly, IBS patients with severe abnormal distension who were uncured by antibiotics but was improved after FMT treatment (Holvoet et al. 2017). Other results also indicated that the cure rate of UC treated with antibiotics was significantly improved after FMT (Keshteli et al. 2017). The treatment of refractory type II CD is very difficult, it’s worth noting that the symptoms of CD disappear completely, and duodenal villi are completely recovered when treated with FMT. In addition, there are also some potential indications for FMT are IBS (El-Salhy and Mazzawi 2018) and CC (Ohkusa et al. 2019). The use of FMT in IBS patients reverses the dysbiosis to normobiosis and reduces the IBS symptoms in about 70% of patients, and is not associated with any serious adverse events (El-Salhy and Mazzawi 2018). The use of FMT in patients with CC immediately alleviates the symptoms, including defecation frequency and feces (Ohkusa et al. 2019). Therefore, when the traditional treatment is ineffective, alternative solutions such as FMT can be considered.
Faecal microbial transplantation may also lead to microbial transmission of adverse infection events and the benefits and risks of FMT in different patient groups are different. In two independent clinical trials, two patients developed extended-spectrum beta-lactamase (ESBL)-producing E. coli bacteremia after receiving FMT, both patients were connected to the same faecal donor through genome sequencing, and one of the patients died (DeFilipp et al. 2019). Therefore, in order to avoid adverse infection, donor screening should be strengthened. For the selection of donors, first of all, effective screening is carried out on the premise of meeting the authoritative standards (Cammarota et al. 2017). In general, spouse or close relatives are ideal FMT donors, but some studies have also shown that unrelated, healthy, but well-screened donors may be more beneficial for genetic related diseases, such as IBD (Kelly et al. 2015).
Dietary interventions
Diet is related to health. The vast majority of a healthy person’s food is absorbed by the small intestine, and dietary residues enter the colon, including complex carbohydrates, protein residues and primary bile acids. They affect the composition and function of the gut microbiome, and keep the colon healthy through fermentation. In a balanced diet, the fermentation of carbohydrates plays a major role in the production of SCFAs. Butyrate, acts as mucosal anti-inflammatory and anti-tumour molecules in colon cells through the regulation of cell metabolism, immune regulation and anti-proliferation. But in an unbalanced diet, protein fermentation and the detoxification of bile acids are increased, which can promote inflammation and damage colon cells, leading to colon cancer (Yang and Yu 2018). In addition, changes in diet affect the distribution of intestinal microbiota. Individuals who consumed more fibre had higher Prevotella sp., while individuals with more amounts of protein and fat in their food had higher Bacteroides sp. These indicate that Prevotella sp. has better fibre degradation ability compared to Bacteroides. As a result, diet affects the composition of the gut microbiome and the patient’s intestinal microbiota is maladjusted, thus affecting the host’s absorption of nutrients, changing the host’s ability to construct an appropriate immune response, and undermining the host’s tolerance to its own intestinal symbiotic bacteria (Marietta et al. 2018). In addition, human intestinal microbiota contains a large number of antibiotic resistance genes (ARGs), and among them, tetracycline resistance genes account for a large proportion. These ARGs can be freely transferred between intestinal bacteria within human colon (Liu et al. 2017b). Some of them are potential pathogens that induce a variety of diseases through the faecal-oral route and endanger human health. The use of antibiotics may help to enrich ARGs in human intestinal microbiota and have long-term effects on human intestinal microbiota (Yang et al. 2016). Again, the more and longer use of antibiotics increase the chances of harbouring, more resistance genes. Through DNA microarray analysis, it has been shown that the ARGs of human intestinal microbiota can be accumulated to adulthood, which create more complex problems with age (Lu et al. 2014). Traditional Chinese medicine has been proven to treat intestinal diseases while avoiding the problem of drug resistance, but the long treatment period is the existing problem.
Dietary fibre
Diet and nutrition heavily affect intestinal microbiota, which ferment dietary fibre to produce SCFAs, which have a variety of health benefits, and metabolites such as trimethylamine and indole propionic acids, that also have a direct impact on health. The foods that contain dietary fibre basically are grains, vegetables, fruits and beans. Many plants and mushroom (Ganoderma lucidum) eaters are resistant to diabetes and obesity (Martel et al. 2017). Pomegranates, nuts and berries have anti-aging effect (Ryu et al. 2016). Among them, soluble and insoluble dietary fibre can be distinguished and most dietary fibre is insoluble, such as cellulose and hemicellulose. Dietary fibre (including probiotics) has a variety of functions, such as reducing bowel passage time, maintaining normal blood cholesterol levels, reducing the risk of coronary heart disease and colorectal cancer, reducing postnatal blood glucose response and preventing harmful colonization (Verspreet et al. 2016).
Dietary soluble corn fibre was significantly correlated with increased calcium absorption in adolescent females, and the diversity of intestinal microbiota was significantly changed. The proportion of Cymbidium increased with the increase of edible soluble corn fibre, and the increase of calcium absorption was positively correlated with the increase of Clostridium and unclassified Clostridium (Whisner et al. 2016). And a high-vegetable/low-protein diet (HV/LP) eases multiple sclerosis.
Evidence from animal models and human experiments indicate that metabolic products produced by intestinal microbiota through dietary sources may affect the health of the host through certain signalling pathways. Diet has a significant impact on the composition and function of intestinal microbiota, and the intestinal microbiota also affects metabolites of dietary components. The bacteria metabolize high-fibre foods to produce succinic acid, which maintains blood sugar balance by activating intestinal glycogenesis. The ability of dietary fibre to improve glucose metabolism was related to the increased abundance of Platycocci (Kovatcheva-Datchary et al. 2015). Secondary bile acids were associated with liver cancer and colon cancer. The mixture of oya pulp and Bacillus coagulans can increase the metabolism of bile acid (Lee et al. 2016).
Dietary components can control colonization of intestinal symbiotic bacteria. According to the many reports, seaweed can affect colonization of the intestinal tract of Bacteroides plebeius DSM17135 (Kearney et al. 2018). Dietary fibre deficiency causes specific bacteria to degrade glycoproteins in intestinal mucus, damage the intestinal barrier and increase pathogen infection. Dietary fibre supplementation (especially fructan and oligogalactose) increases the abundance of Bifidobacteria and Lactobacillus in healthy adult faeces, but does not affect its diversity. A new study has been launched, proposing a novel idea to treat intestinal infection (Rolig et al. 2018). Without antibiotics, the metabolism and physiology of mice can be altered through dietary factors (short-term iron supplementation), in the way they only retain the mutant strain of Citrobacter with weak virulence selectively, thus promoting long-term symbiosis with pathogenic bacteria.
Prebiotics products
The concept of prebiotics has been proposed for 21 years ago, and animal experiments have shown that it can recover the intestinal tract damage, metabolism abnormalities, reduce the incidence of CRC. The supplementation of biogenetic element, short chain oligogalactose (scGOS), long chain oligofructose (lcFOS), m-16v Bifidobacteria increased the proportion of Bifidobacteria for caesarean babies and faecal acetate but decreased the proportion of Enterobacteriaceae, faecal pH (Chua et al. 2017). Thirty seven Australian children aged 2–3 years have higher composition of the children’s microbiome and was significantly different between the dairy based and plant diet (Smith-Brown et al. 2016). The diet increased the food to bacteria ratio, but both the bacterial abundance and the diversity were decreased. Plant diet was positively correlated with the amount of molasses, especially soybean and nut and increased the content of Bacteroides lycopensis.
The number of active rumen coccus was negatively correlated with the intake of fruit, especially apples and pears. In the simulated gastrointestinal experiment, the resistance and survival rate were improved when the rhamnose strain AT195 was precultured in the laboratory with sorbitol/mannose (Succi et al. 2017). Fructooligosaccharides (FOS), a dietary fibre rich in fruits and vegetables, were added to improve blood glucose homeostasis in animal models (Lin et al. 2016). Oligoxylose has the function of prebiotics. The addition of oligoxylose in rice was found to improve the balance of human microbiota (Le Bourgot et al. 2018). However, some butyric acid producing bacteria are reduced by excessive FOS intake, resulting in decreased diversity of bacteria and abnormal blood glucose metabolism.
Inulin is a kind of polyfructose that can be used as prebiotics, sugar substitute, emulsifying agent and fat substitute. Recent studies have shown that inulin and oligofructose can directly regulate mucosal immunity without changing the microbiota. Inulin rich in oligofructose can improve the metabolism of amino acids in plasma and urine of children with CD on a gluten-free diet, thus improving the intestinal condition and permeability (Drabinska et al. 2018). The combination of oligofructose and probiotics also has significant therapeutic effect on functional constipation. Dietary fibre selectively regulates the abundance and/or metabolic activity of intestinal microbiota or can prevent and repair the microbiota imbalance. With the addition of inulin, resistant starch and citrus pectin, the abundance of Bacteroides and Firmicutes in the intestinal microbiota of rats increased, and the abundance of Firmicutes decreased (Ferrario et al. 2017). Oral preparation (OMNi-BiOTiC® Stress Repair) can improve the condition of diarrhea type of IBS patients (Moser et al. 2018). It remarkably increases the diversity of bacteria in the stomach and duodenal membrane, and the elevated number of CD4 (+) T cells were reduced. The concentrations of SCFA, acetic acid and butyric acid in faeces were significantly increased and the concentrations of harmful hyponectin were decreased.
Drug adjustment
Antibiotics
Studies have shown that antibiotic treatment alleviates the effects of dextran sodium sulphate (DSS)-induced colitis and effects on fungi population (Sovran et al. 2018). DSS induced mice when treated with Vancomycin were completely protected from colitis, while colistintreated mice still had a colitis phenotype but were no longer affected by fungi administration. The relative abundance of bacteria and fungi in colistin-treated mice was significantly reduced compared to vancomycin-treated and control mice, indicating that in colistin-sensitive mice, bacteria interacted with the fungus. The beneficial effect of S. boulardii was restored with administration of colistrin-resistant E. coli, but the adverse effects of C. albicans increases. This result implies that the colonization of fungi in the intestine is affected by the bacterial microbiota, which indirectly changes the effect of the fungus on the host, thereby regulating colitis.
Antibiotics that relieve intestinal inflammation have been widely reported, such as rifaximin, otilonium bromide, peppermint oil, mesalazine, chloroquine, secretagogues, μ-Opioid agonist, κ-OR agonist and δ-OR antagonist, Histamine H1 receptor antagonist (ebastine), NK2 receptor antagonist (ibodutant), GABAergic agents (Jiang et al. 2017). Otilonium bromide and peppermint oil have anticonvulsive effects. Mesalazine reduces UC symptoms. Other drugs mainly improve IBS symptoms. Antibiotics use has a non-negligible side effect. Lyer et al. found oxazole compound OxC derived from industrial, dietary and intestinal commensal bacteria can impair the lipid antigen presentation function of CD1d in intestinal ECs, reduce IL-10 production, promote iNKT-mediated colon inflammation and activate the aryl aromatic hydrocarbon receptor (AhR) that triggers a CD1d-dependent intestinal inflammatory response (Iyer et al. 2018).
In addition, if the antibiotic treatment time is transitory, the bacteria develop resistance. Enterobacteriaceae infections in the bloodstream of patients (BSI) show resistance within 48 h of treatment of pseudomonas β-lactams antibiotic (APBL) therapy which is an independent risk factor of CDI (Seddon et al. 2018). Again, it cannot target specific harmful bacteria. Antibiotic use was found to significantly reduce overall survival in patients receiving checkpoint inhibitors for advanced cancer. Considerable conclusion is that, antibiotics do more harm than good for the recovery of intestinal microbiota and curing disease.
Traditional Chinese herbs
Several traditional Chinese medicine formulations and Chinese herbs contain fibre, polyphenols, polysaccharides and other substances, which can regulate intestinal microbiota and alleviate intestinal inflammation. Yupingfeng polysaccharides, consisting mainly of three plants (Astragali radix, Atractylodes macrocephala, Rhizome radix saposhnikoviae), promote the growth and immune activity, improve the steady state of gut microbiota by increasing the Cellulolytic bacteria and Streptococcus sp., and Enterococcus sp. So, the stronger immune activity of Yupingfeng polysaccharides may be related to its ability to improve intestinal microbiota (Sun et al. 2016). Shen Ling Bai Zhu San, which consists of 10 Chinese herbs: Panax ginseng, Atractylodes macrocephala, Wolfiporia cocos, Dioscorea opposita, Dolichos lablab, Semen coicis, Semen nelumbinis, Platycodon grandiflorus, Fructus amomi and Glycyrrhiza uralensis fisch at a ratio of 4 : 4: 4: 4: 3: 2: 2: 2: 2: 4, has been found to regulate the composition of intestinal microbiota in the process of alleviating antibiotic-related diarrhaea (Lv et al. 2017). For example, Sutterella abundance decrease, enrichment of beneficial bacteria, Bacteroides sp. increased significantly. Gegen Qinlian Decoction (GGQLD), Danggui Liuhuang Decoction (DGLHD), Danggui Liuhuang Decoction (DGLHD) and other prescriptions also have different effects on regulating intestinal microbiota, mucosal immunity and inflammatory state. Among them, GGQLD was composed of extracts of Puerariae lobataeradix, Scutellariae radix, Coptidis rhizoma and Glycyrrhizae radix et rhizoma Praeparata cum Melle (Kong et al. 2018). GGQLD is commonly used to treat UC. Studies have shown that GGQLD induces changes in the composition of intestinal microbiota. For example, the relative abundance of beneficial bacteria such as Lachnospiracea incertae sedis, Gemmiger, Bifidobacterium and Faecalibacterium has been significantly increased, while that of harmful bacteria such as F. prausnitzii, Alistipes, Pseudobutyrivibrio and Parabacteroides decreased, these changes in intestinal microbiota are reported to be associated with the antidiabetic effects of GGQLD (Xu et al. 2015). The potential mechanism of Bawei Xileisan in the treatment of UC may be the disruption of TH17 signalling pathway, the induction of TH17/TReg imbalance and the regulation of specific microbiota (Wen et al. 2016). Gwakhyangjeonggisan and Probioticscan combined can alleviate symptoms of IBS by increasing the beneficial gut microbiota (Ko et al. 2013), and Triterpenoid saponins from Gynostemma pentaphyllum herb (GpS) for CRC treatment have also been reported (Chen et al. 2016). GpS may play a crucial role in bringing the disease state of the host to a balance and healthy state through the modulation of the interaction between host and gut microbiota, which may contribute to its preventive efficacy against the tumourigenesis in ApcMin/+mice. Such health beneficial effects of GpS may apply to alleviate other chronic elements associated with inflammatory intestinal environment. These findings provide favourable evidence for the effects of traditional Chinese medicine on the intestinal microbial ecosystem and provide new ideas on its possible mechanism for preventing inflammatory diseases or cancers in the intestinal tract.
Conclusions
This paper reviews the relationship between human intestinal microbiota and several intestinal diseases. Beneficial and harmful bacteria, metabolites and inflammation are all important factors affecting human health. Existing research has confirmed a causal relationship between intestinal microbiota and intestinal diseases, but it has not been thoroughly studied, which can be used as a future research direction. The discovery of bacterial metabolites that can regulate host physiological processes opens up the possibility of many microbial pathways as mediators and potential pharmacological targets for the treatment of intestinal diseases. Many ways including FMT, dietary interventions and drugs receives great attention to achieve the intestinal disease therapeutic purpose through intestinal micro-organisms’ intervention. We need to make significant progress in how the gut microbiome converts dietary and endogenous molecules into metabolites that communicate with the host’s surrounding organs and tissues. Understanding the composition of the microbiome, screening for harmful or beneficial bacteria, and exploring the mechanisms by which diseases are aggravated or alleviated in the course of disease occurrence and development will help us to cope with intestinal diseases by adjusting new therapies of the microbiome. There are few researches on the mechanism of action between intestinal micro-organisms and between intestinal micro-organisms and host. In the future, if one or several related micro-organisms or its key components can be found that can be used as the markers of specific diseases, it will be possible to use intestinal microbiology for the diagnosis, prevention and treatment of diseases.
Acknowledgements
We acknowledge the support of Jilin University. We are thankful to Dr Steven Swendeman for critical reading of the manuscript.
Fundings
This work was supported by general financial grant from the National Natural Science Foundation of China (no. 31670796) to D.Y. and X.F.; and the AHA SDG, Grant/Award (no. 17SDG33410868) to H.W.
Footnotes
Conflict of Interest
The authors declare that they have no competing interests.
References
- Amiri M, Diekmann L, von Köckritz-Blickwede M and Naim HY (2015) The diverse forms of lactose intolerance and the putative linkage to several cancers. Nutrients 7, 7209–7230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur JC, Gharaibeh RZ, Mühlbauer M, Perez-Chanona E, Uronis JM, McCafferty J, Fodor AA and Jobin C (2014) Microbial genomic analysis reveals the essential role of inflammation in bacteria-induced colorectal cancer. Nat Commun 5, 4724–4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcazar JL, Subirats J and Borrego CM (2015) The role of biofilms as environmental reservoirs of antibiotic resistance. Front Microbiol 6, 1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbara G, Grover M, Bercik P, Corsetti M, Ghoshal UC, Ohman L and Rajilić-Stojanović M (2019) Rome foundation working team report on post-infection irritable bowel syndrome. Gastroenterology 156, 46–58.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertesteanu S, Triaridis S, Stankovic M, Lazar V, Chifiriuc MC, Vlad M and Grigore R (2014) Polymicrobial wound infections: pathophysiology and current therapeutic approaches. Int J Pharm 463, 119–126. [DOI] [PubMed] [Google Scholar]
- Bichkaeva FA, Volkova NI, Bichkaev AA, Tretykova TV, Vlasova OS, Nesterova EV, Shengof BA and Baranova NF (2018) Correlation of parameters of carbohydrate metabolism and saturated fatty acids in blood serum in elderly people. Adv Gerontol 31, 231–238. [PubMed] [Google Scholar]
- Blachier F, Beaumont M, Andriamihaja M, Davila A-M, Lan A, Grauso M, Armand L, Benamouzig R and et al. (2017) Changes in the luminal environment of the colonic epithelial cells and physiopathological consequences. Am J Pathol 187, 476–486. [DOI] [PubMed] [Google Scholar]
- Buffie CG, Bucci V, Stein RR, McKenney PT, Ling L, Gobourne A, No D, Liu H et al. (2015) Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature 517, 205–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammarota G, Ianiro G, Tilg H, Rajilić-Stojanović M, Kump P, Satokari R, Sokol H, Arkkila P et al. (2017) European consensus conference on faecal microbiota transplantation in clinical practice. Gut 66, 569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Liu X, An Y, Zhou G, Liu Y, Xu M, Dong W, Wang S et al. (2017) Dysbiosis contributes to chronic constipation development via regulation of serotonin transporter in the intestine. Sci Rep 7, 10322–10322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash BD (2018) Understanding and managing IBS and CIC in the primary care setting. Gastroenterol Hepatol (N Y) 14, 3–15. [PMC free article] [PubMed] [Google Scholar]
- Chai L, Dong Z, Chen A and Wang H (2018) Changes in intestinal microbiota of Bufo gargarizans and its association with body weight during metamorphosis. Arch Microbiol 200, 1087–1099. [DOI] [PubMed] [Google Scholar]
- Chandar AK (2017) Diagnosis and treatment of irritable bowel syndrome with predominant constipation in the primary-care setting: focus on linaclotide. Int J Gen Med 10, 385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. (2018) Short-course therapy for diarrhea-predominant irritable bowel syndrome: understanding the mechanism, impact on gut microbiota, and safety and tolerability of rifaximin. Clin Exp Gastroenterol 11, 335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Brar MS, Leung FC and Hsiao WL (2016) Triterpenoid herbal saponins enhance beneficial bacteria, decrease sulfate-reducing bacteria, modulate inflammatory intestinal microenvironment and exert cancer preventive effects in ApcMin/+ mice. Oncotarget 7, 31226–31242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Chen H, Shu X, Yin Y, Li J, Qin J, Chen L, Peng K et al. (2018) Presence of segmented filamentous bacteria in human children and its potential role in the modulation of human gut immunity. Front Microbiol 9, 1403–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua MC, Ben-Amor K, Lay C, Neo AGE, Chiang WC, Rao R, Chew C, Chaithongwongwatthana S et al. (2017) Effect of synbiotic on the gut microbiota of cesarean delivered infants: a randomized, double-blind, multicenter study. J Pediatr Gastroenterol Nutr 65, 102–106. [DOI] [PubMed] [Google Scholar]
- Culbreath C, Tanner SM, Yeramilli VA, Berryhill TF, Lorenz RG and Martin CA (2015) Environmental-mediated intestinal homeostasis in neonatal mice. J Surg Res 198, 494–501. [DOI] [PubMed] [Google Scholar]
- DeFilipp Z, Bloom PP, Torres Soto M, Mansour MK, Sater MRA, Huntley MH, Turbett S, Chung RT et al. (2019) Drug-resistant E. coli bacteremia transmitted by fecal microbiota transplant. N Engl J Med 381, 2043–2050. [DOI] [PubMed] [Google Scholar]
- Di Martino ML, Campilongo R, Casalino M, Micheli G, Colonna B and Prosseda G (2013) Polyamines: emerging players in bacteria-host interactions. Int J Med Microbiol 303, 484–491. [DOI] [PubMed] [Google Scholar]
- Dishaw LJ, Leigh B, Cannon JP, Liberti A, Mueller MG, Skapura DP, Karrer CR, Pinto MR et al. (2016) Gut immunity in a protochordate involves a secreted immunoglobulin-type mediator binding host chitin and bacteria. Nat Commun 7, 10617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson GP, Lee SM and Mazmanian SK (2016) Gut biogeography of the bacterial microbiota. Nat Rev Microbiol 14, 20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson GP, Ladinsky MS, Yu KB, Sanders JG, Yoo BB, Chou WC, Conner ME, Earl AM et al. (2018) Gut microbiota utilize immunoglobulin A for mucosal colonization. Science 360, 795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnachie E, Schneider A, Mehring M and Enck P (2018) Incidence of irritable bowel syndrome and chronic fatigue following GI infection: a population-level study using routinely collected claims data. Gut 67, 1078–1086. [DOI] [PubMed] [Google Scholar]
- Drabinska N, Krupa-Kozak U, Ciska E and Jarocka-Cyrta E (2018) Plasma profile and urine excretion of amino acids in children with celiac disease on gluten-free diet after oligofructose-enriched inulin intervention: results of a randomised placebo-controlled pilot study. Amino Acids 50, 1451–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberl G, Colonna M, Di Santo JP and McKenzie AN (2015) Innate lymphoid cells. Innate lymphoid cells: a new paradigm in immunology. Science 348, aaa6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Salhy M and Mazzawi T (2018) Fecal microbiota transplantation for managing irritable bowel syndrome. Expert Rev Gastroenterol Hepatol 12, 439–445. [DOI] [PubMed] [Google Scholar]
- Farkas AM, Panea C, Goto Y, Nakato G, Galan-Diez M, Narushima S, Honda K and Ivanov II (2015) Induction of Th17 cells by segmented filamentous bacteria in the murine intestine. J Immunol Methods 421, 104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W, Wu Y, Chen G, Fu S, Li B, Huang B, Wang D, Wang W et al. (2018) Sodium butyrate attenuates diarrhea in weaned piglets and promotes tight junction protein expression in colon in a GPR109A-dependent manner. Cell physio Biochem 47, 1617–1629. [DOI] [PubMed] [Google Scholar]
- Ferrario C, Statello R, Carnevali L, Mancabelli L, Milani C, Mangifesta M, Duranti S, Lugli GA et al. (2017) How to feed the mammalian gut microbiota: bacterial and metabolic modulation by dietary fibers. Front Microbiol 8, 1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer CL, Drake DR, Dawson DV, Blanchette DR, Brogden KA and Wertz PW (2012) Antibacterial activity of sphingoid bases and fatty acids against Gram-positive and Gram-negative bacteria. Antimicrob Agents Chemother 56, 1157–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francavilla R, Piccolo M, Francavilla A, Polimeno L, Semeraro F, Cristofori F, Castellaneta S, Barone M et al. (2019) Clinical and microbiological effect of a multispecies probiotic supplementation in celiac patients with persistent IBS-type symptoms: a randomized, double-blind, placebo-controlled, multicenter trial. J Clin Gastroenterol 53, e117–e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung KY, Cosgrove L, Lockett T, Head R and Topping DL (2012) A review of the potential mechanisms for the lowering of colorectal oncogenesis by butyrate. Br J Nutr 108, 820–831. [DOI] [PubMed] [Google Scholar]
- Gorkiewicz G, Thallinger GG, Trajanoski S, Lackner S, Stocker G, Hinterleitner T, Gülly C and Högenauer C (2013) Alterations in the colonic microbiota in response to osmotic diarrhea. PLoS ONE 8, e55817–e55817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y. (2019) Epithelial cells as a transmitter of signals from commensal bacteria and host immune cells. Front Immunol 10, 2057–2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronke K, Hernández PP, Zimmermann J, Klose CSN, Kofoed-Branzk M, Guendel F, Witkowski M, Tizian C et al. (2019) Interleukin-22 protects intestinal stem cells against genotoxic stress. Nature 566, 249–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hang S, Paik D, Yao L, Kim E, Jamma T, Lu J, Ha S, Nelson BN et al. (2019) Bile acid metabolites control T (H)17 and T(reg) cell differentiation. Nature 576, 143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepworth MR, Monticelli LA, Fung TC, Ziegler CG, Grunberg S, Sinha R, Mantegazza AR, Ma HL et al. (2013) Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature 498, 113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata S-I and Kunisawa J (2017) Gut microbiome, metabolome, and allergic diseases. Allergol Int 66, 523–528. [DOI] [PubMed] [Google Scholar]
- Hoarau G, Mukherjee PK, Gower-Rousseau C, Hager C, Chandra J, Retuerto MA, Neut C, Vermeire S et al. (2016) Bacteriome and mycobiome interactions underscore microbial dysbiosis in familial Crohn’s disease. MBio 7, 10.1128/mBio.01250-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hoeven-Hangoor E, van der Vossen JMBM, Schuren FHJ, Verstegen MWA, de Oliveira JE, Montijn RC and Hendriks WH (2013) Ileal microbiota composition of broilers fed various commercial diet compositions. Poult Sci 92, 2713–2723. [DOI] [PubMed] [Google Scholar]
- Holvoet T, Joossens M, Wang J, Boelens J, Verhasselt B, Laukens D, van Vlierberghe H, Hindryckx P et al. (2017) Assessment of faecal microbial transfer in irritable bowel syndrome with severe bloating. Gut 66, 980–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer SS, Gensollen T, Gandhi A, Oh SF, Neves JF, Collin F, Lavin R, Serra C et al. (2018) Dietary and microbial oxazoles induce intestinal inflammation by modulating aryl hydrocarbon receptor responses. Cell 173, 1123–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Ellabaan MMH, Charusanti P, Munck C, Blin K, Tong Y, Weber T, Sommer MOA et al. (2017) Dissemination of antibiotic resistance genes from antibiotic producers to pathogens. Nat Commun 8, 15784–15784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakiyama G, Muto A, Takei H, Nittono H, Murai T, Kurosawa T, Hofmann AF, Pandak WM et al. (2014) A simple and accurate HPLC method for fecal bile acid profile in healthy and cirrhotic subjects: validation by GC-MS and LC-MS. J Lipid Res 55, 978–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JD, Myers CJ, Harris SC, Kakiyama G, Lee IK, Yun BS, Matsuzaki K, Furukawa M et al. (2019) Bile acid 7 alpha-dehydroxylating gut bacteria secrete antibiotics that inhibit Clostridium difficile: role of secondary bile acids. Cell Chem Biol 26, 27–34.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney SM, Gibbons SM, Erdman SE and Alm EJ (2018) Orthogonal dietary niche enables reversible engraftment of a gut bacterial commensal. Cell Rep 24, 1842–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly CR, Kahn S, Kashyap P, Laine L, Rubin D, Atreja A, Moore T and Wu G (2015) Update on fecal microbiota transplantation 2015: indications, methodologies, mechanisms, and outlook. Gastroenterology 149, 223–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempeneers MA, Ahmed Ali U, Issa Y, van Goor H, Drenth JPH, van Dullemen HM, van Hooft JE, Poen AC et al. (2020) Natural course and treatment of pancreatic exocrine insufficiency in a nationwide cohort of chronic pancreatitis. Pancreas 49, 242–248. 10.1097/MPA.0000000000001473. [DOI] [PubMed] [Google Scholar]
- Keshteli AH, Millan B and Madsen KL (2017) Pretreatment with antibiotics may enhance the efficacy of fecal microbiota transplantation in ulcerative colitis: a meta-analysis. Mucosal Immunol 10, 565–566. [DOI] [PubMed] [Google Scholar]
- Khatri I, Tomar R, Ganesan K, Prasad GS and Subramanian S (2017) Complete genome sequence and comparative genomics of the probiotic yeast Saccharomyces boulardii. Sci Rep 7, 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H-R, Rhee K-J and Eom Y-B (2019) Anti-biofilm and antimicrobial effects of zerumbone against Bacteroides fragilis. Anaerobe 57, 99–106. [DOI] [PubMed] [Google Scholar]
- Ko SJ, Han G, Kim SK, Seo JG, Chung WS, Ryu B, Kim J, Yeo I et al. (2013) Effect of Korean herbal medicine combined with a probiotic mixture on diarrhea-dominant irritable bowel syndrome: a double-blind, randomized, placebo-controlled trial. Evid Based Compl Alternat Med 2013, 824605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolho KL, Korpela K, Jaakkola T, Pichai MV, Zoetendal EG, Salonen A and de Vos WM (2015) Fecal microbiota in pediatric inflammatory bowel disease and its relation to inflammation. Am J Gastroenterol 110, 921–930. [DOI] [PubMed] [Google Scholar]
- Kong H, Wang X-Q, Wang Q-G, Zhao Y, Sun Y, Zhang Y, Xu J-K and Qu H-H (2018) Effect of puerarin on the pharmacokinetics of baicalin in Gegen Qinlian decoction () in Mice. Chin J Integr Med 24, 525–530. [DOI] [PubMed] [Google Scholar]
- Konishi H, Fujiya M, Tanaka H, Ueno N, Moriichi K, Sasajima J, Ikuta K, Akutsu H et al. (2016) Probiotic-derived ferrichrome inhibits colon cancer progression via JNK-mediated apoptosis. Nat Commun 7, 12365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovatcheva-Datchary P, Nilsson A, Akrami R, Lee YS, De Vadder F, Arora T, Hallen A, Martens E et al. (2015) Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of prevotella. Cell Metab 22, 971–982. [DOI] [PubMed] [Google Scholar]
- Kummen M, Holm K, Anmarkrud JA, Nygard S, Vesterhus M, Hoivik ML, Troseid M, Marschall HU et al. (2017) The gut microbial profile in patients with primary sclerosing cholangitis is distinct from patients with ulcerative colitis without biliary disease and healthy controls. Gut 66, 611–619. [DOI] [PubMed] [Google Scholar]
- Le Bourgot C, Apper E, Blat S and Respondek F (2018) Fructo-oligosaccharides and glucose homeostasis: a systematic review and meta-analysis in animal models. Nutr Metab (Lond) 15, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Yoshitsugu R, Kikuchi K, Joe GH, Tsuji M, Nose T, Shimizu H, Hara H et al. (2016) Combination of soya pulp and Bacillus coagulans lilac-01 improves intestinal bile acid metabolism without impairing the effects of prebiotics in rats fed a cholic acid-supplemented diet. Br J Nutr 116, 603–610. [DOI] [PubMed] [Google Scholar]
- Levy M, Kolodziejczyk AA, Thaiss CA and Elinav E (2017) Dysbiosis and the immune system. Nat Rev Immunol 17, 219–232. [DOI] [PubMed] [Google Scholar]
- Li D and Cao W (2016) Bile acid receptor TGR5, NADPH oxidase NOX5-S and CREB mediate bile acid-induced dna damage in Barrett’s esophageal adenocarcinoma cells. Sci Rep 6, 31538–31538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Chen Q, Ma T and Yu X (2017) Targeting reactive nitrogen species suppresses hereditary pancreatic cancer. Proc Natl Acad Sci USA 114, 7106–7111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, van Esch BCAM, Henricks PAJ, Folkerts G and Garssen J (2018) The anti-inflammatory effects of short chain fatty acids on lipopolysaccharide- or tumor necrosis factor α-stimulated endothelial cells via activation of GPR41/43 and inhibition of HDACs. Front Pharmacol 9, 533–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liauw S and Saibil F (2019) Sorbitol: Often forgotten cause of osmotic diarrhea. Can Fam Physician 65, 557–558. [PMC free article] [PubMed] [Google Scholar]
- Lin SH, Chou LM, Chien YW, Chang JS and Lin CI (2016) Prebiotic effects of xylooligosaccharides on the improvement of microbiota balance in human subjects. Gastroenterol Res Pract 2016, 5789232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HN, Wu H, Chen YZ, Chen YJ, Shen XZ and Liu TT (2017a) Altered molecular signature of intestinal microbiota in irritable bowel syndrome patients compared with healthy controls: a systematic review and meta-analysis. Dig Liver Dis 49, 331–337. [DOI] [PubMed] [Google Scholar]
- Liu P, Jia S, He X, Zhang X and Ye L (2017b) Different impacts of manure and chemical fertilizers on bacterial community structure and antibiotic resistance genes in arable soils. Chemosphere 188, 455–464. [DOI] [PubMed] [Google Scholar]
- Liu D, Wei Y, Liu X, Zhou Y, Jiang L, Yin J, Wang F, Hu Y et al. (2018) Indoleacetate decarboxylase is a glycyl radical enzyme catalysing the formation of malodorant skatole. Nat Commun 9, 4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodes MJ, Cong Y, Elson CO, Mohamath R, Landers CJ, Targan SR, Fort M and Hershberg RM (2004) Bacterial flagellin is a dominant antigen in Crohn disease. J Clin Invest 113, 1296–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu N, Hu Y, Zhu L, Yang X, Yin Y, Lei F, Zhu Y, Du Q et al. (2014) DNA microarray analysis reveals that antibiotic resistance-gene diversity in human gut microbiota is age related. Sci Rep 4, 4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig IS, Broere F, Manurung S, Lambers TT, van der Zee R and van Eden W (2018) Lactobacillus rhamnosus GG-derived soluble mediators modulate adaptive immune cells. Front Immunol 9, 1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv W, Liu C, Ye C, Sun J, Tan X, Zhang C, Qu Q, Shi D et al. (2017) Structural modulation of gut microbiota during alleviation of antibiotic-associated diarrhea with herbal formula. Int J Biol Macromol 105, 1622–1629. [DOI] [PubMed] [Google Scholar]
- Mahalhal A, Williams JM, Johnson S, Ellaby N, Duckworth CA, Burkitt MD, Liu X, Hold GL et al. (2018) Oral iron exacerbates colitis and influences the intestinal microbiome. PLoS ONE 13, e0202460–e0202460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeed M, Nagabhushanam K, Arumugam S, Majeed S and Ali F (2018) Bacillus coagulans MTCC 5856 for the management of major depression with irritable bowel syndrome: a randomised, double-blind, placebo controlled, multi-centre, pilot clinical study. Food Nutr Res 62, 10.29219/fnr.v62.1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marietta E, Horwath I and Taneja V (2018) Microbiome, immunomodulation, and the neuronal system. Neurotherapeutics 15, 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel J, Ojcius DM, Chang CJ, Lin CS, Lu CC, Ko YF, Tseng SF, Lai HC et al. (2017) Anti-obesogenic and antidiabetic effects of plants and mushrooms. Nat Rev Endocrinol 13, 149–160. [DOI] [PubMed] [Google Scholar]
- Mills JP, Rao K and Young VB (2018) Probiotics for prevention of Clostridium difficile infection. Curr Opin Gastroenterol 34, 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moor K, Diard M, Sellin ME, Felmy B, Wotzka SY, Toska A, Bakkeren E, Arnoldini M et al. (2017) High-avidity IgA protects the intestine by enchaining growing bacteria. Nature 544, 498–502. [DOI] [PubMed] [Google Scholar]
- Mortha A, Chudnovskiy A, Hashimoto D, Bogunovic M, Spencer SP, Belkaid Y and Merad M (2014) Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science 343, 1249288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser AM, Spindelboeck W, Halwachs B, Strohmaier H, Kump P, Gorkiewicz G and Hogenauer C (2018) Effects of an oral synbiotic on the gastrointestinal immune system and microbiota in patients with diarrhea-predominant irritable bowel syndrome. Eur J Nutr 10.1007/s00394-018-1826-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TL, Grajales-Reyes GE, Wu X, Tussiwand R, Briseno CG, Iwata A, Kretzer NM, Durai V et al. (2016) Transcriptional control of dendritic cell development. Annu Rev Immunol 34, 93–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkusa T, Koido S, Nishikawa Y and Sato N (2019) Gut microbiota and chronic constipation: a review and update. Front Med(Lausanne) 6, 10.3389/fmed.2019.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olin-Sandoval V, Yu JSL, Miller-Fleming L, Alam MT, Kamrad S, Correia-Melo C, Haas R, Segal J et al. (2019) Lysine harvesting is an antioxidant strategy and triggers underground polyamine metabolism. Nature 572, 249–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Mahoney C. (2005) Widening the dimensions of care. Emerg Nurse 13, 18–24. [DOI] [PubMed] [Google Scholar]
- Oppfeldt AM, Dahlerup JF, Christensen LA and Hvas CL (2016) Faecal microbiota transplantation for recurring Clostridium difficile infection in a patient with Crohn’s disease and ileorectal anastomosis. BMJ Case Rep. 10.1136/bcr-2016-217209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm NW, de Zoete MR, Cullen TW, Barry NA, Stefanowski J, Hao L, Degnan PH, Hu J et al. (2014) Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell 158, 1000–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paramsothy S, Paramsothy R, Rubin DT, Kamm MA, Kaakoush NO, Mitchell HM and Castaño-Rodríguez N (2017) Faecal Microbiota transplantation for inflammatory bowel disease: a systematic review and meta-analysis. J Crohns Colitis 11, 1180–1199. [DOI] [PubMed] [Google Scholar]
- Pimentel M. (2018) Evidence-based management of irritable bowel syndrome with diarrhea. Am J Manag Care 24, S35–S46. [PubMed] [Google Scholar]
- Pinto-Sanchez MI, Hall GB, Ghajar K, Nardelli A, Bolino C, Lau JT, Martin FP, Cominetti O et al. (2017) Probiotic Bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: a pilot study in patients with irritable bowel syndrome. Gastroenterology 153, 448–459.e448. [DOI] [PubMed] [Google Scholar]
- Postler TS and Ghosh S (2017) Understanding the holobiont: how microbial metabolites affect human health and shape the immune system. Cell Metab 26, 110–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell N and MacDonald TT (2017) Recent advances in gut immunology. Parasite Immunol 39, 10.1111/pim.12430. [DOI] [PubMed] [Google Scholar]
- Rajilić-Stojanović M, Biagi E, Heilig HGHJ, Kajander K, Kekkonen RA, Tims S and de Vos WM (2011) Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology 141, 1792–1801. [DOI] [PubMed] [Google Scholar]
- Rao SSC, Rattanakovit K and Patcharatrakul T (2016) Diagnosis and management of chronic constipation in adults. Nat Rev Gastroenterol Hepatol 13, 295–305. [DOI] [PubMed] [Google Scholar]
- Reti KL, Tymensen LD, Davis SP, Amrein MW and Buret AG (2015) Campylobacter jejuni increases flagellar expression and adhesion of noninvasive Escherichia coli: effects on enterocytic Toll-like receptor 4 and CXCL-8 expression. Infect Immun 83, 4571–4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard ML, Lamas B, Liguori G, Hoffmann TW and Sokol H (2015) Gut fungal microbiota: the Yin and Yang of inflammatory bowel disease. Inflamm Bowel Dis 21, 656–665. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Nogales A, Algieri F, Garrido-Mesa J, Vezza T, Utrilla MP, Chueca N, Garcia F, Rodriguez-Cabezas ME et al. (2018) Intestinal anti-inflammatory effect of the probiotic Saccharomyces boulardii in DSS-induced colitis in mice: impact on microRNAs expression and gut microbiota composition. J Nutr Biochem 61, 129–139. [DOI] [PubMed] [Google Scholar]
- Rogier EW, Frantz AL, Bruno ME, Wedlund L, Cohen DA, Stromberg AJ and Kaetzel CS (2014) Secretory antibodies in breast milk promote long-term intestinal homeostasis by regulating the gut microbiota and host gene expression. Proc Natl Acad Sci USA 111, 3074–3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolig AS, Sweeney EG, Kaye LE, DeSantis MD, Perkins A, Banse AV, Hamilton MK and Guillemin K (2018) A bacterial immunomodulatory protein with lipocalin-like domains facilitates host–bacteria mutualism in larval zebrafish. eLife 7, 10.7554/eLife.37172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa K, Delgado-Herrera L, Zeiher B, Banderas B, Arbuckle R, Spears G and Hudgens S (2016) Psychometric assessment of the IBS-D Daily Symptom Diary and Symptom Event Log. Qual Life Res 25, 3197–3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostami K, Rostami-Nejad M and Al Dulaimi D (2015) Post gastroenteritis gluten intolerance. Gastroenterol Hepatol Bed Bench 8, 66–70. [PMC free article] [PubMed] [Google Scholar]
- Round JL and Mazmanian SK (2009) The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol 9, 313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu D, Mouchiroud L, Andreux PA, Katsyuba E, Moullan N, Nicolet-Dit-Felix AA, Williams EG, Jha P et al. (2016) Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nat Med 22, 879–888. [DOI] [PubMed] [Google Scholar]
- Schultz BM, Paduro CA, Salazar GA, Salazar-Echegarai FJ, Sebastian VP, Riedel CA, Kalergis AM, Alvarez-Lobos M et al. (2017) A potential role of salmonella infection in the onset of inflammatory bowel diseases. Front Immunol 8, 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddon MM, Bookstaver PB, Justo JA, Kohn J, Rac H, Haggard E, Mediwala KN, Dash S et al. (2018) Role of early de-escalation of antimicrobial therapy on risk of clostridioides difficile infection following enterobacteriaceae bloodstream infections. Clin Infect Dis 69, 414–420. [DOI] [PubMed] [Google Scholar]
- Shapiro H, Kolodziejczyk AA, Halstuch D and Elinav E (2018) Bile acids in glucose metabolism in health and disease. J Exp Med 215, 383–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM, Glickman JN and Garrett WS (2013) The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341, 569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Brown P, Morrison M, Krause L and Davies PS (2016) Dairy and plant based food intakes are associated with altered faecal microbiota in 2 to 3 year old Australian children. Sci Rep 6, 32385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol H, Leducq V, Aschard H, Hang-Phuong P, Jegou S, Landman C, Cohen D, Liguori G et al. (2017) Fungal microbiota dysbiosis in IBD. Gut 66, 1039–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Sun X, Oh SF, Wu M, Zhang Y, Zheng W, Geva-Zatorsky N, Jupp R et al. (2020) Microbial bile acid metabolites modulate gut RORγ(+) regulatory T cell homeostasis. Nature 577, 410–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sovran B, Planchais J, Jegou S, Straube M, Lamas B, Natividad JM, Agus A, Dupraz L et al. (2018) Enterobacteriaceae are essential for the modulation of colitis severity by fungi. Microbiome 6, 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Succi M, Tremonte P, Pannella G, Tipaldi L, Cozzolino A, Romaniello R, Sorrentino E and Coppola R (2017) Pre-cultivation with selected prebiotics enhances the survival and the stress response of Lactobacillus rhamnosus strains in simulated gastrointestinal transit. Front Microbiol 8, 1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Ni X, Song X, Wen B, Zhou Y, Zou F, Yang M, Peng Z et al. (2016) Fermented Yupingfeng polysaccharides enhance immunity by improving the foregut microflora and intestinal barrier in weaning rex rabbits. Appl Microbiol Biotechnol 100, 8105–8120. [DOI] [PubMed] [Google Scholar]
- Svendsen AT, Bytzer P and Engsbro AL (2019) Systematic review with meta-analyses: does the pathogen matter in post-infectious irritable bowel syndrome? Scand J Gastroenterol 54, 546–562. [DOI] [PubMed] [Google Scholar]
- Swidsinski A, Weber J, Loening-Baucke V, Hale LP and Lochs H (2005) Spatial organization and composition of the mucosal flora in patients with inflammatory bowel disease. J Clin Microbiol 43, 3380–3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiagarajah JR, Kamin DS, Acra S, Goldsmith JD, Roland JT, Lencer WI, Muise AM, Goldenring JR et al. (2018) Advances in evaluation of chronic diarrhea in infants. Gastroenterology 154, 2045–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropini C, Moss EL, Merrill BD, Ng KM, Higginbottom SK, Casavant EP, Gonzalez CG, Fremin B et al. (2018) Transient osmotic perturbation causes long-term alteration to the gut microbiota. Cell 173, 1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez Roque M and Bouras EP (2015) Epidemiology and management of chronic constipation in elderly patients. Clin Interv Aging 10, 919–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verspreet J, Damen B, Broekaert WF, Verbeke K, Delcour JA and Courtin CM (2016) A critical look at prebiotics within the dietary fiber concept. Annu Rev Food Sci Technol 7, 167–190. [DOI] [PubMed] [Google Scholar]
- Wang W, Yu J, He Y, Wang Z and Li F (2016) Ambroxol inhibits mucoid conversion of Pseudomonas aeruginosa and contributes to the bactericidal activity of ciprofloxacin against mucoid P. aeruginosa biofilms. APMIS 124, 611–618. [DOI] [PubMed] [Google Scholar]
- Wang L, Hu L, Xu Q, Yin B, Fang D, Wang G, Zhao J, Zhang H et al. (2017) Bifidobacterium adolescentis exerts strain-specific effects on constipation induced by loperamide in BALB/c mice. Int J Mol Sci 18, 318. 10.3390/ijms18020318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingarden AR and Vaughn BP (2017) Intestinal microbiota, fecal microbiota transplantation, and inflammatory bowel disease. Gut Microbes 8, 238–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen J, Teng B, Yang P, Chen X, Li C, Jing Y, Wei J and Zhang C (2016) The potential mechanism of Bawei Xileisan in the treatment of dextran sulfate sodium-induced ulcerative colitis in mice. J Ethnopharmacol 188, 31–38. [DOI] [PubMed] [Google Scholar]
- Whisner CM, Martin BR, Nakatsu CH, Story JA, MacDonald-Clarke CJ, McCabe LD, McCabe GP and Weaver CM (2016) Soluble corn fiber increases calcium absorption associated with shifts in the gut microbiome: a randomized dose-response trial in free-living pubertal females. J Nutr 146, 1298–1306. [DOI] [PubMed] [Google Scholar]
- Windey K, De Preter V and Verbeke K (2012) Relevance of protein fermentation to gut health. Mol Nutr Food Res 56, 184–196. [DOI] [PubMed] [Google Scholar]
- Wong SH, Zhao L, Zhang X, Nakatsu G, Han J, Xu W, Xiao X, Kwong TNY et al. (2017) Gavage of fecal samples from patients with colorectal cancer promotes intestinal carcinogenesis in germ-free and conventional mice. Gastroenterology 153, 1621–1633.e1626. [DOI] [PubMed] [Google Scholar]
- Xie Y, Ding F, Di W, Lv Y, Xia F, Sheng Y, Yu J and Ding G (2020) Impact of a high-fat diet on intestinal stem cells and epithelial barrier function in middle-aged female mice. Mol Med Rep. 10.3892/mmr.2020.10932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Lian F, Zhao L, Zhao Y, Chen X, Zhang X, Guo Y, Zhang C et al. (2015) Structural modulation of gut microbiota during alleviation of type 2 diabetes with a Chinese herbal formula. ISME J 9, 552–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J and Yu J (2018) The association of diet, gut microbiota and colorectal cancer: what we eat may imply what we get. Protein Cell 9, 474–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Guo Z, Qiu C, Li Y, Feng X, Liu Y, Zhang Y, Pang P et al. (2016) Preliminary analysis showed country-specific gut resistome based on 1,267 feces samples. Gene 581, 178–182. [DOI] [PubMed] [Google Scholar]
- Yu J, Feng Q, Wong SH, Zhang D, Liang QY, Qin Y, Tang L, Zhao H et al. (2017) Metagenomic analysis of faecal microbiome as a tool towards targeted non-invasive biomarkers for colorectal cancer. Gut 66, 70–78. [DOI] [PubMed] [Google Scholar]
- Yu W, Su X, Chen W, Tian X, Zhang K, Guo G, Zhou L, Zeng T et al. (2019) Three types of gut bacteria collaborating to improve Kui Jie’an enema treat DSS-induced colitis in mice. Biomed Pharmacother 113, 108751. 10.1016/j.biopha.2019.108751. [DOI] [PubMed] [Google Scholar]
- Zeng H, Umar S, Rust B, Lazarova D and Bordonaro M (2019) Secondary bile acids and short chain fatty acids in the colon: a focus on colonic microbiome, cell proliferation, inflammation, and cancer. Int J Mol Sci 20, 1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M and Qiu S (2019) Activation of GPR120 promotes the metastasis of breast cancer through the PI3K/Akt/NF-κB signaling pathway. Anticancer Drugs 30, 260–270. [DOI] [PubMed] [Google Scholar]
- Zhao L, Huang Y, Lu L, Yang W, Huang T, Lin Z, Lin C, Kwan H et al. (2018) Saturated long-chain fatty acid-producing bacteria contribute to enhanced colonic motility in rats. Microbiome 6, 10.1186/s40168-018-0492-6. [DOI] [PMC free article] [PubMed] [Google Scholar]