Abstract
Background
Autologous monocyte-derived mRNA co-electroporated dendritic cells with mRNA encoding CD40 ligand (CD40L), CD70 and a constitutively activated TLR4 (caTLR4) (referred to as TriMixDC-MEL) have anti-tumor activity in advanced melanoma patients. We investigated the safety and activity of adjuvant TriMixDC-MEL in stage III/IV melanoma patients.
Materials and methods
Forty-one patients were randomly assigned to treatment with TriMixDC-MEL (n = 21) and standard follow-up (n = 20). “Cross-over” was allowed at the time of non-salvageable recurrence. The primary endpoint was the percentage of patients alive and disease-free at 1-year. For a subset of patients, (formalin-fixed paraffin-embedded), tumor tissue samples were available for mRNA expression profiling and PD-L1 immunohistochemical staining.
Results
Baseline characteristics were well balanced. One-year after randomization, 71% of patients in the study arm were alive and free of disease compared to 35% in the control arm. After a median follow-up of 53 months (range 3–67), 23 patients experienced a non-salvageable melanoma recurrence (TriMixDC-Mel arm n = 9 and control arm n = 14).The median time to non-salvageable recurrence was superior in the TriMixDC-MEL arm (median 8 months (range 1–6) vs. not reached; log-rank p 0.044). TriMixDC-MEL-related adverse events (AE) consisted of transient local skin reactions, flu-like symptoms and post-infusion chills. No grade ≥ 3 AE’s occurred. The mRNA expression profiling revealed four genes (STAT2, TPSAB1, CD9 and CSF2) as potential predictive biomarkers.
Conclusion
TriMixDC-MEL id/iv as adjuvant therapy is tolerable and may improve the 1-year disease-free survival rate. Combination of optimized autologous monocyte-derived DC-formulations warrants further investigation in combination with currently approved adjuvant therapy options.
Electronic supplementary material
The online version of this article (10.1007/s00262-020-02618-4) contains supplementary material, which is available to authorized users.
Keywords: TriMixDC-MEL, Melanoma, Adjuvant therapy, Dendritic vaccine
Introduction
Patients diagnosed with melanoma macrometastases to the locoregional lymph nodes (definition by the 2009 American Joint Committee on Cancer [AJCC]) are at high risk of occult systemic metastases and melanoma recurrence following the resection of all lesions [2]. In an analysis of 3307 patients, the 5-year melanoma-specific survival was 59% for AJCC stage IIIB and 40% for stage IIIC melanoma patients [6]. Melanoma recurrence at 5 years has been reported to be 68% for IIIB disease and 89% for stage IIIC disease [15]. Also patients who present with resectable metastases at distant sites such as the skin or lymph nodes (AJCC stage IV-M1a), lung (IV-M1b) or visceral sites (IV-M1c) have a high risk of recurrence and melanoma-related death following resection [14].
Spontaneous anti-melanoma immune responses have been demonstrated against melanocyte differentiation antigens (e.g., tyrosinase, gp100, and melan-A/MART-1) and cancer-testis antigens (e.g., MAGE-A3, NY-ESO-1 and PRAME). Dendritic vaccines loaded against one or more of these MAAs are used to enhance this anti-tumor immunity. We previously demonstrated that the immune-stimulatory capacity of autologous monocyte-derived DCs can be optimized by co-electroporation with mRNA encoding CD40 ligand (CD40L), CD70, and a constitutively activated TLR4 (caTLR4) [4]. TriMixDC-MEL represents a monocyte-derived dendritic cell vaccines (moDC) formulation by electroporation of full-length melanoma-associated antigens (MAA) fused to an HLA class II targeting signal (one out of four MAAs (gp100, tyrosinase, MAGE-A3, or MAGE-C2 fused to DC.LAMP) encoding mRNA. This allows for the cellular processing and presentation of the full range of antigenic peptides within the MAA protein. The electroporation of full length MAA offers the potential benefit for immunization regardless of HLA type and overcomes the HLA-type restriction imposed by peptide vaccines. Additionally, MAA presentation in both HLA class I and class II molecules can be achieved by genetic fusion of the MAA-encoding sequence with a HLA class II-targeting sequence. The optimized TriMixDC-MEL is safe and immunogenic in melanoma patients [16, 21, 22]. In a phase IB study, durable tumor responses were observed in four out of 15 patients with pre-treated advanced melanoma when administrating TriMixDC-MEL both by the intravenous (iv) and intradermal (id) route [21]. The combination of iv and id administration was safe and promising in our previous studies [17, 21]. Adjuvant treatment moDC vaccine combined with interferon alpha-2b resulted in encouraging long-term overall survival rates with 2-year and 4-year survival rates of 93% and 70%, respectively [20].
We here report the results of a randomized phase II clinical trial investigating the effect of adjuvant treatment with TriMixDC-MEL in melanoma patients who had no evidence of disease following the resection of macrometastases.
Patients and methods
Patients
Patients with histologically confirmed, AJCC 2009 stage IIIB/C or IV melanoma, who had no evidence of disease following surgical treatment for macrometastases, were invited to participate in this clinical trial. Key eligibility criteria verified during the screening procedures were: release for clinical use of the TriMixDC-MEL product, age ≥ 18 years; World Health Organization performance status (WHO-PS) of ≤ two; normal hematological, liver and renal function tests; negative serological tests for HIV, syphilis, hepatitis B and hepatitis C, and no evidence for melanoma metastases on whole-body 18FDG-PET/CT. Exclusion criteria included prior treatment with an immune checkpoint inhibitor, history of severe autoimmune disease, primary uveal melanoma, and the need for permanent therapeutic anticoagulation. This trial was approved by the Institutional Ethics Committee of the UZ Brussel (ClinicalTrials.gov identifier: NCT01676779). All patients provided signed informed consent.
TriMixDC-MEL production
In brief, immature dendritic cells (DCs) were generated by culturing monocytes in the presence of 1% autologous plasma, 1000 U/ml GM-CSF, and 500 U/ml IL-4. Following leukapheresis, monocytes were enriched by plastic adherence. On day 6, DCs were harvested and co-electroporated with TriMix-mRNA (CD40L, CD70, and caTLR4 encoding mRNA) and mRNA encoding one of four MAAs (MAGE-A3, MAGE-C2, tyrosinase, or gp100) linked to an HLA class II targeting signal, as reported previously [5]. After electroporation, the four different TriMixDC-MEL cellular constituents (i.e., DCs expressing one of the four antigens) were mixed at equal ratios and cryopreserved. The manufacturing and release of the TriMixDC-MEL requires 4 weeks. The TriMixDCs were thawed 2–3 h before injection. An in-process, as well as quality control of the final product, was carried out, as reported previously [22].
Study design, treatment and assessments
Eligible patients underwent a leukapheresis for the procurement of PBMC that served for the production of the autologous TriMixDC-MEL product. Randomization was performed upon the release for clinical use of the TriMixDC-MEL product, in a 1:1 ratio to immediate adjuvant treatment with TriMixDC DC-MEL or a control arm. Patients on the control arm were allowed to “cross-over” and be treated with TriMixDC-MEL 1 year after randomization, or at the time of non-salvageable relapse. Randomization was stratified according to stage (stage IIIB/C vs. stage IV). TriMixDC-MEL was administered iv (20.106 DCs) and id (4.106 DCs) at two separate sites of the body every 2 weeks for a total of four administrations and a fifth administration after 16 weeks in patients randomized to the treatment arm of the study (figure S1). Patients in the control arm were not allowed to undergo any systemic adjuvant treatment that were available at the time this study was conducted (e.g., interferon-alfa2b).
Safety was assessed continuously throughout the study. Clinical and blood parameters were assessed at each TriMixDC-administration and every 16 weeks in follow-up phase. Adverse events (AEs) were graded for severity according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0).
Tumor evaluations on both study arms were performed by total-body 18-FDG-PET/CT every 16 weeks (week 16, 32 and 48 after the randomization). The primary endpoint was the percentage of patients who were alive and free from melanoma macrometastases at 1 year (12-month disease-free survival rate [1-year DFS]) following randomization. Patients were allowed to undergo local salvage treatments (surgery or stereotactic radiotherapy) for loco-regional melanoma recurrences during the study. If local salvage treatment during the study resulted in a disease-free status that was confirmed at any of the planned assessments, but not earlier than 16 weeks after the salvage treatment, these patients were considered not to have reached the primary endpoint of the study. Patients in the TriMixDC-arm that experienced a salvageable relapse were allowed to finish their TriMixDC treatment after local treatment. Previous experience with moDC-vaccines demonstrated durable relapse-free and overall survival following early relapses salvaged by surgery (< 3 months after start of TriMixDC-MEL administration) [19, 21]. Secondary endpoints were the recurrence-free survival (RFS), non-salvageable recurrence-free survival, distant metastasis free survival (DMFS) and overall survival (OS). RFS was defined as the time from randomization until the date of first recurrence (local, regional or distant metastasis) or last follow-up. OS was defined as the time from randomization until death from any cause or last follow-up. DMFS was defined as the time from randomization until the diagnosis of distant metastasis or last follow-up.
Materials and methods
Formalin-fixed paraffin-embedded (FFPE) tumor tissue samples from 14 patients of the adjuvant treatment arm were available for further analysis. The lesions closest to the randomization date were selected. Two response groups according to 1 year DFS were identified for further analysis: no relapse at 1 year and relapse at 1 year. Tumor cell enrichment was performed by macrodissection of four FFPE sections per sample (5 μm) prior to RNA extraction using the High Pure FFPET RNA isolation kit (Roche, Anderlecht, Belgium). The mRNA expression of 730 targets was analyzed with the nCounter PanCancer Immune Profiling panel (Nanostring Technologies, Seattle, WA, USA) on a Nanostring Analysis System (Nanostring Technologies). This commercial panel was extensively validated in-house for accuracy, repeatability and reproducibility before analyzing the study samples. The counts, generated per molecular ‘barcode’ (gene) by the nCounter system, were normalized using three different normalization methods. For the first normalization method, nSolver software (version 3) was used, correcting for the negative and positive controls as well as for the 40 housekeeping genes (HKG) present in the panel (using the geometric mean) (N40HKG). As second normalization, method (Q10HKG) quantile normalization was applied. In brief, raw data counts were LOG10 transformed and the percentage coefficient of variation (%CV) of the HKG was calculated. The mean of the 10 HKG (DDX50; FCF1; POLR2A; MTMR14; PRPF38A; CNOT4; TLK2; SF3A3; SDHA; ZNF143) with the lowest %CV was calculated and used to subtract from each target gene. In the third normalization method (Q5HKG), quantile normalization was performed using the five overlapping HKG (POLR2A, ABCF1, TBP, SDHA and G6PD) with the study from Ayers et al. [1].
All 14 available samples have been stained for programmed cell death ligand 1 (PD-L1) using the 22C3 PharmDX kit and scored by a qualified pathologist in accordance with the method described by Daud et al. [8].
Statistical analyses
A Fleming-one-stage design was used to calculate the sample size for the experimental arm of this trial. We assumed that in the absence of any adjuvant therapy 35% of patients would have no evidence of disease 12 months after the date of randomization (12-month disease-free survival rate; 12-month DFS%). We would consider the experimental treatment with TriMixDC-MEL to be of sufficient interest if the 12-month DFS% was > 55% arm. Allowing for a probability of Type I Error (alpha) of 10%, and a probability of Type II Error (beta) of 20% (providing a Power [1 − beta] of 80%), a total of 27 patients are required. An equal number of patients would be recruited to the contemporary control arm. Due to a lack of financial means to continue the study, patient recruitment was interrupted in October 2014. One year later, following an interim analysis with a minimal follow-up of 1 year for all randomized patients, it was decided to permanently stop the recruitment of patients.
Estimation of the survival endpoints and percentages (with their respective 95% confidence intervals) were calculated from the date of randomization to date that the endpoint was recorded using Kaplan–Meier estimates. The log-rank test was used to compare the survival between subgroups; hazard ratios were calculated according to the Cox proportional hazards model (SPSS20.0 software, Chicago, Illinois, USA).
The Nanostring mRNA variables were tested for predictive response, as single marker using logistic regression and the area under the curve (AUC) values. RNA expression FPKM (Fragments per Kilobase of transcript per Million mapped reads) and clinical data files were downloaded from the TCGA data portal from patients with skin cutaneous melanoma (date 14MAR2018). Only patients with stage III or IV disease were selected for further analysis since these are most representative to the study population. Overall survival (OS) was calculated using the data available in the clinical data file from TCGA, days until death or days until follow-up. The six promising Nanostring genes (from the Nanostring AUC analysis) were selected based on their ensemble number [ENSG00000170581 (STAT2); ENSG00000164825 (DEFB1); ENSG00000010278 (CD9); ENSG00000164400 (CSF2); ENSG00000240403 (KIR3DL2); ENSG00000172236 (TPSAB1)]. Patients were divided into low or high expression groups using the median expression as cutoff. Multivariate hazard ratio analysis was conducted for all six genes in combination with potential confounding clinical parameters (gender, age at diagnosis, age). PD-L1 expression was statistically evaluated using the nonparametric Wilcoxon test. All normalization and statistical analysis was performed in R (version 3.4.3).
Results
Patients
Between January 2013 and August 2014, 60 patients were screened (Figure S1). Forty-five patients who met the eligibility criteria underwent a leukapheresis for the production of their autologous TriMixDC-MEL product. Production failed in one patient, due to bacterial contamination and three patients were diagnosed with early recurrence of their melanoma prior to the availability of their product. Forty-one patients were randomized (21 were allocated to the TriMixDC-MEL arm and 20 to the control arm of the study). The baseline characteristics were well balanced between both treatment arms (Table 1). The majority of patients on both arms had resected stage IIIC disease, and an equal number of patients on both arms (three) was recruited after the resection of lung metastases. There were a numerically higher number of patients in the TriMixDC-MEL arm with an acral primary melanoma, stage IV-M1C, and who had received prior systemic therapy (interferon).
Table 1.
Baseline characteristics of the patients
| Variable | Control arm | Treatment arm |
|---|---|---|
| Total (male/female) | 20 (11/9) | 21 (11/10) |
| Median age–years (range) | 61 (48–76) | 54 (24–81) |
| Primary site | ||
| Extremities | 10 | 14 |
| Trunk | 4 | 2 |
| Head and neck | 3 | 1 |
| Acral | 0 | 2 |
| Unknown primary | 3 | 2 |
| Ulceration of primary melanoma | 5 | 7 |
| AJCC stage | ||
| IIIB | 2 | 4 |
| IIIC | 14 | 13 |
| IV-M1a | 1 | 0 |
| IV-M1b | 3 | 3 |
| IV-M1c | 0 | 1 |
| Prior therapy | ||
| Surgery | 20 | 21 |
| Adjuvant radiotherapy | 5 | 4 |
| Immunotherapy | ||
| Adjuvant high-dose IFN-α-2b | 1 | 3 |
| DC-vaccination | 0 | 1 |
| Mage.A3/AS15 peptide vaccine | 0 | 1 |
| BRAF V600 Mutation | ||
| Yes | 11 | 10 |
| No | 6 | 7 |
| Unknown | 3 | 4 |
Study treatment disposition
Twenty out of the 21 patients allocated to the TriMixDC-MEL arm of the study received all five planned administrations of TriMixDC-MEL. One patient died due to a melanoma and Treatment unrelated cause 4 weeks after the fourth TriMixDC-MEL administration. Three patients on the control arm received their TriMixDC-MEL administrations upon crossing-over 1 year after randomization. All were free from recurrence and received the planned five administrations.
Safety and tolerability
The safety population consisted of the 21 patients treated on the TriMixDC-MEL arm and the three patients who crossed over from the control arm. Treatment with TriMixDC-MEL was generally well tolerated, and no patient suffered from grade ≥ 3 treatment-related adverse events. The majority of patients (80%) reported swelling and erythema at the site of intradermal DC-injection that persisted for 2–4 days. These local reactions did not need treatment in any patient. Acute chills during the first hour after the intravenous administration of TriMixDC-MEL were observed in four (16%) patients. These were self-limited and resolved within 1 h without special care or medication. Post-treatment grade 1 flu-like symptoms persisting for 2–3 days were reported by four (16%) patients. No grade 3 adverse events were identified (Table 2).
Table 2.
Adverse events of the TriMixDC-Mel treatment (N = 24 patients) in the 21 patients treated on the TriMixDC-MEL arm and the three patients who crossed over from the control arm
| Adverse event | Grade 1 | Grade 2 | Grade 3–4 | ||
|---|---|---|---|---|---|
| # | % | # | % | # | |
| DC injection site local skin reaction | 4 | (16.7) | 14 | (58.3) | 0 |
| Flu-like symptoms | 5 | (20.8) | 0 | (0) | 0 |
| Chills | 3 | (12.5) | 2 | (8.3) | 0 |
| Fatigue | 5 | (20.8) | 1 | (4.2) | 0 |
| Headache | 2 | (8.3) | 0 | (0) | 0 |
| Thrombophlebitis at infusion site | 1 | (4.2) | 0 | (0) | 0 |
| Itch | 1 | (4.2) | 0 | (0) | 0 |
| Myalgia | 0 | (0) | 1 | (4.2) | 0 |
| Fever | 1 | (4.2) | 1 | (4.2) | 0 |
| Transpiration | 1 | (4.2) | 0 | (0) | 0 |
One-year disease-free survival rate, RFS, DMFS OS and crossover patients
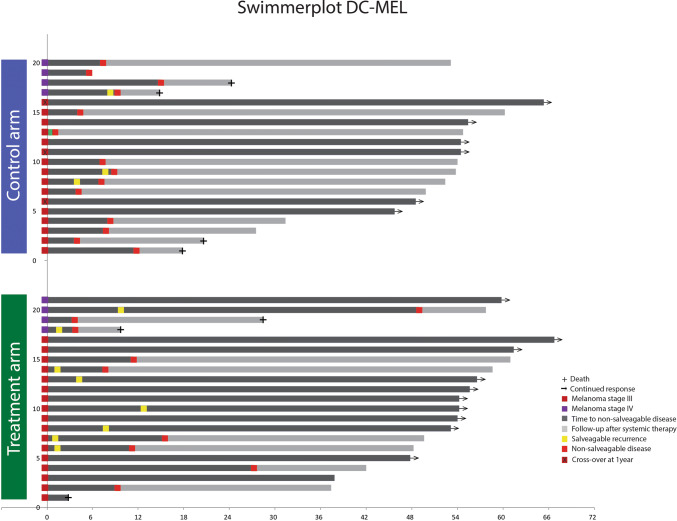
Twenty-six patients were diagnosed with a first melanoma recurrence (12 on the TriMixDC-MEL arm and 14 on the control arm) (Fig. 1). Time to first recurrence was not significantly different between both arms (median 8 months (range 6–8) vs. 13 months (range 0–38); p 0.500; Fig. 2a). There were more early recurrences occurring within the first 3 months on the TriMixDC-MEL arm (n = 4) as compared to the control arm (n = 1) (Figs. 1, 2a). A higher number of first recurrences could be effectively salvaged among patients on the TriMixDC-MEL arm (n = 7) as compared to the control arm. (Two patients in whom surgical salvage at first recurrence was attempted were diagnosed with unresectable disease progression within less than 4 months.) Fourteen (70%) patients on the control arm and nine (43%) patients on the TriMixDC-MEL arm were diagnosed with a non-salvageable melanoma recurrence within the first year following randomization (Fig. 2b). In addition, one patient on the TriMixDC-MEL arm died within the first year to a non-melanoma-related cause. The 1-year disease-free survival rate was 71% for the TriMixDC-MEL arm versus 35% for the control arm. After a median follow-up of 53 months (range 3–67), 23 patients were diagnosed with a non-salvageable melanoma recurrence (nine on the TriMixDC-MEL arm and 14 on the control arm). The time to non-salvageable recurrence or death was superior in the TriMixDC-MEL arm as compared to the control arm (median 8 months (range 1–6) vs. not reached; p 0.044).
Fig. 1.
Swimmer plot
Fig. 2.
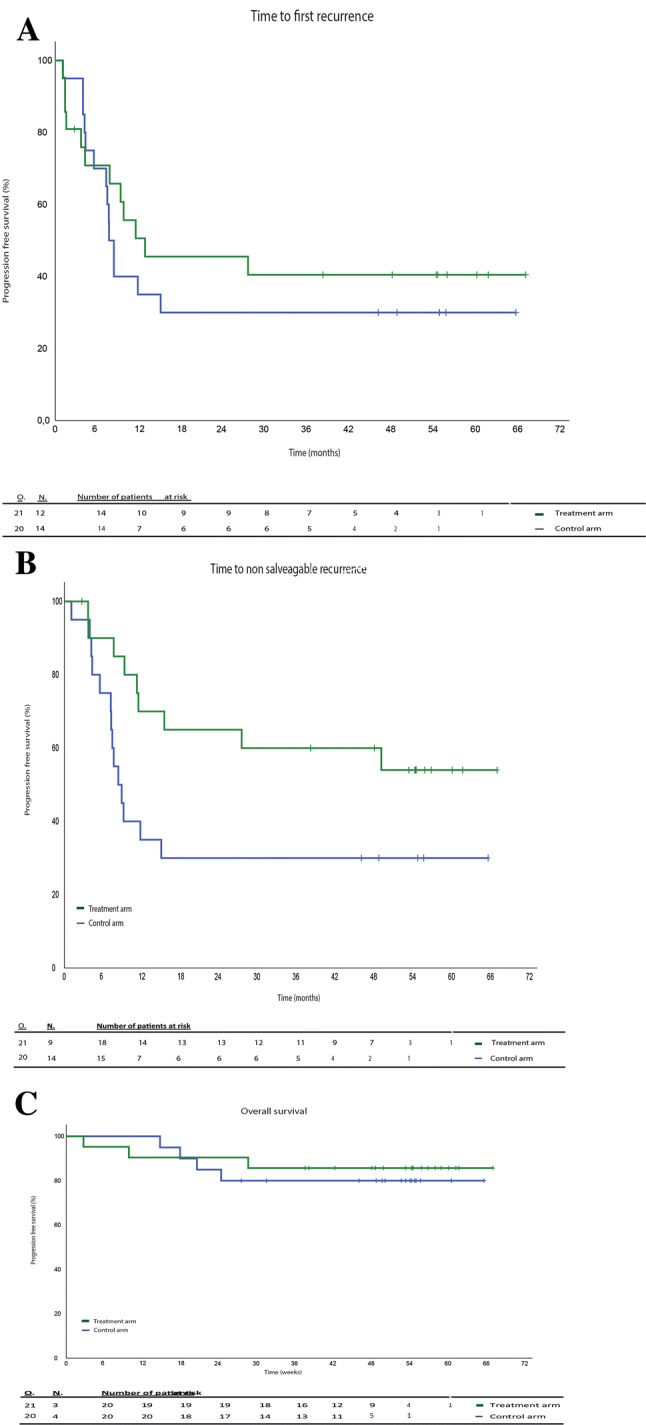
Survival analysis. a Appearance of first recurrence in weeks (p 0.5); b appearance of non-salvageable recurrence (p 0.0042), c overall survival in weeks (p 0.65)
The sites of salvageable and non-salvageable sites of recurrence on both study arms are listed in Supplementary Table S2. No significant difference was found in time to DMFS (p 0.164; estimated median in control arm 15 months (range 0–39), not reached in TriMixDC-MEL arm).
Three patients on the TriMixDC-MEL arm and four patients on the control arm died. At the exception of one 81-year patient who died from the consequences of a cerebrovascular stroke (without evidence of underlying brain metastases in the TriMixDC-MEL arm), all deaths are melanoma related (Fig. 2c). There was no significant difference in overall survival between both study arms. (Median overall survival was not reached in both study arms, p 0.65.)
In total, 3 out of 20 (15%) patients opted to have their DC treatment after 1 year of follow-up (as defined per protocol). Two had a stage IIIc disease at randomization, and one had a stage IIIb disease at randomization. Their median follow-up after randomization was 48 months (stage IIIc 48 and 65 months, stage IIIb patient 54 weeks); none of the three experienced a recurrence. These patients were censored in the Kaplan–Meier curves at 1 year.
Disposition of post-study treatment
Fourteen (65%) patients in the control arm and nine (43%) patients on the TriMixDC-MEL arm needed additional therapy for advanced melanoma. The time to off-study additional systemic treatment for advanced melanoma was significantly shorter on the control arm as compared to the TriMixDC-MEL arm (log rank 0.038). The median time to post-study systemic treatment is 8 months (range 4–12) in the control arm and 11 months (range 4–49) in the TriMixDC-MEL arm.
In the control arm, the first treatment was ipilimumab in seven (35%) patients (of which six (30%) received ipilimumab + dendritic vaccine), one (5%) received pembrolizumab, and four (20%) patients were treated with targeted therapy (combination of dabrafenib and trametinib). Two (10%) patients had repetitive in-transit metastasis that were resected on multiple occasions but eventually systemic therapy (targeted therapy in one (5%) and pembrolizumab in the other (5%)) was required.
In the TriMixDC-MEL treated arm, the first treatment following non-resectable relapse was ipilimumab in four (10%) patients, pembrolizumab in one (5%) patient and targeted therapy in 4 (10%) patients (combination of dabrafenib and trametinib).
mRNA expression analysis
Evaluation of the top 15 genes with the highest AUC value in the TriMixDC-MEL arm revealed six overlapping genes (Table 3, Figure S3) irrespective of the applied normalization method (N40HKG, Q10HKG, Q5HKG). These findings were further investigated using multivariate hazard ratio analysis on melanoma TCGA data (stage III and IV; n = 193). Significant differences in OS could be observed in the patient samples from TCGA based on the DEFB1 and KIR3DL2 gene expression. A low DEFB1 expression and high KIR3DL2 expression corresponded to an improved OS (respectively, p = 0.04 and p = < 0.001) in contrast to TPSAB1, STAT2, CD9 and CSF2.
Table 3.
Overview of the six genes with the highest AUC values in the TriMixDC-MEL arm compared to the AUC values in the control arm
| Gene | Area under the curve (AUC) | ||
|---|---|---|---|
| N40HKG normalization | Q10HKG normalization | Q5HKG normalization | |
| CSF2 | 1 | 0.867 | 0.822 |
| CD9 | 0.844 | 0.800 | 0.844 |
| DEFB1 | 1 | 0.911 | 0.889 |
| KIR3DL2 | 0.911 | 0.889 | 0.844 |
| STAT2 | 0.889 | 0.822 | 0.956 |
| TPSAB1 | 0.889 | 0.844 | 0.867 |
AUC area under the curve
PD-L1 expression analysis
For the TriMixDC-MEL arm, seven out 14 samples were not suitable for diagnostic purposes due to the high level of melanin pigment. No significant differences between the response groups (defined as no relapse at 1 year vs. relapse at 1 year, figure S2) could be detected.
Discussion
This randomized phase II trial suggest that adjuvant administration of the TriMixDC-MEL autologous monocyte-derived dendritic cell therapy can protect patients from a non-salvageable melanoma recurrence following the resection of macrometastases. Due to financial reasons, our study was closed prematurely and a lower number of patients were recruited than foreseen (20 instead of 27). This warrants caution while interpreting the results. Post hoc descriptive comparison indicates a statistical difference between the TriMixDC-MEL arm and control arm with a numeric 36% difference in the 1-year disease-free survival rate between the two study arms (71 vs. 35%). A numerically superior outcome between both study arms was also observed for the secondary endpoint of relapse-free survival; this difference, however, did not reach statistical significance. Of consideration is the higher incidence of early loco-regional relapses in the TriMixDC-MEL arm as compares to the control arm (respectively, four vs. one patient(s) were diagnosed with an early recurrence). Our prior observation made in studies with dendritic cell therapies following the resection of melanoma metastases had indicated a favorable outcome of patients who could be offered surgical salvage for early locoregional recurrences [20]. Observations made in patients affected by metastatic melanoma have also indicated that dendritic cell therapy can be associated with a latency of the therapeutic effect [7]. Therefore, taken into account the limitations of small cohort studies, we opted for 1-year disease-free survival rate as the primary endpoint for our study. This leaves room for further improvement upon the results obtained by considering the potential for combination with anti-melanoma therapies that have an instant anti-tumor effect such as the BRAF-/MEK-inhibitors in BRAF V600 mutant melanoma [11]. Our primary endpoint of 12-month disease-free survival was not affected by the possibility of crossover in the control arm; however, secondary endpoint such as OS and RFS might have been influenced. Given the study design requiring a leukapheresis in all patients prior to randomization, the non-comparative phase II clinical trial design with a primary endpoint not being influenced by cross-over, the demonstrated anti-tumor activity of TriMixDC-MEL in prior studies and the absence of active alternative treatment options, we felt it to be ethical to offer patients in the control arm the possibility of TriMixDC-MEL treatment at 1-year post-randomization or at the diagnosis of a non-salvageable recurrence.
DEFB1 and KIR3DL2 were significantly different in the study population and in TCGA patients. This might hint toward a potential prognostic marker. For TriMixDC-MEL treatment, STAT2, TPSAB1, CD9 and CSF2 were potentially identified in this study as predictive markers. These findings are novel and deserve further investigation. PD-L1 IHC staining was not associated with a predictive value in the current study using only the small subset of informative samples.
Treatment with TriMixDC-MEL was safe and well tolerated with no ≥ grade 3 treatment-related toxicities. The most common adverse event was a local swelling and skin inflammation at the injection site (≤ grade 2) which resolved spontaneously. This safety profile compares favorably with registered therapies for the adjuvant treatment of melanoma, namely interferon alfa-2b, pegylated interferon alfa-2b, ipilimumab, the PD-1 blocking mAb nivolumab and pembrolizumab, or the combination of dabrafenib and trametinib [10, 13].
Comparing outcome data across different dendritic vaccines trials is difficult since all dendritic vaccines are produced differently and baseline prognostic characteristics of the populations may differ, contributing to different results on OS and PFS. The inherent personalized nature of a dendritic vaccine makes large-scale industrial production and subsequent commercialization unattractive. Most of the trials are supported by university hospitals leading to a lack of large multicenter randomized control trials. In January 2016, Bol et al. demonstrated an overall survival benefit from the adjuvant treatment with DC after CLND (complete lymph node dissection) compared to a historical control group (63.6 months vs. 31.0 months, respectively) [3]. This impact on overall survival was also demonstrated by the group of Markowicz et al. with their peptide-loaded DC vaccine obtaining a 3-year OS of 68% compared to 26% in the 22 patients of the matched historical control group. However, their primary endpoint, 3-year PFS rate, was not significantly improved [12]. These data are comparable to our institutional data on long-term survival following the combination of moDC vaccines with INF- alpha-2b. Even though the difference in 1-year disease-free survival rate appears promising in this randomized trial, no difference in median overall survival could be established at the time of this analysis. This could relate to the low number of OS events at the time of this analysis. (Median overall survival is not reached in both cohorts.)
In 2018, the anti-PD-1 monoclonal antibodies nivolumab and pembrolizumab demonstrated to significantly improve RFS when compared, respectively, to ipilimumab or placebo as adjuvant therapy following the resection of melanoma lymph node metastases (with a 12-month RFS of, respectively, 70.5%, and 75.4% for nivolumab and pembrolizumab) [9–11, 18]. Numerically superior 1-year RFS results (88%) were obtained in a BRAF V600 mutant melanoma population with the BRAF/MEK inhibitors dabrafenib plus trametinib [11]. All three treatment options have been registered in this indication and represent the current standard of care. In the TriMixDC-MEL treatment arm, 71% of patients were free from disease at 1 year following treatment. Our study, however, allowed for local salvage treatment, and it should therefore be acknowledged that TriMixDC-MEL would likely be inferior with respect to protecting patients from a first recurrence when compared to the contemporary standard of care adjuvant treatment options. Therefore, combination strategies of TriMixDC-MEL plus an anti-PD-1 or BRAF-/MEK-inhibitor therapy would be of interest for further study. Moreover, the safety profile of TriMixDC-MEL compares favorable with available standard treatment options and would allow for exploring combination strategies.
It should be taken into account that this trial was initiated and recruited patients prior to the approval in 2018 of contemporary standard adjuvant treatment options. Previously, we reported encouraging results for the combination of TriMixDC-MEL and Ipilimumab investigated in a phase II clinical trial in patients with pre-treated advanced melanoma. This combination immunotherapy was found to be safe and resulted in an encouraging rate of durable complete remissions, currently ongoing after more than 6 years of follow-up in seven out of 39 patients treated on this trial [19]. Therefore, combination of TriMixDC-MEL or further optimized autologous monocyte-derived DC-formulations warrant further investigation in combination with currently approved adjuvant therapy options for patients who are at high risk of recurrence following the resection of melanoma metastases.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the patients who participated in the study, their families and caregivers. We would also like to thank Katrien Van den Bossche for assisting us with the data management.
Abbreviations
- %CV
Percentage coefficient of variation
- AJCC
American Joint Committee on Cancer
- AUC
Area under the curve
- CD40-L
CD40 ligand
- CLND
Complete lymph node dissection
- DC
Dendritic cells
- DMFS
Distant metastasis free survival
- FFPE
Formalin-fixed paraffin-embedded
- HKG
Housekeeping genes
- id
Intradermal
- iv
Intravenous
- OS
Overall survival
- RFS
Recurrence-free survival
- TCGA
The Cancer Genome Atlas
- TriMixDC-MEL
Autologous monocyte-derived mRNA electroporated dendritic cells
- WHO-PS
World Health Organization performance status
Authors’ contribution
YJ contributed to first draft, patient treatment, statistical analyses. VK contributed to patient treatment, revision of draft. JC contributed to manufacturing of study drug, revision of draft. KS contributed to analyses of samples, revision of draft, statistical analyses. TS contributed to patient treatment, revision of draft, statistical analyses. P-JVD contributed to analyses of samples, revision of draft, statistical analyses. CH contributed to manufacturing of study drug, revision of draft. LB contributed to patient treatment, revision of draft. MK contributed to analyses of samples, revision of draft, statistical analyses. KT contributed to concept of study, manufacturing of study drug, revision of draft, statistical analyses. BN contributed to concept of study, patient treatment, revision of draft, statistical analyses.
Funding
This study was funded by Rijksinstituut voor Ziekte- en Invaliditeitsverzekering Belgium.
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Compliance with ethical standards
Conflict of interest
Yanina Jansen received travel grant support from BMS, MSD, Pfizer. Vibeke Kruse provided consultation, attended advisory boards, and/or provided lectures for: Roche, Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, Amgen and Sanofi. My institution received honoraria for my contribution. Jurgen Corthals has no conflict of interest. Kelly Schats: KS is an employee of HistoGeneX NV which performs immunohistochemical and molecular testing for pharmaceutical companies as part of (pre-) clinical studies that evaluate new anticancer drugs. There is no other relevant affiliation or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Pieter-Jan Van Dam4: PJ Van Dam is an employee of HistoGeneX NV which performs immunohistochemical and molecular testing for pharmaceutical companies as part of (pre-) clinical studies that evaluate new anticancer drugs. There is no other relevant affiliation or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Teofila Seremet received congresses and advisory fees from Novartis and one preceptorship MSD. Carlo Heirman: CH is an inventor on patents related to TriMix and is employee of eTheRNA ImmunoTherapies and may therefore have a financial interest in the development of TriMix technologies. Lieve Brochez: Advisory Board Incyte 6/2017, Value-based health care project sponsored by Amgen. Mark Kockx: Mark Kockx is the CEO of HistoGeneX NV which performs immunohistochemical and molecular testing for pharmaceutical companies as part of (pre-) clinical studies that evaluate new anticancer drugs. There is no other relevant affiliation or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Kris Thielemans: KT is an inventor on patents related to TriMix and DC technology and is consultant for eTheRNA ImmunoTherapies and may therefore has a financial interest in the development of TriMix technologies. Bart Neyns: Honoraria-Bristol-Myers Squibb; Merck Sharp & Dohme; Novartis; Roche. Consulting or Advisory Role-Bristol-Myers Squibb; Merck Sharp & Dohme; Novartis; Roche. Speakers’ Bureau-Novartis. Travel, Accommodations, Expenses-Amgen; Bristol-Myers Squibb; Merck Sharp & Dohme; Novartis; Roche.
Ethical approval and consent to participate
This trial was approved by the Institutional Ethics Committee of the UZ Brussel (ClinicalTrials.gov identifier: NCT01676779). All patients provided signed informed consent.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ayers M, Lunceford J, Nebozhyn M, Murphy E, Loboda A, Kaufman DR, et al. IFN-γ–related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest. 2017;127(8):2930–2940. doi: 10.1172/JCI91190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balch CM, Gershenwald JE, Soong S, et al. Multivariate analysis of prognostic factors among 2,313 patients with stage III melanoma: comparison of nodal micrometastases versus macrometastases. J Clin Oncol. 2010;28:2452–2459. doi: 10.1200/JCO.2009.27.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bol KF, Aarntzen EHJG, in’t Hout FEM, et al. Favorable overall survival in stage III melanoma patients after adjuvant dendritic cell vaccination. Oncoimmunology. 2016;5:1–8. doi: 10.1080/2162402X.2015.1057673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonehill A, Tuyaerts S, Van Nuffel AMT, et al. Enhancing the T-cell stimulatory capacity of human dendritic cells by co-electroporation with CD40L, CD70 and constitutively active TLR4 encoding mRNA. Mol Ther. 2008;16:1170–1180. doi: 10.1038/mt.2008.77. [DOI] [PubMed] [Google Scholar]
- 5.Bonehill A, Van Nuffel AMT, Corthals J, et al. Single-step antigen loading and activation of dendritic cells by mRNA electroporation for the purpose of therapeutic vaccination in melanoma patients. Clin Cancer Res. 2009;15:3366–3375. doi: 10.1158/1078-0432.CCR-08-2982. [DOI] [PubMed] [Google Scholar]
- 6.Byrd DR, Sondak VK, Soong S, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206. doi: 10.1200/jco.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carrasco J, Van Pel A, Neyns B, et al. Vaccination of a melanoma patient with mature dendritic cells pulsed with MAGE-3 peptides triggers the activity of nonvaccine anti-tumor cells. J Immunol. 2014;180:3585–3593. doi: 10.4049/jimmunol.180.5.3585. [DOI] [PubMed] [Google Scholar]
- 8.Daud AI, Wolchok JD, Robert C, et al. Programmed death-ligand 1 expression and response to the anti-programmed death 1 antibody pembrolizumab in melanoma. J Clin Oncol. 2016;34:4102–4109. doi: 10.1200/JCO.2016.67.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eggermont AMM, Blank CU, Mandala M, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med. 2018;378:1789–1801. doi: 10.1056/NEJMoa1802357. [DOI] [PubMed] [Google Scholar]
- 10.Eggermont AMM, Chiarion-Sileni V, Grob J-J, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med. 2016;375:1845–1855. doi: 10.1056/NEJMoa1611299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Long GV, Hauschild A, Santinami M, et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med. 2017;377:1813–1823. doi: 10.1056/nejmoa1708539. [DOI] [PubMed] [Google Scholar]
- 12.Markowicz S, Nowecki ZI, Rutkowski P, et al. Adjuvant vaccination with melanoma antigen-pulsed dendritic cells in stage III melanoma patients. Med Oncol. 2012;29:2966–2977. doi: 10.1007/s12032-012-0168-1. [DOI] [PubMed] [Google Scholar]
- 13.Mocellin S, Lens MB, Pasquali S, et al. Interferon alpha for the adjuvant treatment of cutaneous melanoma. Cochrane Database Syst Rev. 2013 doi: 10.1002/14651858.cd008955.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ollila DW. Complete metastasectomy in patients with stage IV metastatic melanoma. Lancet Oncol. 2006;7:919–924. doi: 10.1016/S1470-2045(06)70938-X. [DOI] [PubMed] [Google Scholar]
- 15.Romano E, Scordo M, Dusza SW, et al. Site and timing of first relapse in stage III melanoma patients: implications for follow-up guidelines. J Clin Oncol. 2010;28:3042–3047. doi: 10.1200/JCO.2009.26.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Nuffel AMT, Benteyn D, Wilgenhof S, et al. Dendritic cells loaded with mRNA encoding full-length tumor antigens prime CD4 + and CD8 + T cells in melanoma patients. Mol Ther. 2012;20:1063–1074. doi: 10.1038/mt.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Nuffel AMT, Benteyn D, Wilgenhof S, et al. Intravenous and intradermal TriMix-dendritic cell therapy results in a broad T-cell response and durable tumor response in a chemorefractory stage IV-M1c melanoma patient. Cancer Immunol Immunother. 2012;61:1033–1043. doi: 10.1007/s00262-011-1176-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weber J, Mandala M, Del Vecchio M, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med. 2017;377:1824–1835. doi: 10.1056/NEJMoa1709030. [DOI] [PubMed] [Google Scholar]
- 19.Wilgenhof S, Corthals J, Heirman C, et al. Phase II study of autologous monocyte-derived mRNA electroporated dendritic cells (TriMixDC-MEL) plus ipilimumab in patients with pretreated advanced melanoma. J Clin Oncol. 2016;34:1330–1338. doi: 10.1200/JCO.2015.63.4121. [DOI] [PubMed] [Google Scholar]
- 20.Wilgenhof S, Corthals J, Van Nuffel AMT, et al. Long-term clinical outcome of melanoma patients treated with messenger RNA-electroporated dendritic cell therapy following complete resection of metastases. Cancer Immunol Immunother. 2015;64:381–388. doi: 10.1007/s00262-014-1642-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilgenhof S, Van Nuffel AMT, Benteyn D, et al. A phase IB study on intravenous synthetic mRNA electroporated dendritic cell immunotherapy in pretreated advanced melanoma patients. Ann Oncol Off J Eur Soc Med Oncol. 2013;24:2686–2693. doi: 10.1093/annonc/mdt245. [DOI] [PubMed] [Google Scholar]
- 22.Wilgenhof S, Van Nuffel AMT, Corthals J, et al. Therapeutic vaccination with an autologous mRNA electroporated dendritic cell vaccine in patients with advanced melanoma. J Immunother. 2011;34:448–456. doi: 10.1097/CJI.0b013e31821dcb31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.