Abstract
Background
Many anti-angiogenic agents have the potential to modulate the tumor microenvironment and improve immunotherapy. Anlotinib has demonstrated anti-tumor efficacy in non-small cell lung cancer (NSCLC) in third-line clinical trials. However, its roles in immune regulation and potentially synergistic anti-tumor effect in combination with immune checkpoint inhibition remain unclear.
Methods
Here, based on a syngeneic lung cancer mouse model, the intratumoral immunological changes post-anlotinib treatment in the model were assessed. Furthermore, it was tested whether anlotinib could enhance the anti-tumor effect of αPD-1 in vivo.
Results
This study shows that anlotinib increased infiltration of the innate immune cells, including natural killer (NK) cells, and antigen-presenting cells (APC), which include M1-like tumor-associated macrophages (TAM) and dendritic cells (DC), whereas the percentage of M2-like TAM was dramatically reduced. Subsequently, when combined with PD-1/PD-L1 (programmed cell death 1/PD-1 ligand 1) blockade, anlotinib conferred significantly synergistic therapeutic benefits.
Conclusions
Overall, these findings describe a role for anlotinib in the innate immune cells in the tumor microenvironment and a potentially synergistic anti-tumor combination with immune checkpoint inhibition.
Electronic supplementary material
The online version of this article (10.1007/s00262-020-02641-5) contains supplementary material, which is available to authorized users.
Keywords: Anlotinib, PD-1, Tumor immune microenvironment, NK cell, APC
Introduction
Lung cancer is the leading cause of cancer-related death worldwide, and non-small cell lung cancer (NSCLC) accounts for 80–85% of all cases [1, 2]. Approximately 75% of NSCLC cases are diagnosed at an advanced stage, resulting in a 5-year survival rate of < 15% [3]. The identification of tumor oncogenic gene alterations and immunotherapy have revolutionized the treatment paradigm of advanced NSCLC [4, 5]. Although first- and second-line therapy regimens confer great survival benefit to patients with NSCLC, drugs that strongly prolong progression-free survival (PFS) and overall survival (OS) in third-line therapy or third-line therapy and above remain limited.
Anlotinib is an oral multi-targeted tyrosine kinase receptor inhibitor (TKI) that selectively inhibits vascular endothelial growth factor receptor (VEGFR) 1–3, fibroblast growth factor receptor (FGFR) 1–4, platelet-derived growth factor receptor (PDGFR) α/β, c-KIT, and MET [6, 7]. Clinical trials have indicated that anlotinib significantly prolongs the PFS and OS of patients with NSCLC at third-line treatment or third-line treatment and above [8, 9]. Increasing data indicate that anti-angiogenic treatment has beneficial effects on tumor growth but also has favorable effects on reprogramming the immunosuppressive tumor microenvironment, which is due to the fact that angiogenic factors play important roles in immune regulation [10–13]. However, if anlotinib has an effect on the tumor immune microenvironment (TIME), it has yet to be characterized.
Currently, immunotherapy is a very promising therapeutic strategy for cancer. Since the first US Food and Drug Administration (FDA) approval of ipilimumab (anti-CTLA4 [cytotoxic T-lymphocyte–associated protein 4]) for melanoma, several such immune checkpoint blockade (ICB) agents have been approved for patients with advanced cancer, including NSCLC. The recent demonstration of ICB activity of the CTLA4 and PD-1 (programmed cell death 1) pathways in NSCLC have already shown excellent survival benefits, with long-term efficacy and less toxicity [14–17]. Despite the success of immune-based therapies, there is an urgent need for efforts to develop new therapeutic strategy combinations for enhancing the anti-tumor efficacy of ICB in lung cancer, as only about 20% of patients with NSCLC benefit from monotherapy overall [14, 18]. In recent years, the efficacy of anti-PD-1/PD-L1 (PD-1 ligand 1) blockade in combination with anti-angiogenesis has attracted much interest; several preclinical studies have reported promising anti-tumor effects following these combinations [10, 13, 19]. Trials investigating these combinations in patients with advanced NSCLC are ongoing, and the existing data are preliminary but promising [20, 21]. However, there remain challenges to be overcome before the full potential of combined anti-angiogenesis and immunotherapy can be achieved.
In the present study, the effect of anlotinib on the TIME in murine Lewis lung carcinoma (LLC) models, as well as its combination with an anti–PD-1 monoclonal antibody (mAb), was evaluated. It was observed that anlotinib could reprogram the immunosuppressive tumor microenvironment in a manner that augmented anti-cancer innate immune cells, including natural killer (NK) cells, dendritic cells (DC), and non-M2-like macrophages (MΦ). Moreover, potential additive or synergistic anti-tumor efficacy was observed when anlotinib was combined with anti-PD-1 mAb (αPD-1) in the in vivo experiment.
Materials and methods
Cell lines and reagents
LLC (Lewis lung carcinoma) cells were cultured with Dulbecco’s modified Eagle’s medium (Gibco, Waltham, MA, USA) supplemented with 10% fetal bovine serum (Gibco), 100 μg/mL penicillin, and 0.1 mg/mL streptomycin, and were maintained in a humidified chamber at 37 ℃ in a 5% CO2 atmosphere.
Anlotinib was provided by Chia Tai Tianqing Pharmaceutical Group (Nanjing, China). Anti-PD-1 (clone RMP1-14; 10 mg/kg) and isotype control antibodies (clone 2A3; 10 mg/kg) were purchased from Bioxcell (West Lebanon, NH, USA).
Cell proliferation analysis
Cell proliferation was determined using the MTT assay. LLC cells (10,000/well) were cultured in 96-well plates overnight and then treated with anlotinib. After MTT (Alfa Aesar, Haverhill, MA, USA) had been added at the indicated time points and incubated for 4 h, 200 μL dimethyl sulfoxide (DMSO) was added to resuspend the formazan. The absorbance was measured at 490 nm using a spectrometer (Synergy 2; BioTek, Winooski, VT, USA).
Animal experiments
Female C57BL/6 mice (6–8 weeks old) were maintained in an animal facility under pathogen-free conditions. After acclimatization, a total of 1 × 106 LLC cells were injected subcutaneously into the right flanks of the mice. Tumor dimensions were measured by calipers every 3 or 4 days, and tumor volume (mm3) was calculated as follows: (length × width2)/2. Anlotinib (1.5 mg/kg or 2.25 mg/kg) treatment was initiated 7 days after tumor cell inoculation and administered by oral gavage every day for 2 weeks. The αPD-1 was administered 11 days after tumor cell inoculation at 200 μg/mouse by intraperitoneal injection every 5 days. At the indicated days post-inoculation, the tumor-bearing mice were anesthetized, and the serum and tissues were harvested for analysis.
Immunohistochemistry (IHC) analysis
IHC staining was conducted according to the manufacturer’s protocol (Zhongshan Golden Bridge, Beijing, China). Formalin-fixed, paraffin-embedded tissue sections were deparaffinized, and after antigen retrieval, the slides were stained with hematoxylin and eosin (H&E) or with mouse mAbs against Ki67 (#ab16667, 1:500), CD31 (#ab9498, 1:50), VEGFA (#ab52917, 1:100), CXCR4 (#ab181020, 1:500), PD-1 (#ab214421, 1:200), PD-L1 (#ab238697, 1:500), and TIGIT (immunoreceptor tyrosine-based inhibition motif [ITIM] domains, #ab233404, 1:200). All antibodies were purchased from Abcam (Cambridge, UK).
Preparation of tumors for flow cytometry
The tumors were minced and digested in DMEM with collagenase IV (0.5 mg/mL, Sigma, St. Louis, MO, USA), hyaluronidase (1 mg/mL, Sigma), and DNase I (20 mg/mL, Sigma) at 200 rpm for 1 h at 37 ℃, and the suspensions were filtered through sieves. Erythrocytes were lysed in Red Blood Cell Lysis Buffer (Beyotime Biotechnology, Shanghai, China). Then, single cells were blocked with CD16/32 Ab (clone 93, eBioscience, San Diego, CA, USA, #14–0161-85) and stained with the following anti-mouse Abs for 30 min at 4 ℃: CD11b (allophycocyanin, clone M1/70, BioLegend (San Diego, CA, USA, #101212)), Gr-1 (peridinin chlorophyll protein/cyanine 5.5 [PerCP/Cy5.5], clone RB6-8C5, BioLegend, #108428), F4/80 (fluorescein isothiocyanate [FITC], clone BM8, BioLegend, #123108), CD206 (phycoerythrin [PE], clone C068C2, BioLegend, #141706), CD86 (allophycocyanin/Fire™ 750, clone GL-1, BioLegend, #105046), CD11c (PE/Cy7, clone N418, BioLegend, #117318), CD3e (PerCP, clone 145-2c11, BioLegend, #100326), NK1.1 (PE, clone PK136, BioLegend, #108708), CD4 (Alexa Fluor® 488, clone GK1.5, BioLegend, #100423), CD8a (allophycocyanin, clone 53–6.7, BioLegend, #100712), PD-L1 (PE, clone 10F.9G2, BioLegend, #278302), or isotype controls. For intracellular markers, the cells were incubated with 10 μg/mL brefeldin A (PeproTech, Rocky Hill, NJ, USA) for 4 h. After surface staining, the cells were fixed, permeabilized, and stained with the following anti-mouse mAbs for 30 min at 4 ℃: CD107a (PE/Cy7, clone 1D4B, BioLegend, #121620), interferon (IFN)-γ (PE or allophycocyanin, clone XMG1.2, BioLegend, #505808 or #505810), and interleukin (IL)-4 (PE/Cy7, clone 11B11, BioLegend, #504118).
Data were acquired using a BD FACSAria II instrument (BD Biosciences, San Jose, CA, USA) and analyzed using FlowJo software (Tree Star Inc., Ashland, OR, USA).
Statistical analysis
The data were analyzed using GraphPad Prism 8 (GraphPad Software, La Jolla, CA, USA). The values are presented as the mean ± standard error of the mean (SEM). The data were examined using the two-tailed Student’s t test, one-way analysis of variance (ANOVA), or two-way ANOVA with post hoc Bonferroni correction. Differences were considered significant at p < 0.05 and p < 0.01.
Results
Anlotinib induced anti-tumor effects in the LLC tumor models
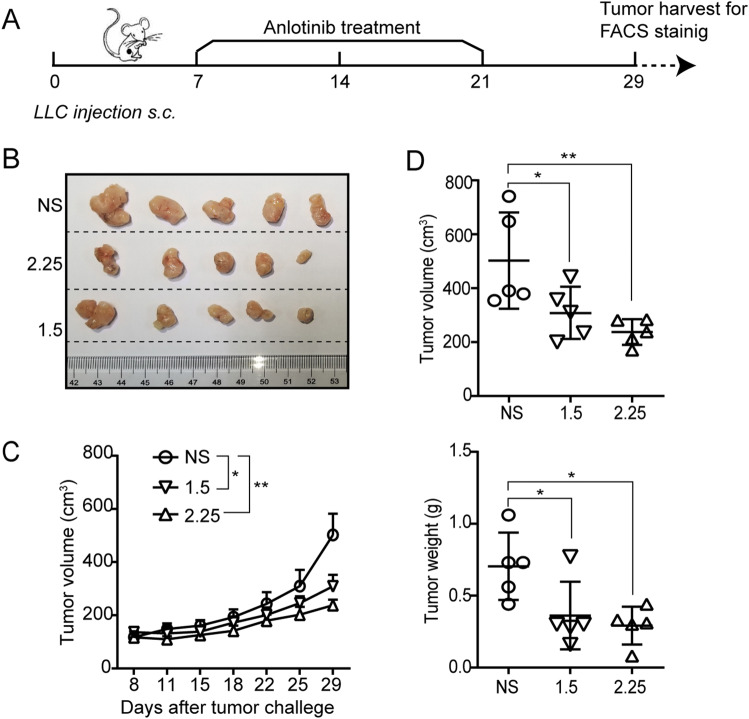
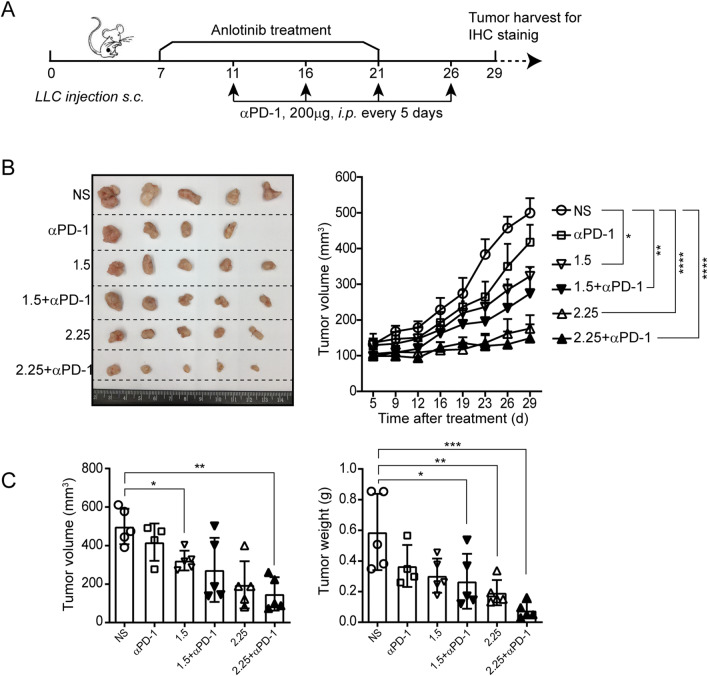
To study the tumor response to anlotinib in lung cancer, anlotinib to LLC-bearing mice were administered 7 days after tumor inoculation on the following schedules: 1.5 or 2.25 mg/kg anlotinib or normal saline (NS) treatment once a day for 2 weeks (Fig. 1a). As expected, anlotinib attenuated tumor growth significantly compared with the control at both dosages (2.25 mg/kg, hereafter referred to as “2.25”, and 1.5 mg/kg, references the recommended dose based on preliminary data [8], hereafter referred to as “1.5”) (Fig. 1b–d). Taken together, these results illustrate the anti-tumor activity of anlotinib in a mouse lung cancer model.
Fig. 1.
Anlotinib delays tumor progression. a Timeline of the animal experiments. b Photographs of tumors from each group. c The tumor growth curves in the mice. d Measurement of the tumor volume and weight. Results are the means ± SEM, n = 5. A two-tailed unpaired Student’s t-test was used for comparisons. For tumor growth curves, statistical analysis was performed using two-way ANOVA with Sidak correction for multiple comparisons. *p < 0.05, **p < 0.01
Anlotinib activated tumor-infiltrating NK cells
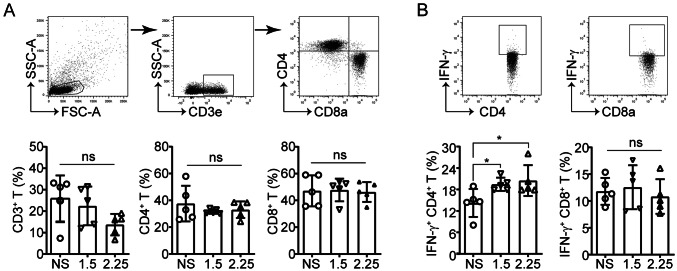
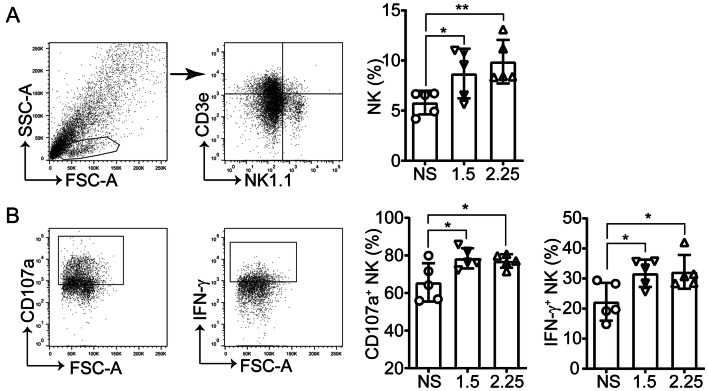
Various published studies have demonstrated that anti-angiogenic agents reprogram the immunosuppressive tumor microenvironment by facilitating immune cell trafficking and function [10, 13, 22]. Accordingly, the intratumoral immunological changes post-anlotinib treatment in the models via flow cytometry were assessed (Fig. 1a). First, lymphocyte, T cell, and NK cell infiltration and activation in the tumors were analyzed. Neither high- nor low-dose anlotinib treatment altered the proportion of CD4+ and CD8+ T cells in the tumors (Fig. 2a). It was noted, however, that anlotinib upregulated IFN-γ expression in CD4+ T cells but not in CD8+ T cells (Fig. 2b). Moreover, consistent with the inhibition of tumor growth, anlotinib significantly increased tumor-infiltrating NK cells (Fig. 3a), accompanied by an increase in the degranulation molecule CD107a and IFN-γ expression (Fig. 3b). These data suggest that, in comparison with the nuanced changes of T cells, NK cells appear to be more sensitive to anlotinib treatment.
Fig. 2.
No difference was observed in the percentages and activation of T cells in each anlotinib treatment group. a Representative dot plots showing T cell (CD3e+), CD4+ T cell (CD3e+CD4+), and CD8+ T cell (CD3e+CD8a+) tumor infiltration (top) and their percentages in the tumor-infiltrating lymphocytes (TIL) (bottom). b Flow cytometry analysis of IFN-γ in CD8+ and CD4+ T cells. Results are the means ± SEM, n = 5; one-way ANOVA. *p < 0.05
Fig. 3.
Anlotinib activates intratumoral NK cells. a Representative dot plots showing NK cell (CD3−NK1.1+) tumor infiltration (left) and the percentages in TIL (right). b Expression of CD107a and IFN-γ in NK cells. Results are the means ± SEM, n = 5, one-way ANOVA. *p < 0.05, **p < 0.01
Anlotinib increased tumor antigen presentation
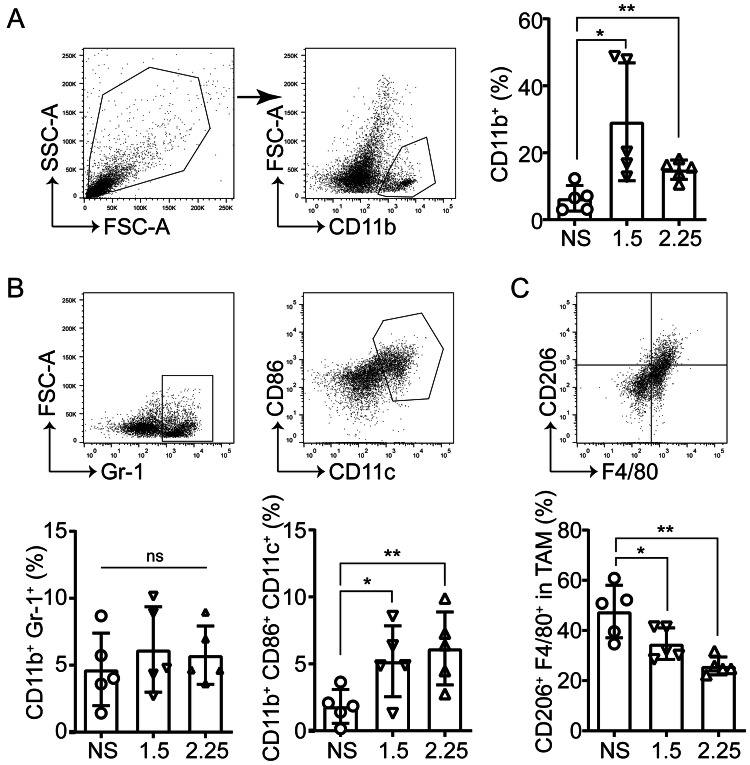
The effects of anlotinib on myeloid cells were analyzed, which could potentially enhance or inhibit the anti-tumor immune response in the TIME, and the myeloid-derived suppressor cells (MDSC), DC, and MΦ were included. It was found that there were significantly increased myeloid cells (measured by CD11b+) in the anlotinib groups compared to the control group (Fig. 4a). Simultaneously, it was observed that the intratumoral MDSC levels in the anlotinib groups remained comparable to that of the control group. However, the anlotinib-treated tumors had relative expansion of CD11c+ CD86+ antigen-presenting cells (APC), which included M1-like tumor-associated macrophages (TAM) and DCs (Fig. 4b). Next, CD206+ (marker of M2-like TAM) cells in the tumors were examined and dramatically reduced percentages of M2-like TAM in the anlotinib groups were found (Fig. 4c). These findings indicate that anlotinib can reprogram the intratumoral immune cell compartment toward increased tumor antigen presentation.
Fig. 4.
Anlotinib increases the intratumoral myeloid cells, including DC and non–M2-like macrophages. a Representative images of the gating strategy for defining myeloid cell (CD11b+) tumor infiltration (left) and the percentages in TIL (right). b Flow cytometry analysis of MDSC (CD11b+Gr-1+) and APC (CD11b+CD11c+CD86+) in TIL. c Percentages of M2-like TAM (CD206+). Results are the means ± SEM, n = 5, one-way ANOVA. *p < 0.05, **p < 0.01
Anlotinib improved the anti-tumor activity of PD-1 blockade
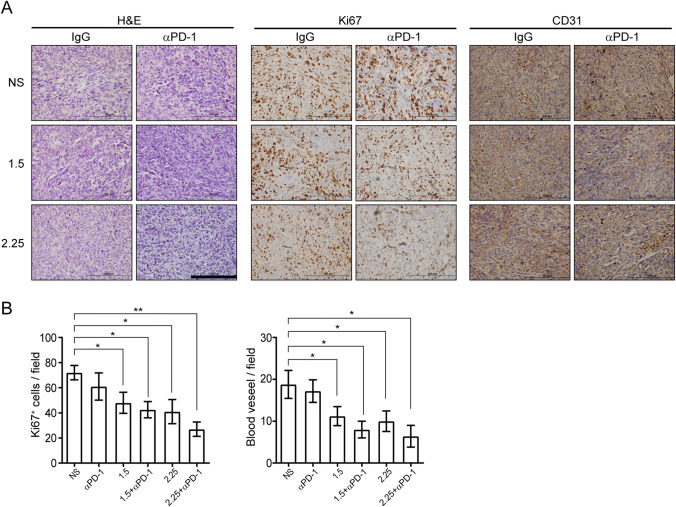
As reported above, anlotinib could convert the TIME from an immune-suppressive to immune-supportive phenotype by stimulating infiltration of the innate immune cells (NK cells and APC). Then, it was tested whether anlotinib could enhance the anti-tumor effect of αPD-1 in vivo. Anlotinib and αPD-1 to LLC-bearing mice were administered on the following schedules: anlotinib treatment, followed by the administration of αPD-1 or immunoglobulin G (IgG) control started on day 11 (Fig. 5a). It was observed that anlotinib plus αPD-1 treatment attenuated tumor growth significantly compared with the αPD-1 or anlotinib monotherapy group, and control group. Moreover, high-dose anlotinib plus αPD-1 treatment caused apparently significantly greater inhibition of tumor growth than low-dose anlotinib (Fig. 5b, c), which differs from the findings of other studies on other anti-angiogenesis agents [10, 13]. The body weight among all groups was not statistically different (data not shown), suggesting that there was no overt toxicity. Subsequently, the intratumoral proliferation and angiogenesis changes post-anlotinib treatment via IHC staining in the models were assessed. Consistent with the observation of tumor inhibition, intratumoral Ki67 and CD31 expressions were markedly decreased in the anlotinib plus αPD-1 group (Fig. 6a, b). Together, these results demonstrate that anlotinib enhances the therapeutic benefit of PD-1 blockade.
Fig. 5.
Anlotinib enhances the anti-tumor activity of PD-1 blockade. a Timeline of the animal experiments; blue arrows indicate injections of αPD-1. b (left) Photographs of tumors from each group; (right) tumor growth curves in the mice. (c)The tumor weights and volumes. Results are the means ± SEM, n = 5. A two-tailed unpaired Student’s t test was used for comparisons. For tumor growth curves, statistical analysis was performed using two-way ANOVA with Sidak correction for multiple comparisons. *p < 0.05, **p < 0.01
Fig. 6.
Combining anlotinib and PD-1 blockade inhibiting tumor proliferation and angiogenesis. a Representative images of H&E, Ki67, and CD31 staining in LLC-derived xenograft tumors. Images all were obtained at × 400 magnification; scale bar = 100 μm. b Quantification of CD31-positive vessels and Ki67-positive cells in the primary tumors. Results are the means ± SEM, n = 5, one-way ANOVA. *p < 0.05, **p < 0.01
Discussion
Various published findings position immune cells as key effectors of anti-angiogenic therapy and support the rationale for co-targeting angiogenesis and immune checkpoints in cancer therapy [10, 13, 19, 22]. Although clinical trials have suggested that anlotinib potentially has an anti-angiogenesis effect for inhibiting disease progression [8, 9], its roles in immune regulation have not been characterized. In the present study, anlotinib promoted the development and deployment of anti-tumor innate immunity in mouse models of lung cancer, increasing intratumoral active NK cell and APC infiltration (Figs. 2, 3, 4). Based on these immune-stimulatory properties, anlotinib potentiated anti-tumor activity when used in combination with PD-1 blockade in the LLC mouse models (Figs. 5, 6).
More precisely, various immune components in the TIME after anlotinib monotherapy were compared, and it was observed that anlotinib could increase active NK cell infiltration, accelerate APC recruitment, and reduce the percentage of M2-like TAM in the TIME (Figs. 3, 4), exhibiting a bona fide innate immune-modulating effect. These immune-stimulatory properties seemed to correlate positively with the anlotinib dose, which differs from the findings of others regarding other anti-angiogenesis agents [10, 13].
Angiogenesis inhibitors are conventionally intended to prune tumor vessels and starve cancer cells using high, tolerable doses in the clinic, which paradoxically may result in aggravating hypoxia and promoting tumor immunosuppression [23]. Previous research has suggested that pericyte coverage and vessel perfusion may be favorable for tumor vessel normalization [23, 24]. Subsequently, an increasing number of studies have suggested that a lower vascular normalization dose, rather than a high anti-vascular/anti-angiogenic dose, could be an effective strategy for tumor vessel normalization [10, 25]. Furthermore, based on the mutual regulation between tumor vessel normalization and the immune-stimulatory pathways [26], lower doses are superior to high doses for eliciting a relatively immune-supportive tumor microenvironment and reengineering the tumor microenvironment for active immunotherapies in the clinical setting [10, 13].
Different from the above anti-VEGF/VEGFR2 mAbs and VEGFR2 TKI, it was found that lower-dose anlotinib was not superior to high-dose anlotinib in tumor microenvironment modulation (Figs. 3, 4). This might be because anlotinib is a multi-targeted TKI; besides VEGFR1–3, it also targets FGFR1–4, PDGFRα/β, c-KIT, MET, RET, aurora-B, and DDR1 (discoidin domain receptor tyrosine kinase 1) [6, 7]. Notably, Tian et al. [26] showed a crucial role of IFN-γ–producing type 1 helper (Th1) cells rather than CD8+ T cells in tumor vessel normalization, where the changes are consistent with these findings (Fig. 2). Therefore, further studies should clarify tumor angiogenic vasculature, such as pericyte coverage and vessel perfusion.
There is growing evidence from preclinical studies that anti-angiogenic agents can enhance anti-tumor efficacy when combined with immunotherapy by altering lymphocyte or macrophage infiltration in a range of tumor types [10, 13, 21, 22]. Here, it was observed that anlotinib potentiated anti-tumor activity when used in combination with PD-1 blockade in LLC mouse models, and that high-dose anlotinib had a more significantly synergistic anti-tumor effect (Figs. 5, 6). In addition, evidence indicating that PD-1 blockade elicits an anti-tumor innate response directly through PD-1 expression on NK cells and myeloid cells (TAM and DCs) have emerged [27–29]. Hence, it appears that coactivation of the innate immune cells was due to the synergistic anti-tumor effects of anlotinib and αPD-1. However, further studies are needed to provide detailed mechanistic evidence.
Aside from PD-1, TIGIT has displayed the potential to reverse NK cell dysfunction in tumors and to boost anti-tumor immunity [30]. No statistically significant differences were found in PD-1 and TIGIT expression after anlotinib treatment (Fig. S1). However, other innate immune checkpoint receptors, such as NKG2A, T cell immunoglobulin- and mucin domain-containing molecule 3 (TIM-3), and lymphocyte activation gene 3 (LAG-3), should to be analyzed further.
Otherwise, PD-L1 expression may upregulated in response to CD4+ T and NK cell-derived IFN-γ (Figs. 2, 3). As the impact of anlotinib on LLC cell PD-L1 expression was not observed in vitro and in vivo (Fig. S2), it is speculated many other intracellular and extracellular signals potentially regulate the changes in PD-L1 expression.
This study has some limitations. Although CCL2, VEGFA, and CXCR4 can modulate the innate immune response [31–34], it was found that they were decreased in the anlotinib treatment groups (Fig. S3), and the present work does not illustrate more detailed mechanisms for the anlotinib-modulated TIME in lung cancer. In addition, other possible regulatory mechanisms, including the innate immune response modulators TLRs and STING, or the induced NK cell ligands [35–37], also warrant further investigation.
In summary, as an oral multi-targeted TKI, anlotinib exhibits efficacy, prolonging PFS and OS in patients with refractory advanced NSCLC [8, 9]. The present results reveal its innate immune-stimulatory properties, especially in stimulating intratumoral active NK cell and APC infiltration. These data provide preclinical evidence that anlotinib reprograms the tumor microenvironment from immuno-suppressive to immune-supportive and broadens the benefit of anti-PD-1 therapy. It should be noted that this work is limited by the capacity of animal models to mimic the human TIME and the hypothesis-generating nature of the study, suggesting that further evaluation in more mouse experimental systems and relative clinical trials are warranted.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- APC
Antigen-presenting cells
- DCs
Dendritic cells
- ICB
Immune checkpoint blockade
- IHC
Immunohistochemistry
- MDSC
Myeloid-derived suppressor cells
- TAM
Tumor-associated macrophage
- TIME
Tumor immune microenvironment
- TKI
Tyrosine kinase receptor inhibitor
Author contributions
YY designed, performed, and analyzed the experiments, and wrote the manuscript. LL, ZJ, and BW performed the experiments. YY, ZJ, and ZP provided the reagents and technical support. ZP designed and edited the manuscript. All the authors were involved in critical revision of the final manuscript.
Funding
This work was supported in part by grants from the National Natural Science Foundation of China (No. 81703786 and No. 81803914) and from the Tianjin Science and Technology Committee (No. 18JCZDJC36700).
Compliance with ethical standards
Conflict of interest
The authors declare no conflicts of interest regarding this article.
Ethical approval
All procedures involving animals were performed according to the Ethics Committee of Tianjin Medical University (Tianjin, China).
Animal source
Female C57BL/6 mice (6–8 weeks old) were purchased from Beijing HFK Bioscience (Beijing, China).
Cell line authentication
LLC (Lewis) murine lung carcinoma cells were purchased via the National Cancer Institute cell bank repository of China, and the cell line was authenticated there. Further, before use, the cell line tested negative for mycoplasma via the Mycoplasma ELISA Kit from Invitrogen (Carlsbad, CA, USA).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chen Z, Fillmore CM, Hammerman PS, Kim CF, Wong KK. Non-small-cell lung cancers: a heterogeneous set of diseases. Nat Rev Cancer. 2014;14(8):535–546. doi: 10.1038/nrc3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. GLobal cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Torre LA, Siegel RL, Jemal A. Lung cancer statistics. Adv Exp Med Biol. 2016;893:1–19. doi: 10.1007/978-3-319-24223-1_1. [DOI] [PubMed] [Google Scholar]
- 4.Kris MG, Johnson BE, Berry LD, Kwiatkowski DJ, Iafrate AJ, Wistuba II, Varella-Garcia M, Franklin WA, Aronson SL, Su PF, Shyr Y, Camidge DR, Sequist LV, Glisson BS, Khuri FR, Garon EB, Pao W, Rudin C, Schiller J, Haura EB, Socinski M, Shirai K, Chen H, Giaccone G, Ladanyi M, Kugler K, Minna JD, Bunn PA. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311(19):1998–2006. doi: 10.1001/jama.2014.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin B, Song X, Yang D, Bai D, Yao Y, Lu N. Anlotinib inhibits angiogenesis via suppressing the activation of VEGFR2, PDGFRbeta and FGFR1. Gene. 2018;654:77–86. doi: 10.1016/j.gene.2018.02.026. [DOI] [PubMed] [Google Scholar]
- 7.Xie C, Wan X, Quan H, Zheng M, Fu L, Li Y, Lou L. Preclinical characterization of anlotinib, a highly potent and selective vascular endothelial growth factor receptor-2 inhibitor. Cancer Sci. 2018;109(4):1207–1219. doi: 10.1111/cas.13536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han B, Li K, Zhao Y, Li B, Cheng Y, Zhou J, Lu Y, Shi Y, Wang Z, Jiang L, Luo Y, Zhang Y, Huang C, Li Q, Wu G. Anlotinib as a third-line therapy in patients with refractory advanced non-small-cell lung cancer: a multicentre, randomised phase II trial (ALTER0302) Br J Cancer. 2018;118(5):654–661. doi: 10.1038/bjc.2017.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han B, Li K, Wang Q, Zhang L, Shi J, Wang Z, Cheng Y, He J, Shi Y, Zhao Y, Yu H, Zhao Y, Chen W, Luo Y, Wu L, Wang X, Pirker R, Nan K, Jin F, Dong J, Li B, Sun Y. Effect of anlotinib as a third-line or further treatment on overall survival of patients with advanced non-small cell lung cancer: the ALTER 0303 Phase 3 randomized clinical trial. JAMA Oncol. 2018;4(11):1569–1575. doi: 10.1001/jamaoncol.2018.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang Y, Yuan J, Righi E, Kamoun WS, Ancukiewicz M, Nezivar J, Santosuosso M, Martin JD, Martin MR, Vianello F, Leblanc P, Munn LL, Huang P, Duda DG, Fukumura D, Jain RK, Poznansky MC. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci U S A. 2012;109(43):17561–17566. doi: 10.1073/pnas.1215397109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mortara L, Benest AV, Bates DO, Noonan DM. Can the co-dependence of the immune system and angiogenesis facilitate pharmacological targeting of tumours? Curr Opin Pharmacol. 2017;35:66–74. doi: 10.1016/j.coph.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 12.De Palma M, Biziato D, Petrova TV. Microenvironmental regulation of tumour angiogenesis. Nat Rev Cancer. 2017;17(8):457–474. doi: 10.1038/nrc.2017.51. [DOI] [PubMed] [Google Scholar]
- 13.Zhao S, Ren S, Jiang T, Zhu B, Li X, Zhao C, Jia Y, Shi J, Zhang L, Liu X, Qiao M, Chen X, Su C, Yu H, Zhou C, Zhang J, Camidge DR, Hirsch FR. Low-dose apatinib optimizes tumor microenvironment and potentiates antitumor effect of PD-1/PD-L1 blockade in lung cancer. Cancer Immunol Res. 2019;7(4):630–643. doi: 10.1158/2326-6066.CIR-17-0640. [DOI] [PubMed] [Google Scholar]
- 14.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhaufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crino L, Blumenschein GR, Jr, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O'Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR, Investigators K. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 16.Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols MC, Cortinovis DL, Leach J, Polikoff J, Barrios C, Kabbinavar F, Frontera OA, De Marinis F, Turna H, Lee JS, Ballinger M, Kowanetz M, He P, Chen DS, Sandler A, Gandara DR, Group OAKS Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee CK, Man J, Lord S, Cooper W, Links M, Gebski V, Herbst RS, Gralla RJ, Mok T, Yang JC. Clinical and molecular characteristics associated with survival among patients treated with checkpoint inhibitors for advanced non-small cell lung carcinoma: a systematic review and meta-analysis. JAMA Oncol. 2018;4(2):210–216. doi: 10.1001/jamaoncol.2017.4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D, Ready N, Gainor J, Aren Frontera O, Havel L, Steins M, Garassino MC, Aerts JG, Domine M, Paz-Ares L, Reck M, Baudelet C, Harbison CT, Lestini B, Spigel DR. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tartour E, Pere H, Maillere B, Terme M, Merillon N, Taieb J, Sandoval F, Quintin-Colonna F, Lacerda K, Karadimou A, Badoual C, Tedgui A, Fridman WH, Oudard S. Angiogenesis and immunity: a bidirectional link potentially relevant for the monitoring of antiangiogenic therapy and the development of novel therapeutic combination with immunotherapy. Cancer Metastasis Rev. 2011;30(1):83–95. doi: 10.1007/s10555-011-9281-4. [DOI] [PubMed] [Google Scholar]
- 20.Manegold C, Dingemans AC, Gray JE, Nakagawa K, Nicolson M, Peters S, Reck M, Wu YL, Brustugun OT, Crino L, Felip E, Fennell D, Garrido P, Huber RM, Marabelle A, Moniuszko M, Mornex F, Novello S, Papotti M, Perol M, Smit EF, Syrigos K, van Meerbeeck JP, van Zandwijk N, Chih-Hsin Yang J, Zhou C, Vokes E. The potential of combined immunotherapy and antiangiogenesis for the synergistic treatment of advanced NSCLC. J Thorac Oncol. 2017;12(2):194–207. doi: 10.1016/j.jtho.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Chen DS, Hurwitz H. Combinations of bevacizumab with cancer immunotherapy. Cancer J. 2018;24(4):193–204. doi: 10.1097/PPO.0000000000000327. [DOI] [PubMed] [Google Scholar]
- 22.Schmittnaegel M, Rigamonti N, Kadioglu E, Cassara A, Wyser Rmili C, Kiialainen A, Kienast Y, Mueller HJ, Ooi CH, Laoui D, De Palma M. Dual angiopoietin-2 and VEGFA inhibition elicits antitumor immunity that is enhanced by PD-1 checkpoint blockade. Sci Transl Med. 2017 doi: 10.1126/scitranslmed.aak9670. [DOI] [PubMed] [Google Scholar]
- 23.Jain RK. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell. 2014;26(5):605–622. doi: 10.1016/j.ccell.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ganss R. Tumour vessel normalization and immune checkpoint blockade: a new synergism. Immunol Cell Biol. 2017;95(6):497–498. doi: 10.1038/icb.2017.30. [DOI] [PubMed] [Google Scholar]
- 25.Hamzah J, Jugold M, Kiessling F, Rigby P, Manzur M, Marti HH, Rabie T, Kaden S, Grone HJ, Hammerling GJ, Arnold B, Ganss R. Vascular normalization in Rgs5-deficient tumours promotes immune destruction. Nature. 2008;453(7193):410–414. doi: 10.1038/nature06868. [DOI] [PubMed] [Google Scholar]
- 26.Tian L, Goldstein A, Wang H, Ching Lo H, Sun Kim I, Welte T, Sheng K, Dobrolecki LE, Zhang X, Putluri N, Phung TL, Mani SA, Stossi F, Sreekumar A, Mancini MA, Decker WK, Zong C, Lewis MT, Zhang XH. Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature. 2017;544(7649):250–254. doi: 10.1038/nature21724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsu J, Hodgins JJ, Marathe M, Nicolai CJ, Bourgeois-Daigneault MC, Trevino TN, Azimi CS, Scheer AK, Randolph HE, Thompson TW, Zhang L, Iannello A, Mathur N, Jardine KE, Kirn GA, Bell JC, McBurney MW, Raulet DH, Ardolino M. Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. J Clin Invest. 2018;128(10):4654–4668. doi: 10.1172/JCI99317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN, Gupta R, Tsai JM, Sinha R, Corey D, Ring AM, Connolly AJ, Weissman IL. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545(7655):495–499. doi: 10.1038/nature22396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim TS, Chew V, Sieow JL, Goh S, Yeong JP, Soon AL, Ricciardi-Castagnoli P. PD-1 expression on dendritic cells suppresses CD8(+) T cell function and antitumor immunity. Oncoimmunology. 2016;5(3):e1085146. doi: 10.1080/2162402X.2015.1085146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bi J, Tian Z. NK cell dysfunction and checkpoint immunotherapy. Front Immunol. 2019;10:1999. doi: 10.3389/fimmu.2019.01999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol. 2018;15(5):325–340. doi: 10.1038/nrclinonc.2018.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Du Four S, Maenhout SK, Niclou SP, Thielemans K, Neyns B, Aerts JL. Combined VEGFR and CTLA-4 blockade increases the antigen-presenting function of intratumoral DCs and reduces the suppressive capacity of intratumoral MDSCs. Am J Cancer Res. 2016;6(11):2514–2531. [PMC free article] [PubMed] [Google Scholar]
- 33.Lu J, Zhong H, Chu T, Zhang X, Li R, Sun J, Zhong R, Yang Y, Alam MS, Lou Y, Xu J, Zhang Y, Wu J, Li X, Zhao X, Li K, Lu L, Han B. Role of anlotinib-induced CCL2 decrease in anti-angiogenesis and response prediction for nonsmall cell lung cancer therapy. Eur Respir J. 2019 doi: 10.1183/13993003.01562-2018. [DOI] [PubMed] [Google Scholar]
- 34.Rivera LB, Meyronet D, Hervieu V, Frederick MJ, Bergsland E, Bergers G. Intratumoral myeloid cells regulate responsiveness and resistance to antiangiogenic therapy. Cell Rep. 2015;11(4):577–591. doi: 10.1016/j.celrep.2015.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sato-Kaneko F, Yao S, Ahmadi A, Zhang SS, Hosoya T, Kaneda MM, Varner JA, Pu M, Messer KS, Guiducci C, Coffman RL, Kitaura K, Matsutani T, Suzuki R, Carson DA, Hayashi T, Cohen EE. Combination immunotherapy with TLR agonists and checkpoint inhibitors suppresses head and neck cancer. JCI Insight. 2017 doi: 10.1172/jci.insight.93397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sen T, Rodriguez BL, Chen L, Corte CMD, Morikawa N, Fujimoto J, Cristea S, Nguyen T, Diao L, Li L, Fan Y, Yang Y, Wang J, Glisson BS, Wistuba II, Sage J, Heymach JV, Gibbons DL, Byers LA. Targeting DNA damage response promotes antitumor immunity through STING-mediated T-cell activation in small cell lung cancer. Cancer Discov. 2019;9(5):646–661. doi: 10.1158/2159-8290.CD-18-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang YX, Chen XT, Guo KY, Li YH, Wu BY, Song CY, He YJ. Sunitinib induces NK-kappaB-dependent NKG2D ligand expression in nasopharyngeal carcinoma and hepatoma cells. J Immunother. 2017;40(5):164–174. doi: 10.1097/CJI.0000000000000168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.