Abstract
Background
An elevated pre-treatment neutrophil to lymphocytes ratio (NLR) is associated with poor prognosis in various malignancies. Optimal cut-off is highly variable across studies and could not be determined individually for a patient to inform his prognosis. We hypothesize that NLR variations could be more useful than baseline NLR to predict progression-free survival (PFS) and overall survival (OS) in patients (pts) receiving anti-PD1 treatment.
Patients and methods
All pts with metastatic renal cell carcinoma (mRCC) and metastatic non-small cell lung cancer (mNSCLC) who received anti-PD1 nivolumab monotherapy in second-line setting or later were included in this French multicentric retrospective study. NLR values were prospectively collected prior to each nivolumab administration. Clinical characteristics were recorded. Associations between baseline NLR, NLR variations and survival outcomes were determined using Kaplan–Meier’s method and multivariable Cox regression models.
Results
161 pts (86 mRCC and 75 mNSCLC) were included with a median follow-up of 18 months. On the whole cohort, any NLR increase at week 6 was significantly associated with worse outcomes compared to NLR decrease, with a median PFS of 11 months vs 3.7 months (p < 0.0001), and a median OS of 28.5 months vs. 18 months (p = 0.013), respectively. In multivariate analysis, NLR increase was significantly associated with worse PFS (HR 2.2; p = 6.10−5) and OS (HR 2.1; p = 0.005). Consistent results were observed in each cohort when analyzed separately.
Conclusion
Any NLR increase at week 6 was associated with worse PFS and OS outcomes. NLR variation is an inexpensive and dynamic marker easily obtained to monitor anti-PD1 efficacy.
Electronic supplementary material
The online version of this article (10.1007/s00262-020-02637-1) contains supplementary material, which is available to authorized users.
Keywords: Neutrophil to lymphocyte ratio (NLR), NLR variation, Anti-PD1, Nivolumab, Renal cell carcinoma, Non-small cell lung cancer
Introduction
Non-small cell lung cancer (NSCLC) is one of the most commonly diagnosed cancer and remains the leading cause of cancer-related death worldwide. Renal cell carcinoma (RCC) accounts for 3% of all malignant disease in adult [1]. Despite progress in surgical resection, targeted therapy and immunotherapy, local and distal recurrences are common and 40% of patients will die from metastasis [2]. Both NSCLC and RCC patients have been offered exciting advances with the development of anti-PD-L1 therapies leading to a major shift in the treatment landscape. Nivolumab, an anti-PD1 checkpoint blocker is the current standard second-line treatment in mNSCLC and mRCC based on phase III randomized clinical trials results [3–5]. At the same time pembrolizumab was approved as the standard of care of first-line treatment in metastatic NSCLC patients with PD-L1 expression ≥ 50% [6]. Combination of anti-PD1 and anti-CTLA4 therapies had demonstrated significant improvement in terms of objective response rate (ORR) and PFS in both localizations and OS in mRCC, leading to the approvement as the new first-line standard of care option for mRCC with intermediate or poor IMDC risk as well as for mNSCLC harboring a high tumor mutational burden [7, 8].
Even though immune checkpoint blockers (ICB) are well-tolerated, by unbalancing the immune system, they may give rise to immune-related adverse events (irAEs) [9]. Almost 10% of patients receiving anti-PD/L1 therapy experience grade ≥ 3 irAEs [10] with some of them requiring immunosuppressive treatment beyond corticosteroid. In addition, when given as monotherapy, 35% and nearly 40% of patients experienced progressive disease in the pivotal trial of mRCC and NSCLC respectively [3–5]. Because of the potential toxicities and the lack of efficacy for many patients, biomarkers are needed for early identification of non-responders, to avoid useless and expensive treatments.
Inflammation and immunity are part of the Hallmarks of Cancer [11]. Chronic inflammation, mediated by cytokines and chemokines has been linked with genetic instability, angiogenesis, cell growth and survival whereas immune system, cytotoxic T and B cells play a major role in detecting and eliminating cancer cells [12–15]. Many biomarkers of inflammation, including C Reactive Protein (CRP) and neutrophil to lymphocyte ratio (NLR) have been investigated as prognostic factors. In various malignancies, an elevated pre-treatment NLR has been associated with poor prognosis [15–18]. Being cohort-dependent and time-dependent, the optimal cut-off for NLR is highly variable across studies. Thus for a given patient we cannot use its baseline NLR value to determine its prognostic.
Few data reported NLR value and/or variations for patients treated with anti-PD-1. Lalani et al. [19] showed that higher NLR at 6 weeks was a stronger predictor of ORR, PFS and OS than baseline NLR in mRCC pts receiving anti-PD1. We hypothesize that NLR variations could be more useful than baseline NLR to predict PFS and OS in patients receiving anti-PD1 in two different cohorts. We also explore the association between Best Overall Response (BOR) and NLR variations in mRCC pts.
Materials and methods
Study design and participants
We performed a multicenter, retrospective analysis in three French institutions of all consecutive patients treated with anti-PD1 nivolumab for metastatic NSCLC or mRCC who consented to participate. Data were collected from patient’s electronic medical records including patients’ clinical and laboratory characteristics, IMDC (International Metastatic Renal Cell Carcinoma Database Consortium risk factors) for mRCC patients, adverse events as well as date of radiological progression, date of death or last follow-up. NLR values defined as the absolute neutrophil count divided by the absolute lymphocyte count were prospectively stored in the patient’s electronic medical records prior each nivolumab administration and retrospectively collected for our study. NLR variation was defined as the variation between the baseline NLR value collected before the first nivolumab administration and the NLR values collected before each nivolumab administrations. Nivolumab was given intravenously every 2 weeks, at the dose of 3 mg/kg for pts included before May 2018 and at a flat dose of 240 mg afterwards. All toxicities were reported according to the Common Terminology Criteria for Adverse Events (CTCAE) of the National Cancer Institute using the v4.03. Patients who received corticosteroids at the time of NLR collection were excluded from this study. Patients with known hematologic disorders or concurrent infection at the time of nivolumab start were excluded from our analysis. According to the good clinical practices, for each time points, patients with a suspected or an active infection did not receive the nivolumab administration. Thus, no confounding parameters falsely elevated the collected blood parameters.
To demonstrate that NLR variations were associated with outcomes with anti-PD1 treatment whatever the tumor type, we decided to consider the same anti-PD1 across the two cohort, to limit the treatment heterogeneity. Indeed, pembrolizumab is only available for 1st line PD-L1+ NSCLC pts but not in mRCC.
Patients gave their oral, informed consent to participation in the study. The study has been declared to the CNIL on the following number: 2215794 v 0.
Outcome
Primary endpoint was the association between NLR variations and PFS and OS in patients treated with anti-PD-1 nivolumab for mNSCLC and mRCC. Tumor response and progression were assessed every 8–12 weeks per RECIST 1.1 (Response evaluation Criteria in Solid Tumor version 1.1). NLR variations were calculated between value from each new nivolumab administration and the baseline value.
Statistical analysis
Qualitative variables were reported as percentage, and quantitative variables as median. We investigated the impact of baseline NLR, NLR variation and others parameters on PFS and OS using Cox regression models, adjusted for ECOG-PS and IMDC group for mRCC cohort. Analyses were done on the whole cohort population after stratification on tumor site. Kruskal–Wallis test was used to compare baseline NLR and NLR variation according to the tumor response and according to the IMDC prognostic score. T test and Wilcoxon tests were used to compare quantitative variables.
PFS was defined as the time interval between first administration of nivolumab to radiographic or clinical progression or death, censored at last follow-up for patients who have not progressed. OS was defined as the time interval between the first administration of nivolumab and death from any cause. Patients who were alive at the time of the final analysis were censored at last follow-up. PFS and OS were evaluated using Kaplan–Meier’s method, and reported with their 95% confidence interval (CI). Survival outcomes were compared using the log-rank test.
All tests were two-sided and a p value of 0.05 or less was considered as statistically significant. All analyses were performed using R software Version 1.0.136.Boston, MA.
Results
Patients’ characteristics and outcomes
One hundred sixty-one patients (86 mRCC and 75 mNSCLC) were enrolled. Baseline characteristics are summarized in Table 1.
Table 1.
Patients’ baseline characteristics
| Characteristics | Renal cell carcinoma (n = 86) | Lung carcinoma (n = 75) | All patients (n = 161) |
|---|---|---|---|
| Age, years—median (range) | 67 (21.6–82) | 65 (31.2–86.7) | 66 (21.6–86.7) |
| Sex ratio, (M:F), n | 67:19 | 47:28 | 114: 47 |
| ECOG-PS, n (%) | |||
| 0–1 | 73 (85%) | 51 (67%) | 124 (77%) |
| 2 | 9 (10.5%) | 20 (27%) | 29 (18%) |
| ≥3 | 3 (3.5%) | 4 (6%) | 7 (5%) |
| Prior lines, n (%) | |||
| 1 | 42 (49%) | 38 (51%) | 80 (49%) |
| 2 | 27 (31%) | 19 (25%) | 46 (29%) |
| ≥3 | 17 (20%) | 18 (24%) | 35 (22%) |
| Metastatic sites, n (%) | |||
| Lung | 58 (67%) | 63 (84%) | 121 (75%) |
| Liver | 16 (19%) | 12 (16%) | 28 (17%) |
| Bone | 35 (41%) | 31 (41%) | 66 (41%) |
| Brain | 3 (4%) | 19 (25%) | 22 (14%) |
| IMDC, n (%) | |||
| Favorable | 24 (28%) | ||
| Intermediate | 46 (53%) | ||
| Poor | 9 (11%) | ||
| Unknown | 7 (8%) | ||
| NLR, median 95% CI | 3.26 (1–37) | 3.4 (1.4–13) | 3.4 (1–37) |
| Albumin g/l, median (range) | 38 (16–47) | 38.4 (29–48) | 38 (16–48) |
| Serum CRP mg/dl, median (range) | 20.20 (1–216) | 24 (1.1–171) | 22 (1–216) |
| Neutrophils/mm3 , median (range) | 4310 (1230–13390) | 4898 (1810–15520) | 4600 (1230–15520) |
| Lymphocytes/mm3, median (range) | 1284 (200–3300) | 1323 (558–2863) | 1320 (200–3300) |
| Platelets/mm3, median (range) | 261,000 (115,000–654,000) | 277,000 (79,000–693,000) | 267,000 (79,000–693,000) |
| PFS (months) 95% CI | 4.6 (3.8–9) | 4.4 (3.5–6.6) | Not evaluated |
| OS (months) 95% CI | 24.7 (18.9–NA) | 16.8 (14.1–27.4) | Not evaluated |
ECOG-PS Eastern Cooperative Oncology Group, IMDC International metastatic renal cell carcinoma data base consortium, NLR neutrophils to lymphocytes ratio, CRP C-reactive protein, PFS progression-free survival, OS overall survival
In the whole cohort, median age was 66 (range 21.6–86.7) and 71% (n = 114) of patients were male. One hundred twenty-four patients (77%) were ECOG-PS 0 or 1 and 49% (n = 80) had received two or more prior therapeutic lines. Most common metastatic sites were lung (75%, n = 121) and bone (41%, n = 66).
In mRCC cohort, IMDC risk group’s distribution was as follow: 28% favorable risk (n = 24), 53% intermediate risk (n = 46) and 11% poor risk (n = 9). After a median follow-up of 24.7 months (range 0.2–47), median PFS and OS were 4.6 months (95% CI 3–8.9) and 24.7 months (95% CI 18.9–NR), respectively.
In mNSCLC cohort, after a median follow-up of 16.8 months (range 0.4–41), median PFS and OS were 4.4 months (95% CI 3.5–6.6) and 16.8 months (95% CI 14.1–27.4), respectively.
Association of NLR variations and survival outcomes in the whole cohort
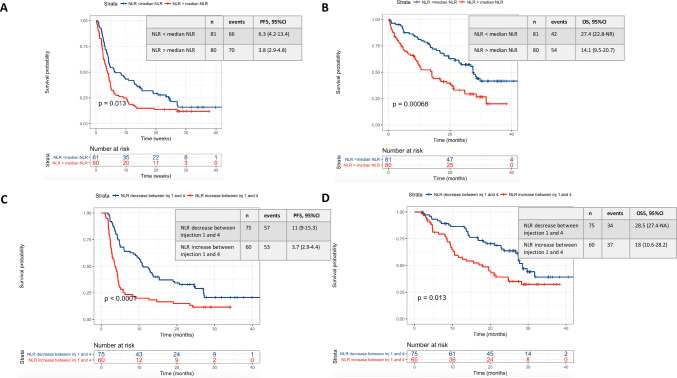
Median NLR at baseline was 3.4 (range 1–37) and 3 (range 0.97–13.4) at week 6. Less than median baseline NLR was significantly associated with a longer median PFS and OS compared to greater or equal to median (data shown in Fig. 1a, b).
Fig. 1.
PFS and OS among all patients according to baseline NLR (a, b) and NLR variations between the first and the fourth administration (c, d). a Is referring to the PFS of patients of the whole cohort according to the baseline NLR value (below or above the median NLR baseline value in the whole cohort). b Is referring to the OS of patients of the whole cohort according to the baseline NLR value (below or above the median NLR baseline value in the whole cohort). c Is referring to the PFS of patients of the whole cohort according to the NLR variation between the first and the fourth nivolumab administration. d Is referring to the OS of patients of the whole cohort according to the NLR variation between the first and the fourth nivolumab administration
NLR variations were explored from baseline (before first administration) to each administration of nivolumab until the date of the first CT-scan (between week 8 and 12). We dichotomized NLR variations into two categories: any increase vs. any decrease. Associations between variations and survival outcomes were analyzed from baseline to each administration until week 6. The better hazard ratio for decrease vs. increase was found to be at week 6 (Supplementary Data Table 1). NLR variations between baseline and week 6 was summarized as varNLRw1-6.
Pts with any NLR decrease at week 6 had a significant better PFS and OS than those with an NLR increase with a median PFS of 11 months (95% CI 9.1–13) vs. 3.7 months (95% CI 2.9–4.4) (p < 0.0001), respectively. Median OS was 28.5 months (95% CI 27.4–NR) vs. 18 months (95% CI 10.6–28.2) (p = 0.013), respectively (Fig. 1c, d). Analyses according to quartiles of NLR variations between baseline and week 6 (varNLRw1-6) confirmed that any increase vs. any decrease was the best predictor of a worse outcome (Supplementary Data Fig. 1).
In univariate analysis, together with varNLRw1-6, an ECOG-PS ≥ 2 and a poor IMDC prognostic score were significantly associated with poor PFS and OS. Hazard Ratio (HR) for PFS were respectively 2.2 (95% CI 1.4–3.1), (p = 0.0001), 2.1 (95% CI 1.4–3.1) (p = 0.001) and 4.1 (95% CI 1.8–9.5), (p = 0.0005) for varNLRw1-6, an ECOG-PS ≥ 2 and a poor IMDC prognostic score. HR for OS were respectively 2.3 (95% CI 1.4–3.8), (p = 0.001), 3 (95% CI 2.4–5.8) (p = 2.10–9) and 4.3 (95% CI 12.4–17), (p = 0.0002) for varNLRw1-6, an ECOG-PS ≥ 2 and a poor IMDC prognostic score (Supplementary Data Fig. 2). Tumor type, number of prior therapeutic lines, sex and age were not associated with survival outcomes. No baseline characteristic was associated with NLR increase (Supplementary Data Fig. 3).
In multivariate analysis, ECOG-PS ≥ 2, high-baseline NLR and positive varNLRw1-6 were independently associated with worse PFS and OS (Table 2).
Table 2.
Association of baseline characteristics and survival outcomes with multivariate Cox regression models
| Multivariate analysis all patients | ||
|---|---|---|
| PFS | OS | |
| HR 95% CI p value | HR 95% CI p value | |
| Median NLR (ref: NLR < median NLR) | 1.4 (1.01–2.1), p = 0.05* | 1.8 (1.2–2.9), p = 0,019* |
| NLR variations (ref: decrease) | 2.2 (1.5–3.4), p = 5.9.10–5* | 2.1 (1.2–3.4), p = 0.005* |
|
ECOS-PS (ref: 0–1) ECOG-PS > 2 |
2.1 (1.4–3.1), p = 0.0004* | 3.2 (1.7–5.7), p = 0.0001* |
| Tumor type (ref: RCC) | 1.02 (0.7–1.5), p = 0.9 | 0.98 (0.8–1.7), p = 0.95 |
| Age (ref : < 65 years) | 0.99 (0.7–1.5), p = 0.98 | 1.25 (0.7–2.1), p = 0.4 |
Multivariate analysis was adjusted for PS-ECOG. Baseline NLR was also included as a covariate for the analysis of NLR kinetics.
ECOG-PS Eastern Cooperative Oncology Group-Performance Status, NLR neutrophils to lymphocytes ratio, PFS Progression-Free Survival, OS Overall Survival, RCC renal cell carcinoma
*p<0.05
Regarding safety, no new safety signal was reported as compared to pivotal phase III trial [3–5]. No significant association was observed between incidence of ≥ grade 3 adverse events and baseline NLR or NLR variations. Among patients who experienced grade 3–4 adverse events, median baseline NLR was 3.4 vs 3.3 for those who did not experience grade 3–4 adverse events (p = 0.9). VarNLRw16 was − 4% for patients experiencing grade 3–4 adverse events vs − 27% for those who did not experience severe toxicities (p = 0.2). In the same way, no significant association was observed between incidence of ≥ grade 3 adverse events and survival outcomes. Median PFS were respectively 6 months (95% CI 2.9–27.2) and 4.3 months (95% CI 3.8–5.1), (p = 0.3) for patients experiencing severe toxicities vs those who did not. Median OS were respectively 13.6 (95% CI 6.7–NR) vs 23 (95% CI 18–28.5), (p = 0.13) for patients experiencing severe toxicities vs those who did not.
Association of NLR variations and survival outcomes in mNSCLC and mRCC cohorts
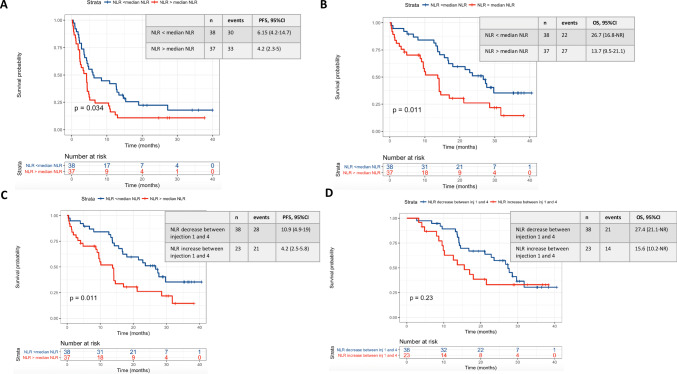
For mNSCLC cohort, median PFS and median OS were respectively 10.9 months (95% CI 4.9–19) vs 4.2 months (95% CI 2.5–5.8) (p < 0.01) and 27.4 months (95% CI 21.1–NR) vs 15.6 months (95% CI 10.2–NR) (p = 0.23) for patients with any NLR decrease vs. increase at week 6 (Fig. 2). In multivariate analysis, any NLR increase at week 6 remained independently associated with worse PFS (HR 2.5, p = 0.002) and OS (HR 2.2, p = 0.049).
Fig. 2.
PFS and OS among lung carcinoma according to baseline NLR (a, b) and NLR variations between the first and the fourth administration (c, d). a Is referring to the PFS of patients of the lung carcinoma cohort according to the baseline NLR value (below or above the median NLR baseline value in the lung carcinoma cohort). b Is referring to the OS of patients of the lung carcinoma cohort according to the baseline NLR value (below or above the median NLR baseline value in the lung carcinoma cohort). c Is referring to the PFS of patients of the lung carcinoma cohort according to the NLR variation between the first and the fourth nivolumab administration. d Is referring to the OS of patients of the lung carcinoma cohort according to the NLR variation between the first and the fourth nivolumab administration
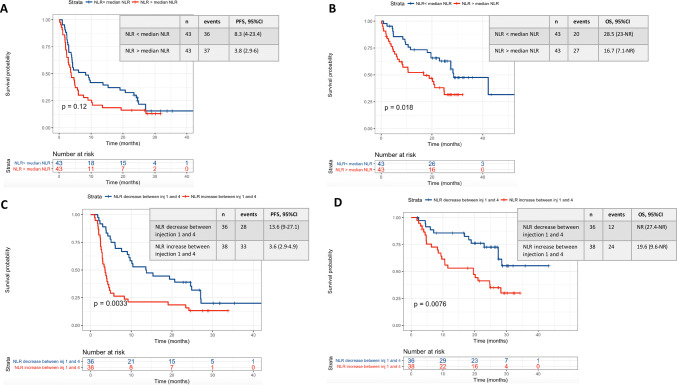
For mRCC cohort, median PFS and median OS were respectively 13.6 months (95% CI 9–27.1) vs. 3.6 months (95% CI 2.9–4.9) (p = 0.003) and NR (27.4–NR) vs. 19.6 months (95% CI 9.6–NR) (p = 0.008) for patients with any NLR decrease vs. increase (Fig. 3). In multivariate analysis, any NLR increase at week 6 remained independently associated with worse PFS (HR 2.2, p = 0.01) and OS (HR 1.7, p = 0.09). Other factors independently associated with worse PFS and OS were ECOG-PS ≥ 2 (HR 5.5, p = 2.5·10–5 and HR 6, p = 0.0001, respectively) and poor IMDC prognostic score (HR 4.2, p = 0.006 and HR 2.5, p = 0.07 respectively) (Supplementary Data Table 2).
Fig. 3.
PFS and OS among renal cell carcinoma according to baseline NLR (a, b) and NLR variations between the first and the fourth administration (c, d). a Is referring to the PFS of patients of the renal cell carcinoma cohort according to the baseline NLR value (below or above the median NLR baseline value in the renal cell carcinoma cohort). b Is referring to the OS of patients of the renal cell carcinoma cohort according to the baseline NLR value (below or above the median NLR baseline value in the renal cell carcinoma cohort). c Is referring to the PFS of patients of the renal cell carcinoma cohort according to the NLR variation between the first and the fourth nivolumab administration. d Is referring to the OS of patients of the renal cell carcinoma cohort according to the NLR variation between the first and the fourth nivolumab administration
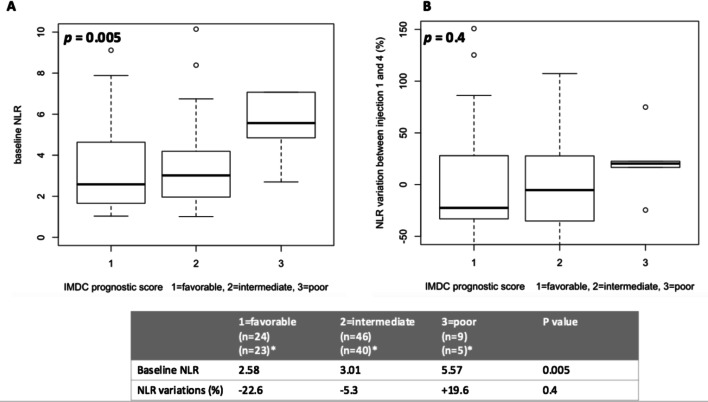
Interestingly, if baseline NLR was significantly higher in the poor IMDC risk group compared to those with favorable or intermediate risk (p = 0.005, Fig. 4), no correlation was identified between varNLRw1-6 and IMDC group (p = 0.4, Fig. 4).
Fig. 4.
Baseline NLR (a) and NLR variations between the first and the fourth administration (b) according to IMDC prognostic group among the renal cell carcinoma cohort. Among the renal cell carcinoma cohort, the baseline NLR value is significantly different between the IMDC prognostic groups. The NLR variation between the first and the fourth nivolumab administration is not significantly different between the three groups. Nonetheless, the poor IMDC group tends to harbor an NLR increased which is not the case of both the favorable and intermediates groups
Association of NLR variation and best overall response in mRCC patients
At Georges Pompidou Hospital, all mRCC pts treated with ICB have a second and blinded radiologic review by an international expert (LF) to confirm RECIST 1.1. 84/86 (97.7%) mRCC patients included in this study had a robust tumor response evaluation using RECIST 1.1 criteria allowing us to evaluate association between NLR variations and BOR.
The overall response rate was 17% (2 CR, 12 PR), and 35% (n = 29) of patients had a stable disease.
Baseline NLR was significantly higher in patients with progressive disease (PD) compared to those with stable disease (SD) or partial and complete response (PR/CR) (p = 0.048, Supplementary Data Fig. 4).
Disease control rate was higher in pts with any NLR decrease at week 6 as compared to pts with any NLR increase, 81% vs 40% (p = 0.0007) respectively.
Discussion
To our knowledge, we report here the largest cohort investigating the prognostic value of early NLR variations and its utility in every practice in patients receiving anti-PD1 monotherapy either for mRCC or mNSCLC. We found the commonly accepted relationship between high-baseline NLR value and poor survival outcomes. As reported in various cohorts of pts treated with chemotherapy, NLR optimal cut-off is hard to define and time- and cohort-dependent, limiting its utility in clinical practice. As mentioned above, Lalani et al. first demonstrated that for mRCC patients treated with anti-PD1/L1, a 25% increase in NLR at 6-weeks was associated with reduced ORR, shorter PFS (p < 0.001) and OS (p = 0.004) being in line with our results [19]. In 2018, Khunger et al. demonstrated that NLR increased in NSCLC non-responders after two cycles of nivolumab by 6.6 as compared to responders (p = 0.03) [20]. Focusing on patients with advanced solid tumors on phase 1 trials of PD-(L)1 inhibitors, Ameratunga et al. showed that NLR reduced over time in responders to immunotherapy: patients with CR/PR had a − 0.09 (p = 0.01) change in transformed NLR per months [21]. Focusing on NSCLC, main studies related to this question (including Bagley et al. [22], Kiriu et al. [23], Diem et al. [24], Nakaya et al. [25], or Shiroyama et al. [26]) identified an elevated pre-treatment NLR as a poor prognostic factor for NSCLC pts receiving nivolumab. Nonetheless, results were not totally unequivocal since baseline NLR could be associated with PFS, or OS, or both PFS and OS, based on the selected study. Main limitation of such studies is the heterogeneity of the NLR cut-off used to stratify the patients : NLR ≥ 5 for Bagley et al. and Suh et al., NLR ≥ 3 for Nakaya et al., NLR > 4 for Shiroyama et al., NLR ≥ median for others studies…which do not permit any use at the individual level in clinical practice.
The question of the putative prognostic value of the NLR variation is less-well studied.
Nakaya et al. have studied NLR variations at several time points (baseline, 2 and 4 weeks) but they did not studied the prognostic value of the NLR variations between the different time points.
No largest publication related to the prognostic value of NLR variations in patients with solid tumors treated with ICB have been published. Focusing on both mRCC and mNSCLC, we aimed to demonstrate that NLR variation is not cohort dependent.
We showed that any NLR increase was associated with worse outcomes. Interestingly, we showed that the baseline NLR value was associated with IMDC prognostic groups but on the opposite, no significant difference was observed between varNLRw1-6 and IMDC, suggesting that this parameter does not add information to IMDC regarding prognosis. Beyond the prognostic value of NLR variation on OS, the association with PFS in the two cohorts and ORR in the mRCC cohort suggests that this parameter might be predictive of anti-PD1 efficacy.
The major strength of our study is to demonstrate that the prognostic and/or predictive value of NLR variations is not cohort-dependent with similar results in both NSCLC and mRCC cohorts. We decided to group these cohorts because pragmatically, nivolumab was EMA-approved first in NSCLC and mRCC allowing us to treat a large cohort of patients as soon as 2016. Second, at the tumor cell-level NSCLC and mRCC share similarities in the abundance of tissue-infiltrating immune and stromal cell population, with high abundance of neutrophils, T-cells, CD8 T-cells and cytotoxic lymphocytes. Nevertheless, they also had major differences: B lineage, myeloid dendritic cells and fibroblast are abundant in NSCLC microenvironment whereas endothelial cells is predominant in RCC microenvironment [22–25] and tumor mutational burden is commonly higher in NSCLC than in RCC [28]. We hypothesize that peripheral immunity, evaluated by NLR variations could be a strong predictor of ICB efficacy, whatever tumor site.
This study has limitations due to its retrospective nature and the small number of pts. Risk for selection bias is also a potential limitation. Moreover, only some pts with mNSCLC had a robust and homogeneous tumor evaluation using RECIST 1.1 criteria even if we believe that iRECIST criteria may have been more relevant to evaluate ICB efficacy [29]. Nevertheless, as survival criteria are certainly more relevant and more robust than ORR, the latter limitation does not impair the importance of our findings. The NLR evaluation time point could be discussed but as shown previously, the better HR for decrease vs increase was found to be at week 6. Lastly, no subgroups analysis stratified by PD-L1 expression or correlation between NLR and tumor mutation burden have been done. Neither PD-L1 expression, nor tumor mutation burden are performed in clinical routine in our country. Nivolumab approval is not based on any companion biomarker, neither for NSCLC nor mRCC. Especially in mRCC, PD-L1 expression is not a good predictive biomarker of nivolumab response.
Our data may have important clinical implications. First NLR, as opposed to many biomarkers such as PD-L1 expression, is an inexpensive and reproducible blood test, could be easily and dynamically assessed before each ICB injection and thus could be a better reflect of immune reinvigoration. Some translational data suggest that a small subset of tumor infiltrating immune cells (TIIC) could recirculate in the blood and is sensitive to checkpoint inhibition [30]. Second, as tumor response is still a challenge under ICB therapy, including pseudo progression [31, 32] or dissociated responses, NLR might help clinicians to decide to stop treatment or to maintain it. In our study, more than 80% of pts with NLR decrease between 1st and 4th injection had a response or SD.
We think that looking at the NLR variations over time may enable to identify patients with the worst outcomes. The link between NLR and ICB response is obvious since NLR is a marker of the balance between inflammation and immunity, thus suggesting that NLR change may have its best predictive value in patients treated with ICB. Solid biological evidences linking NLR and ICB response have recently emerged. A high neutrophils count is associated with the release of pro-tumor substances (as reactive oxygen species, arginase, inflammatory cytokines, tumor or vascular growth factor and metallo-proteases…) whereas a low lymphocytes count is associated with an impaired anti-tumor response, an impaired CD8+ T cell cytotoxicity and CD4+ helper T-cell functions [33]. NLR is thus the expression of a global balance between pro-tumor inflammation and anti-tumor immunity. Recently, Kargl et al. have identified a strong negative correlation between intra tumor neutrophils and CD8+ cellular content in NSCLC. Given that peripheral cells abundance can reflect the TME, this reinforces the link between NLR and ICB response [34]. Dirican et al. [35] also demonstrated a strong negative correlation between a high NLR and CD3+ T-cells (r = 0.623, p = 0.012).
This work needs an external validation in a prospective trial, which will be available with the mRCC randomized phase II trial BIONIKK (NCT02960906) comparing responses to 1st line nivolumab ± ipilimumab and TKI according to molecular group. If validated, these results could lead to an adaptive trial either based on NLR variation at 6 weeks with addition of an immune boost, or treatment intensification with addition of a TKI in pts treated with nivolumab alone, or even based on NLR variation at the time of dissociated response and/or first unconfirmed PD by iRECIST criteria. The concept of immune boost is of particular interest and is already tested in the phase 2 TITAN-RCC (Tailored Immunotherapy Approach with Nivolumab in advanced Renal Cell Carcinoma) (NCT0291772) where an ipilimumab boost is given in case of stability or progressive disease at first time evaluation. First results were recorded during ESMO congress 2019 and showed that ipilimumab boost significantly improved ORR compared to nivolumab monotherapy [29].
Conclusion
Our findings show that NLR variation (increase vs. decrease) at week 6 is significantly associated with survival outcomes PFS and OS. NLR variation could become one of the easiest and cheapest prognostic factors for pts with solid tumors treated with ICB.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
AS, RE, SO and YAV were involved in the study design and concept. AS, YAV, EF, VF, DB, JT, PB, CT were involved in the identification and selection of patients. AS and RE were involved in the statistical analysis. LF was involved in the radiographic evaluation. All authors were involved in the review and editing of the manuscript. All authors read and approved the final manuscript.
Funding
The investigators AS, YA had access to the raw data. All authors approved the final manuscript. The corresponding author had full access to all the data, and takes final responsibility for the manuscript submitted for publication.
Data availability
Not applicable.
Compliance with ethical standards
Conflict of interest
YV, SO, CT and PB: consulting fees from BMS, MSD, Pfizer, Novartis, Ipsen, Roche, Astellas, Sanofi, Janssen. DB: funding to institution for clinical research, advisory role or travel accommodation: Bristol-Meyers Squibb, Pfizer, Roche, Ipsen, MSD, Astra-Zeneca. No potential conflicts of interest were disclosed by the other authors.
Ethics approval and consent to participate
The study was approved by the local institutional review board and was conducted with Good Clinical Practice Guidelines and the Declaration of Helsinki. All patients gave their oral consent. CNIL declaration No 2215794 v 0
Consent for publication
Informed consent for publication has been obtained.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Abe H, Kamai T. Recent advances in the treatment of metastatic renal cell carcinoma. Int J Urol Off J Jpn Urol Assoc. 2013;20(10):944–955. doi: 10.1111/iju.12187. [DOI] [PubMed] [Google Scholar]
- 3.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab vs docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab vs docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab vs everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab vs chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 7.Hammers HJ, Plimack ER, Infante JR, et al. Safety and efficacy of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma: the CheckMate 016 study. J Clin Oncol Off J Am Soc Clin Oncol. 2017;35(34):3851–3858. doi: 10.1200/JCO.2016.72.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hellmann MD, Ciuleanu T-E, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018 doi: 10.1056/NEJMoa1801946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer Oxf Engl. 1990;2016(54):139–148. doi: 10.1016/j.ejca.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 10.Boutros C, Tarhini A, Routier E, et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol. 2016;13(8):473. doi: 10.1038/nrclinonc.2016.58. [DOI] [PubMed] [Google Scholar]
- 11.Hallmarks of Cancer: The next generation: cell. https://www.cell.com/cell/fulltext/S0092-8674(11)00127-9?_returnURL=http%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS0092867411001279%3Fshowall%3Dtrue. Published September 3, 2017. Accessed 3 Sep 2017.
- 12.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bui JD, Schreiber RD. Cancer immunosurveillance, immunoediting and inflammation: independent or interdependent processes? Curr Opin Immunol. 2007;19(2):203–208. doi: 10.1016/j.coi.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Galdiero MR, Bonavita E, Barajon I, Garlanda C, Mantovani A, Jaillon S. Tumor associated macrophages and neutrophils in cancer. Immunobiology. 2013;218(11):1402–1410. doi: 10.1016/j.imbio.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Templeton AJ, McNamara MG, Eruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. JNCI J Natl Cancer Inst. 2014;106(6):dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 16.Marchioni M, Primiceri G, Ingrosso M, et al. The clinical use of the neutrophil to lymphocyte ratio (NLR) in urothelial cancer: a systematic review. Clin Genitourin Cancer. 2016;14(6):473–484. doi: 10.1016/j.clgc.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Bar-Ad V, Palmer J, Li L, et al. Neutrophil to lymphocyte ratio associated with prognosis of lung cancer. Clin Transl Oncol Off Publ Fed Span Oncol Soc Natl Cancer Inst Mex. 2016 doi: 10.1007/s12094-016-1593-y. [DOI] [PubMed] [Google Scholar]
- 18.Vano Y-A, Oudard S, By M-A, et al. Optimal cut-off for neutrophil-to-lymphocyte ratio: fact or fantasy? A prospective cohort study in metastatic cancer patients. PLoS ONE. 2018 doi: 10.1371/journal.pone.0195042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lalani A-KA, Xie W, Martini DJ, et al. Change in Neutrophil-to-lymphocyte ratio (NLR) in response to immune checkpoint blockade for metastatic renal cell carcinoma. J Immunother Cancer. 2018;6(1):5. doi: 10.1186/s40425-018-0315-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khunger M, Patil PD, Khunger A, et al. Post-treatment changes in hematological parameters predict response to nivolumab monotherapy in non-small cell lung cancer patients. PLoS ONE. 2018 doi: 10.1371/journal.pone.0197743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ameratunga M, Chénard-Poirier M, Moreno Candilejo I, et al. Neutrophil-lymphocyte ratio kinetics in patients with advanced solid tumours on phase I trials of PD-1/PD-L1 inhibitors. Eur J Cancer Oxf Engl. 1990;2018(89):56–63. doi: 10.1016/j.ejca.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 22.Bagley SJ, Kothari S, Aggarwal C, et al. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer Amst Neth. 2017;106:1–7. doi: 10.1016/j.lungcan.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 23.Kiriu T, Yamamoto M, Nagano T, et al. The time-series behavior of neutrophil-to-lymphocyte ratio is useful as a predictive marker in non-small cell lung cancer. PLoS ONE. 2018 doi: 10.1371/journal.pone.0193018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diem S, Schmid S, Krapf M, et al. Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer Amst Neth. 2017;111:176–181. doi: 10.1016/j.lungcan.2017.07.024. [DOI] [PubMed] [Google Scholar]
- 25.Nakaya A, Kurata T, Yoshioka H, et al. Neutrophil-to-lymphocyte ratio as an early marker of outcomes in patients with advanced non-small-cell lung cancer treated with nivolumab. Int J Clin Oncol. 2018 doi: 10.1007/s10147-018-1250-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiroyama T, Suzuki H, Tamiya M, et al. Pretreatment advanced lung cancer inflammation index (ALI) for predicting early progression in nivolumab-treated patients with advanced non-small cell lung cancer. Cancer Med. 2018;7(1):13–20. doi: 10.1002/cam4.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Becht E, Giraldo NA, Lacroix L, et al. Estimating the population abundance of tissue-infiltrating immune and stromal cell populations using gene expression. Genome Biol. 2016;17(1):218. doi: 10.1186/s13059-016-1070-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med. 2017;377(25):2500–2501. doi: 10.1056/NEJMc1713444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18(3):e143–e152. doi: 10.1016/S1470-2045(17)30074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.T-cell invigoration to tumour burden ratio associated with anti-PD-1 response.—PubMed—NCBI. https://www.ncbi.nlm.nih.gov/pubmed/?term=T-cell+invigoration+to+tumour+burden+ratio+associated+with+anti-+PD-1+response. Accessed 22 June 2019. [DOI] [PMC free article] [PubMed]
- 31.Chiou VL, Burotto M. Pseudoprogression and immune-related response in solid tumors. J Clin Oncol Off J Am Soc Clin Oncol. 2015;33(31):3541–3543. doi: 10.1200/JCO.2015.61.6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Escudier B, Motzer RJ, Sharma P, et al. Treatment beyond progression in patients with advanced renal cell carcinoma treated with nivolumab in CheckMate 025. Eur Urol. 2017;72(3):368–376. doi: 10.1016/j.eururo.2017.03.037. [DOI] [PubMed] [Google Scholar]
- 33.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30(7):1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 34.Kargl J, Busch SE, Yang GHY, et al. Neutrophils dominate the immune cell composition in non-small cell lung cancer. Nat Commun. 2017;8(1):1–11. doi: 10.1038/ncomms14381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dirican N, Karakaya YA, Gunes S, Daloglu FT, Dirican A. Association of intra-tumoral tumour-infiltrating lymphocytes and neutrophil-to-lymphocyte ratio is an independent prognostic factor in non-small cell lung cancer. Clin Respir J. 2017;11(6):789–796. doi: 10.1111/crj.12417. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.