Abstract
Background
Programmed cell death ligand-1 (PD-L1) and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) play a pivotal role in cancer immunotherapy. Each of these molecules has a membrane-bound receptor form (mPD-L1/mCTLA-4) and a soluble form (sPD-L1/sCTLA-4). However, these prognostic impacts in colorectal cancer (CRC) remain unclear.
Methods
We immunohistochemically scored tumoral mPD-L1/mCTLA-4 expression and quantified preoperative circulating sPD-L1/sCTLA-4 levels using matched serum specimens from 131 patients with pStage I–III CRC. We also examined the association between these statuses and tumor infiltrating lymphocytes (TILs) in these patients.
Results
Elevated levels of mPD-L1, mCTLA-4, sPD-L1 and sCTLA-4 were significantly correlated with poor overall survival (OS) and disease-free survival (DFS). Co-high expression of tumoral mPD-L1 and mCTLA-4 or co-elevated levels of serum sPD-L1 and sCTLA-4 were strongly correlated with poor OS and DFS. Multivariate analysis revealed that both statuses were negative independent prognostic factors for OS [hazard ratio (HR) 3.86, 95% confidence interval (95% CI) 1.71–8.51, p = 0.001; HR 5.72, 95% CI 1.87–14.54, p = 0.004, respectively] and DFS (HR 2.53, 95% CI 1.23–4.95, p = 0.01; HR 6.88, 95% CI 2.42–17.13, p = 0.0008, respectively). Although low expression of tumoral mCTLA-4 was significantly correlated with increased CD8(+) TILs, there was no correlation in any other combination.
Conclusions
We verified the prognostic impacts of mPD-L1, mCTLA-4, sPD-L1 and sCTLA-4 in pStage I–III CRC patients. Dual evaluation of immune checkpoint molecules in primary tissues or preoperative serum could identify a patient population with poor prognosis in these patients.
Electronic supplementary material
The online version of this article (10.1007/s00262-020-02645-1) contains supplementary material, which is available to authorized users.
Keywords: Tumoral membrane expression, Soluble receptors and ligands, PD-L1, CTLA-4, Colorectal Cancer
Introduction
Colorectal cancer (CRC) is the third leading cause of cancer-related death in the United States [1]. Although the standard strategy for cure in CRC patients is curative resection, most of these patients need to receive additional therapy, including chemotherapy and radiotherapy, before or after surgery to increase the curative resection success rate or to reduce the recurrence rate, especially in advanced CRC patients.
Recently, immunotherapy entered the limelight as a fourth cancer treatment for various malignancies, including CRC [2, 3], and emerging evidence has revealed that inhibition of immune checkpoints in the programmed cell death-1 (PD-1)/programmed cell death ligand-1 (PD-L1) and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) pathways play a pivotal role in immune evasion in cancer [4]. Regarding the PD-1/PD-L1 pathway, PD-1 binds to its ligands PD-L1 and PD-L2, and it promotes T cell apoptosis and exhaustion [4, 5]. In contrast, CTLA-4 binds to CD80 and CD86 with greater affinity and avidity than CD28, thus regulating T cell activation to the tumor and preventing self-tolerance [4, 6, 7]. Accumulating evidence from clinical studies demonstrates the favorable effect of immunotherapy that targets these pathways in the treatment of various types of malignancies [2, 8]. Moreover, several studies have shown that combination therapy with the PD-1/PD-L1 and CTLA-4 pathways was more effective than monotherapy at improving prognosis in patients with cancers [9, 10]. Each human PD-L1 and CTLA-4 molecules has two forms: a membrane-bound receptor form (mPD-L1 and mCTLA-4) and a soluble form (sPD-L1 and sCTLA-4). Recent studies demonstrated prognostic impact of these molecules on malignancies [6, 7, 11–17]; however, the prognostic burden of tumoral or soluble immune checkpoint molecules remains unclear in CRC patients.
CD8(+) T cells are cytotoxic and have a crucial role in anti-tumor immunity in CRC [18]. Therefore, they are recognized as a promoter and inhibitor of local tumor immunity in the tumor microenvironment (TME) of CRC, whereas regulatory T (Treg) cells expressed FoxP3, which is a transcription factor, could suppress the immune system against tumor progression [19]. However, the function of FoxP3(+) T cells in CRC is still controversial. One recent study about CRC revealed that Foxp3(+) T cells were classified into two types by the degree of Foxp3 expression levels; Foxp3high and Foxp3low. Foxp3high T cells suppress immune response against cancer cells hence a high infiltration of FOXP3high T cells in the TME is associated with a poor prognosis; however, a high infiltration of Foxp3low T cells did contribute better prognosis for CRC patients [20]. Recent meta-analysis revealed a favorable impact of FoxP3(+) T cell infiltration for prognosis in CRC, which contrasts with previous meta-analyses suggesting the negative impact of tumor-infiltrating FoxP3(+) T cells for oncological outcomes in solid cancers [21, 22]. Furthermore, the association between the two types of immune checkpoint molecule expression in tumor tissues or blood and CD8(+) or FoxP3(+) tumor infiltrative lymphocytes (TILs) is still unclear.
Here, we evaluated tumoral mPD-L1 and mCTLA-4 expression in CRC tissues, and quantified preoperative circulating sPD-L1 and sCTLA-4 levels using matched serum specimens to comprehensively explore the clinical burden of these immune checkpoint forms in pStage I–III CRC patients. Furthermore, we examined TILs in TME using matched CRC tissues to clarify the association between immune checkpoint forms and TILs in curatively resected CRC patients.
Materials and methods
Patients
We enrolled 131 patients with pStage I–III CRC who underwent curative surgical resection between 2013 and 2015 at the Department of Gastrointestinal Surgery, Mie University Hospital (Tsu, Mie, Japan). Total twenty-five patients were deceased due to CRC in this cohort. Detailed information of the tests is provided in Supplementary Methods.
Immunohistochemistry analysis of PD-L1, CTLA-4, CD8, and FoxP3 expression in primary CRC tissue
Formalin-fixed, paraffin-embedded sections (FFPE; 5 µm thick) were prepared from surgical specimens of CRC patients. Further information is provided in Supplementary Methods.
Immunohistochemistry scoring of tumoral mPD-L1 and mCTLA-4 expression in primary CRC tissue
Tumoral mPD-L1 and mCTLA-4 immunoreactivity at the core of the CRC were evaluated according to the intensity and extent of staining. Staining intensity of cytoplasmic PD-L1 and CTLA-4 expression in CRC cells was scored as follows: no staining, 0; weak staining, 1; moderate staining, 2; and strong staining, 3 (Supplementary Figure 1a–h). The extent of staining was scored according to the percentage of CRC cells with cytoplasmic PD-L1 and CTLA-4 expression as follows: 0%, 0; 1%–25%, 1; 26%–50%, 2; 51%–75%, 3 and 76%–100%, 4). The immunohistochemistry scores were calculated by multiplying the intensity and extent of the staining scores (score range 0–12). The slides were evaluated three times by three independent investigators (YuO, YT and YoO) who were blinded to the nature of the specimens and antibodies used.
Immunohistochemistry scoring of PD-L1, CTLA-4, CD8 and FoxP3 (+) T cells in the tumor microenvironment
PD-L1, CTLA-4, CD8 and Foxp3 (+) T cells were counted using a scanner system under an Olympus BX-51 and DP21 with the Cellsens software imaging system, as previously described [14]. Each slide was scanned microscopically, and PD-L1, CTLA-4, CD8 and Foxp3 (+) T cells were photographed at a magnification of 100 × in three representative low-power fields at the tumor margin (Supplementary Figure 2a–d).
Detection of serum sPD-L1 and sCTLA-4 levels by Enzyme-linked immunosorbent assay
Serum sPD-L1 levels were determined using enzyme-linked immunosorbent assay kits for human PD-L1(WLS CLOUD-CLONE CORP, Houston, TX, USA) as previously described [14]. Serum sCTLA-4 levels were determined using enzyme-linked immunosorbent assay kits for human CTLA-4(Life Technologies, Carlsbad, USA) as previously described [13]. Further information is provided in Supplementary Methods.
MSI, KRAS and BRAF status detection
Microsatellite unstable (MSI) analysis was carried out using five mononucleotide repeat microsatellite markers(BAT-25, BAT-26, NR-21, NR-24 and NR-27) in a pentaplex PCR system as previously described [23]. Primer sequences were described previously [24]. KRAS (exons 2 and 3) and BRAF (V600E) mutations were analyzed by pyrosequencing using primers as previously descried [23]. Further information is provided in Supplementary Methods.
Statistical analysis
Statistical analyses were performed using JMP software version 13.2.1(SAS Institute, Cary, NC, USA). Optimal cut-off values for each tumoral membrane expression of immune checkpoints in CRC cells was determined using receiver operating characteristic (ROC) curves for overall survival (OS) with Youden’s index (Supplementary Figure 3a, b). The cut-off values of each soluble form level of immune checkpoint in preoperative serum was also determined using ROC curves for OS with Youden’s index (Supplementary Figure 3c, d). Further information is provided in Supplementary Methods. All P values were two-sided and p < 0.05 was considered statistically significant.
Results
High tumoral mPD-L1 expression was significantly correlated with clinicopathological factors in pStage I–III CRC patients
At first, we evaluated the correlation between tumoral mPD-L1 or mCTLA-4 expression in CRC tissues and clinicopathological factors in pStage I–III CRC patients (Table 1). High tumoral mPD-L1 expression was significantly correlated with well-established local disease-progression factors, including advanced T-stage (p = 0.009), presence of vessel invasion (p = 0.0002), presence of lymphatic invasion (p = 0.03), lymph node metastasis positive (p = 0.01) and advanced TNM stage (p = 0.01). As for the molecular subtype of CRC, patients with low tumoral mPD-L1 expression have significantly more BRAF mutations than those with high tumoral mPD-L1 expression (p = 0.008), and other molecular statuses were not correlated with tissue mPD-L1 expression in CRC tissues. However, patients with high tumoral mCTLA-4 expression have significantly more MSI status than those with low tumoral mCTLA-4 expression (p = 0.056), and none of the clinicopathological factors and molecular subtypes was associated with tumoral mCTLA-4 expression in pStage I–III CRC tissues.
Table 1.
Correlation between clinicopathological variables and tumoral membrane immune checkpoint expression or preoperative serum soluble form levels of immune checkpoints in colorectal cancer patients
| Variable | n | Tumoral mPD-L1 expression | Tumoral mCTLA-4 expression | Serum sPD-L1 levels | Serum sCTLA-4 levels | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High (n = 100) | Low (n = 31) | p value | High (n = 27) | Low (n = 104) | p value | High (n = 25) | Low (n = 106) | p value | High (n = 22) | Low (n = 109) | p value | ||
| Sex | |||||||||||||
| Male | 79 | 63 | 16 | 0.26 | 59 | 0.02* | 0.22 | 20 | 13 | 66 | 0.89 | ||
| Female | 52 | 37 | 15 | # | 8 | 44 | # | 5 | 47 | # | 9 | 43 | # |
| Age (years) | |||||||||||||
| < 69 | 64 | 47 | 17 | 0.44 | 10 | 54 | 0.16 | 10 | 54 | 0.32 | 12 | 52 | 0.55 |
| ≥ 69 | 67 | 53 | 14 | # | 17 | 50 | # | 15 | 52 | # | 10 | 57 | # |
| Histological type | |||||||||||||
| Differentiated | 122 | 94 | 28 | 0.44 | 25 | 97 | 1 | 24 | 98 | 1 | 20 | 102 | 0.65 |
| Undifferentiated | 9 | 6 | 3 | ## | 2 | 7 | ## | 1 | 8 | ## | 2 | 7 | ## |
| Pathological T category | |||||||||||||
| pT1/2 | 62 | 41 | 21 | 0.009* | 13 | 49 | 0.92 | 15 | 47 | 0.15 | 9 | 53 | 0.5 |
| pT3/4 | 69 | 59 | 10 | # | 14 | 55 | # | 10 | 59 | # | 13 | 56 | # |
| Vessel invasion | |||||||||||||
| Present | 72 | 64 | 8 | 0.0002* | 18 | 54 | 0.16 | 11 | 61 | 0.22 | 14 | 58 | 0.36 |
| Absent | 59 | 36 | 23 | # | 9 | 50 | # | 14 | 45 | # | 8 | 51 | # |
| Lymphatic invasion | |||||||||||||
| Present | 64 | 54 | 10 | 0.03* | 15 | 49 | 0.43 | 7 | 57 | 0.01* | 15 | 49 | 0.04* |
| Absent | 67 | 46 | 21 | # | 12 | 55 | # | 18 | 49 | # | 7 | 60 | # |
| Lymph node metastasis | |||||||||||||
| Present | 43 | 38 | 5 | 0.01* | 8 | 35 | 0.68 | 6 | 37 | 0.28 | 8 | 35 | 0.7 |
| Absent | 88 | 62 | 26 | # | 19 | 69 | # | 19 | 69 | # | 14 | 74 | # |
| TNM classification | |||||||||||||
| Stage I/II | 88 | 62 | 26 | 0.01* | 19 | 69 | 0.68 | 19 | 69 | 0.28 | 14 | 74 | 0.7 |
| Stage III | 43 | 38 | 5 | # | 8 | 35 | # | 6 | 37 | # | 8 | 35 | # |
| MSI status | |||||||||||||
| MSS | 123 | 96 | 27 | 0.09 | 23 | 100 | 0.056 | 24 | 99 | 1 | 21 | 102 | 1 |
| MSI | 8 | 4 | 4 | ## | 4 | 4 | ## | 1 | 8 | ## | 1 | 7 | ## |
| KRAS stats | |||||||||||||
| Wild | 69 | 54 | 15 | 0.58 | 18 | 51 | 0.09 | 14 | 55 | 0.71 | 13 | 56 | 0.5 |
| Mutation | 62 | 46 | 16 | # | 9 | 53 | # | 11 | 51 | # | 9 | 53 | # |
| BRAF status | |||||||||||||
| Wild | 124 | 98 | 26 | 0.008* | 25 | 99 | 0.63 | 24 | 100 | 1 | 21 | 103 | 1 |
| Mutation | 7 | 2 | 5 | ## | 2 | 5 | ## | 1 | 6 | ## | 1 | 6 | ## |
Bold font means significant p value
#The median age at surgery was 69 years in this cohort. #Chi square test, ##Fisher test. *p < 0.05
Elevated preoperative serum sPD-L1 or sCTLA-4 levels were significantly correlated with lymphatic invasion in pStage I–III CRC patients
We also evaluated the association between the preoperative serum sPD-L1 or sCTLA-4 levels and clinicopathological factors in pStage I–III CRC patients (Table 1). pStage I–III CRC patients with high preoperative serum sPD-L1 levels had significantly less presence of lymphatic invasion than those with low serum sPD-L1 levels (p = 0.01), while patients with high preoperative serum sCTLA-4 levels had significantly more presence of lymphatic invasion than those with low serum sCTLA-4 levels (p = 0.04). Preoperative serum sPD-L1 levels was not significantly correlated with other clinicopathological factors despite sex differences (p = 0.02) in these patients. Furthermore, none of the other clinicopathological factors was significantly correlated with preoperative serum sCTLA-4 levels in these patients. In addition, both statuses of preoperative serum levels were also not correlated with any molecular subtype of CRC, including KRAS mutation, BRAF mutation, and MSI status in pStage I–III CRC patients.
High tumoral mPD-L1 and/or mCTLA-4 expression in CRC tissues were significantly correlated with shorter OS and DFS in pStage I–III CRC patients
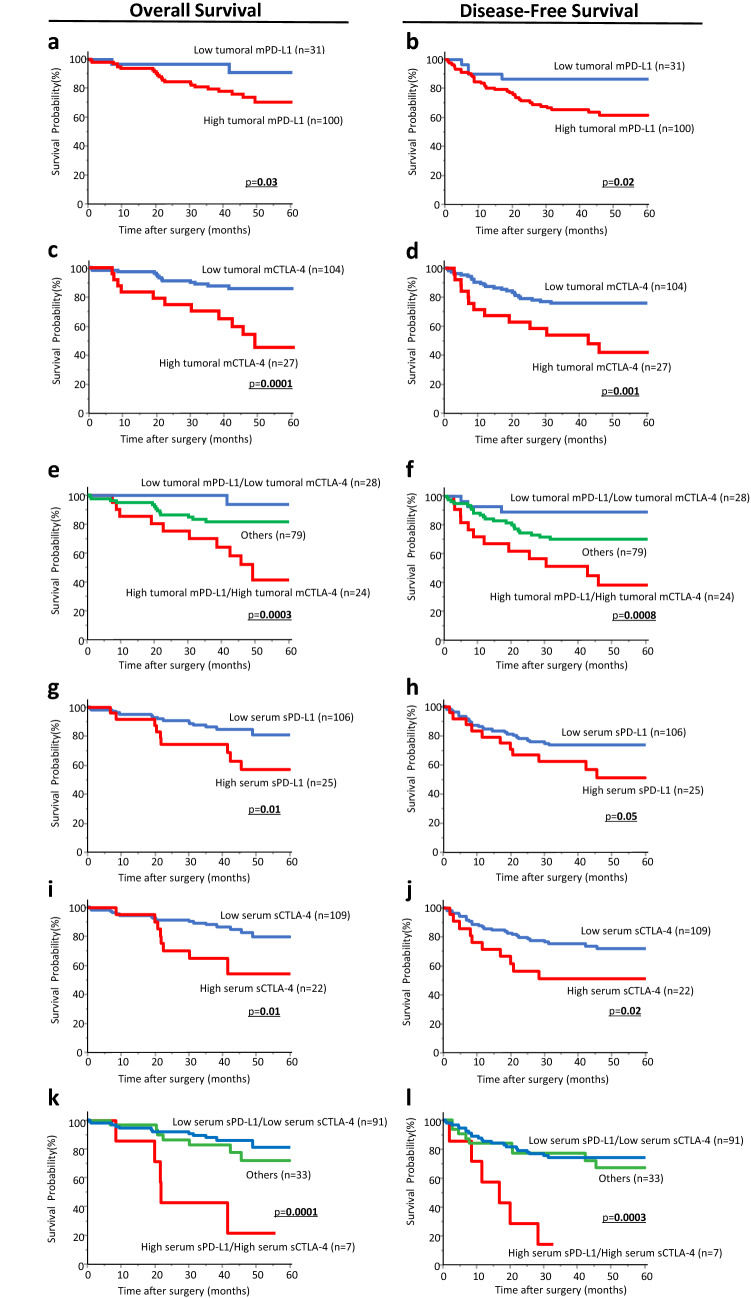
We next performed time-to-event analyses to evaluate the prognostic relevance of tumoral mPD-L1 or mCTLA-4 expression in CRC tissues for OS and DSF in pStage I–III CRC patients. Patients with high tumoral mPD-L1 expression showed shorter OS and DFS than those with low expression (p = 0.03, p = 0.02, respectively; Fig. 1a, b). Likewise, high tumoral mCTLA-4 expression was significantly associated with poor OS and DFS, whereas low expression in these patients was not (p = 0.0001, p = 0.001, respectively; Fig. 1c, d). To further assess the clinical significance of tumoral mPD-L1 and mCTLA-4 expression status, we analyzed the association between the status combined tumoral mPD-L1 expression with tumoral mCTLA-4 expression and various clinicopathological factors in pStage I–III CRC patients (Table 2). Interestingly, patients with high expression of at least either tumoral mPD-L1 or mCTLA-4 in CRC tissues were significantly associated with presence of vessel involvement (high/high vs low/low: p = 0.002; others vs low/low: p = 0.003, Chi square test with Holm adjustment). Other clinicopathological factors, including molecular profiles, were not significantly associated with the dual evaluation of the expression status. It is noteworthy that the status was strongly correlated with poor prognosis for OS and DFS in pStage I–III CRC patients (p = 0.0003, p = 0.0008, respectively; Fig. 1e, f)
Fig. 1.
Kaplan–Meier analysis of patient survival according to tumoral mPD-L1/mCTLA-4 expression in CRC tissues and sPD-L1/sCTLA-4 levels in preoperative serum in pStage I–III CRC patients. a–d Kaplan–Meier analysis of patient survival according to tumoral mPD-L1 or mCTLA-4 expression in CRC tissues. Patients with high tumoral mPD-L1 or mCTLA-4 expression had significantly poorer OS and DFS than those with low expression (tumoral mPD-L1 expression; p = 0.03 and p = 0.02, tumoral mCTLA-4 expression; p = 0.0001 and p = 0.001, respectively). Kaplan–Meier survival curves for OS (e) and DFS (f) of CRC patients based on the co-expression of tumoral mPD-L1 and mCTLA-4. Patients with co-expression of tumoral mPD-L1 and mCTLA-4 expression had much poorer OS and DFS than patients with other values (p = 0.0003 and p = 0.0008, respectively). g–j Kaplan–Meier analysis of patient survival according to preoperative serum sPD-L1 or sCTLA-4 levels in pStage I–III CRC patients. Patients with elevated sPD-L1 or sCTLA-4 levels in preoperative serum had significantly shorter OS and DFS than those without elevated values (serum sPD-L1 levels; p = 0.01 and p = 0.05, serum sCTLA-4 levels; p = 0.01 and p = 0.02, respectively). Kaplan–Meier survival curves for OS (k) and DFS (l) of CRC patients based on co-elevated serum sPD-L1 and sCTLA-4 levels. Patients with co-elevated serum sPD-L1 and sCTLA-4 levels had much poorer OS and DFS than patients with other values (p = 0.0001 and p = 0.0003, respectively)
Table 2.
Correlation between clinicopathological variables and tumoral mPD-L1/mCTLA-4 expression status and preoperative serum sPD-L1/sCTLA-4 levels status in colorectal cancer patients
| Variable | n | Tumoral membrane expression status | p value | Serum soluble form levels status | p value | ||||
|---|---|---|---|---|---|---|---|---|---|
| High/high (n = 24) | Others (n = 79) | Low/low (n = 28) | High/high (n = 7) | Others (n = 33) | Low/low (n = 91) | ||||
| Sex | |||||||||
| Male | 79 | 17 | 48 | 14 | 0.37§# | 4 | 25 | 50 | 0.74§## |
| Female | 52 | 7 | 31 | 14 | 0.39§§# | 3 | 8 | 41 | 1§§## |
| Age (year) | 0.64§§§# | 0.12§§§## | |||||||
| < 69### | 64 | 10 | 37 | 17 | 0.66§# | 4 | 14 | 46 | 1§## |
| ≥ 69 | 67 | 14 | 42 | 11 | 0.52§§# | 3 | 19 | 45 | 1§§## |
| Histological type | 0.42§§§# | 1§§§## | |||||||
| Differentiated | 122 | 23 | 73 | 26 | 1§## | 6 | 32 | 84 | 0.97§## |
| Undifferentiated | 9 | 1 | 6 | 2 | 1§§## | 1 | 1 | 7 | 0.92§§## |
| Pathological T category | 1§§§## | 0.68§§§## | |||||||
| pT1/2 | 62 | 10 | 34 | 18 | 0.91§# | 4 | 16 | 42 | 1§## |
| pT3/4 | 69 | 14 | 45 | 10 | 0.21§§# | 3 | 17 | 49 | 1§§§## |
| Vessel invasion | 0.16§§§ | 1§§§## | |||||||
| Present | 72 | 17 | 48 | 7 | 0.37§# | 4 | 17 | 51 | 1§## |
| Absent | 59 | 7 | 31 | 21 | 0.002§§# | 3 | 16 | 40 | 1§§## |
| Lymphatic invasion | 0.003§§§# | 1§§§## | |||||||
| Present | 64 | 13 | 43 | 8 | 0.98§# | 3 | 16 | 45 | 1§## |
| Absent | 67 | 11 | 36 | 20 | 0.13§§# | 4 | 17 | 46 | 1§§## |
| Lymph node metastasis | 0.057§§§ | 1§§§## | |||||||
| Present | 43 | 8 | 30 | 5 | 0.68§# | 2 | 10 | 31 | 1§## |
| Absent | 88 | 16 | 49 | 23 | 0.41§§# | 5 | 23 | 60 | 1§§## |
| TNM classification | 0.16§§§# | 1§§§## | |||||||
| Stage I/II | 88 | 16 | 49 | 23 | 0.68§# | 5 | 23 | 60 | 1§## |
| Stage III | 43 | 8 | 30 | 5 | 0.41§§# | 2 | 10 | 31 | 1§§## |
| MSI status | 0.16§§§# | 1§§§## | |||||||
| MSS | 123 | 22 | 75 | 26 | 1§## | 7 | 31 | 85 | 1§## |
| MSI | 8 | 2 | 4 | 2 | 1§§## | 0 | 2 | 6 | 1§§## |
| KRAS stats | 1§§§## | 1§§§## | |||||||
| Wild | 69 | 16 | 40 | 13 | 0.34§# | 4 | 19 | 46 | 1§## |
| Mutation | 62 | 8 | 39 | 15 | 0.44§§# | 3 | 14 | 45 | 1§§## |
| BRAF status | 0.704§§§# | 1§§§## | |||||||
| Wild | 124 | 24 | 75 | 25 | 0.57§## | 7 | 31 | 86 | 1§## |
| Mutation | 7 | 0 | 4 | 3 | 0.72§§## | 0 | 2 | 5 | 1§§## |
| 0.75§§§## | 1§§§## | ||||||||
Bold font means significant p value
§High/high vs others, §§high/high vs low/low, §§Others vs low/low, #Chi square test, ##Fisher test, ###The median age at surgery is 69 years in this cohort. p value were adjusted by Holm method
Elevated preoperative serum sPD-L1 and/or sCTLA-4 levels were significantly correlated with poor OS and DFS in pStage I–III CRC patients
Next, we generated Kaplan–Meier survival curves according to preoperative serum sPD-L1 levels to clarify the impacts on oncological outcome in pStage I–III CRC patients. Elevated preoperative serum sPD-L1 levels were significantly correlated with poor prognosis for OS and DFS in these patients (p = 0.01, p = 0.05, respectively; Fig. 1g, h). Notably, consistent findings were also observed for sCTLA-4 levels in preoperative serum, and patients with elevated preoperative serum sCTLA-4 levels showed significantly shorter OS and DFS when compared with those who had decreased serum levels (p = 0.01, p = 0.02, respectively; Fig. 1i, j). We further analyzed the association between the status combined preoperative serum sPD-L1 with serum sCTLA-4 levels and various clinicopathological factors in pStage I–III CRC patients (Table 2). Although none of the clinicopathological factors was associated with the serum soluble form status of immune checkpoints, the group with high preoperative serum levels of both sPD-L1 and sCTLA-4 were strongly associated with poor OS and DFS compared with other groups (p = 0.0001, p = 0.0003, respectively; Fig. 1k, l).
Co-high expression of tumoral mPD-L1 and mCTLA-4 in CRC tissues and co-elevated levels of sPD-L1 and sCTLA-4 in preoperative serum were independent prognostic factors for OS and DFS in pStage I–III CRC patients
To determine the potential of co-high expression of tumoral mPD-L1 and mCTLA-4 in CRC tissues and co-elevated levels of sPD-L1 and sCTLA-4 using preoperative serum as a predictive biomarker of poor OS and DFS in pStage I–III CRC patients, we further performed multivariate Cox’s regression analysis. As for being predictors of OS, the statuses of co-high expression of tumoral mPD-L1 and mCTLA-4 in CRC tissues [hazard ratios(HR) 3.86, 95% confidence interval (95% CI) 1.71–8.51, p = 0.001; Table 3a] and co-elevated levels of sPD-L1 and sCTLA-4 in preoperative serum (HR 5.72, 95% CI 1.87–14.54, p = 0.004; Table 3a) were independent prognostic factors for OS in pStage I–III CRC patients. Furthermore, these two statuses (tumoral expression; HR 2.53, 95% CI 1.23–4.95, p = 0.01, serum levels; HR 6.88, 95% CI 2.42–17.13, p = 0.0008, Table 3b) and the presence of lymph node metastasis (HR 2.16, 95%CI 1.06–4.47, p = 0.03, Table 3b) were independent prognostic factors for poor DFS in pStage I–III CRC patients.
Table 3.
Multivariate analysis for (a) predictors of overall survival and (b) predictors of disease-free survival
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| (a) Multivariate analysis for predictors of overall survival | ||||||
| Sex (male) | 1.35 | 0.6–3.37 | 0.45 | |||
| Age (≥ 69)a | 1.58 | 0.72–3.66 | 0.25 | |||
| Histological type (undifferentiated type) | 3.39 | 0.98–9 | 0.052 | |||
| T classification (pT3/T4) | 2.02 | 0.89–4.95 | 0.09 | |||
| Venous invasion (present) | 1.77 | 0.78–4.34 | 0.17 | |||
| Lymphatic invasion (present) | 1.98 | 0.89–4.68 | 0.09 | |||
| Lymph node metastasis (present) | 1.81 | 0.8–3.99 | 0.14 | |||
| TNM classification(pStage3) | 1.81 | 0.8–3.99 | 0.14 | |||
| MSI status (MSS) | 1.37 | 0.29–24.68 | 0.74 | |||
| KRAS status (wild) | 1.58 | 0.71–3.75 | 0.26 | |||
| BRAF status (wild) | 1.28 | 0.26–22.94 | 0.8 | |||
| Tumoral mPD-L1/mCTLA-4 (high/high) | 4.03 | 1.78–8.87 | 0.001* | 3.86 | 1.71–8.51 | 0.001* |
| Serum sPD-L1/sCTLA-4 (high/high) | 6.12 | 2.01–15.38 | 0.003* | 5.72 | 1.87–14.54 | 0.004* |
| (b) Multivariate analysis for predictors of disease-free survival | ||||||
| Sex (male) | 1.43 | 0.73–2.94 | 0.29 | |||
| Age (≥ 69)a | 1.07 | 0.56–2.04 | 0.82 | |||
| Histological type (undifferentiated type) | 1.82 | 0.54–4.59 | 0.29 | |||
| T classification (pT3/T4) | 2.52 | 1.28–5.31 | 0.006* | 2.15 | 0.98–4.97 | 0.054 |
| Venous invasion (present) | 2.14 | 1.09–4.51 | 0.02* | 1.05 | 0.45–2.52 | 0.9 |
| Lymphatic invasion (present) | 2.26 | 1.17–4.56 | 0.01* | 1.47 | 0.67–3.4 | 0.33 |
| Lymph node metastasis (present) | 2.59 | 1.36–4.94 | 0.004* | 2.16 | 1.06–4.47 | 0.03* |
| TNM classification(pStage3) | 2.59 | 1.36–4.94 | 0.004* | |||
| MSI status (MSS) | 2.33 | 0.5–41.51 | 0.33 | |||
| KRAS status (wild) | 1.17 | 0.62–2.26 | 0.61 | |||
| BRAF status (wild) | 2.33 | 0.5–41.49 | 0.33 | |||
| Tumoral mPD-L1/mCTLA-4 (high/high) | 3.02 | 1.5–5.82 | 0.003* | 2.53 | 1.23–4.95 | 0.01* |
| Serum sPD-L1/sCTLA-4 (high/high) | 5.06 | 1.88–11.48 | 0.003* | 6.88 | 2.42–17.13 | 0.0008* |
Bold font means significant p value
aMedian age at surgery = 69 years. HR hazard ratio. 95% CI 95% confidence intervals. *p < 0.05
No correlation existed between preoperative serum sPD-L1 or sCTLA-4 levels and tumoral mPD-L1 or mCTLA-4 expression or PD-L1 or CTLA-4 (+) TILs in CRC tissues
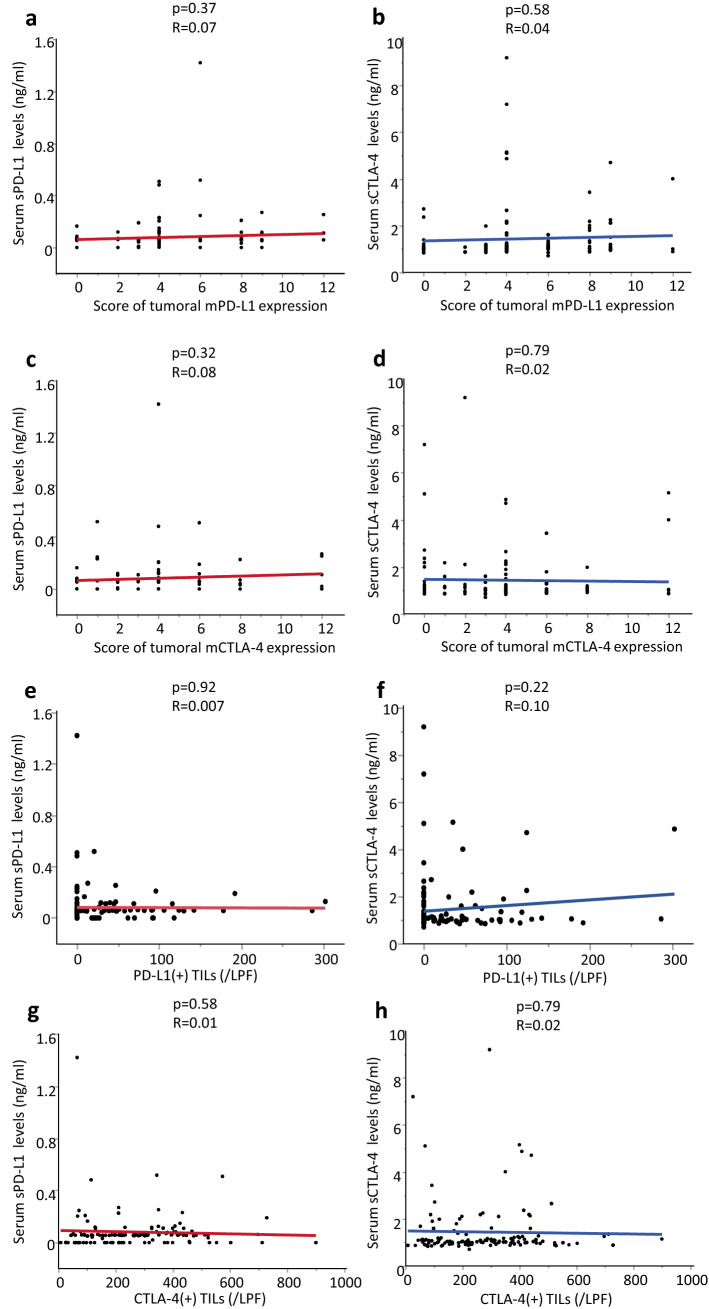
We examined the correlations between preoperative serum levels and tumoral expression of immune checkpoints in CRC tissues (Fig. 2a–d). Furthermore, we also checked whether preoperative serum levels of immune checkpoints were correlated with immune checkpoints positive TILs of CRC patients (Fig. 2e–h). In these analyses, none of any combination showed significant correlation in pStage I–III CRC patients.
Fig. 2.
Correlation between TILs in CRC tissues and levels of tissue or serum immune checkpoints in pStage I–III CRC patients. There was no association between tumoral membrane immune checkpoint expression in CRC tissues and soluble form levels in preoperative serum (a, b) tumoral mPD-L1 expression score; serum sPD-L1 levels: p = 0.37 R = 0.07, serum sCTLA-4 levels: p = 0.58 R = 0.04 (c, d) tumoral mCTLA-4 expression score; serum sPD-L1 levels: p = 0.32 R = 0.08, serum sCTLA-4 levels: p = 0.79 R = 0.02. There was no association between immune checkpoint expression at TILs and soluble form levels in preoperative serum (e, f) the number of PD-L1(+) T cells; sPD-L1: p = 0.92 R = 0.007, sCTLA-4: p = 0.22 R = 0.10, (g, h) the number of CTLA-4(+) T cells; sPD-L1: p = 0.58 R = 0.01, sCTLA-4: p = 0.79 R = 0.02
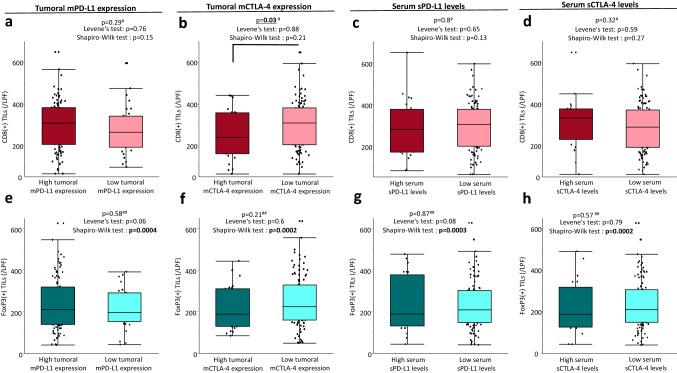
High tumoral mCTLA-4 expression was significantly associated with decreased CD8 positive TILs in CRC tissues
We further examined the correlation between CD8 or FoxP3(+) TILs and various levels of PD-L1 or CTLA-4 in pStage I–III CRC patients. As for CD8(+) TILs, low expression of tumoral mCTLA-4 was significantly correlated with increased CD8(+) TILs (p = 0.03), but other covariates were not correlated with the number of CD8(+) TILs (Fig. 3a–d). In addition, there was no correlation between the number of FoxP3(+) TILs and tumoral expression or serum levels of immune checkpoints in CRC patients (Fig. 3e–h).
Fig. 3.
Correlation between tumoral membrane immune checkpoint expression or soluble form levels of immune checkpoints in preoperative serum and the tumor microenvironment in CRC tissue. a–d As for CD8(+) TILs, low expression of tumoral mCTLA-4 was significantly correlated with increased CD8(+) TILs, and other covariates were not correlated with the number of CD8(+)TILs in pStage I–III CRC patients (tumoral mPD-L1 expression; p = 0.29, tumoral mCTLA-4 expression; p = 0.03, serum sPD-L1 levels; p = 0.8, serum sCTLA-4 levels; p = 0.32). e–h There was no correlation between FoxP3(+) TILs and all of the evaluation items (tumoral mPD-L1 expression; p = 0.58, tumoral mCTLA-4 expression; p = 0.21, serum sPD-L1 levels; p = 0.87, serum sCTLA-4 levels; p = 0.79) in pStage I–III CRC patients. #One-Way ANOVA, ##Mann–Whitney U test
Discussion
“Cancer immunotherapy” was selected as Science’s selection of Breakthrough of the Year 2013 [3], and the PD-1/PD-L1 and CTLA-4 pathways are currently recognized as representative key players of immunotherapy for malignancies among a series of immune checkpoints [4]. Emerging evidence has demonstrated the functional role of the PD-1/PD-L1 and CTLA-4 pathways in various malignancies, and the clinical impacts of tumoral membrane expressions and soluble forms of these markers have also been gradually elucidated [6, 7, 11–17]. The presence of immune checkpoint expression on tumoral membrane helps the tumor escape host immune surveillance [25]. In contrast, the soluble form of immune checkpoints has been considered as a therapeutic target, and Ward et al. demonstrated that blockade of soluble CTLA-4 activity could inhibit metastatic spread in mice [26]. Therefore, these two forms of immune checkpoints are getting attention in the cancer research field.
One major finding of our study was that the statuses for tumoral membrane protein expression of immune checkpoints in CRC tissues were significantly associated with oncological outcome in pStage I–III CRC patients. The role of mPD-L1 in tumor tissues was to inhibit activated T cells by binding with the PD-1 expressed on the T cells [4, 5]. Previous cohort studies showed that high tumoral mPD-L1 expression was significantly associated with several clinicopathological factors associated with disease development, but not CD8(+) T cells in the TME of CRC patients [27, 28]. Our present study also showed that tumoral mPD-L1 expression in CRC tissues was correlated with clinicopathological factors for local progression of primary tumor. Regarding these results, we and several researchers verified that overexpression of mPD-L1 in tumor cells was associated with poor prognosis in various types of malignancies [15, 16]. In contrast, the functional role of mCTLA-4 in CRC tissues remains unclear. CTLA-4 is normally expressed on surfaces of regulator T cells and effector T cells. However, Contardi et al. demonstrated that CTLA-4 was also expressed in tumor cells [29]. Additionally, several researchers previously verified that CTLA-4 was frequently expressed in tumor cells immunohistochemically, and overexpression of mCTLA-4 in tumor cells was associated with worse prognosis in malignancies [6, 7], which are consistent with our results. Recently, Chen et al. demonstrated that tumoral mCTLA-4 expression in breast cancer cells impeded production of cytokines of dendric cells, and suppressed the activity of CD8(+) cells [30]. Considering this evidence, our finding of an inverse correlation between mCTLA-4 expression in CRC tissue and the number of CD8(+) T cells in the TME suggests that increased mCTLA-4 expression inhibits the activity of CD8(+) cells and decreases the number of CD8(+) TILs in the TME of CRC tissues. Furthermore, based on the pivotal role of CTLA-4 in inhibiting T cell activation to the tumor, increased expression of tumoral mCTLA-4 accompanied with decreased CD8(+) TILs in the TME of CRC tissues might be influenced early recurrence and poor oncological outcome in our study.
Another major finding of this study was that the preoperative serum soluble form status of both immune checkpoints was also associated with OS and DFS in pStage I–III CRC patients. Previous studies revealed the function of soluble forms of PD-L1 in vitro and in vivo [11, 12]. sPD-L1 could bind to membrane-bound PD-1, similar to PD-L1 expressed on tumor cells, and inhibit the activation and proliferation of T cells through negative feedback [12]. Furthermore, patients with high serum sPD-L1 levels had higher serum levels of IL-10, which was found to be an independent negative prognostic marker in hepatocellular carcinoma (HCC) patients [31], than those with low serum sPD-L1 levels in the same patient population [11]. Consistent with these evidences, our study also showed that patients with elevated circulating sPD-L1 levels showed a poorer prognosis compared with those without elevated sPD-L1 levels in pStage I–III CRC as with other types of malignancies [11, 14, 17]. On the other hands, sCTLA-4, similar to full-length CTLA-4, can bind to B7 costimulatory ligands on the antigen-presenting cells to prevent B7 from combining with the costimulatory receptor CD28 in T cells, thus inhibiting T-cell responses [32]. Additionally, the blockade of sCTLA-4 activation was associated with secretion of IFN-γ, which enhanced anti-tumor effects [33]. Considering these evidences, the present study showed that the group with high preoperative serum sCTLA-4 levels experienced a statistically poorer prognosis than those with low serum levels in pStage I–III CRC. Interestingly, sPD-L1 and sCTLA-4 showed opposite significant associations with lymphatic invasion of CRC patients. Considering the functional role of circulating sCTLA-4 in inhibiting circulating T-cell responses [32] and our findings that mCTLA-4 expression on tumor cells is significantly associated with a decrease the number of CD8(+) TILs in the TME of CRC tissues, circulating sCTLA-4 also might influence the local involvement of lymphatic invasion via suppression of local tumor immunity in CRC. In contrast, several lines of studies have shown that circulating sPD-L1 binds to membrane-bound PD-1, similar to PD-L1 expressed on tumor cells, and inhibit the activation and proliferation of circulating T cells through negative feedback [12, 32]. However, the effect of circulating sPD-L1 on the immune system in CRC remains unclear, and the logical reason for the significant correlation between the absence of lymphatic invasion and increased sPD-L1 levels cannot be determined based on current evidence. In fact, some studies have shown that the association is inconsistent with our finding [34, 35], and others have demonstrated consistent findings in other types of malignancies [14, 36]. Interestingly, several studies have shown that the concentration of sPD-L1 is elevated in the early stage of malignancies [14, 36] and have suggested that circulating sPD-L1 are potentially involved in the early steps of carcinogenesis. However, to clarify these points, we need further research in a large cohort.
The question of where sPD-L1 derives from remains unresolved. A previous study by Ando and colleagues showed no correlation between tumoral mPD-L1 expression and sPD-L1 levels in the blood in non-small cell lung cancer or gastric cancer [37]. In that study, they showed the same tendency of serum sPD-L1 levels in concordance with tumor regression during the treatment course with immunocheckpoint inhibitors. They also suggested that sPD-L1 levels reflect the total active cancer cells in an individual. Furthermore, other studies showed that soluble forms of immunosuppressive molecules also could regulate T cell activation [12, 32], as described above. The evidence presented in those reports, combined with our findings (shown in Fig. 2), indicate that the physiological roles of tumoral expression of immune checkpoints is to regulate T cells in TME. In addition, the circulating soluble form of immune checkpoints might be secreted from various cells, including total active cancer cells, and travel around the body to regulate circulating T cells in blood.
Accumulating evidence has revealed the different natures of these three molecule statuses, MSI-high, BRAF mutation and KRAS mutation, in CRC [38, 39]. Currently, these statuses are clinically used to decide the chemotherapeutic regimens and immunotherapy in CRC patients. For example, pembrolizumab is more effective for CRC patients with MSI-high than those with MSS status [2], and CRC patients with mutated KRAS do not benefit from anti-EGFR therapy [40]. Furthermore, previous evidence revealed a significant correlation between the BRAF mutation and MSI-high status in CRC [41]. In addition to the clinical impact of these molecular phenotypes, Rosenbaum et al. demonstrated that MSI and BRAF status were associated with tumoral mPD-L1 expression in CRC patients [42]. However, the relationship between the circulating immune checkpoints levels and molecular phenotypes in CRC have never been elucidated. Based on these perspectives, we evaluated the association between molecular phenotypes and PD-L1 or CTLA-4 expression (tumor and soluble) in our study. Interestingly, there was no association between the circulating expression of immune checkpoints and these molecular subtypes in CRC patients. Collectively, these findings suggest that circulating levels of immune checkpoints are not influenced by tumor molecular phenotype in CRC patients.
Several previous studies demonstrated the association between mPD-L1 or mCTLA-4 expression on cancer cells and TILs in the TME for various types of malignancies [14, 25, 28]. However, the influence of circulating soluble forms of immune checkpoints for dysregulation of TME remains unclear. Actually, previous studies showed that the circulating sPD-L1 level was not associated with the number of TILs in HCC and gastric cancer patients [11, 14]. However, to best of our knowledge, there has been no study to demonstrate the correlation between circulating sCTLA-4 levels and the number of TILs in solid cancer. Recently, Shin and coworkers indicated that a local immune response could reflect the degree of TILs in the TME and the systematic immune response could be demonstrated from blood data [43]. Our study showed that clinicopathological factors or the degree of TILs were more associated with tumoral immune checkpoint expression than circulating expression despite the survival impact of both high tumoral membrane and circulating expression of immune checkpoints in CRC patients. Furthermore, our findings also showed no significant correlation between circulating immune checkpoints and local TILs in CRC tissues. Considering these findings, preoperative serum soluble forms of immune checkpoints might reflect the systemic immune response but do not influence the local immune response to induce recurrence in CRC.
Previous evidence demonstrated that mPD-L1 in tumor tissues inhibited activated T cells by binding with the PD-1 expressed T cells [4, 5], and CTLA-4 bound to B7 costimulatory ligands on the antigen-presenting cell to inhibit T-cell responses [32]. Although it is difficult to suspect the role of immunocheckpoints in TME for FoxP3(+) TILs owing to controversial clinical significance of FOXP3(+) TILs as described above, these evidences suggest that PD-L1 expression in the TME could inhibit CD8(+) TILs, and CTLA-4 expression in the TME might decrease the number of CD8(+) TILs via its inhibition effect on T cells. In fact, despite there being no association between PD-L1 expression in the TME and the degree of CD8(+) TILs, the present findings demonstrating that increased expression of CTLA-4 in the TME decreased the number of CD8(+) TILs in CRC might support these hypotheses.
Interestingly, our findings also revealed that dual evaluation using PD-L1 and CTLA-4 factors in both tumoral expression and serum level statuses could identify a population at high risk for oncological outcome in curatively resected CRC patients. Curran et al. demonstrated that combination co-inhibitory receptor blockade could increase TILs in the TME, and those cells appear to increase high levels of inflammatory cytokines [5]. The previous clinical trials revealed synergistic anti-tumor effect of anti-PD-L1 antibody in combination with anti-CTLA-4 antibodies in various malignancies [9, 10]. Furthermore, the strategy of dual immunocheckpoint inhibition is gradually expanding in the CRC field, and Fiegle et al. demonstrated that blockade of PD-L1 and CTLA-4 was effective at preventing tumor growth and liver metastasis in a mouse model of colon cancer, since the combination therapy increased CD8(+) and CD4(+) T cells compared with PD-L1 monotherapy [44]. Preliminary results of a recent clinical trial also showed that nivolumab with low-dose ipilimumab provided a durable clinical benefit with a deepening response and a manageable safety profile in metastatic CRC patients with MSI-High or deficient Mismatch repair [45]. Thus, administration of immune checkpoint inhibitors for both pathways might be a key point for cancer immunotherapy. Although previous studies verified the prognostic value of tumoral immune checkpoints or those soluble forms dissolved in the blood in either PD-L1 or CTLA-4, respectively [6, 7, 11–17], this is the first study to examine the clinical burden of biomarkers that simultaneously included both the immune factors in tumors or matched serum in CRC. Our results showed that biomarkers that combined PD-L1 with CTLA-4 factors might have a stronger prognostic potential than single covariates in pStage I–III CRC patients.
There are several potential limitations to this study. First, although we successfully showed the prognostic impact of immunocheckpoints in the TME and matched preoperative serum in pStage I–III CRC patients, this study was single institution, retrospective study. Second, blood specimens were collected within 2 weeks before surgery, and tissue specimens were collected when the patients received surgery. Although all of enrolled patients did not receive any treatment before surgery, these samples were actually collected different timing. Third, we conducted multivariate analysis including various covariates in relatively small number of enrolled patients, especially small number of cancer-related deaths. Finally, the clinical materials analyzed in this study were solely from patients of Japanese origin. To overcome these limitations, a larger multicenter prospective observation study is needed to clarify our findings in pStage I–III CRC patients.
In conclusion, the status of tumoral membrane expression and preoperative serum soluble form levels of immune checkpoints might be used as a pivotal prognostic biomarker in pStage I–III CRC patients. Furthermore, the evaluations using tumoral expression or serum levels that combined PD-L1 with CTLA-4 factors were more useful to predict oncological outcome than evaluating the factors independently in pStage I–III CRC patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1. Representative images of tumoral mPD-L1 and mCTLA-4 expression in CRC cells. (a–d) PD-L1; (a) absent (b) weak (c) moderate (d) strong, 100 × magnification (e–h) CTLA-4; (e) absent (f) weak (g) moderate (h) strong, 100 × magnification (PDF 490 kb)
Supplementary Figure 2. Representative images of PD-L1, CTLA-4, CD8 and FoxP3 (+) T cells with Cellsens software imaging system in CRC tissue. (a) PD-L1(+) T cells (b) CTLA-4(+) T cells, (c) CD8(+) T cells (d) FoxP3(+) T cells 100 × magnification (PDF 293 kb)
Supplementary Figure 3. Receiver operating characteristic (ROC) curve analysis for overall survival to decide the cut-off value of tumoral and circulating expression of immune checkpoints. (a) mPD-L1 (sensitivity: 0.92, specificity: 0.28, AUC: 0.57, cut-off: 4 (score of expression)) (b) mCTLA-4 (sensitivity: 0.48, specificity: 0.86, AUC: 0.65, cut-off: 6 (score of expression)) (c) sPD-L1 (sensitivity: 0.40, specificity: 0.86, AUC: 0.59, cut-off: 0.08 (ng/ml)) (d) sCTLA-4 (sensitivity: 0.32, specificity: 0.87, AUC: 0.51, cut-off: 1.79 (ng/ml)) (PDF 144 kb)
Acknowledgements
We thank all the patients, their families, and the investigators involved in this study. We also thank Mrs. Yuki Orito and Mrs. Amphone Okada for their excellent technical assistance and R J Frampton from Edanz Group for editing a draft of this manuscript.
Abbreviations
- CRC
Colorectal cancer
- CTLA-4
Cytotoxic T-lymphocyte-associated antigen 4
- DFS
Disease-free survival
- FFPE
Formalin-fixed, paraffin-embedded
- HCC
Hepatocellular carcinoma
- HR
Hazard ratios
- mPD-L1
Membrane-bound PD-L1
- mCTLA-4
Membrane-bound CTLA-4
- MSI
Microsatellite unstable
- MSS
Microsatellite Stable
- OS
Overall survival
- PD-1
Programmed cell death-1
- PD-L1
Programmed cell death ligand-1
- ROC
Receiver operating characteristic
- sPD-L1
Soluble PD-L1
- sCTLA-4
Soluble CTLA-4
- TME
Tumor microenvironment
- Treg cells
Regulatory T cells
- TILs
Tumor infiltrating lymphocytes
- TNM
Tumor–Node–Metastasis
- 95% CI
95% confidence interval
Author contributions
Study conception and design: YuO, YT, YOO, and MK; acquisition and analysis of the data: YuO, YT, YoO, AY, CY, KK, YK, TS, SI, TK, HF, HY, JH, and MO; interpretation of the data: YUO, YT, and YOO; drafting of the manuscript: YUO, YT, YOO, and MK; critical revision of the manuscript: YUO, YT, YOO, AY, CY, KK, YK, TS, SI, TK, HF, HY, JH, MO, and MK.
Funding
The work was partially supported by a Grant in Aid for Scientific Research (16K10533, 18K08592) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
Compliance with ethical standards
Conflict of interest
The authors have no conflicts of interest to disclose.
Consent to participate
Written informed consent was obtained from all patients in accordance with guidelines approved by the Institutional Review Board of Mie University.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yuji Toiyama, Email: ytoi0725@clin.medic.mie-u.ac.jp.
Yoshinaga Okugawa, Email: yoshinaga.okugawa@gmail.com.
References
- 1.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104–117. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 2.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Couzin-Frankel J. Breakthrough of the year 2013. Cancer Immunother Sci. 2013;342:1432–1433. doi: 10.1126/science.342.6165.1432. [DOI] [PubMed] [Google Scholar]
- 4.Goto M, Chamoto K, Higuchi K, et al. Analytical performance of a new automated chemiluminescent magnetic immunoassays for soluble PD-1, PD-L1, and CTLA-4 in human plasma. Sci Rep. 2019;9:10144. doi: 10.1038/s41598-019-46548-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci USA. 2010;107:4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang PY, Guo SS, Zhang Y, et al. Tumor CTLA-4 overexpression predicts poor survival in patients with nasopharyngeal carcinoma. Oncotarget. 2016;7:13060–13068. doi: 10.18632/oncotarget.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang XF, Pan K, Weng DS, et al. Cytotoxic T lymphocyte antigen-4 expression in esophageal carcinoma: implications for prognosis. Oncotarget. 2016;7:26670–26679. doi: 10.18632/oncotarget.8476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antonia S, Goldberg SB, Balmanoukian A, et al. Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncol. 2016;17:299–308. doi: 10.1016/s1470-2045(15)00544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Reilly EM, Oh DY, Dhani N, et al. Durvalumab with or without tremelimumab for patients with metastatic pancreatic ductal adenocarcinoma: a phase 2 randomized clinical trial. JAMA Oncol. 2019 doi: 10.1001/jamaoncol.2019.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang B, Huang T, Wei H, et al. The correlation and prognostic value of serum levels of soluble programmed death protein 1 (sPD-1) and soluble programmed death-ligand 1 (sPD-L1) in patients with hepatocellular carcinoma. Cancer Immunol Immunother CII. 2019;68:353–363. doi: 10.1007/s00262-018-2271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y, Xiao Y, Su M, Zhang R, Ding J, Hao X, Ma Y. Role of soluble programmed death-1 (sPD-1) and sPD-ligand 1 in patients with cystic echinococcosis. Exp Ther Med. 2016;11:251–256. doi: 10.3892/etm.2015.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pistillo MP, Fontana V, Morabito A, et al. Soluble CTLA-4 as a favorable predictive biomarker in metastatic melanoma patients treated with ipilimumab: an Italian melanoma intergroup study. Cancer Immunol Immunother CII. 2019;68:97–107. doi: 10.1007/s00262-018-2258-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shigemori T, Toiyama Y, Okugawa Y, et al. Soluble PD-L1 expression in circulation as a predictive marker for recurrence and prognosis in gastric cancer: direct comparison of the clinical burden between tissue and serum PD-L1 expression. Ann Surg Oncol. 2019;26:876–883. doi: 10.1245/s10434-018-07112-x. [DOI] [PubMed] [Google Scholar]
- 15.Zhang M, Li G, Wang Y, Wang Y, Zhao S, Haihong P, Zhao H, Wang Y. PD-L1 expression in lung cancer and its correlation with driver mutations: a meta-analysis. Sci Rep. 2017;7:10255. doi: 10.1038/s41598-017-10925-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang M, Sun H, Zhao S, Wang Y, Pu H, Wang Y, Zhang Q. Expression of PD-L1 and prognosis in breast cancer: a meta-analysis. Oncotarget. 2017;8:31347–31354. doi: 10.18632/oncotarget.15532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang P, Ouyang S, Wang J, Huang Z, Wang J, Liao L. Levels of programmed death-1 and programmed death ligand-1 in the peripheral blood of patients with oral squamous cell carcinoma and its clinical implications. Hua Xi Kou Qiang Yi Xue Za Zhi. 2015;33:529–533. doi: 10.7518/hxkq.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, Ohtani H. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58:3491–3494. [PubMed] [Google Scholar]
- 19.Martin F, Ladoire S, Mignot G, Apetoh L, Ghiringhelli F. Human FOXP3 and cancer. Oncogene. 2010;29:4121–4129. doi: 10.1038/onc.2010.174. [DOI] [PubMed] [Google Scholar]
- 20.Saito T, Nishikawa H, Wada H, et al. Two FOXP3(+)CD4(+) T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat Med. 2016;22:679–684. doi: 10.1038/nm.4086. [DOI] [PubMed] [Google Scholar]
- 21.Shang B, Liu Y, Jiang S-j, Liu Y. Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: a systematic review and meta-analysis. Sci Rep. 2015;5:15179. doi: 10.1038/srep15179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Idos GE, Kwok J, Bonthala N, Kysh L, Gruber SB, Qu C. The prognostic implications of tumor infiltrating lymphocytes in colorectal cancer: a systematic review and meta-analysis. Sci Rep. 2020;10:3360. doi: 10.1038/s41598-020-60255-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toiyama Y, Tanaka K, Kitajima T, et al. Elevated serum angiopoietin-like protein 2 correlates with the metastatic properties of colorectal cancer: a serum biomarker for early diagnosis and recurrence. Clin Cancer Res. 2014;20:6175–6186. doi: 10.1158/1078-0432.ccr-14-0007. [DOI] [PubMed] [Google Scholar]
- 24.Goel A, Nagasaka T, Hamelin R, Boland CR. An optimized pentaplex PCR for detecting DNA mismatch repair-deficient colorectal cancers. PLoS One. 2010;5:e9393. doi: 10.1371/journal.pone.0009393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim JW, Nam KH, Ahn SH, et al. Prognostic implications of immunosuppressive protein expression in tumors as well as immune cell infiltration within the tumor microenvironment in gastric cancer. Gastric Cancer. 2016;19:42–52. doi: 10.1007/s10120-014-0440-5. [DOI] [PubMed] [Google Scholar]
- 26.Ward FJ, Dahal LN, Wijesekera SK, Abdul-Jawad SK, Kaewarpai T, Xu H, Vickers MA, Barker RN. The soluble isoform of CTLA-4 as a regulator of T-cell responses. Eur J Immunol. 2013;43:1274–1285. doi: 10.1002/eji.201242529. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, Liang L, Dai W, Cai G, Xu Y, Li X, Li Q, Cai S. Prognostic impact of programed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor infiltrating lymphocytes in colorectal cancer. Mol Cancer. 2016;15:55. doi: 10.1186/s12943-016-0539-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masugi Y, Nishihara R, Yang J, et al. Tumour CD274 (PD-L1) expression and T cells in colorectal cancer. Gut. 2017;66:1463–1473. doi: 10.1136/gutjnl-2016-311421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Contardi E, Palmisano GL, Tazzari PL, et al. CTLA-4 is constitutively expressed on tumor cells and can trigger apoptosis upon ligand interaction. Int J Cancer. 2005;117:538–550. doi: 10.1002/ijc.21155. [DOI] [PubMed] [Google Scholar]
- 30.Chen X, Shao Q, Hao S, Zhao Z, Wang Y, Guo X, He Y, Gao W, Mao H. CTLA-4 positive breast cancer cells suppress dendritic cells maturation and function. Oncotarget. 2017;8:13703–13715. doi: 10.18632/oncotarget.14626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chau GY, Wu CW, Lui WY, et al. Serum interleukin-10 but not interleukin-6 is related to clinical outcome in patients with resectable hepatocellular carcinoma. Ann Surg. 2000;231:552–558. doi: 10.1097/00000658-200004000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gu D, Ao X, Yang Y, Chen Z, Xu X. Soluble immune checkpoints in cancer: production, function and biological significance. J Immunother Cancer. 2018;6:132. doi: 10.1186/s40425-018-0449-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ward FJ, Dahal LN, Khanolkar RC, Shankar SP, Barker RN. Targeting the alternatively spliced soluble isoform of CTLA-4: prospects for immunotherapy? Immunotherapy. 2014;6:1073–1084. doi: 10.2217/imt.14.73. [DOI] [PubMed] [Google Scholar]
- 34.Kushlinskii NE, Gershtein ES, Morozov AA, Goryacheva IO, Filipenko ML, Alferov AA, Bezhanova SD, Bazaev VV, Kazantseva IA. Soluble ligand of the immune checkpoint receptor (sPD-L1) in blood serum of patients with renal cell carcinoma. Bull Exp Biol Med. 2019;166:353–357. doi: 10.1007/s10517-019-04349-8. [DOI] [PubMed] [Google Scholar]
- 35.Yang J, Hu M, Bai X, Ding X, Xie L, Ma J, Fan B, Yu J. Plasma levels of soluble programmed death ligand 1 (sPD-L1) in WHO II/III nasopharyngeal carcinoma (NPC): a preliminary study. Medicine (Baltimore) 2019;98:e17231. doi: 10.1097/md.0000000000017231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng Z, Bu Z, Liu X, et al. Level of circulating PD-L1 expression in patients with advanced gastric cancer and its clinical implications. Chin J Cancer Res. 2014;26:104–111. doi: 10.3978/j.issn.1000-9604.2014.02.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ando K, Hamada K, Watanabe M, et al. Plasma levels of soluble PD-L1 correlate with tumor regression in patients with lung and gastric cancer treated with immune checkpoint inhibitors. Anticancer Res. 2019;39:5195–5201. doi: 10.21873/anticanres.13716. [DOI] [PubMed] [Google Scholar]
- 38.Dienstmann R, Mason MJ, Sinicrope FA, et al. Prediction of overall survival in stage II and III colon cancer beyond TNM system: a retrospective, pooled biomarker study. Ann Oncol. 2017;28:1023–1031. doi: 10.1093/annonc/mdx052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taieb J, Le Malicot K, Shi Q, et al. Prognostic value of BRAF and KRAS mutations in MSI and MSS stage III colon cancer. J Natl Cancer Inst. 2017 doi: 10.1093/jnci/djw272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–1765. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 41.Wu M, Kim YS, Ryu H-S, et al. MSI status is associated with distinct clinicopathological features in BRAF mutation colorectal cancer: a systematic review and meta-analysis. Pathol Res Pract. 2020;216:152791. doi: 10.1016/j.prp.2019.152791. [DOI] [PubMed] [Google Scholar]
- 42.Rosenbaum MW, Bledsoe JR, Morales-Oyarvide V, Huynh TG, Mino-Kenudson M. PD-L1 expression in colorectal cancer is associated with microsatellite instability, BRAF mutation, medullary morphology and cytotoxic tumor-infiltrating lymphocytes. Mod Pathol. 2016;29:1104–1112. doi: 10.1038/modpathol.2016.95. [DOI] [PubMed] [Google Scholar]
- 43.Shin SJ, Kim SY, Choi YY, Son T, Cheong JH, Hyung WJ, Noh SH, Park CG, Kim HI. Mismatch repair status of gastric cancer and its association with the local and systemic immune response. Oncologist. 2019;24:e835–e844. doi: 10.1634/theoncologist.2018-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fiegle E, Doleschel D, Koletnik S, Rix A, Weiskirchen R, Borkham-Kamphorst E, Kiessling F, Lederle W. Dual CTLA-4 and PD-L1 blockade inhibits tumor growth and liver metastasis in a highly aggressive orthotopic mouse model of colon cancer. Neoplasia. 2019;21:932–944. doi: 10.1016/j.neo.2019.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Overman MJ, Lonardi S, Wong KYM, et al. Nivolumab (NIVO) + low-dose ipilimumab (IPI) in previously treated patients (pts) with microsatellite instability-high/mismatch repair-deficient (MSI-H/dMMR) metastatic colorectal cancer (mCRC): long-term follow-up. J Clin Oncol. 2019;37:635. doi: 10.1200/jco.2019.37.4_suppl.635. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Representative images of tumoral mPD-L1 and mCTLA-4 expression in CRC cells. (a–d) PD-L1; (a) absent (b) weak (c) moderate (d) strong, 100 × magnification (e–h) CTLA-4; (e) absent (f) weak (g) moderate (h) strong, 100 × magnification (PDF 490 kb)
Supplementary Figure 2. Representative images of PD-L1, CTLA-4, CD8 and FoxP3 (+) T cells with Cellsens software imaging system in CRC tissue. (a) PD-L1(+) T cells (b) CTLA-4(+) T cells, (c) CD8(+) T cells (d) FoxP3(+) T cells 100 × magnification (PDF 293 kb)
Supplementary Figure 3. Receiver operating characteristic (ROC) curve analysis for overall survival to decide the cut-off value of tumoral and circulating expression of immune checkpoints. (a) mPD-L1 (sensitivity: 0.92, specificity: 0.28, AUC: 0.57, cut-off: 4 (score of expression)) (b) mCTLA-4 (sensitivity: 0.48, specificity: 0.86, AUC: 0.65, cut-off: 6 (score of expression)) (c) sPD-L1 (sensitivity: 0.40, specificity: 0.86, AUC: 0.59, cut-off: 0.08 (ng/ml)) (d) sCTLA-4 (sensitivity: 0.32, specificity: 0.87, AUC: 0.51, cut-off: 1.79 (ng/ml)) (PDF 144 kb)