Abstract
Cervical cancer is the most common malignant tumor of the genital tract in females worldwide. Persistent human papillomavirus (HPV) infection is closely associated with the occurrence of cervical cancer. No licensed therapeutic HPV vaccines for cervical cancer are currently available. In our previous study, we demonstrated that the vaccine containing the HPV16 E7 43-77 peptide and the adjuvant unmethylated cytosine-phosphate-guanosine oligodeoxynucleotide elicited significant prophylactic and therapeutic effects on cervical cancer. In the current study, we comprehensively evaluated the effect of the vaccine on systemic immune responses and the tumor microenvironment (TME) in a mouse model of cervical cancer. The results showed that the administration of the vaccine induced a significant increase in splenic IFN-γ-producing CD4 and CD8 T cells as well as tumor infiltrating CD4 and CD8 T cells. Moreover, marked decreases in splenic MDSCs and Tregs as well as intratumoral MDSCs, Tregs and type 2-polarized tumor-associated macrophages were observed in the vaccine group. The profile of cytokines, chemokines and matrix metalloproteinases (MMPs) in the TME revealed significantly increased expression of IL-2, IL-12, TNF-α, IFN-γ, CCL-20, CXCL-9, CXCL-10 and CXCL-14 and decreased expression of IL-6, IL-10, TGF-β, CCL-2, CCL-3, CCL-5, CXCL-8, MMP-2, MMP-9 and VEGF in the vaccine group. The expression of the cell proliferation indicator Ki67, apoptosis regulatory protein p53 and angiogenesis marker CD31 was significantly decreased in the vaccine group. In conclusion, the vaccine reversed tolerogenic systemic and local TME immunosuppression and induced robust antitumor immune responses, which resulted in the inhibition of established implanted tumors.
Electronic supplementary material
The online version of this article (10.1007/s00262-020-02651-3) contains supplementary material, which is available to authorized users.
Keywords: Cervical cancer, Human papillomavirus, Therapeutic vaccine, Tumor microenvironment, Immunosuppression
Introduction
Cervical cancer is the most common malignant tumor of the genital tract in females with an estimated 569,847 new cases and 311,365 deaths in 2018 worldwide, which represents a major global health challenge [1]. Persistent infection with high risk human papillomavirus (HPV), a major carcinogenic pathogen of human beings, is closely related to the occurrence and the development of cervical cancer [2]. Among the high-risk subtypes of HPV, type 16 (53.5%) and type 18 (17.2%) account for approximately 70% of all cervical cancer cases [3]. Vaccines targeting HPV 16 and HPV 18 have the potential to prevent most deaths in unscreened women with cervical cancer and to substantially reduce the anxiety and costs related to the detection, diagnosis, and treatment of precancerous lesions and cervical cancer. To date, three licensed prophylactic HPV vaccines, a bivalent vaccine (Cervarix, GlaxoSmithKline, GSK), tetravalent vaccine (Gardasil, Merck Sharp & Dohme, MSD) and nine-valent vaccine (Gardasil 9, MSD), are currently available to prevent common HPV infection [4]. Despite the encouraging progress in the field of prevention, there are still a wide range of emergent issues to be resolved. First, vaccine introduction and widespread application in low- and middle-income countries is impeded by sociocultural, financial and political hurdles [5]. In addition, current prophylactic HPV vaccines do not eliminate existing HPV infections or interfere with precancerous lesion progression to malignancy [6]. Therefore, it is urgently necessary to develop safe and efficacious therapeutic vaccines to clear established HPV infection and even cervical cancer.
The high-risk HPV oncoproteins E6 and E7 play important roles in initiating and maintaining HPV-associated malignancies. These oncoproteins are continuously expressed on HPV-infected cells and HPV-transformed tumor cells but not normal cells, which makes these two oncoproteins as excellent target antigens for T cell-mediated immunotherapeutic strategies.
Cellular immunity is necessary for clearing HPV-infected and HPV-transformed tumor cells. HPV-specific CD8 cytotoxic T lymphocytes (CTLs) are considered critical for the immune defense against cervical cancer. However, the function of CTLs may be blunted by systemic and local immunosuppressive environments associated with tumor growth. A series of clinical trials [7–9] showed that the immune system is unable to completely eradicate the tumor despite the presence of HPV-specific T cells in HPV-associated neoplastic tissue, which suggests the possible existence of systemic immunosuppression and an immunosuppressive tumor microenvironment (TME) that significantly influence the efficacy of therapeutic vaccines and clinical outcome. Therefore, an ideal therapeutic vaccine for cervical cancer is capable of inducing robust cellular immunity as well as overcoming or reversing the cancer-associated systemic and local immunosuppressive environment.
In our previous study, we demonstrated that single administration of a therapeutic vaccine containing the HPV16 E7 43-77 peptide, which contains both a CTL epitope (E7 49-57) and two Th epitopes (E7 50-62 and E7 43-77), and the adjuvant unmethylated cytosine-phosphate-guanosine oligodeoxynucleotide (CpG ODN) in C57BL/6 mice bearing cervical cancer induced the complete clearance of tumors 24 days after vaccination [10]. In the current study, to investigate the effect of the vaccine on the TME, we chose an end point of 10 days after vaccination in the murine tumor model when the tumors are available in all the mice. We further evaluated the antitumor efficacy of the vaccine, specifically the effect of the vaccine on the systemic immune responses, as well as the TME of cervical cancer. The results showed that the vaccinations induced an increase in systemic and local CD4 and CD8 T cells and a decrease in immunosuppressive cells such as myeloid-derived suppressor cells (MDSCs), regulatory T cells (Tregs), and M2-polarized tumor-associated macrophages (M2-TAMs), which may ultimately result in the efficient control of established implanted tumors. Because the vaccine strategy used in this study offers significant therapeutic effects, these data provide a solid foundation for future clinical trials.
Materials and methods
TC-1 cell culture
The TC-1 cell line (Beijing Beina Chuanglian Biotechnology Institute, Beijing, China) was derived from primary lung epithelial cells from C57BL/6 mice and were cotransformed with HPV16 E6, HPV16 E7 and c-Ha-ras oncogenes. TC-1 cells were maintained in Roswell Park Memorial Institute 1640 (Biological Industries, Kibbutz Beit Haemek, Israel) medium supplemented with 10% fetal bovine serum (Biological Industries, Kibbutz Beit Haemek, Israel), 400 μg/ml G418 (Genview, Tallahasses, FL, USA), 100 U/ml penicillin and 100 μg/ml streptomycin (Biological Industries, Kibbutz Beit Haemek, Israel) at 37 °C in humidified air containing 5% CO2.
Peptide and adjuvant
The HPV16 E7 peptide (E7 43-77: GQAEPDRAHYNIVTFCCKCDSTLRLCVQSTHVDIR) was synthesized at GL Biochem (Shanghai) Ltd. (Shanghai, China). The purity of the peptide was determined by high-pressure liquid chromatography and was found to be greater than 95%. The peptide was used at a dose of 50 μg/mouse and was stored at − 20 °C if not used immediately. CpG ODN 1826 (5′-TCCATGACGTTCCTGACGTT-3′) was provided by Sangon Biotech (Shanghai) Co. Ltd. (Shanghai, China) and was used at a dose of 20 μg/mouse.
Animal experiments
Female C57BL/6 mice aged 6–8 weeks were obtained from Liaoning Changsheng Biotechnology Co. Ltd. (Benxi, China), housed in a specific pathogen-free environment and treated in accordance with the guidelines for the proper use and care of animals established by the Institutional Animal Care and Use Committee of the China Medical University.
All the mice were injected subcutaneously into the right flank with a single-cell suspension of 5 × 105 TC-1 cells (in 100 μl of PBS) for tumor formation at day 0. Four days after tumor cell inoculation, the mice were randomly divided into four groups. The three experimental groups were immunized subcutaneously in the left flank with (1) 20 μg of CpG alone, (2) 50 μg of HPV16 E7 peptide alone, or (3) vaccine containing 20 μg of CpG and 50 μg of E7 peptide in a total volume of 100 μl. The control group was immunized subcutaneously with 100 μl of PBS. Tumor volume was measured using an electronic caliper (Pro’skit, Shanghai, China) to determine the largest (a) and the smallest (b) superficial diameters of the tumor and then calculated according to the formula V = 0.5 × a × b2.
Preparation of single-cell suspensions
At day 14, the mice were euthanized by cervical dislocation. To prepare the splenocyte suspensions, mouse spleens were harvested and mashed on a stainless steel mesh, and the dissociated cells were passed through a 100 μm cell strainer. The tumors were cut into small pieces using a blade and incubated in 2 mg/ml Collagenase D (Roche, Diagnostics, Indianapolis, IN, USA) and 0.4 mg/ml DNase (Sigma-Aldrich, St. Louis, MO) at 37 °C for 90 min, after which the tumor cells were filtered using a 100 μm strainer to obtain a single-cell suspension.
Flow cytometry
The following Abs (eBioscience, San Diego, CA, USA) were used for surface staining: FITC-conjugated anti-mouse CD4, PerCP-conjugated anti-mouse CD8a, PerCP/Cy5.5-conjugated anti-mouse CD11b, APC-conjugated anti-mouse Gr-1, PE-conjugated anti-mouse F4/80, FITC-conjugated anti-mouse CD86, and the respective isotype Abs.
Splenocytes (1 × 106) were cultured with Phorbol 12- myristate 13-acetate (PMA, 1 μg/ml, Sigma-Aldrich, St. Louis, MO, USA) and Ionomycin (50 μg/ml, Sigma-Aldrich, St. Louis, MO, USA) stimulation for 5 h. GolgiPlug (BD Biosciences, San Diego, CA, USA) was added to the cultures to inhibit protein secretion. Cells were stained with the surface markers CD4 and CD8a, fixed and stained with the intracellular marker PE-conjugated anti-mouse IFN-γ (eBioscience, San Diego, CA, USA) and analyzed by flow cytometry.
Intracellular APC-conjugated anti-mouse CD206 (eBioscience, San Diego, CA, USA) and IFN-γ staining was performed using the Cytofix/Cytoperm kit according to the manufacturer’s instructions (BD Biosciences, San Diego, CA, USA). For intranuclear staining of Foxp3, cells were fixed and permeabilized using the Foxp3/Transcription Factor Staining Buffer Set according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA, USA) and then stained with PE-conjugated anti-mouse Foxp3 (eBioscience, San Diego, CA, USA). All samples were acquired using a BD LSRFortessa flow cytometer (BD Biosciences, USA). The raw data were analyzed using FlowJo Flow Cytometry Analysis Software (Treestar, Ashland, OR, USA).
Quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR)
Total cellular RNA was isolated from tumor cells using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The extracted RNA was used for reverse transcription using a Reverse Transcription System (Promega, Madison, WI, USA). The SYBR qPCR Master Mix (Vazyme, Nanjing, China) was used to perform the PCR analysis. Specific RT-PCR primers are listed in Table 1. The mouse β-actin gene expression value was used as an endogenous control to normalize the expression of the genes of interest. The 2−ΔΔCt method was used to express the ratio between the gene of interest and the internal reference gene (β-actin).
Table 1.
Specific RT-PCR primers
| Gene | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|
| β-actin | CATCCGTAAAGACCTCTATGCCAAC | ATGGAGCCACCGATCCACA |
| IL-2 | AAGCTCTACAGCGGAAGCAC | TCATCGAATTGGCACTCAAA |
| IL-6 | AGCAGCATCACCTTCGCTTAG | GTGTCCAGATATTGGCATGGG |
| IL-10 | AGGATGCACATCAAAAGGCTT | GGCCTCGGTTAGGAAGGATAC |
| IL-12 | CAATCACGCTACCTCCTCTTTT | CAGCAGTGCAGGAATAATGTTTC |
| TNF-α | CCAACATGCTGATTGATGACACC | GAGAATGCCAATTTTGATTGCCA |
| TGF-β | AATGGTACCGTCAGTGCTGGAAATA | TGGCTCATGTTGCAGAGGCTA |
| IFN-γ | ACTCMGTGGCATAGATGTGGMG | GACGCTTATGTTGTTGCTGATGG |
| CCL-2 | TAAAAACCTGGATCGGAACCAAA | GCATTAGCTTCAGATTTACGGGT |
| CCL-3 | TGAGAGTCTTGGAGGCAGC | ATGCAGGTGGCAGGAATG |
| CCL-5 | CCCTGTCATTGCTTGCTCT | ATGCTGATTTCTTGGGTTTG |
| CCL-20 | CGACTGTTGCCTCTCGTACAT | AGCCCTTTTCACCCAGTTCT |
| CXCL-8 | GATTCACCTCAAGAACATCCAGA | GGACACCTTTTAGCATCTTTTGG |
| CXCL-9 | CTTGAGCCTAGTCGTGATAAC | CCAGCTTGGTGAGGTCTATC |
| CXCL-10 | CCACGTGTTGAGATCATTGC | AGTAGCAGCTGATGTGACC |
| CXCL-14 | TGGTTATCGTCACCACCAAG | TCTCTCAACTGGCCTGGAGT |
| MMP-2 | CATCGTAGTTGGCTGTGGTCG | GTCTTCCCCTTCACTTTCCTG |
| MMP-9 | GCAGAGGCATACTTGTACCG | TGATGTTATGATGGTCCCACTTG |
| VEGF | GCACATAGAGAGAATGAGCTTCC | CTCCGCTCTGAACMGGCT |
Immunohistochemistry
Formalin-fixed and paraffin-embedded tissue was cut into 5 µm sections and stained with hematoxylin and eosin (H&E) for histopathological examination. For immunohistochemistry, antigen retrieval was performed with EDTA buffer at pH = 9.0. Endogenous peroxidase activity and nonspecific binding were blocked using UltraSensitive S-P kits (Maixin Biotechnology, Fuzhou, Fujian, China) according to the manufacturer’s instructions. The slides were then incubated overnight at 4 °C with the following primary antibodies: CD4, CD8, Ki67, p53 and CD31 (rabbit polyclonal antibodies, ABclonal Biotech Co, Ltd, Wuhan, China, 1:200 dilution). This step was followed by the incubation with biotinylated secondary antibodies and streptavidin–horseradish peroxidase (Maixin Biotechnology, Fuzhou, Fujian, China). After incubation, 3,3′-diaminobenzidine-tetrahydrochloride was applied as chromogen (Maixin Biotechnology, Fuzhou, Fujian, China). Finally, the sections were lightly counterstained in hematoxylin and coverslipped. Negative controls were prepared by omitting the primary antibodies.
Evaluation of immunohistochemical staining intensity
The staining intensity was scored as follows: no staining (0), weak staining (1), moderate staining (2) and strong staining (3). The positive proportion of stained tumor cells was defined as follows: 0 (0%), 1 (1–10%), 2 (11–50%), 3 (51–80%), and 4 (> 80%). The staining results were semiquantitatively assessed by multiplying the staining intensity by the percentage of positively stained tumor cells. Two experienced pathologists scored the slides, and disagreements were resolved by consensus.
Statistical analysis
GraphPad Prism software version 5.0 (GraphPad Software Inc., San Diego, CA, USA) was used to perform all statistical tests and prepare the graphs. Statistically significant differences were determined by one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. The data are expressed as the mean ± standard deviation (SD). A value of p < 0.05 was considered to be statistically significant.
Results
Antitumor effect of the vaccine
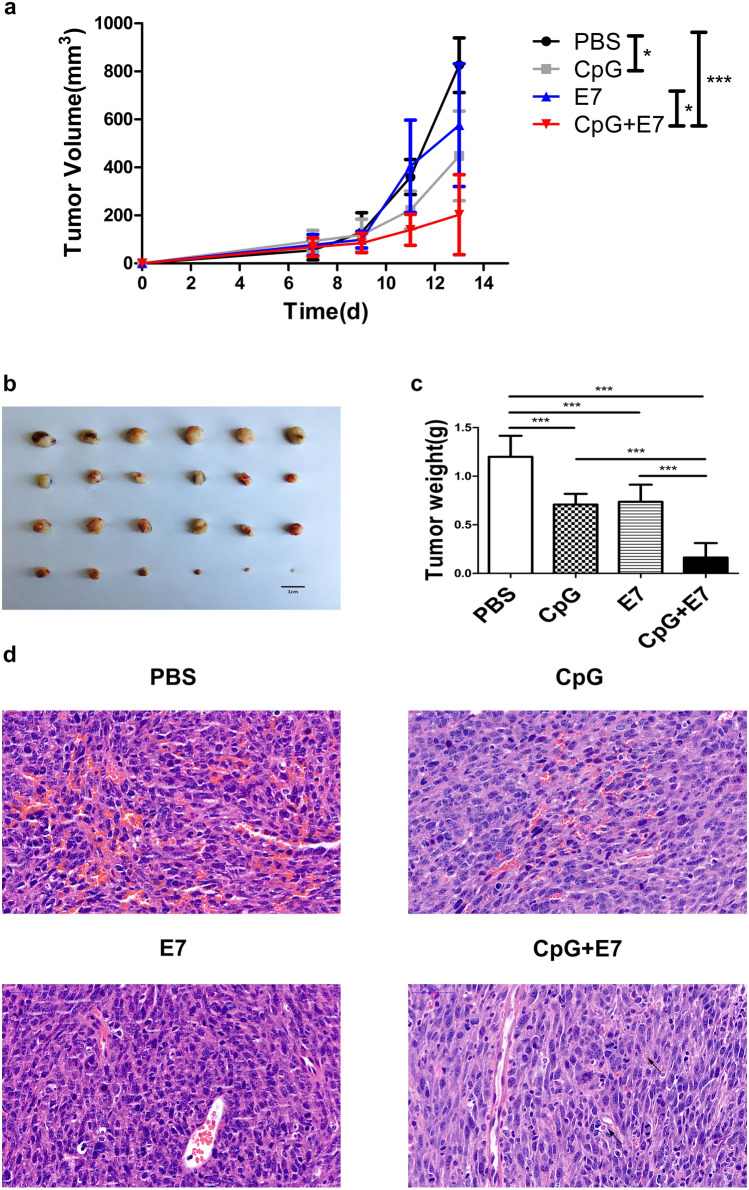
The antitumor effect induced by vaccination was confirmed by the tumor size, tumor weight, and the tumor histopathological analysis results in the C57BL/6 murine tumor model (Fig. 1a–d). Compared to the PBS group, the administration of CpG alone, HPV E7 peptide alone and the vaccine containing the HPV16 E7 43-77 peptide with the adjuvant CpG ODN (the combination of CpG and HPV E7 peptide was referred to as the vaccine) resulted in inhibition of tumor growth (Fig. 1b), as indicated by 45.78%, 30.23% and 75.44% reductions in tumor volume (Fig. 1a), respectively, as well as 40.97%, 38.61% and 86.39% reductions in tumor weight, respectively (Fig. 1b, c). The vaccine significantly inhibited tumor growth compared to that of CpG alone or peptide alone (Fig. 1a–c). To further evaluate the antitumor efficacy of the therapeutic vaccine, tumor sections were stained with H&E. Histopathological analysis indicated that the blood vessel density of the vaccine group was significantly lower than that of the other three groups, and scattered apoptotic cells only appeared in the vaccine group. The apoptotic cells are highlighted by arrows. (Fig. 1d).
Fig. 1.
Antitumor efficacy of the vaccine containing the HPV E7 43-77 peptide and CpG ODN in vivo. a Tumor growth curves in tumor-bearing mice. TC-1 cells (5 × 105) were subcutaneously injected into C57BL/6 mice at day 0, followed by subcutaneous administration of PBS, CpG, E7 peptide and CpG + E7 peptide at day 4. Photography of tumors which were excised from the mice at day 14 and tumor weights (grams) in the four groups are shown in b and c, respectively. d Representative images of H&E-stained tumor sections. Scale bar = 50 μm. The data are shown as the mean ± SD (n = 6). The asterisks indicate statistically significant differences between the control group and experimental groups, as determined by one-way ANOVA followed by Tukey’s multiple comparison test (*p < 0.05, ***p < 0.001)
Effect of the vaccine on the systemic immune responses
At day 10 following the administration of the vaccine, mouse spleens were harvested, and splenocytes were subjected to flow cytometry analysis to assess the frequency of CD4+ IFN-γ+ T cells, CD8+ IFN-γ+ T cells, Tregs and MDSCs. The percentage of IFN-γ-producing CD4+ T cells significantly increased from 1.16% in control mice to 3.63% in vaccine-inoculated mice (S1a-b, p < 0.05). Moreover, significant increases in the percentage of IFN-γ-producing CD8+ T cells were observed in mice inoculated with the vaccine (S1c-d, p < 0.01). The administration of the vaccine significantly reduced the percentage of MDSCs (S1e-f; control group: 3.19% ± 1.14% vs. vaccine group: 1.58% ± 0.11%; p < 0.05) and Tregs (S1g-h; control group: 3.07% ± 0.19% vs. vaccine group: 1.94% ± 0.57%; p < 0.01) in the spleen.
Effect of the vaccine on immune cells in the TME
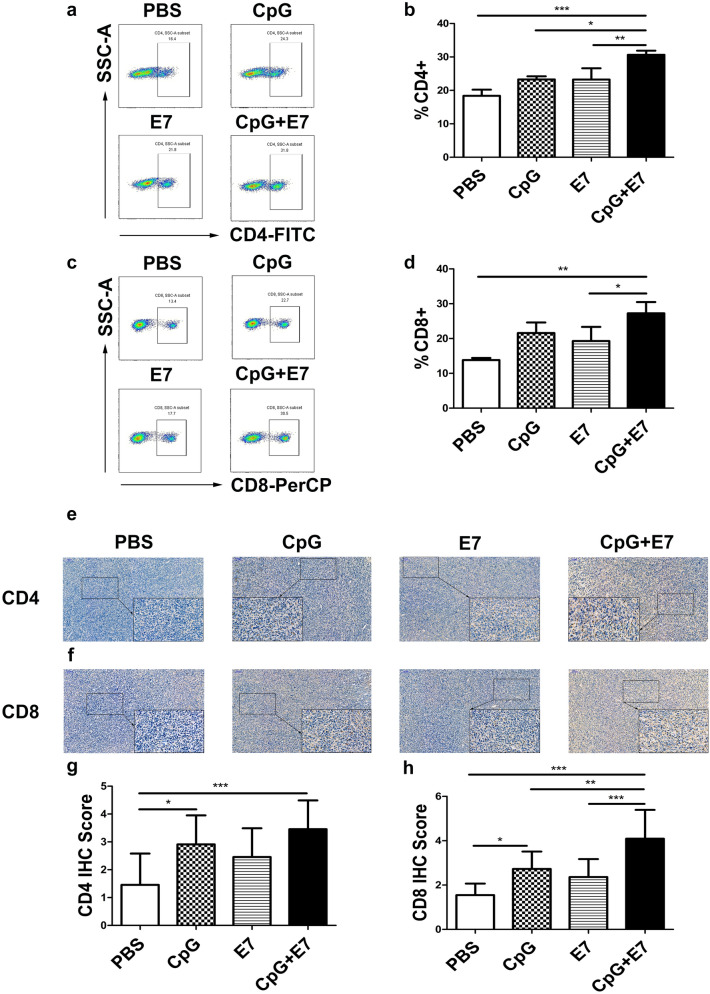
We next examined the effects of the vaccine on immune cells in the TME of cervical cancer. The numbers of infiltrating CD4 T cells and CD8 T cells within the tumor were assessed by flow cytometry. Administration of the vaccine significantly expanded the populations of infiltrating CD4 and CD8 T cells (expressed as a percentage of the total viable cells within the tumor) in the tumor, increasing their percentages from 18.37% and 13.80% in the control group to 30.67% (p < 0.001) and 27.27% (p < 0.01) in the vaccine group, respectively (Fig. 2a–d). Moreover, inoculation with the vaccine induced a significant increase in CD4 T cells compared to that of CpG alone (Fig. 2b, p < 0.05) and E7 peptide alone (Fig. 2b, p < 0.01), and a significant increase in CD8 T cells compared to that of E7 peptide alone (Fig. 2d, p < 0.05). The distribution of intratumoral CD4 and CD8 T cells was assessed by immunohistochemistry. The expression of CD4 and CD8 was assessed by determining the percentage of positive cells and the staining intensity. As shown in Fig. 2e, f, the positive staining intensity and percentage of CD4 and CD8 cells in the CpG and vaccine groups indicated higher expression than in the control group. In addition, immunohistochemical analysis showed that the increase in the population of CD8 cells in the tumor of the vaccine group was significantly different from that of the CpG group (Fig. 2h, p < 0.01) and the E7 peptide alone group (Fig. 2h, p < 0.001).
Fig. 2.
The effect of the vaccine on CD4 and CD8 cells in the TME. a–d The vaccine increased the number of infiltrating CD4 and CD8 T cells in the tumor. Representative flow cytometry scatter plots from one mouse (one out of three mice) showing tumor-infiltrating a CD4 and c CD8 T cells. Flow cytometry data showing tumor-infiltrating CD4 and CD8 T cells is represented as a bar graph expressed as b %CD4+ T cells and d %CD8+ T cells. The distribution of intratumoral e CD4 and f CD8 T cells was assessed by immunohistochemistry. The densities of g CD4 and h CD8 T cells in the vaccine group and CpG alone group were significantly higher than those in the control group. The data are depicted as the mean ± SD (n = 3). The significance of the data was evaluated by one-way ANOVA followed by Tukey’s multiple comparison test (*p < 0.05, **p < 0.01, ***p < 0.001)
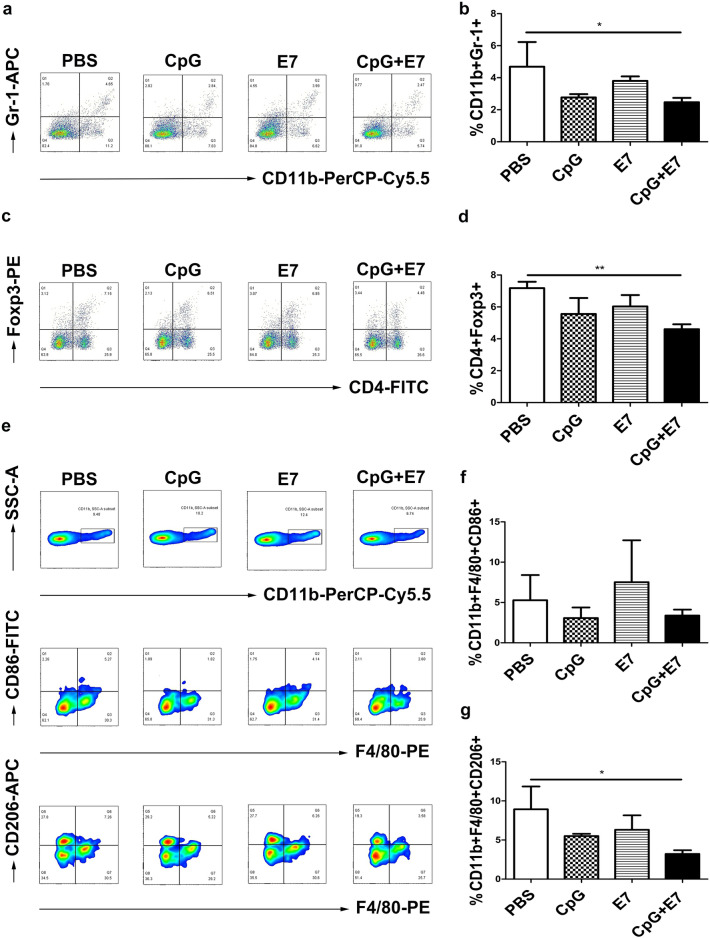
Vaccine administration resulted in a 47.33% (Fig. 3a, b, p < 0.05) decrease in intratumoral MDSCs compared to that of control mice. Moreover, the vaccine significantly reduced the percentage of Tregs compared to that of control mice (Fig. 3c, d; control: 7.18% ± 0.40% vs. CpG + E7: 4.61% ± 0.30%; p < 0.01). The percentage of intratumoral M2-TAMs (CD11b + F4/80 + CD206 +) decreased from an average of 9.24% in control mice to 3.22% in mice inoculated with the vaccine (Fig. 3e, g, p < 0.05). There was no significant difference in the proportion of M1-TAMs (CD11b+ F4/80+ CD86+) between the experimental groups and the control group. In summary, the vaccine and CpG ODN alone significantly increased intratumoral CD4 and CD8 T cells, while the vaccine substantially reduced immunosuppressive cells including Tregs, MDSCs and M2-TAMs within the TME.
Fig. 3.
The effect of the vaccine on immunosuppressive cells in the TME. The vaccine reduced the number of intratumoral immunosuppressive cells, including a, b MDSCs; c, d Tregs; e, g M2-TAMs. Representative flow cytometry scatter plots from one mouse (one out of three mice) showing intratumoral a MDSCs, c Tregs, e M1-TAMs, and M2-TAMs. Flow cytometry data showing tumor-infiltrating MDSCs, Tregs, M1-TAMs and M2-TAMs is represented as a bar graph expressed as b %CD11b+ Gr-1+ cells, d %CD4+ Foxp3+ cells, f %CD11b+ F4/80+ CD86+ cells and g %CD11b+ F4/80+ CD206+ cells. The data are depicted as the mean ± SD (n = 3). The significance of the data was evaluated by one-way ANOVA followed by Tukey’s multiple comparison test (*p < 0.05, **p < 0.01)
Effect of the vaccine on cytokines, chemokines and matrix metalloproteinases (MMPs) in the TME
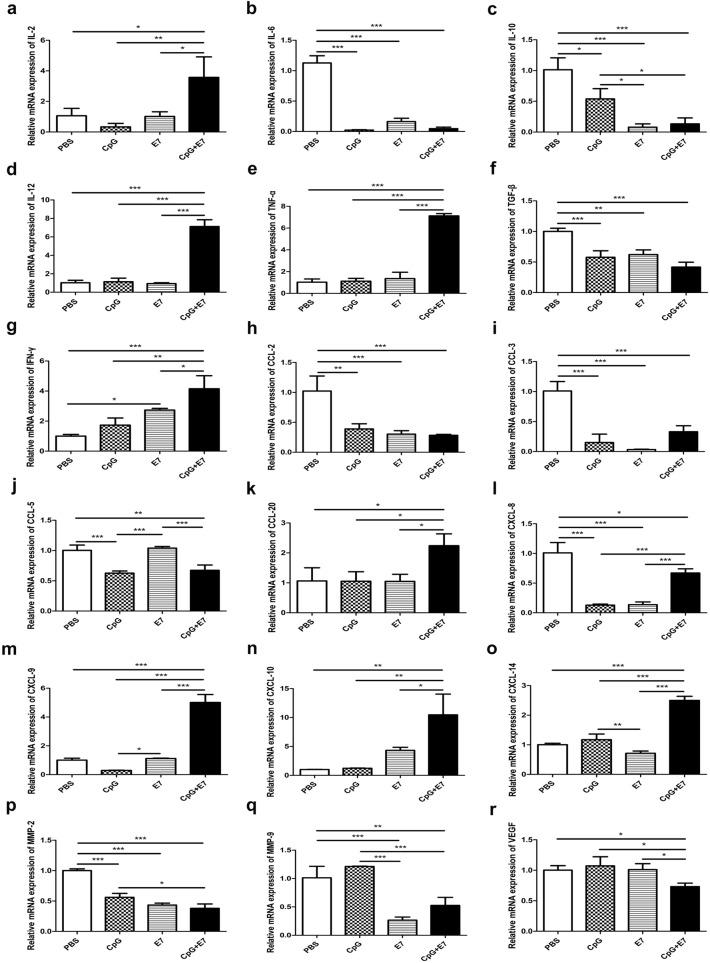
The TME is the primary location of the interaction between tumor cells and the host immune system. Different immune cell subsets are recruited to the TME via interactions between chemokines and chemokine receptors, and these cell populations have distinct effects on tumor progression and therapeutic outcomes [11]. Therefore, we used qRT-PCR to quantify the mRNAs encoding a panel of cytokines, chemokines and MMPs in the TME. The expression of transcripts for IL-2, IL-12, TNF-α, IFN-γ, CC-chemokine ligand (CCL)-20, CXC-chemokine ligand (CXCL)-9, CXCL-10 and CXCL-14 was significantly higher in tumor tissue from mice inoculated with the vaccine than in samples from control mice. The relative expression levels of IL-2, IL-12, TNF-α and IFN-γ in the vaccine group were significantly increased by 2.5-fold (Fig. 4a, p < 0.05), sixfold (Fig. 4d, p < 0.001), sixfold (Fig. 4e, p < 0.001) and threefold (Fig. 4g, p < 0.001), respectively, compared to those in the control group. In addition, CCL-20, CXCL-9, CXCL-10 and CXCL-14 mRNA expression levels in the tumor tissue of vaccine-inoculated mice were increased by onefold (Fig. 4k, p < 0.05), fourfold (Fig. 4m, p < 0.001), ninefold (Fig. 4n, p < 0.01) and 1.5-fold (Fig. 4o, p < 0.001), respectively, compared to those of control mice. Furthermore, the single use of E7 resulted in a significant increase in the relative expression of intratumoral IFN-γ (Fig. 4g, p < 0.05) compared to that of control mice.
Fig. 4.
The relative expression of cytokines, chemokines and MMPs. The relative expression levels of a IL-2, b IL-6, c IL-10, d IL-12, e TNF-α, f TGF-β, g IFN-γ, h CCL-2, i CCL-3, j CCL-5, k CCL-20, l CXCL-8, m CXCL-9, n CXCL-10, o CXCL-14, p MMP-2, q MMP-9, and r VEGF in the tumor tissue are shown. All PCR data were calculated relative to β-actin and represent the average ± SD of triplicate samples. The significance of the data was evaluated by one-way ANOVA followed by Tukey’s multiple comparison test (*p < 0.05, **p < 0.01, ***p < 0.001)
The transcription levels of IL-6, IL-10, TGF-β, CCL-2, CCL-3, CCL-5, CXCL-8, MMP-2, MMP-9 and VEGF were significantly lower in the vaccine group than in the control group. The relative mRNA levels of IL-6, IL-10 and TGF-β in the tumor tissue of vaccinated mice were reduced by 95.86% (Fig. 4b, p < 0.001), 86.84% (Fig. 4c, p < 0.001) and 58.33% (Fig. 4f, p < 0.001), respectively, compared to those of control mice. Moreover, the mRNA expression of CCL-2, CCL-3, CCL-5, CXCL-8, MMP-2, MMP-9 and VEGF in the vaccine group was significantly reduced by 72.31% (Fig. 4h, p < 0.001), 67.33% (Fig. 4i, p < 0.001), 32.91% (Fig. 4j, p < 0.01), 33.66% (Fig. 4l, p < 0.05), 62% (Fig. 4p, p < 0.001), 48.03% (Fig. 4q, p < 0.01) and 26.94% (Fig. 4r, p < 0.05), respectively, compared to that in the control group. Moreover, CpG alone resulted in significantly decreased expression of intratumoral IL-6, IL-10, TGF-β, CCL-2, CCL-3, CCL-5, CXCL-8 and MMP-2 compared to that in control mice. Likewise, the relative expression of IL-6, IL-10, TGF-β, CCL-2, CCL-3, CXCL-8, MMP-2 and MMP-9 in mice inoculated with the E7 peptide alone significantly decreased compared to that of the control group. We also examined the relative mRNA expression levels of IL-4, CCL-12, CCL-19, CCL-21, MMP-3 and MMP-7, but the differences did not reach statistical significance (data not shown).
Effect of the vaccine on cellular proliferation, apoptosis and angiogenesis in tumors
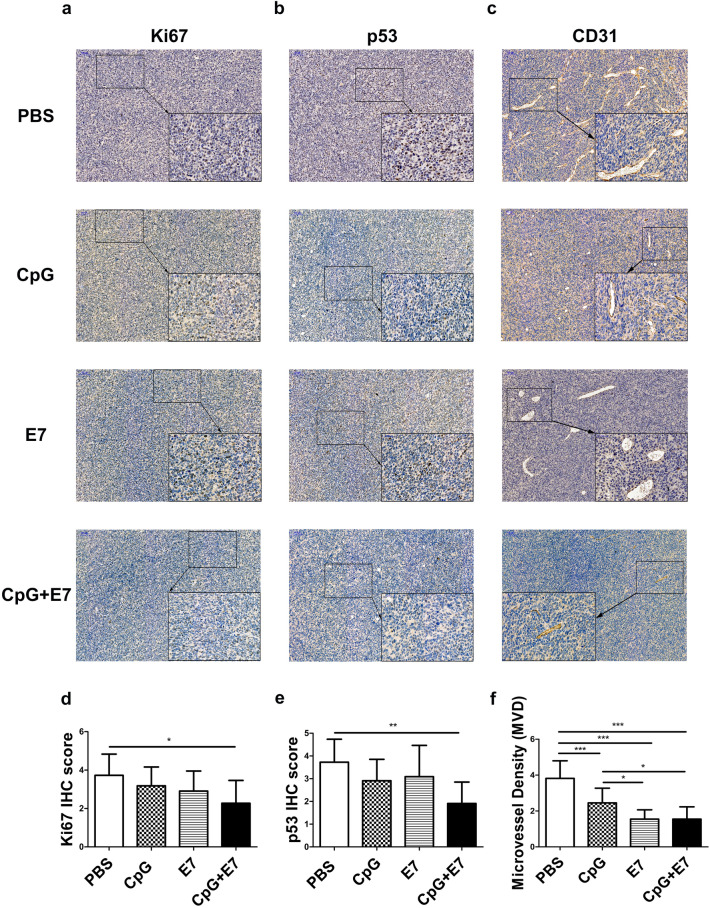
The cell proliferation indicator Ki67 and apoptosis regulatory protein p53 are primarily expressed in the nucleus of tumor cells, and positive immunohistochemical staining is exhibited as yellow to brown. As shown in Fig. 5a, b, d, e, we observed a significant decrease in the number of Ki67- and p53-immunopositive cells in the vaccine group. CD31 staining was used to evaluate microvessel density (MVD). All capillaries expressing membranous CD31 were counted at 400× magnification. We found a significantly less MVD in the vaccine group (1.55 ± 0.69) than in the control group (3.82 ± 0.98) (Fig. 5c, f), which suggests that the vaccine reduced the MVD in the tumors. In addition, CpG alone or the E7 peptide alone significantly decreased the MVD by 35.71% and 59.52%, respectively, compared to that of the control group (Fig. 5c, f).
Fig. 5.
Effect of the vaccine on cellular proliferation, apoptosis and vessel density in tumors. Fewer a, d Ki67+ and b, e p53+ cells were quantified in mice treated with the vaccine compared to the control mice. c, f The expression of CD31 was significantly decreased in the vaccine group compared to the control group. Representative images of a Ki67, b p53 and c CD31 staining are shown. Images are shown at 100× magnification. Scale bars = 100 μm. Insert images are at 400× magnification and scale bars = 50 μm. The data are depicted as the mean ± SD. The significance of the data was evaluated by one-way ANOVA followed by Tukey’s multiple comparison test (*p < 0.05, **p < 0.01, ***p < 0.001)
Discussion
Tumor-specific CD8 T cells and CD4 T cells play pivotal roles in antitumor immunity [12]. The simultaneous induction of these two cell subsets by a single vaccine would be highly efficacious. In our previous study, the increased cellular immunity mediated by CTLs and Th1 cells was observed 24 days after inoculation with the vaccine containing both CD4 and CD8 T cell epitopes [10]. In the present study, we measured immune responses 10 days after vaccination and showed the significantly increased IFN-γ-producing CD8 and CD4 T cells in the spleen, which suggested that the vaccine induced robust systemic cellular immunity within 10 days. In particular, activated CD4 Th1 cells produce several cytokines, such as IL-2, TNF-α and IFN-γ, which are essential for the induction of cell-mediated immunity against tumors [13]. The increased number of intratumoral CD4 T cells as well as the increased levels of IL-2, TNF-α and IFN-γ (Fig. 2a, e, 4a, e, g) in the vaccine group indicated that the vaccine induced local Th1-polarized CD4 T cells, which promote potent antitumor effects.
A bottleneck for cancer immunotherapy is the presence of immunosuppressive cells, such as MDSCs, Tregs, and TAMs, which cause the subversion of antitumor immunity in the TME and promote tumor growth and metastasis [14]. MDSCs are a heterogeneous population of bone marrow-derived immature myeloid cells [15], which are broadly identified as CD11b+ Gr-1+ cells in mice [16] and are divided into two different types: polymorphonuclear MDSCs (PMN-MDSCs) and monocytic MDSCs (M-MDSCs) [17]. CCL-2 and CCL-5 are the main chemokines implicated in M-MDSC migration to tumors [17, 18]. Moreover, PMN-MDSCs are recruited primarily by CXC chemokines such as CXCL-8 [17]. Our results showed that reduced levels of intratumoral CCL-2, CCL-5 and CXCL-8 (Fig. 4h, j, l) in the vaccine group. The therapeutic blockade of these chemokines induced by vaccination may have a significant impact on the recruitment of MDSCs into tumors and may explain the reduced number of MDSCs in the tumor, as shown in vaccine group by flow cytometry (Fig. 3b). It was demonstrated that naive T cells are particularly sensitive to MDSC-mediated suppression [19]. Spontaneous and vaccine-induced T cell responses are curtailed by MDSCs [20]. Therefore, MDSCs are a major type of immunosuppressive cell that may lead to immune evasion and progression in some cancers [21]. The increased frequency of circulating granulocytic MDSCs is associated with tumor burden and recurrence in early and locally advanced cervical cancer patients [22]. In this study, decreased levels of MDSCs in the spleen (S1f) and tumors (Fig. 3b) induced by the vaccine indicates a reduction in the inhibition of MDSC-mediated systemic and local T cell responses.
Tregs are a functionally mature subpopulation of T cells that play an indispensable role in immune homeostasis and self-tolerance [23]. The recruitment and activation of Tregs at tumor sites is associated with poor prognosis [24–27]. It has been demonstrated that increased numbers of Tregs are present at the cervical tumor site and in the lymph nodes of patients with cervical intraepithelial neoplasias or cervical cancer [28]. Tregs are recruited to the TME via interactions between chemokines and chemokine receptors including CCL5-CCR5, CCL22-CCR4, and CCL28-CCR10, and contribute to the antitumor immunosuppression, immune evasion and tumor progression [29, 30]. Our results indicate that mice inoculated with the vaccine had a significant decrease in splenic and intratumoral Tregs. In addition, the level of CCL-5 in the tumor tissue of the vaccine group was significantly reduced, which indicates that the vaccine might deplete Tregs in the TME by blocking CCL-5. Tregs suppress a variety of immune cells, including effector T cells, natural killer (NK) cells, monocytes and dendritic cells (DCs), through cell–cell contact or the production of immunosuppressive cytokines, such as IL-10 and TGF-β [31]. The cytokines IL-10 and TGF-β play an important role in MDSC-mediated [16], Treg-mediated [31] and M2-TAM-mediated [32] antitumor immunosuppression. The vaccine induced in a significant decrease in intratumoral IL-10 and TGF-β levels (Fig. 4c, f) compared to those of control mice, which suggested that the vaccine might abrogate the inhibitory effect of these immunosuppressive cells on a variety of immune cells by reducing IL-10 and TGF-β levels to achieve prominent antitumor effects.
TAMs are the most abundant immune cells in tumors and may differentiate into proinflammatory type 1-polarized tumor-associated macrophages (M1-TAMs) or immunosuppressive M2-TAMs depending on the local microenvironment [33]. At 10 days after vaccine inoculation, we observed significantly lower relative expression of M2-related cytokines such as IL-10 and TGF-β [32] (Fig. 4c, f) and higher gene expression of M1-related chemokines such as CXCL-9 and CXCL-10 [33] (Fig. 4m, n) in tumor tissue from mice inoculated with the vaccine than in samples from control mice. Furthermore, the percentage of intratumoral M2-TAMs in the vaccine group was significantly decreased. These results may explain the mechanism by which the vaccine induces antitumor effects.
The development of cervical cancer is a multistep process based directly or indirectly on cell proliferation [34, 35]. Ki67 is present in all active stages of the cell cycle, including the G1, S, and G2 phases and mitosis, but Ki67 is not present in the quiescent cells at G0 phase. This protein is mainly located in the nucleolar cortex during interphase, recruited to condensed chromosomes during mitosis and has two protein isoforms with molecular weights of 345 and 395 kDa [36]. Ki67 expression is strongly associated with tumor growth and tumor cell proliferation, and this protein is widely used in routine pathological investigations as a tumor proliferation index marker [37]. Numerous studies have documented that increased Ki67 expression in squamous-cell carcinoma of the cervix is associated with poor prognosis [34, 38, 39]. In the present study, we observed a decrease in the percentage of Ki67-positive cells in the vaccine group (Fig. 5d), which might result from the inhibition of tumor cell proliferation or a vaccine-induced increase in the killing of tumor cells. Decreased Ki67 expression in the vaccine group led us to hypothesize that this group would have improved survival if we continuously investigated the survival of the immunized animals.
Loss of normal p53 function, either by mutation or by inactivation of wild-type p53 is closely related to the carcinogenesis of many human malignancies [34, 40, 41]. The HPV E6 protein binds to p53 for degradation via the ubiquitin pathway, preventing apoptosis and enabling potentially transformed cells to replicate an initiate cervical carcinogenesis [42, 43]. Furthermore, there are some reports showing that p53 overexpression was correlated with poor prognosis [44], higher tumor stage [45] and deep stromal invasion [46] in patients with cervical cancer. In the present study, immunohistochemical analysis showed that decreased p53 expression in mice treated with the vaccine (Fig. 5e) may be potentially related to the decreased proliferation of tumor cells and the reduced viral gene load.
Angiogenesis is not only a prerequisite for growth but also related to the metastasis of cervical cancer [47]. One frequently quantified aspect of tumor vasculature is MVD, which is an independent prognostic indicator and plays a role in predicting recurrence and survival in patients with cervical carcinoma [48]. In our study, MVD was determined by measuring expression of the CD31 marker by immunohistochemistry. CD31 is one of the best-known immunohistochemical markers for vascular endothelial cells [47]. The results of our study showed that the expression of CD31 markers was lower in the vaccine group (Fig. 5f) than in the control group, which suggested decreased vascularity within the vaccine group. The findings also indicated that the vaccine might play a vital role in antiangiogenic treatment of cervical cancer. The various cells recruited by the tumor can not only foster an immunosuppressive microenvironment that promotes tumor expansion and progression but also support angiogenesis by the production of proangiogenic molecules [49]. MDSCs have been shown to promote angiogenesis in part through the production of VEGF and MMP-9 in various tumor models [50, 51]. Tregs secrete high levels of cytokines and growth factors, including IL-10, IL-4, IL-13, TGF-β1, GM-CSF and CSF-1, which drive tumor angiogenesis [52]. TAMs were found to drive tumor angiogenesis and progression in a spontaneous model of cervical cancer through the production of MMP-9 [53]. Combining the existing experimental results, the number of MDSCs, Tregs and M2-TAMs in the vaccine group was significantly reduced, and the relative mRNA levels of VEGF, MMP-9, IL-10 and TGF-β in the vaccine group were also significantly reduced. We hypothesized that this might be the mechanism behind the reduction in intratumoral vessel density.
Conclusion
In this study, we comprehensively determined the effect of a therapeutic vaccine for cervical cancer on systemic immune responses and the TME in an animal model. We proved that the vaccine could enhance cellular immunity as well as decrease the number of immunosuppressive cells including MDSCs, Tregs and M2-TAMs which contribute to removing the obstruction to antitumor immune responses and promoting immune-mediated tumor regression. The present study also demonstrated that vaccination inhibited cell proliferation, p53 expression and angiogenesis in tumors. In conclusion, the vaccine reverted tolerogenic systemic and local TME immunosuppression and induced robust antitumor immune responses, which led to the eradication of established implanted tumors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. 1 Effect of the vaccine on systemic immune responses. The vaccine increased the percentage of a,b IFN-γ-producing CD4 and c,d IFN-γ-producing CD8 T cells; and decreased the percentage of e,f MDSCs and g,h Tregs in the spleen. Representative scatter plots from one mouse (out of three mice) showing a IFN-γ-producing CD4 T cells, c IFN-γ-producing CD8 T cells, e MDSCs and g Tregs. Flow cytometry data showing splenic IFN-γ-producing CD4 and CD8 T cells, MDSCs and Tregs is represented as a bar graph expressed as b %CD4+IFN-γ+ cells, d %CD8+IFN-γ+ cells, f %CD11b+Gr-1+ cells and h %CD4+Foxp3+ cells. The data are depicted as the mean±SD (n=3). The significance of the data was evaluated by one-way ANOVA followed by Tukey’s multiple comparison test (*p<0.05, **p<0.01).
Abbreviations
- ANOVA
Analysis of variance
- CCL
CC-chemokine ligand
- CXCL
CXC-chemokine ligand
- CTLs
Cytotoxic T lymphocytes
- CpG ODN
Unmethylated cytosine-phosphate-guanosine oligodeoxynucleotide
- DCs
Dendritic cells
- H&E
Hematoxylin and eosin
- HPV
Human papillomavirus
- M1-TAMs
Type 1-polarized tumor-associated macrophages
- M2-TAMs
Type 2-polarized tumor-associated macrophages
- MMPs
Matrix metalloproteinases
- MDSCs
Myeloid-derived suppressor cells
- MVD
Microvessel density
- M-MDSC
Monocytic MDSC
- NK
Natural killer
- PMN-MDSC
Polymorphonuclear MDSC
- qRT-PCR
Quantitative real-time reverse transcription polymerase chain reaction
- Tregs
Regulatory T cells
- TME
Tumor microenvironment
Authors’ contribution
XW, YC and YY designed the experiments; YC, YY, JS and YA carried out experiments; YC analyzed the data and wrote the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81472439), and the Natural Science Foundation of Liaoning Province (No. 20180550760).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
The study was approved by the Institutional Animal Care and Use Committee of the China Medical University.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Burd EM. Human papillomavirus and cervical cancer. Clin Microbiol Rev. 2003;16(1):1–17. doi: 10.1128/cmr.16.1.1-17.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castellsague X. Natural history and epidemiology of HPV infection and cervical cancer. Gynecol Oncol. 2008;110(3 Suppl 2):S4–7. doi: 10.1016/j.ygyno.2008.07.045. [DOI] [PubMed] [Google Scholar]
- 4.Kim HJ, Kim HJ. Current status and future prospects for human papillomavirus vaccines. Arch Pharmacal Res. 2017;40(9):1050–1063. doi: 10.1007/s12272-017-0952-8. [DOI] [PubMed] [Google Scholar]
- 5.Wigle J, Coast E, Watson-Jones D. Human papillomavirus (HPV) vaccine implementation in low and middle-income countries (LMICs): health system experiences and prospects. Vaccine. 2013;31(37):3811–3817. doi: 10.1016/j.vaccine.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoppe-Seyler K, Bossler F, Braun JA, Herrmann AL, Hoppe-Seyler F. The HPV E6/E7 oncogenes: key factors for viral carcinogenesis and therapeutic targets. Trends Microbiol. 2018;26(2):158–168. doi: 10.1016/j.tim.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, Drijfhout JW, Wafelman AR, Oostendorp J, Fleuren GJ, Offringa R, van der Burg SH, Melief CJ. Phase I immunotherapeutic trial with long peptides spanning the E6 and E7 sequences of high-risk human papillomavirus 16 in end-stage cervical cancer patients shows low toxicity and robust immunogenicity. Clin Cancer Res. 2008;14(1):169–177. doi: 10.1158/1078-0432.CCR-07-1881. [DOI] [PubMed] [Google Scholar]
- 8.van Poelgeest MI, Welters MJ, van Esch EM, Stynenbosch LF, Kerpershoek G, van van Persijn van Meerten EL, van den Hende M, Lowik MJ, Berends-van der Meer DM, Fathers LM, Valentijn AR, Oostendorp J, Fleuren GJ, Melief CJ, Kenter GG, van der Burg SH. HPV16 synthetic long peptide (HPV16-SLP) vaccination therapy of patients with advanced or recurrent HPV16-induced gynecological carcinoma, a phase II trial. J Transl Med. 2013;11:88. doi: 10.1186/1479-5876-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, Essahsah F, Fathers LM, Offringa R, Drijfhout JW, Wafelman AR, Oostendorp J, Fleuren GJ, van der Burg SH, Melief CJ. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. New Engl J Med. 2009;361(19):1838–1847. doi: 10.1056/NEJMoa0810097. [DOI] [PubMed] [Google Scholar]
- 10.Yang Y, Che Y, Zhao Y, Wang X. Prevention and treatment of cervical cancer by a single administration of human papillomavirus peptide vaccine with CpG oligodeoxynucleotides as an adjuvant in vivo. Int Immunopharmacol. 2019;69:279–288. doi: 10.1016/j.intimp.2019.01.024. [DOI] [PubMed] [Google Scholar]
- 11.Nagarsheth N, Wicha MS, Zou W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat Rev Immunol. 2017;17(9):559–572. doi: 10.1038/nri.2017.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Burg SH, Ressing ME, Kwappenberg KM, de Jong A, Straathof K, de Jong J, Geluk A, van Meijgaarden KE, Franken KL, Ottenhoff TH, Fleuren GJ, Kenter G, Melief CJ, Offringa R. Natural T-helper immunity against human papillomavirus type 16 (HPV16) E7-derived peptide epitopes in patients with HPV16-positive cervical lesions: identification of 3 human leukocyte antigen class II-restricted epitopes. Int J Cancer. 2001;91(5):612–618. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1119>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 13.Dosset M, Godet Y, Vauchy C, Beziaud L, Lone YC, Sedlik C, Liard C, Levionnois E, Clerc B, Sandoval F, Daguindau E, Wain-Hobson S, Tartour E, Langlade-Demoyen P, Borg C, Adotevi O. Universal cancer peptide-based therapeutic vaccine breaks tolerance against telomerase and eradicates established tumor. Clin Cancer Res. 2012;18(22):6284–6295. doi: 10.1158/1078-0432.CCR-12-0896. [DOI] [PubMed] [Google Scholar]
- 14.Kalathil SG, Thanavala Y. High immunosuppressive burden in cancer patients: a major hurdle for cancer immunotherapy. CII. 2016;65(7):813–819. doi: 10.1007/s00262-016-1810-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Investig. 2015;125(9):3356–3364. doi: 10.1172/JCI80005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar V, Patel S, Tcyganov E, Gabrilovich DI. The Nature of Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Trends Immunol. 2016;37(3):208–220. doi: 10.1016/j.it.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475(7355):222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arina A, Schreiber K, Binder DC, Karrison TG, Liu RB, Schreiber H. Adoptively transferred immune T cells eradicate established tumors despite cancer-induced immune suppression. J Immunol. 2014;192(3):1286–1293. doi: 10.4049/jimmunol.1202498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arina A, Bronte V. Myeloid-derived suppressor cell impact on endogenous and adoptively transferred T cells. Curr Opin Immunol. 2015;33:120–125. doi: 10.1016/j.coi.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331(6024):1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 22.Liang Y, Lu B, Zhao P, Lu W. Increased circulating GrMyeloid-derived suppressor cells correlated with tumor burden and survival in locally advanced cervical cancer patient. J Cancer. 2019;10(6):1341–1348. doi: 10.7150/jca.29647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133(5):775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 24.Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, Harris AL, Banham AH. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006;24(34):5373–5380. doi: 10.1200/JCO.2006.05.9584. [DOI] [PubMed] [Google Scholar]
- 25.Sasada T, Kimura M, Yoshida Y, Kanai M, Takabayashi A. CD4 + CD25 + regulatory T cells in patients with gastrointestinal malignancies: possible involvement of regulatory T cells in disease progression. Cancer. 2003;98(5):1089–1099. doi: 10.1002/cncr.11618. [DOI] [PubMed] [Google Scholar]
- 26.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10(9):942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 27.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, Kepner J, Odunsi T, Ritter G, Lele S, Chen YT, Ohtani H, Old LJ, Odunsi K. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA. 2005;102(51):18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu MY, Kuo TY, Ho HN (2011) Tumor-infiltrating lymphocytes contain a higher proportion of FOXP3(+) T lymphocytes in cervical cancer. Journal of the Formosan Medical Association = Taiwan yi zhi 110 (9):580-586. 10.1016/j.jfma.2011.07.005 [DOI] [PubMed]
- 29.Nishikawa H (2014) [Regulatory T cells in cancer immunotherapy]. [Rinsho ketsueki] The Japanese journal of clinical hematology 55 (10):2183-2189 [PubMed]
- 30.Elkord E, Alcantar-Orozco EM, Dovedi SJ, Tran DQ, Hawkins RE, Gilham DE. T regulatory cells in cancer: recent advances and therapeutic potential. Exp Opin Biol Ther. 2010;10(11):1573–1586. doi: 10.1517/14712598.2010.529126. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt A, Oberle N, Krammer PH. Molecular mechanisms of treg-mediated T cell suppression. Front Immunol. 2012;3:51. doi: 10.3389/fimmu.2012.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sawa-Wejksza K, Kandefer-Szerszen M. Tumor-associated macrophages as target for antitumor therapy. Arch Immunol Ther Exp. 2018;66(2):97–111. doi: 10.1007/s00005-017-0480-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Dalen FJ, van Stevendaal M, Fennemann FL, Verdoes M, Ilina O (2018) Molecular repolarisation of tumour-associated macrophages. Molecules 24 (1). 10.3390/molecules24010009 [DOI] [PMC free article] [PubMed]
- 34.Hanprasertpong J, Tungsinmunkong K, Chichareon S, Wootipoom V, Geater A, Buhachat R, Boonyapipat S. Correlation of p53 and Ki-67 (MIB-1) expressions with clinicopathological features and prognosis of early stage cervical squamous cell carcinomas. J Obstetr Gynaecol Res. 2010;36(3):572–580. doi: 10.1111/j.1447-0756.2010.01227.x. [DOI] [PubMed] [Google Scholar]
- 35.Hahn WC, Weinberg RA. Rules for making human tumor cells. New Engl J Med. 2002;347(20):1593–1603. doi: 10.1056/NEJMra021902. [DOI] [PubMed] [Google Scholar]
- 36.Yang C, Zhang J, Ding M, Xu K, Li L, Mao L, Zheng J. Ki67 targeted strategies for cancer therapy. Clin Transl Oncol. 2018;20(5):570–575. doi: 10.1007/s12094-017-1774-3. [DOI] [PubMed] [Google Scholar]
- 37.Li LT, Jiang G, Chen Q, Zheng JN. Ki67 is a promising molecular target in the diagnosis of cancer (review) Mol Med Rep. 2015;11(3):1566–1572. doi: 10.3892/mmr.2014.2914. [DOI] [PubMed] [Google Scholar]
- 38.Garzetti GG, Ciavattini A, Lucarini G, Goteri G, de Nictolis M, Muzzioli M, Fabris N, Romanini C, Biagini G. MIB 1 immunostaining in stage I squamous cervical carcinoma: relationship with natural killer cell activity. Gynecol Oncol. 1995;58(1):28–33. doi: 10.1006/gyno.1995.1179. [DOI] [PubMed] [Google Scholar]
- 39.Ho DM, Hsu CY, Chiang H. MIB-1 labeling index as a prognostic indicator for survival in patients with FIGO stage IB squamous cell carcinoma of the cervix. Gynecol Oncol. 2000;76(1):97–102. doi: 10.1006/gyno.1999.5663. [DOI] [PubMed] [Google Scholar]
- 40.Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991;253(5015):49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- 41.Levine AJ, Momand J, Finlay CA. The p53 tumour suppressor gene. Nature. 1991;351(6326):453–456. doi: 10.1038/351453a0. [DOI] [PubMed] [Google Scholar]
- 42.Munger K, Scheffner M, Huibregtse JM, Howley PM. Interactions of HPV E6 and E7 oncoproteins with tumour suppressor gene products. Cancer Surv. 1992;12:197–217. [PubMed] [Google Scholar]
- 43.Crosbie EJ, Einstein MH, Franceschi S, Kitchener HC. Human papillomavirus and cervical cancer. Lancet. 2013;382(9895):889–899. doi: 10.1016/S0140-6736(13)60022-7. [DOI] [PubMed] [Google Scholar]
- 44.Avall-Lundqvist EH, Silfversward C, Aspenblad U, Nilsson BR, Auer GU. The impact of tumour angiogenesis, p53 overexpression and proliferative activity (MIB-1) on survival in squamous cervical carcinoma. Eur J Cancer. 1997;33(11):1799–1804. doi: 10.1016/s0959-8049(97)00161-5. [DOI] [PubMed] [Google Scholar]
- 45.Huang LW, Chou YY, Chao SL, Chen TJ, Lee TT. p53 and p21 expression in precancerous lesions and carcinomas of the uterine cervix: overexpression of p53 predicts poor disease outcome. Gynecol Oncol. 2001;83(2):348–354. doi: 10.1006/gyno.2001.6397. [DOI] [PubMed] [Google Scholar]
- 46.Shiohara S, Shiozawa T, Miyamoto T, Feng YZ, Kashima H, Kurai M, Suzuki A, Konishi I. Expression of cyclins, p53, and Ki-67 in cervical squamous cell carcinomas: overexpression of cyclin A is a poor prognostic factor in stage Ib and II disease. Virchows Archiv. 2005;446(6):626–633. doi: 10.1007/s00428-005-1252-0. [DOI] [PubMed] [Google Scholar]
- 47.Dellas A, Moch H, Schultheiss E, Feichter G, Almendral AC, Gudat F, Torhorst J. Angiogenesis in cervical neoplasia: microvessel quantitation in precancerous lesions and invasive carcinomas with clinicopathological correlations. Gynecol Oncol. 1997;67(1):27–33. doi: 10.1006/gyno.1997.4835. [DOI] [PubMed] [Google Scholar]
- 48.Tomao F, Papa A, Rossi L, Zaccarelli E, Caruso D, Zoratto F, Panici PB, Tomao S (2014) Angiogenesis and antiangiogenic agents in cervical cancer. Oncotargets Ther 7 [DOI] [PMC free article] [PubMed]
- 49.Rahma OE, Hodi FS. The Intersection between tumor angiogenesis and immune suppression. Clin Cancer Res. 2019;25(18):5449–5457. doi: 10.1158/1078-0432.CCR-18-1543. [DOI] [PubMed] [Google Scholar]
- 50.Pan PY, Wang GX, Yin B, Ozao J, Ku T, Divino CM, Chen SH. Reversion of immune tolerance in advanced malignancy: modulation of myeloid-derived suppressor cell development by blockade of stem-cell factor function. Blood. 2008;111(1):219–228. doi: 10.1182/blood-2007-04-086835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, Matrisian LM, Carbone DP, Lin PC. Expansion of myeloid immune suppressor Gr+ CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6(4):409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 52.Rivera LB, Bergers G. Intertwined regulation of angiogenesis and immunity by myeloid cells. Trends Immunol. 2015;36(4):240–249. doi: 10.1016/j.it.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pahler JC, Tazzyman S, Erez N, Chen YY, Murdoch C, Nozawa H, Lewis CE, Hanahan D. Plasticity in tumor-promoting inflammation: impairment of macrophage recruitment evokes a compensatory neutrophil response. Neoplasia. 2008;10(4):329–340. doi: 10.1593/neo.07871. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. 1 Effect of the vaccine on systemic immune responses. The vaccine increased the percentage of a,b IFN-γ-producing CD4 and c,d IFN-γ-producing CD8 T cells; and decreased the percentage of e,f MDSCs and g,h Tregs in the spleen. Representative scatter plots from one mouse (out of three mice) showing a IFN-γ-producing CD4 T cells, c IFN-γ-producing CD8 T cells, e MDSCs and g Tregs. Flow cytometry data showing splenic IFN-γ-producing CD4 and CD8 T cells, MDSCs and Tregs is represented as a bar graph expressed as b %CD4+IFN-γ+ cells, d %CD8+IFN-γ+ cells, f %CD11b+Gr-1+ cells and h %CD4+Foxp3+ cells. The data are depicted as the mean±SD (n=3). The significance of the data was evaluated by one-way ANOVA followed by Tukey’s multiple comparison test (*p<0.05, **p<0.01).