Abstract
The PD-1-targeting IgG4 antibody pembrolizumab has significant anti-tumor activity in a proportion of stage IV melanoma patients. A subset of patients develop anti-drug antibodies (ADA) which can form immune complexes (IC) with pembrolizumab. Although IC can induce powerful, Fc-mediated, immune-regulatory effects, their functional impact during pembrolizumab treatment is unclear. The functional effects of IC generated in vitro using pembrolizumab and patient-derived ADA was, therefore, investigated. Screening identified a patient whose trough serum samples from three treatment cycles contained both ADA with neutralizing activity and low levels of pembrolizumab. This patient responded well to therapy over 2 years and had ongoing, infusion-related, hypersensitivity reactions despite the later absence of detectable ADA. The components of IC were mimicked by forming a complex of pembrolizumab by absorption onto a solid phase with or without subsequent exposure to the ADA+ patient sera. Complexes comprised of pembrolizumab alone significantly inhibited TLR4 (LPS)-driven IL-10 production by PBMC and stimulated the generation of reactive oxygen species by granulocytes. In contrast, soluble and solid-phase F(ab´)2 fragments of pembrolizumab had no effect demonstrating the requirement for cross-linked Fc regions. IC containing pembrolizumab and ADA could additionally induce complement and NK activation. The results of this study demonstrate that, when oligomerized, the Fc region of pembrolizumab alone can provide immuno-regulatory signals. Furthermore, IC containing both pembrolizumab and patient-generated ADA can induce additional signals. These Fc-mediated signals may modulate both hypersensitivity reactions and anti-tumor responses associated with pembrolizumab therapy.
Keywords: Pembrolizumab, Anti-drug antibodies, Immune complex, Fc, IgG4
Introduction
The advent of immune checkpoint inhibitors (ICI) such as the PD-1-specific monoclonal antibodies (mAb) pembrolizumab and nivolumab has revolutionized the treatment of several malignancies [1]. However, many patients do not respond to these agents and much remains unclear regarding the factors modulating their effectiveness.
Pembrolizumab was manufactured as a fully humanized mAb to minimize the development of anti-drug antibodies (ADA) [2]. Such ADA have the potential to neutralize pembrolizumab binding and, therefore, its efficacy. Additionally, ADA binding to pembrolizumab would generate immune complexes (IC) which could potentially increase pembrolizumab clearance and/or induce immune-related adverse events (irAEs) [3, 4].
The level of pembrolizumab–ADA IC formation in treated patients is unclear. A low incidence of ADA has been reported (< 3%) and no association between ADA detection and drug efficacy was observed [2, 5]. However, these studies primarily utilized bridging ADA assays which have a number of well-recognized limitations with respect to ADA detection [6]. IC can induce a wide range of hypersensitivity reactions and it is of note that 30–40% of patients receiving pembrolizumab exhibit immune-related adverse effects (irAE) [7, 8].
Pembrolizumab increases T cell responses by binding to, and thereby blocking signalling through, PD-1. However, the oligomerization of antibodies resulting from IC formation or binding to cell surface antigen can result in the delivery of additional signals through their Fc regions [3, 9–12]. These result primarily from the subsequent triggering of the complement cascade and/or clustering of Fc receptors (FcR) which generates powerful immune-regulatory signals [13, 14]. Consequently, both nivolumab and pembrolizumab were designed as IgG4 isotype which cannot trigger complement and/or cell-mediated target destruction [15]. Although generally regarded as a relatively weak stimulator of all FcR signaling, a number of studies have reported that oligomerized IgG4 can, in some contexts, induce strong Fc-mediated signaling [10, 15–20]. Studies on the impact that FcR signaling may have on the efficacy of anti-PD-1 mAb have been limited but suggest that their engagement with the macrophage FcR can impair their effectiveness [19, 21–23]. The ability of oligomerised pembrolizumab to induce Fc-mediated signaling has, however, not been analysed to date. Manufactured IgG, even of the same isotype, can have differing post-translational modifications that modulate properties such as Fc receptor binding [24, 25]. Consequently, the functional properties of pembrolizumab cannot necessarily be extrapolated from the limited data available using other IgG4 and/or PD-1-specific antibodies.
The functional effects of IC are dependent on not only their size but also the isotypes present, as they have distinct abilities to activate both complement and each of the FcR subtypes [13]. Patient-generated ADA are typically polyclonal [26, 27] and, therefore, pembrolizumab–ADA IC will effectively present a complex of pembrolizumab IgG4 in combination with a complex of different ADA isotypes. Therefore, it is necessary to compare complexes of pembrolizumab alone with pembrolizumab–ADA IC to determine the relative contributions pembrolizumab and ADA isotypes make to any observed effects. It has become clear that antibody bound to even a low-density cell surface antigen can rapidly oligomerize [11, 12]. Therefore, analysing the functional effects of in vitro-generated pembrolizumab complexes is also relevant to understanding the potential Fc-mediated effects of pembrolizumab bound to cell surface PD-1.
The immunomodulatory effects of either pembrolizumab complexes or pembrolizumab–ADA IC are not well understood. In this study, we report the detection of ADA in a pembrolizumab-treated melanoma patient and investigate the in vitro functional effects of both pembrolizumab complexes and IC. The complexes and IC were generated in vitro by absorption onto a solid phase. Although the resulting IC are likely to represent only the largest subset of IC generated in vivo [28], the use of patient-derived ADA allows replication of an exact in vivo isotype repertoire. This study is the first to our knowledge to show that pembrolizumab can induce strong Fc-mediated immunomodulatory effects both as an oligomer and as an IC.
Methods
Cell and serum preparation
Blood was collected from normal donors and patients following informed consent according to Northern B Health and Disability Ethics Committee (New Zealand) guidelines. Serum was obtained by incubation (2 h/RT) and then centrifugation (1200×g/20 min) of blood. Peripheral blood mononuclear cells (PBMC) and granulocytes were obtained by centrifugation of blood over Ficoll/Paque (Amersham Pharmacia Biotech, Uppsala, Sweden). Contaminating red blood cells were removed by lysis with NH4Cl. NK cells were enriched (40–55% CD56+) from PBMC by negative selection using CD14, CD19 and CD3 mAb, in combination with Goat–anti-mouse-IgG-coated Dynabeads (Invitrogen). Monocytes were purified by positive selection using CD14 MicroBeads (Miltenyi Biotec, Germany). All experiments were performed in RPMI (Sigma, St Louis, MO) supplemented with 10% FCS (Invitrogen, Auckland, New Zealand)].
Antibodies and flow cytometry
Stocks of therapeutic monoclonals were obtained from injection vials of rituximab (MabThera, Roche), nivolumab (OPDIVO, Bristol-Myers Squibb) and pembrolizumab (Keytruda, Merck). F(ab´)2 fragments of pembrolizumab were prepared using solid-phase pepsin according to the manufacturer’s instructions (Thermo Scientific Pierce) and PD-1 binding activity confirmed by ELISA. Pembrolizumab and nivolumab were biotinylated and conjugated to AlexaFluor-488 as described previously [29]. CD25-PE, CD16-PE and CD56-APC were obtained from BD Biosciences. Flow cytometric analysis was performed on a Beckman Coulter FC500 MPL flow cytometer, and results are expressed as mean fluorescence intensity (MFI).
Detection of pembrolizumab and anti-drug antibodies (ADA)
Pembrolizumab levels were analysed by ELISA using a modification of our previously described ELISA methodology [29]. Briefly, ELISA plates (Maxisorp, Nunc) coated with 100 µL/well rhPD-1 (250 ng/mL, R&D Systems) were used to capture pembrolizumab from patient sera and pembrolizumab standard (doubling dilutions from 100 ng/ml). Serum was diluted one in 1000–4000 (trough) or one in 12,000 (post) prior to analysis. Bound pembrolizumab was detected using goat anti-human IgG biotin [Fc-specific F(ab´)2, Sigma] in combination with streptavidin–HRP (Sigma) and TMB+ substrate (Dako). Based on levels in normal donor serum, the limit of the blank was 2.12 ng/ml and the limit of detection was 2.28 ng/ml. The inter-assay (< 20%) and intra-assay (< 10%) coefficients of variation (CV) were calculated as described previously [29].
ADA were detected using the affinity capture elution (ACE) assay and the homogeneous mobility shift assay (HMSA) we have described previously [29]. In both assays, samples undergo initial acid dissociation then neutralization. The only modifications were the therapeutic monoclonal antibodies utilized and the use of an AdvanceBio SEC (2.7 µm, 7.8 × 300 mm) column (Agilient) for the HMSA. For each ACE assay, control serums were used to define a normalized signal-to-noise (s/n) ratio defined as arbitrary units [29]. The LOB and LOD were 1.15 AU and LOB = 1.2 AU, respectively. The intra-assay and inter-assay CV were calculated using data from patient #1and were 15% and 6.5%, respectively.
The neutralizing capacity of ADA+ serum was determined using a competitive ligand binding (CLB) assay as previously described [30]. In brief, serum samples were diluted one in 50 then spiked with an equal volume of either pembrolizumab or nivolumab at 25 ng/ml. Following incubation (30 min/37 °C), the amount of pembrolizumab or nivolumab that remained available to bind PD-1 in each spiked sample was determined using the ELISA described above.
Formation of antibody complexes and immune complexes
Antibody complexes were formed by the absorption of monoclonal IgG onto ELISA plates (Costar, Corning Incorporated). Wells were incubated (2 h/37 °C) with 100 µl antibody or F(ab´)2 at 10 μg/ml in PBS. Following washing (PBS), wells were either (1) used directly or (2) blocked (0.5% BSA/0.1% Tween 20 in PBS) prior to washing and IC formation by incubation (1 h/4 °C) with either control (pembrolizumab naïve, healthy donor) serum or ADA+ patient#1 serum diluted 1/50 in 10 mM EDTA/0.2% BSA/PBS. Wells were then further washed.
NK activation assay
Enriched NK cells (200 µl/well, 1.25 × 106/ml) in media containing 25 ng/ml IL-2 (R&D Systems) were added to ELISA plates coated with/without complexes and IC as described above. Following incubation (20 h/37 °C), antigen expression on CD56+ NK cells was determined using double-labelling (CD25-PE or CD16-PE vs CD56-APC) in combination with flow cytometry. IFNγ levels in culture supernatant were determined by ELISA (R&D Systems) and lytic activity analysed using flow cytometry in combination with Carboxyfluorescein succinimidyl ester (CFSE) labelling of effectors and propidium iodide detection of target cell death.
Complement deposition assay
ELISA plates coated with either nil, antibody complexes or IC as described above were incubated (2 min/37 °C) with a complement source (fresh human serum diluted 1/10 in HBSS) before addition of cold wash buffer. Following washing, C3b was detected by sequential incubations (1 h/37 °C) with C3b/C3bi monoclonal (1/6000 in 0.2% BSA/PBS, Biolegend) and HRP-conjugated Goat–anti-mouse Ig (Sigma). Color was developed using TMB+ substrate.
IL-10 release assay
Cryopreserved PBMC or purified monocytes (3 × 105/well) were added to ELISA plates that were either uncoated or coated with antibody complexes as described above. Uncoated wells were additionally supplemented with either nil, pembrolizumab or rituximab at 50 μg/ml. Following incubation (1 h/37 °C), either media or LPS (100 ng/ml) was added and cultures were incubated a further 24 h. Culture supernatants were then harvested and IL-10 levels determined by sandwich ELISA (R&D Systems).
ROS production by granulocytes
Granulocytes (1 × 106/ml) were incubated (15 min/37 °C) with ROS indicator Dihydrorhodamine 123 (Invitrogen, 5 μM) and then 200 μl was added per well of an ELISA plate either uncoated or coated with antibody complexes as described above. Uncoated wells were additionally supplemented with either nil, pembrolizumab or rituximab at 50 μg/ml or PMA (80 ng/ml) as a positive control. Following incubation (1 h/37 °C), the MFI of green fluorescent rhodamine 123 was determined by flow cytometry.
Results
Analysis of drug and ADA levels
Serum was collected from patients receiving pembrolizumab as treatment for stage IV melanoma. Samples were collected immediately pre (trough) and, in some cases, immediately post each 3-weekly cycle of infusion with patients first entering the study at different cycles (1–9) of their treatment.
Eight patients received > three cycles of treatment and in seven pembrolizumab levels were > 20 μg/ml (range 23–86 μg/ml) for all trough samples collected at ≥ cycle 4 (data not shown). However, one patient (Patient#1) did not have detectable trough levels of pembrolizumab present from the time they entered the study (cycle 3) through to cycle 7 (Fig. 1a). After cycle 7, the pembrolizumab levels slowly increased reaching 40 μg/ml at cycle 13. The post-infusion samples from patient #1 at cycles 3–7 had pembrolizumab levels between 44 and 59 μg/ml (data not shown).
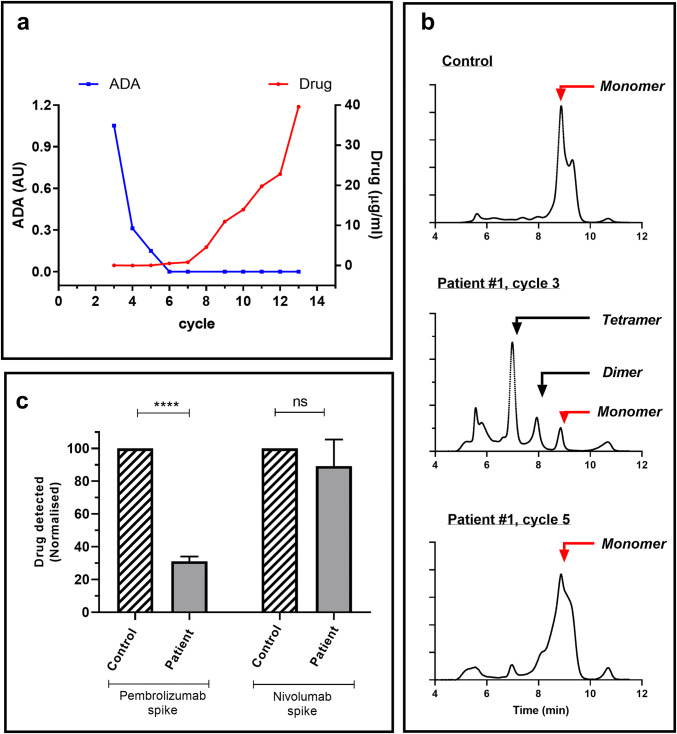
Fig. 1.
Levels of pembrolizumab and ADA in patient#1. Serum was collected from patient#1 immediately prior to each 3-weekly cycle of infusion (trough). Collection of consecutive samples commenced at cycle#3 when the patient entered the study (a) trough pembrolizumab and ADA levels at each treatment cycle. Pembrolizumab levels (μg/ml) were determined by ELISA. ADA were analysed by ACE and levels expressed as AU (signal/background). b Chromatograms obtained following ADA analysis of control and patient serum using HMSA. Data shown are from (1) a normal control (2) patient#1 cycle 3 and (3) patient#1, cycle 5. Based on MW, the position of IgG monomer, dimers and tetramers is indicated (c) Neutralizing capacity of ADA in serum from cycle 3. Serums from patient#1 and a normal control were spiked with either pembrolizumab or nivolumab and then, following incubation, the level of drug present able to bind to PD-1 was determined by ELISA. Data from individual experiments (n = 4) were normalized relative to the concentration observed in the control serum (defined as 100%). Pooled data are shown as mean ± SEM and asterisks indicate significant differences between indicated control and patient data following analysis by ANOVA using the Sidak multiple comparison analysis test
The levels of ADA in trough sera from all patients were analysed using an ACE methodology. ADA were detected only in sera from patient #1 (Fig. 1a). They were highest in the first sample collected (cycle 3), then steadily declined and were undetectable from cycle 6 (Fig. 1a). Patient ADA were detected in both pre- and post-infusion samples from cycle 5 thereby demonstrating the assays drug tolerance as the post-sample contained high levels of pembrolizumab (43 μg/ml, data not shown).
HMSA provides an alternative method of ADA analysis which detects the increased molecular weight (MW) of fluorescent-pembrolizumab resulting from ADA binding. When added to control serum, fluorescent-pembrolizumab appears as a single monomeric peak in the associated chromatogram (Fig. 1b). In contrast, when added to trough serum from patient #1 (cycle 3), the AF-pembrolizumab is detected predominantly in the form of higher MW complexes (≥ 300 kD). The proportion of high MW complexes then declines over subsequent treatment cycles.
Clinical features and treatment response of patient #1
Patient #1, a 59-year-old white male presented in 2017 with a CT scan revealing innumerable bilateral pulmonary and three small right-sided intracerebral metastases. Biopsy confirmed metastatic melanoma (BRAF V600E wild-type). He had previously had a nodular melanoma on his upper left back excised in 2011. He then began pembrolizumab treatment (2 mg/kg3-weekly) and reported increased cough, fatigue and mild dyspnea. These symptoms worsened following his second infusion and a CT scan (4 weeks after baseline scan) revealed grade 3 pneumonitis, concurrent reduction in lung tumour burden and a mixed response in the brain, with both new lesions and resolution of old lesions. Pembrolizumab was delayed 2 weeks and the patient was started on dexamethasone followed by high-dose prednisone (1 mg/kg) tapered over 4 weeks. On pembrolizumab restart (cycle 3), the patient developed a grade 3 infusion hypersensitivity reaction characterized by a flushed face, tachycardia and hypotension. Infusion was, therefore, paused, intravenous hydrocortisone and chlorphenamine were administered and then infusion was reinitiated at a slower rate. He was, therefore, pre-medicated (100 mg hydrocortisone, 10 mg loratadine) prior to subsequent infusions administered at a slower rate. He developed a community-acquired pneumonia following the sixth pembrolizumab infusion, received antibiotics and had a 1-week delay in cycle 7. He was considered in a partial remission radiographically from melanoma in February 2018, with two residual pulmonary lesions (< 10 mm) and complete resolution of brain metastasis. As of October 2019, the patient received 43 infusions of pembrolizumab, and stopped further therapy. A January 2020 CT showed ongoing stability of the lung lesions and no evidence of progression. He continued to experience low-grade infusion reactions and ongoing grade 1 fatigue and macular rash throughout his treatment, which improved off treatment. Additionally, the patient uses short- and long-acting beta agonists for persistent reactive airways disease that occurred uniformly during the second week following each infusion.
Neutralizing capacity of ADA
A CLB assay was used to assess the neutralizing capacity of patient#1 sera (cycle 3) which contained no detectable drug and the highest level of ADA. Consistent with the presence of neutralizing ADA the addition of a pembrolizumab spike to patient#1 serum resulted in significantly lower drug signal than that observed following spiking of control serum (Fig. 1c). The specificity of the inhibitory effect was confirmed by the demonstration that the patient and control serums showed similar levels of drug binding following the addition of a nivolumab spike.
Effect of IC on complement activation
We investigated whether pembrolizumab complexes or IC could modulate complement activation. Antibody complexes were generated by coating therapeutic antibodies onto plates and IC formed by further incubating them with either nil, control serum or ADA+ patient#1 serum. Plates were then exposed to complement and C3b deposition analysed. The human IgG1 monoclonal rituximab which is a known strong inducer of both complement and FcR activation was used as a positive control in all experiments. As expected [13] rituximab complexes triggered high levels of deposition whilst no deposition was induced by complexes of pembrolizumab or nivolumab (Fig. 2). However, IC formed following incubation of pembrolizumab with ADA+ serum triggered significant C3b deposition. The specificity of this effect was demonstrated by the observation that IC resulting from incubation of nivolumab–IC with ADA+ serum or pembrolizumab–IC with control sera did not induce C3b deposition.
Fig. 2.
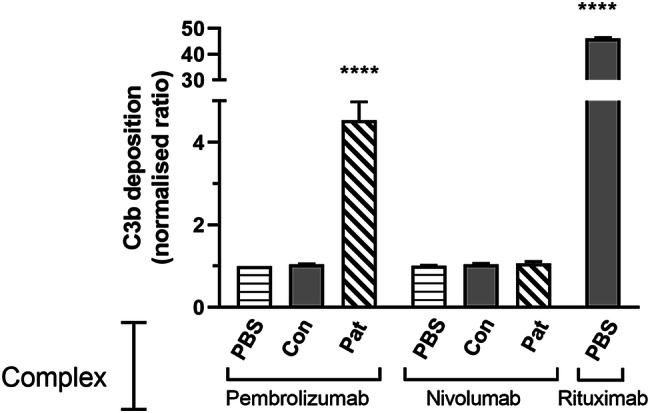
Effect of IC on complement activation. IC were formed by first coating wells with pembrolizumab, nivolumab or rituximab then further incubating wells with either PBS, control serum (Con) or patient#1 trough serum from cycle 3 (Pat). Following exposure to fresh serum, C3b/iC3b deposition was analysed using a C3b/iC3b-specific antibody in combination with colourimetric detection. Data from individual experiments (n = 3) were normalized relative to that observed in control wells coated with pembrolizumab + PBS. The OD in control wells (0.07–0.08) was used to define the baseline ratio = 1. Pooled data are shown as mean ± SEM and asterisks indicate values significantly different from the control following analysis by ANOVA using the Dunnet multiple comparison analysis test
Effect of IC on NK activation
Stimulation of NK cells via FcR engagement promotes NK activation in response to IL-2 [31, 32]. Such activation is characterized by decreased CD16 expression, which disassembles the immune synapse [33] as well as increased CD25 and changes in cytotoxicity and IFN release. We, therefore, investigated whether pembrolizumab complexes or IC could induce NK activation.
NK cells incubated with IL-2 alone expressed high levels of CD16 but only a small proportion were CD25+ (Fig. 3). However, incubation with Rituximab complexes resulted in a significantly increased proportion of CD25+ NK cells and significantly reduced CD16 expression. In contrast, soluble rituximab had no significant effect (data not shown). Pembrolizumab complexes also had no significant effect demonstrating that the effect of IC on NK cells is isotype dependent. However, IC formed following incubation of pembrolizumab complexes with ADA+ serum significantly increased the proportion of CD25+ NK cells and significantly decreased CD16 expression. These results suggest that IC comprising pembrolizumab + ADA provide the necessary isotypes for NK cell activation. Specificity was further demonstrated by the observation that IC resulting from incubation of nivolumab complexes with ADA+ serum or pembrolizumab complexes with control sera did not significantly modulate CD25 or CD16 expression. Analysis of supernatants from the same cultures demonstrated similar changes in IFNγ release, namely that unlike pembrolizumab complexes, rituximab complexes and pembrolizumab–ADA IC induced increased release. Activation of NK cells with IL-2 and oligomerized rituximab has been reported to reduce subsequent cytotoxicity against K562 [34]. We observed the same impairment of cytoxicity following activation with rituximab complexes. A similar effect was observed with pembrolizumab–ADA IC but pembrolizumab complexes alone did not induce a change in cytotoxicity.
Fig. 3.
Effect of IC on NK activation. IC were formed by first coating wells with pembrolizumab, nivolumab or rituximab then further incubating wells with either PBS, control serum (Con) or patient#1 trough serum from cycle 3 (Pat). NK cells were then added to the plates together with IL-2 and, following 24-h incubation, cells and supernatants were separately collected for analysis. a, b Cultures from four separate experiments were analysed to determine a levels of CD16 expression (MFI) and the proportion of CD25+ cells (%) within the gated CD56+ NK population as determined by flow cytometry (b) Levels of IFNγ in supernatants as determined by ELISA. Data from individual experiments (n = 4) were normalized relative to that observed in NK cells from control wells coated with PBS alone. The percentage of CD25+ NK cells (1.3–5.5%) and the level of IFNγ (0.1–7.7 ng/ml) detected in control wells were used to define the respective baseline ratio = 1. The MFI of NK cell CD16 expression (70-92) in control wells was used to define the baseline expression = 100%. Pooled data are shown as mean ± SEM and asterisks indicate values significantly different from the control following analysis by ANOVA using the Dunnet multiple comparison analysis test. c Lytic activity of activated NK cells. Cytotoxicity was assessed in a 4 h killing assay using the sensitive erythromyeloid cell line K562 as a target at a 10:1 effector to target ratio. Data are shown as % specific lysis and are from a representative experiment of two performed. All NK preparations were CD3neg, CD14neg, CD19neg and 40–56% CD56Pos
Effect of antibody complexes on IL-10 release
Stimulation of FcR by IC can modulate IL-10 release [10, 18, 35]. The ability of pembrolizumab complexes to modulate the release of IL-10 by PBMC was, therefore, assessed.
Incubation of PBMC in media alone resulted in low levels of IL-10 release (Fig. 4a). The presence of rituximab, pembrolizumab or pembrolizumab–F(ab´)2 complexes did not significantly modulate IL-10 release. As expected the addition of LPS to PBMC alone induced high levels of IL-10. This release was not significantly modulated by the presence of soluble forms of either rituximab or pembrolizumab. However, the presence of rituximab or pembrolizumab complexes significantly reduced IL-10 release. Complexes formed with the F(ab´)2 fragments of pembrolizumab did not modulate IL-10 release thereby confirming the Fc dependence of this effect. Purified monocytes gave the same responses as those observed with PBMC (Fig. 4b).
Fig. 4.

Effect of antibody complexes on IL-10 release. a PBMC or b monocytes were added to 96-well plates pre-coated with either nil or complexes (IC) of rituximab, pembrolizumab or pembrolizumab F(ab´)2 fragments. A number of nil-coated wells were additionally supplemented with soluble forms of pembrolizumab or rituximab. Plates were then incubated for 1 h prior to addition of either media or media plus LPS. Following a further 24-h incubation, supernatants were collected and IL-10 levels determined. Levels in each experiment were normalized relative to levels in nil-coated wells containing cells + LPS (1.3–7.7 ng/ml, defined as 100%) and pooled data from a 5 PBMC experiments and b three monocyte experiments are shown as mean ± SEM. Statistical analysis was performed using repeated measures analysis of variance of raw data in combination with Dunnett’s post hoc test and asterisks indicate values significantly different from those in uncoated wells containing cells + LPS
Effect of antibody complexes on granulocyte activation
ROS generation is an early marker of granulocyte activation by IC [4]. The ability of pembrolizumab–IC to induce ROS release by circulating neutrophils was, therefore, assessed.
Granulocytes incubated in the presence of soluble IgG alone had little detectable ROS generation whilst the presence of rituximab complexes strongly induced ROS production (Fig. 5). Pembrolizumab complexes similarly induced ROS although at a lower level. Complexes formed with the F(ab´)2 fragments of pembrolizumab did not modulate IL-10 release thereby confirming the Fc dependence of this effect.
Fig. 5.
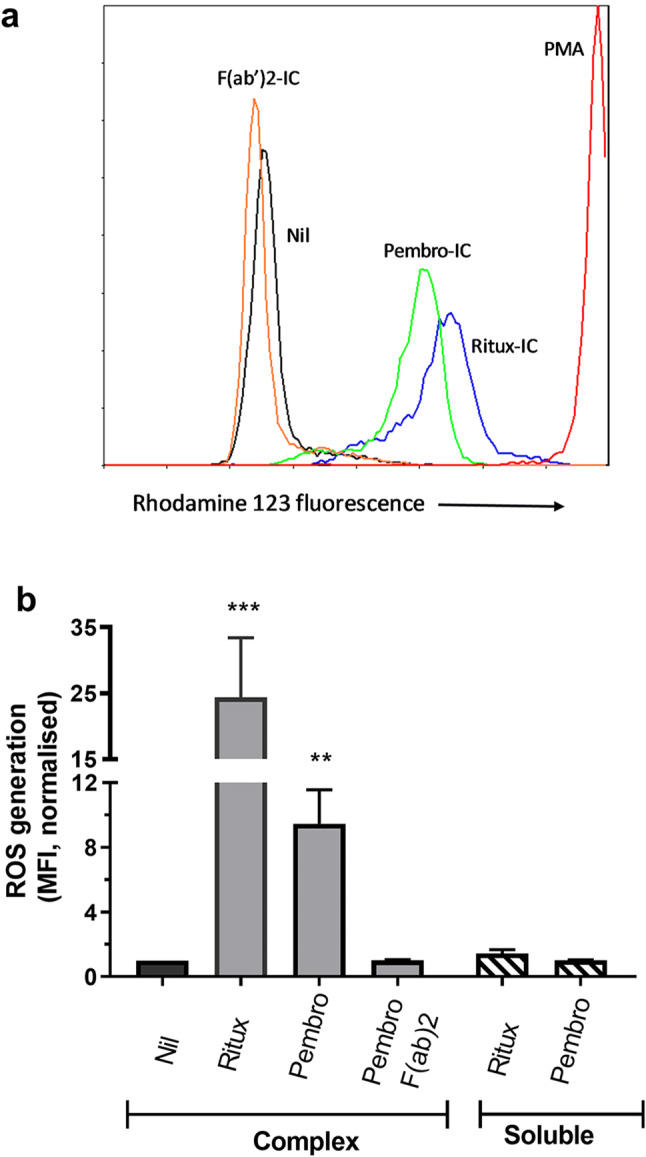
Effect of antibody complexes on ROS production by granulocytes. Granulocytes loaded with ROS indicator (DHR) were added to 96-well pre-coated with either nil or complexes (IC) of rituximab, pembrolizumab or pembrolizumab F(ab´)2 fragments. A number of nil-coated wells were additionally supplemented with soluble forms of pembrolizumab or rituximab. Plates were then incubated for 1 h prior to flow cytometric analysis of rhodamine 123 staining. a Representative histograms of rhodamine 123 staining observed following culture under the indicated conditions. Data are from a single experiment of five performed. b Histogram of the relative MFI of rhodamine 123 staining. Data for each experiment are normalized relative to the MFI of nil-treated granulocytes. Pooled data from five experiments shown as mean ± SEM and were analysed by repeated measures analysis of variance. Asterisks indicate values significantly different from those observed in the respective control cultures. Granulocyte purity was > 90% in all preparations based on flow cytometric analysis of both scatter and CD66b expression
Discussion
The oligomerization of pembrolizumab resulting from either ADA-mediated IC formation or binding to cell surface PD-1 can potentially result in the delivery of additional immunoregulatory signals through its Fc regions [3, 9–12]. The functional impact of pembrolizumab oligomerization was, therefore, investigated using patient-derived ADA to generate IC.
Using both HMSA and ACE assays, a patient was identified who early in their treatment had high ADA levels associated with an absence of detectable drug. Over three subsequent cycles of treatment, there was a steady decline and eventual disappearance of detectable ADA. This pattern is consistent with that from other studies of patients receiving therapeutic monoclonals, although it remains unclear why detectable ADA levels decline [36, 37]. The inability to detect ADA does not preclude their continued presence. It has become increasingly clear that changes in ADA affinities, isotypes and/or concentration over successive cycles of exposure may result in an ADA repertoire that’s undetectable using current assays [38]. In this regard, it is notable that drug levels in patient#1 were low and then only slowly increased despite the absence of detectable ADA. This suggests the continued presence of ADA and, therefore, IC at levels which increase drug clearance and/or inhibit drug detection. This possibility is supported by the ongoing infusion reactions and irAEs experienced by patient#1 as even small amounts of IgG aggregates or IC have the ability to trigger a range of immunoregulatory responses [39]. However, the formation of such IC in patients has not yet been demonstrated and merits investigation. The presence of circulating soluble PD-1 provides an alternative explanation for decreased pembrolizumab detection. However, this possibility is unlikely given its reported low levels in patient serum and the size of the IC detected by HMSA. The FcR/complement binding ability of crosslinked Fc regions is dependent not only on the isotype but also, in the case of therapeutics, the level of post-translational modifications introduced during manufacture [15, 24, 25]. To date, functional studies on the Fc-mediated effects of either IgG4 complexes or PD-1 antibodies have been limited [17–20]. Reports that engagement of macrophage FcR by PD-1 antibodies results in reduced efficacy [13, 19, 21, 22] has led to the suggestion that abrogating the FcR binding ability of PD-1 antibodies would improve efficacy [13]. However, little is currently known concerning the impact such FcR binding may have on the function of other immune populations.
In the current study, we provide the first demonstration that an IgG4 antibody, when oligomerised, can both activate un-primed circulating neutrophils and inhibit monocyte IL-10 release. Activation of neutrophils by IgG complexes has been reported to contribute to anaphylaxis [4] and, therefore, such activation may contribute to the hypersensitivity reactions observed in patient#1. It has been reported that IgG4 antibodies, including an anti-PD-1 mAb, when oligomerised, induce IL-10 release by macrophages [17–19]. In this study, we demonstrate that monocytes respond differently, in that pembrolizumab complexes, in common with those of IgG1 rituximab, did not directly induce IL-10 release but rather inhibited TLR4 ligand (LPS)-induced IL-10 release. This is consistent with previous reports that monocyte TLR4 signalling can be regulated by Fc engagement [10, 35, 40]. The potential importance of this pathway is underlined by reports that melanoma-induced TLR4 signalling can convert monocytes into a PD-L1+ immunosuppressive population [41, 42].
The pembrolizumab-specific ADA generated by patient#1 are likely to be polyclonal and include a range of isotypes [26, 27]. Therefore, their presence in IC would potentially provide additional complement/FcR activation to that provided by pembrolizumab alone. In the current study, we demonstrate that pembrolizumab complexes alone could not induce either complement or NK activation. However, the specific binding of ADA to pembrolizumab resulted in an IC with the capacity to induce both processes. Because the ADA are neutralizing such complement activation would not result in killing of PD-1+ T cells in vivo but would result in both deposition of activation products and the release of anaphylatoxins. These have the potential to contribute to both tumor immunity and hypersensitivity [14]. NK cells play an important role in cancer immune surveillance. Therefore, the formation of IC by ADA and pembrolizumab has the potential to increase NK-mediated anti-tumour responses.
There are a number of limitations to this study. First, the ADA from only one patient were analysed and it is possible those ADA are not representative of ADA occurrence in other patients. Additionally, immobilization of pembrolizumab on a solid surface was used as a model of IC. This approach is widely used in the literature [17, 18, 32, 43], but does not re-create the range of IC structures observed in fluid phase. Limited studies on the size of IC generated in ADA+ patients following infusion with a therapeutic mAb have been performed but indicate that a range of sizes results including very large (> 1 × 106 kDa) complexes [28]. It is likely that immobilized IC utilized in the current study most closely resemble the largest subset of in vivo generated IC. As some of the functional effects of IC increase with their size then it must be acknowledged that the magnitude of functional effects observed in this study may not be induced by smaller IC. A similar caveat applies when extending the effects observed using solid-phase pembrolizumab complexes to the structures formed in vivo. However, the size and structure of the in vivo IC is difficult to determine or replicate being dependent on the ratio of ADA and therapeutic drug [44]. Additionally, the limited amount of sample available from patient #1 precluded either purification of ADA or extensive evaluation of optimal formation of IC in the fluid phase. Identification and study of further ADA+ patients are, therefore, required. A major advantage of the current study is that the ADA utilized were patient generated and, therefore, reflect the in vivo repertoire of ADA isotypes.
The results of this study demonstrate that ADA generated in response to pembrolizumab infusions can result in IC capable of not only directly activating neutrophils and the complement system but also modulating the response of NK cells and monocytes to other stimuli. These processes may contribute to both hypersensitivity and anti-tumour responses and, therefore, their contribution to clinical responses merits further investigation. The demonstration that oligomerised pembrolizumab alone can directly influence both neutrophil and monocyte function has not been previously reported. These findings, together with those of other studies [17–20], suggest that complexes formed by the binding of pembrolizumab to PD-1+ T cells have the potential to deliver signals to multiple cell types via FcR engagement. The issue of whether the overall effect of such signals is to enhance or inhibit the therapeutic effects of PD-1 blockade requires further investigation. Such studies will inform the development of more effective therapeutic approaches.
Acknowledgements
The authors would like to thank the staff of the Christchurch Oncology Research Unit for their invaluable assistance and particularly acknowledge the contribution of the Research Coordinator, Tracey King.
Abbreviations
- ACE
Affinity capture elution assay
- ADA
Anti-drug antibodies
- FcR
Fc receptors
- HMSA
Homogeneous mobility shift assay
- IC
Immune complexes
- ICI
Immune checkpoint inhibitors
Author contributions
BH, JM, MS and MC were involved in the study concept and design. BH, JM, LG and LB performed the research and analysed the data. BH and JM drafted the manuscript, and all authors were involved in critical revision of the manuscript.
Funding
This work was supported by grants from the Bone Marrow Cancer Research Trust (116020.01), and a University of Otago Research Fund administered by Mr Jeremy Simcock, Department of Surgery (110819.01). MC was supported by the Mackenzie Charitable Foundation and LB was supported by a University of Otago Scholarship.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval and ethical standards
The study protocol conformed to the ethical guidelines of the Declaration of Helsinki and was approved by the Northern B Health and Disability Ethics Committee, New Zealand (Approval # 16/NTB/139).
Informed consent
All patients and healthy blood donors gave written informed consent prior to blood sample collection.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350–1355. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davda J, Declerck P, Hu-Lieskovan S, Hickling TP, Jacobs IA, Chou J, et al. Immunogenicity of immunomodulatory, antibody-based, oncology therapeutics. J Immunother Cancer. 2019;7(1):105–114. doi: 10.1186/s40425-019-0586-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krishna M, Nadler SG. Immunogenicity to biotherapeutics—the role of anti-drug immune complexes. Front Immunol. 2016;7(21):1–13. doi: 10.3389/fimmu.2016.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jonsson F, de Chaisemartin L, Granger V, Gouel-Cheron A, Gillis CM, Zhu Q, et al. An IgG-induced neutrophil activation pathway contributes to human drug-induced anaphylaxis. Sci Transl Med. 2019;11(500):1–14. doi: 10.1126/scitranslmed.aat1479. [DOI] [PubMed] [Google Scholar]
- 5.van Vugt MJH, Stone JA, De Greef R, Snyder ES, Lipka L, Turner DC, et al. Immunogenicity of pembrolizumab in patients with advanced tumors. J Immunother Cancer. 2019;7(1):212. doi: 10.1186/s40425-019-0663-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jensen PEH, Warnke C, Ingenhoven K, Piccoli L, Gasis M, Hermanrud C, et al. Detection and kinetics of persistent neutralizing anti-interferon-beta antibodies in patients with multiple sclerosis. Results from the ABIRISK prospective cohort study. J Neuroimmunol. 2019 doi: 10.1016/j.jneuroim.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 7.El Osta B, Hu F, Sadek R, Chintalapally R, Tang SC. Not all immune-checkpoint inhibitors are created equal: meta-analysis and systematic review of immune-related adverse events in cancer trials. Crit Rev Oncol Hematol. 2017 doi: 10.1016/j.critrevonc.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Eigentler TK, Hassel JC, Berking C, Aberle J, Bachmann O, Grunwald V, et al. Diagnosis, monitoring and management of immune-related adverse drug reactions of anti-PD-1 antibody therapy. Cancer Treat Rev. 2016 doi: 10.1016/j.ctrv.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Wang XY, Wang B, Wen YM. From therapeutic antibodies to immune complex vaccines. NPJ Vaccines. 2019;4(2):1–8. doi: 10.1038/s41541-018-0095-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swisher JF, Feldman GM. The many faces of FcgammaRI: implications for therapeutic antibody function. Immunol Rev. 2015;268(1):160–174. doi: 10.1111/imr.12334. [DOI] [PubMed] [Google Scholar]
- 11.Diebolder CA, Beurskens FJ, de Jong RN, Koning RI, Strumane K, Lindorfer MA, et al. Complement is activated by IgG hexamers assembled at the cell surface. Science. 2014;343(6176):1260–1263. doi: 10.1126/science.1248943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strasser J, de Jong RN, Beurskens FJ, Wang G, Heck AJR, Schuurman J, et al. Unraveling the macromolecular pathways of IgG oligomerization and complement activation on antigenic surfaces. Nano Lett. 2019;19(7):4787–4796. doi: 10.1021/acs.nanolett.9b02220. [DOI] [PubMed] [Google Scholar]
- 13.Chen X, Song X, Li K, Zhang T. FcgammaR-binding is an important functional attribute for immune checkpoint antibodies in cancer immunotherapy. Front Immunol. 2019;10(292):1–13. doi: 10.3389/fimmu.2019.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reis ES, Mastellos DC, Ricklin D, Mantovani A, Lambris JD. Complement in cancer: untangling an intricate relationship. Nat Rev Immunol. 2018;18(1):5–18. doi: 10.1038/nri.2017.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beers SA, Glennie MJ, White AL. Influence of immunoglobulin isotype on therapeutic antibody function. Blood. 2016;127(9):1097–1101. doi: 10.1182/blood-2015-09-625343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dudek S, Weissmuller S, Anzaghe M, Miller L, Sterr S, Hoffmann K, et al. Human Fcgamma receptors compete for TGN1412 binding that determines the antibody’s effector function. Eur J Immunol. 2019;49(7):1117–1126. doi: 10.1002/eji.201847924. [DOI] [PubMed] [Google Scholar]
- 17.Bianchini R, Roth-Walter F, Ohradanova-Repic A, Flicker S, Hufnagl K, Fischer MB, et al. IgG4 drives M2a macrophages to a regulatory M2b-like phenotype: potential implication in immune tolerance. Allergy. 2019;74(3):483–494. doi: 10.1111/all.13635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swisher JF, Haddad DA, McGrath AG, Boekhoudt GH, Feldman GM. IgG4 can induce an M2-like phenotype in human monocyte-derived macrophages through FcgammaRI. MAbs. 2014;6(6):1377–1384. doi: 10.4161/19420862.2014.975657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang T, Song X, Xu L, Ma J, Zhang Y, Gong W, et al. The binding of an anti-PD-1 antibody to FcgammaRIota has a profound impact on its biological functions. Cancer Immunol Immunother. 2018;67(7):1079–1090. doi: 10.1007/s00262-018-2160-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holland M, Hewins P, Goodall M, Adu D, Jefferis R, Savage CO. Anti-neutrophil cytoplasm antibody IgG subclasses in Wegener’s granulomatosis: a possible pathogenic role for the IgG4 subclass. Clin Exp Immunol. 2004;138(1):183–192. doi: 10.1111/j.1365-2249.2004.02566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lo Russo G, Moro M, Sommariva M, Cancila V, Boeri M, Centonze G, et al. Antibody-Fc/FcR interaction on macrophages as a mechanism for hyperprogressive disease in non-small cell lung cancer subsequent to PD-1/PD-L1 blockade. Clin Cancer Res. 2019;25(3):989–999. doi: 10.1158/1078-0432.CCR-18-1390. [DOI] [PubMed] [Google Scholar]
- 22.Arlauckas SP, Garris CS, Kohler RH, Kitaoka M, Cuccarese MF, Yang KS, et al. In vivo imaging reveals a tumor-associated macrophage-mediated resistance pathway in anti-PD-1 therapy. Sci Transl Med. 2017;9(389):1–10. doi: 10.1126/scitranslmed.aal3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dahan R, Sega E, Engelhardt J, Selby M, Korman AJ, Ravetch JV. FcgammaRs modulate the anti-tumor activity of antibodies targeting the PD-1/PD-L1 axis. Cancer Cell. 2015;28(3):285–295. doi: 10.1016/j.ccell.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Kang H, Larson NR, White DR, Middaugh CR, Tolbert T, Schoneich C. Effects of glycan structure on the stability and receptor binding of an IgG4-Fc. J Pharm Sci. 2019;109(1):677–689. doi: 10.1016/j.xphs.2019.10.036. [DOI] [PubMed] [Google Scholar]
- 25.Duivelshof BL, Jiskoot W, Beck A, Veuthey JL, Guillarme D, D'Atri V. Glycosylation of biosimilars: recent advances in analytical characterization and clinical implications. Anal Chim Acta. 2019;1089(December):1–18. doi: 10.1016/j.aca.2019.08.044. [DOI] [PubMed] [Google Scholar]
- 26.Cassotta A, Mikol V, Bertrand T, Pouzieux S, Le Parc J, Ferrari P, et al. A single T cell epitope drives the neutralizing anti-drug antibody response to natalizumab in multiple sclerosis patients. Nat Med. 2019;25(9):1402–1407. doi: 10.1038/s41591-019-0568-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Schouwenburg PA, Kruithof S, Votsmeier C, van Schie K, Hart MH, de Jong RN, et al. Functional analysis of the anti-adalimumab response using patient-derived monoclonal antibodies. J Biol Chem. 2014;289(50):34482–34488. doi: 10.1074/jbc.M114.615500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Laken CJ, Voskuyl AE, Roos JC, Stigter van Walsum M, de Groot ER, Wolbink G, et al. Imaging and serum analysis of immune complex formation of radiolabelled infliximab and anti-infliximab in responders and non-responders to therapy for rheumatoid arthritis. Ann Rheum Dis. 2007;66(2):253–256. doi: 10.1136/ard.2006.057406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hock BD, McKenzie JL, Goddard L, Smith SM, McEntyre CJ, Keating PE. Discrimination of anti-drug antibodies with neutralizing capacity in infliximab- and adalimumab-treated patients: comparison of the homogeneous mobility shift assay and the affinity capture and elution assay. Ther Drug Monit. 2018;40(6):705–715. doi: 10.1097/FTD.0000000000000553. [DOI] [PubMed] [Google Scholar]
- 30.Hock BD, Stamp LK, Hayman MW, Keating PE, Helms ET, Barclay ML. Development of an ELISA-based competitive binding assay for the analysis of drug concentration and antidrug antibody levels in patients receiving adalimumab or infliximab. Ther Drug Monit. 2016;38(1):32–41. doi: 10.1097/FTD.0000000000000229. [DOI] [PubMed] [Google Scholar]
- 31.Harris DT, Travis WW, Koren HS. Induction of activation antigens on human natural killer cells mediated through the Fc-gamma receptor. J Immunol. 1989;143(7):2401–2406. [PubMed] [Google Scholar]
- 32.Duggan MC, Campbell AR, McMichael EL, Opheim KS, Levine KM, Bhave N, et al. Co-stimulation of the Fc receptor and interleukin-12 receptor on human natural killer cells leads to increased expression of cd25. Oncoimmunology. 2018;7(2):e1381813. doi: 10.1080/2162402X.2017.1381813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Srpan K, Ambrose A, Karampatzakis A, Saeed M, Cartwright ANR, Guldevall K, et al. Shedding of CD16 disassembles the NK cell immune synapse and boosts serial engagement of target cells. J Cell Biol. 2018;217(9):3267–3283. doi: 10.1083/jcb.201712085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Capuano C, Romanelli M, Pighi C, Cimino G, Rago A, Molfetta R, et al. Anti-CD20 therapy acts via FcgammaRIIIA to diminish responsiveness of human natural killer cells. Cancer Res. 2015;75(19):4097–4108. doi: 10.1158/0008-5472.CAN-15-0781. [DOI] [PubMed] [Google Scholar]
- 35.Popat RJ, Hakki S, Thakker A, Coughlan AM, Watson J, Little MA, et al. Anti-myeloperoxidase antibodies attenuate the monocyte response to LPS and shape macrophage development. JCI Insight. 2017;2(2):e87379. doi: 10.1172/jci.insight.87379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vultaggio A, Nencini F, Carraresi A, Pratesi S, Moverare R, Eriksson C, et al. IgG4 anti-infliximab in treated patients: clinical impact and temporal evolution. Allergy. 2018;73(11):2172–2181. doi: 10.1111/all.13471. [DOI] [PubMed] [Google Scholar]
- 37.Vande Casteele N, Gils A, Singh S, Ohrmund L, Hauenstein S, Rutgeerts P, et al. Antibody response to infliximab and its impact on pharmacokinetics can be transient. Am J Gastroenterol. 2013;108(6):962–971. doi: 10.1038/ajg.2013.12. [DOI] [PubMed] [Google Scholar]
- 38.Egging D, Verhagen J, Laat-Arts K, Wit B, Boekel TV, Buurman M, et al. Sensitivity and drug tolerance of antidrug antibody assays in relation to positive control characteristics. Bioanalysis. 2018;10(16):1289–1306. doi: 10.4155/bio-2018-0091. [DOI] [PubMed] [Google Scholar]
- 39.Ahmadi M, Bryson CJ, Cloake EA, Welch K, Filipe V, Romeijn S, et al. Small amounts of sub-visible aggregates enhance the immunogenic potential of monoclonal antibody therapeutics. Pharm Res. 2015;32(4):1383–1394. doi: 10.1007/s11095-014-1541-x. [DOI] [PubMed] [Google Scholar]
- 40.Shalova IN, Kajiji T, Lim JY, Gomez-Pina V, Fernandez-Ruiz I, Arnalich F, et al. CD16 regulates TRIF-dependent TLR4 response in human monocytes and their subsets. J Immunol. 2012;188(8):3584–3593. doi: 10.4049/jimmunol.1100244. [DOI] [PubMed] [Google Scholar]
- 41.Fleming V, Hu X, Weller C, Weber R, Groth C, Riester Z, et al. Melanoma extracellular vesicles generate immunosuppressive myeloid cells by upregulating PD-L1 via TLR4 signaling. Cancer Res. 2019;79(18):4715–4728. doi: 10.1158/0008-5472.CAN-19-0053. [DOI] [PubMed] [Google Scholar]
- 42.Wen ZF, Liu H, Gao R, Zhou M, Ma J, Zhang Y, et al. Tumor cell-released autophagosomes (TRAPs) promote immunosuppression through induction of M2-like macrophages with increased expression of PD-L1. J Immunother Cancer. 2018;6(1–51):1–16. doi: 10.1186/s40425-018-0452-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pahl JHW, Koch J, Gotz JJ, Arnold A, Reusch U, Gantke T, et al. CD16A activation of NK cells promotes NK cell proliferation and memory-like cytotoxicity against cancer cells. Cancer Immunol Res. 2018;6(5):517–527. doi: 10.1158/2326-6066.CIR-17-0550. [DOI] [PubMed] [Google Scholar]
- 44.Hoffmann E, Jordan G, Lauer M, Ringler P, Kusznir EA, Rufer AC, et al. Generation, characterization, and quantitative bioanalysis of drug/anti-drug antibody immune complexes to facilitate dedicated in vivo studies. Pharm Res. 2019;36(129):1–15. doi: 10.1007/s11095-019-2661-0. [DOI] [PMC free article] [PubMed] [Google Scholar]