Abstract
Recently, the effectiveness of anti-programmed death 1 (PD-1) antibody therapy in the treatment of renal cell carcinoma (RCC) has been established. Nevertheless, efficacy has been reported to be limited to only 10–30% of patients. To develop more effective immunotherapy for RCC, we analyzed the immunological characteristics in RCC tissues by immunohistochemistry (IHC). We prepared a tissue microarray that consisted of tumor tissue sections (1 mm in diameter) from 83 RCC patients in Kanagawa Cancer Center between 2006 and 2015. IHC analysis was performed with antibodies specific to immune-related (CD8 and Foxp3) and immune checkpoint (programmed death ligand 1 (PD-L1) and 2 (PD-L2), B7-H4 and galectin-9) molecules. The numbers and proportions of positively stained tumor cells or immune cells were determined in each section. From multivariate analysis of all 83 patients, higher galectin-9 expression was detected as a factor associated with worse overall survival (OS) (P = 0.029) and that higher stage and higher B7-H4 expression were associated with worse progression-free survival (PFS) (P < 0.001 and P = 0.021, respectively). Similarly, in multivariate analysis of 69 patients with clear cell RCC, though not statistically significant, there was a trend for association between higher galectin-9 expression and worse OS (P = 0.067), while higher stage was associated with worse PFS (P < 0.001). This study suggests that higher galectin-9 expression is an independent adverse prognostic factor of OS in RCC patients. Therefore, to develop more effective personalized immunotherapy to treat RCC, it may be important to target not only PD-1/PD-L1, but also other immune checkpoint molecules such as galectin-9.
Electronic supplementary material
The online version of this article (10.1007/s00262-020-02608-6) contains supplementary material, which is available to authorized users.
Keywords: Renal cell carcinoma, Galectin-9, Programmed death ligand 1, Immunohistochemistry, Immune checkpoint molecule
Introduction
According to the global cancer statistics in 2018, approximately 403,000 people are newly diagnosed with renal cell carcinoma (RCC) and 175,000 patients die from the disease each year [1]. In general, RCC patients undergo surgical nephrectomy if indicated. But in case of metastasis or relapse, they typically receive drug therapies such as molecular targeted medicine or immune checkpoint inhibitor (ICI). Although molecular targeted agents have been used as the first-line of RCC treatment for a long time, ICI, anti-programmed death 1 (PD-1) antibody, has recently become available as an additional treatment choice for RCC [2–4].
ICI is innovative medicine which takes advantage of tumor immunity, but its efficacy has been reported to be limited to only 10–30% of patients in various types of cancers. In addition, ICI therapy requires so large amount of costs that it should not be recommended to choose this treatment for all cancer patients from the viewpoint of healthcare economy. Therefore, the careful selection of patients for whom immunotherapy will be effective, so-called “personalized immunotherapy”, is necessary. Of note, in patients with various types of cancers, the expression patterns of programmed death ligand 1 (PD-L1) have been reported to be a predictive marker of response to immunotherapy with anti-PD-1 antibody [5–7]. For example, positive PD-L1 expression in tumor cells assessed by the immunohistochemical assay has been shown to be significantly associated with better responses to treatment with anti-PD-1 antibody in patients with melanoma and non-small cell lung carcinoma [5–7]. In contrast, however, the clinical efficacy of anti-PD-1 antibody therapy was reported to be independent of PD-L1 expression in RCC [4]. Thus, more comprehensive understanding of tumor immunity including other immunological characteristics might be essential to get greater effects of immunotherapy for RCC.
RCC has been reported to show unique immunological characteristics different from other cancers. For example, it has been demonstrated that high densities of CD8+ T cells in the invasive margins of tumors are related to worse survival in RCC [8]. In addition, RCC typically has abundant blood flow due to angiogenesis, and recruit regulatory T cells (Tregs), which are associated with polyclonal CD8+ T cells with a limited cytotoxic capability [9] and worse prognosis [10, 11]. Furthermore, several reports suggest that it is difficult to predict the prognosis from the data of PD-L1 expression alone in RCC [12–15].
Since not only the expressions of PD-1 or PD-L1, but also those of other immune-related molecules such as programmed death ligand 2 (PD-L2), B7-H4, and galectin-9/T cell immunoglobulin and mucin domain-containing protein 3 (TIM-3) in tumor tissues are known to be related to cancer oncogenesis and progression [16–19], detailed analysis of the expression patterns of other immune-related molecules might be essential to develop more effective “personalized immunotherapy.” However, few studies have done a comprehensive analysis of immune-related molecules in RCC. In this study, we intended to clarify the significance of the expressions of several immune-related molecules, especially immune-suppressive ones including various immune checkpoints and Tregs, in tumor microenvironment of RCC to comprehensively understand tumor immunity and improve the efficacy of immunotherapy for RCC.
Patients and methods
Patients
Between 2006 and 2015, 85 patients with RCC underwent radical surgery in Kanagawa Cancer Center. Among them, we selected 83 patients whose tumor tissues were available. Two patients were excluded because their tumor tissues were not preserved. This study was approved by the Institutional Review Board of Kanagawa Cancer Center (2014-64). Informed consent for the study was obtained from all participants.
This was a retrospective cohort study of individuals recorded in the Kanagawa Cancer Center database who underwent radical surgery between 2006 and 2015 and who were followed up until October 12, 2017. Progression-free survival (PFS) was defined as the period from the date of surgery to the date of disease progression or death due to any cause. If disease progression or death had not occurred at the time of the last follow-up, PFS was considered to have been censored at that time. Overall survival (OS) was defined as the period from the date of surgery to the date of death from any cause. If death had not occurred at the time of the last follow-up, OS was considered to have been censored at that time.
Immunohistochemistry (IHC) staining
We prepared a tissue microarray (TMA), which consisted of 162 tissue cores (1 mm in diameter) of formalin-fixed, paraffin-embedded tumor tissues from 83 patients. Two different tissue cores were provided from 79 patients, whereas only one tissue core was prepared from the remaining four patients due to the limited availability of tumor tissues. TMA blocks were built from representative tumor areas, which were identified and selected with hematoxylin eosin stained sections. IHC analyses were performed with antibodies specific to immune-related (CD8 and Foxp3) and immune checkpoint (PD-L1, PD-L2, B7-H4 and galectin-9) molecules. The primary antibodies used for IHC were as follows: anti-CD8 (1:50; rabbit polyclonal; Abcam. Cambridge, UK), anti-Foxp3 (1:100; SP97; rabbit monoclonal; SPRING Bio, Pleasanton, CA), anti-PD-L1 (1:100; E1L3N; rabbit monoclonal; Cell Signaling, Danvers, MA), anti-PD-L2 (1:100; D7U8C; rabbit monoclonal; Cell Signaling), anti-B7-H4 (1:500; EP1165; rabbit monoclonal; Abcam) and anti-galectin-9 (1:500; rabbit polyclonal; Life Span, Seattle, WA). For IHC, the microarray sections (4 μm thick) were mounted on glass slides, heat-treated for 15 min, and then incubated with each antibody for 30 min, followed by their corresponding secondary antibodies for 30 min, using a HISTOSTAINER (Nichirei Biosciences Inc., Tokyo, Japan). This automated system used 3,3′-diaminobenzidine as the chromogen (Nichirei Biosciences Inc.).
Evaluation of IHC staining
We determined the numbers and proportions of positively stained tumor cells or immune cells in each section. The observations and measurements were conducted separately by two medical doctors (a pathologist and a urologist) in a blind manner. If the evaluations were different between them, the sections were reviewed jointly, and consensus results were obtained. We calculated the average of two tissues from the same patient if two different tissue cores were available (79 patients), whereas the result in one tissue was shown if only one tissue core could be prepared due to the limited availability of tumor tissues (4 patients). The patients were stratified into “high” and “low” groups by using the 1% of PD-L1 expression as the cutoff value, because this value (1%) has been frequently used as the cutoff of PD-L1 expression in various cancers, such as non-small cell lung cancer [20] and RCC [21]. In addition, the patients were also stratified into “high” and “low” groups by setting the median value of each of other molecules (CD8, FoxP3, PD-L2, B7-H4, galectin 9) as the cutoff.
Statistical analysis
Statistical analysis was performed by EZR software (version 2.4-0) (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria) [22]. Stage (stage I and II vs stage III and IV) and grade (grade 1 and 2) were combined to provide adequate numbers to run analyses. The Kaplan–Meier method was used to calculate OS and PFS, and differences in survival rates between groups were compared using the log-rank test. Spearman’s rank correlation coefficient was used to evaluate the correlations between each molecule. Univariate and multivariate Cox hazard models were used to calculate unadjusted and adjusted hazard ratios (HR) and 95% confidence intervals (95% CI). All P values calculated were two-sided. P values < 0.05 were considered statistically significant.
Results
Characteristics of the immune-related or immune checkpoint molecules
Table 1 shows the characteristics of 83 patients with RCC and 69 patients with clear cell RCC (ccRCC) enrolled in this study. All stages of RCC patients [stage I, n = 35 (42.2%); stage II, n = 6 (7.2%); stage III, n = 23 (27.7%); stage IV, n = 19 (22.9%)] were included. One patient underwent neoadjuvant molecularly targeted therapy consisting of oral administration of sorafenib (400 mg twice daily) for 5 weeks before surgery.
Table 1.
Patients' characteristics and the rates of positive expression for each molecule
| Baseline characteristics | RCC (n = 83) | Clear cell RCC (n = 69) |
|---|---|---|
| n (%) | n (%) | |
| Age (median) | 66 (38–85) | 66 (38–85) |
| Gender | ||
| Female | 26 (31.3) | 21 (30.4) |
| Male | 57 (68.7) | 48 (69.6) |
| Observation period (month, median and range) | 51 (3–113) | 48 (3–113) |
| Prior therapy | 1 (1.2) | 1 (1.4) |
| Stage | ||
| I | 35 (42.2) | 30 (43.5) |
| II | 6 (7.2) | 4 (5.8) |
| III | 23 (27.7) | 18 (26.1) |
| IV | 19 (22.9) | 17 (24.6) |
| Pathology | ||
| Clear | 69 (83.1) | 69 (100) |
| Papillary | 9 (10.8) | |
| Granular | 3 (3.6) | |
| Chemophobe | 1 (1.2) | |
| Sarcomatoid | 1 (1.2) | |
| Grade | ||
| G1 | 4 (4.8) | 4 (5.8) |
| G2 | 32 (38.6) | 30 (43.5) |
| G3 | 36 (43.4) | 33 (47.8) |
| unknown | 11 (13.2) | 2 (2.9) |
| CD8 expression | ||
| Low | 43 (51.8) | 35 (50.7) |
| High | 40 (48.2) | 34 (49.3) |
| Foxp3 expression | ||
| Low | 50 (60.2) | 38 (55.1) |
| High | 33 (39.8) | 31 (44.9) |
| PD-L1 expression | ||
| Low | 59 (71.1) | 56 (81.2) |
| High | 24 (28.9) | 13 (18.8) |
| PD-L2 expression | ||
| Low | 59 (71.1) | 53 (76.8) |
| High | 24 (28.9) | 16 (23.2) |
| B7-H4 expression | ||
| Low | 50 (60.2) | 36 (52.2) |
| High | 33 (39.8) | 33 (47.8) |
| Galectin-9 expression | ||
| Low | 59 (71.1) | 50 (72.5) |
| High | 24 (28.9) | 19 (27.5) |
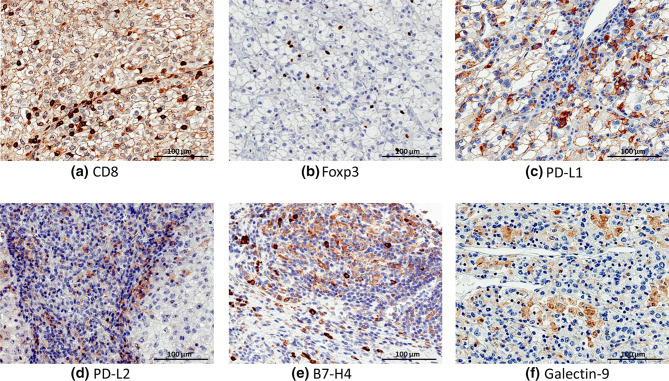
A total of 162 tissue fragments from 83 patients were analyzed by IHC with antibodies specific to CD8, Foxp3, PD-L1, PD-L2, B7-H4 and galectin-9. The representative IHC staining patterns of each molecule in cancer tissues are shown in Fig. 1. CD8 or FoxP3 was positive in some of tumor-infiltrating lymphocytes, whereas PD-L1 or PD-L2 was positive mainly in tumor cells. B7-H4 was strongly positive in some of tumor-infiltrating macrophages, which were morphologically distinguished from lymphocytes by the presence of broad cytoplasm, and was faintly positive in tumor cells. Galectin-9 was positive in cytosol of tumor cells.
Fig. 1.
Representative staining patterns of each immune-related molecule. Immunohistochemical images of the expression of each immune-related molecule in clear cell RCC tissues at 400 × optical magnification; scale bar, 100 µm; a CD8, b Foxp3, c PD-L1, d PD-L2, e B7-H4, and f galectin-9
The rates of patients with PD-L1 expression < 1% and ≥ 1% in tumor cells were 71.1% and 28.9%, respectively. Expression of the other molecules (Foxp3, CD8, PD-L2, B7-H4 and galectin-9) was detected in tumor infiltrating immune cells and/or cancer cells at different frequencies. The rates of tissues positive for expression of each molecule are shown in Table 1. When evaluated by Spearman’s rank correlation coefficient, there was a slight correlation between PD-L1 and PD-L2 (r = 0.434) in 83 RCC patients (Supplementary Table 1) and between CD8 and PD-L2 (r = 0.518) in 69 ccRCC patients (Supplementary Table 2). However, no significant correlations were found between other combinations (Supplementary Table 1 and 2).
Prognostic significance of each clinicopathological factor in RCC
In order to evaluate the prognostic value of expression of each immune-related or immune checkpoint molecule and clinical factors, we applied univariate Cox regression models to compare the clinical outcomes (Table 2). From analysis of all of 83 patients with RCC, higher stage and higher galectin-9 expression were associated with worse OS (P = 0.019 and P = 0.013, respectively), whereas higher stage, higher grade, and higher B7-H4 expression were associated with worse PFS (P < 0.001, P = 0.002 and P = 0.010, respectively). In order to estimate the firmness of the prognostic value of each selected factor, Cox multivariate regression analysis was performed by adjusting by stage (I, II vs III, IV) and grade (1, 2 vs 3). As shown in Table 2, this analysis revealed that higher galectin-9 expression was associated with worse OS (P = 0.029, HR 4.025, 95% CI 1.157–14.000), and higher stage and higher B7-H4 expression were associated with worse PFS (P < 0.001, HR 9.871, 95% CI 2.858–34.090; P = 0.021, HR 2.538, 95% CI 1.150–5.603, respectively).
Table 2.
Cox univariate and multivariate analysis of prognostic parameters for OS and PFS in patients with RCC (n = 83)
| Prognostic parameter | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Overall survival | ||||||
| Stage (I, II vs III, IV) | 6.083 | 1.346–27.49 | 0.019 | 3.102 | 0.630–15.260 | 0.164 |
| Grade (1, 2 vs 3) | 2.917 | 0.773–11.010 | 0.114 | 2.149 | 0.544–8.483 | 0.275 |
| CD8 (low vs high) | 2.717 | 0.835–8.841 | 0.097 | |||
| Foxp3 (low vs high) | 1.535 | 0.511–4.609 | 0.445 | |||
| PD-L1 (low vs high) | 1.783 | 0.582–5.460 | 0.311 | |||
| PD-L2 (low vs high) | 1.034 | 0.318–3.360 | 0.955 | |||
| B7-H4 (low vs high) | 1.894 | 0.636–5.640 | 0.251 | |||
| Galectin-9 (low vs high) | 4.150 | 1.357–12.690 | 0.013 | 4.025 | 1.157–14.000 | 0.029 |
| Progression-free survival | ||||||
| Stage (I, II vs III, IV) | 12.54 | 3.790–41.490 | < 0.001 | 9.871 | 2.858–34.090 | < 0.001 |
| Grade (1, 2 vs 3) | 4.077 | 1.706–9.743 | 0.002 | 2.117 | 0.850–5.273 | 0.107 |
| CD8 (low vs high) | 1.527 | 0.744–3.133 | 0.248 | |||
| Foxp3 (low vs high) | 1.354 | 0.646–2.835 | 0.422 | |||
| PD-L1 (low vs high) | 0.857 | 0.367–1.997 | 0.720 | |||
| PD-L2 (low vs high) | 0.897 | 0.396–2.030 | 0.794 | |||
| B7-H4 (low vs high) | 2.664 | 1.267–5.600 | 0.010 | 2.538 | 1.150–5.603 | 0.021 |
| Galectin-9 (low vs high) | 1.376 | 0.639–2.966 | 0.415 | |||
HR hazard ratio, CI confidence interval
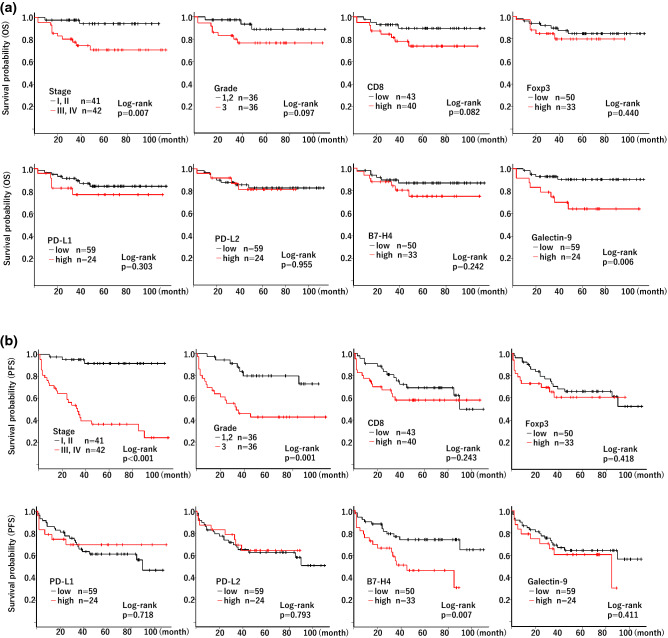
Figure 2a, b shows the Kaplan–Meier analysis of OS and PFS, respectively, for each factor in the 83 patients with RCC. Higher stage (stage III or IV) and higher galectin-9 expression were associated with worse OS (P = 0.007 and P = 0.006, respectively, by log-rank analysis), whereas grade, CD8, Foxp3, PD-L1, PD-L2 and B7-H4 had no significant association with OS (P = 0.097, P = 0.082, P = 0.440, P = 0.303, P = 0.955 and P = 0.242, respectively, by log-rank analysis). In contrast, higher stage, higher grade, and higher B7-H4 expression were associated with worse PFS (P < 0.001, P = 0.001, and P = 0.007, respectively, by log-rank analysis), whereas CD8, Foxp3, PD-L1, PD-L2 and galectin-9 had no significant association with PFS (P = 0.243, P = 0.418, P = 0.718, P = 0.793 and P = 0.411, respectively, by log-rank analysis).
Fig. 2.
Kaplan–Meier analysis of OS and PFS in RCC patients. Kaplan–Meier analysis of OS (a) and PFS (b) was performed according to stage, grade, and each molecule expression in RCC patients (n = 83). The patients were divided into two groups by stage (stage I and II vs stage III and IV) and grade (grade 1 and 2 vs grade 3). They were also stratified into “high” and “low” groups by setting the expression ratio of 1% (PD-L1) or the median value of each molecule (CD8, FoxP3, PD-L2, B7-H4, galectin 9) as the cutoff. Log-rank P value was shown
We further analyzed OS in the subgroups separated by the stages (lower stage subgroup consisting of stage I and II; higher stage subgroup consisting of stage III and IV) or the grades (lower grade subgroup consisting of grade 1 and 2; higher grade subgroup consisting of grade 3). Interestingly, galectin-9 expression showed a prognostic significance only in the higher stage (stage III and IV; P = 0.008) or higher grade (grade 3; P = 0.004) subgroups, but not in the lower stage (stage I and II; P = 0.454) or lower grade (grade 1 and 2; P = 0.780) subgroups in the cohort of RCC patients (Supplementary Fig. 1). These findings suggested that galectin-9 expression showed a prognostic significance only in advanced stage of RCC.
Prognostic significance of each clinicopathological factor in clear cell RCC
We also analyzed the prognostic significance of each of clinicopathological factor in 69 patients with ccRCC. In Cox univariate analysis, higher stage, higher PD-L1 expression and higher galectin-9 expression were associated with worse OS (P = 0.028, P = 0.013 and P = 0.017, respectively), whereas higher stage, higher grade and higher B7-H4 expression were associated with worse PFS (P < 0.001, P = 0.002 and P = 0.043, respectively) (Table 3). In order to estimate the firmness of the prognostic value of each selected factor, Cox multivariate regression analysis was performed by adjusting by stage (I, II vs III, IV) and grade (1, 2 vs 3). As shown in Table 3, this analysis revealed that though not statistically significant, there was a trend for association between higher galectin-9 expression and worse OS (P = 0.067, HR 3.330, 95% CI 0.921–12.040) and that higher stage was associated with worse PFS (P < 0.001, HR 9.987, 95% CI 2.881–34.610).
Table 3.
Cox univariate and multivariate analysis of prognostic parameters for OS and PFS of patients with clear cell RCC (n = 69)
| Prognostic parameter | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Overall survival | ||||||
| Stage (I, II vs III, IV) | 5.474 | 1.198–25.010 | 0.028 | 3.062 | 0.579–16.190 | 0.188 |
| Grade (1, 2 vs 3) | 3.002 | 0.796–11.330 | 0.105 | 1.565 | 0.354–6.929 | 0.555 |
| CD8 (low vs high) | 2.278 | 0.685–7.573 | 0.179 | |||
| Foxp3 (low vs high) | 1.437 | 0.460–4.495 | 0.533 | |||
| PD-L1 (low vs high) | 4.418 | 1.374–14.210 | 0.013 | 2.122 | 0.538–8.366 | 0.282 |
| PD-L2 (low vs high) | 1.664 | 0.501–5.527 | 0.406 | |||
| B7-H4 (low vs high) | 1.637 | 0.519–5.162 | 0.400 | |||
| Galectin-9 (low vs high) | 4.043 | 1.283–12.740 | 0.017 | 3.330 | 0.921–12.040 | 0.067 |
| Progression-free survival | ||||||
| Stage (I, II vs III, IV) | 12.450 | 3.744–41.420 | < 0.001 | 9.987 | 2.881–34.610 | < 0.001 |
| Grade (1, 2 vs 3) | 4.194 | 1.727–10.190 | 0.002 | 2.155 | 0.835–5.564 | 0.113 |
| CD8 (low vs high) | 1.444 | 0.686–3.038 | 0.333 | |||
| Foxp3 (low vs high) | 1.052 | 0.489–2.265 | 0.896 | |||
| PD-L1 (low vs high) | 1.968 | 0.787–4.921 | 0.148 | |||
| PD-L2 (low vs high) | 1.234 | 0.520–2.926 | 0.634 | |||
| B7-H4 (low vs high) | 2.248 | 1.025–4.932 | 0.043 | 2.227 | 0.982–5.050 | 0.055 |
| Galectin-9 (low vs high) | 1.471 | 0.658–3.287 | 0.347 | |||
HR hazard ratio, CI confidence interval
Figure 3a, b shows the Kaplan–Meier analysis of OS and PFS, respectively, for each factor in 69 patients with ccRCC. Higher stage (stage III or IV), higher PD-L1 expression, and higher galectin-9 expression were associated with worse OS (P = 0.013, P = 0.006 and P = 0.009, respectively, by log-rank analysis), whereas grade, CD8, Foxp3, PD-L2 and B7-H4 had no significant association with OS (P = 0.087, P = 0.165, P = 0.529, P = 0.398 and P = 0.393, respectively, by log-rank analysis). In contrast, higher stage, higher grade, and higher B7-H4 expression were associated with worse PFS (P < 0.001, P = 0.001, and P = 0.037, respectively, by log-rank analysis), whereas CD8, Foxp3, PD-L1, PD-L2 and galectin-9 had no significant association with PFS (P = 0.328, P = 0.895, P = 0.137, P = 0.631 and P = 0.341, respectively, by log-rank analysis).
Fig. 3.
Kaplan–Meier analysis of OS and PFS in clear cell RCC patients. Kaplan–Meier analysis of OS (a) and PFS (b) was performed according to stage, grade, and each molecule expression in clear cell RCC patients (n = 69). The patients were divided into two groups by stage (stage I and II vs stage III and IV) and grade (grade 1 and 2 vs grade 3). They were also stratified into “high” and “low” groups by setting the expression ratio of 1% (PD-L1) or the median value of each molecule (CD8, FoxP3, PD-L2, B7-H4, galectin 9) as the cutoff. Log-rank P value was shown
We also analyzed OS in the subgroups of ccRCC patients, separated by the stages (lower stage subgroup consisting of stage I and II; higher stage subgroup consisting of stage III and IV) or the grades (lower grade subgroup consisting of grade 1 and 2; higher grade subgroup consisting of grade 3). Similar to the data in RCC patients, galectin-9 expression showed a prognostic significance only in the higher stage (stage III and IV; P = 0.012) or higher grade (grade 3; P = 0.007) subgroups, but not in the lower stage (stage I and II; P = 0.483) or lower grade (grade 1 and 2; P = 0.716) subgroups in the cohort of ccRCC patients as well (Supplementary Figure 2). These findings demonstrated that galectin-9 expression showed a prognostic significance only in advanced stage of ccRCC.
Discussion
It has recently been reported that not only the expressions of PD-1 or PD-L1, but also those of other immune checkpoint molecules such as galectin-9/TIM-3, PD-L2, and B7-H4 in tumor tissues are related to cancer progression [16–19]. In order to develop more effective personalized immunotherapy for RCC, immune-related molecules in RCC tissues should be examined in more detail. Therefore, we comprehensively examined the expression of immune-related and immune checkpoint molecules in RCC.
In the current study, Cox regression analysis did not show a significant association between PD-L1 expression and OS in all 83 patients with RCC or in 69 patients with ccRCC. In contrast, higher galectin-9 expression was significantly associated with worse OS in 83 RCC patients. Similarly, in 69 ccRCC patients, though not statistically significant, there was a trend for association between higher galectin-9 expression and worse OS. These results suggested that clinical outcomes in RCC cannot be simply affected by the PD-L1 expression alone; other factors, such as galectin-9 expression in tumor tissues, are also important to consider. Therefore, to facilitate the development of personalized immunotherapy to treat RCC, it might be important to target not only PD-1/PD-L1, but also other immune checkpoint molecules such as galectin-9.
Galectin-9 is a member of the animal lectin family, and has evolutionary conserved carbohydrate recognition domains that possess a high binding affinity to β-galactosides. Galectin-9 has recently received more attention as a new prognostic biomarker in many types of cancers. For example, higher galectin-9 expression was reported to be associated with better clinical outcomes in malignant melanoma [23], breast cancer [24], cervical squamous cell carcinoma [25] and hepatocellular carcinoma [26]. In contrast, regarding RCC, Fu et al. [27] reported that galectin-9 expression was positively associated with tumor size, Fuhrman grade, and necrosis, and was determined to be an independent adverse prognostic factor for OS and recurrence-free survival (RFS) by the multivariate Cox regression analysis with 196 ccRCC patients undergoing nephrectomy. Similarly, the current study demonstrated that higher galectin-9 expression is significantly associated with worse OS in RCC patients. Of note, interestingly and additionally, our subgroup analyses revealed that galectin-9 shows a prognostic significance only in patients with aggressive tumors with higher stage (stage III and IV) or higher grade (grade 3), but not in those with lower stage (stage I and II) or lower grade (grade 1 and 2) in the cohort of RCC patients as well as ccRCC patients (Supplementary Figure 1 and 2). We thus think that this study could provide more detailed information on the significance of galectin-9 expression as the diagnostic and therapeutic target in RCC.
Recently, the prognostic significance of tumor-associated macrophages (TAMs) expressing galectin-9 has been paid attention. For example, Qi et al. [28] reported that the frequency of galectin-9-expressing TAMs, which increased with tumor stage and grade, predicted poor OS and recurrence-free survival in urothelial carcinoma. Similarly, Li et al. [29] showed that high infiltration of galectin-9-expressing Kupffer cells was correlated with worse prognosis in HBV-associated hepatocellular carcinoma patients. Although not examined in the current study, the prognostic role of galectin-9-expressing TAMs in RCC remains to be elucidated in future studies.
Galectin-9 has been reported to inhibit T cell function through the direct interaction with its ligand, TIM-3, on T cells [30]. Interestingly, a recent study showed that the percentage of tumor-infiltrating CD8+ T cells co-expressing PD-1 and TIM-3 was associated with an aggressive phenotype and a larger tumor size at diagnosis, a higher risk of relapse, and a poorer 36-month OS in RCC [31]. Considering that intratumoral TIM-3+PD1+CD8+ T cells may be a critical mediator of an aggressive phenotype in RCC, it is possible that higher expression of galectin-9 inhibits the immune responses to RCC through the TIM-3 pathway. Further studies are needed to clarify the precise role and mechanism of galectin-9 in RCC.
In the current study, Cox multivariate analysis revealed that higher expression of B7-H4 was significantly associated with worse PFS in 83 RCC patients (P = 0.021). B7-H4 is a B7 family coregulatory ligand which expresses typically in the antigen-presenting cells and aberrantly in several human malignancy. B7-H4 expression has been suggested to be associated with tumor aggressiveness and adverse clinical features in cancers, including RCC [17]. In addition, it has recently been reported that B7-H4 might be a potential therapeutic target for the treatment of cancer [32]. Notably, based on our finding that high B7-H4 expression showed a significant association only with worse PFS, but not with worse OS, the prognostic impact of B7-H4 might be different from that of galectin-9 in RCC patients, although more remains to be done to obtain conclusive results.
PD-1 (CD279) is a co-inhibitory molecule and a member of the CD28 family [33]. PD-1 functions in the late period in the activation of naïve T cells and is involved in the exhaustion of effector T cells. Therefore, it is suggested that PD-L1 may play an important role in chronic inflammation such as viral infection or exposure to tumors [34], and that tumor cells can escape from immune responses by inactivating T cells through PD-1/PD-L1/2 pathways [35]. Although many studies have been performed to clarify the prognostic implications of PD-L1 expression, the results have been diverse. In some tumors, high PD-L1 expression correlated with poor survival [36–38], while in other types of cancers the relationship was either not present [39], or was even associated with better outcomes [40, 41].
There have been several reports published regarding the association between PD-L1 expression and clinical outcomes in RCC. For example, Leite et al. [12] reported that PD-L1 expression assessed by IHC was related to negative outcomes in 155 patients with ccRCC. Similarly, Shin et al. [13] analyzed the expression of PD-L1 by IHC in 425 RCC patients and showed that it was significantly associated with adverse features of tumors, as well as worse PFS and cancer specific survival in ccRCC, but not in papillary RCC. Moreover, meta-analysis of 1323 RCC patients indicated that higher PD-L1 expression was a negative prognostic factor in RCC [14]. Collectively, these studies indicate that PD-L1 expression may be a negative prognostic factor in RCC, although there have been contradictory results. Kim et al. [15] reported that in the assessment of nine markers (BAP1, PBRM1, p56, PTEN, TGase2, PD-L1, CA9, PSMA, Ki-67) by IHC in 351 RCC patients, PD-L1 was not associated with OS and RFS, whereas Ki-67 and p56 were identified as significant independent prognostic factors in OS and RFS. Similarly, our findings also suggest that it is difficult to predict the prognosis of RCC from the data of PD-L1 expression alone. This may be because PD-L1 expression changes dynamically according to circumstances over time [42]. In fact, the clinical efficacy of anti-PD-1 antibody therapy was reported to be independent of PD-L1 expression in RCC [4].
Since the aim of this study was to comprehensively examine the immune-related molecules, especially immune-suppressive ones, in tumor microenvironment of RCC, we studied the expression of Foxp3, a representative molecular marker for Tregs [10]. Although analysis of CD4 expression might also be potentially informative, we did not examine CD4 expression in this study, because CD4 T cells contain two functionally different subsets, helper T (Th) cells and Tregs, which help and suppress the activity of other immune cells, respectively [10], and availability of tumor tissue sections was limited. TIM3, a receptor of galectin-9, has been shown to be expressed not only on CD4+ Th1 cells and CD8+ T cells but also on Tregs in cancers, such as ovarian [43] and head and neck [44] carcinoma, and affects the number and function of Tregs in mouse model [45]. Unexpectedly, however, this study could not find a significant correlation between Foxp3 and galectin-9 in RCC tissues, when evaluated by Spearman’s rank correlation coefficient, suggesting that the prevalence of Tregs within tumors might be affected by other immune-related factors.
There are some limitations in this analysis. First, TMA consisted of two separated regions selected from the same tumor tissues. However, since RCC tissues often show intra-tumor heterogeneity, all the characteristics of the cancer tissues may not have been covered in this analysis. Second, the expression of immune-related molecules may change depending on the disease stage and clinical course, but we analyzed samples obtained only at surgery in this study. Future studies and validation with a larger patient sample size are required.
Conclusion
In this study, Cox multivariate regression analysis revealed that higher galectin-9 expression was an independent factor of worse OS in 83 RCC patients (P = 0.029). In addition, in multivariate analysis of 69 patients with ccRCC, though not statistically significant, there was a trend for association between higher galectin-9 expression and worse OS (P = 0.067). In order to develop more effective personalized immunotherapy, it might be important to target not only PD-1/PD-L1, but also other immune checkpoint molecules such as galectin-9.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure 1. Kaplan–Meier analysis of OS in the subgroups of RCC patients separated by stage or grade. Kaplan–Meier analysis of OS was performed according to galectin-9 expression in the subgroups of RCC patients (n=83) separated by stage or grade. The patients were separated by the stage to (a) lower stage subgroup consisting of stage I and II and (b) higher stage subgroup consisting of stage III and IV. They were also separated by the grade to (c) lower grade subgroup consisting of grade 1 and 2 and (d) higher grade subgroup consisting of grade 3. The patients were divided into “high” and “low” groups in each subgroup by setting the median value of galectin-9 expression as the cutoff. Log-rank p value was shown. (PDF 239 kb)
Figure 2. Kaplan–Meier analysis of OS in the subgroups of clear cell RCC patients separated by stage or grade. Kaplan–Meier analysis of OS was performed according to galectin-9 expression in the subgroups of clear cell RCC patients (n=69) separated by stage or grade. The patients were separated by the stage to (a) lower stage subgroup consisting of stage I and II and (b) higher stage subgroup consisting of stage III and IV. They were also separated by the grade to (c) lower grade subgroup consisting of grade 1 and 2 and (d) higher grade subgroup consisting of grade 3. The patients were divided into “high” and “low” groups in each subgroup by setting the median value of galectin-9 expression as the cutoff. Log-rank p value was shown. (PDF 237 kb)
Acknowledgements
This study was supported by Kanagawa Cancer Center Hospital-Research Institute Joint Study.
Abbreviations
- ccRCC
Clear cell renal cell carcinoma
- CSS
Cancer-specific survival
- ICI
Immune checkpoint inhibitor
- IHC
Immunohistochemistry
- OS
Overall survival
- PD-1
Programmed death 1
- PD-L1
Programmed death ligand 1
- PD-L2
Programmed death ligand 2
- PFS
Progression-free survival
- RCC
Renal cell carcinoma
- RFS
Recurrence-free survival
- TIM-3
T cell immunoglobulin and mucin domain-containing protein 3
- TMA
Tissue microarray
Author contributions
RJ, TK, and TS were involved in conceptualization; RJ, TK, TY, MY, AH, TT, NM, KM, SU, MK, MY, YN, YM, and TS contributed to data acquisition and methodology; RJ, TK, MS, TY, and TS contributed to formal analysis and investigation; RJ contributed to writing—original draft preparation; TK, MS, and TS contributed to writing—review and editing; RJ and TS contributed to funding acquisition; and all authors read and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
The authors have no conflicts of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Takeshi Kishida, Email: kishidat@kcch.jp.
Tetsuro Sasada, Email: tsasada@kcch.jp.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab for metastatic renal cell carcinoma: results of a randomized phase II trial. J Clin Oncol. 2015;33:1430–1437. doi: 10.1200/JCO.2014.59.0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weber JS, D'Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375–384. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 6.Daud AI, Wolchok JD, Robert C, et al. Programmed death-ligand 1 expression and response to the anti-programmed death 1 antibody pembrolizumab in melanoma. J Clin Oncol. 2016;34:4102–4109. doi: 10.1200/JCO.2016.67.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 8.Fridman WH, Zitvogel L, Sautes-Fridman C, Kroemer G. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol. 2017;14:717–734. doi: 10.1038/nrclinonc.2017.101. [DOI] [PubMed] [Google Scholar]
- 9.Giraldo NA, Becht E, Vano Y, et al. Tumor-infiltrating and peripheral blood T-cell immunophenotypes predict early relapse in localized clear cell renal cell carcinoma. Clin Cancer Res. 2017;23:4416–4428. doi: 10.1158/1078-0432.CCR-16-2848. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017;27:109–118. doi: 10.1038/cr.2016.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polimeno M, Napolitano M, Costantini S, et al. Regulatory T cells, interleukin (IL)-6, IL-8, vascular endothelial growth factor (VEGF), CXCL10, CXCL11, epidermal growth factor (EGF) and hepatocyte growth factor (HGF) as surrogate markers of host immunity in patients with renal cell carcinoma. BJU Int. 2013;112:686–696. doi: 10.1111/bju.12068. [DOI] [PubMed] [Google Scholar]
- 12.Leite KR, Reis ST, Junior JP, Zerati M, Gomes Dde O, Camara-Lopes LH, Srougi M. PD-L1 expression in renal cell carcinoma clear cell type is related to unfavorable prognosis. Diagn Pathol. 2015;10:189. doi: 10.1186/s13000-015-0414-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shin SJ, Jeon YK, Kim PJ, Cho YM, Koh J, Chung DH, Go H. Clinicopathologic analysis of PD-L1 and PD-L2 expression in renal cell carcinoma: association with oncogenic proteins status. Ann Surg Oncol. 2016;23:694–702. doi: 10.1245/s10434-015-4903-7. [DOI] [PubMed] [Google Scholar]
- 14.Iacovelli R, Nole F, Verri E, et al. Prognostic role of PD-L1 expression in renal cell carcinoma. a systematic review and meta-analysis. Target Oncol. 2016;11:143–148. doi: 10.1007/s11523-015-0392-7. [DOI] [PubMed] [Google Scholar]
- 15.Kim SH, Park WS, Park EY, Park B, Joo J, Joung JY, Seo HK, Lee KH, Chung J. The prognostic value of BAP1, PBRM1, pS6, PTEN, TGase2, PD-L1, CA9, PSMA, and Ki-67 tissue markers in localized renal cell carcinoma: a retrospective study of tissue microarrays using immunohistochemistry. PLoS ONE. 2017;12:e0179610. doi: 10.1371/journal.pone.0179610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simon I, Zhuo S, Corral L, Diamandis EP, Sarno MJ, Wolfert RL, Kim NW. B7-H4 is a novel membrane-bound protein and a candidate serum and tissue biomarker for ovarian cancer. Cancer Res. 2006;66:1570–1575. doi: 10.1158/0008-5472.CAN-04-3550. [DOI] [PubMed] [Google Scholar]
- 17.Krambeck AE, Thompson RH, Dong H, et al. B7–H4 expression in renal cell carcinoma and tumor vasculature: associations with cancer progression and survival. Proc Natl Acad Sci USA. 2006;103:10391–10396. doi: 10.1073/pnas.0600937103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen L, Qian Y, Wu W, et al. B7-H4 is a prognostic biomarker for poor survival in patients with pancreatic cancer. Hum Pathol. 2017;66:79–85. doi: 10.1016/j.humpath.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 19.Thijssen VL, Heusschen R, Caers J, Griffioen AW. Galectin expression in cancer diagnosis and prognosis: a systematic review. Biochim Biophys Acta. 2015;1855:235–247. doi: 10.1016/j.bbcan.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819–1830. doi: 10.1016/S0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 21.Yeong J, Zhao Z, Lim JCT, et al. PD-L1 expression is an unfavourable prognostic indicator in Asian renal cell carcinomas. J Clin Pathol. 2020 doi: 10.1136/jclinpath-2019-206092. [DOI] [PubMed] [Google Scholar]
- 22.Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transpl. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kageshita T, Kashio Y, Yamauchi A, et al. Possible role of galectin-9 in cell aggregation and apoptosis of human melanoma cell lines and its clinical significance. Int J Cancer. 2002;99:809–816. doi: 10.1002/ijc.10436. [DOI] [PubMed] [Google Scholar]
- 24.Irie A, Yamauchi A, Kontani K, et al. Galectin-9 as a prognostic factor with antimetastatic potential in breast cancer. Clin Cancer Res. 2005;11:2962–2968. doi: 10.1158/1078-0432.CCR-04-0861. [DOI] [PubMed] [Google Scholar]
- 25.Liang M, Ueno M, Oomizu S, Arikawa T, Shinonaga R, Zhang S, Yamauchi A, Hirashima M. Galectin-9 expression links to malignant potential of cervical squamous cell carcinoma. J Cancer Res Clin Oncol. 2008;134:899–907. doi: 10.1007/s00432-008-0352-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang ZY, Dong JH, Chen YW, Wang XQ, Li CH, Wang J, Wang GQ, Li HL, Wang XD. Galectin-9 acts as a prognostic factor with antimetastatic potential in hepatocellular carcinoma. Asian Pac J Cancer Prev. 2012;13:2503–2509. doi: 10.7314/apjcp.2012.13.6.2503. [DOI] [PubMed] [Google Scholar]
- 27.Fu H, Liu Y, Xu L, Liu W, Fu Q, Liu H, Zhang W, Xu J. Galectin-9 predicts postoperative recurrence and survival of patients with clear-cell renal cell carcinoma. Tumour Biol. 2015;36:5791–5799. doi: 10.1007/s13277-015-3248-y. [DOI] [PubMed] [Google Scholar]
- 28.Qi Y, Chang Y, Wang Z, et al. Tumor-associated macrophages expressing galectin-9 identify immunoevasive subtype muscle-invasive bladder cancer with poor prognosis but favorable adjuvant chemotherapeutic response. Cancer Immunol Immunother. 2019;68:2067–2080. doi: 10.1007/s00262-019-02429-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li H, Wu K, Tao K, et al. Tim-3/galectin-9 signaling pathway mediates T-cell dysfunction and predicts poor prognosis in patients with hepatitis B virus-associated hepatocellular carcinoma. Hepatology. 2012;56:1342–1351. doi: 10.1002/hep.25777. [DOI] [PubMed] [Google Scholar]
- 30.Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, Strom TB, Kuchroo VK. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6:1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 31.Granier C, Dariane C, Combe P, et al. Tim-3 expression on tumor-infiltrating PD-1(+)CD8(+) T cells correlates with poor clinical outcome in renal cell carcinoma. Cancer Res. 2017;77:1075–1082. doi: 10.1158/0008-5472.CAN-16-0274. [DOI] [PubMed] [Google Scholar]
- 32.Podojil JR, Miller SD. Potential targeting of B7-H4 for the treatment of cancer. Immunol Rev. 2017;276:40–51. doi: 10.1111/imr.12530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 35.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 36.Ohigashi Y, Sho M, Yamada Y, et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res. 2005;11:2947–2953. doi: 10.1158/1078-0432.CCR-04-1469. [DOI] [PubMed] [Google Scholar]
- 37.Wu C, Zhu Y, Jiang J, Zhao J, Zhang XG, Xu N. Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochem. 2006;108:19–24. doi: 10.1016/j.acthis.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 38.Hamanishi J, Mandai M, Iwasaki M, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci USA. 2007;104:3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Droeser RA, Hirt C, Viehl CT, et al. Clinical impact of programmed cell death ligand 1 expression in colorectal cancer. Eur J Cancer. 2013;49:2233–2242. doi: 10.1016/j.ejca.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 40.Schalper KA, Velcheti V, Carvajal D, Wimberly H, Brown J, Pusztai L, Rimm DL. In situ tumor PD-L1 mRNA expression is associated with increased TILs and better outcome in breast carcinomas. Clin Cancer Res. 2014;20:2773–2782. doi: 10.1158/1078-0432.CCR-13-2702. [DOI] [PubMed] [Google Scholar]
- 41.Lipson EJ, Vincent JG, Loyo M, et al. PD-L1 expression in the Merkel cell carcinoma microenvironment: association with inflammation, Merkel cell polyomavirus and overall survival. Cancer Immunol Res. 2013;1:54–63. doi: 10.1158/2326-6066.CIR-13-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16:275–287. doi: 10.1038/nrc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bu M, Shen Y, Seeger WL, An S, Qi R, Sanderson JA, Cai Y. Ovarian carcinoma-infiltrating regulatory T cells were more potent suppressors of CD8(+) T cell inflammation than their peripheral counterparts, a function dependent on TIM3 expression. Tumour Biol. 2016;37:3949–3956. doi: 10.1007/s13277-015-4237-x. [DOI] [PubMed] [Google Scholar]
- 44.Liu Z, McMichael EL, Shayan G, Li J, Chen K, Srivastava R, Kane LP, Lu B, Ferris RL. Novel effector phenotype of Tim-3(+) regulatory T cells leads to enhanced suppressive function in head and neck cancer patients. Clin Cancer Res. 2018;24:4529–4538. doi: 10.1158/1078-0432.CCR-17-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu JF, Wu L, Yang LL, Deng WW, Mao L, Wu H, Zhang WF, Sun ZJ. Blockade of TIM3 relieves immunosuppression through reducing regulatory T cells in head and neck cancer. J Exp Clin Cancer Res. 2018;37:44. doi: 10.1186/s13046-018-0713-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 1. Kaplan–Meier analysis of OS in the subgroups of RCC patients separated by stage or grade. Kaplan–Meier analysis of OS was performed according to galectin-9 expression in the subgroups of RCC patients (n=83) separated by stage or grade. The patients were separated by the stage to (a) lower stage subgroup consisting of stage I and II and (b) higher stage subgroup consisting of stage III and IV. They were also separated by the grade to (c) lower grade subgroup consisting of grade 1 and 2 and (d) higher grade subgroup consisting of grade 3. The patients were divided into “high” and “low” groups in each subgroup by setting the median value of galectin-9 expression as the cutoff. Log-rank p value was shown. (PDF 239 kb)
Figure 2. Kaplan–Meier analysis of OS in the subgroups of clear cell RCC patients separated by stage or grade. Kaplan–Meier analysis of OS was performed according to galectin-9 expression in the subgroups of clear cell RCC patients (n=69) separated by stage or grade. The patients were separated by the stage to (a) lower stage subgroup consisting of stage I and II and (b) higher stage subgroup consisting of stage III and IV. They were also separated by the grade to (c) lower grade subgroup consisting of grade 1 and 2 and (d) higher grade subgroup consisting of grade 3. The patients were divided into “high” and “low” groups in each subgroup by setting the median value of galectin-9 expression as the cutoff. Log-rank p value was shown. (PDF 237 kb)