Abstract
On-treatment steroids for countering immune checkpoint inhibitor-induced inflammatory responses (irAEs) are a hallmark of cancer immunotherapy. However, the suppressive nature of steroids has raised questions regarding their ability to compromise the function of the ‘proliferative burst’ of effector T cells induced by immune checkpoint antibodies. We investigated the effector functions and the co-inhibitory receptor profile of stimulated peripheral blood mononuclear cells (PBMCs) pre-treated with prednisone and dexamethasone alone or in the presence of anti-PD-1/CTLA-4 antibodies. Also, clinical analysis of a patient who exhibited irAEs following combination (anti-PD-1/CTLA-4) in the presence of glucocorticoids was done. We found that prednisone in contrast to dexamethasone did not compromise T cell cytokine production (IL-2, IFN-γ and TNF-α) and proliferation in the absence or presence of anti-PD-1/CTLA-4 antibodies, when a physiological concentration was used. Neither single prednisone treatment nor co-treatment with checkpoint inhibitors impacted the expression of co-inhibitory receptors PD-1, CTLA-4, TIM-3 and LAG-3. In contrast, dexamethasone treatment promoted downregulation of LAG-3 expression by T cells. In addition, co-treatment of PD-1 + Jurkat cells with prednisone and/or dexamethasone with anti-PD-1 before stimulation significantly reduced SHP-2 phosphorylation, indicative of increased T cell function. Our findings hereby demonstrate a differential steroid effect on T cell function, which should be taken into consideration for patients undergoing immunotherapy. Also, the clinical analysis of a patient who exhibited irAEs following combination (anti-PD-1/CTLA-4) therapy indicated complete metabolic response in the presence of glucocorticoids. Therefore, concomitant use of prednisone does not appear to interfere with the function of immune checkpoint blockade.
Electronic supplementary material
The online version of this article (10.1007/s00262-020-02555-2) contains supplementary material, which is available to authorized users.
Keywords: Prednisone, Dexamethasone, Immune check points, T cells
Introduction
In cancer and chronic viral infections, cytotoxic T lymphocytes (CTLs) are persistently exposed to the tumor or viral antigens. These chronic antigen signals often lead to the deterioration of CTL function. During this process, CTLs undergo a hierarchical loss of effector functions such as cytokine production, proliferation and cytotoxicity [1]. Instead, CTLs express multiple co-inhibitory receptors (immune checkpoints), which compromise patient’s immunity to mounting effective CTL response [1]. Functionally compromised effector T cells, termed exhausted T cells, are also graded according to the number of inhibitory receptors (e.g., PD-1, CTLA-3, TIM-3, LAG-3, CD160, 2B4, CD39) they co-express [2, 3], and co-inhibitory receptors such as PD-1 have been shown to be more indicative of this state compared to others [1]. In recent years, immune checkpoint blockade has been at the forefront of immunotherapy. Monoclonal antibodies (mAbs) targeting PD-1 PD-L1, and CTLA-4 have become standard of care for an increasing number of cancers [4, 5]. In addition to melanoma, promising treatment outcomes have been reported for other cancers including lung cancer, renal cell carcinoma and bladder cancer [6–8]. Furthermore, clinical trials for the use of immunotherapy to treat other cancers such as head and neck, hematological, central nervous system and gastrointestinal are underway [9–11]. These milestones indicate the great promise held by these mAbs in the era of immunotherapy. Thus, immune checkpoint blockade has provided a new key pillar for cancer treatment with potential long-term durable immune responses in some patients. Thus, an increasing number of patients will be treated with these mAbs with a chance of developing toxicities mainly due to immune dysregulation. The expression of co-inhibitory receptors by CTLs and their ligands by antigen-presenting cells has evolved to mediate immune tolerance and contain immunopathology in response to inflammation and microbial invasion [12]. Consequently, immune checkpoint blockade as an immunotherapy strategy breaks this barrier and enhances the risk of adverse immune reactions. The efficacy/safety of immune checkpoint inhibitors has been of concern, and several toxic reactions termed immune-related adverse events (irAEs) have been reported [13, 14]. The incidence and severity of toxicity depend on the targeted co-inhibitory receptors. In general, irAEs associated with anti-PD-1 antibodies (nivolumab or pembrolizumab) are less common than for the anti-CTLA-4 blocking antibody (ipilimumab) [15, 16].
When it comes to irAEs, any tissue or organ can be affected such as the skin, colon, endocrine glands, pancreas, liver and lungs [17–19]. Thus, risk assessments prior to the initiation of immunotherapy and management of irAEs are vital for patients. Normally, in the event of irAEs, immunosuppressive treatment will be initiated immediately, and depending on the severity, immunotherapy is temporarily or permanently discontinued [20, 21]. Corticosteroids are now prescribed as first-line immunosuppressive agents to manage immunotherapy-related irAEs [22, 23]. However, concomitant use of corticosteroids and immune checkpoint inhibitors is still a controversial clinical issue. Presently due to the perception that corticosteroids may antagonize the therapeutic effects of immune checkpoint inhibitors, many clinical trials exclude cancer patients who are on corticosteroids from immunotherapy [24, 25]. Several studies have demonstrated that the administration of steroids at the start of treatment (daily prednisone-equivalent dose ≥ 10 mg for at least 1 day within 28 days after immune checkpoint inhibitor initiation) impacts the effectiveness of immune checkpoint inhibitor therapy [26–29]. Nevertheless, results from a few studies suggest that steroid on-treatment with immune checkpoint inhibitors does not affect overall response rates, progression-free survival and overall survival, suggesting heterogeneity of steroid-associated immunomodulation [30, 31]. Despite extensive animal studies on concomitant use of corticosteroids and immune checkpoint inhibitors, the results are mixed and inconclusive [32, 33]. Therefore, we decided to investigate the effects of two commonly used corticosteroids (prednisone and dexamethasone) on inhibitory receptor expression and T cell function using peripheral blood mononuclear cells (PBMCs) from healthy individuals. As proof of concept, we have included clinical data from a metastatic melanoma patient treated with immune checkpoint inhibitors, who also required steroid therapy for the management of immune-related toxicity.
Materials and methods
Study population
PBMC samples from > 20 healthy individuals were used for the ex vivo studies. The appropriate Institutional Review Boards at the University of Alberta approved the studies, IRB #Pro00046064. All study participants gave written informed consent to participate in this study.
Cell culture and reagents
2.5 × 105 PBMCs were plated per well in a 96 U-bottomed plate and stimulated with 100 ng/ml staphylococcal enterotoxin B (SEB) (Sigma) or soluble anti-CD3 (1.5 μg/ml) and anti-CD28 (0.5 μg/ml; BD Biosciences) for 72 h at 37 °C, 5% CO2 in the presence or absence of pembrolizumab (5 μg/ml), ipilimumab (5 μg/ml) and/or prednisone (1 and 2 μg/ml) or dexamethasone (0.25–0.5 μg/ml). For prednisone, a typical dose for irAEs is 1 mg/kg body weight. For a 70 kg individual, the physiological volume of drug distribution would be 4.65 × 10–6 mol/l which is ~ 1 μg/ml. With respect to dexamethasone, a typical dose for symptomatic brain metastases is 16 mg daily. Assuming the same physiological volume of distribution, the concentration will be 10–6 molar concentrations ≥ 0.5 μg/ml. For some experiments, PBMCs were stimulated in the presence of pembrolizumab and ipilimumab alone or in combination with prednisone and dexamethasone. Unstimulated and untreated PBMCs were used as negative controls throughout the study.
Flow cytometry
Cells were stained with the following surface and intracellular antibodies: CD3 (SK7), CD4 (RPA-T4), CD8 (SK1 and RPA-T8), CTLA-4 (BNI3), TIM-3 (F38-2E2), LAG-3 (3DS223H), PD-1 (EH12.1), TIGIT (MBSA43), (BD Biosciences), CD160 (BY55), Ki67 (20Raj1, ThermoFisher), CD244 (eBioDM244). LIVE/DEAD® Fixable Aqua Dead Cell Stain Kit, (ThermoFisher Scientific) was used to exclude dead cells. T cell proliferation was performed according to our previous report [34]. Intracellular cytokine staining (ICS) following stimulation with α-CD3 and α-CD28 was conducted according to our previous report [35]. Cells were acquired using an LSR FortessaSORP flow cytometer (BD Biosciences) and analyzed with FlowJo software.
ELISA
Cell culture supernatants were collected 72 h after PBMCs stimulation and used for measurement of IL-2, IFN-γ and TNF-α production by ELISA (R&D Systems).
PD-1/PD-L1 inhibitor screening bioluminescent cell-based assay
Recombinant Jurkat T cells expressing firefly luciferase gene under the control of NFAT with constitutive expression of PD-1 and Chinese Hamster Ovary (CHO) cells constitutively expressing PD-L1 and an engineered TCR activator were purchased from BPS Bioscience. To measure the potency and effect of prednisone or dexamethasone on PD-1/PDL-1 interaction, Jurkat cells were co-cultured with CHO cells according to manufacturer’s instructions. Briefly, 2.5 × 105 Jurkat cells were pre-incubated with varying concentrations of prednisone, dexamethasone or monoclonal antibodies (anti-PD-1, PD-L1 or CTLA-4) for 30 min at 37 °C followed by co-culture with 4 × 104 CHO cells per well for 6 h at 37 °C. After stimulation, the luciferase reagent was added to measure NFAT activity.
Jurkat T cell stimulation and Phospho-SHP-2 (Y542) ELISA
Jurkat T cells were pre-treated with human pembrolizumab (10 μg/ml), pednisone (1 μg/ml), dexamethasone (0.5 μg/ml) or a combination of the three for 48 h. Treated Jurkat T cells were co-cultured with recombinant CHO cells at a ratio of 10:1 for 3 h at 37 °C. Jurkat cell lysates were collected, and phosphorylation of the Src homology region SHP-2 was measured using ELISA (R&D Systems).
T cell stimulation and Phospho-SHP-2 (Y542) ELISA
PBMCs were stimulated with anti-CD3 (3ug/ml) and anti-CD28 (1 μg/ml) at a density of 5 × 106 cells/ml in a 24-well plate, with or without anti-PD-1 (10 μg/ml), prednisone (1 μg/ml), dexamethasone (0.5 μg/ml) or a combination for 3 h. Cells were harvested and lysed using Cell Extraction Buffer (Thermo Scientific) supplemented with protease inhibitors (cOmplete Protease Inhibitor Cocktail, Roche) and 1 mM PMSF (Sigma). Briefly, stimulated PBMCs were washed twice in ice-cold PBS and lysed at a density of 1 × 107 cells/ml of cell extraction buffer for 30 min on ice followed by centrifugation at 13,000 rpm for 15 min. Cell lysates were collected and SHP-2 phosphorylation was assessed using the Phospho-SHP-2 (Y542) ELISA kit (R&D Systems) according to manufacturer’s instructions. Unstimulated PBMCs were used as negative controls.
Statistical analysis
Statistical analyses were performed using the Mann–Whitney nonparametric test for paired groups and the Kruskal–Wallis test for unmatched groups. One-way ANOVA was used to analyze more than two groups. P values less than 0.05 were considered statistically significant.
Results
Prednisone exhibits differential effects on IL-2, IFN-γ and TNF-α production in vitro
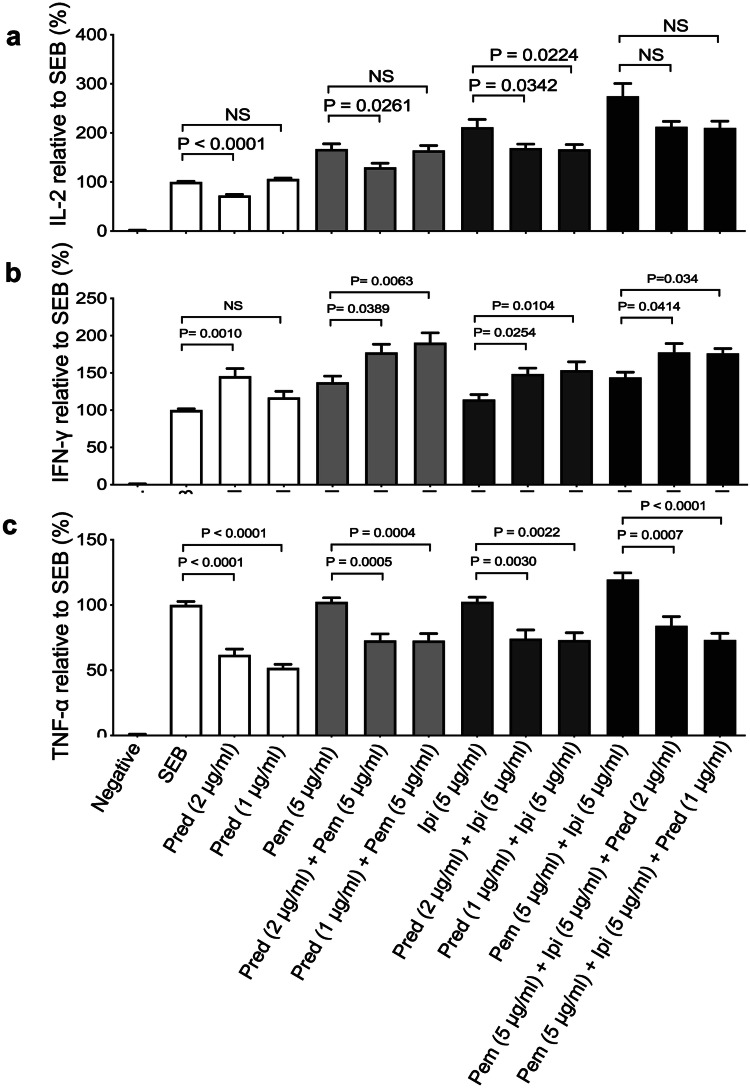
We developed an in vitro assay to mimic T cell exhaustion for examining the effects of corticosteroids on immune functions as we have reported elsewhere [34, 36]. The effects of steroids prednisone and dexamethasone were analyzed in parallel in the same experiments in the absence/presence of pembrolizumab, ipilimumab alone or their combination. For our studies, we used a physiologically relevant dose of prednisone (1 μg/ml) and a higher concentration for comparison (2 μg/ml). Consistently we found that prednisone (1 μg/ml) had no significant effect on IL-2 production by PBMCs stimulated with SEB. However, a twofold increase in the concentration of prednisone (2 μg/ml) significantly reduced IL-2 production (Fig. 1a). As anticipated, treatment with pembrolizumab enhanced IL-2 production (Fig. 1a), but it remained unchanged in the presence of prednisone (1 μg/ml). Of note, using 2 μg/ml prednisone significantly impaired IL-2 production (Fig. 1a). Similar to pembrolizumab, treatment of PBMCs with ipilimumab enhanced IL-2 production. In contrast to pembrolizumab, prednisone impaired the enhanced IL-2 production by ipilimumab even at the physiological relevant concentration (Fig. 1a). Although pembrolizumab and ipilimumab combined elicited a synergistic effect on IL-2 production; this was unaffected by prednisone cotreatment (1 or 2 μg/ml) (Fig. 1a). We also observed that prednisone at a physiologically relevant concentration (1 μg/ml) had no significant effect on IL-2 production (Supplementary Fig. 1a).
Fig. 1.
Production of IL-2, IFN-γ and TNF-α by PBMCs stimulated and treated with indicated concentrations of prednisone (pred), anti-PD-1 (pembrolizumab (pem)), anti-CTLA-4 (ipilimumab (ipi)) alone, or in combination. a Plots showing fold concentration of IL-2, b IFN-γ and c TNF-α in cell culture supernatants from PBMCs stimulated by SEB (100 ng/ml) plus the indicated treatments compared to SEB stimulated only control cells. PBMCs were stimulated for 72 h. Data from four different experiments. Error bars represent mean ± SEM. P values obtained by ordinary one-way ANOVA followed by Dunnett’s multiple comparisons test. Unstim, unstimulated cells
This was the case for IFN-γ production; however, at the higher concentration (2 μg/ml), it increased this cytokine (Fig. 1b). We found that when prednisone at either 1 or 2 μg/ml concentrations was combined with pembrolizumab, ipilimumab or a combination of the two resulted in increased IFN-γ production (Fig. 1b). In contrast, TNF-α production was significantly reduced by prednisone alone even at the physiologically relevant concentration (Fig. 1c). This impaired TNF-α production even in the presence of pembrolizumab, ipilimumab or their combination was evident (Fig. 1c). Thus, our data indicate that prednisone at recommended physiological concentration does not impact IL-2 production, increases IFN-γ but reduces TNF-α production in vitro.
Prednisone alone or in combination with immune checkpoint inhibitors does not impact expression of inhibitory receptors on T cells
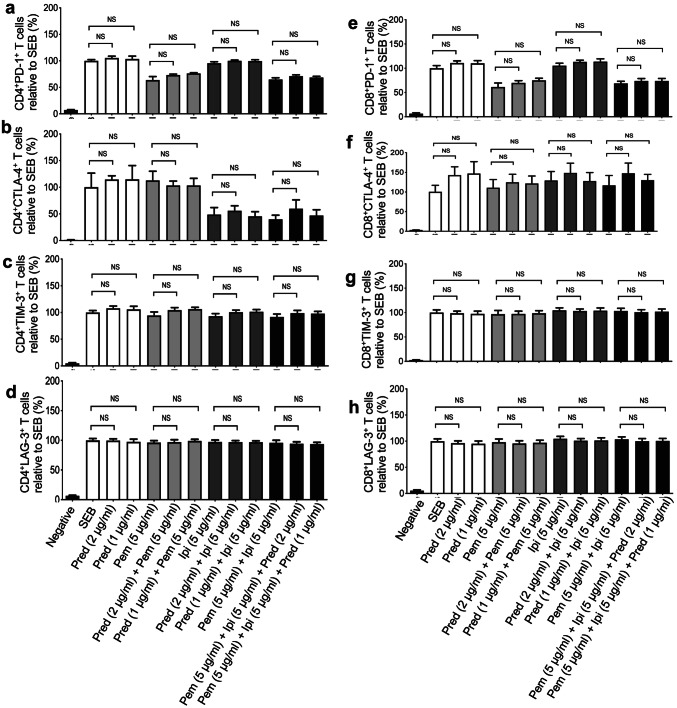
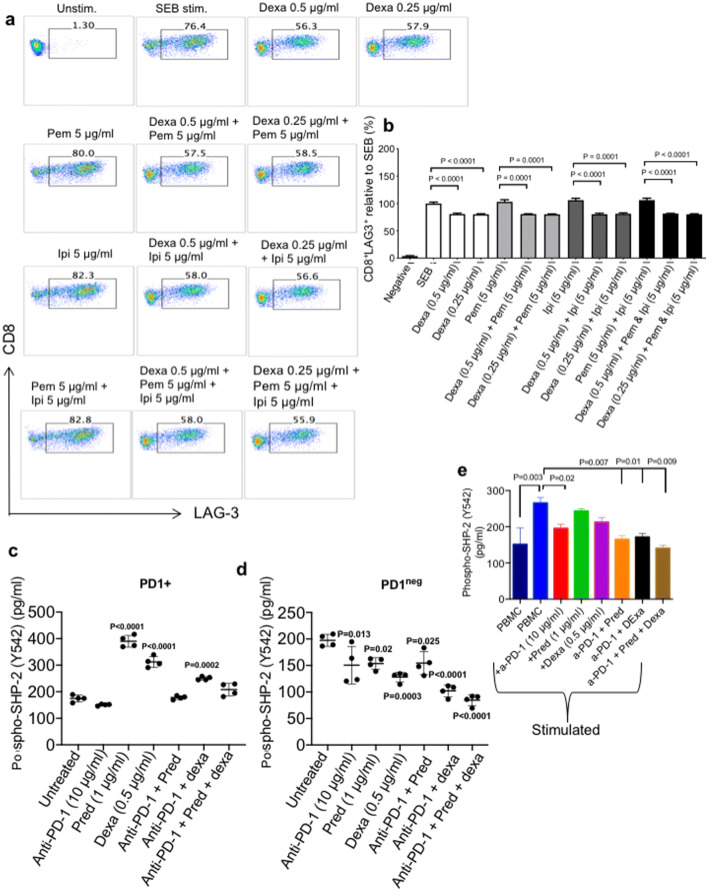
Based on the observation that prednisone modulates cytokine production by PBMCs, we hypothesized that expression of co-inhibitory receptors might be affected by this treatment. We thereby investigated the expression of PD-1, TIM-3, CTLA-4 and LAG-3 by both CD4+ and CD8+ T cells following 72 h stimulation with SEB in the presence or absence of prednisone (1–2 μg/ml) and pembrolizumab or ipilimumab. Despite the fact that pembrolizumab and ipilimumab treatment downregulated the expression of their target co-inhibitory receptors PD-1 and CTLA-4, respectively, we did not find any significant change in the expression of other co-inhibitory receptors on either CD4+ T cells (Fig. 2a–d) or CD8+ T cells (Fig. 2e–h) in the presence of prednisone. Similar results were observed for the co-expression of these co-inhibitory receptors (data not shown).
Fig. 2.
Prednisone treatment does not affect expression of PD-1, CTLA-4, TIM-3 and LAG-3 by SEB stimulated CD4+ and CD8+ T cells. a Cumulative data showing expression of PD-1 on CD4+ T cells, b CTLA-4 on CD4+ T cells, c Tim-3 on CD4+ T cells and d LAG-3 on CD4+ T cells. e Cumulative data showing expression of PD-1 on CD8+ T cells, f CTLA-4 on CD8+ T cells, g Tim-3 on CD8+ T cells and h LAG-3 on CD8+ T cells in response to SEB stimulation (100 ng/ml) plus the indicated treatments compared to stimulated-only control cells. PBMCs were stimulated for 72 h. Data from four independent experiments shown. Error bars represent mean ± SEM. P values obtained by ordinary one-way ANOVA followed by Dunnett’s multiple comparisons test. Unstim, unstimulated cells, prednisone (pred), anti-PD-1 (pembrolizumab (pem)), a-CTLA-4 (ipilimumab (ipi))
Prednisone at the physiological concentration does not inhibit T cell proliferation in vitro
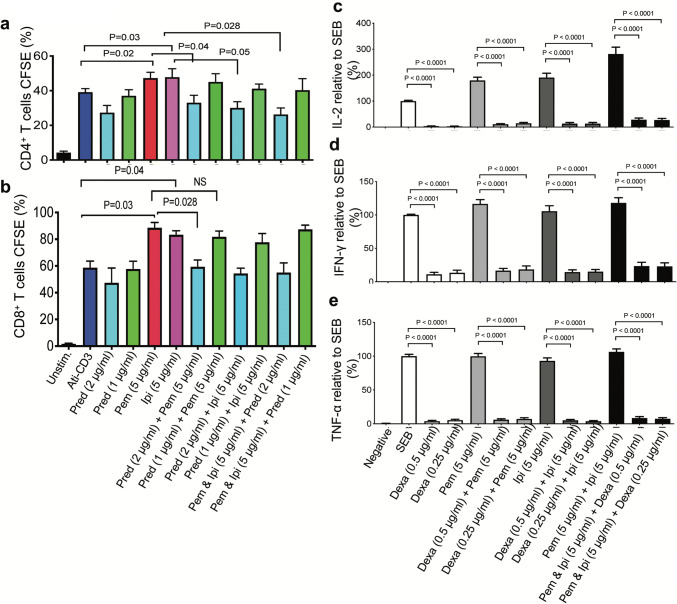
We performed a proliferation assay using CFSE as described elsewhere [34]. As expected, we found that both pembrolizumab and ipilimumab significantly enhanced CD4+ T cell proliferation when total PBMCs were stimulated with anti-CD3 for 4 days (Fig. 3a). Although we noticed that prednisone at 2 μg/ml significantly reduced CD4+ T cell proliferation regardless of the presence or the absence of immune checkpoint inhibitors, at the physiologically relevant concentration it did not exhibit a significant suppressive effect on proliferation of CD4+ (Fig. 3a) and CD8+ T cells (Fig. 3b) in the presence of pembrolizumab and ipilimumab. Thus, our data indicates that prednisone at 1 μg/ml does not impair T cell proliferation in vitro.
Fig. 3.
T cell proliferation in response to prednisone treatment and cytokines (IL-2, IFN-γ and TNF-α) production in response to dexamethasone. a CFSE dilution by CD4+ and b CD8+ T cells in response to PBMC stimulation by anti-CD3 (1 μg/ml) plus indicated treatments for 96 h. Data from four independent experiments shown. Error bars represent mean ± SD. P values obtained by Mann–Whitney nonparametric testing for CFSE analysis. (c) Cumulative data showing fold concentration of IL-2, (d) IFN-γ and (e) TNF-α in cell culture supernatants from PBMCs stimulated with SEB (100 ng/ml) plus the indicated treatments compared to SEB stimulated only control cells. PBMCs were stimulated for 72 h. Data from four independent experiments shown. Error bars represent mean ± SEM. P values obtained by ordinary one-way ANOVA followed by Dunnett’s multiple comparisons test. Unstim, unstimulated cells, dexamethasone (dexa), anti-PD-1 (pembrolizumab (pem)), a-CTLA-4 (ipilimumab (ipi))
Prednisone does not interfere with PD-1:PDL-1 interactions using a cell-based assay system
Although based on our observations prednisone does not impact the expression levels of co-inhibitory receptors including PD-1 on both CD4+ and CD8+ T cells, we decided to investigate if it interferes with PD-1/PDL-1 interactions using a cell-based assay system. In this assay, Jurkat T cells that constitutively express PD-1 were co-cultured with CHO cells that constitutively express PDL-1. Following the co-culture, NFAT activity luminescence was measured and analyzed in the presence or absence of either anti-PDL-1 antibody (research grade) or pembrolizumab. As expected, we observed that both anti-PDL-1 and pembrolizumab significantly enhanced luminescence activity by blocking PD-1:/PDL-1 interactions (Supplementary Fig. 1b); however, prednisone at either 2 or 1 μg/ml concentrations did not interfere with the action of either a-PD-L1 or pembrolizumab in the cell based assay (Supplementary Fig. 1b). Therefore, our results suggest that prednisone does not directly impact PD-1/PDL-1 interaction in this system.
Dexamethasone significantly inhibits production of cytokines IL-2, TNF-α and IFN-γ in vitro
As discussed above, similar studies were performed in parallel using physiologically relevant concentrations of dexamethasone (0.25–0.5 μg/ml) and/or immune checkpoint inhibitors. We found that dexamethasone significantly reduced IL-2 (Fig. 3c), IFN-γ (Fig. 3d) and TNF-α (Fig. 3e) production in vitro. Although pembrolizumab and ipilimumab individually or in combination enhanced IL-2 and IFN-γ production, addition of dexamethasone significantly impaired cytokine production even in the presence of these immune checkpoint inhibitors in vitro (Fig. 3e, d). While pembrolizumab and ipilimumab did not enhance TNF-α production, the presence of dexamethasone further reduced production of this cytokine in vitro (Fig. 3e). Thus, our results suggest that dexamethasone may impact T cell function by suppressing cytokine production.
Differential effects of dexamethasone on co-inhibitory receptor expression
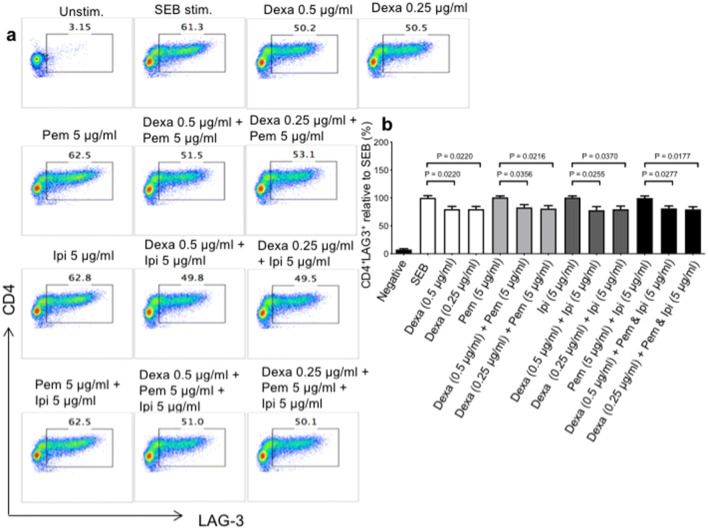
Although prednisone did not have any substantial effects on the expression levels of co-inhibitory receptors, we found that dexamethasone at the relevant physiological concentrations significantly reduced LAG-3 expression on both CD4+ (Fig. 4a, b) and CD8+ T cells (Fig. 5a, b). Reduction in LAG-3 expression by dexamethasone was not dose-dependent and occurred regardless of the presence/absence of pembrolizumab or ipilimumab. However, dexamethasone had no effects on the expression levels of PD-1, CTLA-4 and TIM-3 on CD4+ T cells (Supplementary Fig. 1c–e), and PD-1 and CTLA-4 on CD8+ T cells (Supplementary Fig. 2a–c). However, dexamethasone reduced the expression of TIM-3 on CD8+ T cells when PBMCs were co-treated with pembrolizumab, but not ipilimumab (Supplementary Fig. 2c). These observations show that dexamethasone treatment differentially impacts the expression of co-inhibitory receptors by CD4+ and CD8+ T cells.
Fig. 4.
Dexamethasone treatment impacts the expression of LAG-3 by CD4+ T cells. PBMCs were treated with the indicated concentrations of dexamethasone (dexa), anti-PD-1 (pembrolizumab (pem)), a-CTLA-4 (ipilimumab (ipi)) alone or in combination and stimulated with SEB for 72 h. a Representative FACS plots showing expression of LAG-3 by gated CD4+T cells in response to dexamethasone treatment and SEB stimulation. b Cumulative data of LAG-3 expression by CD4+ T cells. Data from 4 independent experiments shown. Error bars represent mean ± SEM. P values obtained by ordinary one-way ANOVA followed by Dunnett’s multiple comparisons test. Unstim, unstimulated cells
Fig. 5.
Dexamethasone treatment impacts the expression of LAG-3 by CD8+ T cells. PBMCs were treated with the indicated concentrations of dexamethasone (dexa), anti-PD-1 (pembrolizumab (pem)), a-CTLA-4 (ipilimumab (ipi)) alone or in combination and stimulated with SEB for 72 h. a Representative FACS plots showing expression of LAG-3 by gated CD8+ T cells in response to dexamethasone treatment and SEB stimulation. b Cumulative data of LAG-3 expression by CD8+ T cells. Data from four independent experiments shown. Error bars represent mean ± SEM. P values obtained by ordinary one-way ANOVA followed by Dunnett’s multiple comparisons test. Unstim, unstimulated cells. c Plots showing phosphorylation of the SHP-2 tyrosine phosphatase Y542 by PD-1 + Jurkat cells stimulated with CHO cells which constitutively express PD-L + and a TCR activator. d PD-1negJurkat cells were pre-treated with the indicated steroids or anti-PD-1 (pembrolizumab) alone or in combination for 48 h. Jurkat cells were co-cultured with CHO cells for 3 h at a ratio of 10:1. Cell lysates were collected followed by measurement of Y542 phosphorylation by ELISA. e PBMCs were pre-treated with the indicated steroids or anti-PD-1 (pembrolizumab) alone or in combination for 3 h. Cell lysates were collected followed by measurement of Y542 phosphorylation by ELISA. Data from four experiments shown. Error bars represent mean ± SD. P values obtained by ordinary one-way ANOVA followed by Dunnett’s multiple comparisons test
Dexamethasone does not interfere with PD-1/PDL-1 interactions in a cell-based assay system
Next, we decided to investigate its possible interference with PD-1/PDL-1 interactions. As anticipated, we observed that both anti-PD-L1 and pembrolizumab significantly enhanced luminescence activity due to interference with PD-1/PDL-1 interaction (Supplementary Fig. 2d); however, dexamethasone at either 0.5 or 0.25 μg/ml did not influence the action of a-PD-L1 or pembrolizumab in this cell-based assay (Supplementary Fig. 2d). Therefore, our data suggest that dexamethasone does not directly impact PD-1/PDL-1 interactions in vitro.
Treatment of Jurkat cells with prednisone or dexamethasone results in increased SHP-2 phosphorylation, which is reversed by anti-PD-1/CTLA-4 treatment
We also investigated whether prednisone or dexamethasone treatment can influence PD-1 signaling in the presence or absence of pembrolizumab. The recruitment of the Src-homology 2 domain-containing protein tyrosine phosphatase (SHP-2) to the cytoplasmic tail of PD-1 occurs in response to interaction with PD-L1 [37]. Furthermore, SHP-2 recruitment upon PD-1 engagement is synonymous with its inhibitory function [38]. In this regard, we assessed the phosphorylation of the SHP-2 tyrosine kinase, Y542 [39], in response to stimulation of PD-1 + Jurkat cells pre-treated with either anti-PD-1, prednisone or dexamethasone before co-culture with PD-L1 + CHO cells for 3 h. Treatment of PD1 + Jurkat cells with physiological prednisone (1 μg/ml) promoted a threefold increase in SHP-2 phosphorylation compared to cells treated with anti-PD-1 (Fig. 5c). Also, SHP-2 phosphorylation by PD-1 + Jurkat cells treated with dexamethasone (0.5 μg/ml) was increased in comparison with anti-PD-1-treated cells, although at a lesser extent than prednisone (twofold, (Fig. 5c)). As expected, treatment with anti-PD-1 (10 μg/ml) led to a slight reduction in SHP-2 phosphorylation compared to no treatment, (≈ 175 pg/ml compared to ≈ 150 pg/ml, Fig. 5c). However, co-treatment with anti-PD-1 and prednisone/dexamethasone or both induced significant reduction of SHP-2 phosphorylation by Jurkat cells (Fig. 5c). These observations indicate that steroid treatment promotes PD-1-PD-L1 interaction with concomitant SHP-2 phosphorylation, which is countered by anti-PD-1 treatment. This occurrence was dependent on PD-1 expression/signaling as treatment of PD-1 negative Jurkat cells with prednisone resulted in decreased SHP-2 phosphorylation, which was unaffected by anti-PD-1 treatment (Fig. 5d). These results suggest that anti-PD-1 can reverse the inhibitory effect of steroid-induced SHP-2 phosphorylation in Jurkat cells, which may potentially promote increased functionality. To determine whether this is the case for human primary cells, we stimulated total PBMCs from healthy individuals with anti-CD3/CD28 (3 μg/ml and 1 μg/ml) for 3 h. As anticipated, interaction of PD-1/PDL-1 in stimulated PBMCs resulted in SHP-2 recruitment, which was partially abrogated by anti-PD-1 antibody (10 μg/ml) (Fig. 5e). Although the cell-based assay showed that prednisone (1 μg/ml) or dexamethasone (0. 5 μg/ml) alone could increase SHP-2 phosphorylation in Jurkat-cells (Fig. 5c), this was not the case when human PBMCs were tested (Fig. 5e). The interesting observation was that neither prednisone nor dexamethasone or their combination interfered with the abrogation of SHP-2 phosphorylation by anti-PD-1 antibody (Fig. 5e).
Case report
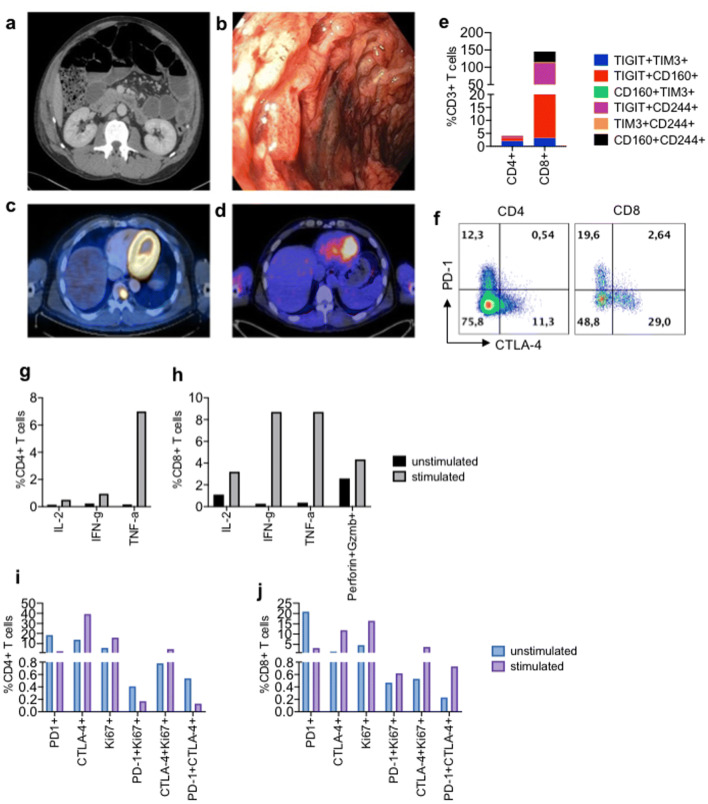
A 53-year-old male with a diagnosis of metastatic melanoma. In the spring of 2018, he presented with recurrent disease (biopsy-proven) in the form of an isolated metastatic deposit within the third thoracic vertebrae. With no contraindications to treatment with immune checkpoint inhibitors, the patient was offered the combination of ipilimumab and nivolumab. In dramatic fashion, on the fourth day of the first cycle of treatment our patient developed a profound dynamic ileus (Fig. 6a), for which conservative measures failed to improve the situation. Computed tomography failed to demonstrate a mechanical obstruction, and in light of the temporal association between initiating treatment with immune checkpoint inhibitors and the development of the patient’s symptoms, a presumptive diagnosis of immune-related ileus was made (Fig. 6b), after which treatment with high-dose glucocorticoids was initiated (methylprednisolone, 2 mg/kg prednisone-equivalent). Approximately 36 h after beginning treatment with methylprednisolone, the patient’s bowel functions resumed, after which pan colitis with severe diarrhea developed. Glucocorticoids were continued following the development of diarrhea, and over a period of several days the consistency of stool and frequency of bowel movements improved. The patient was subsequently transitioned from parental to oral corticosteroids, with a taper lasting 11 weeks. In total, the patient required glucocorticoids for more than 11 of the 12 weeks between the initiation of immune checkpoint inhibitors and re-staging imaging, during which time no further treatment with immune checkpoint inhibitors was administered. Despite this, upon re-imaging a complete metabolic response to treatment was observed (Fig. 6c and d).
Fig. 6.
Clinical data of the case study patient. a On day 4 of cycle 1 treatment with ipilimumab (anti-CTLA-4) and nivolumab (anti-PD-1) a profound a dynamic ileus developed; transverse contrast-enhanced CT abdomen. b Endoscopic imaging of large bowel mucosa demonstrating a severe, hemorrhagic pancolitis. c Baseline tumor imaging; transverse CT/PET imaging demonstrates an intensely FDG-avid (biopsy-proven) metastatic lesion involving the ninth thoracic vertebral body. d Complete metabolic response 24 weeks following single infusion of ipilimumab and nivolumab. e Percentage co-expression of the indicated inhibitory receptors by CD4+ and CD8+ T cells. f Representative flow cytometry plots showing expression of PD-1/CTLA-4 on both CD4+ and CD8+ T cells. g Expression of IL-2, IFN-γ and TNF-α by CD4+ T cells. h Expression of IL-2, IFN-γ, TNF-α, granzyme B and perforin by CD8+ T cells after 6-h stimulation with soluble anti-CD3/CD28. i, j Data showing expression of PD-1, CTLA-4 and Ki67 by CD4+ and CD8+ T cells, respectively, after 6-h stimulation with soluble anti-CD3/CD28
Peripheral blood T cells from the case study patient co-expressed multiple inhibitory receptors and expressed moderate levels of cytokines and cytotoxic mediators in response to TCR stimulation
Due to this dramatic clinical response, we investigated the function of the T cell fraction of PBMCs from this patient based on inhibitory receptor expression and IL-2, IFN-γ and TNF-α, perforin and granzyme B expression. We found CD8+ T cells, co-expressed TIGIT, CD160, CD244 (2B4) and TIM-3 (Fig. 6e). The frequency of PD-1 + CTLA-4 + expressed by CD4+ and CD8+ T cells was quite low; however, we observed substantial expression level of PD-1 or CTLA-4 by CD8+ and CD4+ T cells, respectively, (Fig. 6f). T cells from the patient expressed moderate levels of IFN-γ, TNF-α and low levels of IL-2 (Fig. 6g, h). As observed with inhibitory receptor co-expression, CD8+ T cells co-expressed higher percentages of cytokines compared to CD4+ T cells (Fig. 6g, h). These CD8+ T cells also co-expressed approximately 4% granzyme B and perforin in response to TCR stimulation (Fig. 6h). The frequency of both Ki67 + peripheral blood CD4+ and CD8+ increased by threefold in response to TCR stimulation (Fig. 6i, j). These observations indicate that treatment with checkpoint inhibitors followed by corticosteroid treatment, promoted the compensatory expression of multiple co-inhibitory receptors by T cells, particularly the CD8+ T cells population, which, were moderately functional based on the expression of cytokines and cytotoxic mediators.
Discussion
The administration of steroids for managing cancer-associated symptoms, particularly nausea and pain, has been widely practiced for decades. It is therefore not surprising, how over four decades ago, a number of studies were carried out to investigate how this treatment influenced the activity of human lymphocyte populations (4). The general consensus from these early studies was that steroid-induced anti-inflammation impacts lymphocyte function/numbers. With the advent of immune checkpoint blockade strategies and the ensuing restoration of ‘exhausted’ T cell function, the impact of such treatments on immune cells is urgently required to be addressed. Furthermore, the use of steroid therapy to counter immune checkpoint blockade-associated irAEs also warranted investigating the impact of steroid treatment on the function of T cells and other immune cells. Due to the anti-inflammatory nature of steroids, it is of concern that interaction with the ‘proliferative burst’ of anti-tumor T cells induced after blockade of PD-1 and CTLA-4 inhibitory pathways will compromise the function of the latter. This notion has been supported by recent studies, which showed that early steroid use correlated with poor disease outcome and mechanistically, by modulating the profile of peripheral blood immune cells [26, 29]. Other studies have demonstrated that steroids affect the function of T cells in several ways, ranging from induction of apoptosis to T helper 2 or regulatory T cell skewing [41].
From our observations, cytokine production and T cells proliferation were not affected in the presence of physiological prednisone concentration or/and anti-PD-1 and anti-CTLA-4) treatments. Reduced IL-2 production and T cell proliferation in response to a twofold higher prednisone concentration (2 μg/ml) indicates that there may be a correlation between T cell dysfunction and exposure to non-physiological prednisone levels. However, the increased production of IFN-γ by PBMCs treated with 2 μg/ml of prednisone, in the presence or absence of checkpoint inhibitors; and reduced TNF-α production, when both concentrations were used, shows that prednisone, instead, may have differential effects on various facets of T cell function.
In contrast, we found that dexamethasone had an inhibitory effect on all T cell functions examined, which could not be overcome by immune checkpoint blockade. Although prednisone and dexamethasone are both synthetic corticosteroids with highly similar chemical structure, dexamethasone differs by the presence of a methyl group at the 16-alpha position and of a fluorine atom at the 9-alpha position [42]. Such structural differences influence the drug potency and may explain different effects on immune cells as we have reported. Our observation is consistent with a report showing that dexamethasone suppressed cytokine production and induced apoptosis in T cells [43]. This was not surprising, as dexamethasone is a long-acting steroid and more potent anti-inflammatory than prednisone [42]. However, whether prednisone and dexamethasone have differential effects on immune checkpoints requires further in vivo studies. It has been reported that T cell affinity predicts sensitivity to corticosteroids. As such, CD8+ T cells recognizing neoantigens stemming from mutated tumor cells exhibit higher affinity compared to CD8+ T cells recognizing self-antigens [44]. One can speculate that the effects of corticosteroids on T cells vary in patients depending on the magnitude of neoantigens. Indeed, patients with lower mutation burden will exhibit poorer antitumor responses compared with those with higher mutation burden when on immune checkpoint therapies [45]. In agreement, a recent study has reported that low affinity memory CD8+ T cells are mainly impaired by methylprednisolone [46]. In particular, such inhibitory effects on the clinical outcomes of immune checkpoint blockers are dose and time dependent as late corticosteroids treatment was associated with better therapeutic outcome [46].
Steroids are widely used to manage both cancer symptoms and checkpoint blockade-associated irAEs, but their effect on the expression of coinhibitory receptors has not been extensively investigated. In spite of the inherent anti-inflammatory effects on some T cell functions, in our hands, prednisone treatment did not affect the expression of all coinhibitory receptors analyzed. The ability of dexamethasone to promote PD-1 and CTLA-4 expression by activated T cells has been described [43, 47]. In both studies however, increased PD-1 and CTLA-4 expression was achieved using high, non-physiological concentrations of dexamethasone (10–7 M). Our results from dexamethasone treatment using a physiological concentration (equivalent to 10–6 M) showed selective downregulation of LAG-3 and TIM-3 (induced by anti-PD-1 and anti-CTLA-4 co-treatment).
Although prednisone and dexamethasone do not appear to directly interfere with PD-1-PD-L1 interaction, we have demonstrated that both steroids can exert their anti-inflammatory effects by promoting the phosphorylation of the SHP-2 tyrosine kinase, Y542 using a cell-based assay. Surprisingly, these corticosteroids did not enhance SHP-2 phosphorylation when PBMCs were tested instead of the cell-based assay. The binding of PD-1 to its ligand, PD-L1, induces recruitment of SHP-2 to tyrosine residues within the immunoreceptor tyrosine-based switch motif (ITSM) in the cytoplasmic tail [48]. The recruitment of SHP-2 to PD-1 results in de-phosphorylation of the TCR zeta chain and subsequent inhibition of downstream signaling events. Of significance is the ability of anti-PD-1 to counter prednisone-mediated Y542 phosphorylation to levels comparable to anti-PD-1 single treatment in the cell-based assay system. Additionally, we observed that neither prednisone nor dexamethasone or their combination interfered with the effects of anti-PD-1 antibody on SHP-2 phosphorylation in PBMCs. This observation ‘mirrors’ the negative impact of baseline steroid treatment on checkpoint blockade and implies that T cell function is highly dependent on the nature and concentration of the steroid administered. This observation corresponds with increased IFN-γ production associated with anti-PD-1/anti-CTLA-4 and prednisone co-treated PBMCs. This needs to be taken into consideration that the cell-based assay is completely a different system compared to human PBMCs, which can explain the observed differences. Although prednisone and dexamethasone appear to have similar effects on SHP-2 phosphorylation, their differential impact on T cell effector functions suggests different pathways are modulated by these corticosteroids. Further studies are required to better understand their immunomodulatory roles.
Our case study demonstrated dramatic responses to steroid treatment for irAEs (induced by anti-PD-1/CTLA-4 combination), which correlated with a favorable overall response. Although one of the limitations of this study was our accessibility to patient samples at various stages of treatment, we did observe that their peripheral blood CD4+ and CD8+ T cells underwent activation and expressed higher, albeit moderate levels of cytokines and cytotoxic mediators in response to TCR stimulation, irrespective of multiple co-inhibitory receptor expression. This increased T cell function possibly played a role in the development of pan colitis with severe diarrhea that occurred in response to steroid treatment.
We have demonstrated, similar to previous studies, that the anti-inflammatory activity of long-acting steroids such as dexamethasone compromises T cell function. However, the interaction between T cells and prednisone alone and in the context of checkpoint blockade may not always correlate with reduced function. Of relevance to cancer immunotherapy is our observation that anti-PD-1 checkpoint blockade can potentially mediate restoration of steroid-treated T cells based on reduced SHP-2 (Y542) phosphorylation. In particular, concomitant use of prednisone does not appear to interfere with the function of immune checkpoint blockers. Therefore, our observations have important implications for modifying steroid regimens for cancer immunotherapy. A major limitation of our study has been using an in vitro system and lack of access to a large patient cohort. Therefore, additional animal and clinical studies with larger cohorts are required to determine the differential effects of prednisone versus dexamethasone and the appropriate dosage/timing of such medications in patients on immune checkpoint blockers.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank the University of Alberta Faculty of Medicine and Dentistry’s Flow cytometry facility, which has received financial support from the faculty of Medicine and Dentistry and the Canadian Foundation for Innovation (CFI) awards to contributing investigators.
Abbreviations
- CTLA-4
Cytotoxic T lymphocyte-associated protein 4
- irAEs
Immune-related adverse events
- LAG-3
Lymphocyte activation gene 3
- NFAT
Nuclear factor of activated T cells
- PBMCs
Peripheral blood mononuclear cells
- PD-1
Programmed cell death-1
- PD-L1
Programmed cell death-ligand 1
- SEB
Staphylococcus enterotoxin B
- SHP-2
Src homology region 2-domain phosphatase 2
- TIGIT
T cell immunoreceptor with Ig and ITIM domains
- TIM-3
T-cell immunoglobulin and mucin-domain containing-3
Authors’ contributions
IO performed SHP-2, luminescence, some of the ELISA assays, processed sample related to the case study, analyzed the data and wrote the manuscript. LX conducted most of the ELISA assays, CFSE, and the effects of prednisone and dexamethasone on co-inhibitory receptors expression. JW performed clinical study on the patient and contributed in conceiving the original idea. SE conceived the original idea, designed and supervised all of the research, assisted in data analysis and wrote the manuscript.
Funding
This work was supported by the Canadian Institutes of Health Research (CIHR), a CIHR New Investigator Salary Award (360929), an a CIHR Foundation Scheme Grant (353953) (all to S.E.). Alberta Cancer Foundation also supported the clinical study (to J.W. and S.E).
Compliance with ethical standards
Conflict of interest
The authors declare no competing interests.
Consent for publication
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
Ethics approval and consent to participate
The appropriate Institutional Review Boards at the University of Alberta approved the studies, IRB #Pro00046064.
Informed consent
All study participants gave written informed consent to participate in this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Okoye IS, Houghton M, Tyrrell L, Barakat K, Elahi S. Coinhibitory receptor expression and immune checkpoint blockade: Maintaining a balance in CD8+T cell responses to chronic viral infections and cancer. Front Immunol. 2017 doi: 10.3389/fimmu.2017.01215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DAA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thommen DS, Schreiner J, Muller P, et al. Progression of lung cancer is associated with increased dysfunction of T cells defined by coexpression of multiple inhibitory receptors. Cancer Immunol Res. 2015;3(12):1344–1355. doi: 10.1158/2326-6066.CIR-15-0097. [DOI] [PubMed] [Google Scholar]
- 4.Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908–918. doi: 10.1016/S1470-2045(15)00083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Callahan MK, Flaherty CR, Postow MA. Checkpoint blockade for the treatment of advanced melanoma. Cancer Treat Res. 2016;167:231–250. doi: 10.1007/978-3-319-22539-5_9. [DOI] [PubMed] [Google Scholar]
- 6.Hakenberg OW. Nivolumab for the treatment of bladder cancer. Expert Opin Biol Ther. 2017 doi: 10.1080/14712598.2017.1353076. [DOI] [PubMed] [Google Scholar]
- 7.Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019 doi: 10.1056/NEJMoa1816714. [DOI] [PubMed] [Google Scholar]
- 8.Antonia SJ, López-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016 doi: 10.1016/S1470-2045(16)30098-5. [DOI] [PubMed] [Google Scholar]
- 9.Economopoulou P, Agelaki S, Perisanidis C, Giotakis EI, Psyrri A. The promise of immunotherapy in head and neck squamous cell carcinoma. Ann Oncol. 2016 doi: 10.1093/annonc/mdw226. [DOI] [PubMed] [Google Scholar]
- 10.Bachireddy P, Burkhardt UE, Rajasagi M, Wu CJ. Haematological malignancies: at the forefront of immunotherapeutic innovation. Nat Rev Cancer. 2015 doi: 10.1038/nrc3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Myint ZW, Goel G. Role of modern immunotherapy in gastrointestinal malignancies: a review of current clinical progress. J Hematol Oncol. 2017 doi: 10.1186/s13045-017-0454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15:486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marrone KA, Ying W, Naidoo J. Immune-related adverse events from immune checkpoint inhibitors. Clin Pharmacol Ther. 2016 doi: 10.1002/cpt.394. [DOI] [PubMed] [Google Scholar]
- 14.Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139–148. doi: 10.1016/j.ejca.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 15.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in advanced melanoma. N Engl J Med. 2015 doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 16.El Osta B, Hu F, Sadek R, Chintalapally R, Tang SC. Not all immune-checkpoint inhibitors are created equal: Meta-analysis and systematic review of immune-related adverse events in cancer trials. Crit Rev Oncol Hematol. 2017 doi: 10.1016/j.critrevonc.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Hofmann L, Forschner A, Loquai C, et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur J Cancer. 2016;60:190–209. doi: 10.1016/j.ejca.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 18.Wozniak S, Mackiewicz-Wysocka M, Krokowicz L, Kwinta L, Mackiewicz J. Febrile neutropenia in a metastatic melanoma patient treated with ipilimumab—case report. Oncol Res Treat. 2015;38:105–108. doi: 10.1159/000377650. [DOI] [PubMed] [Google Scholar]
- 19.Sibaud V, Meyer N, Lamant L, Vigarios E, Mazieres J, Delord JP. Dermatologic complications of anti-PD-1/PD-L1 immune checkpoint antibodies. Curr Opin Oncol. 2016;28:254–263. doi: 10.1097/CCO.0000000000000290. [DOI] [PubMed] [Google Scholar]
- 20.Ahmed T, Pandey R, Shah B, Black J. Resolution of ipilimumab induced severe hepatotoxicity with triple immunosuppressants therapy. BMJ Case Rep. 2015 doi: 10.1136/bcr-2014-208102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horvat TZ, Adel NG, Dang T-O, et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol. 2015;33:3193–3198. doi: 10.1200/JCO.2015.60.8448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncol. 2016 doi: 10.1001/jamaoncol.2016.1051. [DOI] [PubMed] [Google Scholar]
- 23.Harmankaya K, Erasim C, Koelblinger C, Ibrahim R, Hoos A, Pehamberger H, Binder M. Continuous systemic corticosteroids do not affect the ongoing regression of metastatic melanoma for more than two years following ipilimumab therapy. Med Oncol. 2011 doi: 10.1007/s12032-010-9606-0. [DOI] [PubMed] [Google Scholar]
- 24.Pawarode A, D’Souza A, Pasquini MC, et al. Phase 2 study of pembrolizumab during lymphodepleted state after autologous hematopoietic cell transplantation in multiple myeloma patients. Blood. 2017;130:339. [Google Scholar]
- 25.Garant A, Guilbault C, Ekmekjian T, Greenwald Z, Murgoi P, Vuong T. Concomitant use of corticosteroids and immune checkpoint inhibitors in patients with hematologic or solid neoplasms: a systematic review. Crit Rev Oncol Hematol. 2017 doi: 10.1016/j.critrevonc.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 26.Arbour KC, Mezquita L, Long N, et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol. 2018;36:2872–2878. doi: 10.1200/JCO.2018.79.0006. [DOI] [PubMed] [Google Scholar]
- 27.Martínez Bernal G, Mezquita L, Auclin E, et al. Baseline corticosteroids (CS) could be associated with absence of benefit to immune checkpoint inhibitors (ICI) in advanced non-small cell lung cancer (NSCLC) patients. Ann Oncol. 2017 doi: 10.1093/annonc/mdx380.025. [DOI] [Google Scholar]
- 28.Della Corte CM, Morgillo F. Early use of steroids affects immune cells and impairs immunotherapy efficacy. ESMO Open. 2019 doi: 10.1136/esmoopen-2018-000477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fucà G, Galli G, Poggi M, et al. Modulation of peripheral blood immune cells by early use of steroids and its association with clinical outcomes in patients with metastatic non-small cell lung cancer treated with immune checkpoint inhibitors. ESMO Open. 2019 doi: 10.1136/esmoopen-2018-000457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schadendorf D, Wolchok JD, Stephen Hodi F, et al. Efficacy and safety outcomes in patients with advanced melanoma who discontinued treatment with nivolumab and ipilimumab because of adverse events: a pooled analysis of randomized phase II and III trials. J Clin Oncol. 2017 doi: 10.1200/JCO.2017.73.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weber JS, Larkin JMG, Schadendorf D, et al. Management of gastrointestinal (GI) toxicity associated with nivolumab (NIVO) plus ipilimumab (IPI) or IPI alone in phase II and III trials in advanced melanoma (MEL) J Clin Oncol. 2018 doi: 10.1200/jco.2017.35.15_suppl.9523. [DOI] [Google Scholar]
- 32.Giles AJ, Hutchinson M-KND, Sonnemann HM, et al. Dexamethasone-induced immunosuppression: mechanisms and implications for immunotherapy. J Immunother Cancer. 2018;6:51. doi: 10.1186/s40425-018-0371-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maxwell R, Luksik AS, Garzon-Muvdi T, et al. Contrasting impact of corticosteroids on anti-PD-1 immunotherapy efficacy for tumor histologies located within or outside the central nervous system. Oncoimmunology. 2018 doi: 10.1080/2162402X.2018.1500108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okoye I, Namdar A, Xu L, Crux N, Elahi S. Atorvastatin downregulates co-inhibitory receptor expression by targeting Ras-activated mTOR signalling. Oncotarget. 2017;8:98215–98232. doi: 10.18632/oncotarget.21003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elahi S, Dinges WL, Lejarcegui N, Laing KJ, Collier AC, Koelle DM, McElrath MJ, Horton H. Protective HIV-specific CD8(+) T cells evade T(reg) cell suppression. Nat Med. 2011;17:989–995. doi: 10.1038/nm.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ganesan A, Ahmed M, Okoye I, et al. Comprehensive in vitro characterization of PD-L1 small molecule inhibitors. Sci Rep. 2019 doi: 10.1038/s41598-019-48826-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. 2004;173:945–954. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- 38.Hui E, Cheung J, Zhu J, et al. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science (80-) 2017 doi: 10.1126/science.aaf1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lorenz U. SHP-1 and SHP-2 in T cells: two phosphatases functioning at many levels. Immunol Rev. 2009 doi: 10.1111/j.1600-065X.2008.00760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu DTY, Clements PJ, Paulus HE, Peter JB, Levy J, Barnett EV. Human lymphocyte subpopulations. Effect of corticosteroids. J Clin Investig. 1974 doi: 10.1172/JCI107591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Libert C, Dejager L. How steroids steer T cells. Cell Rep. 2014 doi: 10.1016/j.celrep.2014.04.041. [DOI] [PubMed] [Google Scholar]
- 42.Boland EW. Clinical comparison of the newer anti-inflammatory corticosteroids. Ann Rheum Dis. 1962 doi: 10.1136/ard.21.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xing K, Gu B, Zhang P, Wu X. Dexamethasone enhances programmed cell death 1 (PD-1) expression during T cell activation: An insight into the optimum application of glucocorticoids in anti-cancer therapy. BMC Immunol. 2015 doi: 10.1186/s12865-015-0103-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yarchoan M, Johnson BA, Lutz ER, Laheru DA, Jaffee EM. Targeting neoantigens to augment antitumour immunity. Nat Rev Cancer. 2017 doi: 10.1038/nrc.2016.154. [DOI] [PubMed] [Google Scholar]
- 45.Khalil DN, Smith EL, Brentjens RJ, Wolchok JD. The future of cancer treatment: Immunomodulation, CARs and combination immunotherapy. Nat Rev Clin Oncol. 2016 doi: 10.1038/nrclinonc.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tokunaga A, Sugiyama D, Maeda Y, Warner AB, Panageas KS, Ito S, Togashi Y, Sakai C, Wolchok JD, Nishikawa H. Selective inhibition of low-affinity memory CD8+ T cells by corticosteroids. J Exp Med. 2019 doi: 10.1084/jem.20190738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xia M, Gasser J, Feige U. Dexamethasone enhances CTLA-4 expression during T cell activation. Cell Mol Life Sci. 1999 doi: 10.1007/s000180050403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salmond RJ, Alexander DR. SHP2 forecast for the immune system: fog gradually clearing. Trends Immunol. 2006;27:154–160. doi: 10.1016/j.it.2006.01.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.