Abstract
Combination immunotherapy targeting the PD-1 and CTLA-4 checkpoint inhibitor pathways provides substantial clinical benefit in patients with advanced-stage cancer but at the risk of dose-limiting inflammatory and autoimmune toxicity. The delicate balance that exists between unleashing tumor killing and promoting systemic autoimmune toxicity represents a major clinical challenge. We hypothesized that targeting anti-CTLA-4 so that it perfuses tumor-draining lymph nodes would provide a significant therapeutic advantage and developed an injectable hydrogel with controlled antibody release characteristics for this purpose. Injection of hydrogel-encapsulated anti-CTLA-4 at a peri-tumor location (MC-38 tumor model) produced dose-dependent antitumor responses and survival that exceeded those by anti-CTLA-4 alone (p < 0.05). Responses to 100 µg of targeted anti-CTLA-4 also equaled or exceeded those observed with a series of systemic injections delivering 600 µg (p < 0.05). While preserving antitumor activity, this approach resulted in serum anti-CTLA-4 exposure (area under the curve) that averaged only 1/16th of that measured with systemic therapy. Consistent with the marked differences in systemic exposure, systemic anti-CTLA-4 stimulated the onset of autoimmune thyroiditis in iodide-exposed NOD.H-2h4 mice, as measured by anti-thyroglobulin antibody titer, while hydrogel-encapsulated anti-CTLA-4 had a minimal effect (p ≤ 0.01). At the same time, this targeted low-dose anti-CTLA-4 approach synergized well with systemic anti-PD-1 to control tumor growth and resulted in a high frequency of complete responders that were immune to tumor re-challenge at a distant site. We conclude that targeted and controlled delivery of low-dose anti-CTLA-4 has the potential to improve the benefit–risk ratio associated with combination checkpoint inhibitor therapy.
Keywords: CTLA-4, Checkpoint inhibitor blockade, Hydrogel, Peri-tumor injection, Autoimmune toxicity, NOD.H-2h4
Introduction
Cancer therapy has entered a new era with an increasing number of immunotherapy regimens targeting immune checkpoint inhibitors as first-line therapy. However, given the number of immunosuppressive pathways that can be co-opted to suppress tumor responses, it is not surprising that individual approaches are effective in only a minority of patients. As such, combination therapies are of particular interest including those targeting blockade of more than one checkpoint inhibitor and checkpoint inhibitor blockade in combination with vaccination, chemotherapy, radiation, and small-molecule inhibitors [1–4]. Of these, combining the systemic administration of an anti-CTLA-4 monoclonal antibody (mAb) with mAbs targeting anti-PD-1 stands out as a central approach. Clinical results indicate that this combination yields a significantly greater response and progress-free survival rate compared to monotherapy [5–9]. While anti-PD-1 and anti-CTLA-4 each target distinct pathways, their combination produces a synergistic effect that activates novel antitumor mechanisms not observed when either is used alone [10, 11]. However, along with the induction of a superior clinical response, this combination also produces a significant increase in severe inflammatory/autoimmune toxicity. The delicate balance that exists between unleashing tumor killing and promoting immune-related adverse events (irAEs) represents a major obstacle when combining immune checkpoint inhibitors [5–9, 12, 13].
As such, the current investigation focuses on the administration of anti-CTLA-4, a checkpoint inhibitor therapy associated with a less favorable benefit-to-risk ratio and a significant increase in toxicity when combined with anti-PD-1 [12–14]. We hypothesized that the targeted and controlled administration of low-dose anti-CTLA-4 could be used to activate regulatory T cells within tumor-draining lymph nodes (TDLN) while limiting high-dose systemic exposure and the activation of autoimmune responses at distant organ sites. We employed an injectable and self-polymerizing hyaluronic acid (HA)-based hydrogel to encapsulate and deliver anti-CTLA-4. A peri-tumor injection site was employed as a simple approach for targeting tumor-responsive T cells that are concentrated within TDLN and capable of producing a systemic abscopal antitumor response [15, 16]. Ultimately, the goal was to produce a reagent that would not only reduce systemic exposure and toxicity, but also synergize with the systemic administration of anti-PD-1. In this preclinical evaluation, a subcutaneous MC-38 tumor implantation model was employed and the antitumor effects of targeted-controlled low-dose anti-CTLA-4 were compared to that of a standard regimen of systemic anti-CTLA-4. In addition, in order to determine the impact of this targeted low-dose approach on the induction of autoimmune toxicity, we measured serum thyroglobulin antibody levels to assess the impact on the induction of autoimmune thyroiditis using NOD.H-2h4 mice. NOD.H-2h4 mice develop spontaneous thyroiditis, a process accelerated by dietary iodide supplementation [17], and both the frequency and severity of the thyroiditis are susceptible to anti-CTLA-4 blockade [18, 19]. Our findings suggest a promising approach for improving the benefit–risk ratio associated with conventional delivery of anti-CTLA-4 therapy.
Materials and methods
Animals
Six- to eight-week old C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME) or the UCLA Department of Radiation Oncology Mouse Facility and housed at the UCLA Division of Laboratory Animal Medicine facility. A colony of NOD.H-2h4 mice (breeding pairs of NOD.Cg-H2h4/DilTacUmmJ from The Jackson Laboratory) was bred at the UCLA Division of Laboratory Animal Medicine facility and housed at UCLA CFAR Humanized Mouse Core facility under laminar flow conditions with sterile food, water, and caging. All protocols and procedures were approved by the UCLA Animal Research Committee.
Reagents
Mouse colorectal cancer cell line, MC-38, was from the Division of Cancer Treatment and Diagnosis Tumor Repository, National Cancer Institute. Thiolated carboxymethyl HA (CMHA-S) and poly-(ethylene glycol)-diacrylate (PEGDA) were from Lineage Cell Therapeutics (Alameda, CA, USA). Anti-CTLA-4 (clone # 9H10) and anti-PD-1 (clone # BE0146) were from BioXcell (West Lebanon, NH, USA). Purified mouse CTLA-4 protein was from BioLegend (San Diego, CA, USA), and FITC-labeled anti-PD-1 was from Invitrogen (Carlsbad, CA). FITC-labeled dextrans were from Sigma-Aldrich (St. Louis, MO). Purified bovine hyaluronidase (HAse) was from MP Biomedicals (Santa Ana, CA). Labeled mAb for flow cytometry included anti-mouse CD3 and CD152 (anti-CTLA-4) from BD Biosciences (San Jose, CA) along with anti-CD4 and CD8 from BioLegend.
Hydrogel formulation and in vitro assessment of retention and release characteristics
CMHA-S and PEGDA were individually dissolved in degassed deionized water (pH 7.4) to prepare solutions of 1.25% (w/v) and 6% (w/v), respectively. Molecules to be incorporated, either Dextran-FITC (5 µg in 24 µl/gel), anti-CTLA-4 (25–100 µg in 24 µl/gel) or FITC-labeled anti-PD-1 (5 µg in 24 µl/gel), were mixed with PEGDA solution (30 µl/gel), and the resulting mixtures were added to CMHA-S (96 µl/gel) with thorough mixing to initiate gelation. Final component concentrations within standard 150 µl hydrogels were 0.8% w/v for CMHA-S and 1.2% w/v for PEGDA.
For in vitro assessment, 150 µl of hydrogel solution containing an incorporated target molecule was allowed to undergo gelation (20 min in 14-ml round-bottom tube) followed by addition of either 1 ml (for incorporated molecules ≤ 5 µg/gel) of 10 ml (for incorporated molecules of 5–50 µg/gel) of release media (PBS with 1% BSA, 0.5 mM EDTA) and incubation at 37 °C. At indicated times, media were completely recovered from each tube and replaced by fresh pre-warmed media. Exogenous HAse (1000 units) was added to release media after the final sample collection to release any remaining target molecule from the hydrogel matrix as previously described [20]. The quantity of Dextran-FITC or FITC-labeled anti-PD-1 recovered at each time point was determined using a fluorescence plate reader (Bio-Rad Laboratories, Hercules, CA, USA), and recovered anti-CTLA-4 was measured using a binding-specific ELISA.
Retention of Dextran-FITC by hydrogels after injection in vivo
Hydrogels formulated with Dextran-FITC (20, 150 or 500 kDa) were implanted into the upper flank of C57BL/6 mice by subcutaneous injection and excised 4 h later. Dextran-FITC remaining in the recovered hydrogels was recovered by incubating in 1 ml of release media at 37 °C in the presence of HAse (1000 units) until hydrogels were lysed. The amount of recovered Dextran-FITC was determined by fluorescent plate reader using stock preparations to generate standard curves.
Binding-specific ELISA for quantitation of functional anti-CTLA-4 antibody
To measure intact anti-CTLA antibody with binding activity, wells were coated with mouse recombinant CTLA-4 protein (2 mg/ml) in coating buffer (Sigma-Aldrich) overnight at 4 °C, washed and then incubated with test samples (after a 1:100 dilution) for 2 h at room temperature. Wells were washed and then incubated with horseradish peroxidase-conjugated affinity-purified F(ab′)2 fragment goat anti-hamster IgG (H + L) (Jackson ImmunoResearch, West Grove, PA, USA) for 1 h followed by detection with TMB Substrate Solution (Thermo Fisher Scientific, Waltham, MA) for 30 min. Stop solution (Thermo Fisher Scientific) was added, and optical densities were read at 450 nm using a microplate reader (Bio-Rad Laboratories). Serial dilutions of anti-CTLA-4 obtained from the same manufacturing lot were used to generate standard curves and anti-CTLA-4 concentration calculated based on measured optical density at 450 nm.
Tumor model and determination of treatment responses to anti-CTLA-4
C57BL/6 mice were implanted with MC-38 cells (3 × 105/mouse) by subcutaneous injection into the right upper flank. Six days after implantation, all animals with palpable tumors were randomly divided into control and treatment groups (12 mice/group). Control mice received no therapy, while mice in the experimental groups received anti-CTLA-4 administered by either intraperitoneal (i.p) injection (200 µg in PBS) on days 6, 9, and 12 (total dose 600 µg) after tumor implantation or by subcutaneous injection at a peri-tumoral location with (1) anti-CTLA4 (50 µg) diluted in PBS or (2) anti-CTLA4 (25–100 µg) that had been incorporated into a 150 µl hydrogel on days 6 and 11 (total dose 50–200 µg). Hydrogel injections occurred between 4 and 5 min after final mixing, while the solution was still in a liquid state. Tumor volumes were measured by digital caliper every 3–4 days up to day 28, and survival rate was determined over 40 days. Mice with tumor volumes > 3000 mm3 were considered terminal and euthanized.
Characterization of TDLN and tumor-infiltrating lymphocytes (TIL)
C57BL/6 mice that were treated with peri-tumor injections of hydrogel-encapsulated anti-CTLA-4 (50 µg/dose) on days 6 and 11 after tumor implantation, or untreated tumor-bearing animals, were killed on day 13 to recover axillary, brachial, and inguinal TDLN, contralateral lymph nodes (CLLN), and tumor. Single-cell suspensions were prepared by mechanical disaggregation for cell counts, stained with fluorochrome-conjugated mAbs and results acquired with a LSR II flow cytometer (Becton–Dickinson, Franklin Lakes, NJ).
Assessment of autoimmune toxicity using NOD.H-2h4 mice
Female NOD.H-2h4 mice were given drinking water containing sodium iodide (0.05% w/v, Sigma) starting at the age of 8–11 weeks to accelerate and synchronize the spontaneous onset of autoimmunity [17]. Control mice received no additional therapy. In the experimental groups, mice were started on treatment at 11 weeks with either hydrogel-encapsulated anti-CTLA-4 (50 µg/dose) by subcutaneous injection into the right flank on treatment days 0, 5, and 10 (total dose = 150 µg) or systemic administration of anti-CTLA-4 (200 µg/dose) on treatment days 0, 3, 6, 9, and 12 (total dose = 1000 µg) by i.p. injection. Serum samples were collected at 11 weeks (prior to anti-CTLA-4) and 15 weeks of age, and the levels of anti-thyroglobulin autoantibody were assessed by ELISA. Mouse thyroglobulin protein was prepared from BALB/C mouse thyroid glands (BioIVT, Westbury, NY) as described previously [21] and used to coat ELISA wells (1.5 µg/ml). After an overnight incubation (4 °C), wells were washed and blocked, and then diluted serum samples (1:100) were added for 2 h at room temperature. Wells were washed and then incubated with secondary antibody, horseradish peroxidase-conjugated goat anti-mouse IgG (Sigma-Aldrich), for 1 h followed by washing and incubation with TMB Substrate Solution (Thermo Fisher Scientific) for 15 min. Stop solution (Thermo Fisher Scientific) was added, and optical densities were read at 450 nm on a microplate reader (Bio-Rad Laboratories).
Calculations and statistical analysis
The cumulative amounts of Dextran-FITC and checkpoint inhibitor antibody recovered at each time point were calculated as a percentage of the total recovery (summation of values at all time points including recovery after digestion with HAse). Reported fluorescent O.D. and ELISA values represent the average of replicate determinations. Tumor volume (mm3) was calculated by multiplying measured width, height, and depth of the tumor. Tumor growth (size) is reported as the mean ± SE for the tumor volume measured from all animals, and comparisons between groups over time were performed using a two-way ANOVA with repeated measures. A Bonferroni correction was applied to post hoc comparisons between individual treatment groups. A Fisher’s exact test was used to compare the frequency of responders, based on tumor size < 500 mm3, when comparing single and combination therapy. Survival curves were compared using a log-rank test. T tests were applied for single-point comparisons between groups when applicable. p < 0.05 was considered significant and adjusted by Bonferroni correction for post hoc comparisons.
Results
Hydrogel retention and release characteristics
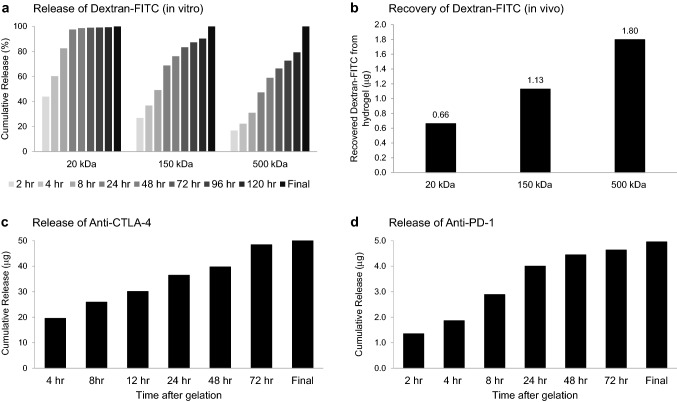
An injectable HA-based hydrogel with controlled release characteristics was optimized to deliver an initial bolus of anti-CTLA-4 at the injection site followed by a sustained drug delivery over a period of days. As shown in Fig. 1, a formulation consisting of 0.8% w/v of CMHA-S and 1.2% w/v of PEGDA appeared ideally suited for mAb delivery with an intrinsic gelation time of approximately 5 min after mixing, allowing for injection and rapid polymerization in situ, and the capacity to control the release of large molecules. In both in vitro and in vivo testing, the release rate for Dextran-FITC was inversely related to the molecular weight (Fig. 1a/b). When incorporating the 20 kDa molecules, ≥ 60% was released within the first 4 h in vitro. In contrast, only 36.8% and 22.3% of the 150 and 500 kDa Dextran-FITC, respectively, were released at 4 h. While release of the 20 kDa construct was essentially complete within 24 h, measurable levels of the 150 and 500 kDa constructs still remained within hydrogels after 5 days. Similarly, when hydrogels containing the different-sized Dextran-FITC were injected into the subcutaneous tissue of C7BL/6 mice and recovered 4 h later, there was a significant difference in the retained Dextran-FITC based on molecular weight (p < 0.001). In vitro release kinetics for anti-CTLA-4 were determined using a similar approach (Fig. 1c). In order to assure that released antibody remained functional, a binding-based ELISA that employed mouse CTLA-4 protein as the target antigen was used. Approximately 95% of anti-CTLA-4 was gradually released over a period of 3 days. Similar release kinetics were observed for anti-PD-1/FITC suggesting a generalized effect on mAb release (Fig. 1d). When administered to mice by subcutaneous injection, functional antibody was still detected from hydrogels harvested 3 days later (data not shown), correlating with the in vitro kinetics.
Fig. 1.
Hydrogel release characteristics. a, b Hydrogels containing 5 µg of Dextran-FITC with different molecular weights (20–500 kDa) were prepared. For in vitro experiments (a), hydrogels were incubated in 1 ml release media at 37 °C with the media collected and replaced with fresh media at indicated time points, HAse (1000 U) was added after 120 h to promote lysis and the release of any retained molecules for “Final” measurement. Recovery at each time point was normalized as a percentage of total antibody recovered. A significant impact of molecular weight on the release over time was observed (p < 0.001, two-way ANOVA with repeated measures). For in vivo experiments (b), hydrogels prepared under the same conditions were implanted into the flank of C57BL/6 mice by subcutaneous injection and excised 4 h later. Dextran-FITC remaining in excised hydrogels was recovered by incubating in release media containing HAse (1,000 U) at 37 °C. A significant impact of molecular weight on the retention of Dextran-FITC was observed (p < 0.001, two-way ANOVA). All experiments were performed in duplicate. c, d In vitro release profiles were also determined for hydrogels incorporating 50 µg of anti-CTLA-4 (c) or 5 µg of anti-PD-1/FITC (d) with the release media collected and replaced with fresh media at indicated time points. HAse (1000 U) was added after 72 h to promote hydrogel lysis and the release of any remaining antibody for “Final” measurement. Measurements represent the mean of duplicate hydrogels
Antitumor effect from targeted administration of low-dose anti-CTLA-4
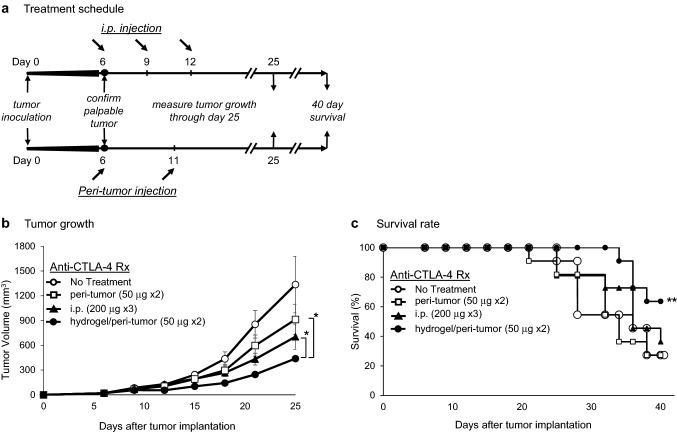
Having developed a mechanism for controlled delivery of anti-CTLA-4, we next assessed whether a low dose of hydrogel-encapsulated anti-CTLA-4 delivered by a peri-tumor injection could mediate significant antitumor activity. As shown in treatment schedule Fig. 2a, C57BL/6 mice bearing palpable subcutaneous MC-38 tumors were treated with one of three anti-CTLA-4 regimens: (1) standard systemic administration with 3 doses (200 µg each, i.p. injection) at days 6, 9, and 12 after tumor implantation; (2) low-dose peri-tumor injection in PBS (50 µg each, subcutaneous injection) at days 6 and 11; or (3) low-dose peri-tumor injection of hydrogel-encapsulated anti-CTLA-4 (50 µg each, subcutaneous injection) at days 6 and 11. Positive treatment effects were observed in all cases, but the magnitude was different for each route of administration. As demonstrated in Fig. 2b, peri-tumoral administration of hydrogel-encapsulated anti-CTLA-4 induced the most efficient tumor regression among all treatment groups (p < 0.001) with the day 25 tumor volume reduced to 32.9 ± 24.2% (mean ± SE) of control values. The response to targeted administration of anti-CTLA-4 alone, in the absence of a hydrogel, was significantly less. In this setting, the day 25 tumor volume was reduced to 55.9 ± 46.0% of control values (p < 0.001 compared to hydrogel-encapsulated anti-CTLA-4). High-dose systemic therapy was effective and reduced tumor volume to 47.9 ± 37.9% of control values (p < 0.001 compared to control), but this effect was also significantly less than that resulting from low-dose therapy with hydrogel-encapsulated anti-CTLA-4 (p < 0.001). A similar pattern was observed with respect to survival (Fig. 2c) in which there was a significant survival advantage in animals treated with hydrogel-encapsulated anti-CTLA-4 compared to the untreated control group (p < 0.05), a trend toward a survival advantage when animals were treated with systemic administration, but no survival advantage in animals that received a targeted injection of low-dose anti-CTLA-4 prepared in PBS.
Fig. 2.
Antitumor activity of anti-CTLA-4; comparing systemic to peri-tumor administration. a C57BL/6 mice implanted with MC-38 cells (3.5 × 105) by subcutaneous injection into the right upper flank had palpable tumor confirmed on day 6 prior to initiation of therapy. For systemic administration, mice received anti-CTLA-4 (200 µg by i.p. injection) on days 6, 9, and 12. For subcutaneous peri-tumor injection, mice received anti-CTLA-4 (50 μg) formulated in either PBS or incorporated into a hydrogel and administered on days 6 and 11. b Tumor volume (mm3) was determined every 3–4 days over a course of 25 days (11 mice/group, ± SE) with a significant difference between groups identified (p < 0.001; two-way ANOVA with repeated measures). Tumor size at day 25 was significantly less in animals receiving peri-tumor injection of hydrogel-encapsulated anti-CTLA-4 when compared to animals receiving peri-tumor injection in PBS or systemic administration (*p < 0.001). c Survival rates of the four groups were compared over a 40-day period. **p < 0.05 compared to untreated and peri-tumor injection in PBS groups; log rank test
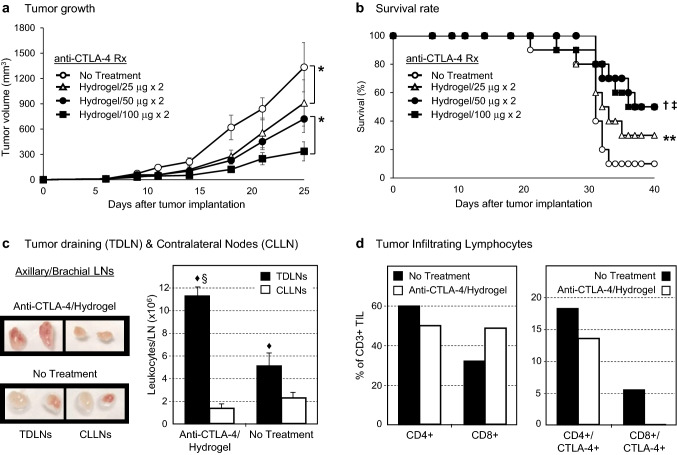
When examined in more detail, the targeted administration of hydrogel-encapsulated anti-CTLA-4 was associated with a dose-dependent antitumor effect at total doses that were only 1/12th (25 µg × 2), 1/6th (50 µg × 2), or 1/3rd (100 µg × 2) of the standard systemic dose (Fig. 3a). Tumor volumes at day 25 were reduced on average to 68.2%, 54.0%, and 25.2% of the untreated control tumor size (p < 0.001 comparing untreated control to 25 µg dose and comparing 50–100 µg dose). Similarly, there was a dose-dependent survival advantage when compared to untreated animals (Fig. 3b; 25 µg, p = 0.07; 50 µg, P = 0.005; 100 µg, p = 0.01). The biologic effects of targeted hydrogel-encapsulated anti-CTLA-4 were further assessed by recovering TDLN, CLLN, and TIL for analysis at 7 days after initiation of treatment with the 50 µg dose (day 13 after tumor implantation). As expected (Fig. 3c), TDLNs demonstrated an innate response to tumor implantation, even in the absence of treatment, with 5.1 ± 1.2 × 106 mononuclear cells recovered from TDLNs as compared to only 2.2 ± 0.6 × 106 cells recovered from CLLNs (p ≤ 0.05). Targeted administration of hydrogel-encapsulated anti-CTLA-4 further expanded the cell numbers within TDLN to 11.3 ± 0.8 × 106 cells (122% increase compared to untreated TDLN; p ≤ 0.01) without any impact on cell counts recovered from CLLN (1.3 ± 0.5 × 106; no difference from untreated CLLN). These regional effects on TDLN were linked to dynamic changes in the characteristics of TIL including a relative increase in the percentage of CD3 + /CD8 + T cells and a reduction in the CD4 + /CTLA-4 + and CD8 + /CTLA-4 + subsets (Fig. 3d). These data suggest that targeted peri-tumor injection of low-dose anti-CTLA-4, when delivered with an injectable hydrogel, preferentially activates TDLN, promotes the accumulation of activated T cells within tumors, and produces potent antitumor effects that translate into survival advantages that were equal to or more effective than conventional systemic administration.
Fig. 3.
Dose-dependent antitumor activity of hydrogel-encapsulated anti-CTLA-4. C57BL/6 mice bearing palpable subcutaneous MC-38 tumors, confirmed by palpation on day 6, received either no treatment (control) or peri-tumor subcutaneous injections of hydrogels formulated with different doses of anti-CTLA-4 (25, 50 or 100 µg) on days 6 and 11. a Tumor volume (mm3) was measured every 3–4 days over a course of 25 days (10 mice/group, ± SE). A significant overall impact of anti-CTLA-4 dose on growth of tumor over times was observed (p < 0.001, two-way ANOVA with repeated measures), and significant differences between groups were identified (*p < 0.001; two-way ANOVA with repeated measures). b Survival rates of the four groups were compared over a 40-day period. A significant overall impact of anti-CTLA-4 dose on tumor growth over time was observed (p < 0.001, two-way ANOVA with repeated measures), and significant differences between treatment groups were identified (†p = 0.005 50 µg vs. control; ‡p = 0.01 100 µg vs. control; **p = 0.07 25 µg vs. control; log rank test). c Axillary, brachial, and inguinal tumor-draining lymph nodes (TDLNs) and contralateral lymph nodes (CLLN) were recovered on day 13 from animals that received hydrogel-encapsulated anti-CTLA-4 (50 µg/dose) or untreated controls (3 animals/group). The nodal size (representative images shown, 1.75 × magnification) and cell counts were always greater in TDLNs as compared to contralateral nodes regardless of treatment (♦p ≤ 0.05; t test). In addition, leukocyte counts from the TDLNs were dramatically higher in treated animals as compared to untreated controls (§p < 0.01). In contrast, there was no significant difference in cell counts in CLLNs regardless of treatment group. d Tumors were also recovered on day 13 (3 animals/group), pooled, and used to prepare single-cell preparations for analysis by flow cytometry. Treatment with hydrogel-encapsulated anti-CTLA-4 resulted in a shift in TILs toward a CD8 + phenotype and a reduction in CTLA-4 expressing CD4 + and CD8 + T cells (mean % positive in pooled cells)
Impact of targeted administration of anti-CTLA-4 on the induction of autoantibodies
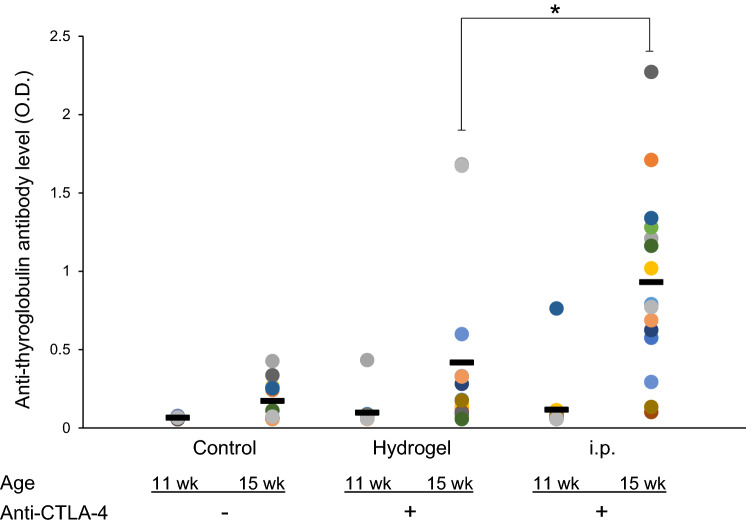
We hypothesized that the targeted and controlled delivery of anti-CTLA-4 would produce less systemic exposure and therefore reduce the magnitude of autoimmune toxicity. The induction and severity of autoimmune thyroiditis were assessed using the NOD.H-2h4 mouse model in which spontaneous thyroiditis is accelerated when the drinking water is supplemented with sodium iodide. In this model, the severity of thyroiditis is exacerbated with administration of anti-CTLA-4, an effect that correlates with the production of autoantibody directed against thyroglobulin [18, 19]. As shown in Fig. 4, and consistent with spontaneous development of autoimmune thyroiditis in this model, there is a modest increase between weeks 11 and 15 in the level of thyroglobulin autoantibody in control animals. However, following systemic i.p. administration of anti-CTLA-4, the level of thyroglobulin autoantibody dramatically increases over the same time interval and is significantly higher than the control group (week 15 vs week 11, OD = 0.93 ± 0.15 vs. 0.17 ± 0.12 (means ± SE), respectively, p < 0.001). By comparison, targeted low-dose delivery of anti-CTLA-4 using a hydrogel resulted in week 15 thyroglobulin autoantibody levels that were not significantly different than in control mice (week 15 OD = 0.42 ± 0.14 vs. 0.17 ± 0.12, respectively, p > 0.1). The week 15 thyroglobulin autoantibody levels were significantly higher following i.p. administration compared to administration by hydrogel (OD = 0.93 ± 0.15 vs. 0.42 ± 0.14, respectively, p = 0.01).
Fig. 4.
The route of anti-CTLA-4 administration impacts autoantibody (to thyroglobulin) levels in an autoimmune thyroiditis model. NOD.H-2h4 mice received drinking water supplemented with sodium iodide starting at 8 weeks of age. From 11 weeks of age, mice were treated with either subcutaneous injections of hydrogel-encapsulated anti-CTLA-4 (3 doses of 50 µg; 150 µg total) or systemic (i.p.) administration of anti-CTLA-4 (5 doses of 200 µg; 1000 µg total). Serum was collected from mice at age of 11 (pre-treatment) and 15 weeks for determination of anti-thyroglobulin antibody by ELISA. N = 15 in control and i.p. groups, 14 in hydrogel; group. Data for individual mice are shown in different colors. Bars indicate mean levels of thyroglobulin autoantibody. *p = 0.01; t test
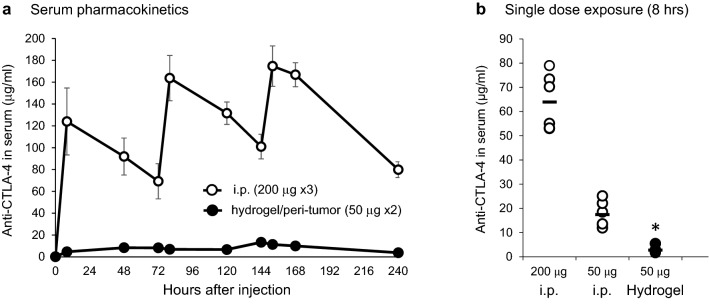
Serum levels of anti-CTLA-4 following systemic versus targeted administration
Consistent with reduced autoimmune toxicity, we hypothesized that targeted administration of low-dose anti-CTLA-4 would avoid high serum peak and overall drug exposure that occurs following systemic administration. Following the administration schedule in Fig. 2a, serum levels of anti-CTLA-4 were measured at various time points by ELISA. As shown in Fig. 5a, the serum levels of anti-CTLA-4 following i.p. injection (200 µg × 3) were significantly higher at each time point than hydrogel injection (50 µg × 2). The highest serum levels at 152 h were 174.7 ± 18.5 µg/ml following i.p. injection and only 11.39 ± 1.11 µg/ml (means ± SE) following administration by hydrogel (ratio of i.p. vs. hydrogel concentration = 15:1, p < 0.01). The overall difference in serum exposure, as measured by the ratio of the two areas under the curve (AUC) calculations, was 16:1. To address whether the observed differences in serum exposure were due solely to the amount of mAb administered, animals were given either 200 µg or 50 µg of anti-CTLA-4 by systemic i.p. injection or 50 µg by targeted peri-tumor hydrogel injection and serum concentration measured 8 h later (Fig. 5b). Consistent with the fourfold difference in dose, serum levels in animals receiving a 50 µg i.p. dose averaged only 27.2% (17.4 ± 2.0 µg/ml) of that measured in animals receiving 200 µg i.p. (64.0 ± 17.4 µg/ml). However, when encapsulated in hydrogel and administered as a peri-tumor injection of 50 µg, the serum level was only 4.4% of that measured following the 200 µg systemic dose (2.8 ± 0.5 µg/ml). Controlled release using hydrogel-encapsulated anti-CTLA-4 results in much lower systemic exposure than predicted by dose difference alone.
Fig. 5.
Serum exposure to anti-CTLA-4 Ab; comparing systemic to peri-tumor administration. a C57BL/6 mice were treated with either peri-tumor injection of hydrogel/anti-CTLA-4 (50 µg; day 0 and 5) or i.p. injection of anti-CTLA-4 (200 µg; day 0, 3 and 9). Serum was collected at various time points (8, 48, 72, 80, 120, 144, 152, 168, 240 h after the first treatment) and anti-CTLA-4 concentration measured by ELISA. Data displayed as means ± SE, N = 6 mice/group. b To assess if the difference in peak serum levels was solely related to the reduced injection dose, C57BL/6 mice received either a single systemic i.p. injection of anti-CTLA-4 at a dose of 50 µg or 200 µg, or a peri-tumor injection of hydrogel/anti-CTLA-4 containing 50 µg. Serum was collected at 8 h after drug administration and anti-CTLA-4 concentration measured by ELISA. Data displayed as means ± SE, N = 6 mice/group (*p < 0.001 comparing hydrogel/anti-CTLA-4 to either systemic dose)
Targeted anti-CTLA-4 therapy enhances antitumor efficacy mediated by systemic anti-PD-1
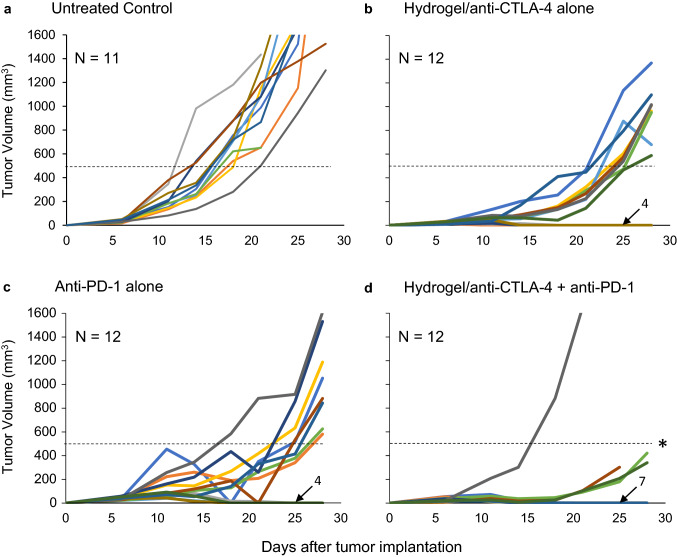
Having demonstrated that targeted administration of low-dose anti-CTLA-4 induces an effective antitumor response while limiting autoimmune toxicity, we asked whether it would still enhance antitumor efficacy when administered in combination with systemic anti-PD-1 (Fig. 6). Following a similar treatment regimen to that shown in Fig. 2a, tumor-bearing mice were treated with either peri-tumor injection of hydrogel formulated with anti-CTLA-4 alone, i.p. injection of anti-PD-1 alone following the same injection schedule as detailed for i.p. administration of anti-CTLA-4, or the combination of hydrogel formulated with anti-CTLA-4 and i.p. injection of anti-PD-1. Tumor growth in control animals that received no therapy is also shown. Monotherapy with either anti-CTLA-4 alone or anti-PD-1 alone exhibited similar effects on tumor growth, with lasting tumor regression in 33% of treated mice and delayed development of larger tumors (> 500 mm3) in remaining mice. On the other hand, when treated with combination therapy, the majority of mice (58%) experienced lasting tumor regression and only one mouse developed tumor greater than 500 mm3. As such, combination therapy significantly reduced the development of large tumors (p < 0.005; combination vs. either monotherapy). All mice that experienced lasting tumor regression (4 per monotherapy group, 7 in combination therapy group) were re-challenged with a 50% higher dose of tumor inoculation on the opposite side of the original tumor at day 40. No palpable tumors developed in these animals. These results suggest that targeted peri-tumor administration of anti-CTLA-4, using a controlled hydrogel delivery system, synergizes with systemic anti-PD-1 therapy and results in a high level of systemic protection as documented by the rejection of tumor challenge at a distant site.
Fig. 6.
Tumor regression by monotherapy vs combination therapy. C57BL/6 mice were implanted with MC-38 cells (3.5 × 105) by subcutaneous injection and treatment initiated on day 6 after confirmation of palpable tumor. Tumor size was determined in three dimensions at intervals to calculate tumor volumes and construct tumor growth curves. a Untreated control. Tumor growth in control animals that received tumor without administration of any checkpoint inhibitor antibody. b Monotherapy with hydrogel-encapsulated anti-CTLA-4. Mice were treated with peri-tumor injections of hydrogel-encapsulated anti-CTLA-4 (50 µg) on days 6 and 11. c Monotherapy with anti-PD-1. Mice were treated with i.p. injection of anti-PD-1 (100 µg) on days 6, 9, and 12. d Combination therapy. Mice were treated with peri-tumor injection of hydrogel formulated with anti-CTLA-4 (50 µg) at days 6 and 11 and i.p. injection of anti-PD-1 (100 µg) on days 6, 9, and 12. Each line represents tumor growth in a single mouse. The number of animals with no palpable tumor at day 28 is indicated with an arrow. *p < 0.005; combination therapy versus either monotherapy
Discussion
Tumor immunotherapy has evolved over time from a focus on antigen-specific vaccines to a broader attack on checkpoint inhibitor pathways that shield both normal tissue and tumors from host immunity [22, 23]. Monoclonal antibodies targeting CTLA-4, PD-1 and PD-L1 represent a cadre of reagents with broad clinical activity against melanoma, lung cancer, renal cancer, and other tumor types [3, 4]. While capable of mediating antitumor responses as single agents, there is significant interest in combination therapy to boost both the frequency and magnitude of responses [1–4]. Simultaneously targeting CTLA-4 and PD-1 produces a synergistic biologic effect that activates novel antitumor mechanisms [10, 11] and yields a significantly greater clinical response and progress-free survival rate compared to monotherapy [5–9]. In the CheckMate 067 trial, progression-free survival in previously untreated melanoma patients increased from 2.9 months with ipilimumab alone to 11.5 months in those treated with ipilumumab plus nivolumab [5]. Similarly, the combined use of nivolumab and ipilimumab proved superior to chemotherapy for previously untreated non-small cell lung cancer, while nivolumab alone did not [5, 24]. Unfortunately, the potential for inflammatory and immune related adverse events is also magnified by combination therapy [12, 14]. In the CheckMate 067 trial, the occurrence of Grade 3 or 4 treatment-related adverse events increased from 27.3% in patients treated with anti-CTLA-4 alone to 55.0% in those receiving combined anti-CTLA-4 plus anti-PD-1 [5]. The work presented here directly addresses this problematic aspect of systemic checkpoint inhibitor therapy and the risk for unleashing irAEs. In this study, hydrogel-encapsulated anti-CTLA-4 was administered as a peri-tumor injection to target TDLN and perfuse them with a sustained release of therapeutic antibody. This approach takes advantage of the unique enrichment of both tumor-responsive and regulatory T cells that occurs within TDLNs [25–28] and was shown to activate TDLNs and alter the phenotype of TIL in a manner consistent with the known effects of anti-CTLA-4 therapy [25, 29, 30]. This targeted administration was associated with a dramatic reduction in serum anti-CTLA-4 levels, as compared to systemic administration, while producing equal or superior tumor control in a dose-dependent manner. More importantly, targeted administration of anti-CTLA-4 limited the stimulation of autoimmune thyroiditis while still synergizing with systemic anti-PD-1 to control tumor growth.
The administration of immune modulators via a peri-tumor injection, including anti-CTLA-4, is a well-established approach for stimulating protective antitumor immunity. In preclinical studies, efficacy relies upon the heightened frequency of tumor-specific T cells that reside within TDLN and on the capacity for treatment to induce the systemic circulation of activated T cells that control tumor growth, including metastatic disease, and results in protective immune memory [25–30]. In this report, a self-polymerizing HA-based hydrogel was employed as a mechanism to provide sustained and targeted delivery of anti-CTLA-4. This formulation rapidly polymerizes at the site of injection and produces a mechanism for sustained perfusion of TDLN. When compared to administration of anti-CTLA-4 alone (in PBS), encapsulating the same dose of anti-CTLA-4 within the hydrogel produced a superior tumor response; suggesting that the mode and/or rate of delivery are important determinants of the response. Hyaluronic acid is a natural component of the extracellular matrix with good biocompatibility and limited risk for immunogenicity [20, 31]. The HA and PEGDA reagents used in this study were admixed at a ratio optimized for localized administration and to control antibody release, but are otherwise the same as those recently approved for human use (Renevia®) [32]. Hydrogel-encapsulated anti-CTLA-4 administered in this manner produced a dose-dependent response capable of achieving equal or better tumor control and survival than systemic dosing while requiring only a fraction of the dose. This dose dependency recapitulates the known response to systemic anti-CTLA-4 [32, 33].
As toxicity is also dose-related, a primary goal of targeted therapy is to employ dose reduction as a mechanism to reduce systemic toxicity [25–27]. The irAEs associated with anti-CTLA-4 represent off-target effects from immune activation including both inflammatory and organ-specific autoimmune toxicity [14]. Other mouse models employing low-dose targeted anti-CTLA-4 have demonstrated a reduction in the release of liver enzymes and serum IL-6 as compared to the effects of systemic therapy [25, 34]. In this study, subcutaneous administration of low-dose anti-CTLA-4 dramatically reduced both peak and sustained serum exposure to a level lower than otherwise expected based on dose and antibody bioavailability [35]. This may reflect the impact from using a controlled release hydrogel or a significant first-pass effect due to binding within TDLN. On average, serum exposure following the administration of an effective dose of hydrogel-encapsulated anti-CTLA-4 was only 1/16th of that documented following standard systemic therapy. Organ-specific autoimmunity is stimulated by endogenous antigen presentation, which occurs primarily in organ-draining lymph nodes, but is typically kept in check by the local induction of antigen-specific regulatory T cells [36]. In this setting, we hypothesized that low-dose targeted therapy would reduce the frequency/severity of autoimmune toxicity occurring at a distant site. To test this hypothesis, we employed NOD.H-2h4 mice that spontaneously develop autoimmune thyroiditis and thyroglobulin autoantibodies, a process accelerated by dietary iodide supplementation [17]. Autoimmune thyroiditis in NOD.H-2h4 mice is regulated by checkpoint inhibitors and regulatory T cells [18]. According to Sharma et al. [19], the incidence of disease, severity of the mononuclear cell infiltration of the thyroid, and the thyroglobulin autoantibody levels in NOD.H-2h4 mice are all significantly increased when animals are exposed to systemic anti-CTLA-4. As such, this model provided an opportunity to assess the impact of checkpoint inhibitor blockade on features of autoimmune toxicity that are similar to those occurring in patients [37]. Consistent with our hypothesis, systemic anti-CTLA-4 stimulated a significant increase in serum thyroglobulin autoantibody levels in this model, while peri-tumor administration resulted in thyroglobulin autoantibody levels similar to those in controls not treated with anti-CTLA-4. These findings, when combined with the impact on tumor growth and survival, imply a positive impact on the clinical benefit/risk ratio.
Given the unique synergism that occurs when systemic anti-CTLA-4 and anti-PD-1 are administered together, and the low serum exposure that occurs following targeted delivery of anti-CTLA-4, it was important to ask whether targeted administration would synergize with systemic anti-PD-1. Indeed, while monotherapy with either anti-CTLA-4 or anti-PD-1 alone significantly slowed tumor growth, combining targeted peri-tumor anti-CTLA with systemic anti-PD-1 resulted in the majority of animals experiencing lasting tumor regression. In addition, animals that experienced complete tumor regression were protected from tumor re-challenge at a contralateral site. This synergism between a targeted peri-tumor injection and systemic therapy could pave the way for a number of different approaches to combination therapy.
In conclusion, our findings add to a growing literature and document the capacity for targeted peri-tumor administration of immune modulators to elicit systemic antitumor immunity and control tumor growth [25–28, 34]. By targeting anti-CTLA-4 toward TDLN, this approach activated tumor-specific T cell responses at a fraction of the required systemic dose. The use of a hydrogel-encapsulated anti-CTLA-4, specifically designed to act as a reservoir for controlled antibody release over several days, further enhanced potency and was associated with a lower-than-expected systemic exposure. The fact that targeted administration mimicked systemic administration with respect to efficacy and dose-responsiveness is significant, as is the capacity for targeted administration to synergize with systemic anti-PD-1. These features imply that the activation of T cell responses from TDLN is sufficient (as compared to systemic exposure) and likely represents the dominant response pathway for anti-CTLA-4. In this setting, one would hypothesize that systemic exposure to anti-CTLA-4 is not required and more associated with toxicity than efficacy. The testing of systemic versus targeted administration of anti-CTLA-4 in the autoimmune thyroiditis model further supported this—autoimmune toxicity at a distant organ only occurred with systemic therapy. The logical conclusion of this work is that targeted administration of anti-CTLA-4, especially in the setting of a controlled release formulation, has considerable promise for enabling combination immunotherapies while limiting toxicity, resulting in a significant impact in the overall benefit-to-risk ratio.
Acknowledgements
The authors thank Michael Onorato, Ph.D., and BioTime Inc., for providing hydrogel reagents for use in the study. The UCLA CFAR grant 5P30 AI028697 and the UCLA AIDS Institute for animal service at the UCLA CFAR Humanized Mouse Core provided key consultation and resources for the generation and maintenance of the NOD.H-2h4 colony.
Abbreviations
- AUC
Area under the curve
- CLLN
Contralateral lymph nodes
- CMHA-S
Thiolated carboxymethyl HA
- HA
Hyaluronic acid
- HAse
Hyaluronidase
- i.p.
Intraperitoneal
- irAEs
Immune-related adverse events
- mAb
Monoclonal antibody
- PEGDA
Poly-(ethylene glycol)-diacrylate
- TDLN
Tumor-draining lymph nodes
- TIL
Tumor-infiltrating lymphocytes
Authors' Contributions
AH collected and performed the primary analysis of all data. AH, SMM, BR, and MDR participated in study design, interpretation, writing, and review of the manuscript. TIZ participated in discussions of study findings and manuscript review.
Funding
Support provided, in part, through an investigator-initiated research contract and provision of reagents from BioTime Inc. (now called Lineage Cell Therapeutics, Alameda, CA). Study design, collection and analysis of data, interpretation and writing of the manuscript were independent from the financial sponsor.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Compliance with ethical standards
Conflict of interest
Drs. Roth and Harui report grants and non-financial support from BioTime Inc. during the conduct of the study and institutional submission of a patent application. Dr. Zarembinski was previously a Senior Director, Alliance Management and External R&D Collaborations, BioTime Inc., Alameda, CA. Professors McLachlan and Rapoport declare no conflict of interest.
Ethics approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ledford H. Cocktails for cancer with a measure of immunotherapy. Nature. 2016;532(7598):162–164. doi: 10.1038/532162a. [DOI] [PubMed] [Google Scholar]
- 2.Rotte A. Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J Exp Clin Cancer Res. 2019;38(1):255. doi: 10.1186/s13046-019-1259-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marshall HT, Djamgoz MBA. Immuno-Oncology: Emerging Targets and Combination Therapies. Front Oncol. 2018;8:315. doi: 10.3389/fonc.2018.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seliger B. Combinatorial approaches with checkpoint inhibitors to enhance anti-tumor immunity. Front Immunol. 2019;10:999. doi: 10.3389/fimmu.2019.00999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, Ferrucci PF, Hill A, Wagstaff J, Carlino MS, Haanen JB, Maio M, Marquez-Rodas I, McArthur GA, Ascierto PA, Long GV, Callahan MK, Postow MA, Grossmann K, Sznol M, Dreno B, Bastholt L, Yang A, Rollin LM, Horak C, Hodi FS, Wolchok JD. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hodi FS, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Cowey CL, Lao CD, Schadendorf D, Wagstaff J, Dummer R, Ferrucci PF, Smylie M, Hill A, Hogg D, Marquez-Rodas I, Jiang J, Rizzo J, Larkin J, Wolchok JD. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19(11):1480–1492. doi: 10.1016/S1470-2045(18)30700-9. [DOI] [PubMed] [Google Scholar]
- 7.Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim SW, Carcereny Costa E, Park K, Alexandru A, Lupinacci L, de la Mora JE, Sakai H, Albert I, Vergnenegre A, Peters S, Syrigos K, Barlesi F, Reck M, Borghaei H, Brahmer JR, O'Byrne KJ, Geese WJ, Bhagavatheeswaran P, Rabindran SK, Kasinathan RS, Nathan FE, Ramalingam SS. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med. 2019;381(21):2020–2031. doi: 10.1056/NEJMoa1910231. [DOI] [PubMed] [Google Scholar]
- 8.Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK, Plimack ER, Barthélémy P, Porta C, George S, Powles T, Donskov F, Neiman V, Kollmannsberger CK, Salman P, Gurney H, Hawkins R, Ravaud A, Grimm MO, Bracarda S, Barrios CH, Tomita Y, Castellano D, Rini BI, Chen AC, Mekan S, McHenry MB, Wind-Rotolo M, Doan J, Sharma P, Hammers HJ, Escudier B, CheckMate 214 Investigators Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277–1290. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Overman MJ, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M, Morse MA, Van Cutsem E, McDermott R, Hill A, Sawyer MB, Hendlisz A, Neyns B, Svrcek M, Moss RA, Ledeine JM, Cao ZA, Kamble S, Kopetz S, André T. Durable clinical benefit with nivolumab plus ipilimumab in dna mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol. 2018;36(8):773–779. doi: 10.1200/JCO.2017.76.9901. [DOI] [PubMed] [Google Scholar]
- 10.Das R, Verma R, Sznol M, Boddupalli CS, Gettinger SN, Kluger H, Callahan M, Wolchok JD, Halaban R, Dhodapkar MV, Dhodapkar KM. Combination therapy with anti-CTLA-4 and anti-PD-1 leads to distinct immunologic changes in vivo. J Immunol. 2015;194(3):950–959. doi: 10.4049/jimmunol.1401686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei SC, Levine JH, Cogdill AP, Zhao Y, Anang NAS, Andrews MC, Sharma P, Wang J, Wargo JA, Pe'er D, Allison JP. Distinct cellular mechanisms underlie anti-CTLA-4 and anti-PD-1 checkpoint blockade. Cell. 2017;170(6):1120–1133.e17. doi: 10.1016/j.cell.2017.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, Rathmell WK, Ancell KK, Balko JM, Bowman C, Davis EJ, Chism DD, Horn L, Long GV, Carlino MS, Lebrun-Vignes B, Eroglu Z, Hassel JC, Menzies AM, Sosman JA, Sullivan RJ, Moslehi JJ, Johnson DB. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4(12):1721–1728. doi: 10.1001/jamaoncol.2018.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lebbé C, Meyer N, Mortier L, Marquez-Rodas I, Robert C, Rutkowski P, Menzies AM, Eigentler T, Ascierto PA, Smylie M, Schadendorf D, Ajaz M, Svane IM, Gonzalez R, Rollin L, Lord-Bessen J, Saci A, Grigoryeva E, Pigozzo J. Evaluation of two dosing regimens for nivolumab in combination with ipilimumab in patients with advanced melanoma: results from the phase IIIb/IV CheckMate 511 Trial. J Clin Oncol. 2019;37(11):867–875. doi: 10.1200/JCO.18.01998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, Fraga M, Shabafrouz K, Ribi C, Cairoli A, Guex-Crosier Y, Kuntzer T, Michielin O, Peters S, Coukos G, Spertini F, Thompson JA, Obeid M. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16(9):563–580. doi: 10.1038/s41571-019-0218-0. [DOI] [PubMed] [Google Scholar]
- 15.Hebb JPO, Mosley AR, Vences-Catalán F, Rajasekaran N, Rosén A, Ellmark P, Felsher DW. Administration of low-dose combination anti-CTLA4, anti-CD137, and anti-OX40 into murine tumor or proximal to the tumor draining lymph node induces systemic tumor regression. Cancer Immunol Immunother. 2018;67(1):47–60. doi: 10.1007/s00262-017-2059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellmark P, Mangsbo SM, Furebring C, Norlén P, Tötterman TH. Tumor-directed immunotherapy can generate tumor-specific T cell responses through localized co-stimulation. Cancer Immunol Immunother. 2017;66(1):1–7. doi: 10.1007/s00262-016-1909-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braley-Mullen H, Sharp GC, Medling B, Tang H. Spontaneous autoimmune thyroiditis in NOD.H-2h4 mice. J Autoimmun. 1999;12(3):157–165. doi: 10.1006/jaut.1999.0272. [DOI] [PubMed] [Google Scholar]
- 18.Xue H, Wang W, Shan Z, Li Y, Li Y, Teng X, Gao Y, Fan C, Teng W. Dynamic changes of CD4+CD25 + regulatory T cells in NOD.H-2h4 mice with iodine-induced autoimmune thyroiditis. Biol Trace Elem Res. 2011;143(1):292–301. doi: 10.1007/s12011-010-8815-x. [DOI] [PubMed] [Google Scholar]
- 19.Sharma R, Di Dalmazi G, Caturegli P. Exacerbation of autoimmune thyroiditis by CTLA-4 blockade: a role for IFNγ-induced indoleamine 2, 3-dioxygenase. Thyroid. 2016;8:1117–1124. doi: 10.1089/thy.2016.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harui A, Roth MD. Employing a glutathione-s-transferase-tag and hyaluronidase to control cytokine retention and release from a hyaluronic acid hydrogel matrix. J Biomater Appl. 2019;34(5):631–639. doi: 10.1177/0885328219867974. [DOI] [PubMed] [Google Scholar]
- 21.Chen C-R, Hamidi S, Braley-Mullen H, Nagayama Y, Bresee C, Aliesky HA, Rapoport B, McLachlan SM. Antibodies to thyroid peroxidase arise spontaneously with age in NOD.H-2h4 mice and appear after thyroglobulin antibodies. Endocrinology. 2010;151(9):4583–4593. doi: 10.1210/en.2010-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butterfield LH. Cancer vaccines. BMJ. 2015;350:h988. doi: 10.1136/bmj.h988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fritz JM, Lenardo MJ. Development of immune checkpoint therapy for cancer. J Exp Med. 2019;216(6):1244–1254. doi: 10.1084/jem.20182395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, Felip E, van den Heuvel MM, Ciuleanu TE, Badin F, Ready N, Hiltermann TJN, Nair S, Juergens R, Peters S, Minenza E, Wrangle JM, Rodriguez-Abreu D, Borghaei H, Blumenschein GR, Jr, Villaruz LC, Havel L, Krejci J, Corral Jaime J, Chang H, Geese WJ, Bhagavatheeswaran P, Chen AC, Socinski MA, CheckMate 026 Investigators First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med. 2017;376(25):2415–2426. doi: 10.1056/NEJMoa1613493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fransen MF, van der Sluis TC, Ossendorp F, Arens R, Melief CJ. Controlled local delivery of CTLA-4 blocking antibody induces CD8+ T-cell-dependent tumor eradication and decreases risk of toxic side effects. Clin Cancer Res. 2013;19(19):5381–5389. doi: 10.1158/1078-0432.CCR-12-0781. [DOI] [PubMed] [Google Scholar]
- 26.Fransen MF, Sluijter M, Morreau H, Arens R, Melief CJ. Local activation of CD8 T cells and systemic tumor eradication without toxicity via slow release and local delivery of agonistic CD40 antibody. Clin Cancer Res. 2011;17(8):2270–2280. doi: 10.1158/1078-0432.CCR-10-2888. [DOI] [PubMed] [Google Scholar]
- 27.Narumi K, Miyakawa R, Shibasaki C, Henmi M, Mizoguchi Y, Ueda R, Hashimoto H, Hiraoka N, Yoshida T, Aoki K. Local administration of GITR agonistic antibody induces a stronger antitumor immunity than systemic delivery. Sci Rep. 2019;9(1):5562. doi: 10.1038/s41598-019-41724-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marabelle A, Kohrt H, Sagiv-Barfi I, Ajami B, Axtell RC, Zhou G, Rajapaksa R, Green MR, Torchia J, Brody J, Luong R, Rosenblum MD, Steinman L, Levitsky HI, Tse V, Levy R. Depleting tumor-specific Tregs at a single site eradicates disseminated tumors. J Clin Invest. 2013;123(6):2447–2463. doi: 10.1172/JCI64859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Selby MJ, Engelhardt JJ, Johnston RJ, Lu LS, Han M, Thudium K, Yao D, Quigley M, Valle J, Wang C, Chen B, Cardarelli PM, Blanset D, Korman AJ. Preclinical development of Ipilimumab and Nivolumab combination immunotherapy: mouse tumor models, in vitro functional studies, and cynomolgus macaque toxicology. PLoS ONE. 2016;11(9):e0161779. doi: 10.1371/journal.pone.0161779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ribas A, Comin-Anduix B, Economou JS, Donahue TR, de la Rocha P, Morris LF, Jalil J, Dissette VB, Shintaku IP, Glaspy JA, Gomez-Navarro J, Cochran AJ. Intratumoral immune cell infiltrates, FoxP3, and indoleamine 2,3-dioxygenase in patients with melanoma undergoing CTLA4 blockade. Clin Cancer Res. 2009;15(1):390–399. doi: 10.1158/1078-0432.CCR-08-0783. [DOI] [PubMed] [Google Scholar]
- 31.Trombino S, Servidio C, Curcio F, Cassano R. Strategies for hyaluronic acid-based hydrogel design in drug delivery. Pharmaceutics. 2019;11(8):E407. doi: 10.3390/pharmaceutics11080407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blackshear CP, Flacco JS, Vistnes SM, Chung NN, Irizarry D, Brett EA, Yen DJ, Momeni A, Longaker MT, Wan DC. Cell-based soft tissue reconstruction in a hydrogel scaffold. Ann Plast Surg. 2017;79(6):618–622. doi: 10.1097/SAP.0000000000001194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, Waterfield W, Schadendorf D, Smylie M, Guthrie T, Jr, Grob JJ, Chesney J, Chin K, Chen K, Hoos A, O'Day SJ, Lebbé C. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11(2):155–164. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 34.van Hooren L, Sandin LC, Moskalev I, Ellmark P, Dimberg A, Black P, Tötterman TH, Mangsbo SM. Local checkpoint inhibition of CTLA-4 as a monotherapy or in combination with anti-PD1 prevents the growth of murine bladder cancer. Eur J Immunol. 2017;47(2):385–393. doi: 10.1002/eji.201646583. [DOI] [PubMed] [Google Scholar]
- 35.Viola M, Sequeira J, Seiça R, Veiga F, Serra J, Santos AC, Ribeiro AJ. Subcutaneous delivery of monoclonal antibodies: how do we get there? J Control Release. 2018;286:301–314. doi: 10.1016/j.jconrel.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 36.Wheeler KM, Samy ET, Tung KS. Cutting edge: normal regional lymph node enrichment of antigen-specific regulatory T cells with autoimmune disease-suppressive capacity. J Immunol. 2009;183(12):7635–7638. doi: 10.4049/jimmunol.0804251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ryder M, Callahan M, Postow MA, Wolchok J, Fagin JA. Endocrine-related adverse events following ipilimumab in patients with advanced melanoma: a comprehensive retrospective review from a single institution. Endocr Relat Cancer. 2014;21(2):371–381. doi: 10.1530/ERC-13-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.