Abstract
Background
Bladder cancer is diagnosed by the use of several biomarkers, including survivin. This protein has an important role in the cancer progression by controlling the rate of cell apoptosis. Findings show that there is no survivin in normal tissues, whereas the level of survivin expression increases in tumor cells.
Design
The purpose of this study was to specify the reactive antibodies to survivin protein as a biomarker to determine the bladder cancer stage with ELISA method and using GNPs conjugated with survivin antibody. The serum and urine samples of patients with bladder cancer were collected among those referred to Sina Hospital, Tehran, Iran. The survivin protein level was measured in the serum and urine by ELISA technique and in the urine by GNPs conjugated with survivin.
Results
Based on the results of ELISA, the serum and urinary levels of survivin increased significantly in T3 and T4 stages of the disease (high grades), compared with the healthy individuals. Also, using conjugated GNPs, survivin protein was detected in the urine specimens of patients at all grades (low and high grades).
Conclusion
Our findings showed that using the ELISA technique, the increased level of survivin could be identified in high grades of bladder cancer, but using anti-survivin antibody-conjugated GNPs, bladder cancer can be detected in early stages. The applied method was found to be a rapid tool, dependent on visible color changes and colorimetric detection, without any need for reader devices.
Keywords: Bladder cancer, Survivin, Gold nanoparticles, Colorimetric detection, LSPR
Introduction
Bladder cancer can be considered as the most common malignancy in the genitourinary tract [1]. In humans, it is the fifth most common cancer, and among men, it is the fourth most common cancer. According to the previous reports from the USA, more than 70,000 people were affected by bladder cancer in 2016, and more than 16,000 people died due to this disease [2, 3].
Cystoscopy has been used as a gold standard for bladder cancer diagnosis for more than 80 years; nevertheless, this procedure is invasive, unpopular and expensive. Even after cystoscopy, some patients (5.5%) may develop urinary tract infections. Moreover, urine cytology is used for the diagnosis of bladder cancer with acceptable level of specificity and sensitivity. But diagnostic sensitivity of this method for low-grade cancer is not acceptable (30%). Also, it should be noted that the accuracy of this test is dependent on the pathologist’s skills [4–7].
Biomarkers such as survivin, genetic loci such as 9p21 locus and natural autoantibodies can be useful in early diagnosis, treatment and reduction in mortality in cancer patients [8, 9]. Survivin is mainly expressed in bladder cancer. Survivin, with a molecular weight of 16.5 kDa and 142 amino acids, is described as a member of the inhibitor of apoptosis protein (IAP) family. This family of proteins contributes to the inhibition of caspase-3, 7 and 9. Findings indicate that survivin expression is usually in embryonic and non-differentiated tissues, while its overexpression is in many solid cancers such as bladder cancer. However, it is rarely present in normal and differentiated tissues. This protein is a multi-functional protein that leads to suppressing apoptosis in response to apoptotic stimuli, the regulation of cell division and also promotion of angiogenesis. The level of expression of this protein is related to higher rates of bladder cancer, and some of the treatments for this cancer have led to a decrease in survivin levels in the urine [5, 10–12].
Recently, the use of nanoscale materials, especially gold nanoparticles (GNPs), has progressed vastly in various biomedical fields, because of their unique and special physicochemical, optical and electronic properties [13–15]. The gold nanoparticle solution displays different colors according to the particles’ size and shape. This color spectrum is related to a situation called surface plasmon resonance (SPR). It is known that these free electrons are responsible for the optical and conductive properties of these particles. In the case of metal nanoparticles, the motion of these electrons is localized and leads to strong absorption, which is called localized surface plasmon resonance (LSPR). LSPR is responsible for creating different colors of the nanoparticle solution. Spherical gold nanoparticles show different colors, including red, purple and blue, depending on the size of the core and the aggregation of particles (network formation). It has been determined that the solution of nanoparticles with a size of about 20 nm is red. The aggregation of these particles leads to redshifting of frequency and change in color from red to blue, which can be useful in identifying various analytics with high sensitivity, based on colorimetric detection [13, 16, 17].
Therefore, the purpose of this study was synthesizing anti-survivin-coated gold nanoparticles to be used as a new technique for the diagnosis of bladder cancer and comparing this technique with ELISA method used for detection of this cancer. This new method is used according to the aggregation of GNPs and might be used as a faster, high accuracy and inexpensive diagnostic tool which does not require complicated instrument and can be examined by the naked eye and used as a rapid test in bladder cancer.
Patients, materials and methods
Reagents
Human survivin ELISA kit was purchased from Abcam Company (ab183361 lot: GR278827-1 UK). Chloroauric acid (HAuCl4) and polyethylene glycol 20,000 were prepared from (Sigma-Aldrich, Philadelphia, USA). K2CO3 and sodium citrate were from (Merck, Darmstadt, Germany). N-(3-Dimethylaminopropyl)-N-ethylcarbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS) were prepared (Thermo Scientific Company, Iran, Tehran). Anti-survivin antibody was supplied by Abcam, EP2880Y-ab76424, UK.
Study design and data collection
Serum and urine samples were obtained from 30 healthy individuals (8 females and 22 males) and 41 patients with bladder cancer (12 females and 29 males) who signed the consent form and attended the Sina Hospital, Tehran, Iran. The clinical features of the patients were examined by staging systems, TNM classification, by the assessment of the primary tumor (T) extent, by considering regional lymph node metastasis (N) presence, absence or extent and also by distance metastasis (M) presence, absence or extent. The growth of the tumor in the bladder wall and its influence on adjacent tissue were determined by sorting Tx, T0, Ta, T1, T2, T3 and T4 [18].
Measurement of survivin in samples by ELISA test
Serum and urine samples were stored at − 80 °C for short-term storage until analysis. (Urine samples were collected before cystoscopy.) Urine samples were centrifuged (at 200 g for 10 min) to remove debris. EILSA was performed for detecting the survivin protein (ab183361, lot: GR278827-1, Abcam, UK); protocol was used according to the manufacturer’s instructions for the determination of the quantity of survivin protein. Briefly, 50 µl survivin standards and serum and urine samples were added to the wells. Then, 50 µl antibody cocktail was added to the wells. After incubating at room temperature for 1 h and washing three times, 100 µl TMB was added to each well. For 10 min, the plate was incubated on a plate shaker at room temperature. Then, substrate solution was added to the wells. After 1-min shake, the absorption of each well was determined at 450 nm by an ELISA plate reader (Bio-Rad, Hercules, California, USA). The levels of survivin protein were reported pg/ml. All experiments were carried out in triplicate.
Preparation of gold nanoparticles and conjugated with anti-survivin antibody
Gold nanoparticles (25 nm) were synthesized with citrate reduction reaction of HAuCl4 using the Frens method [19]. This method in our previous work was expressed in detail [20]. In short, 1% HAuCl4 solution (25 ml) was adjusted on a hot plate with a magnetic stirrer, and when the solution got boiled, 1% trisodium citrate (1 ml) was added to the solution quickly. Covalent binding of nanoparticles to antibodies could be mediated linkers such as EDC/NHS [20]. Then, a combination of the 0.5 mM EG6 –COOH(HS(CH2)11(OCH2CH2)6OCH2CO2H) and the 0.5 mM EG3 –OH(HS(CH2)11(OCH2CH2)3OH) was added to suspension of HAuCl4 with a ratio of 1:10. The combined solution containing carboxylated GNPs was centrifuged at room temperature after 25 h in order to apart the unbound and carboxylated GNPs and carboxylate-based self-assembled monolayers on GNPs. For the removal of the unbound GNPs, the pellet was suspended again in 0.1 M phosphate-buffered saline (pH 7.4). Next, 100 ml of 0.1 M EDC was mixed with 10 ml of carboxylated GNPs, followed by adding 100 μl of 0.1 M NHS in order to activate the GNP solution as a coupling agent. After that, 100 g ml−1 anti-survivin polyclonal Ab (1 ml) was added to the EDC/NHS-activated GNP solution. To remove the unbound anti-survivin pAb, the solution was centrifuged for 50 min at 14 000 RPM after 24 h. In the end stage, the obtained anti-survivin pAb-conjugated GNPs (GNPs–survivin pAb) were suspended again in 0.1 M phosphate-buffered saline (5 ml) (pH 7.4).
Using anti-survivin antibody-conjugated GNPs for detection of survivin
Survivin levels in patients with bladder cancer and in healthy control subjects of the study were examined with anti-survivin antibody-conjugated GNPs. For data collection from the patients and healthy urine sample, 10-mL medistream urine was collected and centrifuged at 3000 RPM for 5 min. A supernatant liquid was obtained and divided into aliquots, and then, the samples were frozen at − 20 °C until final diagnosis. The test was carried out with adding 20 µL of urine sample to 80 µL GNPs-antibody-conjugated solution. The results were read after 15 min. Each urine sample was analyzed at least twice on two different occasions.
Statistical analysis
The experiments were conducted in triplicate and individually repeated at least twice. All experiments were represented in the form of mean values ± SEM (standard error of mean). Data analysis was conducted by GraphPad Prism software (version 6.01). Obtained data were assessed using Mann–Whitney and Kruskal–Wallis test. P values were statistically significant (< 0.05).
Results
Serum and urine levels of survivin protein
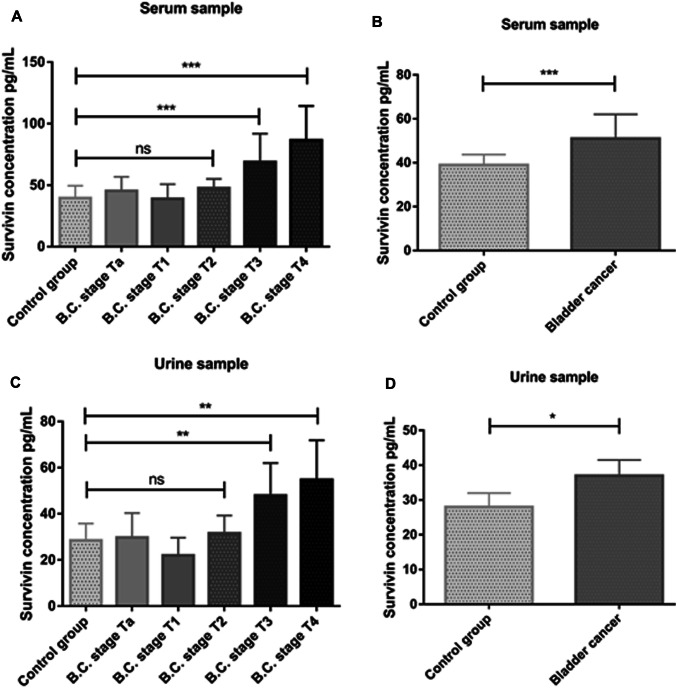
In this study, serum and urine levels of survivin with enzyme-linked immunosorbent assay method were determined after collection of serum and urine samples from bladder cancer patients and healthy individuals. According to staging classification of bladder cancer, patients were classified into Ta–T1–T2–T3 and T4 groups (Ta, n = 8; T1, n = 8; T2, n = 11; T3, n = 8 and T4, n = 6). Statistical analysis of serum level showed a significant increase in serum concentrations of T3 and T4 compared with healthy control group (P = 0.0002), but Ta, T1 and T3 groups did not show any significant increase (Fig. 1a). In general, according to these data, the comparison of the group of bladder cancer patients with healthy control group shows a significant difference in survivin concentration and indicated that the level of this protein in patients group was higher than in the healthy group. Also, analysis of urine samples showed that the levels of this protein in T3 and T4 groups were significantly higher than in the healthy group, and in general, the survivin urine concentration in this disease was increased in comparison with healthy individuals (Fig. 1c, d). Comparison of survivin concentration in serum samples with urine samples shows that increased serum level of this protein (P = 0.0002) was higher than urine (P = 0.019) (Fig. 1b, d). Protein level in bladder cancer patients was higher than that of healthy subjects. Also, according to data of ELISA test, serum and urine levels in patients with low-grade bladder cancer were significantly lower than that in patients with high-grade bladder cancer. The statistical descriptions of serum and urine levels of survivin in bladder cancer and healthy control groups are shown in Table 1.
Fig. 1.
Serum and urine survivin concentrations. a Significant difference was seen in serum survivin concentrations of T3 and T4 groups in comparison with healthy control, b serum survivin concentration in bladder cancer group was significantly increased compared to healthy control, c significant difference was seen in urine survivin concentrations of T3 and T4 groups in comparison with healthy control, d urine survivin concentration in bladder cancer group was significantly increased compared to healthy control. *P < 0.05; **P < 0.01; ***P < 0.001
Table 1.
The statistical descriptions of serum and urine levels of survivin in groups
| ELISA test | Healthy control | Patient | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | 95% CI lower–upper | Median | Mean | SD | 95% CI lower–upper | Median | |
| Urine survivin | 28.50 | 7.357 | 25.75–31.25 | 28.00 | 35.93 | 15.80 | 30.94–40.91 | 37.00 |
| Serum survivin | 39.53 | 9.96 | 35.81–43.26 | 39.00 | 55.32 | 22.92 | 48.08–62.55 | 51.0030 |
SD Std. deviation
Preparation of the GNPs and conjugation with anti-survivin antibody
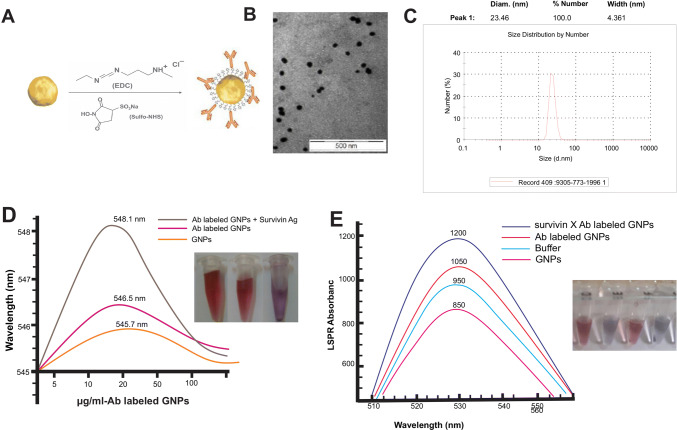
The GNPs were synthesized and conjugated with anti-survivin antibody and then characterized by ultraviolet absorption spectroscopy and TEM (Fig. 2b–e). To determine the distribution of the particles, the GNPs were measured by DLS. Based on DLS results, the average hydrodynamic diameter over 90% of GNPs was ~ 25 nm. After conjugation, to increase the stability of the nanoparticles, GNPs were pegylated with PEG 20,000. Zetasizer ZS was used to determine the stability of the GNPs. The approximate zeta potential of the bare GNPs was about 44 3 mV. GNPs zeta potential was reduced (27 ± 2 mV) after conjugation and pegylation. In the determination of the value of soluble compounds by X-ray diffraction (XRD), there was 71% GNPs and 60% sodium chloride concentration.
Fig. 2.
Synthesis and characterization of the unconjugated GNPs and anti-survivin Ab-conjugated GNPs. a Schematic image of antibody binding to gold nanoparticles via EDC/NHS linkers, b transmission electron microscopy (TEM) image of unconjugated gold nanoparticles (GNPs), c GNPs size distribution by dynamic light scattering (DLS), d wavelength changes in the GNPs before and after conjugation with anti-survivin antibody, e LSPR absorbance of bare GNPs, buffer, Ab-labeled GNPs and survivin-Ab-labeled GNPs
Detection of surviving protein with anti-survivin antibody-conjugated GNPs
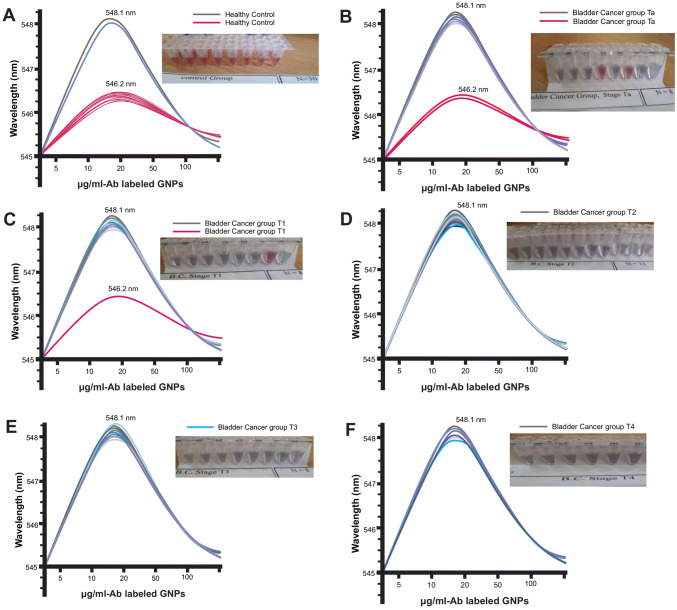
For detection of survivin protein with anti-survivin antibody-conjugated GNPs, after the separation of medistream urine, the amount of 20 μl of urine sample was added to 80 μl of gold nanoparticle solution conjugated with anti-survivin antibody. Finally, results were obtained by changing the color of the solution (from red to gray) visible with the naked eye (Table 2) and also by changing the absorption of the gold nanoparticle solution (Fig. 3) due to the antibody binding to the survivin antigen. The sensitivity and specificity of test are 92.68% and 93.33%, respectively (healthy controls = 30; patients, n = 41; false positive = 2; false negative = 3). According to the results, the color change observed by the naked eye was exactly compatible with changes in LSPR peak wavelength.
Table 2.
The characterization of detection of survivin protein with anti-survivin antibody-conjugated GNPs in urine samples
| Groups | Number per group | Frequency (n) | Percent (%) | ||
|---|---|---|---|---|---|
| Negative | Positive | Negative | Positive | ||
| Healthy control | 30 | 28 | 2 | 93.3 | 6.7 |
| Bladder cancer | 41 | 2 | 39 | 4.9 | 95.1 |
| Subgroups of bladder cancer | |||||
| B.C. stage Ta | 8 | 2 | 6 | 25 | 75 |
| B.C. stage T1 | 8 | 1 | 7 | 12.5 | 87.5 |
| B.C. stage T2 | 11 | 0 | 11 | 0 | 100 |
| B.C. stage T3 | 8 | 0 | 8 | 0 | 100 |
| B.C. stage T4 | 6 | 0 | 6 | 0 | 100 |
Negative, no change in the color of the GNPs solution; positive, change in the color of the GNPs solution from red to gray
Fig. 3.
The absorption curve of anti-survivin Ab-conjugated GNPs solution after adding urine samples. Change in color and absorption curve of positive and negative samples; a healthy control, b bladder cancer stage Ta, c bladder cancer stage T1, d bladder cancer stage T2, e bladder cancer stage T3, f bladder cancer stage T4
Discussion
Diagnosis of bladder cancer, based on different biomarkers, particularly urine-based biomarkers, is necessary, as urine samples are suitable noninvasive diagnostic tools. It has been reported that the sensitivity of survivin test is higher than that of cytology test, especially in patients diagnosed with low-grade bladder cancer. BioDot test and ELISA are commercially available to detect and measure survivin, although these tests have not been approved by FDA [4, 21, 22]. Considering the high recurrence and mortality of this cancer, research is needed to propose suitable methods with acceptable sensitivity and specificity for early diagnosis.
According to the previous reports, 90% of patients who had bladder cancer of non-muscle invasive type present with Ta and T1 stages of bladder cancer [23], which necessitates the use of proper diagnostic methods for diagnosis at low stages. On the other hand, nanotechnology has major impacts on medicine, with great potentials for cancer diagnosis and treatment. Nanoparticles can interact with biomolecules inside and at the surface of cells. One of the most studied and suitable types of nanoparticles in nanotechnology is GNP, which is widely used in different fields of medicine and biology due to its unique features including biocompatibility, simple synthesis and size-dependent optical and electronic properties [13, 14, 24–27]. These nanoparticles have been strongly studied in the colorimetric detection of different analytes such as protein, DNA and ions including mercury (Hg2+) and lead (Pb2+) [28]. Colorimetric detection using GNPs is on the basis of the aggregation of GNPs in the presence of target analyte and GNP solution color change (red to blue-gray) that can be observed by the naked eye, with no need to use complicated technical tools [26, 27]. Gold nanoparticles also have a variety of therapeutic applications in medical science such as diagnosis and drug delivery. They have been shown to reduce neuronal damage and improve neurological deficits in neurodegenerative diseases. In the previous study, we examined the possible effects of these particles on experimental autoimmune encephalomyelitis (EAE), a mouse model of multiple sclerosis (MS) disease, and observed positive effects, including a decrease in the severity of the disease by these nanoparticles [29, 30].
Hyaluronidase (HAase) is one of the bladder cancer markers, which can be detected in urine. The HAase activity was studied using GNPs based on the aggregation of these particles and finally color change in the solution. In this method, the enzyme activity is characterized with high sensitivity within a shorter period, and the results are quickly determined without any need for complex technical equipment. This method is suggested as a valuable method in diagnosis of bladder cancer [31]. Generally, these particles are conjugated to aptamer [26, 32, 33] or specific antibodies [17, 34] for increasing the specificity and sensitivity of colorimetric detection based on GNPs.
Conjugation of GNPs is possible with antibodies and biomolecules through covalent bonds [20]. Di Pasqua reported conjugation of GNPs with anti-E. coli O157:H7 antibodies [35]. In the previous study, we increased the detection sensitivity of prostate-specific antigen (PSA) by combining GNPs conjugated with anti-PSA antibody. Conjugated GNPs aggregation in the presence of PSA antigen, based on antibody–antigen interactions, caused the change in the solution color (red to gray) [17].
Findings of the present study showed that anti-survivin antibody-conjugated GNPs could be used to detect survivin protein. Survivin measurement in patients with bladder cancer, based on ELISA technique, indicated that identification of this biomarker is more sensitive at higher grades of bladder cancer, compared with lower grades. Also, identification is more sensitive in urine samples in comparison with serum samples. With the use of GNPs conjugated with specific antibodies, which exhibit aggregation characteristics in the presence of target antigens and cause subsequent changes in the solution color, survivin can be detected at low grades in urine samples. The proposed method does not require any sophisticated equipment and can be carried out by observing the change in the solution color by the naked eye. In fact, it can be applied as a new approach to detect survivin biomarker in the urine of bladder cancer patients, even at low grades of the disease.
Acknowledgements
This study was supported by Immunology Research Center, Iran University of Medical Sciences, Tehran, Iran (Grant No. IR.IUMS.REC.1394.26402).
Authors’ contribution
MHJ and TA were involved in the research; MHJ and MM performed the research; HN and RN analyzed the data; MHJ and RN wrote the manuscript; and MHJ performed the statistical analysis.
Data availability statement
Research data are not shared.
Compliance with ethical standards
Conflict of interest
The authors declared that they have no conflict of interest.
Ethics approval
All methods in this study were conducted based on the ethical standards of the local ethics committee of Iran University of Medical Sciences and also based on the 1964 Helsinki Declaration, its recent amendments, or comparable ethical standards (Grant No. IR.IUMS.REC.1394.26402).
Informed consent
All authors are aware of and agree to the content of the manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chang Y, Xu J, Zhang Q. Microplate magnetic chemiluminescence immunoassay for detecting urinary survivin in bladder cancer. Oncol Lett. 2017;14(4):4043–4052. doi: 10.3892/ol.2017.6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiner AB et al (2018) Discrepancies in staging, treatment, and delays to treatment may explain disparities in bladder cancer outcomes: an update from the National Cancer Data Base (2004–2013). In: Urologic oncology: seminars and original investigations. Elsevier [DOI] [PubMed]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 4.Schmitz-Dräger BJ, et al. Molecular markers for bladder cancer screening, early diagnosis, and surveillance: the WHO/ICUD consensus. Urol Int. 2015;94(1):1–24. doi: 10.1159/000369357. [DOI] [PubMed] [Google Scholar]
- 5.Margulis V, Lotan Y, Shariat SF. Survivin: a promising biomarker for detection and prognosis of bladder cancer. World J Urol. 2008;26(1):59–65. doi: 10.1007/s00345-007-0219-y. [DOI] [PubMed] [Google Scholar]
- 6.D’Costa JJ, et al. A systematic review of the diagnostic and prognostic value of urinary protein biomarkers in urothelial bladder cancer. Bladder Cancer. 2016;2(3):301–317. doi: 10.3233/BLC-160054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan WS, et al. Novel urinary biomarkers for the detection of bladder cancer: a systematic review. Cancer Treat Rev. 2018;69:39–52. doi: 10.1016/j.ctrv.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 8.Rivandi M, et al. The 9p21 locus: a potential therapeutic target and prognostic marker in breast cancer. J Cell Physiol. 2018;233(7):5170–5179. doi: 10.1002/jcp.26332. [DOI] [PubMed] [Google Scholar]
- 9.Ebrahimnezhad S, et al. Current status and prospective regarding the therapeutic potential of natural autoantibodies in cancer therapy. J Cell Physiol. 2017;232(10):2649–2652. doi: 10.1002/jcp.25765. [DOI] [PubMed] [Google Scholar]
- 10.Ning S, et al. siRNA-mediated down-regulation of survivin inhibits bladder cancer cell growth. Int J Oncol. 2004;25(4):1065–1136. [PubMed] [Google Scholar]
- 11.Kiu K-T, et al. Expression of survivin in bladder cancer cell lines using quantitative real-time polymerase chain reaction. Urol Sci. 2014;25(1):19–21. [Google Scholar]
- 12.Cui X, et al. NF-κB suppresses apoptosis and promotes bladder cancer cell proliferation by upregulating survivin expression in vitro and in vivo. Sci Rep. 2017;7:40723. doi: 10.1038/srep40723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alex S, Tiwari A. Functionalized gold nanoparticles: synthesis, properties and applications—a review. J Nanosci Nanotechnol. 2015;15(3):1869–1894. doi: 10.1166/jnn.2015.9718. [DOI] [PubMed] [Google Scholar]
- 14.Zhou J, et al. Functionalized gold nanoparticles: synthesis, structure and colloid stability. J Colloid Interface Sci. 2009;331(2):251–262. doi: 10.1016/j.jcis.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Shukla R, et al. Biocompatibility of gold nanoparticles and their endocytotic fate inside the cellular compartment: a microscopic overview. Langmuir. 2005;21(23):10644–10654. doi: 10.1021/la0513712. [DOI] [PubMed] [Google Scholar]
- 16.Boisselier E, Astruc D. Gold nanoparticles in nanomedicine: preparations, imaging, diagnostics, therapies and toxicity. Chem Soc Rev. 2009;38(6):1759–1782. doi: 10.1039/b806051g. [DOI] [PubMed] [Google Scholar]
- 17.Jazayeri M, et al. Enhanced detection sensitivity of prostate-specific antigen via PSA-conjugated gold nanoparticles based on localized surface plasmon resonance: GNP-coated anti-PSA/LSPR as a novel approach for the identification of prostate anomalies. Cancer Gene Ther. 2016;23(10):365. doi: 10.1038/cgt.2016.42. [DOI] [PubMed] [Google Scholar]
- 18.Skinner DG. Current state of classification and staging of bladder cancer. Cancer Res. 1977;37(8 Part 2):2838–2842. [PubMed] [Google Scholar]
- 19.Frens G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat Phys Sci. 1973;241(105):20–22. [Google Scholar]
- 20.Jazayeri MH, et al. Various methods of gold nanoparticles (GNPs) conjugation to antibodies. Sens Bio-Sens Res. 2016;9:17–22. [Google Scholar]
- 21.Gleichenhagen J, et al. Evaluation of a new survivin ELISA and UBC® rapid for the detection of bladder cancer in urine. Int J Mol Sci. 2018;19(1):226. doi: 10.3390/ijms19010226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Hakim TFA, et al. Value of urinary survivin as a diagnostic marker in bladder cancer. Anal Quant Cytopathol Histpathol. 2014;36(3):121–127. [PubMed] [Google Scholar]
- 23.Mbeutcha A, et al. Current status of urinary biomarkers for detection and surveillance of bladder cancer. Urol Clin. 2016;43(1):47–62. doi: 10.1016/j.ucl.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Cai W, et al. Applications of gold nanoparticles in cancer nanotechnology. Nanotechnol Sci Appl. 2008;1:17. doi: 10.2147/NSA.S3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang L, et al. Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther. 2008;83(5):761–769. doi: 10.1038/sj.clpt.6100400. [DOI] [PubMed] [Google Scholar]
- 26.Gopinath SC, Lakshmipriya T, Awazu K. Colorimetric detection of controlled assembly and disassembly of aptamers on unmodified gold nanoparticles. Biosens Bioelectron. 2014;51:115–123. doi: 10.1016/j.bios.2013.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saha K, et al. Gold nanoparticles in chemical and biological sensing. Chem Rev. 2012;112(5):2739–2779. doi: 10.1021/cr2001178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jazayeri MH, et al. Colorimetric detection based on gold nano particles (GNPs): an easy, fast, inexpensive, low-cost and short time method in detection of analytes (protein, DNA, and ion) Sens Bio-Sens Res. 2018;20:1–8. [Google Scholar]
- 29.Aghaie T, et al. Gold nanoparticle and polyethylene glycol in neural regeneration in the treatment of neurodegenerative diseases. J Cell Biochem. 2019;120(3):2749–2755. doi: 10.1002/jcb.27415. [DOI] [PubMed] [Google Scholar]
- 30.Aghaie T, et al. Gold nanoparticles and polyethylene glycol alleviate clinical symptoms and alter cytokine secretion in a mouse model of experimental autoimmune encephalomyelitis. IUBMB Life. 2019;71(9):1313–1321. doi: 10.1002/iub.2045. [DOI] [PubMed] [Google Scholar]
- 31.Nossier AI, et al. Direct detection of hyaluronidase in urine using cationic gold nanoparticles: a potential diagnostic test for bladder cancer. Biosens Bioelectron. 2014;54:7–14. doi: 10.1016/j.bios.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 32.Huang C-C, et al. Aptamer-modified gold nanoparticles for colorimetric determination of platelet-derived growth factors and their receptors. Anal Chem. 2005;77(17):5735–5741. doi: 10.1021/ac050957q. [DOI] [PubMed] [Google Scholar]
- 33.Medley CD, et al. Gold nanoparticle-based colorimetric assay for the direct detection of cancerous cells. Anal Chem. 2008;80(4):1067–1072. doi: 10.1021/ac702037y. [DOI] [PubMed] [Google Scholar]
- 34.Jonoush ZA, et al. Localized surface plasmon resonance biosensor for detection of serum prostate specific antigen in prostate cancer patients. Biosci Biotechnol Res Asia. 2016;13(4):2273–2279. [Google Scholar]
- 35.Di Pasqua AJ, et al. Preparation of antibody-conjugated gold nanoparticles. Mater Lett. 2009;63(21):1876–1879. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Research data are not shared.