Abstract
MMP12 is mainly secreted by macrophages, is involved in macrophage development, and decomposes the extracellular matrix. Herein, we investigated whether macrophages would change in the intestinal tumor microenvironment after MMP12 knockout. ApcMin/+;MMP12−/−mice were obtained by crossbreeding ApcMin/+ mice with MMP12 knockout mice (MMP12−/− mice). The data showed that the number and volume of intestinal tumors were significantly increased in ApcMin/+;MMP12−/− mice compared with ApcMin/+ mice. Additionally, the tumor biomarkers CA19-9, CEA, and β-catenin appeared relatively early in intestinal tumors in ApcMin/+;MMP12−/− mice. The results demonstrated that knocking out MMP12 accelerated the tumor growth and pathological process. On further investigation of its mechanism, the proportions of M2 macrophages in the spleen and among peritoneal macrophages were significantly up-regulated in ApcMin/+;MMP12−/− mice. Expression of M2 macrophage-related genes was up-regulated in tumor and peritoneal macrophages. The M2-related cytokine levels of IL-4 and IL-13 were increased in the serum of ApcMin/+;MMP12−/−mice. In vitro, bone marrow-derived M2 macrophages were obtained by treating bone marrow cells with IL-4 and IL-13, and these M2 macrophages secreted cytokines being changed. This finding reveals the crucial role of MMP12 in macrophage development and provides a new target for the control of macrophage polarization. Knocking out MMP12 causes intestinal M2 macrophage accumulation in tumor microenvironment, promoting the growth of intestinal tumors in ApcMin/+ mice.
Electronic supplementary material
The online version of this article (10.1007/s00262-020-02538-3) contains supplementary material, which is available to authorized users.
Keywords: MMP12, ApcMin/+ mice, M2 macrophage, Tumor growth
Introduction
Matrix metalloproteinases (MMPs), which are Zn-dependent endoproteinhydrolases with activity dependent on calcium ions, are involved in the development of animal morphological changes, collagens, and cardiovascular systems. Currently, more than 20 kinds of these enzymes have been found [1]. MMPs also mediate tissue homeostasis and regulate tumor growth, tissue remodeling, inflammation, tumor invasion, and metastasis [2].
Matrix metalloproteinase-12 (MMP12), also called macrophage metalloelastase or macrophage elastase, was first identified as an elastolytic MMP secreted by inflammatory macrophages in 1975 [3–5].Mice with MMP12 homozygously knocked out exhibit abnormal macrophage physiology, decreased sensitivity to cigarette smoke, reduced tissue inflammation, and decreased litter size [6, 7]. A recent study also showed that inhibiting extracellular MMP12 could be a new strategy for antiviral treatment [8].
MMP12 is also expressed in many cancers, including intestinal cancer, gastric cancer, lung cancer, and liver cancer [9–12]. MMP12 would promote tumor invasion and metastasis by extensively degrading extracellular matrix and vascular components [13, 14], while other reports have indicated that MMP12 inhibits tumor growth [15, 16]. As known well, MMP12 is expressed not only in macrophages but also in tumor cells. Importantly, macrophages are involved in antitumor immunity, which means that macrophages and tumor cells both affect tumor expansion or invasion. Therefore, we should determine the role of MMP12 expression by macrophages or tumor cells, which one is most important for tumor growth. Recent studies have found that macrophages in the tumor microenvironment can secrete a variety of cytokines to inhibit or promote tumor growth, and tumor-derived cytokines affect M1 to M2 transformation, interfering with antitumor immunity.
Our previous data showed that knocking out MMP12 induced myeloid-derived suppressor cell accumulation among myeloid cells, contributing to tumor growth in MMP12 knockout mice [17]. Recently, we again found that knocking out MMP12 affects the number of macrophages in the blood and white adipose tissue of mice; therefore, we investigated whether knocking out MMP12 induces M2 macrophage accumulation in the tumor microenvironment.
Traditionally, macrophages can be divided into classically activated M1 macrophages and alternatively activated M2 macrophages [18]. M1-type macrophages mainly produce pro-inflammatory factors, while M2-type macrophages mainly secrete anti-inflammatory factors to inhibit the inflammatory reaction and maintain internal environmental stability in a tissue [19, 20]. Jay W. Heinecke found that MMP12 deletion can alter the infiltration and polarization of macrophages and that knocking out MMP12 contributes to macrophage differentiation into the M2 phenotype in high-fat diet-fed mice [21].
In this study, we found that the growth of intestinal tumors is accelerated when MMP12 knocked out in ApcMin/+ mice. Because MMP12−/− mouse is systemic knockout, the effect of knocking out MMP12 in tumor cells cannot be excluded. According to the previous reports and studies, MMP12 in tumor cells promotes tumor development, so we think that knocking out MMP12 should therefore inhibit tumor growth. However, we observed that knocking out MMP12 promoted intestinal tumor growth in mice, so we hypothesized that knocking down MMP12 expression promotes tumor growth is because MMP12 in macrophages not MMP12 in tumor cells. Therefore, we investigated the role of MMP12 in macrophages and how MMP12 affects tumor growth.
Our results demonstrated that knocking out MMP12 promoted tumor growth by affecting macrophage polarization and M2 macrophage accumulation in tumor microenvironment. This study is useful for exploring the role of macrophages in antitumor immunity and indicates that MMP12 plays an important role in balancing the M1 to M2 transformation of macrophages.
Materials and methods
Animal
B6.129X-Mmp12tm1Sds/J mice (No. 004855, http://jaxmice.jax.org/strain/004855.html) and C57BL/6 J-ApcMin/+ mice (ApcMin/+) were purchased from The Jackson Laboratory (USA). C57 (C57BL/6 J) mice were purchased from Guangdong Medical Laboratory Animal Center. The production license number was SCXK (Guangdong) 2017-0125. ApcMin/+; MMP12−/−mice were obtained by crossbreeding ApcMin/+ mice with MMP12 knockout mice, which develop spontaneous intestinal adenoma. The genotypes were confirmed by PCR. The mice were bred in an SPF environment. Feed treated with 60 Co irradiation was purchased from the Guangdong Medical Animals Center. Drinking water was autoclaved. The room temperature was maintained at 24 ± 2 °C, and the humidity was maintained between 40% and 60%. The noise level was less than 60 db.
Identification of genotypes
The APC gene mutation primer sequences were as follows: P1, 5′-TTCTGAGAAAGACAGAAGTTA-3′ and P2, 5′-GCCATCCCTTCACGTTAG-3′; the PCR product size was 340 bp. The wild-type (WT) primer sequences were as follows: P3, 5′-TTCCACTTTGGCATAAGGC-3′ and P4, 5′-GCCATCCCTTCACGTTAG-3′; the PCR product size was 600 bp (Fig. S1). PCR conditions were as follows: denature at 94 °C for 3 min; 94 °C for 30 s; 55 °C for 1 min; and 72 °C for 1 min for 35 cycles; and extension at 72 °C for 2 min. The MMP12 knockout (mutation-type) primers: P1, 5′-CACGAGACTAGTGAGACGTG-3′ and P2, 5′-ACATCCTCACGCTTCATGTC-3′; the PCR product size was 1400 bp. The wild-type (WT) primer sequences were as follows: P3, 5′-GCTAGAAGCAACTGGGCAAC-3′ and P4, 5′-ACATCCTCACGCTTCATGTC-3′; the PCR product size was 1064 bp (Fig. S1). PCR conditions were as follows: denature at 94 °C for 3 min; 94 °C for 1 min; 60 °C for 2 min; and 72 °C for 2 min for 35 cycles; and 72 °C for 5 min. Gel electrophoresis was performed with a 1.2% agarose gel and evaluated with a gel imaging system (GboxGyngene system, UK).
ApcMin/+;MMP12-/-mouse model
Homozygous ApcMin/+ mice did not survive through the embryonic stage, and only a heterozygous line of ApcMin/+ mice with a genotype of Min ± could be maintained. Male ApcMin/+ mice and female MMP12−/− mice were bred at approximately 8 weeks of age in the same cage at a ratio of 1:3, and no more than four mice were housed in one cage as shown in Fig. S1.
Measuring the number and size of intestinal adenomas
ApcMin/+ and ApcMin/+;MMP12−/− mice were selected at 9 weeks, 15 weeks, and 24 weeks(n = 7–9 in each group). The mice were sacrificed by cervical dislocation, the entire intestine was isolated, and the small intestine was separated from the intestine region, which was divided into three sections on average. The small intestine was rinsed with PBS and spread on filter paper. Then, the intestinal tissue was spread evenly with cotton swabs and placed in 10% formalin. Under a stereo microscope, the intestine was imaged, and the longest and shortest diameters of each adenoma were measured with software to calculate the tumor volume. The following formula was used: volume = 4/3πab2 (where a is half of the long diameter, and b is half of the short diameter). After the completion of tumor counting, each of the intestinal tissue samples was placed in 70% ethanol and stored.
Antibodies
The following flow cytometry antibodies such as PerCP Cy5.5-conjugated anti-mouse CD45 (clone 30-F11), FITC-conjugated anti-mouse/human CD11b (clone M1/70), BV421-conjugated anti-mouse F4/80 (clone BM8), and APC-conjugated anti-mouse CD206 (MMR, clone C068C2) were purchased from BioLegend Inc. The following antibodies, such as anti-CD34 (BA0532) and anti-CD68 (BA3638) antibodies were purchased from BOSTER (Wuhan, China); anti-CA19-9 (GT210101), anti-Ki67 (GT210102) and anti-CD45 (GM074202) antibodies were purchased from Genetech (Shanghai, China); anti-CEA (AB38016) antibodies purchased from Absci; anti-β-catenin (610154) antibodies purchased from BD, were used for immunohistochemistry. The following antibodies are used for immunofluorescence: an anti-MMP12 antibody (ab61157) purchased from Abcam, and an anti-F4/80 antibody (14-4801-81) purchased from eBioscience. The following antibodies were used for Western blotting: anti-Arg1 (Ab91279), and anti-Ym1 (ab93034) antibodies purchased from Abcam; anti-GAPDH (5174P) and anti-β-actin (4970S) antibodies were purchased from Cell Signaling Technology Inc (CST). ADAB chromogenic kit (8059S) was purchased from CST. Red blood cell lysis buffer (GAS-010) was purchased from Life Technologies. Immunohistochemical staining secondary antibodies were purchased from Zhongshan Golden Bridge, China, while the secondary antibodies were purchased from Life Technologies. Blood cell collection was performed using ACD anticoagulant tubes, which were purchased from Sigma-Aldrich.
Immunohistochemistry
Intestinal tissue was fixed in paraffin, and serial sections were used for hematoxylin and eosin (H&E) staining or immunohistochemical staining. Primary antibodies against CD34, CD68, Ki67, CA19-9, CEA, β-catenin and CD45 were incubated overnight at 1:100 at 4 °C. A secondary antibody conjugated with HRP (goat anti-mouse/rabbit IgG) was diluted 1:100 and incubated at 37 °C for 1 h, DAB staining was performed for 1 min, and hematoxylin counterstaining was performed for 20–60 s.
Immunofluorescence
Tissue sections were dewaxed, blocked in 3% BSA at 37 °C for 1 h, and incubated with a primary antibody or a negative control overnight at 4 °C. After washing in PBS, the sections were incubated with a FITC-labeled anti-rat IgG antibody at 37 °C for 1 h. Co-staining was performed with DAPI (500 ng/mL) for 2 min; next, it was observed under a confocal microscope.
Flow cytometry
A 24-week-old ApcMin/+ and ApcMin/+; MMP12−/− mice were sacrificed by cervical dislocation. The spleen, peritoneal macrophages, and tumors were made into single-cell suspensions, and 100 μL of each suspension was added into tubes. Then, PerCP Cy5.5-conjugated anti-CD45, FITC-conjugated anti-CD11b, BV421-conjugated anti-F4/80, and APC-conjugated anti-CD206 antibodies were added into the tubes, which were mixed and incubated for 30 min at room temperature in the dark. Next, red blood cell lysis was performed to eliminate red blood cells, and 1 mL of 0.5% BSA was added to each tube for washing. Finally, 1 mL of a 1% precooled paraformaldehyde solution was added, and the solution was mixed well and stored at 2–8 °C in the dark. A BD FACS antoTM II system was used for detection within 12 h.
Western blotting
Intestinal tumor tissue samples were isolated from ApcMin/+ and ApcMin/+; MMP12−/−mice. Tissue cells were treated with a RIPA cell lysis buffer containing 1/100 phenylmethylsulfonyl fluoride (PMSF), and the proteins were quantified by using BCA kit. Protein were denatured, loaded for SDS-PAGE electrophoresis, transferred to a PVDF membrane, blocked, and incubated with specific primary antibodies overnight at 4 °C. After washing with TBST, a secondary antibody was incubated at 37 °C for 1 h, washed, and then chemiluminescence was developed. The experiment was repeated 3 times.
Primary culture of peritoneal macrophages
24-week-old ApcMin/+ and ApcMin/+; MMP12−/− mice were sterilized with 75% ethanol after cervical dislocation. The mouse abdomen was opened while taking care to avoid cutting the peritoneum, injected with 5 mL of PBS, and gently massaged for approximately 30 s; after 5 min, a syringe was used to collect the peritoneal fluid. The peritoneal wash fluid was transferred into a 15-mL sterile tube. After centrifugation at 1000 rpm for 10 min at 4 °C, the supernatant was removed, and the cells were resuspended in DMEM with 10% FBS. The cells were incubated in a dish at 37 °C for 2 h, and then the medium was removed. The adherent cells were considered peritoneal macrophages.
Isolation of bone marrow cells and induced M2 macrophage
Bone marrow was extracted to prepare a single-cell suspension, and then red blood cell was lysis, washing, cell counting, etc., were performed. The cells were resuspended in DMEM with 10% FBS and 1% penicillin/streptomycin and treated with 50 ng/mL M-CSF. On the third day, the cells were washed with PBS 3 times. On the seventh day, drug stimulation was performed as follows: M2 macrophage differentiation drug administration consisted of 20 ng/mL IL-4 and 10 ng/mL IL-13.
Quantitative real-time PCR
Total RNA was extracted from intestinal tumors in ApcMin/+ and ApcMin/+; MMP12−/− mice with Trizol; RNA was quantified by a NanoDrop nucleic acid protein analyzer. RNA was reverse transcribed into cDNA according to the reverse transcription protocol. The cDNA was used as a template, and GAPDH or β-actin was used as an internal reference for PCR amplification using Takara’s qPCR SYBR Green kit. The 20-μL PCR reaction volumes included 10 μL of SYBR Green Master Mix, 1 μL of forward primer, 1 μL of reverse primer, 1 μL of cDNA, and 7 μL of ddH2O. The following procedure was used: denature at 94 °C for 5 min; denature at 94 °C for 30 s, annealing at 60 °C for 30 s; 72 °C for 30 s; 40 cycles; 72 °C for 5 min. Data were analyzed based on the dissolution curve and Ct value, and the data were measured according to the 2−ΔΔCt method. All PCR primers were purchased from Shanghai Sangon Biotechnology Inc, China, and listed in Table S1.
Cytokine array
The medium of M2 macrophage culture (supernatant) derived from ApcMin/+ and ApcMin/+; MMP12−/− mice were detected with the cytokines array kit (R&D, ARY006, USA); then, using Images J to analyze the results. The detection procedure, in brief, the bio-film conjugated antibodies was immersed with PBS buffer, add 15-μL cytokines antibodies mix into samples, then incubated prepared samples on a shaker for 1 h at room temperature, next incubated bio-film with prepared samples in a shaker overnight at 4 °C, washing, and incubated the secondary antibody (diluted streptavidin-HRP) for 30 min on a shaker, washing, and develop with ECL chemiluminescence.
Statistical analysis
All data were analyzed by GraphPad Prism 5.0 software and are presented as . A two-tailed t test was used to determine whether there was a statistically significant difference between two groups. P < 0.05 was considered statistically significant; *P < 0.05, **P < 0.01 and ***P < 0.001.
Results
Knocking out MMP12 promote intestinal tumor growth in ApcMin/+ mice
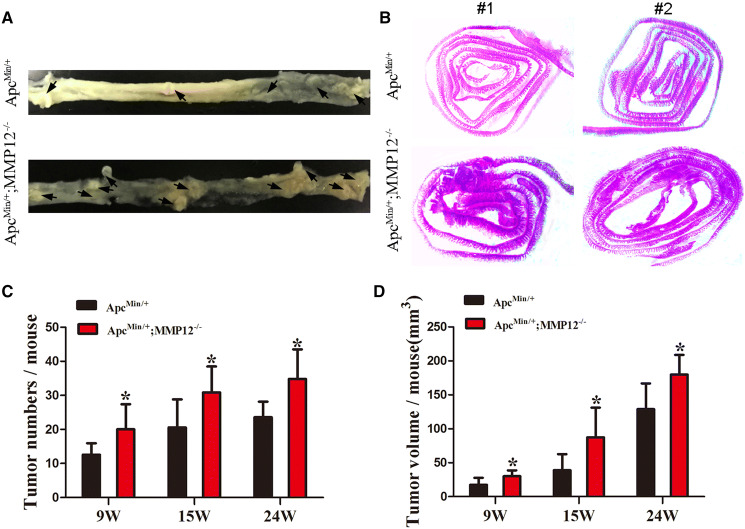
To investigate whether knocking out MMP12 promotes tumor growth, 9-, 15-, and 24-week-old ApcMin/+; MMP12−/− mice and ApcMin/+ mice were sacrificed. We found that the ApcMin/+; MMP12−/−mice had increased numbers of intestinal tumors compared with the ApcMin/+ mice (Fig. 1a). The intestines from the mice in the two groups were embedded and sectioned, and H&E staining was carried out. Under a stereo microscope, the ApcMin/+;MMP12−/− mouse intestinal adenomas were densely distributed in the intestine, and the tumor numbers were higher, but the intestinal adenomas of the ApcMin/+ mice were more sparsely distributed and fewer in number (Fig. 1b). Furthermore, using imaging software to measure the long and short diameters of the tumor, the results showed that the number of intestinal adenomas in the ApcMin/+; MMP12−/− mice was significantly higher than that in the ApcMin/+ mice. There were significant differences in the number and volume of tumors at the above-mentioned weeks of age (Fig. 1c, d, *P < 0.05), indicating that knocking out MMP12 can increase the number and volume of tumors in intestine of ApcMin/+ mice. Therefore, knocking out MMP12 promotes intestinal tumor growth in ApcMin/+ mice.
Fig. 1.
Knocking out MMP12 accelerates intestinal tumor growth in ApcMin/+ mice. a Representative image showed intestinal tumor numbers of ApcMin/+; MMP12−/− mice and ApcMin/+ mice. Black arrows indicated the intestinal tumor, b Representative images of intestinal tumors stained with H&E showed that more tumors appeared in the intestines of ApcMin/+; MMP12−/− mice than in those of ApcMin/+ mice, c Intestinal tumor numbers were more in ApcMin/+; MMP12−/− mice than in ApcMin/+ mice at 9, 15, and 24 weeks old (*P < 0.05), d The intestinal tumor volume was increased in ApcMin/+; MMP12−/− mice compared with ApcMin/+ mice at 9, 15, and 24 weeks old (*P < 0.05). Each group, the mice number, n = 7–9
The pathological process is accelerated in intestinal tumors of ApcMin/+;MMP12−/− mice
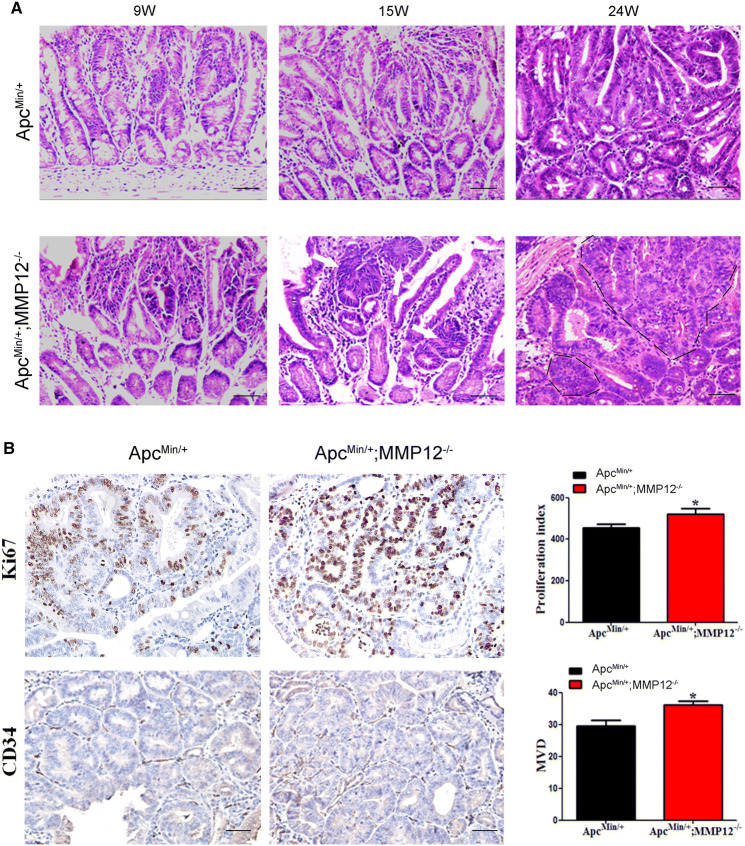
As shown in the above, knocking out MMP12 promotes the growth of intestinal tumors in ApcMin/+ mice. To further confirm the pathological process changes in intestinal tumors in the two groups of mice, we performed H&E staining of intestinal tissue samples from mice at 9, 15, and 24 weeks old and observed the pathological processes in the intestine of ApcMin/+ mice and ApcMin/+;MMP12−/−mice. At 9 weeks, the ApcMin/+ mice presented glandular hyperplasia but no atypical inflammatory cell infiltration in the lumen; at 15 weeks, the ApcMin/+ mice exhibited glandular atypia with tightly arranged, irregular crypts, and at 24 weeks, the glands of the ApcMin/+ mice were disordered and multilayered, and there was an obvious loss of normal intestine villi, and crypt irregularity was aggravated (Fig. 2a, up and Fig. S3A); we further used to score immunohistochemistry results to confirm the difference (S Fig. 3A). At 9 weeks, the ApcMin/+;MMP12−/− mice had abnormal glandular glands, an irregular shape, a tight arrangement, and irregular crypts; at 15 weeks, the intestinal glands in the ApcMin/+;MMP12−/− mice exhibited island cancerization (as indicated by the white arrow shown in Fig. 2a, down), and epithelial cells had begun to develop pathological mitosis, in which larger adenomas have the same cell wall and begin to fuse with each other. At 24 weeks, large-area fusion occurred among glands, cancer cells broke through the basement membrane and exhibited obvious invasive behavior, and glandular epithelial cells had increased pathological mitosis and a significant increase in atypia (Fig. 2a, down line, black line). Therefore, knocking out MMP12 accelerated the pathological process of intestinal tumors in ApcMin/+ mice.
Fig. 2.
Pathological features of ApcMin/+; MMP12−/− mice compared with those of ApcMin/+ mice. a H&E staining was used to show that tumor pathological features are present more tumor cells and tumor cells appear earlier in ApcMin/+;MMP12−/− mice than in ApcMin/+ mice, as shown by the white arrow and black line at 24 weeks (Fig. a, left, down), b Immunohistochemical staining for Ki67 and CD34 in intestinal tumor tissue samples from ApcMin/+;MMP12−/− mice compared with those from ApcMin/+ mice is shown. Microvascularization and the cell proliferation ratio were increased in ApcMin/+;MMP12−/− mice compared with ApcMin/+ mice (*P < 0.05, n = ×6400). Each group, the mice number, n = 7–9
To detect the proliferation of intestinal tumor cells, immunohistochemistry was carried out with an anti-Ki67 antibody (cell proliferation marker) in ApcMin/+;MMP12−/− mice and ApcMin/+ mice at 24 weeks old. The results showed that the Ki67 expression ratio in the ApcMin/+;MMP12−/−mice was higher than that in the ApcMin/+ mice, indicating that the ApcMin/+;MMP12−/− mice had a higher tumor cell proliferation ratio than the ApcMin/+ mice (Fig. 2b, *P < 0.05).
The angiogenic ability of a tumor is also an important factor in tumor growth evaluation. An anti-CD34 antibody was used as an angiogenic marker to stain intestinal tumor tissue samples from ApcMin/+;MMP12−/− mice and ApcMin/+ mice at 24 weeks. There was a significant difference in CD34 expression between ApcMin/+;MMP12−/−and ApcMin/+mouse intestinal tumors (Fig. 2b, *P < 0.05).
Knocking out MMP12 causes intestinal cancer markers to appear early and at a high level
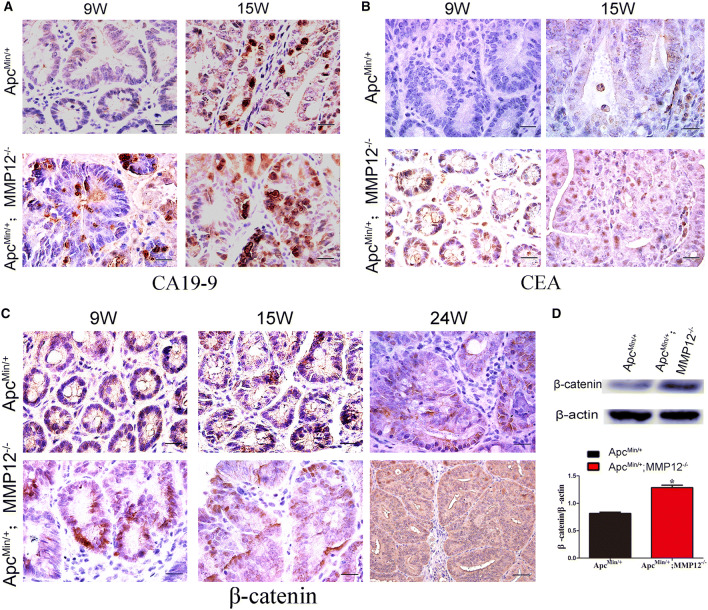
CA19-9 is a cell surface mucin-type carbohydrate protein, also known as gastrointestinal cancer antigen, used as a biomarker for the diagnosis of gastrointestinal tumors. Immunohistochemical staining showed that tumors in ApcMin/+;MMP12−/− mice expressed CA19-9 at 9 weeks, but there was almost no expression in ApcMin/+ mice intestinal tumors at the same age, indicating that knocking out MMP12 could promote the expression of CA19-9 in ApcMin/+mouse intestinal tumors (Fig. 3a). CEA is also called embryonic carcinogenic antigen. CEA secreted by developing cancer cells enters the blood and bodily fluids and is one of the earliest biomarkers used for tumor diagnosis. The Chinese Colorectal Cancer Treatment and Treatment Regulations (2015 Edition) state that colorectal cancer patients must be tested for CEA and CA19-9 expression before diagnosis and during efficacy evaluation and tumor monitoring. Our results showed that CEA expression in ApcMin/+;MMP12−/−mouse intestinal tumors occurred earlier and was higher than that in ApcMin/+mouse intestinal tumors (Fig. 3b). Therefore, knocking out MMP12 caused early carcinogenesis to occur in the ApcMin/+mouse intestine.
Fig. 3.
Knocking out MMP12 promotes intestinal tumorigenesis in ApcMin/+ mice. a Immunohistochemistry results showed that CA19-9 (tumor marker) was present at a higher level in intestinal tumors of ApcMin/+;MMP12−/− mice than that of ApcMin/+ mice at different ages (9 weeks and 15 weeks), b CEA also as tumor marker expression in intestinal tumors in ApcMin/+; MMP12−/− mice was increased compared with that in intestinal tumors in ApcMin/+ mice at 9 weeks and 15 weeks, c The expression of β-catenin in intestinal tumors in ApcMin/+; MMP12−/−mice was higher than that in intestinal tumors in ApcMin/+ mice at 9 weeks, 15 weeks and 24 weeks. The score of β-catenin expression was shown in S Fig. 3B, d Western blotting was used to detect the expression of β-catenin in intestinal tumor tissue samples from ApcMin/+;MMP12−/−mice and ApcMin/+ mice (*P < 0.05). Each group, the mice number, n = 4
Wnt/β-catenin signaling activation by APC gene mutation is the main pathway activated in familial intestinal polyp syndrome. This pathway promotes the development of intestinal adenoma in ApcMin/+ mice. The accumulation of β-catenin is also positively correlated with the severity of colorectal cancer. Immunohistochemistry results showed that ApcMin/+;MMP12−/−mouse intestinal epithelial cells expressed more β-catenin in the cell nucleus than ApcMin/+mouse intestinal epithelial cells (Fig. 3c, the score of β-catenin expression in tissue shown in S Fig. S3B).Western blotting also showed that the ApcMin/+;MMP12−/−mouse intestine exhibited a significantly higher β-catenin expression level than the ApcMin/+mouse intestine (Fig. 3d, *P < 0.05). Therefore, knocking down MMP12 expression would promote the translocation of β-catenin into the nucleus; although the underlying reason remains unknown, the results at least indicate that the Wnt/β-caten in signaling pathway is activated after knocking out MMP12.
M2 macrophage infiltration increased in intestinal tumors of MMP12 knockout mice
MMP12 is mainly expressed on macrophages, and our previously published data show that MDSCs accumulate in the tumor environment [17]. Recently, we again found that macrophage numbers increase when MMP12 is knocked out. To further investigate whether knocking out MMP12 changes macrophage numbers in the tumor microenvironment, immunohistochemistry was carried out on intestinal tissue samples from ApcMin/+mice and ApcMin/+;MMP12−/− mice at 24 weeks. First, leukocyte and macrophage infiltration levels were determined with anti-CD45 and anti-CD68 antibodies. The results showed that the positive cell rates of CD45 (leukocyte molecular marker) and CD68 (macrophage molecular marker) in the ApcMin/+;MMP12−/− mouse samples were significantly increased compared with those in the ApcMin/+ mouse samples (Fig. S2, **P < 0.01 and ***P < 0.001, respectively), showing that knocking out MMP12 enhances the accumulation of macrophages in the tumor microenvironment. One question make us interesting that if the lymphocyte subtype invaded the tumor tissue with some different or change, the flow cytometry results indicated that CD3 + , CD4 + , and CD8 + cells did not have any significant difference in the tumor tissue (Fig. S4).
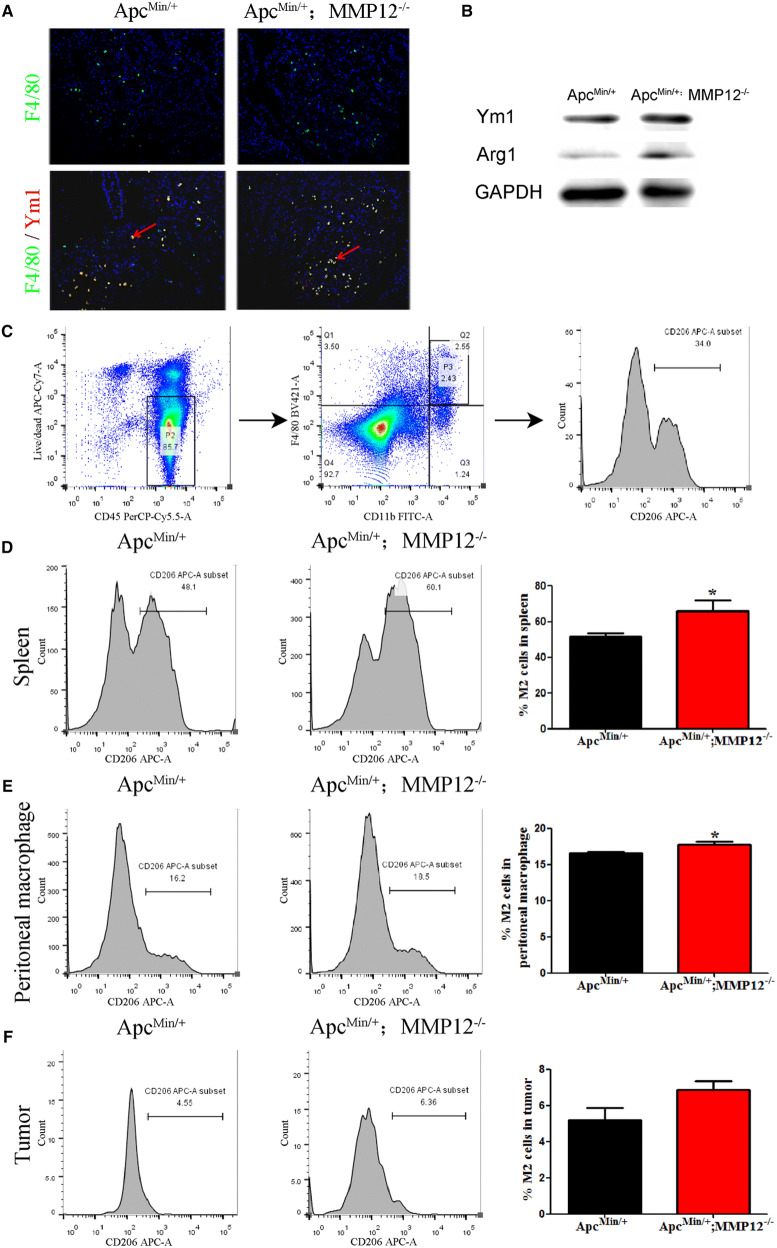
Next, immunofluorescence co-localization was used to detect the macrophages subtype M2 in ApcMin/+ mice and ApcMin/+;MMP12−/−mice, and the results showed that more double-positive cells were present in the ApcMin/+;MMP12−/− mice (Ym1 + and F4/80 + cells) than in the ApcMin/+ mice, as shown in Fig. 4a. Furthermore, western blotting data also supported the changes of M2 macrophages in the ApcMin/+;MMP12−/− mice at 24 weeks. The expression of M2-type macrophage markers (Arg1 and Ym1) was significantly increased in the ApcMin/+;MMP12−/− mice compared with the ApcMin/+ mice (Fig. 4b and Fig. S3C).
Fig. 4.
M2 macrophage numbers increase in mice when MMP12 knock out. a Immunofluorescence results showed that double-positive cells (F4/80 and Ym1, M2 macrophage biomarkers) appeared more in intestinal tumors of ApcMin/+;MMP12−/− mice than those of ApcMin/+ mice. They were located in the tumor microenvironment, b Western blotting results indicated that the levels of the M2 characteristic Ym1 and Arg1 proteins were increased in ApcMin/+;MMP12−/− mice compared with ApcMin/+ mice, the score of protein expression in S Fig. 3C, c Flow cytometry was carried out to detect the M2 macrophage changes, the gating of M2 macrophage gating in flow cytometry experiment, d–f M2 macrophages are increased in the spleen and peritoneal macrophages in ApcMin/+;MMP12−/− mice(*P < 0.05). M2 macrophage in tumor tissues has no statistically significance. Each group, the mice number, n = 5
Further, flow cytometry was performed to detect changes of M2 macrophages in mice. CD206 is an M2-type macrophage marker. The results showed that the proportions of M2-subtype macrophages in the spleen and among peritoneal macrophages of ApcMin/+;MMP12−/− mice were significantly increased (Fig. 4d, e, *P < 0.05). However, the M2 macrophages had no statistically significance in tumor tissues (Fig. 4f).
The M2 macrophage biomarker mRNA increased in peritoneal macrophages and tumors of ApcMin/+;MMP12−/−mice
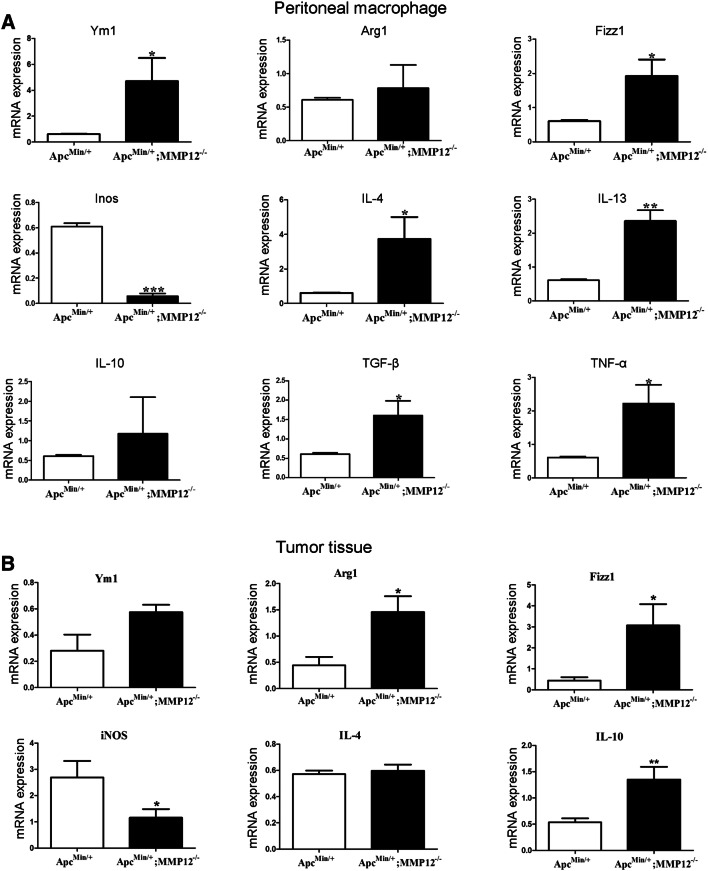
The antitumor immune function of macrophages in the tumor microenvironment is completely different from that in normal tissues. M1 macrophages and M2 macrophages are usually classified according to their different biological functions. M1 macrophages secrete pro-inflammatory molecules including IL-23 and CCL-2 that play an important role in defense against invading pathogens. M2 macrophages play inflammatory roles in tissue repair and tumor progression, by releasing IL-4, MMPs, and other cytokines. The above data show that knocking out MMP12 increases the number of M2 macrophages infiltrating into the tumor microenvironment. So we speculated that the expression of M2-related genes should change in peritoneal macrophages or tumor tissue. Sorting macrophage from tumors, then measuring mRNA level of M2 and M1 genes, which should be done, but it failed because the macrophage was too few to sorting. Thus, RT-qPCR was used to detect M2-related genes in peritoneal macrophages and tumors. Total mRNA was extracted from ApcMin/+and ApcMin/+;MMP12−/−mouse peritoneal macrophages and tumor tissue at 24 weeks, and there are nine related genes, including Ym1, Inos, IL-4 and TNF-α, evaluated for mRNA expression. Compared with the ApcMin/+ mouse, the ApcMin/+;MMP12−/−mouse peritoneal macrophages had up-regulated expression of Ym1, Arg1, and Fizzl (M2 macrophage characteristic factors) but reduced expression of iNOS (M1 macrophage characteristic factor). IL-4, IL-13, TGF-β, and TNF-α mRNA expression in the ApcMin/+;MMP12−/− mice was also up-regulated, as shown in Fig. 5a. Similarly, the expression of the M2-related genes (Arg1, Fizzl, and IL-10) in the tumor tissue of the ApcMin/+;MMP12−/− mice was up-regulated, but that of iNOS was down-regulated; most data indicated that the those M2-related genes are increased in the tumor microenvironment(Fig. 5b, *P < 0.05 and **P < 0.01).
Fig. 5.
Knocking out MMP12 up-regulates M2 macrophage-associated genes. a M2 and M1 macrophages secrete specific cytokines or express related a few specific genes. Therefore, we detected the mRNA expression of related cytokines or genes in peritoneal macrophages, and the results showed that the M2-related cytokines and genes were expressed at high levels, but the M1-related cytokines were expressed at low levels in peritoneal macrophages, b At the same time, M2 macrophages will infiltrate into tumor tissue, so they were also evaluated with RT-qPCR. The mRNA levels of M2-related cytokines and genes in ApcMin/+;MMP12−/− mouse intestinal tumors were increased compared with ApcMin/+ mice (*P < 0.05, **P < 0.01). Here, we try to isolate the M2 macrophage from tumor tissue by flow sorting, but it failed. Each group, the mice number, n = 5
The IL-4, IL-13, and IL-10 level in serum were increased when MMP12 knocked out, which induced M2 macrophage production
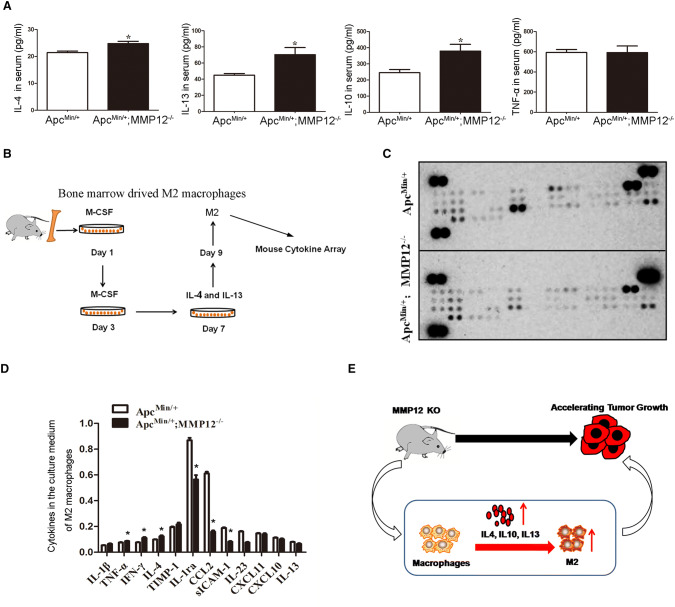
The cytokine levels in mice bearing tumor would alter, so cytokine levels were detected in the serum when MMP12 knocked out mice bearing mice, finding that IL-4, IL-10, and IL-13 expression were up-regulated, as shown in Fig. 6a. As well known, IL4, IL10, and IL13 had been reported would induce M2 macrophage. Here, we used IL-4 and IL-13 to induce M2 macrophages derived from bone marrow, then detecting M2 macrophage medium using cytokines array as shown in Fig. 6b. These M2 macrophages induced from ApcMin/+;MMP12−/−mice and ApcMin/+−mice bone marrow would secrete some cytokines, so the medium of M2 macrophage culture was screened with forty cytokines array. The grayscale analysis and statistical analysis of the bands were performed using ImageJ software. The results showed that the IL-4, INF-γ and TNF-α levels were significantly increased, meanwhile, IL-1ra, CCL-2, sICAM-1, and IL-23 levels were significantly decreased (Fig. 6c, d), indicating the cytokine populations lost their balance and affect immune capacity. Lastly, the schematic was drawn as shown in Fig. 6e. Knocking out MMP12 caused increases in cytokine levels, especially IL-4 and IL-13, induced M2 macrophage production, MM12 knock out would affect immune capacity and promote the growth of intestinal tumors in ApcMin/+ mice.
Fig. 6.
Increased serum cytokine levels induce M2 macrophage production. a Serum samples from ApcMin/+;MMP12−/−mice and control mice were assessed with an ELISA kit. The results showed that IL-4, IL-10, and IL-13 levels were increased in the ApcMin/+;MMP12−/−mice(*P < 0.05), b The schematic diagram shows how we used IL-4 and IL-13 to induce M2 macrophages derived from bone marrow, c and d Cytokines array data showed that cytokine levels increased or decreased in medium of M2 macrophages derived from ApcMin/+;MMP12−/−mice and ApcMin/+ mice, indicating the MMP12 knock out affect immune capacity. E. A schematic diagram shows that knocking out MMP12 would induce increased M2 macrophage accumulation in the tumor microenvironment and accelerated tumor growth. Each group, the mice number, n = 4
Discussion
The ApcMin/+;MMP12−/−mouse model was established in this study. It was used to investigate the effect of MMP12 on spontaneous intestinal adenocarcinoma in mice with an APC mutation. A series of results showed that knocking out MMP12 could increase the growth of intestinal tumors in ApcMin/+; MMP12−/−mice compared with ApcMin/+ mice from 9 to 24 weeks old. The number and volume of intestinal tumors were increased significantly in the ApcMin/+;MMP12−/− mice, and pathological process of the tumors was accelerated. Furthermore, the microvascular density and cell proliferation ratio in the ApcMin/+;MMP12−/− mouse intestinal tumors were increased.
M2 macrophage accumulation in the tumor microenvironment of the ApcMin/+;MMP12−/−mice was increased, contributing to tumor growth in the intestine. Knocking out MMP12 induced M2 macrophage accumulation in mice spleen and peritoneal macrophages, suggesting that MMP12 regulates M2 macrophages to affect tumor growth.
It is believed that the inactivation of the tumor suppressor genes APC and p53 or the activation of the oncogenes Ras and c-Myc cause colorectal tissue to transform from normal mucosa to heterotypic crypts, colon polyps, adenomas, or even adenocarcinomas [22–25]. Our study showed that knocking out MMP12 induced an increase in M2 macrophage numbers, and it regulates intestinal tumor by impacting antitumor immunity.
We used the clinical tumor markers as CEA and CA19-9 to assess the degree of malignancy of intestinal tumors [26–29]. The results showed that ApcMin/+;MMP12−/− mice had earlier expression of CA19-9 and CEA in adenomas compared with ApcMin/+ mice(9 weeks old), and the positive ratio was significantly increased in the ApcMin/+;MMP12−/− mice. All data demonstrated that knocking out MMP12 promoted growth and carcinogenesis in ApcMin/+ mice. In addition, APC mutations in mice occur, Wnt signaling is activated, GSK-3β degradation is suppressed, and β-catenin is protected from degradation. β-catenin translocates into the nucleus and binds to the downstream TCF4/LEF transcription factor to activate the protooncogene, leading to the formation of multiple adenomas [30, 31]. The results showed that in intestinal epithelial cells, β-catenin entered the nucleus earlier and at a significantly higher level in ApcMin/+;MMP12−/−mice compared with ApcMin/+ mice, indicating that knocking out MMP12 activated the Wnt/β-catenin signaling pathway and accelerated the development of intestinal tumors in ApcMin/+ mice.
CRC development is closely related to the tumor microenvironment. Macrophages play a key role in the tumor microenvironment and are the main immune cells infiltrated by the tumor microenvironment. Once macrophages infiltrate peripheral tissues, they will further activate and polarize depending on the local tissue microenvironment [32]. Indifferent microenvironments, macrophages can be polarized into two subtypes: M1 and M2 macrophages. M1 macrophages have anti-infection and antitumor immune responses; M2 macrophages contribute to tumor cell proliferation and tumor angiogenesis [33–35].We found that M2 macrophage infiltration was increased in spleen, peritoneal macrophages and tumor tissue in the ApcMin/+;MMP12−/− mice. Our data indicate that ApcMin/+;MMP12−/− mice exhibit macrophages transforming into an M2-type phenotype under the effect of MMP12 knockout, but we did not know what factor leads to this polarization; we speculate that knocking out MMP12 leads to bone marrow development disorder, increasing M2 macrophages in mice.
Tumor-associated macrophages (TAM) are generated in the tumor microenvironment or are recruited from the bone marrow or other immune organs. TAMs are polarized into M1 macrophages under stimulation with factors such as LPS and then participate in the Th1-type immune response, killing pathogens and tumor cells and ultimately inhibiting tumors. At the same time, some cytokines, such as IL-13 and IL-4, exist, and TAMs are polarized into M2 macrophages, inhibiting the inflammatory reaction, and promoting the development of tumor [18, 36, 37]. Our data showed that IL-4 and IL-13 levels were up-regulated in the serum of ApcMin/+;MMP12−/− mice compared to that of ApcMin/+ mice, and this change induced cultured primary bone marrow cells into M2 macrophages. We noted that the induced M2 macrophages down-regulated CCL-2 expression significantly, while CCL-2 could contribute to tumor growth [38, 39]; it does not support the tumor growth when MMP12 knocks out. Here, we did not know that the detailed plot of cytokine storm occurs when MMP12 is knocked out in mice, but we are sure that MMP12 knock out cause cytokines disorders, which affect M2 macrophages development.
In conclusion, our results showed that knocking out MMP12 causes the accumulation of M2 macrophages in mice, contributing to tumor growth, and increasing the serum levels of inflammatory cytokines induces an increase in M2 macrophage polarization. Therefore, it is useful to explore macrophage functions in antitumor immunity by targeting MMP12.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Guangdong Provincial Key Laboratory of Malignant Tumor Epigenetics and Gene Regulation (Sun Yat-Sen Memorial Hospital) for helping with flow cytometry. We also thank Ting Luo (Guangdong Provincial Experimental Animal Monitoring Institute) for helping with routine tests.
Authors contribution
JCL and LJW were involved in study design and conception. MMY, QL, and TN were involved in data collection, analysis, and interpretation. JBK, XHZ, LBJ, HBL, CLQ, QQZ, and XDH were involved in sample collection and treatment. MMY and JCL were involved in statistical analysis. JCL and LJW were involved in manuscript writing and critical revisions.
Funding
This work was supported by Grants from the National Natural Science Foundation of China (Grant ID: 81773118 and 31771578) and Innovative Foundation of Guangdong Pharmaceutical University (2016KCXTD019).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All of the experiments with mice were approved by the Guangdong Pharmacology University Animal Ethics Committee, and compliance with ethical standards.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Iyer RP, et al. The history of matrix metalloproteinases: milestones, myths, and misperceptions. Am J Physiol Heart Circ Physiol. 2012;303(8):H919–H930. doi: 10.1152/ajpheart.00577.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141(1):52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Werb Z, Gordon S. Elastase secretion by stimulated macrophages. Characterization and regulation. J Exp Med. 1975;142(2):361–377. doi: 10.1084/jem.142.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banda MJ, Werb Z. Mouse macrophage elastase. Purification and characterization as a metalloproteinase. Biochem J. 1981;193(2):589–605. doi: 10.1042/bj1930589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White RR, et al. Partial purification and characterization of mouse peritoneal exudative macrophage elastase. Biochim Biophys Acta. 1980;612(1):233–244. doi: 10.1016/0005-2744(80)90297-1. [DOI] [PubMed] [Google Scholar]
- 6.Hautamaki RD, et al. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science. 1997;277(5334):2002–2004. doi: 10.1126/science.277.5334.2002. [DOI] [PubMed] [Google Scholar]
- 7.Shipley JM, et al. Metalloelastase is required for macrophage-mediated proteolysis and matrix invasion in mice. Proc Natl Acad Sci U S A. 1996;93(9):3942–3946. doi: 10.1073/pnas.93.9.3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marchant DJ, et al. A new transcriptional role for matrix metalloproteinase-12 in antiviral immunity. Nat Med. 2014;20(5):493–502. doi: 10.1038/nm.3508. [DOI] [PubMed] [Google Scholar]
- 9.Kerkela E, et al. Expression of human macrophage metalloelastase (MMP-12) by tumor cells in skin cancer. J Invest Dermatol. 2000;114(6):1113–1119. doi: 10.1046/j.1523-1747.2000.00993.x. [DOI] [PubMed] [Google Scholar]
- 10.Zhao X, et al. Identification of LIFR, PIK3R1, and MMP12 as novel prognostic signatures in gallbladder cancer using network-based module analysis. Front Oncol. 2019;9:325. doi: 10.3389/fonc.2019.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roman J. On the “TRAIL” of a killer: MMP12 in lung cancer. Am J Respir Crit Care Med. 2017;196(3):262–264. doi: 10.1164/rccm.201704-0668ED. [DOI] [PubMed] [Google Scholar]
- 12.Klupp F, et al. Serum MMP7, MMP10 and MMP12 level as negative prognostic markers in colon cancer patients. BMC Cancer. 2016;16:494. doi: 10.1186/s12885-016-2515-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ng KT, et al. Overexpression of matrix metalloproteinase-12 (MMP-12) correlates with poor prognosis of hepatocellular carcinoma. Eur J Cancer. 2011;47(15):2299–2305. doi: 10.1016/j.ejca.2011.05.032. [DOI] [PubMed] [Google Scholar]
- 14.Ella E, et al. Matrix metalloproteinase 12 promotes tumor propagation in the lung. J Thorac Cardiovasc Surg. 2018;155(5):2164–2175. doi: 10.1016/j.jtcvs.2017.11.110. [DOI] [PubMed] [Google Scholar]
- 15.Yang W, et al. Human macrophage metalloelastase gene expression in colorectal carcinoma and its clinicopathologic significance. Cancer. 2001;91(7):1277–1283. doi: 10.1002/1097-0142(20010401)91:7<1277::AID-CNCR1129>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 16.Kerkela E, et al. Metalloelastase (MMP-12) expression by tumour cells in squamous cell carcinoma of the vulva correlates with invasiveness, while that by macrophages predicts better outcome. J Pathol. 2002;198(2):258–269. doi: 10.1002/path.1198. [DOI] [PubMed] [Google Scholar]
- 17.Li J, et al. Myeloid-derived suppressor cells accumulate among myeloid cells contributing to tumor growth in matrix metalloproteinase 12 knockout mice. Cell Immunol. 2018;327:1–12. doi: 10.1016/j.cellimm.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis MJ, et al. Macrophage M1/M2 polarization dynamically adapts to changes in cytokine microenvironments in Cryptococcus neoformans infection. MBio. 2013;4(3):e00264–13. doi: 10.1128/mBio.00264-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liamina SV, et al. M1 and M2 macrophage phenotypes functional activity as essential components in innate immune response assessment. Ross FiziolZhIm I M Sechenova. 2012;98(8):1030–1035. [PubMed] [Google Scholar]
- 21.Lee JT, et al. Macrophage metalloelastase (MMP12) regulates adipose tissue expansion, insulin sensitivity, and expression of inducible nitric oxide synthase. Endocrinology. 2014;155(9):3409–3420. doi: 10.1210/en.2014-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brody H. Colorectal cancer. Nature. 2015;521(7551):S1. doi: 10.1038/521S1a. [DOI] [PubMed] [Google Scholar]
- 23.Bradner JE. Cancer: an essential passenger with p53. Nature. 2015;520(7549):626–627. doi: 10.1038/nature14390. [DOI] [PubMed] [Google Scholar]
- 24.Orr-Weaver TL, Weinberg RA. A checkpoint on the road to cancer. Nature. 1998;392(6673):223–224. doi: 10.1038/32520. [DOI] [PubMed] [Google Scholar]
- 25.Powell SM, et al. APC mutations occur early during colorectal tumorigenesis. Nature. 1992;359(6392):235–237. doi: 10.1038/359235a0. [DOI] [PubMed] [Google Scholar]
- 26.Song Y, et al. Clinical usefulness and prognostic value of red cell distribution width in colorectal cancer. Biomed Res Int. 2018;2018:9858943. doi: 10.1155/2018/9858943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahboob S, et al. A novel multiplexed immunoassay identifies CEA, IL-8 and prolactin as prospective markers for Dukes’ stages A-D colorectal cancers. Clin Proteom. 2015;12(1):10. doi: 10.1186/s12014-015-9081-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu PP, et al. Detection and clinical significance of DLC1 gene methylation in serum DNA from colorectal cancer patients. Chin J Cancer Res. 2011;23(4):283–287. doi: 10.1007/s11670-011-0283-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Formica V, et al. Role of CA19.9 in predicting bevacizumab efficacy for metastatic colorectal cancer patients. Cancer Biomark. 2009;5(4):167–175. doi: 10.3233/CBM-2009-0101. [DOI] [PubMed] [Google Scholar]
- 30.Dow LE, et al. Apc restoration promotes cellular differentiation and reestablishes crypt homeostasis in colorectal cancer. Cell. 2015;161(7):1539–1552. doi: 10.1016/j.cell.2015.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li VS, et al. Wnt signaling through inhibition of beta-catenin degradation in an intact Axin1 complex. Cell. 2012;149(6):1245–1256. doi: 10.1016/j.cell.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Leonard F, et al. Macrophage polarization contributes to the anti-tumoral efficacy of mesoporous nanovectors loaded with albumin-bound paclitaxel. Front Immunol. 2017;8:693. doi: 10.3389/fimmu.2017.00693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jackute J, et al. Distribution of M1 and M2 macrophages in tumor islets and stroma in relation to prognosis of non-small cell lung cancer. BMC Immunol. 2018;19(1):3. doi: 10.1186/s12865-018-0241-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Helm O, et al. M1 and M2: there is no “good” and “bad”—how macrophages promote malignancy-associated features in tumorigenesis. Oncoimmunology. 2014;3(7):e946818. doi: 10.4161/21624011.2014.946818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mills CD. M1 and M2 macrophages: oracles of health and disease. Crit Rev Immunol. 2012;32(6):463–488. doi: 10.1615/CritRevImmunol.v32.i6.10. [DOI] [PubMed] [Google Scholar]
- 36.Natoli G, Monticelli S. Macrophage activation: glancing into diversity. Immunity. 2014;40(2):175–177. doi: 10.1016/j.immuni.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 37.Mantovani A, et al. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23(11):549–555. doi: 10.1016/S1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 38.Loberg RD, et al. Targeting CCL2 with systemic delivery of neutralizing antibodies induces prostate cancer tumor regression in vivo. Cancer Res. 2007;67(19):9417–9424. doi: 10.1158/0008-5472.CAN-07-1286. [DOI] [PubMed] [Google Scholar]
- 39.Mizutani K, et al. The chemokine CCL2 increases prostate tumor growth and bone metastasis through macrophage and osteoclast recruitment. Neoplasia. 2009;11(11):1235–1242. doi: 10.1593/neo.09988. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.