Abstract
Background
Tumor-infiltrating lymphocytes (TILs) and their subsets contribute to breast cancer prognosis. We investigated the prognostic impact of CD3+, CD8+ and FOXP3+ TILs in patients with early intermediate/high-risk breast cancer treated with adjuvant anthracycline-based chemotherapy within two randomized trials conducted by our Group.
Methods
We examined 1011 patients (median follow-up 130.9 months) and their tumors for total, stromal (s) and intratumoral (i) CD3, CD8 and FOXP3 lymphocyte density (counts/mm2) on tissue-microarray cores by immunohistochemistry. Morphological sTIL density on whole H&E-stained sections was also evaluated.
Results
The majority of TILs were CD3+. Total CD3 and CD8, sCD3 and sCD8, iCD3 and iCD8, sFOXP3 and iFOXP3 were strongly correlated (Spearman’s rho values > 0.6). High individual lymphocytic subsets and sTIL density were strongly associated with high tumor grade, higher proliferation and HER2-positive and triple-negative tumors (all p values < 0.001). Higher sTIL density (10% increments), high density of almost each individual marker and all-high profiles conferred favorable prognosis. However, when adjusted for sTIL density, stromal and intratumoral lymphocytic subsets lost their prognostic significance, while higher sTIL density conferred up to 15% lower risk for relapse. Independently of sTIL density, higher total CD3+ and CD8+ TILs conferred 35% and 28% lower risk for relapse, respectively.
Conclusions
Stromal and intratumoral CD3+, CD8+ and FOXP3+ TIL density do not seem to add prognostic information over the morphologically assessed sTIL density, which is worth introducing in routine histology reports.
Electronic supplementary material
The online version of this article (10.1007/s00262-020-02557-0) contains supplementary material, which is available to authorized users.
Keywords: Breast cancer, TILs, CD3, CD8, FOXP3
Introduction
In cancer, aberrant host immune response fails to eradicate or control, and may even promote, tumor development [1]. In histology, immune cells in the tumor microenvironment are considered indicative of the host immune response. In recent years, efforts have been made to decipher the diverse cellular composition of tumor-immune infiltrates in neoplasms [2]. The majority of the cells are lymphocytes, which cannot be correctly segregated from other mononuclear cells in hematoxylin- and eosin-stained sections and are collectively reported under the term “tumor-infiltrating lymphocytes” (TILs) [3].
Although TILs have long been observed in breast tumors, the prognostic potential of this marker and its predictive role have only gained momentum in the last decade [4–6]. The different types of immune cells may contribute in different ways to tumor biology. The adaptive immune system includes lymphocytes with a cytotoxic antineoplastic effect, particularly CD8+ T lymphocytes, but also lymphocytes playing immunosuppressive and, possibly, tumor-promoting roles, such as T-regulatory cells (T-regs) which are FOXP3 positive [7, 8]. Although the anti-tumor effect of cytotoxic T lymphocytes is broadly accepted, their prognostic significance in breast cancer seems to depend on molecular subtypes [9], while the prognostic impact of FOXP3+ cells in breast carcinomas is controversial [10, 11]. All T cell subtypes express CD3, which is a specific T cell marker and part of the T cell receptor complex on mature T lymphocytes. CD3 protein [12, 13] and mRNA [14] expression have only rarely been investigated in adjuvantly treated breast cancer and were associated with better prognosis. CD3 immunostaining may aid in better defining intratumoral TILs. Intratumoral TILs are in contact with tumor cells and are distinguished from stromal TILs that are dispersed in the stroma between the cancerous nests. Intratumoral TILs may better reflect host anti-tumor activity; however, these are much less studied than stromal TILs because, when morphologically assessed, they display greater inter-observer variability [3].
In the above context, the aim of the present study was to evaluate the prognostic impact of stromal and intratumoral CD3+, CD8+ and FOXP3+ lymphocytes, and their association with standard clinicopathological parameters in a cohort of early intermediate/high-risk breast cancer patients who received anthracycline-based adjuvant chemotherapy in the context of two randomized trials [15, 16].
Patients, tumors and methods
Patients and tumors
Tumor material from 1.011 patients who participated in two randomized phase III clinical trials conducted by the Hellenic Cooperative Oncology Group (HeCOG) was retrieved from the HeCOGbiological material repository. The previously published trials, HE10/97 (ACTRN-12611000506998 [15]) and HE10/00 (ACTRN12609001036202 [16]), included patients with intermediate/high-risk operable breast cancer treated with adjuvant chemotherapy using epirubicin, paclitaxel and CMF, except for one arm of the HE10/97 trial, where no paclitaxel was administered. Patients with HER2-positive tumors had not received trastuzumab, which was approved for adjuvant treatment in 2005, after completion of recruitment in these trials. Treatment schedules for the two trials are shown in Supplementary Table S1. Tumors were examined on 51 low-density tissue microarrays (TMAs) that included 1.5 mm cores, 2 per tumor, and were constructed as previously described [17]. Breast cancer subtypes were centrally assessed with immunohistochemistry for ER, PgR, HER2, Ki67 and HER2 fluorescent-in-situ-hybridization where needed [17]. Stromal TIL density (sTIL density) had been assessed for infiltrative tumors on whole H&E sections as an average of all evaluated X100 fields per tumor, as previously published for an extended cohort that included the present one [18].
Immunohistochemistry (IHC)
Three 3 μm ΤΜΑ sections were used for the evaluation of CD3, CD8 and FOXP3 protein expression. The staining procedures for CD3 (clone PS1, mouse monoclonal antibody, code NCL-L-CD3-PS1, Novocastra/Leica Biosystems, Newcastle, UK), CD8 (clone C8/144B, mouse monoclonal antibody, code M7103, Dako, Glostrup, Denmark) and FOXP3 (clone SP97, rabbit monoclonal antibody, code M3974, Spring Bioscience, Fremont, CA) were performed using a Bond III autostainer (Leica Microsystems, Wetzlar, Germany), as described in Supplementary Table S2.
IHC evaluation
The evaluation of CD3+, CD8+ and FOXP3+ was conducted by two expert breast pathologists (T. Koletsa, I. Kostopoulos), blinded to patient clinical and survival data. Membranous staining was evaluated for CD3 and CD8 and nuclear for FOXP3. IHC-positive cells were counted in the tumor stroma (sCD3, sCD8, sFOXP3) and in the intratumoral areas attached to malignant cells (iCD3, iCD8, iFOXP3), adhering to recommendations for distinguishing the two tumor compartments [3]. However, stromal and intratumoral lymphocyte distinction is difficult in stromal poor and in single-cell infiltrating tumors (e.g., lobular carcinomas). Therefore, the total number of positive lymphocytes within the tumor area was also considered (total CD3, CD8, FOXP3). Positive cells were counted in four high power fields (X400) covering the entire area of each 1.5 mm diameter core (four values/core were recorded for each marker). The density of positive cells in each tumor compartment was assessed as the ratio of cell counts per mm2 surface [19]. With this approach, total tumor area, stromal area and the area occupied by malignant cells were recorded as 10% increments of the total core area on matched H&E TMA slides (examples are shown in Supplementary Figure S1). The surface of each compartment in mm2 was calculated based on the percentage of the recorded area % and the total core surface (1.76625mm2 for 1.5 mm cores). For each marker, stromal cell counts per core were then divided by the respective stromal surface, intratumoral cell counts by the malignant cell surface and total tumor cell counts by the total tumor surface to assess the density of lymphocytic subsets, as recommended for any TILs [20]. Average values were used for tumors evaluable on multiple cores.
The obtained values were distributed among an extremely wide range, while multiple outliers were identified for each lymphocytic subset, accounting for a significant percentage of the total sample in each case (Supplementary Figure S2). These outliers were natural (could not be attributed to technical reasons). Including the outliers in the continuous lymphocytic subset variables contributed to skewed analyses that were statistically inaccurate. Omitting them from the analysis would lead to misinterpretation of the results. Therefore, we considered the upper quartile (75th percentile) of each distribution to be an appropriate threshold for the classification of tumors into high and low counts/mm2.
Statistical methods
A total of 1,011 breast cancer patients treated with adjuvant chemotherapy were included in the statistical analysis (Fig. 1). Continuous variables are presented as median values (range) and categorical variables as frequencies (%). Spearman’s correlations were used for associations of continuous variables, with rho values > 0.6 indicating strong correlations (https://www.bmj.com/about-bmj/resources-readers/publications/statistics-square-one/11-correlation-and-regression). The chi-square or Fisher’s exact tests (where appropriate) were used for group comparisons of categorical data and the Wilcoxon rank-sum test for comparisons of categorical with continuous data.
Fig. 1.
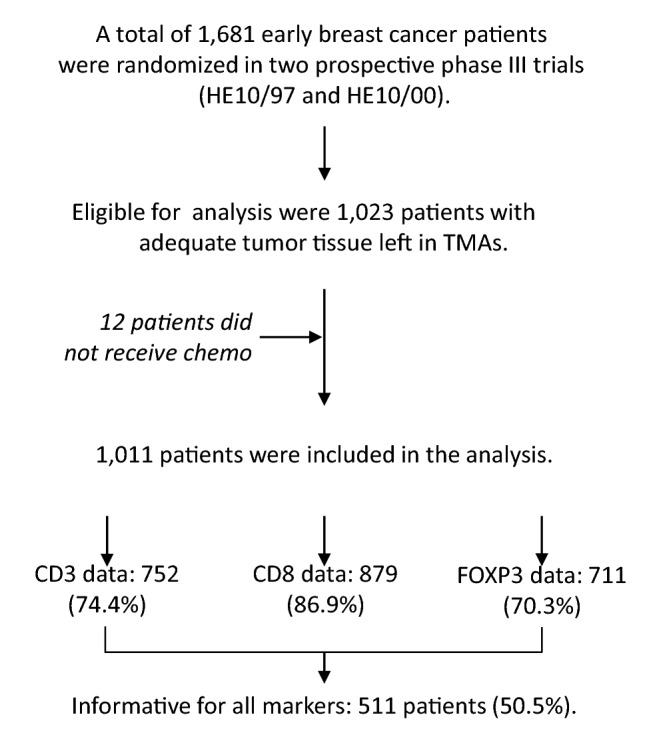
Study outline and informative markers. REMARK diagram
For all analyses, sTIL density was used as a continuous variable, as recommended [3]. For associations with clinicopathological variables and with patient disease-free survival (DFS) and overall survival (OS), lymphocytic subsets were analyzed as categorical variables, based on the aforementioned rationale. Lymphocytic subsets were used as continuous variables for inter-marker correlations and for hierarchical clustering (Ward method), in an attempt to evaluate the profiles rather than ratios of these markers.
OS was defined as the time (in months) from the date of breast cancer diagnosis to the date of patient death or last contact. DFS was defined as the time (in months) from the date of breast cancer diagnosis to the date of documented first relapse, patient death without prior documented disease progression or last contact, whichever occurred first. Patients without events were censored at the date of last contact. Survival curves were estimated using the Kaplan–Meier method and compared across groups with the log-rank test. The associations between the examined factors and the progression/mortality rates were evaluated with hazard ratios estimated with univariate and multivariate Cox proportional hazard regression models.
In multivariate analyses, we estimated the effect (HR) of each lymphocytic subset marker and of sTIL density (10% increments) adjusted for the effect of menopausal status (premenopausal, postmenopausal), tumor size (≤ 2, 2–5, > 5 cm), nodal status (0–3, ≥ 4 positive lymph nodes), histological grade (I–II, III–IV), radiation therapy (yes, no) and breast cancer subtypes (luminal-A, luminal-B, luminal-HER2, HER2-enriched, triple-negative breast cancer [TNBC]). The type of breast surgery (breast-conserving, modified radical mastectomy) was not included in the models because of its association with tumor size and nodal status.
The present IHC data were also compared to CD3, CD8 and FOXP3 mRNA expression data obtained from tumors in the same cohort, as previously published by our group [14]. This analysis served for descriptive comparisons between mRNA and protein expression for the three lymphocytic subsets, as well as for the discussion of the present findings in the context of different methods for the assessment of the host immune response.
All analyses were conducted in the entire cohort and were based on the total follow-up time. Additionally, subgroup analyses were performed by breast cancer subtype. The statistical analyses were completed using the SAS software (SAS for Windows, version 9.3, SAS Institute Inc., Cary, NC). Statistical significance was set at two-sided p = 0.05.
Results
Selected characteristics of the 1,011 patients and tumors examined are presented in Table 1. Briefly, most patients were over 50 years old, had tumors of intermediate size [2–5 cm] and more than 4 metastatic lymph nodes, while the predominant clinical subtype was luminal-B.
Table 1.
Patient and tumor characteristics
| Parameter | N (%) |
|---|---|
| Age (years) | |
| Median | 52.7 |
| Min–max | 22–79 |
| Menopausal status (at diagnosis) | |
| Postmenopausal | 548 (54.2) |
| Premenopausal | 463 (45.8) |
| Breast surgery | |
| Modified radical mastectomy | 694 (68.6) |
| Breast-conserving surgery | 315 (31.2) |
| Unknown | 2 (0.2) |
| Tumor size (cm) | |
| ≤ 2 | 302 (29.8) |
| 2–5 | 580 (57.4) |
| > 5 | 129 (12.8) |
| Nodal status | |
| 0–3 | 408 (40.4) |
| ≥ 4 | 603 (59.6) |
| Paclitaxel treatment | |
| Yes | 866 (85.6) |
| No | 145 (14.4) |
| Adjuvant radiation therapy | |
| Yes | 770 (76.2) |
| No | 214 (21.2) |
| Not reported | 27 (2.6) |
| Adjuvant hormonal therapy | |
| Yes | 804 (79.6) |
| No | 205 (20.2) |
| Not reported | 2 (0.2) |
| Histological grade | |
| I–II | 496 (49.0) |
| III | 511 (50.6) |
| Not reported | 4 (0.4) |
| ER/PgR status (informative N = 962) | |
| Positive | 737 (76.6) |
| Negative | 225 (23.4) |
| HER2 status (informative N = 976) | |
| Positive | 232 (23.8) |
| Negative | 744 (76.2) |
| Ki67 (N = 945) | |
| Median | 25 |
| Min–max | 0–98 |
| Subtypes | |
| Luminal-A | 273 (27.0) |
| Luminal-B | 327 (32.4) |
| Luminal-HER2 | 123 (12.2) |
| HER2-enriched | 103 (10.2) |
| TNBC | 122 (12.0) |
| Unknown | 63 (6.2) |
Immunohistochemical findings
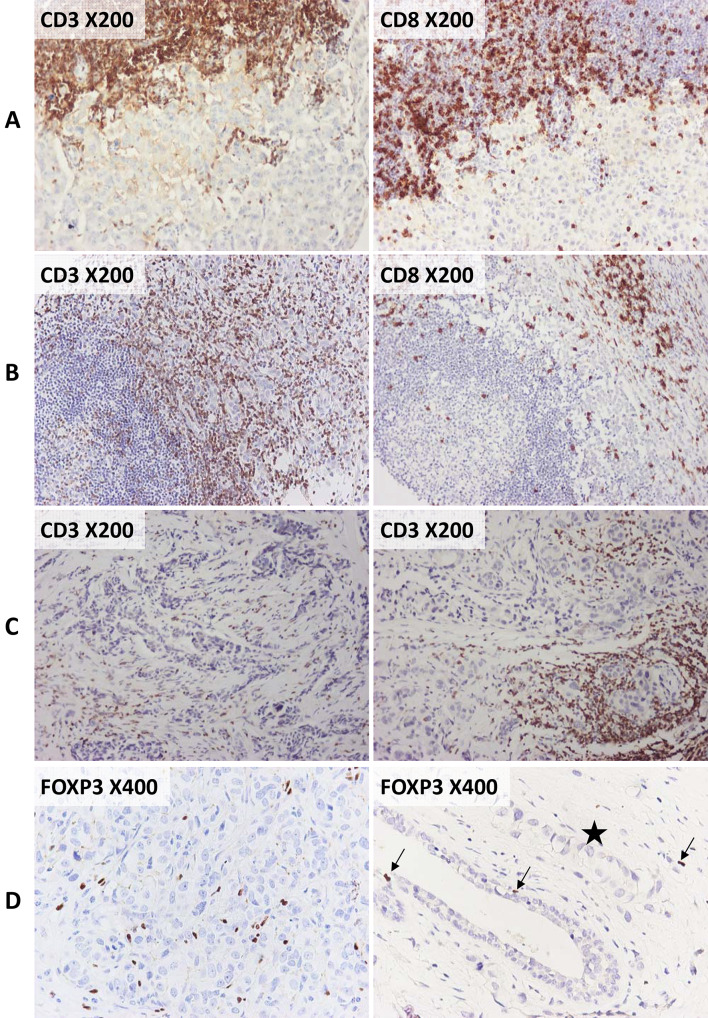
The most common phenotype of stromal TILs pertained to CD3+ T lymphocytes that outnumbered CD8+ cytotoxic T cells (Fig. 2a, b). Lymphocytic infiltrates in tumors with scanty stroma could not easily be delineated as stromal or intratumoral, because they were in contact with the neoplastic cells. In 56 tumors, we observed heterogeneous TIL distribution between the two cores or even in the same core (Fig. 2c). High numbers of sFOXP3+ cells were present in a minority of tumors (Fig. 2d). Nuclear FOXP3 positivity was found in a few single malignant cells of six breast carcinomas. Overall, FOXP3+ cells were absent in non-neoplastic ducts or terminal ductal–lobular units, with the exception of three cases that presented scarce positive cells (Fig. 2d). In 37 tumors, we observed lymphocytes without CD3 immunoreactivity at the invasive tumor front, close to the adipose tissue, or in association with tertiary lymphoid structures. The majority of such non-T lymphocytes were PAX5+ B cells, with few CD138+ plasma cells, as revealed upon routine IHC (data not shown).
Fig. 2.
Examples of CD3, CD8 and FOXP3 immunoreactivity in the examined tumors. Markers and original microphotograph magnifications as indicated. a, b CD3+ outnumbered CD8+ lymphocytes in all areas of the tumor, as shown in the invasive front (a) and around tertiary lymphoid structures (b). c Distribution patterns of CD3+ lymphocytes were not homogeneous in different areas of the same tumor, as shown in two different TMA cores (core 1 on the left, core 2 on the right). D In comparison to CD3+ and CD8+, FOXP3+ cells were poorly represented. A tumor with a relatively high number of FOXP3+ cells is shown on the left. On the right, rare FOXP3+ cells (arrows) are indicated in a normal duct and in the surrounding stroma, in the presence of tumor (asterisk)
In line with the observed immunophenotypes, counts/mm2 for sCD3were higher than for sCD8 and much higher than for sFOXP3 (Supplementary Figure S2). For example, median and the upper quartile cutoff values (in parentheses) for sCD3 were 118.2 (318.8), for sCD8 66.1 (171.7) and for sFOXP3 17.4 (54.9). The respective values for iCD3 were 1.4 (7.5), for iCD8 4.1 (13.2) and for iFOXP3 0 (1.4). Strong positive correlations were obtained for sCD3 and sCD8 (Spearman’s rho 0.883), iCD3 and iCD8 (rho 0.659), sFOXP3 and iFOXP3 (rho 0.633), as well as for total CD3 and CD8 (rho 0.884). Intermediate correlations (rho values between 0.4 and 0.5) were observed between sTIL density and sCD3, sCD8 and sFOXP3. Total CD3, CD8 and FOXP3 were weakly correlated with the respective mRNA values (Supplementary Table S3). Statistically, all lymphocytic subsets as binary variables were significantly associated with each other (all chi-square test p values < 0.001) and with sTIL density (Wilcoxon rank-sum p values < 0.001).
Association of TIL subsets with clinicopathological characteristics
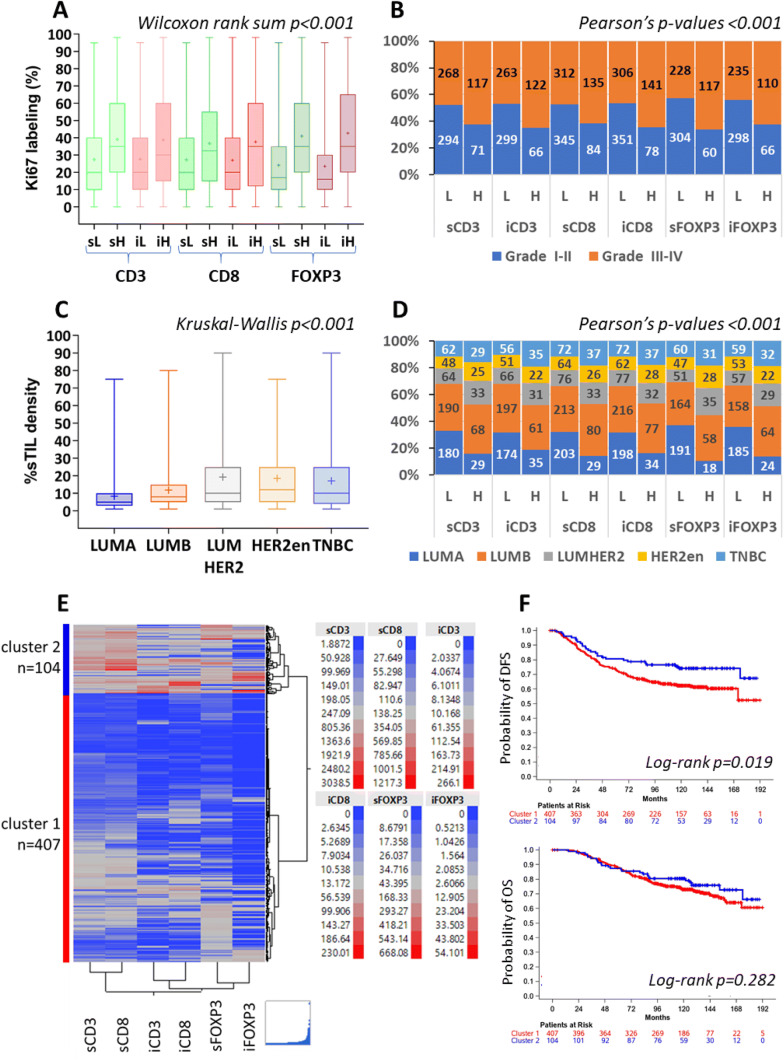
Tumors with higher counts/mm2 of all lymphocytic subsets—total, stromal and intratumoral—had significantly higher Ki67 labeling index (Fig. 3a) and were more frequently of higher histological grade (Fig. 3b). Higher sTIL density was strongly associated with high grade (Wilcoxon rank-sum p < 0.001) but only weakly correlated with Ki67 labeling (Spearman’s rho 0.295). High counts/mm2 of sFOXP3 were observed in 30.1% (88/292) of patients with 0–3 positive nodes but in 21.2% (89/419) of those with ≥ 4 positive nodes (chi-square p = 0.007); such an association was not observed for the rest of the lymphocytic subsets and for sTIL density. Total CD3 (p = 0.035) and sTIL density (p = 0.005) were higher in small tumors and lower in tumors > 5 cm (Supplementary Table S4).
Fig. 3.
Properties of individual lymphocytic subsets and sTIL density in breast carcinomas. High lymphocytic subset counts/mm2 were associated with higher proliferation rate (shown in a) and with high tumor grade (shown in b). Higher sTIL density in HER2-positive tumors irrespectively of hormone receptor positivity and in TNBC (c). Similar distribution patterns were observed for individual lymphocytic subsets, as indicated in d. Hierarchical clustering of continuous counts/mm2 yielded two distinct clusters, cluster 1 with low density of all lymphocytic subsets and cluster 2 with high density, respectively (e). Associations of these clusters with patient DFS and OS are shown in f. sL, sH: stromal low, stromal high; iL, iH: intratumoral low and high, respectively; LUMA: luminal-A; LUMB: luminal-B; LUMHER2: luminal-HER2; HER2-en: HER2-entiched
sTIL density and all lymphocytic subsets were consistently significantly lower in ER-positive tumors and significantly higher in HER2-positive tumors (luminal-HER2 and HER2-enriched) and in TNBC (Fig. 3c, d). Thus, in luminal-A, luminal-B, luminal-HER2, HER2-enriched and TNBC, the rate of high sCD8 was 12.5%, 27.3%, 30.3%, 28.9% and 33.9%, respectively; the rate of high sFOXP3 was 8.6%, 26.1%, 40.7%, 37.3% and 34.1%, respectively; and the median sTIL density was 5, 8, 10, 12 and 10, respectively.
Lymphocytic subsets and sTIL density on patient outcome
Within a median follow-up time of 130.9 months (range 0.1–218.8), 325 deaths (32.1%) and 402 relapses (39.8%) occurred. Median OS was 194.5 (95% CI 184.9–NE) months and median DFS 184.2 (95% CI 171.9–NE) months. Within the first 5 years of follow-up, 152 deaths (15.0%) and 270 relapses (26.7%) occurred. Standard clinical parameters associated with patient outcome were menopausal status, size, nodal status, type of surgery and clinical subtypes, as expected (Supplementary Table S5).
In the entire cohort, higher sTIL density and high counts/mm2 of sCD3, total CD3, sCD8, iCD8, total CD8 and sFOXP3 were all significant predictors of longer DFS (Kaplan–Meier curves and log-rank test results in Supplementary Figure S3) and conferred reduced risk of relapse (univariate Cox regression results in Table 2). Out of these markers, high total CD3, high sFOXP3 and higher sTIL density were similarly significantly associated with longer OS and conferred lower risk of death (Table 2).
Table 2.
Cox univariate regression with respect to DFS and OS for CD3, CD8, FOXP3 and sTIL density
| DFS | OS | |||||
|---|---|---|---|---|---|---|
| Events/total | HR (95% CI) | p value | Events/total | HR (95% CI) | p value | |
| Entire cohort | ||||||
| Total CD3 | ||||||
| High | 53/188 | 0.61 (0.45–0.82) | 0.001 | 46/188 | 0.71 (0.51–0.99) | 0.042 |
| Low | 226/564 | Reference | – | 176/564 | Reference | – |
| iCD3 | ||||||
| High | 60/188 | 0.79 (0.60–1.05) | 0.11 | 52/188 | 0.94 (0.69–1.29) | 0.71 |
| Low | 219/564 | Reference | – | 170/564 | Reference | – |
| sCD3 | ||||||
| High | 58/188 | 0.71 (0.53–0.94) | 0.019 | 51/188 | 0.82 (0.60–1.13) | 0.22 |
| Low | 221/564 | Reference | – | 171/564 | Reference | – |
| total CD8 | ||||||
| High | 70/218 | 0.71 (0.54–0.92) | 0.009 | 63/218 | 0.84 (0.63–1.11) | 0.22 |
| Low | 278/661 | Reference | – | 218/661 | Reference | – |
| iCD8 | ||||||
| High | 68/219 | 0.68 (0.52–0.89) | 0.004 | 61/219 | 0.83 (0.62–1.10) | 0.19 |
| Low | 280/660 | Reference | – | 220/660 | Reference | – |
| sCD8 | ||||||
| High | 73/219 | 0.74 (0.57–0.95) | 0.020 | 62/219 | 0.81 (0.61–1.08) | 0.15 |
| Low | 275/660 | Reference | – | 219/660 | Reference | – |
| total FOXP3 | ||||||
| High | 56/175 | 0.80 (0.60–1.08) | 0.15 | 48/175 | 0.90 (0.65–1.24) | 0.52 |
| Low | 205/536 | Reference | – | 161/536 | Reference | – |
| iFOXP3 | ||||||
| High | 63/176 | 0.98 (0.74–1.30) | 0.88 | 52/176 | 1.03 (0.75–1.41) | 0.84 |
| Low | 198/535 | Reference | – | 157/535 | Reference | – |
| sFOXP3 | ||||||
| High | 51/177 | 0.67 (0.49–0.91) | 0.011 | 41/177 | 0.67 (0.48–0.95) | 0.024 |
| Low | 210/534 | Reference | – | 168/534 | Reference | – |
| sTIL density* | 0.87 (0.80–0.95) | 0.001 | 0.88 (0.80–0.97) | 0.009 | ||
| Luminal-B | ||||||
| Total CD3 | ||||||
| High | 18/68 | 0.47 (0.28–0.78) | 0.004 | 16/68 | 0.52 (0.30–0.91) | 0.022 |
| Low | 86/190 | Reference | – | 68/190 | Reference | – |
| sCD3 | ||||||
| High | 20/68 | 0.56 (0.34–0.92) | 0.022 | 19/68 | 0.77 (0.46–1.29) | 0,33 |
| Low | 84/190 | Reference | – | 65/190 | Reference | – |
| iCD8 | ||||||
| High | 22/77 | 0.56 (0.35–0.88) | 0.013 | 20/77 | 0.69 (0.42–1.13) | 0,14 |
| Low | 99/216 | Reference | – | 78/216 | Reference | – |
| sTIL density* | 0.81 (0.66–0.98) | 0.034 | 0.81 (0.65–1.01) | 0.061 | ||
| Luminal-HER2 | ||||||
| Total CD3 | ||||||
| High | 8/38 | 0.35 (0.16–0.78) | 0.010 | 8/38 | 0.42 (0.19–0.94) | 0.034 |
| Low | 27/59 | Reference | – | 23/59 | Reference | |
| total CD8 | ||||||
| High | 9/36 | 0.39 (0.19–0.82) | 0.012 | 8/36 | 0.36 (0.17–0.78) | 0.010 |
| Low | 36/73 | Reference | – | 33/73 | Reference | – |
| iCD8 | ||||||
| High | 9/32 | 0.47 (0.23–0.98) | 0.044 | 8/32 | 0.47 (0.22–1.01) | 0.054 |
| Low | 36/77 | Reference | – | 33/77 | Reference | – |
| sCD8 | ||||||
| High | 8/33 | 0.40 (0.18–0.85) | 0.018 | 7/33 | 0.36 (0.16–0.81) | 0.013 |
| Low | 37/76 | Reference | – | 34/76 | Reference | – |
| total FOXP3 | ||||||
| High | 6/31 | 0.34 (0.14–0.83) | 0.018 | 6/31 | 0.44 (0.18–1.09) | 0.077 |
| Low | 26/55 | Reference | – | 22/55 | Reference | – |
| sFOXP3 | ||||||
| High | 7/35 | 0.34 (0.15–0.79) | 0.012 | 7/35 | 0.42 (0.18–1.00) | 0.049 |
| Low | 25/51 | Reference | – | 21/51 | Reference | – |
| sTIL density* | 0.74 (0.59–0.92) | 0.006 | 0.74 (0.58–0.93) | 0.010 | ||
| HER2-enriched | ||||||
| iCD8 | ||||||
| High | 5/28 | 0.34 (0.13–0.87) | 0.025 | 4 /28 | 0.41 (0.14–1.19) | 0.10 |
| Low | 28/62 | Reference | – | 22/62 | Reference | – |
| TNBC | ||||||
| sFOXP3 | ||||||
| High | 7/31 | 0.38 (0.17–0.87) | 0.021 | 6/31 | 0.35 (0.14–0.85) | 0.021 |
| Low | 29/60 | Reference | – | 27/60 | Reference | – |
*10% increments; significant results are shown in bold
With respect to breast cancer subtypes, none of the lymphocytic markers was prognostic in the luminal-A group. Favorable prognosticators for DFS were high total CD3, high sCD3, high iCD8 and higher sTIL density in patients with luminal-B tumors; high total CD3, high total CD8, high sCD8, high iCD8, high total FOXP3, high sFOXP3 and higher sTIL density in patients with luminal-HER2 tumors; high iCD8 in the HER2-enriched group; and high sFOXP3 in the TNBC group (Table 2). Most of these markers were also associated with reduced risk of death for the respective subtypes.
For multivariate analysis, in the entire cohort, the above significant lymphocytic subsets and sTIL density were initially examined as single markers upon adjustment for selected clinicopathological parameters. The favorable significance was retained for CD3 and CD8 markers, while it remained only as a trend for sFOXP3 (Supplementary Table S6). However, when sTIL density was included in the models, only a trend toward longer DFS was detected for sCD3 and iCD8, while sCD8 and the FOXP3 subsets were rendered insignificant; in comparison, high total counts/mm2 remained significantly favorable for CD3 and marginally so for CD8 (Table 3). Higher sTIL density for each 10% increment conferred 13–15% less risk of relapse over high sCD3, sCD8 and iCD8 and of death over total CD3. High total CD3 and high total CD8 conferred 35% and 28% lower risks of relapse, respectively. In the sFOXP3 model, neither this marker nor sTIL density remained independent prognosticators for DFS (Wald’s p = 0.26 and p = 0.18, respectively) or OS (p = 0.52 and p = 0.19, respectively). In comparison, nodal status and, occasionally, subtypes remained independently significant for DFS and OS (notes in Table 3).
Table 3.
Cox multivariate regression analysis for DFS and OS in the entire cohort and in patients with luminal-B tumors for the entire follow-up time
| Parameter | Events/total | HR (95% CI) | p value |
|---|---|---|---|
| Entire cohort | |||
| DFS | |||
| Model 1* | |||
| Stromal TIL density (10% increments) | 0.87 (0.77–0.97) | 0.013 | |
| sCD3 | |||
| High | 43/151 | 0.73 (0.51–1.04) | 0.077 |
| Low | 186/498 | Reference | – |
| Model 2** | |||
| Stromal TIL density (10% increments) | 0.85 (0.76–0.94) | 0.003 | |
| sCD8 | |||
| High | 54/175 | 0.83 (0.60–1.13) | 0.24 |
| Low | 228/572 | Reference | – |
| Model 3*** | |||
| Stromal TIL density (10% increments) | 0.86 (0.77–0.96) | 0.005 | |
| iCD8 | |||
| High | 54/184 | 0.75 (0.55–1.03) | 0.072 |
| Low | 228/563 | Reference | – |
| Model 4**** | |||
| Stromal TIL density (10% increments) | 0.93 (0.84–1.03) | 0.18 | |
| sFOXP3 | |||
| High | 41/147 | 0.80 (0.55–1.18) | 0.26 |
| Low | 172/463 | Reference | – |
| Model 5+ | |||
| Stromal TIL density (10% increments) | 0.88 (0.78–0.99) | 0.027 | |
| total CD3 | |||
| High | 40/153 | 0.65 (0.45–0.94) | 0.023 |
| Low | 189/496 | Reference | – |
| Model 6++ | |||
| Stromal TIL density (10% increments) | 0.86 (0.78–0.96) | 0.006 | |
| total CD8 | |||
| High | 50/176 | 0.72 (0.52–1.00) | 0.048 |
| Low | 232/571 | Reference | – |
| OS | |||
| Model 1≠ | |||
| Stromal TIL density (10% increments) | 0.93 (0.83–1.04) | 0.19 | |
| sFOXP3 | |||
| High | 33/147 | 0.87 (0.57–1.33) | 0.52 |
| Low | 136/463 | Reference | – |
| Model 2≠≠ | |||
| Stromal TIL density (10% increments) | 0.87 (0.77–0.99) | 0.035 | |
| total CD3 | |||
| High | 33/153 | 0.72 (0.48–1.09) | 0.12 |
| Low | 146/496 | Reference | – |
| Luminal-B tumors | |||
| DFS | |||
| Model 1a | |||
| Stromal TIL density (10% increments) | 1.00 (0.96–1.02) | 0.71 | |
| sCD3 | |||
| High | 11/51 | 0.42 (0.21–0.82) | 0.012 |
| Low | 73/172 | Reference | – |
| Model 2b | |||
| Stromal TIL density (10% increments) | 0.58 (0.33–1.02) | 0.058 | |
| iCD8 | |||
| High | 16/64 | 0.93 (0.62–1.40) | 0.74 |
| Low | 84/192 | Reference | – |
| Model 3c | |||
| Stromal TIL density (10% increments) | 1.02 (0.77–1.34) | 0.90 | |
| total CD3 | |||
| High | 8/50 | 0.26 (0.12–0.58) | < 0.001 |
| Low | 76/173 | Reference | – |
| OS | |||
| Model 4d | |||
| Stromal TIL density (10% increments) | 0.96 (0.69–1.32) | 0.78 | |
| total CD3 | |||
| High | 6/50 | 0.30 (0.12–0.72) | 0.007 |
| Low | 60/173 | Reference | – |
Significant results are shown in bold. Notes on independently significant clinicopathological parameters for each model: entire cohort: adjusting for menopausal status, tumor size, nodal status, histological grade, radiation therapy and subtypes, the following clinicopathological parameters retained their statistical significance (p < 0.050) in the respective multivariate analyses: DFS: *nodal status (p < 0.001), **nodal status (p < 0.001), subtypes (p = 0.040), ***nodal status (p < 0.001) and subtypes (p = 0.045), ****nodal status (p < 0.001),+nodal status (p < 0.001), + +nodal status (p < 0.001) and subtypes (p = 0.036). OS: ≠nodal status (p < 0.001), ≠≠nodal status (p < 0.001), tumor size (p = 0.034) and subtypes (p = 0.006). Luminal-B subgroup: Adjusting for menopausal status, tumor size, nodal status, histological grade and radiation therapy, the following clinicopathological parameters retained their statistical significance (p < 0.050) in the respective multivariate analyses: DFS:anone, bnodal status (p = 0.011), cnodal status (p = 0.044). OS:dnodal status (p = 0.031)
Multivariate models for breast cancer subtypes were hampered by small group sizes and by the limited number of events (the models were pursued only for patients with luminal-B tumors). High sCD3 and total CD3 remained significantly favorable for patients with luminal-B tumors for DFS, and total CD3 for OS, even after adjustment for sTIL density (Table 3).
Profiling of lymphocytic subsets
Hierarchical clustering of continuous stromal and intratumoral CD3, CD8 and FOXP3 values returned two distinct tumor groups. Tumors in cluster 1 exhibited low counts/mm2 for the profiled markers (all-low cluster), while those in cluster 2 exhibited high counts/mm2 for all markers (all-high cluster) (Fig. 3e). Among the 511 profiled tumors, 20.4% were grouped together in the all-high cluster 2. The associations of these clusters reflected those of single markers: the all-high cluster 2 was strongly associated with higher Ki67 labeling index (Wilcoxon rank-sum p < 0.001), high histological grade (chi-square p < 0.001) and HER2-positive subtypes and TNBC (p = 0.006). In comparison to patients with all-low tumors, patients with all-high tumors experienced longer DFS (Fig. 3f) and had lower risk of relapse (HR 0.61, 95%CI 0.41–0.93, Wald’s p = 0.020). No effect was noticed with this profiling for OS (p = 0.28). Upon adjustment for the clinicopathological parameters, the all-high cluster 2 lost its favorable prognostic significance for DFS (p = 0.41) along with sTIL density (p = 0.12). Nodal status was the only parameter that remained significant in this model (p < 0.001). Due to the very limited number of patients, subgroup analyses were not performed with the profiling results.
Discussion
The present study investigates markers of host anti-tumor immunity in breast carcinomas from patients who were treated with adjuvant anthracycline-based chemotherapy in two randomized trials and were followed up to almost 20 years. The main findings include: stromal CD3 and CD8 lymphocytic subsets as prevalent players in anti-tumor immunity compared to FOXP3 infiltrates; favorable prognostic impact of stromal and/or intratumoral CD3, CD8 and FOXP3 at high density, either as single markers or in profiles; and, importantly, prognostic redundancy of compartmentalized lymphocytic subsets upon adjustment for the morphologically assessed sTIL density, which is relevant to the translation of the present data in breast cancer diagnostics.
The study was performed on TMA cores using cell counts/mm2 for the individual phenotype subsets, which has recently been proposed as a valid method for the evaluation of lymphocytic subpopulations in breast cancer despite TMA limitations [19, 21]. B cells and their derivatives, i.e., plasma cells, were observed in few cases only, in association with tertiary lymphoid structures where these cells are primarily generated [8]. As previously described [3, 21], the bulk of the TILs was located in the stroma of breast carcinomas and the main TIL component was composed of sCD3+ T lymphocytes. The observed correlations between all examined subsets and sTIL density were similar to those previously reported [22]. As can be inferred from our data, despite that we did not address CD4+ cells for direct comparison, more than half of CD3+ cells were cytotoxic CD8+ cells. CD3+ and CD8+ infiltrates appeared interdependent, both in the stromal and the intratumoral compartment. This is in line with previously reported predominantly cytotoxic cells in tumor-infiltrating T lymphocytes [23]. However, it is rather contrary to the predominant helper T cells observed in carcinomas in situ [19, 21], reflecting the different properties of in situ and invasive tumors in summoning host defense. In line with previous reports, [21, 23, 19], FOXP3+ were underrepresented compared to CD3+ and CD8+ cells, in our series by more than one order of magnitude. Considering the immunosuppressive roles generally attributed to FOXP3 [7, 8], the low rates of such cells and the prevalence of cytotoxic CD8+ cells seem consistent with tumor-combating efforts by the host.
In fact, the endogenous anti-tumor attempt is reflected in all aspects of the non-specific sTIL and the more specific lymphocytic subset densities, as described and reviewed in the literature: tumors with high sTIL density were also high CD8+ [20]; all examined lymphocytic subsets tended to coexist in high numbers in both the stroma and the tumor cell area [21, 23]; all of them, individually and profiled, were associated with aggravating tumor characteristics, such as high grade and proliferation [24–26] and HER2-positive and triple-negative disease [23– 26]. Particularly with respect to breast cancer subtypes, the density of T cell subsets substantially differed between the low-proliferating luminal-A and the more aggressive HER2-positive tumors, including luminal-HER2. The latter featured the highest sCD3, sCD8 and sFOXP3 densities, irrespective of hormone receptor positivity, which appears to contradict the as yet largest review on T cell markers per subtype [27]. The problem is that in most studies dealing with the evaluation of immune cell markers in breast cancer subtypes, the method for subtype calling was not taken into account despite that, for example, clinical (IHC4) and gene expression (e.g., PAM50) HER2 subtypes were not directly comparable [28, 29]. Highly proliferating tumors, irrespective of ER/PgR or HER2 positivity, may attract T cells, cytotoxic T cells in particular, possibly because proliferation is related to higher levels of genomic aberrations and, therefore, to the production of neoantigens [30]. Perhaps, adding proliferation index to the known associations between TILs and breast cancer subtypes may aid in the development of algorithms for characterizing the status of host anti-tumor immune response, which needs to be taken into account in breast cancer therapeutics.
The prognostic implications of sTIL density and lymphocytic subsets have been adequately reviewed in the literature, although more often in the neoadjuvant than in the adjuvant setting [23, 31]. We have previously shown the favorable prognostic impact of sTIL density in adjuvantly treated patients in clinical trials by our group [18], which is here presented for patients treated in the pre-trastuzumab era (a part of the previously studied population). In extension of these findings, here we show that high density of sCD3, sCD8, iCD8 and sFOXP3, as well as high density of total CD3 and CD8, was favorable prognosticators in the adjuvant setting. However, compartmentalized stromal and intratumoral lymphocytic subsets did not offer prognostic information on sTIL density. This is consistent with previous reports on redundant lymphocytic markers in breast cancer prognosis, particularly concerning FOXP3 and CD8 (reviewed in [21, 23]). This redundancy may be explained if we consider the biology of the tumor immune contexture and the endless list of technical and methodology issues, some of which are discussed below.
From the biological aspect, none of the CD3, CD8 and FOXP3 immunophenotypes can be considered as a surrogate of the extreme heterogeneity and functional diversity of these lymphocytic populations in the tumor microenvironment, as they are deciphered by more recent methods, e.g., single-cell RNA sequencing [32] or proteomics [33], or with multiplex immunofluorescence on paraffin Sects. [34]. FOXP3 per se is a non-specific marker, since it may be expressed by several immune cell types [35, 36] and also by cancer cells [37], while its mRNA expression in breast cancer cells may be higher than in T-regs [38]. In addition, the different antibodies used for FOXP3IHC yield different staining patterns [11, 37, 39–41] and hence non-comparable results. FOXP3+ subsets may have distinct biological effects compared to CD3+ and CD8+, dependent on the hormone receptor status of breast cancer [42], as tangentially indicated by our results as well. However, without further dissection of the FOXP3 population, which may be considered a limitation of the present study, the respective biological ground cannot be adequately discussed.
Methodology issues for the assessment of intratumoral and stromal lymphocytes and their subsets may also contribute to contradictory or non-comparable results of the prognostic evaluation of lymphocytic subsets: for example, higher iCD8 counts may be a predictor for unfavourable [43] but higher iCD8 counts/mm2 for favorable prognosis, as in the present series. Counting stromal lymphocytes as intratumoral in tumors where this distinction is difficult will also yield misleading results. The design of multivariate analysis models may also impact the interpretation of each marker, as we have clearly shown here and as reported elsewhere [22, 23]. As can be inferred from both the present and our previous study examining the same lymphocytic subsets at the mRNA level [14], the results obtained by different methods for the same marker are not directly comparable. Lastly, almost all studies examining immunophenotypic markers on large tumor series are performed on TMAs, while sTIL density is assessed on whole sections. Apart from the extremes, i.e., tumors with 0–5% or > 50% sTIL density, tumors with 10–40% sTIL density may be heterogeneous for this marker [18, 20]; however, this heterogeneity is not reflected in the returned averaged value of sTIL density. Plausibly, the density of lymphocytic subsets may also be heterogeneous, as shown here, but this parameter could not be reliably assessed due to the selective core sampling for TMA construction.
We have also shown that assessing total rather than compartmentalized CD3 and CD8 density may add prognostic information independently of sTIL density in the adjuvant setting. Assessing lymphocytic subset counts within the entire tumor area alleviates the difficulties in stromal and intratumoral delineation, although we fully agree that it certainly dilutes the density of each lymphocytic subset and cannot be regarded as a solid marker on biological grounds (21). A novel information here concerns the independently favorable prognostic effect of CD3 in the hormone receptor positive, HER2-negative luminal-B tumors of higher proliferation, but not in luminal-A tumors of low proliferation. Unfortunately, the limited number of the remaining subtype groups and the very limited number of events precluded the interpretation of the examined markers in these subgroups.
In conclusion, this study confirms that in operable early stage breast carcinomas, lymphocytic infiltrates are mostly composed of T cells, the majority of which are cytotoxic T lymphocytes. Tumors with higher sTIL density tend to have higher numbers of stromal and intratumoral CD3+, CD8+ and FOXP3+ lymphocytes that are distributed, following the patterns of sTIL density, among breast carcinomas. The individual stromal and intratumoral lymphocytic subset markers may not offer prognostic information on sTIL density in the adjuvant setting, while the less specific and, perhaps, biologically irrelevant total CD3 and, occasionally, CD8 density may do so. The immune contexture in breast carcinomas and its assessment remain fields with too many unknowns, as discussed for respective biological and methodological limitations. In this context, it appears that less specific markers offer more information than the more specific but still partly understood ones. In this sense, introducing the morphological assessment of sTIL density in histology reports would be a first step toward the evaluation of host anti-tumor immune response on a broad basis, as widely recommended. The context of adding CD3 or CD8 IHC in the diagnostic routine of breast cancer still needs to be determined and validated.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are indebted to all patients and their families for their trust and participation in the Hellenic Cooperative Oncology Group trials and for the provision of biological material for research purposes. The authors would also like to thank Eneida Jaupaj for the collection of tissue samples and Maria Moschoni for data coordination.
Abbreviations
- DFS
Disease-free survival
- HER
Human epidermal growth factor receptor
- IHC
Immunohistochemistry
- mRNA
Messenger RNA
- OS
Overall survival
- TILs
Tumor-infiltrating lymphocytes
- TMA
Tissue microarray
- TNBC
Triple-negative breast cancer
- T-regs
T-regulatory cells
Author contributions
TK, VK, GAK, KTK and IK contributed to conceptualization. TK, VK, GAK and KM contributed to data analysis. TK, VK, SC, KC, AI and IK contributed to method implementation and evaluation. SC, GAK, KM and KTK contributed to material and data management. TK, FZ, MS, GP, APB, CC, GX, GZ, KP, EP, AK, HPK, DB, CM, VV, NA, IK, HG and GF collected resources (patient material and clinical data). TK, VK, GAK, KM, SC and GF prepared the original draft. All authors reviewed and edited the manuscript.
Funding
The study was supported by a research grant from Amgen Ltd. and by an internal Hellenic Cooperative Oncology Group (HeCOG) translational research grant (HE TRANS_BR). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Compliance with ethical standards
Conflict of interest
Pentheroudakis G: Advisory Role: Roche, Honoraria: Roche, Speaker bureau: Roche, Grants: Amgen; Christodoulou C: Advisory Role: Merck, Genesis Pharmaceuticals, Pfizer, Novartis, Roche, AstraZeneca, Bristol Myers Squibb. Honoraria: Roche, Bristol Myers Squibb. Travel: AstraZeneca, Sanofi; Koutras A: Advisory Role: Roche, Genesis, AstraZeneca. Travel: Novartis, Sanofi-Aventis, Astellas, Merck Serono, Bristol Myers Squibb, Pfizer, Genesis, Amgen. Gogas H: Advisory Role: Bristol-Myers Squibb, MSD Oncology, Amgen, Novartis, Roche, Pierre-Fabre, Honoraria: Bristol-Myers Squibb, MSD Oncology, Roche, Amgen, Novartis, Research Funding (institution): Bristol-Myers Squibb, Roche, MSD Oncology, Travel: Roche, Bristol-Myers Squibb; Fountzilas G: Advisory Board of Pfizer, Sanofi and Roche. Honoraria from Astra-Zeneca. The rest of the authors declare no conflict of interest.
Ethical approval
The present translational study was approved by the Bioethics Committee of the Aristotle University of Thessaloniki School of Medicine (#77/June 10, 2014) and by the Institutional Review Board of Papageorgiou Hospital of Thessaloniki (#725/May 10, 2013).
Informed consent
Patients had signed a study-specific written informed consent before randomization, which in addition to giving consent for the trial allowed for the use of biological material for future research purposes.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Triantafyllia Koletsa and Vassiliki Kotoula have contributed equally to this work.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Galon J, Angell HK, Bedognetti D, Marincola FM. The continuum of cancer immunosurveillance: prognostic, predictive, and mechanistic signatures. Immunity. 2013;39:11–26. doi: 10.1016/j.immuni.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 3.Salgado R, Denkert C, Demaria S, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an international TILs working group 2014. Ann Oncol. 2015;26:259–271. doi: 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denkert C, Loibl S, Noske A, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clinical Oncol Off J Am Soc Clin Oncol. 2010;28:105–113. doi: 10.1200/JCO.2009.23.7370. [DOI] [PubMed] [Google Scholar]
- 5.Dieci MV, Criscitiello C, Goubar A, et al. Prognostic value of tumor-infiltrating lymphocytes on residual disease after primary chemotherapy for triple-negative breast cancer: a retrospective multicenter study. Ann Oncol Off J Eur Soc Med Oncol ESMO. 2014;25:611–618. doi: 10.1093/annonc/mdt556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salgado R, Denkert C, Demaria S, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an international TILs working group 2014. Ann Oncol Off J Eur Soc Med Oncol ESMO. 2015 doi: 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varn FS, Mullins DW, Arias-Pulido H, Fiering S, Cheng C. Adaptive immunity programmes in breast cancer. Immunology. 2017;150:25–34. doi: 10.1111/imm.12664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gu-Trantien C, Loi S, Garaud S, et al. CD4(+) follicular helper T cell infiltration predicts breast cancer survival. J Clin Investig. 2013;123:2873–2892. doi: 10.1172/JCI67428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ali HR, Provenzano E, Dawson SJ, et al. Association between CD8+ T-cell infiltration and breast cancer survival in 12,439 patients. Ann Oncol. 2014;25:1536–1543. doi: 10.1093/annonc/mdu191. [DOI] [PubMed] [Google Scholar]
- 10.Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Lee AH, Ellis IO, Green AR. An evaluation of the clinical significance of FOXP3+ infiltrating cells in human breast cancer. Breast Cancer Res Treat. 2011;127:99–108. doi: 10.1007/s10549-010-0987-8. [DOI] [PubMed] [Google Scholar]
- 11.West NR, Kost SE, Martin SD, Milne K, Deleeuw RJ, Nelson BH, Watson PH. Tumour-infiltrating FOXP3(+) lymphocytes are associated with cytotoxic immune responses and good clinical outcome in oestrogen receptor-negative breast cancer. Br J Cancer. 2013;108:155–162. doi: 10.1038/bjc.2012.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rathore AS, Kumar S, Konwar R, Srivastava AN, Makker A, Goel MM. Presence of CD3+ tumor infiltrating lymphocytes is significantly associated with good prognosis in infiltrating ductal carcinoma of breast. Indian J Cancer. 2013;50:239–244. doi: 10.4103/0019-509X.118744. [DOI] [PubMed] [Google Scholar]
- 13.Castaneda CA, Mittendorf E, Casavilca S, et al. Tumor infiltrating lymphocytes in triple negative breast cancer receiving neoadjuvant chemotherapy. World J Clin Oncol. 2016;7:387–394. doi: 10.5306/wjco.v7.i5.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsiatas M, Kalogeras KT, Manousou K, et al. Evaluation of the prognostic value of CD3, CD8, and FOXP3 mRNA expression in early-stage breast cancer patients treated with anthracycline-based adjuvant chemotherapy. Cancer Med. 2018;7:5066–5082. doi: 10.1002/cam4.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fountzilas G, Skarlos D, Dafni U, et al. Postoperative dose-dense sequential chemotherapy with epirubicin, followed by CMF with or without paclitaxel, in patients with high-risk operable breast cancer: a randomized phase III study conducted by the Hellenic Cooperative Oncology Group. Ann Oncol. 2005;16:1762–1771. doi: 10.1093/annonc/mdi366. [DOI] [PubMed] [Google Scholar]
- 16.Fountzilas G, Dafni U, Gogas H, et al. Postoperative dose-dense sequential chemotherapy with epirubicin, paclitaxel and CMF in patients with high-risk breast cancer: safety analysis of the Hellenic Cooperative Oncology Group randomized phase III trial HE 10/00. Ann Oncol. 2008;19:853–860. doi: 10.1093/annonc/mdm539. [DOI] [PubMed] [Google Scholar]
- 17.Fountzilas G, Dafni U, Bobos M, et al. Differential response of immunohistochemically defined breast cancer subtypes to anthracycline-based adjuvant chemotherapy with or without paclitaxel. PLoS ONE. 2012;7:e37946. doi: 10.1371/journal.pone.0037946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kotoula V, Chatzopoulos K, Lakis S, et al. Tumors with high-density tumor infiltrating lymphocytes constitute a favorable entity in breast cancer: a pooled analysis of four prospective adjuvant trials. Oncotarget. 2016;7:5074–5087. doi: 10.18632/oncotarget.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beguinot M, Dauplat MM, Kwiatkowski F, Lebouedec G, Tixier L, Pomel C, Penault-Llorca F, Radosevic-Robin N. Analysis of tumour-infiltrating lymphocytes reveals two new biologically different subgroups of breast ductal carcinoma in situ. BMC cancer. 2018;18:129. doi: 10.1186/s12885-018-4013-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hendry S, Salgado R, Gevaert T, et al. Assessing tumor-infiltrating lymphocytes in solid tumors: a practical review for pathologists and proposal for a standardized method from the international immunooncology biomarkers working group: Part 1: assessing the host immune response, tils in invasive breast carcinoma and ductal carcinoma in situ, metastatic tumor deposits and areas for further research. Adv Anat Pathol. 2017;24:235–251. doi: 10.1097/PAP.0000000000000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dieci MV, Radosevic-Robin N, Fineberg S, et al. Update on tumor-infiltrating lymphocytes (TILs) in breast cancer, including recommendations to assess TILs in residual disease after neoadjuvant therapy and in carcinoma in situ: a report of the international immuno-oncology biomarker working group on breast cancer. Semin Cancer Biol. 2018;52:16–25. doi: 10.1016/j.semcancer.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Bottai G, Raschioni C, Losurdo A, et al. An immune stratification reveals a subset of PD-1/LAG-3 double-positive triple-negative breast cancers. Breast Cancer Res. 2016;18:121. doi: 10.1186/s13058-016-0783-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pruneri G, Vingiani A, Denkert C. Tumor infiltrating lymphocytes in early breast cancer. Breast. 2018;37:207–214. doi: 10.1016/j.breast.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 24.Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, Ellis IO, Green AR. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol: Off J Am Soc Clin Oncol. 2011;29:1949–1955. doi: 10.1200/JCO.2010.30.5037. [DOI] [PubMed] [Google Scholar]
- 25.Loi S. Tumor-infiltrating lymphocytes, breast cancer subtypes and therapeutic efficacy. Oncoimmunology. 2013;2:e24720. doi: 10.4161/onci.24720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stanton SE, Adams S, Disis ML. Variation in the incidence and magnitude of tumor-infiltrating lymphocytes in breast cancer subtypes: a systematic review. JAMA Oncol. 2016 doi: 10.1001/jamaoncol.2016.1061. [DOI] [PubMed] [Google Scholar]
- 27.Stanton SE, Adams S, Disis ML. variation in the incidence and magnitude of tumor-infiltrating lymphocytes in breast cancer subtypes: a systematic review. JAMA Oncol. 2016;2:1354–1360. doi: 10.1001/jamaoncol.2016.1061. [DOI] [PubMed] [Google Scholar]
- 28.Bastien RR, Rodriguez-Lescure A, Ebbert MT, et al. PAM50 breast cancer subtyping by RT-qPCR and concordance with standard clinical molecular markers. BMC Med Genom. 2012;5:44. doi: 10.1186/1755-8794-5-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prat A, Carey LA, Adamo B, Vidal M, Tabernero J, Cortes J, Parker JS, Perou CM, Baselga J. Molecular features and survival outcomes of the intrinsic subtypes within HER2-positive breast cancer. J Natl Cancer Inst. 2014 doi: 10.1093/jnci/dju152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thorsson V, Gibbs DL, Brown SD, et al. The immune landscape of cancer. Immunity. 2018;48(812–30):e14. doi: 10.1016/j.immuni.2018.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Savas P, Salgado R, Denkert C, Sotiriou C, Darcy PK, Smyth MJ, Loi S. Clinical relevance of host immunity in breast cancer: from TILs to the clinic. Nature reviews Clin Oncol. 2016;13:228–241. doi: 10.1038/nrclinonc.2015.215. [DOI] [PubMed] [Google Scholar]
- 32.Zhang L, Zhang Z. recharacterizing tumor-infiltrating lymphocytes by single-cell RNA sequencing. Cancer Immunol Res. 2019;7:1040–1046. doi: 10.1158/2326-6066.CIR-18-0658. [DOI] [PubMed] [Google Scholar]
- 33.Wagner J, Rapsomaniki MA, Chevrier S, et al. A single-cell atlas of the tumor and immune ecosystem of human breast cancer. Cell. 2019;177(1330–45):e18. doi: 10.1016/j.cell.2019.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parra ER, Uraoka N, Jiang M, et al. Validation of multiplex immunofluorescence panels using multispectral microscopy for immune-profiling of formalin-fixed and paraffin-embedded human tumor tissues. Sci Rep. 2017;7:13380. doi: 10.1038/s41598-017-13942-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roncador G, Brown PJ, Maestre L, et al. Analysis of FOXP3 protein expression in human CD4+CD25+ regulatory T cells at the single-cell level. Eur J Immunol. 2005;35:1681–1691. doi: 10.1002/eji.200526189. [DOI] [PubMed] [Google Scholar]
- 36.Vadasz Z, Toubi E. FoxP3 Expression in macrophages, cancer, and B cells-is it real? Clin Rev Allerg Immunol. 2017;52:364–372. doi: 10.1007/s12016-016-8572-5. [DOI] [PubMed] [Google Scholar]
- 37.Takenaka M, Seki N, Toh U, et al. FOXP3 expression in tumor cells and tumor-infiltrating lymphocytes is associated with breast cancer prognosis. Mol Clin Oncol. 2013;1:625–632. doi: 10.3892/mco.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karanikas V, Speletas M, Zamanakou M, Kalala F, Loules G, Kerenidi T, Barda AK, Gourgoulianis KI, Germenis AE. Foxp3 expression in human cancer cells. J Transl Med. 2008;6:19. doi: 10.1186/1479-5876-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, Harris AL, Banham AH. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol Off J Am Soc Clin Oncol. 2006;24:5373–5380. doi: 10.1200/JCO.2006.05.9584. [DOI] [PubMed] [Google Scholar]
- 40.Droeser RA, Obermann EC, Wolf AM, Wallner S, Wolf D, Tzankov A. Negligible nuclear FOXP3 expression in breast cancer epithelial cells compared with FOXP3-positive T cells. Clin Breast Cancer. 2013;13:264–270. doi: 10.1016/j.clbc.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 41.Zhang C, Xu Y, Hao Q, et al. FOXP3 suppresses breast cancer metastasis through downregulation of CD44. Int J Cancer. 2015;137:1279–1290. doi: 10.1002/ijc.29482. [DOI] [PubMed] [Google Scholar]
- 42.Qian F, Qingping Y, Linquan W, Xiaojin H, Rongshou W, Shanshan R, Wenjun L, Yong H, Enliang L. High tumor-infiltrating FoxP3(+) T cells predict poor survival in estrogen receptor-positive breast cancer: a meta-analysis. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2017;43:1258–1264. doi: 10.1016/j.ejso.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 43.Catacchio I, Silvestris N, Scarpi E, Schirosi L, Scattone A, Mangia A. Intratumoral, rather than stromal, CD8+ T cells could be a potential negative prognostic marker in invasive breast cancer patients. Transl Oncol. 2019;12:585–595. doi: 10.1016/j.tranon.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.