Abstract
In view of the relatively limited efficacy of immunotherapies targeting the PD-1–PD-L1 axis in triple-negative breast cancer (TNBC) and of published reports on tumor-promoting roles of TNFR2+ tumor-infiltrating lymphocytes (TNFR2+ TILs), we determined the incidence of TNFR2+ TILs in TNBC patient tumors, their association with disease outcome and relations with PD-1+ TILs. Using a cohort of treatment-naïve TNBC patients with long follow-up (n = 70), we determined the presence of TNFR2+ TILs and PD-1+ TILs by immunohistochemistry. TILs (≥ 1% of cellular mass) and TNFR2+ TILs (≥ 1% of total TILs) were detected in 96% and 74% of tumors, respectively. The presence of TILs at > 5% of tumor cell mass (“Positive TILs”), as well as of positive TNFR2+ TILs (> 5%), was independently associated with good prognosis, and combination of both parameters demonstrated superior outcome relative to their lower levels. PD1+ TILs (> 5/hot spot) were detected in 63% of patients. High levels of PD-1+ TILs (> 20/hot spot) showed an unfavorable disease outcome, and in their presence, the favorable outcome of positive TNFR2+ TILs was ablated. Thus, TNFR2+ TILs are strongly connected to improved prognosis in TNBC; these findings suggest that TNFR2+ TILs have favorable effects in TNBC patients, unlike the tumor-promoting roles attributed to them in other cancer systems. Overall, our observations propose that the TNFR2+ TIL subset should not be targeted in the course of TNBC therapy; rather, its beneficial impacts may become into power when anti-PD-1 regimens—that may potentiate immune activities—are administered to TNBC patients.
Electronic supplementary material
The online version of this article (10.1007/s00262-020-02549-0) contains supplementary material, which is available to authorized users.
Keywords: Programmed cell death protein 1 (PD-1), Triple-negative breast cancer (TNBC), Tumor-infiltrating lymphocytes (TILs), Tumor necrosis factor receptor 2 (TNFR2)
Introduction
The poor clinical outcome of triple-negative breast cancer patients (TNBC; referred to as “basal/basal-like” in genomic analyses) [1, 2] has put forward the need to identify novel therapeutic modalities in this aggressive disease subtype. In this context, immune checkpoint blockades (ICBs)—mainly those targeting the immune checkpoint programmed cell death protein 1 (PD-1) and its ligand (PD-L1)—have been recently considered and introduced in therapy of TNBC patients [3–6]; however, the relatively limited success of ICBs in TNBC suggests that complex immune mechanisms, act at the tumor site, having a strong impact on immune activation.
The presence of tumor-infiltrating lymphocytes (TILs) in TNBC patient tumors is speculated to attest for potential anti-tumor activities that took place at the beginning of the malignancy process, and accordingly, they were substantially associated with improved survival ([7–9], and more). Suppression of such activities at later stages due to inhibitory immune checkpoints may lead to recurrence and poor prognosis in TNBC patients. However, so far non-conclusive findings were described on the associations of PD-1+ TILs with prognosis in TNBC (e.g., [10–13]). These findings may reflect the dynamic nature of the immune contexture in the tumors: The expression of PD-1 by TILs may indicate that they have been activated; however, PD-1 expression by TILs may indicate that these cells are already exhausted or have been immune-suppressed by their interactions with PD-L1, expressed by the tumor cells or most importantly, by immune cells [6, 14–16].
In view of these findings, there is a great need to better identify the roles of different TIL subsets that reside in TNBC tumors and their relations with PD-1-expressing TILs. Accordingly, we were interested to explore the presence and clinical relevance of a T cell subpopulation that expresses TNFR2, one of the two receptors of tumor necrosis factor α (TNFα), to TNBC. TNFα itself was strongly and causatively connected to poor prognosis in many malignancies including TNBC [17–19]. Accordingly, TNFα and its two receptors, TNFR1 and TNFR2, were proposed as potential targets for therapy in cancer [20–22]. Of the two receptors, TNFR2 may be an ideal target for therapy because its expression by T cells was connected to increased malignancy in several tumor systems, and it has a restricted expression pattern [22–28]. However, TNFR2 is subject to complex regulatory modes: It is expressed by several T cell subsets, it is activated mainly by membranous but also by soluble TNFα, and it is expressed in a secreted form that regulates immune activities [23, 26, 29–32]. These findings raise the need to carefully determine the contribution of TNFR2-expressing TILs to disease course in cancer in general, and in the context of this research, to TNBC progression.
Thus, in this study, we determined the contents of TNFR2+ TILs and their association with patient survival in a TNBC cohort. Tumors were obtained from treatment-naïve patients, many of which having a long follow-up time. Thus, our analyses reflected the lymphocyte landscape before any treatment could have modified the equilibrium between immune subsets, and the long follow-up time enabled us to have a broader view of the relevance of TNFR2+ TILs to disease progression. Our research provides novel findings demonstrating a significant association of TNFR2+ TILs with improved TNBC patient survival, which was abrogated in tumors containing high levels of PD-1+ TILs.
Therefore, in contrast to reports in other cancer types suggesting that TNFR2+ TILs should be abrogated as a measure of cancer therapy, our findings propose that in TNBC the TNFR2+ TIL subset should be kept intact; particularly, when anti-PD-1 therapies are administered to TNBC patients, it is possible that the overall beneficial impact of TNFR2+ TILs on disease progression may become stronger.
Materials and method
Immunohistochemistry (IHC)
The study included a retrospective cohort of 70 adjuvant-treated TNBC patients (clinicopathological characteristics are provided in Table 1). ASCO/CAP guidelines were followed to determine TNBC status, confirmed by board-certified pathologists. Formalin-fixed paraffin-embedded tumor sections (4 µm) were stained by hematoxylin and eosin (H&E). The expression levels of estrogen receptors, progesterone receptors and HER2 by tumor cells were determined by antibodies used in routine diagnosis hospital tests. PD-1 expression was determined by antibody clone NAT105 (Cell Marque, Rocklin, CA), which is widely used in the clinic. TNFR2 expression was determined by Novus Biological antibodies (Cat# NBP1-88139; Littleton, CO) that demonstrated high specificity in protein arrays (based on Company’s data) and was compared at study setup stage to a non-relevant isotype-matched control (Data not shown). Tonsil and kidney tissues were used as positive controls for PD-1 and TNFR2 staining, respectively. CC1 antigen retrieval solution (Ventana) was used for heat-induced antigen retrieval in alkaline conditions followed by counterstaining with hematoxylin solution. Staining patterns were detected by DAB detection system (Ventana).
Table 1.
Clinicopathological characteristics of TNBC patients
| Patient number | n = 70 |
| Age (years) | Mean 53; range 29–85 |
| Median follow-up time (years) | Median 4.85; range 0–20 |
| Number (n) | Percent (%) | |
|---|---|---|
| Histologic type | ||
| IDC | 64 | 91.4 |
| Metaplastic | 6 | 8.6 |
| Tumor stage | ||
| pT1 | 39 | 55.7 |
| pT2 | 27 | 38.6 |
| pT3 | 4 | 5.7 |
| Lymph node involvement | ||
| pN0 | 48 | 68.6 |
| pN1 | 19 | 27.1 |
| pNx | 3 | 4.3 |
| Tumor grade | ||
| 2 | 9 | 12.9 |
| 2–3 | 8 | 11.4 |
| 3 | 51 | 72.8 |
| ND | 2 | 2.9 |
The table provides information on the clinicopathological characteristics of TNBC tumors included in the current study
IDC Invasive ductal carcinoma, ND not determined
Determination of TIL localization and staining patterns
This stage was performed by certified breast pathologists of the Sheba Medical Center, accompanied by research coordinators from the Cancer Research Center, in a blind manner. H&E staining was used to assess TIL percentages out of the entire biopsy cell mass, according to recommendations of the “International TILs Working Group” [33]. TNFR2+ TILs and PD-1+ TILs were envisioned in high power field (HPF) view (× 400). Generally, TILs demonstrating membranous/cytoplasmic-granular TNFR2 expression were dispersed in the entire area of biopsies, and their percentage out of total TILs in the specimen was determined. PD-1+ TILs were relatively sparse; however, in some tumors they were uncountable; to avoid the impact of tumor size on the results, and in view of the fact that PD-1+ TILs were localized in defined hot spot areas, they were numbered in hot spots using HPF view of the entire biopsy (range 1–28 hot spots/biopsy) and the data presented demonstrate the maximal number of PD-1+ TILs in hot spots, in each patient. This approach agrees with other studies in which lymphocyte numbers were assessed in defined biopsy areas (particularly in relatively rare populations such as PD-1+ TILs) [8, 10, 11, 13, 34, 35].
Statistical analyses
Statistical analysis of the 70 patient cohort data was performed using MATLAB® and Statistics Toolbox Release 2016b, The MathWorks, Inc., and the LogRank package by Cardillo G. (2008 version) for the LogRank test (mathworks/fileexchange/22317). Kaplan–Meier analyses were used to determine survival outcomes, where groups were compared by LogRank statistics. Overall survival (OS) was defined as the time from diagnosis to death of any cause. Recurrence-free survival (RFS) was defined as the time from diagnosis to any recurrence or death of any cause. Univariate Cox regression was used to determine the impact of different parameters on survival. p ≤ 0.05 was considered significant.
Analyses of METABRIC patient dataset
An authorized METABRIC patient dataset version [36] provided information on gene expression levels and clinical characteristics of 331 basal patients, classified according to the PAM50 annotation file of the dataset. Low cellularity specimens contained less than 40% tumor DNA. TNFRSF1B (TNFR2) probe: ILMN_1764788. Associations with survival were depicted by Kaplan–Meier plots, where p values were calculated by Gehan–Breslow–Wilcoxon test. p ≤ 0.05 was considered significant.
Results
TIL levels are significantly associated with improved survival in TNBC patient tumors
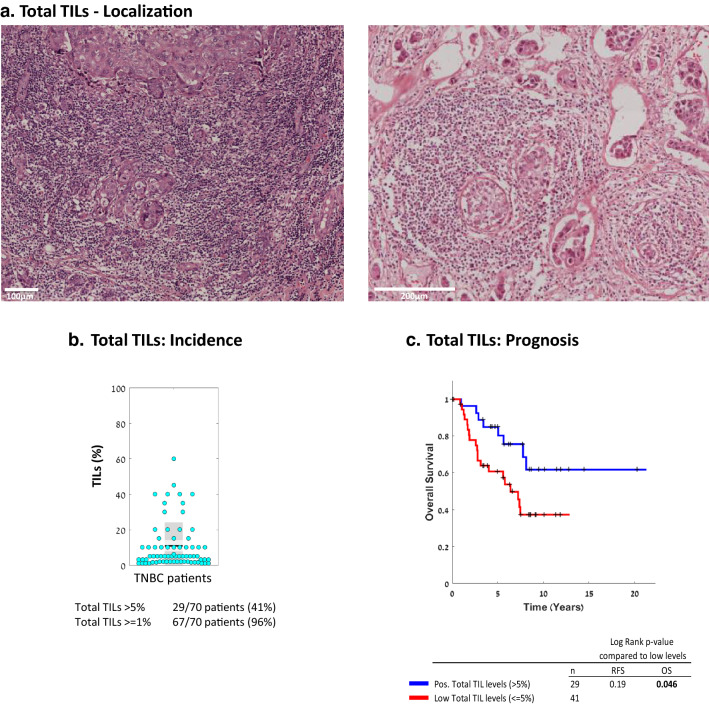
First, we determined the extent of TILs presence in 70 primary tumor samples of treatment-naïve TNBC patients with relatively long follow-up, of up to 20 years. In most TNBC patient tumors (96%), the levels of TILs were ≥ 1% of the cell mass in the tumors, and in 41% of the patients, they were > 5% (Fig. 1a, b). In line with other studies in the field, the 5% TIL level was used as cutoff above which tumors were considered positive for TIL presence. When TILs consisted > 5% of the cell mass in the biopsy, their presence was significantly associated with improved patient OS (p value 0.046; HR = 0.44 [CI 0.21–0.91]) (Fig. 1c; RFS plot is demonstrated in Supplementary Fig. 1a). Lymphocytes were located in adjacent normal tissues in only 16% of the patients (data not shown).
Fig. 1.
The presence of TILs in TNBC patient tumors is significantly associated with improved survival. The presence of TILs was determined in the 70-patient TNBC cohort used in our study. a Representative images of TIL localization in two patient tumors, demonstrated by H&E staining. b Percentage of TILs in each patient tumor (=dot), out of the total cellular tumor mass. Black line, Mean; Light gray box, Standard deviation; Dark grey box, SEM at 95% confidence interval. c OS Kaplan-Meier plot comparing patients with “Positive” (> 5%) vs. “Low” (≤ 5%) TIL levels. The corresponding RFS Kaplan-Meier plot is provided in Supplementary Figure 1a. +, Censored. p values of OS and RFS analyses are provided in the respective Figures
The presence of TNFR2+ TILs in TNBC patient tumors is significantly associated with good prognosis
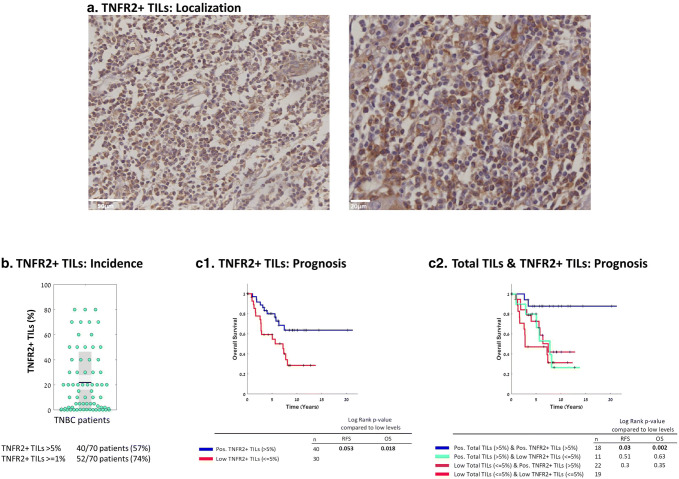
TNFR2+ TILs in TNBC patient tumors had a dispersed localization at the entire tumor area (Fig. 2a). In 74% and 57% of the tumors, the total TIL population included at least 1% and > 5% TNFR2+ TILs out of the total TIL mass, respectively (Fig. 2b). Many tumors had small incidence of TNFR2+ TILs in the total TIL population, and others had up to 80% of TNFR2+ TILs (Fig. 2b). Notably, although many tumors contained only low percentages of TNFR2+ TILs, this lymphocyte subset was significantly associated with better OS in analyses of the entire patient cohort, comparing patients having > 5% TNFR2+ TILs and patients with TNFR2+ levels ≤ 5% (Fig. 2c1; OS, p value 0.018; HR = 0.17 [CI 0.06–0.45]; TNFR2+ TILs were close to significantly associated with RFS (p value 0.053; HR = 0.29 [CI 0.12–0.7]); RFS plot is demonstrated in Supplementary Fig. 1a). Moreover, TNFR2+ TILs were significantly associated with better OS also when they were analyzed at additional cutoffs: p value 0.037 when the 10% cutoff was used, and p value 0.048 when the 15% cutoff was determined (data not shown).
Fig. 2.
TNFR2+ TILs are present in the majority of TNBC patient tumors, and are significantly associated with improved survival. a Representative images of TNFR2+ TILs of two patient tumors, demonstrated by IHC. b Percentages of TNFR2+ TILs in each patient tumor (=dot), out of the total TIL mass. Graph parameters are as in Fig. 1. c OS Kaplan-Meier plots comparing patients with TNFR2+ TILs at levels determined “Positive” (Pos; > 5%) vs. “Low” (≤ 5%) (c1), and comparing different combinations of Total TILs & TNFR2+ TILs (c2). The RFS Kaplan-Meier plot corresponding to Part c1 is provided in Supplementary Figure 1b. +, Censored. p values of OS and RFS analyses are provided in the respective Figures
As these findings on TNFR2+ TILs are the first to be reported in TNBC, we asked whether similar associations could be envisioned in other patient cohorts analyzing TNBC/basal patients. Using the METABRIC dataset, which included 331 basal patients, we noted significant associations of high TNFR2 levels with better patient survival (Supplementary Fig. 2) in two analyses: The first analysis used tumors of the whole patient cohort (n = 331; p value 0.0247), and the second analysis used tumors enriched for components of tumor microenvironment, such as immune cells (n = 56; p value 0.04). Altogether, these findings support the IHC cohort results, connecting the presence of TNFR2+ TILs with good prognosis in TNBC patients.
To follow-up on the fact that total TILs and TNFR2+ TILs were each independently significantly associated with improved patient survival (Figs. 1c and 2c1, respectively), we performed subgroup analysis, uncoupling the effect of each parameter. We found that the survival of patients having > 5% total TIL infiltrates that contained > 5% TNFR2+ TILs was superior over low levels of both (≤ 5% total TILs and ≤ 5% TNFR2+ TILs (Fig. 2c2) (OS, p value 0.002; HR = 0.15 [CI 0.05–0.45] and RFS, p value 0.03; HR = 0.31 [CI 0.12–0.8]). Notably, the other two combinations—either > 5% total TILs and low TNFR2+ TILs (≤ 5%) or ≤ 5% total TILs and > 5% TNFR2+ TILS—did not provide better outcome over the combination of their lower levels (≤ 5% total TILs and ≤ 5% TNFR2+ TILs) (Fig. 2c2). These findings suggest that the beneficial effects of TILs on survival depend on substantial presence of TNFR2+ TILs in the tumors.
In TNBC patient tumors, prognosis is connected to the extent of PD-1+ TILs located in the tumors
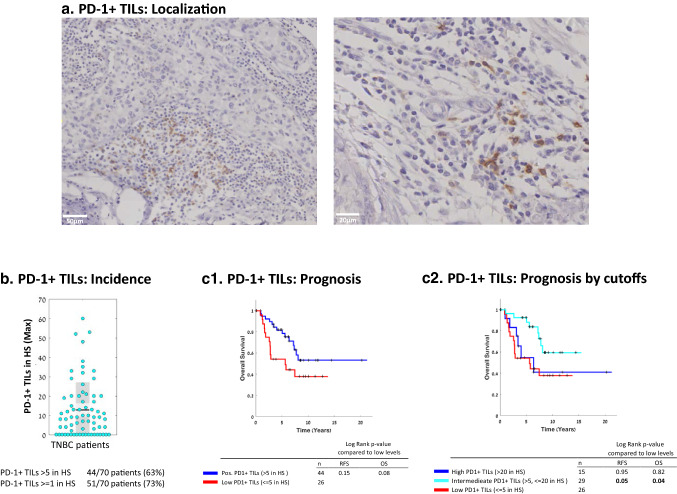
Next, in view of our interest in identifying the interplay between immune-regulating lymphocyte subsets, we determined the associations of PD-1+ TILs with survival in our TNBC cohort, and their relationships with TNFR2+ TILs. As reported by others (e.g., [13, 16, 37]), we observed that PD-1+ TILs were relatively sparse and were mostly localized in defined hot spots (HS) (Fig. 3a; more information on hot spots is provided in “Materials and method”). PD-1+ TILs (≥ 1/HS) were detected in 73% of TNBC patients included in our cohort, and 63% of the patients had > 5 PD-1+ TILs/HS (Fig. 3b).
Fig. 3.
PD-1+ TILs are present in the majority of TNBC patient tumors, and are connected to patient survival in a level-dependent manner. a Representative images of PD-1+ TILs (localized at hotspots, HS) of two patient tumors, determined by IHC. b Maximal numbers of PD-1+ TILs/HS in each patient tumor (=dot). Graph parameters are as in Fig. 1. c OS Kaplan-Meier plots comparing patients with PD-1+ TILs at levels determined “Positive” (Pos; > 5/HS) vs. “Low” (≤ 5/HS) (c1), and comparing patients with PD-1+ TILs at different cutoffs (c2). The RFS Kaplan-Meier plot corresponding to Part c1 is provided in Supplementary Figure 1c. +, Censored. Inter Intermediate; HS Hotspot. p values of OS and RFS analyses are provided in the respective Figures
Analysis of the associations between PD-1+ TILs and patient survival revealed that the prognostic outcome tended to be better when the tumors included > 5 PD-1+ TILs/HS, but the difference was not statistically significant (OS, p value 0.08 and RFS, p value 0.15) (Fig. 3c1; RFS plot is demonstrated in Supplementary Fig. 1c). However, when the cohort was partitioned according to different PD-1+ cutoffs, we found that the associations of PD-1+ TILs with survival were influenced by their incidence in the tumors. Specifically, in patients with low PD-1+ TILs (≤ 5/HS) and with high PD-1+ TILs (> 20/HS) survival rates were low, whereas significantly improved survival was noted in tumor containing PD-1+ TILs at intermediate levels (> 5, ≤ 20/HS) (OS, p value = 0.04; HR = 0.37 [CI 0.16–0.87] and RFS, p value 0.05) (Fig. 3c2). These findings suggest that PD-1+ TILs at intermediate levels represent T cells that have been activated, a process followed by elevated PD-1 expression levels; such a condition may contribute to improved patient survival, whereas high levels of PD-1+ TILs may include T cells that have already transitioned toward exhaustion/immune suppression and thus are connected to poor prognosis, as will be discussed below.
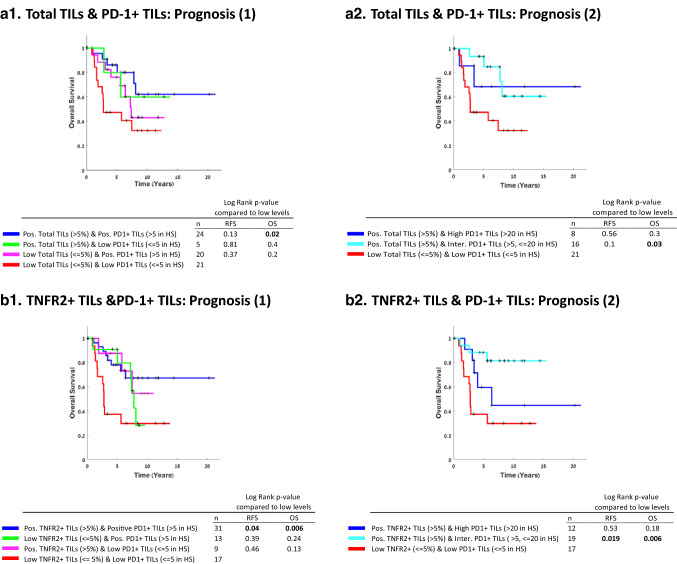
Further evidence of a dynamic and mixed phenotype of PD-1+ TILs in TNBC tumors was provided when a subgroup analysis was performed to determine the relations between the presence of TILs in general (total TILs) and of PD-1+ TILs in the tumors. Figure 4a1, a2 demonstrates that the beneficial effects of TIL presence in TNBC tumors were influenced by the extent of PD-1+ TILs in the tumors. In tumors that had TIL infiltrates at > 5% levels with PD-1+ TILs at intermediate levels (> 5, ≤ 20/HS), but not at high levels (> 20/HS), patient survival was significantly better compared to tumors containing low levels of TIL infiltrates (≤ 5%) with low levels of PD-1+ TILs (≤ 5/HS) (OS, p value 0.03; HR = 0.3 [CI 0.11–0.8]) (Fig. 4a2). These findings suggest that it is important that the TIL population will include PD-1+ TILs at levels that may reflect an active state that can promote anti-tumor activities, as in the intermediate levels detected in our study.
Fig. 4.
The beneficial impact of TNFR2+ TILs on survival of TNBC patients depends on levels of PD1+ TILs. OS Kaplan-Meier plots comparing patients based on combinations of Total TILs & PD-1+ TILs, at different cutoffs (a), and comparing patients based on combinations of TNFR2+ TILs & PD-1+ TILs, at different cutoffs (b). +, Censored. Pos Positive; Inter Intermediate; HS Hotspot. p values of OS and RFS analyses are provided in the Figure
Unfavorable levels of PD-1+ TILs counteract the favorable effects of TNFR2+ TILs on disease outcome
The dynamic nature of PD-1+ TILs was then questioned in the context of TNFR2+ TILs, which on their own were significantly associated with improved clinical outcome (Fig. 2c). Uncoupling the effect of each parameter (Fig. 4b1) showed that only combination of both positive TNFR2+ and PD-1+ TILs (> 5% for both) resulted in better survival compared to combined presence of these parameters at low levels (≤ 5 for both) (OS, p value = 0.006, HR = 0.21 [CI 0.07–0.58] and RFS, p = 0.04, HR = 0.34 [CI 0.13–0.87]). Importantly, subgroup analysis revealed that combined presence of TNFR2+ TILs with intermediate—but not high—levels of PD-1+ TILs (TNFR2+ TILs > 5% & PD-1+ TILs > 5, ≤ 20/HS), demonstrated significantly better prognosis than the presence of low TNFR2+ TILs at ≤ 5% levels, combined with low levels of PD-1+ TILs (≤ 5/HS) (OS, p value 0.006; HR = 0.19 [CI 0.06–0.57] and RFS, p value 0.019; HR = 0.26 [CI 0.09–0.71]) (Fig. 4b2). These results suggest that the beneficial activities of TNFR2+ TILs could be strengthened when PD-L1+ TILs were still at an active state (intermediate levels), but were ablated when PD-1+ TILs were present at unfavorable levels.
Hazard ratio analysis indicates that TNFR2+ TILs are a protective element in TNBC patients
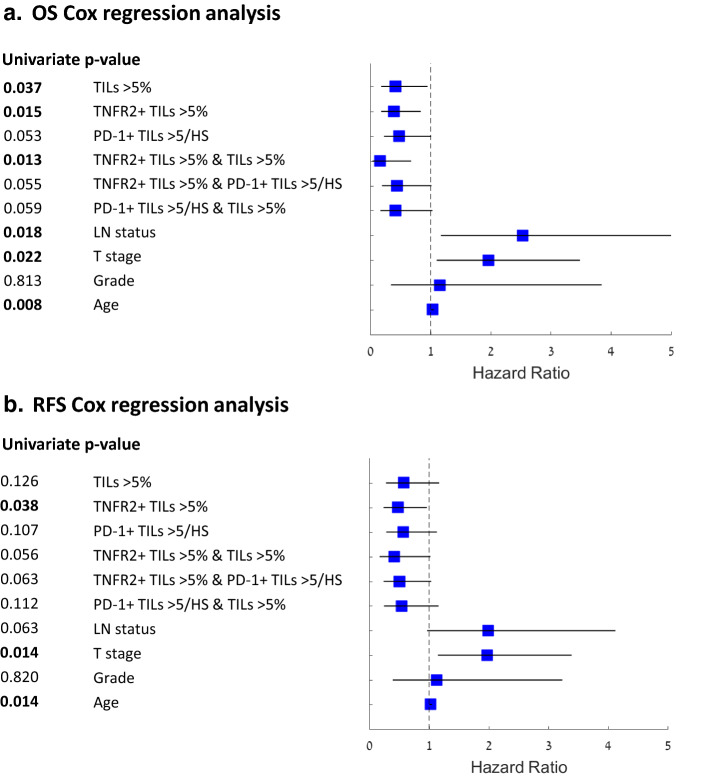
In parallel to Pearson correlation analysis, demonstrating that all the tested clinical parameters were independent (data not shown), univariate Cox proportional hazards regression analysis was performed in order to determine whether any of the above TIL populations may have a protective role in TNBC progression, relative to other parameters known to influence prognosis (Fig. 5). Lymph node status, T stage and age were significantly associated with worse OS, demonstrating that this cohort is representative of TNBC patients (OS, p values 0.018, 0.022 and 0.008, respectively). Here, the presence of either TILs in general (> 5%), TNFR2+ TILs (> 5%), or total TILs and TNFR2+ TILs (each > 5%) has demonstrated significantly protective roles in TNBC (OS, p values 0.037, 0.015 and 0.013, respectively). The PD-1+ TIL subset, alone or in combination with total TILs or with TNFR2+ TILs, provided protective values, but they were only close to significant (p values 0.053 and 0.059, respectively), further reflecting the mixed and dynamic nature of this lymphocyte subset that was revealed in our previous analyses (Figs. 3, 5).
Fig. 5.
Total TILs, TNFR2+ TILs and their combination have a beneficial survival effect in TNBC. Univariate Cox proportional hazard regression for OS and RFS shown as forest plots. Pos Positive; HS Hotspot; LN Lymph nodes. Hazard ratios (squares) and 95% confidence intervals (horizontal lines) are shown for each parameter
Discussion
In this study, we have identified for the first time a subset of TNFR2+ lymphocytes which is substantially associated with improved survival in TNBC patients. Despite the fact that many of the tumors contained only low percentages of TNFR2+ TILs, the impact of such cells was strong enough to support their significant association with better disease outcome in the entire cohort. Moreover, the beneficial survival effect of total TILs was brought into play mainly when TNFR2+ TILs were positioned in the tumors. These findings, together with the analysis of stroma (possibly TILs)-enriched basal tumors of the METABRIC dataset, strongly support the protective roles of TNFR2+ TILs in TNBC.
Our study has also inquired this TNFR2+ TIL subset in the context of the PD-1+ TIL subpopulation in TNBC patient tumors. The expression of PD-1, per se, may exemplify a dynamic process along T cell activation: PD-1 is up-regulated in activated T cells, but its interaction with PD-L1-expressing cells leads to termination of the activation process. Thus, it is possible that PD-L1 expression characterizes T cells that have just been activated or alternatively, T cells that are exhausted or suppressed, depending on the time at which PD-1 is expressed [14, 15]. Our research suggests that if PD-1+ TIL levels are too low, there are not enough activated T cells, and thus, patient survival is poor. In contrast, if PD-1+ TIL levels are too high, it is possible that many of the activated T cells are already exhausted or immune-suppressed, contributing again to reduced survival. However, when PD-1+ TIL levels are intermediate, this may be the exact situation in which T cells are at the peak of their activation state, in which they exert anti-tumor immune activities, and thus may be connected with improved survival.
This hypothesis is supported by our findings demonstrating that in tumors having > 5% TILs, containing PD-1+ TILs at intermediate levels, prognosis was relatively good. Furthermore, such intermediate levels of PD-1+ TILs acted alongside with TNFR2+ TILs and had a superior favorable effect on survival compared to low levels, whereas the beneficial effect of TNFR2+ TILs was lost when PD-1+ TILs were present in the tumors at low or high levels. Here, it is interesting to note that the subpopulation of TNFR2+ TILs was associated with improved patient survival at several cutoffs used. This is in marked contrast to PD-1+ TILs, whose correlation with survival did not demonstrate a stable trend at different cutoffs, probably reflecting the fact that PD-1 expression may signify different lymphocyte activation states in a kinetics-dependent manner. These findings suggest that TNFR2+ TILs may be a more reliable marker of immune status in TNBC than PD-1+ TILs, when efforts are done to associate types of immune infiltrates with prognosis.
Our study provides novel findings on the presence of TNFR2+ TILs in TNBC patient tumors and demonstrates that they may have beneficial roles in TNBC, in contrast to findings in other tumor cell systems [22–28]. When coming to address the phenotype of these TNFR2+ TILs in TNBC, it is important to consider the fact that tumor biopsies were sampled prior to chemotherapy. Current findings in the field propose that because survival rates are determined after chemotherapy, they partly reflect the outcome of chemotherapy-induced effects: Chemotherapy was reported to promote the expression of neo-antigens and thus to elevate the expansion of Teffs, and in parallel, it reduces/ablates the immune-suppressive activities of Tregs [38, 39]. As chemotherapy is the most conventional therapy given to TNBC patients, such chemotherapy-mediated effects may affect the phenotype and roles of TNFR2+ TILs in the immune contexture.
Recent publications indicate that mainly two T cell subpopulations express TNFR2:
T conventional and T effector cells (Teffs), where the latter cell type is connected to elevated anti-tumor activities [30, 32, 40–42]. Such cells may act against the tumor cells if given the proper conditions to do so; in this context, chemotherapy-driven exposure of neo-antigens, combined with reduced presence of Tregs, may give the Teffs just the right conditions to eliminate tumor cells. Overall, such activities of Teffs may well explain our findings on the significant association of TNFR2+ TILs with improved patient survival.
-
FOXP3+ T regulatory cells (Tregs) that have strong suppressive activities and in many studies were found to contribute to increased tumor growth [8, 12, 22–28]. Tregs are relatively sensitive to chemotherapy and may be preferentially ablated by the treatments given to TNBC patients [38, 39]. Under such conditions, other T cell subsets (e.g., TNFR2+ Teffs or TNFR2-Teffs) can be highly activated by chemotherapy-driven exposure to neo-antigens and exert preferential propagation, leading to improved clinical outcome [38]. Moreover, it is possible that TNFR2+ Tregs restrain pro-inflammatory processes that in many malignancies, including TNBC, are strongly connected to increased tumor progression [20]. Together, these effects may give rise to the significant association of TNFR2+ Tregs with better disease outcome in TNBC.
Thus, although the favorable roles of TNFR2+ Tregs in TNBC prognosis may seem counterintuitive, our findings may reflect the complex nature of the immune contexture and its dynamic change in the course of malignancy and chemotherapy. Here, it is important to note that several publications demonstrated that in TNBC patients the general population of Tregs was considerably associated with improved survival (these studies did not analyze TNFR2+ FOXP3+ TILs) (e.g., [43, 44]), further supporting our findings.
As noted above, we have identified TNFR2+ TILs as a protective lymphocyte subset in TNBC; however, these findings differ from studies of other tumor systems, suggesting that TNFR2+ TILs have detrimental roles because they exert suppressive activities of immune functions [22–28]. Moreover, our observations indicate that the potentially protective roles of TNFR2+ TILs in TNBC are counteracted in tumors that contain high levels of PD-1+ TILs. Our findings emphasize the need to perform a study that will be dedicated to kinetics analyses that will determine the phenotype and roles of TNFR2+ TILs in TNBC, as well as of PD-1+ TILs and their direct impact on TNFR2+ TILs during TNBC progression. Indeed, in ongoing studies that we have now initiated, we aim to determine additional such immune-related aspects by using marker analyses of TNBC patient tumors, studies of immune subpopulations in TNBC animal models and in vitro experiments of TNBC cells.
Overall, the significant association of TNFR2+ TILs with better disease outcome in TNBC patients may have important clinical implications. TNFR2 is activated by TNFα, which through NF-κB activation leads to increased survival of lymphocytes that express this receptor [45]. The very strong evidence for TNFα-induced pro-metastatic activities in TNBC has led researchers to suggest that TNFα should be considered as a therapeutic target in this type of disease. However, our study proposes that along with the many detrimental activities of TNFα, it may also activate a beneficial TIL subset that expresses TNFR2. If substantiated, these findings would indicate that therapies directed at inhibiting TNFα activities, or at ablating TNFR2+ TILs, should be well considered in TNBC, particularly if the TNFR2+ TIL subset consists of Teffs. Altogether, our findings shed light on TNFR2+ TILs in TNBC patients and raise important considerations regarding their inhibition, mainly when immunotherapies that target PD-1 are offered to TNBC patients in order to strengthen immune activation.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- ICB
Immune checkpoint blockade
- OS
Overall survival
- PD-1
Programmed cell death protein 1
- RFS
Recurrence-free survival
- Teff
T effector cell
- TIL
Tumor-infiltrating lymphocyte
- TNBC
Triple-negative breast cancer
- TNFα
Tumor necrosis factor α
- TNFR2
Tumor necrosis factor receptor 2
- Treg
T regulatory cell
Author contributions
MD was responsible for coordinating the study at the Sheba Medical Center. She organized the data and was responsible for all survival and statistical analyses and participated in manuscript preparation. DN contributed to setting up the criteria for pathological analyses, participated in determining pathological results and was the expert pathologist who determined the pathological parameters. SKE coordinated cohort assembly and sample collection and also gathered the clinical data at Sheba Medical Center. NO calibrated technical IHC settings and participated in data assessment. TB participated in data organization and in scanning of IHC images. IM participated in pathological assessments and participated in determining pathological results. DMS participated in collecting the clinical data and in scanning the IHC images. AP performed all slide preparations and IHC staining. NBL is an expert breast pathologist who assisted in pathological assessments. LA contributed to sample preparation and IHC. SW participated in conceptual design of dataset analyses. CK performed dataset analyses. ENG is the Deputy Head the Breast Oncology Institute at Sheba Medical Center and participated in clinical data interpretation. BK is the Head of the Breast Oncology Institute at Sheba Medical Center and participated in study design. IB is the Head of the Institute of Pathology at Sheba Medical Center and participated at the conception stages of the study. ABB is the principal investigator, responsible for the entire study at all stages (conception, design, data accumulation and interpretation), as well as manuscript preparation.
Funding
This study was supported by Helmholtz-Israel Cooperation in Personalized Medicine and by Federico Foundation.
Compliance with ethical standards
Conflict of interest
The authors declare that they do not have a financial relationship with the organizations that sponsored the research or that supported participation in conferences. The authors also declare that they do not have any non-financial competing interests.
Ethical approval and informed consent
The authors declare that the study was performed in accordance with the current laws and ethical standards of the country in which it was performed (Israel), and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Institutional Review Board of Sheba Medical Center (Approval No. 8736-11-SMC), with full exemption for consent form for anonymized samples. All samples were anonymized as defined in the study protocol.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Maya Dadiani, Daniela Necula and Smadar Kahana-Edwin have equal first authors.
Nino Oren and Tamir Baram have equal second authors.
References
- 1.Waks AG, Winer EP. Breast cancer treatment: a review. JAMA. 2019;321:288–300. doi: 10.1001/jama.2018.19323. [DOI] [PubMed] [Google Scholar]
- 2.Valentin MD, da Silva SD, Privat M, Alaoui-Jamali M, Bignon YJ. Molecular insights on basal-like breast cancer. Breast Cancer Res Treat. 2012;134:21–30. doi: 10.1007/s10549-011-1934-z. [DOI] [PubMed] [Google Scholar]
- 3.Choi SH, Chang JS, Koo JS, Park JW, Sohn JH, Keum KC, Suh CO, Kim YB. Differential prognostic impact of strong PD-L1 expression and 18F-FDG uptake in triple-negative breast cancer. Am J Clin Oncol. 2018;41:1049–1059. doi: 10.1097/COC.0000000000000426. [DOI] [PubMed] [Google Scholar]
- 4.Schmid P, Adams S, Rugo HS, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379:2108–2121. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 5.Voorwerk L, Slagter M, Horlings HM, et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: the TONIC trial. Nat Med. 2019;25:920–928. doi: 10.1038/s41591-019-0432-4. [DOI] [PubMed] [Google Scholar]
- 6.Emens LA, Cruz C, Eder JP, et al. Long-term clinical outcomes and biomarker analyses of atezolizumab therapy for patients with metastatic triple-negative breast cancer: a phase 1 study. JAMA Oncol. 2019;5:74–82. doi: 10.1001/jamaoncol.2018.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.AiErken N, Shi HJ, Zhou Y, Shao N, Zhang J, Shi Y, Yuan ZY, Lin Y. High PD-L1 expression is closely associated with tumor-infiltrating lymphocytes and leads to good clinical outcomes in Chinese triple negative breast cancer patients. Int J Biol Sci. 2017;13:1172–1179. doi: 10.7150/ijbs.20868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bottai G, Raschioni C, Losurdo A, et al. An immune stratification reveals a subset of PD-1/LAG-3 double-positive triple-negative breast cancers. Breast Cancer Res. 2016;18:121. doi: 10.1186/s13058-016-0783-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loi S, Drubay D, Adams S, et al. Tumor-infiltrating lymphocytes and prognosis: a pooled individual patient analysis of early-stage triple-negative breast cancers. J Clin Oncol. 2019;37:559–569. doi: 10.1200/JCO.18.01010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muenst S, Soysal SD, Gao F, Obermann EC, Oertli D, Gillanders WE. The presence of programmed death 1 (PD-1)-positive tumor-infiltrating lymphocytes is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2013;139:667–676. doi: 10.1007/s10549-013-2581-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brockhoff G, Seitz S, Weber F, Zeman F, Klinkhammer-Schalke M, Ortmann O, Wege AK. The presence of PD-1 positive tumor infiltrating lymphocytes in triple negative breast cancers is associated with a favorable outcome of disease. Oncotarget. 2018;9:6201–6212. doi: 10.18632/oncotarget.23717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Byun KD, Hwang HJ, Park KJ, Kim MC, Cho SH, Ju MH, Lee JH, Jeong JS. T-cell immunoglobulin mucin 3 expression on tumor infiltrating lymphocytes as a positive prognosticator in triple-negative breast cancer. J Breast Cancer. 2018;21:406–414. doi: 10.4048/jbc.2018.21.e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeong J, Lim JCT, Lee B, et al. Prognostic value of CD8+ PD-1+ immune infiltrates and PDCD1 gene expression in triple negative breast cancer. J Immunother Cancer. 2019;7:34. doi: 10.1186/s40425-019-0499-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bardhan K, Anagnostou T, Boussiotis VA. The PD1:PD-L1/2 pathway from discovery to clinical implementation. Front Immunol. 2016;7:550. doi: 10.3389/fimmu.2016.00550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thommen DS, Schumacher TN. T cell dysfunction in cancer. Cancer Cell. 2018;33:547–562. doi: 10.1016/j.ccell.2018.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noske A, Mobus V, Weber K, et al. Relevance of tumour-infiltrating lymphocytes, PD-1 and PD-L1 in patients with high-risk, nodal-metastasised breast cancer of the German Adjuvant Intergroup Node-positive study. Eur J Cancer. 2019;114:76–88. doi: 10.1016/j.ejca.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Hamaguchi T, Wakabayashi H, Matsumine A, Sudo A, Uchida A. TNF inhibitor suppresses bone metastasis in a breast cancer cell line. Biochem Biophys Res Commun. 2011;407:525–530. doi: 10.1016/j.bbrc.2011.03.051. [DOI] [PubMed] [Google Scholar]
- 18.Gari HH, DeGala GD, Lucia MS, Lambert JR. Loss of the oncogenic phosphatase PRL-3 promotes a TNF-R1 feedback loop that mediates triple-negative breast cancer growth. Oncogenesis. 2016;5:e255. doi: 10.1038/oncsis.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qiao Y, He H, Jonsson P, Sinha I, Zhao C, Dahlman-Wright K. AP-1 is a key regulator of proinflammatory cytokine TNFalpha-mediated triple-negative breast cancer progression. J Biol Chem. 2016;291:5068–5079. doi: 10.1074/jbc.M115.702571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marelli G, Sica A, Vannucci L, Allavena P. Inflammation as target in cancer therapy. Curr Opin Pharmacol. 2017;35:57–65. doi: 10.1016/j.coph.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Martinez-Reza I, Diaz L, Garcia-Becerra R. Preclinical and clinical aspects of TNF-alpha and its receptors TNFR1 and TNFR2 in breast cancer. J Biomed Sci. 2017;24:90. doi: 10.1186/s12929-017-0398-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen X, Oppenheim JJ. Targeting TNFR2, an immune checkpoint stimulator and oncoprotein, is a promising treatment for cancer. Sci Signal. 2017;10:1–3. doi: 10.1126/scisignal.aal2328. [DOI] [PubMed] [Google Scholar]
- 23.Chen X, Subleski JJ, Kopf H, Howard OM, Mannel DN, Oppenheim JJ. Cutting edge: expression of TNFR2 defines a maximally suppressive subset of mouse CD4+CD25+FoxP3+ T regulatory cells: applicability to tumor-infiltrating T regulatory cells. J Immunol. 2008;180:6467–6471. doi: 10.4049/jimmunol.180.10.6467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang LY, Lin YC, Chiang JM, et al. Blockade of TNF-alpha signaling benefits cancer therapy by suppressing effector regulatory T cell expansion. Oncoimmunology. 2015;4:e1040215. doi: 10.1080/2162402X.2015.1040215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ham B, Wang N, D'Costa Z, et al. TNF receptor-2 facilitates an immunosuppressive microenvironment in the liver to promote the colonization and growth of hepatic metastases. Cancer Res. 2015;75:5235–5247. doi: 10.1158/0008-5472.CAN-14-3173. [DOI] [PubMed] [Google Scholar]
- 26.Torrey H, Butterworth J, Mera T, et al. Targeting TNFR2 with antagonistic antibodies inhibits proliferation of ovarian cancer cells and tumor-associated Tregs. Sci Signal. 2017;1:1–12. doi: 10.1126/scisignal.aaf8608. [DOI] [PubMed] [Google Scholar]
- 27.Torrey H, Khodadoust M, Tran L, Baum D, Defusco A, Kim YH, Faustman DL. Targeted killing of TNFR2-expressing tumor cells and Tregs by TNFR2 antagonistic antibodies in advanced Sezary syndrome. Leukemia. 2019;33:1206–1218. doi: 10.1038/s41375-018-0292-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vanamee ES, Faustman DL. TNFR2: A novel target for cancer immunotherapy. Trends Mol Med. 2017;23:1037–1046. doi: 10.1016/j.molmed.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 29.Chen X, Wu X, Zhou Q, Howard OM, Netea MG, Oppenheim JJ. TNFR2 is critical for the stabilization of the CD4+Foxp3+ regulatory T. cell phenotype in the inflammatory environment. J Immunol. 2013;190:1076–1084. doi: 10.4049/jimmunol.1202659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen X, Subleski JJ, Hamano R, Howard OM, Wiltrout RH, Oppenheim JJ. Co-expression of TNFR2 and CD25 identifies more of the functional CD4+FOXP3+ regulatory T cells in human peripheral blood. Eur J Immunol. 2010;40:1099–1106. doi: 10.1002/eji.200940022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Govindaraj C, Scalzo-Inguanti K, Madondo M, Hallo J, Flanagan K, Quinn M, Plebanski M. Impaired Th1 immunity in ovarian cancer patients is mediated by TNFR2+ Tregs within the tumor microenvironment. Clin Immunol. 2013;149:97–110. doi: 10.1016/j.clim.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Jiang Y, Chen J, Bi E, et al. TNF-alpha enhances Th9 cell differentiation and antitumor immunity via TNFR2-dependent pathways. J Immunother Cancer. 2019;7:28. doi: 10.1186/s40425-018-0494-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salgado R, Denkert C, Demaria S, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26:259–271. doi: 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McIntire PJ, Zhong E, Patel A, Khani F, D'Alfonso TM, Chen Z, Shin SJ, Ginter PS. Hotspot enumeration of CD8+ tumor-infiltrating lymphocytes using digital image analysis in triple-negative breast cancer yields consistent results. Hum Pathol. 2019;85:27–32. doi: 10.1016/j.humpath.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 35.McIntire PJ, Irshaid L, Liu Y, Chen Z, Menken F, Nowak E, Shin SJ, Ginter PS. Hot spot and whole-tumor enumeration of CD8(+) tumor-infiltrating lymphocytes utilizing digital image analysis is prognostic in triple-negative breast cancer. Clin Breast Cancer. 2018;18:451–458.e1. doi: 10.1016/j.clbc.2018.04.019. [DOI] [PubMed] [Google Scholar]
- 36.Curtis C, Shah SP, Chin SF, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486:346–352. doi: 10.1038/nature10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ren X, Wu H, Lu J, Zhang Y, Luo Y, Xu Q, Shen S, Liang Z. PD1 protein expression in tumor infiltrated lymphocytes rather than PDL1 in tumor cells predicts survival in triple-negative breast cancer. Cancer Biol Ther. 2018;19:373–380. doi: 10.1080/15384047.2018.1423919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Emens LA, Middleton G. The interplay of immunotherapy and chemotherapy: harnessing potential synergies. Cancer Immunol Res. 2015;3:436–443. doi: 10.1158/2326-6066.CIR-15-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ladoire S, Arnould L, Apetoh L, Coudert B, Martin F, Chauffert B, Fumoleau P, Ghiringhelli F. Pathologic complete response to neoadjuvant chemotherapy of breast carcinoma is associated with the disappearance of tumor-infiltrating foxp3+ regulatory T cells. Clin Cancer Res. 2008;14:2413–2420. doi: 10.1158/1078-0432.CCR-07-4491. [DOI] [PubMed] [Google Scholar]
- 40.Yan F, Du R, Wei F, et al. Expression of TNFR2 by regulatory T cells in peripheral blood is correlated with clinical pathology of lung cancer patients. Cancer Immunol Immunother. 2015;64:1475–1485. doi: 10.1007/s00262-015-1751-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williams GS, Mistry B, Guillard S, et al. Phenotypic screening reveals TNFR2 as a promising target for cancer immunotherapy. Oncotarget. 2016;7:68278–68291. doi: 10.18632/oncotarget.11943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen X, Hamano R, Subleski JJ, Hurwitz AA, Howard OM, Oppenheim JJ. Expression of costimulatory TNFR2 induces resistance of CD4+FoxP3− conventional T cells to suppression by CD4+FoxP3+ regulatory T cells. J Immunol. 2010;185:174–182. doi: 10.4049/jimmunol.0903548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.West NR, Kost SE, Martin SD, Milne K, Deleeuw RJ, Nelson BH, Watson PH. Tumour-infiltrating FOXP3(+) lymphocytes are associated with cytotoxic immune responses and good clinical outcome in oestrogen receptor-negative breast cancer. Br J Cancer. 2013;108:155–162. doi: 10.1038/bjc.2012.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee S, Cho EY, Park YH, Ahn JS, Im YH. Prognostic impact of FOXP3 expression in triple-negative breast cancer. Acta Oncol. 2013;52:73–81. doi: 10.3109/0284186X.2012.731520. [DOI] [PubMed] [Google Scholar]
- 45.Wang J, Ferreira R, Lu W, et al. TNFR2 ligation in human T regulatory cells enhances IL2-induced cell proliferation through the non-canonical NF-kappaB pathway. Sci Rep. 2018;8:12079. doi: 10.1038/s41598-018-30621-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.