Abstract
Patients with pediatric cancers such as neuroblastoma (NB) are often unresponsive to checkpoint blockade immunotherapy. One major factor in pediatric tumor resistance to immunotherapy is considered to be the low mutation rate of pediatric tumors. Another factor may be the overexpression of additional inhibitory pathways. While analyzing the RNA-sequencing database TARGET, we found that human NB tumors overexpress immune checkpoint molecule CD200. To determine its significance and impact on tumor immune microenvironment, we analyzed 49 cases of previously untreated, surgically removed NB tumors using immunohistochemistry and multi-color flow cytometry (FACS). We found that CD200 is overexpressed in more than 90% of NB tumors. In the tumor microenvironment of NB, CD200 is mainly overexpressed in CD45− NB tumor cells, while its cognate receptor (CD200R) is mainly expressed in HLA-DR+CD14+ myeloid cells and CD11c+ dendritic cells. Low-level expression of CD200R is also observed in tumor-infiltrating CD4+ and CD8+ T cells. In NB tumors with higher CD200 expression (CD200high), we observed lower numbers of HLA-DR+CD14+ myeloid cells and less tumor-infiltrating CD4+ and CD8+ T cells. Moreover, we found that CD4+ and CD8+ T cells produced less IFN-γ and/or TNF-α in CD200high NB tumors. Thus, CD200–CD200R pathway appears to downregulate anti-tumor immunity in the tumor microenvironment of NB tumors, and blockade of this pathway may be beneficial for NB patients.
Keywords: CD200, CD200R, Neuroblastoma, Tumor immune microenvironment, Tumor-infiltrating lymphocyte
Introduction
Immunotherapy based on checkpoint blockade has achieved significant success in a subset of adult patients with advanced cancer. However, clinical trials using checkpoint inhibitors such as anti-PD-1 antibody in pediatric cancer patients failed to yield satisfactory outcome [1, 2]. It is assumed that pediatric cancer cells such as neuroblastoma (NB) have relatively low mutation rates compared to certain adult cancer cells [3]; therefore, available neoantigens that can be recognized by T cells are scarce. However, pediatric cancer cells often express a number of unique but shared tumor antigens that can be recognized by T cells [4], and examination of pediatric tumor tissues often suggests the presence of infiltrating T cells [5, 6]. Thus, an alternative explanation could be that additional inhibitory pathways other than PD1–PDL1 and CTLA4-B7 play a role in immune tolerance in pediatric tumors.
CD200 (also known as OX-2) is a member of the Ig superfamily (IgSF) of proteins and shares structural similarities with checkpoint molecules such as CD47, PD-1 and CTLA4. CD200 is expressed in a variety of normal tissues including B and activated T lymphocytes [7–11]. CD200 receptor (CD200R), the cognate receptor for CD200, is also an IgSF protein [12]. CD200R is mainly expressed in myeloid cells such as macrophages, neutrophils and mast cells [13], and CD200–CD200R interaction is mainly involved in regulating the functions of myeloid lineages of cells [14–16]. Although CD200R expression is mainly found in macrophages and neutrophils, further research has revealed lower levels of CD200R expression in dendritic cells (DCs) and some subsets of T cells [13, 17, 18], suggesting additional functions for CD200R signaling in regulating these cell types.
Recent studies have revealed that CD200 is overexpressed in a variety of human cancer cells including human melanoma [19], ovarian cancer [20], myeloid leukemia [21], some B cell malignancies [22] and a majority of endocrine malignancies such as small cell lung carcinoma [23]. In the tumor microenvironment (TME), tumor-associated myeloid cells (TAMCs), including tumor-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs) and tumor-associated dendritic cells (TADCs), have been shown to be the major lineages of cells expressing CD200R [24]. Accumulating evidence [24–26] suggests that CD200–CD200R interaction may be important in regulating the TME. In the past decade, reports suggesting an association between CD200–CD200R pathway and prognosis of human cancer patients [21, 22] have caused an explosion of interest in these molecules and their interactions. Today, clinical trials of patients with advanced human cancer such as multiple myeloma and chronic lymphocytic leukemia are underway based on blockade of this pathway using antibodies [27].
In this study, we examined whether pediatric tumors such as NB have an active CD200–CD200R pathway in tumor microenvironment. We found that CD200 is overexpressed in more than 90% of NB tumors. In the tumor microenvironment of NB, CD200 is mainly overexpressed in CD45− NB tumor cells, while its cognate receptor, i.e., CD200R is mainly present in CD14+ myeloid cells and dendritic cells. Moreover, in NB tumors with higher CD200 expression (CD200high), we observed lower numbers of CD14+ myeloid cells, less tumor-infiltrating CD4+ and CD8+ T cells. Notably, CD4+ and CD8+ T cells in CD200high NB tumors produced less IFN-γ and/or TNF-α.
Materials and methods
TARGET data analysis
The TARGET initiatively provides sequencing data and de-identified clinical information of pediatric cancer patients (available through the NIH GDC Data Portal: https://portal.gdc.cancer.gov/). The RNA-seq FPKM (Fragments Per Kilobase of transcript per Million fragments mapped) data of total 148 NB patients were accessed and analyzed in this study.
Patients and tumors
Forty-nine freshly resected NB tumor samples from previously untreated patients (Table 1) were used for the analyses in this study. Other freshly removed, malignant tumor samples from patients with hepatoblastoma (HB), Wilms tumor (WT), adrenocortical carcinoma (ACC) and rhabdomyosarcoma (RS) were used as controls in this study. All diagnoses were pathologically confirmed. The study was conducted with the informed consent from the patients’ legal guardians and approved by the ethics committee of Shanghai Children's Medical Center (SCMC) affiliated with Shanghai Jiao Tong University School of Medicine.
Table 1.
Characteristics of NB patients
| Factors | Quantity/percentage |
|---|---|
| Male/female | n = 29 (59.2%)/n = 20 (40.8%) |
| Age (months) | |
| < 18 | n = 20 (40.8%) |
| ≥ 18 | n = 29 (59.2%) |
| (INSS) Stage | |
| Stage 1 | n = 6 (12.2%) |
| Stage 2 | n = 15 (30.6%) |
| Stage 3 | n = 20 (40.8%) |
| Stage 4/4S | n = 8 (16.3%) |
| Grade of differentiation | |
| Differentiated | n = 21 (42.8%) |
| Poorly differentiated/undifferentiated | n = 23 (47%) |
| Unknown | n = 5 (10.2%) |
| MYCN status | |
| Amplified | n = 2 (4.1%) |
| Not amplified | n = 44 (89.8) |
| Unknown | n = 3 (6.1%) |
| Risk group | |
| Low | n = 10 (20.4%) |
| Intermediate | n = 23 (46.9%) |
| High | n = 16 (32.7%) |
Immunohistochemistry
Frozen tissue sections of NB and other pediatric tumors were used for immunohistochemistry (IHC). Immunostaining was performed on tumor tissue sections using 2 ug/ml of mouse antihuman CD200 mAb (OX-104; BioLegend) or respective isotype control mAb (Mouse IgG1; BioLegend) at 4 °C overnight. Ab binding was detected using anti-mouse peroxidase-conjugated GTVision reagent, and diaminobenzidine was used as chromogen (Gene Tech, Shanghai). Tissue sections were counterstained with hematoxylin, after which, slides were photographed on high-power fields under a microscope by randomly selecting tissue areas.
Tumor digestion and preparation of mononuclear cells
Surgically removed tumor samples were washed with PBS, and non-tumor tissues and necrotic tissues were carefully removed. Tumor tissues were cut into about 1 mm3 pieces and were digested in RPMI 1640 (Gibco) containing 0.2% collagenase type II and IV (Solarbio), 0.5 mg/ml hyaluronidase (Solarbio), 0.02 mg/ml DNase (Solarbio) and 1% BSA (Solarbio) at 37 °C for 1 h in a shaker at the speed of 100 rpm. Viable cells were obtained after filtration and Ficoll Hypaque separation (Lymphoprep; Stem Cell Technologies) and were used for further multicolor flow cytometry analyses.
Antibodies and multicolor flow cytometry
FITC-, PerCP-, PE-Cy7, eV605-, APC-eF780-, PerCP-Cy5.5-, PE-, APC-, BV510-, APC-Cy7-, V500-, BV421-, eF450-labeled antibodies to CD45 (clone HI30), IL-2 (MQ1-17H12), TNF-α (MAb11), IFN-γ (B27), CD200 (0X-104), PD-1 (MIH4), CD11c (B-ly6), CD14 (MφP9), HLA-DR (G46-6), CD123 (9F5), BTLA (J168-540) were purchased from BD Biosciences; CD4 (RPA-T4, SK3), CD8a (RPA-T8), CD200 Receptor (OX108), CD28 (CD28.2), OX40 (ACT35), 4-1BB (4B4), LAG-3 (3DS223H), ICOS (ISA-3) and Foxp3 (236A/E7) were purchased from eBioscience, and CD45 (HI30) was purchased from BioLegend. The isotype-matched control Abs were purchased from BD Biosciences, eBioscience or BioLegend, respectively. For staining of cell surface markers, single cell suspension was stained with various antibodies in staining buffer (PBS with 1% FBS) and incubated on ice for 30 min. After washing with staining buffer, cells were fixed in 1% paraformaldehyde in PBS. For the detection of intracellular cytokines, cells were stimulated in vitro with lymphocytes activation cocktail (BD Biosciences) and/or lipopolysaccharides (Sigma) for 4 h in a 37 °C humidified CO2 incubator. Intracellular cytokine staining procedure was performed according to recommended protocol in Cytofix/Cytoperm™ Fixation/Permeabilization Solution kit (BD Biosciences) to detect IFN-γ, TNF-α and IL-2. A Foxp3/Transcription Factor Staining Buffer Set kit (eBioscience) was used to detect Foxp3 expression. Cells were analyzed on a BD FACS Canton flow cytometer, and collected data were analyzed using the FlowJo software (Tree Star, Ashland, OR).
Statistical analysis
Two-tailed Student’s t test and Mann–Whitney U test were used for statistical analysis. A p value < 0.05 was considered of significance.
Results
Tumor tissues of human neuroblastoma overexpress CD200
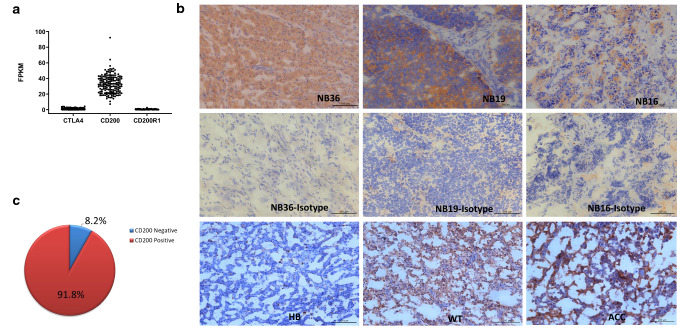
To understand the tumor immune microenvironment of pediatric cancers, we examined immune gene expression levels based on RNA-sequencing data from the TARGET (Therapeutically Applicable Research to Generate Effective Treatments) database. As shown in Fig. 1a, NB tumors express significantly higher levels of the immune checkpoint molecule CD200, when compared to its cognate receptor CD200R and another checkpoint molecule CTLA4. To validate this finding and its potential significance, we examined 49 cases of surgically removed NB tumors from previously untreated patients by IHC and flow cytometry. As detailed in Table 1, the patients represent different risk groups (low risk: n = 10; intermediate risk: n = 23; and high risk: n = 16), which is classified according to the guidelines of Children’s Oncology Group (COG). The classification parameters include age, N-MYC amplification, pathological diagnosis and the International Neuroblastoma Staging System (INSS). As shown in Fig. 1b, IHC revealed three modes of CD200 expression in NB tumors: in some cases, CD200 was expressed predominantly all over the tissues (NB36); in other cases, CD200 was strongly expressed in areas where NB tumor cells aggregated (NB19) or in a scattered manner associated with NB tumor cells (NB16). We also examined CD200 expression in other pediatric cancer types and found that CD200 was occasionally expressed in other cancer types such as Wilms tumors (WT) and adrenocortical carcinoma (ACC) but absent in hepatoblastoma (HB) (Fig. 1b, lower panel). Overall, we found that CD200 was predominantly expressed in more than 90% of NB tumors (Fig. 1c).
Fig. 1.
IHC analysis of CD200 expression in NB and other pediatric tumors. a Fragments Per Kilobase of transcript per Million fragments mapped (FPKM) of CTLA4, CD200 and CD200R1 in neuroblastoma (NB) patient samples. RNA-seq data from TARGET database (n = 148) were analyzed. b Frozen sections of NB, HB, WT and ACC tumors were used to detect CD200 expression by IHC. The original images were taken on high-power fields (200 ×) under a microscope. c Overall expression of CD200 in NB tumor samples (n = 49). Percentages were calculated based on the results obtained from IHC study
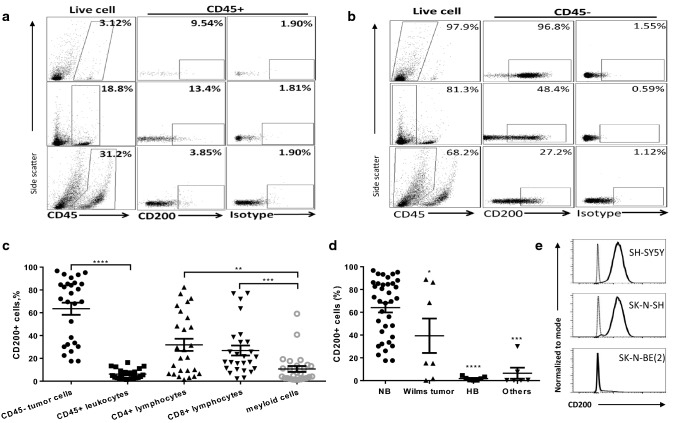
In order to further determine the cell-type-specific expression of CD200 in NB tumor microenvironment, we analyzed the surgically removed NB tumors from previously untreated patients by flow cytometry. As shown in Fig. 2a, b, low levels of CD200 (6.2 ± 4.3%) were detected in CD45+ immune cells, while on average more than 60% of CD45− cells (63.5 ± 27%) in NB tumors expressed CD200 (Fig. 2c). Among CD45+ immune cells, CD200 was mainly detected in tumor-infiltrating CD4+ (31.8 ± 27%) and CD8+ (26.9 ± 22%) T cells, while the majority of tumor-infiltrating CD14+ myeloid cells expressed CD200 at low percentages (10.7 ± 13%) (Fig. 2c). We compared CD200 expression in tumors from NB and other pediatric cancers and found that total tumor cells from NB had the highest CD200 expression (64.1 ± 25%); about 40% of WT cases (3/7) expressed CD200, while CD200 was rarely expressed in HB and other tumor types (Fig. 2d). Predominant expression of CD200 in CD45− tumor cells suggests that CD200 is mainly expressed in NB tumor cells. Indeed, flow cytometry analysis revealed that 2 out of 3 human NB cell lines (SH-SY5Y, SK-N-SH) were 100% CD200-positive, while in another cell line SK-N-BE(2), only a subset of cells (5%) were CD200-positive (Fig. 2e).
Fig. 2.
Flow cytometry analysis of CD200 expression in neuroblastoma and other pediatric tumors. a CD200 expression in CD45+ cells in NB tumors. Single cell suspensions of NB tumors were stained for respective biomarkers and analyzed by flow cytometry. Representative data obtained from three independent NB tumors were shown. b CD200 expression in CD45− cells in NB tumors. Representative data were obtained from the same NB tumors presented in a. c Summary of CD200 expression in various cell subsets in NB tumors (n = 27). Percentages of CD200-positive cells among each subset of cells in NB tumors were determined by flow cytometry. **p < 0.01, ***p < 0.001, ****p < 0.0001 by Mann–Whitney U test. d Summary of CD200 expression in NB, WT, HB and other pediatric tumors (ACC and rhabdomyosarcoma, RS). Percentages of CD200-positive cells among CD45− tumor cells in NB tumors versus other tumor types were determined by flow cytometry. *p < 0.05, ***p < 0.001, ****p < 0.0001 by Mann–Whitney U test. e CD200 expression in NB tumor cell lines. The cell lines were originally purchased from ATCC. Flow cytometry was used to determine CD200 expression. Thin lines represent isotype control, while thick lines represent CD200 staining
Expression of CD200 in NB tumors regulates tumor immune microenvironment
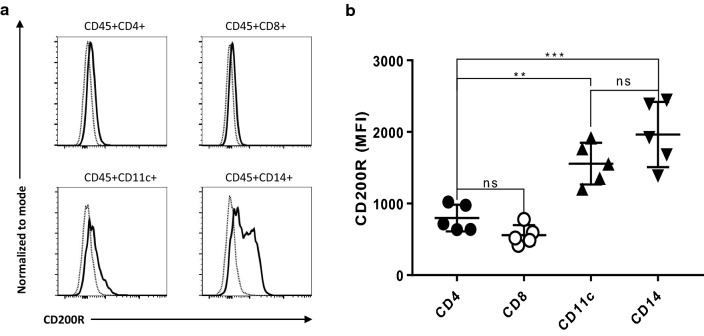
Previous studies suggest that tumor-expressed CD200 affects tumor immune microenvironment through interaction with CD200R-positive cells [24–26]. We therefore examined CD200R expression in the TME of NB using flow cytometry. As shown in Fig. 3, we found that CD200R+ cells mainly lie in CD45+CD4−CD8−CD14+ population (Fig. 3a), consistent with the observations in mouse models that tumor-associated myeloid cells are the major population of cells that express CD200R [24]. As demonstrated in Fig. 3a, b, CD200R was also detected in tumor-infiltrating CD4+ and CD8+ T cells. However, the mean fluorescence intensity (MFI) of CD200R in T cells (CD4+: 798 ± 186; CD8+: 559 ± 138) were much lower than in myeloid cells (CD14+: 1964 ± 454; CD11c+: 1557 ± 291). It appears that CD4+ T cells expressed higher levels of CD200R than CD8+ T cells (Fig. 3b). However, the difference was not significant.
Fig. 3.
CD200R expression in the TME of NB. a CD200R expression in different immune cell subsets in NB tumors. Flow cytometry was used to determine CD200R expression. Dotted thin lines represent isotype control, while thick lines represent CD200 staining. b Summary of CD200R expression in different subsets of immune cells. Mean fluorescence intensity (MFI) of CD200R in different immune cell subsets from five NB tumors was used for the analysis. **p < 0.01, ***p < 0.001 by Mann–Whitney U test
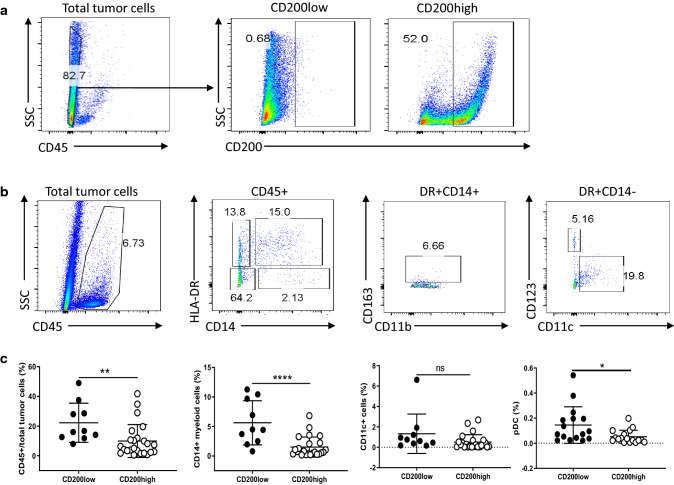
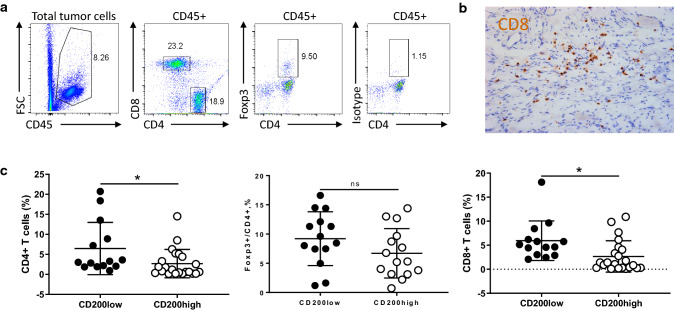
Next, we examined whether immune cell populations might differ in NB tumors that expressed higher levels of CD200 (> 30% of CD45− tumor cells express CD200) versus those with low levels of CD200 (< 15% of CD45− tumor cells express CD200) using flow cytometry (Fig. 4a, b). As shown in Fig. 4c, we found that CD200low tumors contained higher numbers of CD45+ leukocytes (22.2 ± 13 vs 9.9 ± 11) and higher ratios of HLA-DR+CD14+ myeloid cells (5.6 ± 3.7 vs 1.5 ± 1.7). We did not find significant differences in CD11c+ dendritic cells (1.3 ± 1.9 vs 0.5 ± 0.7) between CD200low and CD200high NB tumors. However, the percentages of pDC (DR+CD14−CD11c−CD123+) were higher in CD200low NB tumors than CD200high NB tumors (0.14 ± 0.14 vs 0.05 ± 0.05). We found that CD200low NB tumors had higher percentages of CD4+ (6.4 ± 6.5 vs 2.7 ± 3.5%) and CD8+ T cells (5.9 ± 4.1 vs 2.6 ± 3.2%) compared to CD200high NB tumors (Fig. 5). However, we did not detect a significant difference in CD4+FoxP3+ Treg cell populations (9.2 ± 4.5 vs 6.7 ± 4.2%) between CD200low and CD200high NB tumors (Fig. 5). Thus, the expression levels of CD200 in NB tumors significantly affected the compositions of immune cells in the TME.
Fig. 4.
CD200 expression affects tumor-associated myeloid cells in the TME of NB. a Representative NB tumor samples with low or high expression of CD200. Samples with < 15% CD200+ cells among the CD45− population were determined as CD200low; samples with > 30% CD200+ cells among the CD45− population were determined as CD200high. b Flow cytometry analysis of tumor-associated myeloid cells in NB tumors. The representative gating strategies for subsets of myeloid cells, i.e., CD45+DR+CD14+ tumor-associated macrophage (TAMs), CD45+CD14+DR− myeloid-derived suppressor cells (MDSCs), CD45+DR+CD14−CD11c+ dendritic cells (DCs) and CD45+DR+CD14−CD11c−CD123+ plasmacytoid DCs (pDCs) were shown. c Summary of different subsets of myeloid cells in NB tumors. Differences of total CD45+ leukocytes, TAMs, DCs and pDC between CD200low (n = 10) and CD200high NB (n = 24) samples were shown. *p < 0.05, **p < 0.01, ****p < 0.0001 by Student’s t test
Fig. 5.
CD200 expression affects tumor-infiltrating T cells in the TME of NB. a Flow cytometry analysis of tumor-infiltrating T cells in the TME of NB. The representative gating strategies for three subsets of T cells, i.e., CD45+CD4+ T cells, CD45+CD4+Foxp3+ regulatory T cells (Tregs) and CD45+CD8+ T cells were shown. b A representative IHC image of tumor-infiltrating CD8+ T cells was shown. c Summary of different subsets of T cells in NB tumors. Differences of CD4+ T cells (n = 36), Foxp3+ CD4+ cells (n = 29) and CD8+ T cells (n = 36) between CD200low and CD200high NB samples were shown. *p < 0.05 by Student’s t test
NB tumor expression of CD200 regulates T cell effector functions in the TME
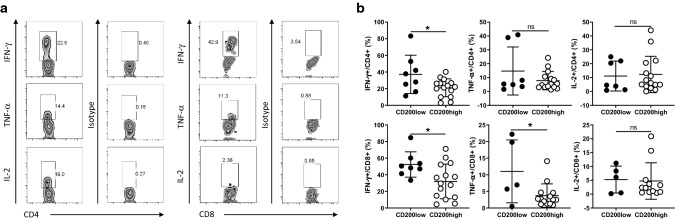
Because NB tumor cells overexpress CD200, and tumor-infiltrating T cells express low levels of CD200R, we hypothesized that tumor-expressed CD200 might directly affect T cell effector functions via interaction with CD200R on T cells in the TME. To test this hypothesis, we analyzed tumor-infiltrating CD4+ and CD8+ T cells for their expression of cytokines including IFN-γ, TNF-α and IL-2 using flow cytometry and compared whether T cell cytokine production differed between CD200-high and CD200-low tumors. As shown in Fig. 6a, we frequently detected all three cytokines in tumor-infiltrating CD4+ T cells. In the case of CD8+ T cells, we detected a high number of them producing IFN-γ, a moderate number producing TNF-α, and much fewer number of cells producing IL-2. Thus, it appears that CD8+ T cells had a more differentiated effector phenotype. We observed that the frequencies of IFN-γ-producing CD4+ T cells were significantly higher in CD200low tumors than CD200high tumors (37.1 ± 23 vs 21 ± 10.7%). For tumor-infiltrating CD8+ T cells, frequencies of both IFN-γ (52.4 ± 15.2 vs 31.9 ± 20.8%) and TNF-α-producing T cells (11.1 ± 9.4 vs 3.7 ± 3.5%) were higher in CD200low tumors (Fig. 6b). Thus, it appears that the effector functions of tumor-infiltrating T cells were inhibited by expression of CD200 in NB tumors.
Fig. 6.
Cytokine-producing T cells in CD200low and CD200high NB tumors. a Flow cytometry analysis of cytokine-producing T cells in the TME of NB. The representative images of IFN-γ-, TNF-α- and IL-2-producing CD4+ and CD8+ tumor-infiltrating lymphocytes (TILs) were shown. b Summary of cytokine-producing T cells in NB tumors. Differences of IFN-γ-, TNF-α- and IL-2-producing CD4+ and CD8+ T cells between CD200low and CD200high NB samples were shown. *p < 0.05 by Student’s t test
Other co-stimulatory and co-inhibitory molecules in NB tumor-infiltrating T cells
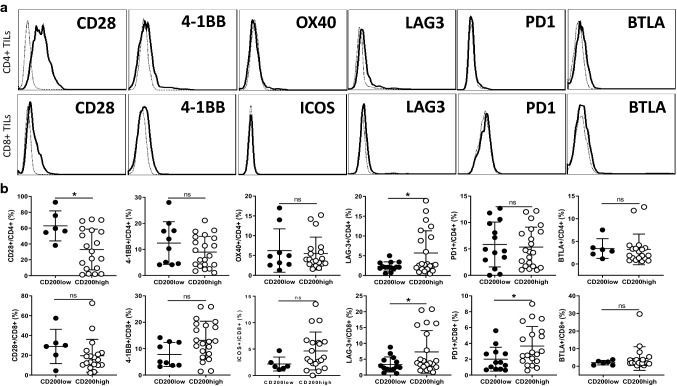
To determine whether CD200–CD200R interaction in NB tumors affects other co-stimulatory or co-inhibitory pathways in tumor-infiltrating T cells, we examined the expression levels of co-stimulatory molecules (CD28, 4-1BB, ICOS and OX40) and co-inhibitory molecules (Lag-3, PD-1 and BTLA) in TILs. As shown in Fig. 7a, we found that tumor-infiltrating CD4+ T cells mainly express CD28, 4-1BB, Lag-3 and BTLA, while the expression of OX40 and PD-1 was low or undetectable. CD8+ TILs mainly express CD28, 4-1BB, Lag-3 and BTLA, while PD-1 and ICOS were low or undetectable. Interestingly, in NB tumors with higher CD200 expression, CD4+ TILs expressed lower CD28 (32.9 ± 25 vs 62.8 ± 18%) and higher Lag3 (5.7 ± 5.6 vs 2.3 ± 1.1%), while CD8+ TILs expressed higher Lag3 (7.3 ± 6.7 vs 3.3 ± 2.3%) and PD-1(3.7 ± 2.4 vs 2 ± 1.5%) (Fig. 7b). Thus, tumor-infiltrating T cells in tumors with higher expression of CD200 had a phenotype of more T cell exhaustion, as reflected by expression of higher Lag-3 and/or PD-1.
Fig. 7.
Co-stimulatory and co-inhibitory molecules in T cells in CD200low and CD200high NB tumors. a Flow cytometry analysis of co-stimulatory and co-inhibitory molecules in tumor-infiltrating T cells in the TME of NB. The representative histograms for different co-stimulatory and co-inhibitory molecules are shown. Thick lines represent expression of respective co-stimulatory/co-inhibitory molecules, while thin dotted lines represent isotype control. b Summary for expression of co-stimulatory and co-inhibitory molecules in infiltrating T cells in NB tumors. Percentages of co-stimulatory/co-inhibitory molecules in T cells in the TME of CD200low and CD200high NB tumors were shown. *p < 0.05 by Student’s t test
Discussion
In this study, we report that human NB tumors overexpress the immune checkpoint molecule CD200. IHC and flow cytometry confirmed that CD200 was mainly overexpressed in CD45− NB cells. Previously, CD200 was found to be overexpressed in a variety of human cancers including melanoma [19], ovarian cancer [20], some B cell malignancies [22], endocrine malignancies such as small cell lung carcinoma [23] and glioblastoma [28]. Although CD200 expression was found in two NB cell lines previously [29], its expression and significance in primary NB tumors remain elusive. In this study, we found that CD200 is overexpressed in more than 90% of NB tumors, a major type of pediatric cancer.
A critical question is what is the significance and role of CD200 overexpression in these tumors. So far, controversial results have been reported in the literature. A study in 2006 suggested that CD200 mRNA expression in myeloma cells is associated with decreased survival of patients [22]. However, this result was later challenged by another report, which showed that loss of CD200 protein expression on myeloma cells was correlated with a clinically more aggressive disease [30]. CD200 expression in acute myeloid leukemia (AML) was reported to be associated with poor prognosis [21]. However, CD200 expression in chronic lymphocytic leukemia (CLL) was reported to be associated with better prognosis [31]. Similarly, CD200 was found to be associated with tumor grading and metastasis in bladder cancer [32], while in breast cancer CD200 was shown to be present in early-stage but not metastatic breast tumors [33]. Thus, it appears that tumor CD200 plays differential roles in human cancers depending on the tumor type. In the case of NB tumors, we found that higher numbers of DR+ macrophages and IFN-γ + T lymphocytes are present in CD200low tumors relative to CD200high tumors. Therefore, NB tumor-expressed CD200 appears to inhibit anti-tumor immunity and should play a pro-tumor role in this particular tumor type. However, since the current anti-CD200 antibody worked the best only for frozen tumor tissues but not paraffin-embedded tumor tissues, we were not able to perform a retrospective analysis of previously achieved NB samples. Therefore, our current study does not allow us to draw a conclusion on the relationship between NB-expressed CD200 and survival.
A notable observation in this study is that CD200low tumors contains significantly higher numbers of CD14+ myeloid cells compared to CD200high tumors. The myeloid cells were mainly of the HLA-DRhigh cells, and few of them expressed CD163 (Fig. 4a). Thus, the myeloid cells in the TME of NB tumors are mainly of the anti-tumor M1 phenotype [34]. The expansion of myeloid cells in CD200low tumors is consistent with the observation that myeloid cells are the major cell types that express CD200R (Fig. 3). These results support a cellular model that tumor-expressed CD200 interacts with CD200R on tumor-associated myeloid cells and inhibits their expansion and functions. This model is supported by our recent mouse study [26] that showed CD200-positive tumors grown in CD200R-deficient mice contained higher numbers of CD11b+Ly6C+ myeloid cells. This model may also explain why tumors exhibit accelerated or reduced growth in the absence of CD200–CD200R interaction [35, 36]. Expansion of M2 macrophages and MDSCs enhances tumor-associated inflammation/angiogenesis [37, 38] and inhibits tumor-specific T cell responses [39], leading to tumor invasion and metastasis [40, 41]. In contrast, expansion of M1 macrophages will lead to tumor growth inhibition due to their direct anti-tumor effects and induction of tumor-specific T cell responses [34].
Dendritic cells (DCs) play key roles in induction of anti-tumor T cell responses. In this study, we observed that CD200R was expressed at high levels in NB tumor-associated DCs (Fig. 3). This observation suggests that NB tumor-expressed CD200 can directly affect DC expansion and function. Although CD11c+ DC expansion was not significant in CD200low tumors, we observed significantly higher numbers of plasmacytoid DC (pDC) in CD200low tumors than in CD200high tumors (Fig. 4). These observations, together with fact that numbers of T cells in CD200low tumors were significantly higher than in CD200high tumors, suggest that in CD200low NB tumors diminished CD200–CD200R interaction between tumor cells and DC is in favor of a stronger T cell response. This point is supported by the observation that a tumor-derived vaccine containing CD200 inhibits immune activation [42].
In addition to HLA-DR+CD14+ macrophages and DCs, we also observed that tumor-infiltrating T cells had significant expression of CD200R (Fig. 3). This observation strongly suggests that tumor-expressed CD200 can directly interfere with T cell effector function via CD200R. The higher numbers of IFN-γ- and/or TNF-α-producing T cells in CD200low tumors (Fig. 6) support this notion. This conclusion is also supported by the observations from in vitro co-culture experiments using allogeneic lymphocytes and CD200-positive cancer cells such as melanoma cells, where blockade of CD200–CD200R interaction increases IFN-γ production by T cells [19, 29, 43, 44].
In this work, we examined a number of other co-stimulatory and co-inhibitory pathways in NB tumors. We found that TILs from NB tumors express high levels of CD28 and significant levels of 4-1BB, low levels of Lag-3 and BTLA, and a lack of PD-1 expression. These results suggest that infiltrating T cells in NB tumors are not functionally exhausted, but rather functionally restrained, likely by the CD200–CD200R interaction. This result may explain why PD-1/PD-L1-based checkpoint blockade therapy does not work in treating neuroblastoma [1, 2], and suggest that CD200 may be an alternative checkpoint pathway to be blocked, as proposed for other solid tumors and B-cell malignancies [45].
In summary, we have found that CD200 is overexpressed in more than 90% of NB tumors. In the TME of NB, CD200 is mainly overexpressed in CD45− NB tumor cells, while its cognate receptor (CD200R) is mainly expressed in DR+CD14+ macrophages and dendritic cells. Moreover, in CD200high NB tumors, we observed lower numbers of CD14+ myeloid cells, less tumor-infiltrating CD4+ and CD8+ T cells, and CD4+ and CD8+ T cells produced less IFN-γ and/or TNF-α in CD200high NB tumors. Thus, CD200–CD200R pathway appears to downregulate anti-tumor immunity in NB tumors, and blockade of this pathway using anti-CD200 antibody [27] may be beneficial for NB patients.
Acknowledgements
This study was supported by the grants from the National Natural Science Foundation of China (Grant Nos. 81800118, 81773039) and Project of Shanghai Science and Technology Committee (Grant Nos. 17441903200, 17411950402).
Abbreviations
- ACC
Adrenocortical carcinoma
- AML
Acute myeloid leukemia
- ATCC
American Type Culture Collection
- BSA
Bovine serum albumin
- CD200R
CD200 receptor
- CLL
Chronic lymphocytic leukemia
- DC
Dendritic cell
- EMEM
Eagle's Minimum Essential Medium
- FBS
Fetal bovine serum
- FPKM
Fragments Per Kilobase of transcript per Million fragments mapped
- HB
Hepatoblastoma
- IgSF
Immunoglobulin superfamily
- IHC
Immunohistochemistry
- INSS
International Neuroblastoma Staging System
- MDSC
Myeloid-derived suppressor cell
- MFI
Mean fluorescence intensity
- NB
Neuroblastoma
- pDC
Plasmacytoid dendritic cell
- RS
Rhabdomyosarcoma
- TADC
Tumor-associated dendritic cell
- TAM
Tumor-associated macrophage
- TAMC
Tumor-associated myeloid cell
- TARGET
Therapeutically Applicable Research to Generate Effective Treatments
- TIL
Tumor-infiltrating lymphocyte
- TME
Tumor microenvironment
- Treg
Regulatory T cell
- WT
Wilms tumor
Author contributions
XB conceived and designed the experiments. CX and HZ performed the experiments and analyzed the data. XB, HZ and CX wrote the manuscript. JZ arranged the panels for flow cytometry. MX and SG prepared the patient samples. MY and JM diagnosed and provided patients’ information. YL and PZ helped to obtain and analyze RNA-seq data. XM, JT and CP provided assistance with revising the manuscript. All authors read and approved the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Min Xu, Email: jackxm1236@126.com.
Hua Zhu, Email: zhu-hua@scmc.com.cn.
References
- 1.Park JA, Cheung NV. Limitations and opportunities for immune checkpoint inhibitors in pediatric malignancies. Cancer Treat Rev. 2017;58:22–33. doi: 10.1016/j.ctrv.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wedekind MF, Denton NL, Chen CY, Cripe TP. Pediatric cancer immunotherapy: opportunities and challenges. Paediatr Drugs. 2018;20:395–408. doi: 10.1007/s40272-018-0297-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, Carter SL, Stewart C, Mermel CH, Roberts SA, Kiezun A, Hammerman PS, McKenna A, Drier Y, Zou L, Ramos AH, Pugh TJ, Stransky N, Helman E, Kim J, Sougnez C, Ambrogio L, Nickerson E, Shefler E, Cortes ML, Auclair D, Saksena G, Voet D, Noble M, DiCara D, Lin P, Lichtenstein L, Heiman DI, Fennell T, Imielinski M, Hernandez B, Hodis E, Baca S, Dulak AM, Lohr J, Landau DA, Wu CJ, Melendez-Zajgla J, Hidalgo-Miranda A, Koren A, McCarroll SA, Mora J, Crompton B, Onofrio R, Parkin M, Winckler W, Ardlie K, Gabriel SB, Roberts CWM, Biegel JA, Stegmaier K, Bass AJ, Garraway LA, Meyerson M, Golub TR, Gordenin DA, Sunyaev S, Lander ES, Getz G. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499:214–218. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hont AB, Cruz CR, Ulrey R, O'Brien B, Stanojevic M, Datar A, Albihani S, Saunders D, Hanajiri R, Panchapakesan K, Darko S, Banerjee P, Fortiz MF, Hoq F, Lang H, Wang Y, Hanley PJ, Dome JS, Bollard CM, Meany HJ. Immunotherapy of relapsed and refractory solid tumors with ex vivo expanded multi-tumor associated antigen specific cytotoxic T lymphocytes: a phase I study. J Clin Oncol. 2019;37:2349–2359. doi: 10.1200/JCO.19.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mina M, Boldrini R, Citti A, Romania P, D'Alicandro V, De Ioris M, Castellano A, Furlanello C, Locatelli F, Fruci D. Tumor-infiltrating T lymphocytes improve clinical outcome of therapy-resistant neuroblastoma. Oncoimmunology. 2015;4:e1019981. doi: 10.1080/2162402X.2015.1019981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu H, Gu S, Yin M, Shi M, Xin C, Zhu J, Wang J, Huang S, Xie C, Ma J, Pan C, Tang J, Xu M, Bai XF. Analysis of infantile fibrosarcoma reveals extensive T-cell responses within tumors: implications for immunotherapy. Pediatr Blood Cancer. 2018;65:e26813. doi: 10.1002/pbc.26813. [DOI] [PubMed] [Google Scholar]
- 7.Koning N, Swaab DF, Hoek RM, Huitinga I. Distribution of the immune inhibitory molecules CD200 and CD200R in the normal central nervous system and multiple sclerosis lesions suggests neuron–glia and glia–glia interactions. J Neuropathol Exp Neurol. 2009;68:159–167. doi: 10.1097/NEN.0b013e3181964113. [DOI] [PubMed] [Google Scholar]
- 8.Ragheb R, Abrahams S, Beecroft R, Hu J, Ni J, Ramakrishna V, Yu G, Gorczynski RM. Preparation and functional properties of monoclonal antibodies to human, mouse and rat OX-2. Immunol Lett. 1999;68:311–315. doi: 10.1016/s0165-2478(99)00060-7. [DOI] [PubMed] [Google Scholar]
- 9.Dick AD, Broderick C, Forrester JV, Wright GJ. Distribution of OX2 antigen and OX2 receptor within retina. Investig Ophthalmol Vis Sci. 2001;42:170–176. [PubMed] [Google Scholar]
- 10.Rosenblum MD, Olasz EB, Yancey KB, Woodliff JE, Lazarova Z, Gerber KA, Truitt RL. Expression of CD200 on epithelial cells of the murine hair follicle: a role in tissue-specific immune tolerance? J Investig Dermatol. 2004;123:880–887. doi: 10.1111/j.0022-202X.2004.23461.x. [DOI] [PubMed] [Google Scholar]
- 11.Wright GJ, Jones M, Puklavec MJ, Brown MH, Barclay AN. The unusual distribution of the neuronal/lymphoid cell surface CD200 (OX2) glycoprotein is conserved in humans. Immunology. 2001;102:173–179. doi: 10.1046/j.1365-2567.2001.01163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barclay AN, Wright GJ, Brooke G, Brown MH. CD200 and membrane protein interactions in the control of myeloid cells. Trends Immunol. 2002;23:285–290. doi: 10.1016/s1471-4906(02)02223-8. [DOI] [PubMed] [Google Scholar]
- 13.Wright GJ, Cherwinski H, Foster-Cuevas M, Brooke G, Puklavec MJ, Bigler M, Song Y, Jenmalm M, Gorman D, McClanahan T, Liu MR, Brown MH, Sedgwick JD, Phillips JH, Barclay AN. Characterization of the CD200 receptor family in mice and humans and their interactions with CD200. J Immunol. 2003;171:3034–3046. doi: 10.4049/jimmunol.171.6.3034. [DOI] [PubMed] [Google Scholar]
- 14.Jenmalm MC, Cherwinski H, Bowman EP, Phillips JH, Sedgwick JD. Regulation of myeloid cell function through the CD200 receptor. J Immunol. 2006;176:191–199. doi: 10.4049/jimmunol.176.1.191. [DOI] [PubMed] [Google Scholar]
- 15.Hoek RM, Ruuls SR, Murphy CA, Wright GJ, Goddard R, Zurawski SM, Blom B, Homola ME, Streit WJ, Brown MH, Barclay AN, Sedgwick JD. Down-regulation of the macrophage lineage through interaction with OX2 (CD200) Science. 2000;290:1768–1771. doi: 10.1126/science.290.5497.1768. [DOI] [PubMed] [Google Scholar]
- 16.Snelgrove RJ, Goulding J, Didierlaurent AM, Lyonga D, Vekaria S, Edwards L, Gwyer E, Sedgwick JD, Barclay AN, Hussell T. A critical function for CD200 in lung immune homeostasis and the severity of influenza infection. Nat Immunol. 2008;9:1074–1083. doi: 10.1038/ni.1637. [DOI] [PubMed] [Google Scholar]
- 17.Rijkers ES, de Ruiter T, Baridi A, Veninga H, Hoek RM, Meyaard L. The inhibitory CD200R is differentially expressed on human and mouse T and B lymphocytes. Mol Immunol. 2008;45:1126–1135. doi: 10.1016/j.molimm.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 18.Rygiel TP, Meyaard L. CD200R signaling in tumor tolerance and inflammation: a tricky balance. Curr Opin Immunol. 2012;24:233–238. doi: 10.1016/j.coi.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Petermann KB, Rozenberg GI, Zedek D, Groben P, McKinnon K, Buehler C, Kim WY, Shields JM, Penland S, Bear JE, Thomas NE, Serody JS, Sharpless NE. CD200 is induced by ERK and is a potential therapeutic target in melanoma. J Clin Investig. 2007;117:3922–3929. doi: 10.1172/JCI32163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moreaux J, Veyrune JL, Reme T, De Vos J, Klein B. CD200: a putative therapeutic target in cancer. Biochem Biophys Res Commun. 2008;366:117–122. doi: 10.1016/j.bbrc.2007.11.103. [DOI] [PubMed] [Google Scholar]
- 21.Tonks A, Hills R, White P, Rosie B, Mills KI, Burnett AK, Darley RL. CD200 as a prognostic factor in acute myeloid leukaemia. Leukemia. 2007;21:566–568. doi: 10.1038/sj.leu.2404559. [DOI] [PubMed] [Google Scholar]
- 22.Moreaux J, Hose D, Reme T, Jourdan E, Hundemer M, Legouffe E, Moine P, Bourin P, Moos M, Corre J, Mohler T, De Vos J, Rossi JF, Goldschmidt H, Klein B. CD200 is a new prognostic factor in multiple myeloma. Blood. 2006;108:4194–4197. doi: 10.1182/blood-2006-06-029355. [DOI] [PubMed] [Google Scholar]
- 23.Love JE, Thompson K, Kilgore MR, Westerhoff M, Murphy CE, Papanicolau-Sengos A, McCormick KA, Shankaran V, Vandeven N, Miller F, Blom A, Nghiem PT, Kussick SJ. CD200 expression in neuroendocrine neoplasms. Am J Clin Pathol. 2017;148:236–242. doi: 10.1093/ajcp/aqx071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L, Liu JQ, Talebian F, El-Omrani HY, Khattabi M, Yu L, Bai XF. Tumor expression of CD200 inhibits IL-10 production by tumor-associated myeloid cells and prevents tumor immune evasion of CTL therapy. Eur J Immunol. 2010;40:2569–2579. doi: 10.1002/eji.201040472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Talebian F, Liu JQ, Liu Z, Khattabi M, He Y, Ganju R, Bai XF. Melanoma cell expression of CD200 inhibits tumor formation and lung metastasis via inhibition of myeloid cell functions. PLoS ONE. 2012;7:e31442. doi: 10.1371/journal.pone.0031442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu JQ, Talebian F, Wu L, Liu Z, Li MS, Wu L, Zhu J, Markowitz J, Carson WE, 3rd, Basu S, Bai XF. A critical role for cd200r signaling in limiting the growth and metastasis of CD200+ melanoma. J Immunol. 2016;197:1489–1497. doi: 10.4049/jimmunol.1600052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahadevan D, Lanasa MC, Farber C, Pandey M, Whelden M, Faas SJ, Ulery T, Kukreja A, Li L, Bedrosian CL, Zhang X, Heffner LT. Phase I study of samalizumab in chronic lymphocytic leukemia and multiple myeloma: blockade of the immune checkpoint CD200. J Immunother Cancer. 2019;7:227. doi: 10.1186/s40425-019-0710-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moertel CL, Xia J, LaRue R, Waldron NN, Andersen BM, Prins RM, Okada H, Donson AM, Foreman NK, Hunt MA, Pennell CA, Olin MR. CD200 in CNS tumor-induced immunosuppression: the role for CD200 pathway blockade in targeted immunotherapy. J Immunother Cancer. 2014;2:46. doi: 10.1186/s40425-014-0046-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siva A, Xin H, Qin F, Oltean D, Bowdish KS, Kretz-Rommel A. Immune modulation by melanoma and ovarian tumor cells through expression of the immunosuppressive molecule CD200. Cancer Immunol Immunother. 2008;57:987–996. doi: 10.1007/s00262-007-0429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alapat D, Coviello-Malle J, Owens R, Qu P, Barlogie B, Shaughnessy JD, Lorsbach RB. Diagnostic usefulness and prognostic impact of CD200 expression in lymphoid malignancies and plasma cell myeloma. Am J Clin Pathol. 2012;137:93–100. doi: 10.1309/AJCP59UORCYZEVQO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.D'Arena G, Valvano L, Vitale C, Coscia M, Statuto T, Bellesi S, Lamorte D, Musto P, Laurenti L, D'Auria F. CD200 and prognosis in chronic lymphocytic leukemia: conflicting results. Leuk Res. 2019;83:106169. doi: 10.1016/j.leukres.2019.106169. [DOI] [PubMed] [Google Scholar]
- 32.Rexin P, Tauchert A, Hanze J, Heers H, Schmidt A, Hofmann R, Hegele A. The immune checkpoint molecule CD200 is associated with tumor grading and metastasis in bladder cancer. Anticancer Res. 2018;38:2749–2754. doi: 10.21873/anticanres.12517. [DOI] [PubMed] [Google Scholar]
- 33.Clark DA, Dhesy-Thind S, Ellis P, Ramsay J. The CD200-tolerance signaling molecule associated with pregnancy success is present in patients with early-stage breast cancer but does not favor nodal metastasis. Am J Reprod Immunol. 2014;72:435–439. doi: 10.1111/aji.12297. [DOI] [PubMed] [Google Scholar]
- 34.Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14:399–416. doi: 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Erin N, Podnos A, Tanriover G, Duymus O, Cote E, Khatri I, Gorczynski RM. Bidirectional effect of CD200 on breast cancer development and metastasis, with ultimate outcome determined by tumor aggressiveness and a cancer-induced inflammatory response. Oncogene. 2015;34:3860–3870. doi: 10.1038/onc.2014.317. [DOI] [PubMed] [Google Scholar]
- 36.Rygiel TP, Karnam G, Goverse G, van der Marel AP, Greuter MJ, van Schaarenburg RA, Visser WF, Brenkman AB, Molenaar R, Hoek RM, Mebius RE, Meyaard L. CD200–CD200R signaling suppresses anti-tumor responses independently of CD200 expression on the tumor. Oncogene. 2012;31:2979–2988. doi: 10.1038/onc.2011.477. [DOI] [PubMed] [Google Scholar]
- 37.Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, Matrisian LM, Carbone DP, Lin PC. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6:409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 38.Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 39.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mantovani A, Schioppa T, Porta C, Allavena P, Sica A. Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev. 2006;25:315–322. doi: 10.1007/s10555-006-9001-7. [DOI] [PubMed] [Google Scholar]
- 42.Xiong Z, Ampudia-Mesias E, Shaver R, Horbinski CM, Moertel CL, Olin MR. Tumor-derived vaccines containing CD200 inhibit immune activation: implications for immunotherapy. Immunotherapy. 2016;8:1059–1071. doi: 10.2217/imt-2016-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McWhirter JR, Kretz-Rommel A, Saven A, Maruyama T, Potter KN, Mockridge CI, Ravey EP, Qin F, Bowdish KS. Antibodies selected from combinatorial libraries block a tumor antigen that plays a key role in immunomodulation. Proc Natl Acad Sci USA. 2006;103:1041–1046. doi: 10.1073/pnas.0510081103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wong KK, Khatri I, Shaha S, Spaner DE, Gorczynski RM. The role of CD200 in immunity to B cell lymphoma. J Leukoc Biol. 2010;88:361–372. doi: 10.1189/jlb.1009686. [DOI] [PubMed] [Google Scholar]
- 45.Gorczynski RM, Zhu F. Checkpoint blockade in solid tumors and B-cell malignancies, with special consideration of the role of CD200. Cancer Manag Res. 2017;9:601–609. doi: 10.2147/CMAR.S147326. [DOI] [PMC free article] [PubMed] [Google Scholar]