Abstract
Interleukin-33 (IL-33) regulates innate and acquired immune response to pathogens, self-antigens and tumors. IL-33 effects on tumors depend on the dose and mode of administration along with the type of malignancy. We studied the effects of IL-33 on the development of primary and metastatic melanoma induced by B16-F1 cell line in C57BL/6 mice. Intraperitoneally applied IL-33 restricts primary tumor growth. When administered intranasally 3 days prior to the intravenous injection of the tumor cells, IL-33 promoted growth of B16-F1 melanoma metastases, while B16-F10 gave massive metastases independently of IL-33. To mimic natural dissemination, we next used a limited number (5 × 104) of B16-F1 cells intravenously followed by application of IL-33 intraperitoneally. IL-33 increased the size of metastases (10.96 ± 3.96 mm2) when compared to the control group (0.86 ± 0.39 mm2), without changing incidence and number of metastases. IL-33 increased expression of ST2 on both tumor and immune cells in metastases. Also, IL-33 enhanced eosinophils and anti-tumor NK cells in the lung. The striking finding was reduced cytotoxicity of CD8+ T cells derived from metastatic lung of IL-33 injected mice. IL-33 reduced the percentage of TNF-α+ and IFN-γ+ CD8+ T cells while increasing the frequency of CD8+ T cells that express inhibitory molecules (PD-1, KLRG-1 and CTLA-4). There was a significant accumulation of CD11b+Gr-1+ myeloid suppressor cells and FoxP3+, IL-10+ and CTLA-4+ regulatory T cells in the metastatic lung of IL-33 injected mice. The relevance of IL-33 for melanoma metastases was also documented in a significantly increased level of serum IL-33 in stage III melanoma patients.
Keywords: Melanoma, IL-33, Metastases, CD8+ T cells
Introduction
Melanoma is one of the most aggressive skin cancers, characterized by fast growth and early dissemination of metastatic cells [1]. The lung is one of the most common sites for metastatic spread of melanoma accompanied with low survival rate [2]. Novel immunotherapeutic strategies involve the use of CTLA-4 blocking agents and anti-PD-1 antibodies, as well as selective BRAF inhibitors in the treatment of metastatic disease [3]. Resistance of melanoma cells to chemotherapy and available biologic therapy, as well as their capability to manipulate immune response or even use it for tumor progression, are the main reasons for limited therapeutic options, in particular in metastatic disease [4].
Interleukin-33 (IL-33) is a multifunctional cytokine that performs biological functions through binding to IL-33 receptor complex, composed of membrane-bound ST2 molecule and IL-1 receptor accessory protein [5]. IL-33 participates in various inflammatory and autoimmune diseases with pathological or protective role depending on cellular and cytokine context [6, 7].
The role of IL-33 in the growth and metastasis of malignant tumors is unclear. Previously, we found that the deletion of the ST2 molecule was associated with increased cytotoxic activity of NK cells and enhanced Th1 and Th17 immune response thus contributing to suppression of mouse breast cancer growth and metastasis [8]. Further, exogenous IL-33 decreases NK cell cytotoxicity, promotes the accumulation of immunosuppressive cells in the tumor microenvironment and increases the density of blood vessels thus contributing to tumor progression [9]. Also, IL-33 is involved in mammary tumor growth by facilitating the expression of pro-angiogenic VEGF in tumor cells and attenuating tumor necrosis [10]. IL-33 in human pancreatic carcinoma cells acts as an important mediator of inflammation that promotes carcinogenesis [11]. Elevated systemic IL-33 in patients with gastric cancer is associated with tumor progression mediated by Th2 immune response [12]. IL-33 is implicated in the adenoma-carcinoma transition of human colorectal cancer through regulation of angiogenesis and metastasis by promoting inflammation and remodeling of the tumor microenvironment [13, 14]. IL-33 induces glucose uptake in NSCLC cells, thus stimulating their growth and metastasis, while at the same time creating immunosuppressive tumor microenvironment [15, 16]. Nevertheless, several studies have reported the protective role of IL-33 in tumor progression. Brunner et al. [17] observed that the infiltration of IL-33 positive CD8+ T cells in hepatocellular carcinoma was associated with prolonged patient survival. The expression of IL-33 in tumor tissue is inversely associated with NSCLC progression [18]. Also, IL-33 restores immunosurveillance against metastatic tumors by upregulating antigen processing machinery [19].
Most studies indicate anti-tumor effects of IL-33 on melanoma. Kim et al. [20] reported that ectopic expression of IL-33 in B16-F10 melanoma reduces primary tumor growth by enhancing the accumulation of type 2 innate lymphoid cells with potent anti-tumor activity. In addition, induced tumoral expression of IL-33 promotes optimal anti-melanoma immune response mediated by IFN-γ producing CD8+ T and NK cells [21]. Also, the IL-33/ST2 axis restores activation and maturation of myeloid dendritic cells in established B16-F10 primary tumor, and thereby the magnitude of anti-tumor immunity [22]. More recently, primary melanoma growth was significantly limited in mice challenged with irradiated B16-IL-33 cells [23]. The transgenic expression of IL-33 in the lungs inhibits B16 tumor growth and metastasis by enhancing cytotoxic capacities of CD8+ T and NK cells [24]. Intranasal IL-33 administration inhibits B16-F10 metastasis by enhancing the tumoricidal activity of eosinophils [25].
Apart from the restrictive effect of IL-33 on primary tumor growth, we observed that IL-33 exhibited quite opposite effects on murine melanoma metastasis. Intraperitoneal or intranasal application of IL-33 markedly promoted growth of pulmonary metastases of low metastatic B16-F1 melanoma cell variant, thus questioning its previously implicated beneficial effects or even therapeutic potential. Our data suggest that the systemic application of IL-33 promotes the growth of pulmonary B16-F1 metastatic colonies by attenuating acquired immune response mediated by CD8+ T cells and creating immunosuppressive metastatic microenvironment.
Materials and methods
Murine melanoma models and IL-33 treatments
For the assessment of tumor growth, C57BL/6 mice (8–12 weeks old) were injected subcutaneously with 0.8 × 106 B16-F1 or B16-F10 cells. Recombinant mouse IL-33 (eBioscience, San Diego, CA, USA), 0.4 µg per dose, dissolved in 100 µl of PBS, was injected intraperitoneally 5 times, every other day, starting from day 0 of the experiment (Fig. 1a) [25]. The control group consisted of mice injected with PBS. Mice survival was monitored daily, and tumor growth was measured every other day using a caliper.
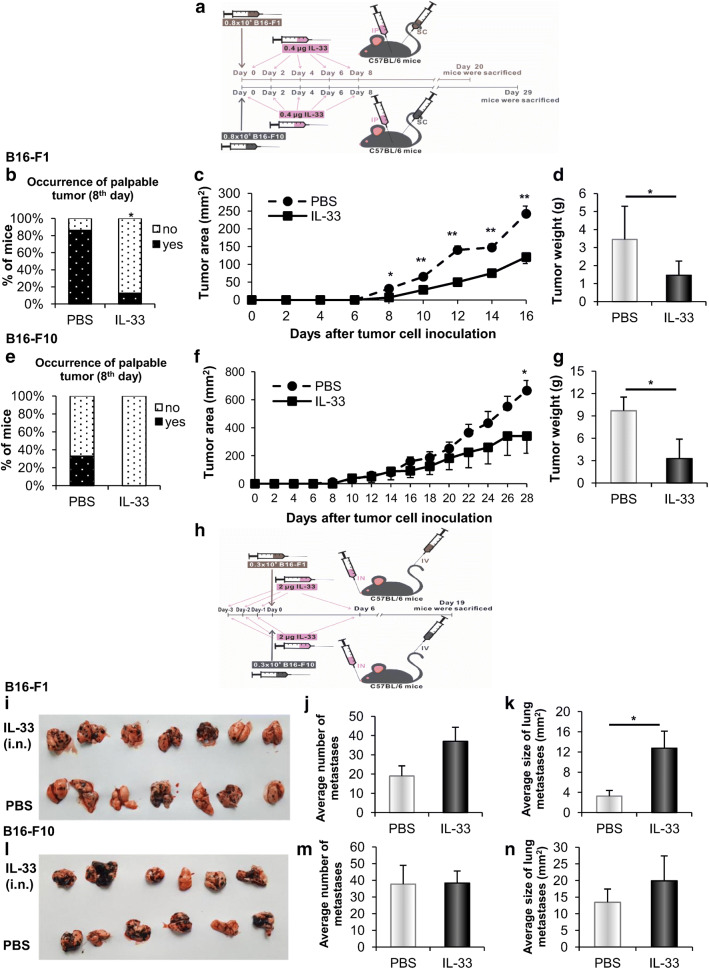
Fig. 1.
IL-33 restricts primary tumor and promotes the growth of pulmonary metastatic colonies. a C57BL/6 mice (n = 6–8) were injected subcutaneously with 0.8 × 106 B16-F1 or B16-F10 melanoma cells and treated intraperitoneally with IL-33 or PBS. b–d Occurrence of palpable B16-F1 melanoma, tumor diameter measured every other day, and tumor weight measured after necropsy in indicated groups. e–g Occurrence of palpable B16-F10 melanoma, tumor diameter and weight in indicated groups. h Mice (n = 6–7) were treated intranasally with IL-33 or PBS and then injected intravenously with 0.3 × 106 B16-F1 or B16-F10 melanoma cells. i–k Macroscopic photos of lungs, the average number of metastases enumerated by light microscope, and the average size of metastases measured using ImageJ software from B16-F1 melanoma-bearing mice injected with IL-33 or PBS. l–n Macroscopic photos of lungs, average number and average size of metastases from B16-F10 melanoma-bearing mice injected with IL-33 or PBS. Data are presented as mean ± SE of all mice in each group, *p < 0.05, **p < 0.01. IP: intraperitoneal; SC: subcutaneous; IN: intranasal; IV: intravenous
For experimental metastasis assays, mice were injected intravenously with 0.3 × 106 B16-F1 or B16-F10 cells. IL-33 treatment (2 µg/20 µl PBS per dose) consisted of 4 intranasal instillations: 3 consecutive days before and on day 6 after tumor cell injection [25]. The control group received PBS. Mice were euthanized on day 19 after tumor cell inoculation (Fig. 1h).
In other experiments, we injected a relatively small number of B16-F1 cells (0.5 × 105) intravenously, as this size of inoculum more closely mimics conditions of natural tumor cell dissemination. Recombinant mouse IL-33 (0.4 µg/100 µl PBS per dose) was injected intraperitoneally 5 times, every other day, starting from day 0 of the experiment. The control group consisted of mice injected with PBS. Mice were sacrificed on the 18th day of the experiment (Fig. 2a).
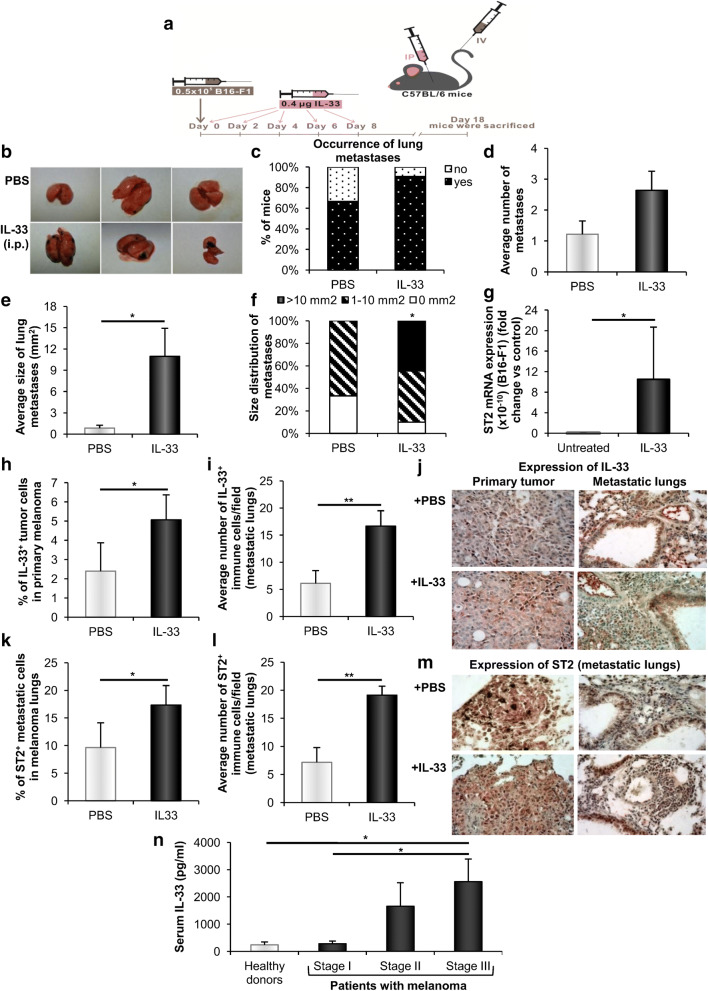
Fig. 2.
Systemic application of IL-33 increases pulmonary melanoma metastases. a Mice (n = 9–11) were injected intravenously with 0.5 × 105 B16-F1 and treated intraperitoneally with IL-33 or PBS. b Representative photographs of lungs from melanoma-bearing mice injected with IL-33 or PBS. c, d Occurrence and average number of metastases. e, f Average size and size distribution of metastases. g Relative mRNA ST2 expression in B16-F1 cells. GAPDH mRNA was used as an internal control. Data are presented as mean ± SE fold of control in B16-F1 cells. h, i Immunohistochemical analysis of IL-33 expression in B16-F1 primary tumor cells and immune cells in metastatic lungs. j Representative photographs of IL-33 expression (magnification at × 400). k, l Immunohistochemical analysis of ST2 expression in B16-F1 metastatic cells and immune cells in metastatic lungs. m Representative images of ST2 expression (magnification at × 400). n Determination of IL-33 serum levels in melanoma patients and healthy volunteers (n = 20–30 per group). Data are presented as mean ± SE of all samples in each group, *p < 0.05, **p < 0.01. IP: intraperitoneal; IV: intravenous
Quantification of lung metastasis
Lungs were collected after euthanasia of mice and fixed in 4% formalin. Hematoxylin–eosin staining was performed using paraffin-embedded melanoma-bearing lung sections. To avoid missing micrometastases, stained sections from at least three different levels were examined for the presence of metastases. Metastases were enumerated by a light microscope, and metastatic areas were quantified using ImageJ software.
Immunohistochemistry
Deparaffinized tissue sections of primary B16-F1 tumor and lung tissue were incubated with anti-mouse IL-33 (sc-98660, Santa Cruz Biotechnology, Santa Cruz, CA, USA) or ST2 antibody (PA5-20077, Thermo Fisher Scientific Inc.). As negative control, the primary antibody was omitted. Staining was visualized by using Rabbit specific HRP/AEC (ABC) Detection IHC Kit (ab64260, Abcam, Cambridge, UK). Sections were photomicrographed with a digital camera mounted on a light microscope (Olympus BX51, Japan), digitized, and analyzed. The IL-33 and ST2-positive cells were determined by counting in five randomly selected fields (at magnification 400 ×). The data were summarized as the mean percentage or number of positive cells.
Isolation of lung leukocytes
Eighteen days after B16-F1 (0.5 × 105) melanoma cell injection, IL-33 or PBS treated mice were sacrificed and lungs were perfused by injection of saline to the right heart ventricle. Lungs were dissected and processed by mechanical disruption with scissors, followed by enzymatic digestion using RPMI with 1 mg/ml collagenase I, 1 mM EDTA and 2% FBS (all from Sigma-Aldrich). The digested lung tissue was then incubated with 4 ml of trypsin and DNase I (Rosche Diagnostic), followed by passing through a 40-μm nylon filter. Single-cell suspensions were then used for flow cytometry.
Flow cytometric analyses
To analyze the presence and phenotype of leukocytes in metastatic lung, the following fluorochrome-conjugated monoclonal antibodies were used: anti-mouse Siglec-F, CD11b, CD3, CD8, CD4, PD-1, CD69, KLRG-1, CTLA-4, CD11b, Gr-1, NKp46, CD49b (BD Biosciences/Miltenyi Biotec GmbH, Bergisch Gladbach, Germany/Biolegend, San Diego, CA, USA/Invitrogen) or their respective isotype controls. For intracellular staining, the cells were stimulated with phorbol 12-myristate 13-acetate (Sigma-Aldrich), ionomycin (Sigma-Aldrich) and Golgi Stop (BD Biosciences). Subsequently, the cells were fixed and permeabilized using Cytofix/Cytoperm kit (BD Biosciences) and labeled with anti-mouse TNF-α, IFN-γ, IL-10 and FoxP3 (BD Biosciences/Biolegend/R&D Systems Inc.). Isotype controls were included to set gates. The cells were analyzed with FACSCalibur Flow Cytometer (BD Biosciences), and the analysis was conducted with FlowJo (Tree Star).
Real-time PCR analysis
Total RNA was extracted from untreated B16-F1 cells or treated with 200 ng/ml of recombinant mouse IL-33 for 48 h, or from frozen lungs derived from melanoma-bearing mice treated with IL-33 or PBS according to the manufacturer’s instructions. First-strand cDNA was synthesized by RevertAid H Minus First Strand cDNA Synthesis Kit (Thermo Fisher Scientific Inc., Waltham, MA, USA). Power SYBR MasterMix (Applied Biosystems, Waltham, MA, USA) was used for performing qRT-PCR and mRNA specific primers for IL-33, ST2, MHC-I H-2b, TAP1b, Foxp3, IL-10, TGF-β and Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as a housekeeping gene (Table 1). Relative expression of genes was calculated according to the formula 2−(Ct-CtGAPDH), where Ct is the cycle threshold of the gene of interest and CtGAPDH is the cycle threshold value of the housekeeping gene (GAPDH).
Table 1.
Primers used for qRT-PCR analysis
| IL-33 | 5′-TCCTTGCTTGGCAGTATCCA-3′ | 5′-TGCTCAATGTGTCAACAGACG-3′ |
|---|---|---|
| ST2 | 5′-GCAATTCTGACACTTCCCATG-3′ | 5′-ACGATTTACTGCCCTCCGTA-3′ |
| MHC-I H-2b | 5′-GCGAGGGTGGCTCTCACACG-3′ | 5′-TCAGGGTGAGGGGCTCAGGC-3′ |
| TAP1b | 5′-CAAACCAGCCCAAAGTCCAG-3′ | 5′-AGAAGAACCGTCCGAGAAGC-3′ |
| Foxp3 | 5′-TGGTTTACTCGCATGTTCGC-3′ | 5′-CCCACCTTTTCTTGGTTTTG-3′ |
| IL-10 | 5′-CCCTGGGTGAGAAGCTGAAG-3′ | 5′-CACTGCCTTGCTCTTATTTTCACA-3′ |
| TGF-β | 5′-ATTCCTGGCGTTACCTTG-3′ | 5′-CTGTATTCCGTCTCCTTGGTT-3′ |
| GAPDH | 5′-AACTTTGGCATTGTGGAAGG-3′ | 5′-CACATTGGGGGTAGGAACAC-3′ |
Isolation of CD8+ T cells
CD8+ T cells were purified from the lungs of melanoma-bearing mice injected with IL-33 or PBS using a magnetic cell separation kit (Miltenyi Biotec Inc., Auburn, CA, USA) as previously described [26]. Cell suspensions containing CD8+ T cells were then used in cytotoxicity assays.
Cytotoxicity assay
Cytotoxic potential of CD8+ T cells was analyzed in a real-time cytotoxicity assay with an xCELLigence RTCA (Real-Time Cell Analyzer) DP (Dual Plate) Instrument (ACEA Biosciences, San Diego, CA, USA) according to manufacturer’s recommendations. B16-F1 cells (1 × 104/well) were seeded in E-plate 16 and used as target cells. After 34 h, isolated CD8+ T cells (ratio of target to effector cells T:E = 1:10) derived from the lung were added in the plates as effector cells. During the next 48 h of real-time monitoring, the cytotoxic potential of CD8+ T cells was determined by measuring the B16-F1 cell index. For plotting purposes, the percentage of cytolysis was calculated using the formula: Percentage of cytolysis = ((Cell Index no effector − Cell Index effector)/Cell Index no effector) × 100.
Detection of serum levels of IL-33 in melanoma patients
Seventy-two melanoma patients were recruited from the Clinics of Dermatovenerology and Plastic Surgery, Military Medical Academy, Belgrade, Serbia. The eighth edition of the American Joint Committee on Cancer (AJCC) and TNM melanoma staging and classification were used. Sera samples were obtained from these patients before the treatment. Sera samples from 29 healthy volunteers with no evidence of illness were used as control samples. Serum IL-33 levels were determined using ELISA kits (R&D Systems Inc., Minneapolis, MN, USA) specific for human IL-33, according to the manufacturer’s instructions.
Statistical analysis
The data were analyzed using statistical package SPSS, version 21. The normality of distribution was tested by the Kolmogorov–Smirnov test. The two-tailed Student’s t test, Fisher’s exact test or nonparametric Mann–Whitney rank-sum test were used. The results were considered significantly different when p < 0.05 and highly significantly different when p < 0.01.
Results
IL-33 attenuates primary tumor and enhances hematogenous metastases
To investigate the effects of IL-33 on primary tumor growth, we initially used B16-F1 cells with low metastatic potential and B16-F10 highly metastatic variant (Fig. 1a) [27]. On day 8 after tumor inoculation, the frequency of B16-F1 palpable tumor was markedly lower in IL-33 injected mice (Fig. 1b). Treatment with IL-33 was also associated with a significant reduction of melanoma growth (Fig. 1c). Further, the tumor weight measured on day 20 was markedly lower in IL-33 treated mice in comparison to the untreated mice (Fig. 1d). The reduction in melanoma growth, as well as the tumor weight, was also observed in B16-F10 tumors (Fig. 1e–g). These results showed a restrictive effect of IL-33 on primary melanoma growth.
The lungs are common and often the first clinically apparent site of visceral melanoma metastasis [28]. Given the fact that intranasal therapy might be a useful approach, delivering therapeutics directly into the lungs [29] and potentially decreasing the establishment of metastases, we proceeded to observe the effect of intranasal instillation of IL-33 (Fig. 1h). While B16-F10 gave massive metastases without any significant difference between IL-33 injected and control mice (Fig. 1l–n), we unexpectedly observed that IL-33 administered with B16-F1 cells with lower metastatic potential induced a significant increase in the size of metastases (Fig. 1k). However, the increase in the number of lung metastases did not reach statistical significance (Fig. 1i, j). In contrast to the restrictive effect on primary melanoma growth, it appeared that IL-33 enhanced melanoma metastases.
Intraperitoneal IL-33 enhances ST2 expression in tumor and immune cells and stimulates growth of pulmonary B16-F1 metastatic colonies
Further, we used a lower number (5 × 104) of B16-F1 cells with lower metastatic potential compared to the B16-F10 cell line to investigate whether intraperitoneal treatment with IL-33, as a convenient option for the treatment of metastatic cancer, could affect the establishment of lung metastases (Fig. 2a). While the difference in the incidence (10/11; 90.9% vs. 6/9; 66.7%, p < 0.05) and number (2.64 ± 0.62 vs. 1.22 ± 0.43, p < 0.05) of metastases did not reach statistical significance, there was a striking difference in the size of lung metastases (Fig. 2b–f). IL-33 treatment led to about tenfold increase in the average size of lung metastases in comparison to untreated mice (Fig. 2e). Moreover, about half of IL-33 injected mice had metastases larger than 10 mm2, while in the control group we did not detect any metastases of the same size (Fig. 2f).
Quantitative RT-PCR analysis did not reveal constitutive or IL-33 induced IL-33 mRNA expression in B16-F1 cells (data not shown). There was very low constitutive ST2 mRNA expression in B16-F1 cells, which was significantly increased by IL-33 treatment (Fig. 2g). We also analyzed the expression of IL-33 and ST2 in the primary tumor and metastatic lungs by immunohistochemistry. In IL-33 treated mice, the percentage of IL-33+ tumor cells was increased in primary melanoma (Fig. 2h, j), but not in melanoma lungs (data not shown). In addition, the number of IL-33+ immune cells in metastatic lungs was increased (Fig. 2i, j), while there was no difference in the number of IL-33+ immune cells in primary melanoma (data not shown). IL-33 treatment did not change the percentage of ST2+ tumor cells in primary melanoma (data not shown), while increasing the percentage of ST2+ metastatic cells (Fig. 2k, m). The increased number of ST2+ immune cells was observed only in the lungs of melanoma-bearing mice treated with IL-33 (Fig. 2l, m).
Serum IL-33 increases in stage III melanoma patients
To investigate the relevance of observed IL-33 effects on murine melanoma for corresponding human pathology, we determined concentrations of serum IL-33 in melanoma patients. The patients with developed regional lymph node metastases (stage III) had significantly increased serum levels of IL-33 in comparison to the patients without lymph node metastases (stage I) or healthy donors (Fig. 2n), indicating pro-metastatic role of IL-33. However, there was no increase in IL-33 serum level of stage I patients when compared to healthy control.
IL-33 enhances eosinophils and anti-tumor NK cells
A recent study showed that eosinophil-mediated anti-melanoma immunity induced by IL-33 limited tumor growth and metastasis [25]. Our data revealed that IL-33 enhanced the total number of lung leukocytes (data not shown), with predominance of Siglec-F+CD11b+ eosinophils (Fig. 3a, b). Analysis of intracellular molecules expression showed that IL-33 reduced the percentage of eosinophils that produce TNF-α, while at the same time increased the total number of eosinophils in melanoma-bearing, but not in naive mice (Fig. 3c, d). IL-33 also reduced the percentage of IL-10 positive eosinophils, but without significant effect on the total number of these cells (Fig. 3e, d).
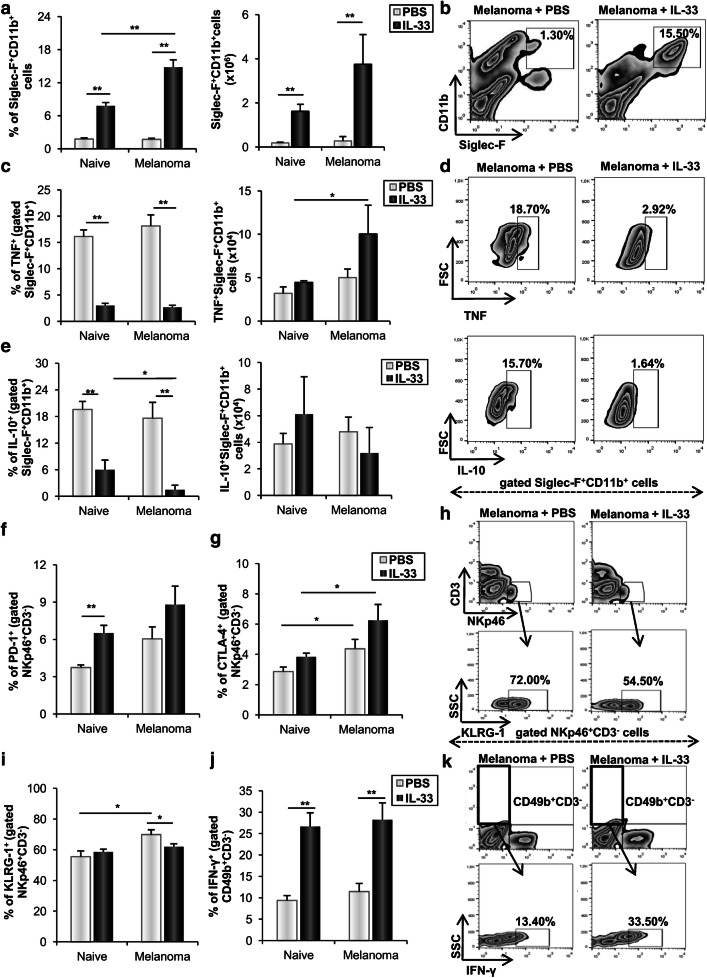
Fig. 3.
IL-33 enhances eosinophils and anti-tumor NK cells in metastatic lungs. Mice were injected intravenously with 0.5 × 105 B16-F1 and treated intraperitoneally with IL-33 or PBS. a The percentage and the total number of Siglec-F+CD11b+ eosinophils. b Representative plots show Siglec-F+CD11b+ eosinophils in IL-33 injected and untreated mice. c–e The frequencies and total number of TNF-α and IL-10 positive Siglec-F+CD11b+ eosinophils. Representative plots show eosinophils expressing TNF-α and IL-10 in mice treated with IL-33 or PBS. f, g The percentage of NKp46+CD3− NK cells expressing PD-1 and CTLA-4. h, i The frequencies of KLRG-1 positive NKp46+CD3− NK cells. Representative plots illustrate NKp46+CD3− NK cells expressing KLRG-1 in mice treated with IL-33 or PBS. j, k The percentage CD49b+CD3− NK cells expressing IFN-γ was additionally demonstrated by representative plots. Data are presented as mean ± SE of all mice in each group (n = 6), *p < 0.05, **p < 0.01
Subsequent analysis of cellular make-up of metastatic tissue revealed that IL-33 did not affect the percentage of NKp46+CD3− NK cells (data not shown), while it significantly increased the percentage of PD-1 positive NK cells in naive mice (Fig. 3f). However, this difference did not reach statistical significance in melanoma-bearing mice. Further, mice challenged with melanoma exhibited a higher percentage of CTLA-4+ NK cells, independently of IL-33 (Fig. 3g). The percentage of Killer cell lectin-like receptor subfamily G member 1 (KLRG1) expressing NK cells was enhanced in melanoma-bearing mice. Interestingly, IL-33 decreased the frequency of these cells in melanoma-bearing, but not in naive mice (Fig. 3i, h). Also, IL-33 increased the percentage of IFN-γ positive CD49b+CD3− NK cells in tumor independent manner (Fig. 3j, k). Our results point out that IL-33 stimulated the growth of melanoma pulmonary metastases despite the stimulation of innate immune response.
IL-33 attenuates CD8+ T cells cytotoxicity
IL-33 does not affect the percentage but reduces the cytotoxic capacity of CD8+ T cells
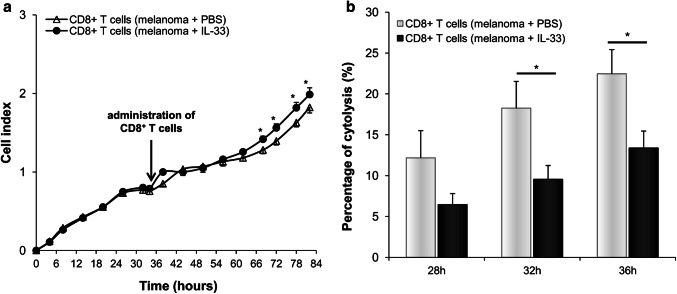
Given the importance of CD8+ T cells in anti-melanoma immune response, we initially tested the effects of IL-33 on the cytotoxic capacities of lung CD8+ T cells from melanoma-bearing mice against tumor cells. Cytotoxic activity of CD8+ T effector cells was determined indirectly by measuring the B16-F1 cell index, as target cells. There was a significant increase in the B16-F1 cell index following co-cultivation with CD8+ T cells of melanoma-bearing mice treated with IL-33 in comparison with untreated mice (Fig. 4a), thus indicating decreased cytotoxicity of effector cells. Cytolytic activity of CD8+ T cells derived from melanoma-bearing mice treated with IL-33 was significantly lower starting from 32 h after effector cells were added (Fig. 4b).
Fig. 4.
IL-33 reduces the cytotoxic capacity of CD8+ T cells. a B16-F1 cells, as target cells, were incubated with lung CD8+ T cells derived from melanoma-bearing mice injected with IL-33 or PBS. The cell index of B16-F1 cells was monitored by the xCELLigence system. b Cytolytic activity of CD8+ effector T cells was calculated as described in Material and method at 28, 32 and 36 h after effector cells were added and showed as the percentage of B16-F1 cytolysis. Data are presented as mean ± SE of all mice in each group (n = 6), *p < 0.05
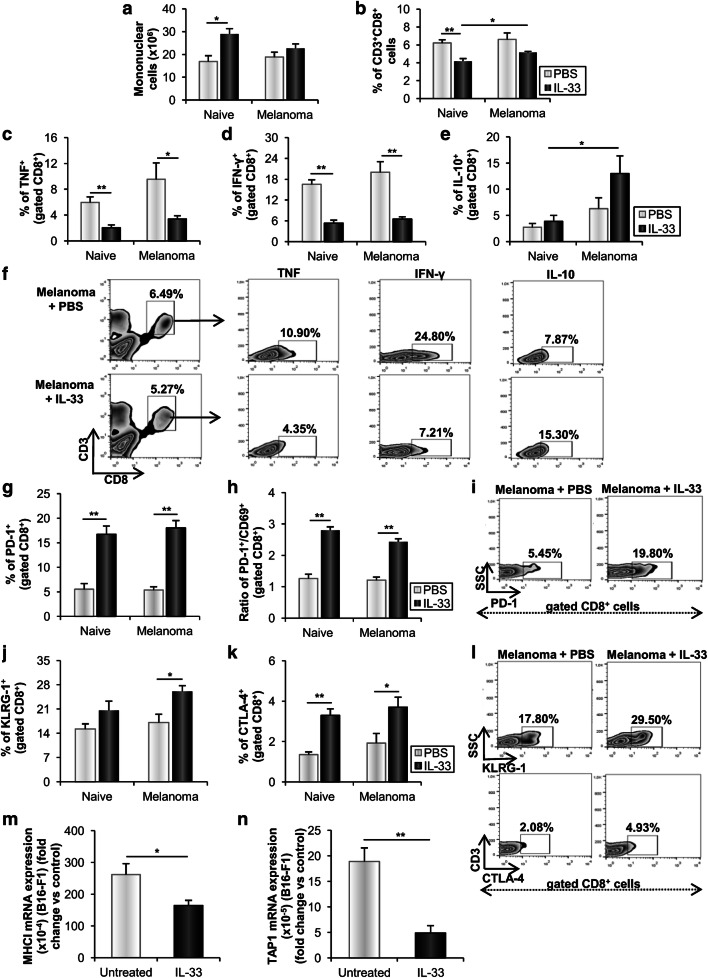
To further assess the effect of IL-33 on CD8+ T effector cells, we analyzed the presence and phenotype of these cells in metastatic lung. IL-33 did not affect the total number of mononuclear cells and the percentage of CD3+CD8+ T cells in melanoma-bearing mice (Fig. 5a, b). However, IL-33 reduced the percentage of lung CD8+ T cells expressing pro-inflammatory cytokines TNF-α and IFN-γ in tumor independent manner (Fig. 5c, d, f). In addition, in the presence of metastatic melanoma IL-33 enhanced the frequency of IL-10 positive CD8+ T cells (Fig. 5e, f).
Fig. 5.
IL-33 affects the expression of TNF-α, IFN-γ and IL-10 and enhances the expression of inhibitory receptors among CD8+ T cells and decreases the immunogenicity of B16-F1 cells. a, b Total number of lung mononuclear cells and percentage of CD3+CD8+ T cells. c–f The frequencies of lung CD8+ T cells expressing TNF-α, IFN-γ and IL-10 were additionally demonstrated by representative plots. g, h The percentage of PD-1+CD8+ T cells and ratio of PD-1+ versus CD69+CD8+ T cells. i Representative plots illustrate PD-1 expression among CD8+ T cells. j, k The percentage of lung KLRG-1+CD8+ T cells and CTLA-4+CD8+ T cells. l Representative plots illustrate the expression of KLRG-1 and CTLA-4 among CD8+ T cells. Data are presented as mean ± SE of all mice in each group (n = 6). m, n Relative mRNA MHC I and TAP1 expression in B16-F1 cells. GAPDH mRNA was used as an internal control. Data are presented as mean ± SE fold of control in B16-F1 cells. *p < 0.05, **p < 0.01
IL-33 stimulates the expression of inhibitory receptors on CD8+ T cells
IL-33 significantly increased the percentage of PD-1+CD8+ T cells in both naive and melanoma-bearing mice (Fig. 5g, i). In addition, IL-33 significantly increased the frequency of cytotoxic T cells expressing early activation receptor CD69 in melanoma-bearing, but not in naive mice (data not shown). However, the ratio of PD-1+ versus CD69+ cytotoxic T cells was markedly enhanced in both IL-33 treated naive and melanoma-bearing mice (Fig. 5h). Next, IL-33 increased the expression of a marker of cellular differentiation and co-inhibitory receptor KLRG-1 among CD8+ T cells in melanoma-bearing mice, indicating lower cytotoxic capacity of these cells (Fig. 5j, l). Also, IL-33 increased the percentage of CD8+ T cells expressing inhibitory receptor CTLA-4 in both naive and melanoma-bearing mice (Fig. 5k, l) further contributing to the impaired tumoricidal potential of cytotoxic T cells. These data indicated lower cytotoxic activity of lung CD8+ T cells from metastatic IL-33 treated mice.
In addition, IL-33 downregulated MHC I and transporter associated with antigen processing 1 (TAP1) mRNA expression in B16-F1 cells (Fig. 5m, n), suggesting that IL-33 reduced immunogenicity of B16-F1 cells and their recognition by CD8+ T cells.
IL-33 creates immunosuppressive microenvironment by enhancing the accumulation of Tregs and MDSCs in metastases
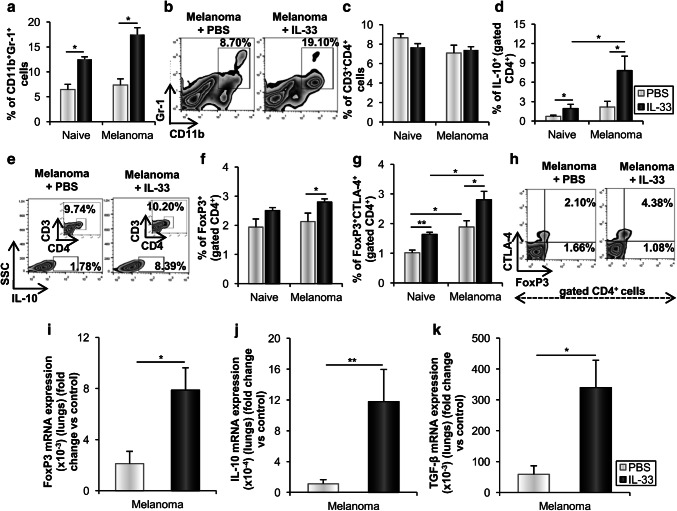
To investigate whether IL-33 affects immunosuppressive cells in metastatic lung, we next analyzed the presence of MDSCs and regulatory T cells. IL-33 enhanced the frequency of CD11b+Gr-1+ MDSCs in both naive and melanoma-bearing mice (Fig. 6a, b). IL-33 did not affect the percentage of CD3+CD4+ cells (Fig. 6c), but the analysis of the expression of intracellular molecules showed that IL-33 significantly increased the percentage of IL-10 positive CD4+ T lymphocytes in both naive and melanoma-bearing mice (Fig. 6d, e). It appears that IL-33 increased the percentage of FoxP3+CD4+ regulatory T cells in melanoma-bearing, but not in naive mice (Fig. 6f). However, the percentage of FoxP3 and CTLA-4 double-positive CD4+ regulatory T cells was significantly higher in melanoma-bearing mice, which was further enhanced by IL-33 (Fig. 6g, h). Transcriptional profiling of metastatic lungs showed increased FoxP3, IL-10 and TGF-β mRNA expression in IL-33 treated mice (Fig. 6i–k).
Fig. 6.
IL-33 enhances the accumulation of MDSCs and Tregs in the metastatic lungs. a, b The percentage of CD11b+Gr-1+ MDSCs. Representative plots are shown in melanoma-bearing IL-33 treated and untreated mice. c, d The percentage of CD3+CD4+ T cells and IL-10+CD4+ T cells. e Representative plots show the expression of IL-10 among CD4+ T cells in melanoma-bearing IL-33 and untreated mice. f, g The percentage of FoxP3+CD4+ Tregs and CTLA-4+FoxP3+CD4+ Tregs. h Representative plots illustrate expression of FoxP3+ and CTLA-4+ among CD4+ T cells. Data are presented as mean ± SE of all mice in each group (n = 6). i–k Expression of mRNA FoxP3, IL-10 and TGF-β in metastatic lungs. GAPDH mRNA was used as an internal control. Data are presented as mean ± SE fold of control. *p < 0.05, **p < 0.01
Discussion
Recent studies revealed that IL-33 limited primary melanoma growth primarily through CD8+ T and NK cells [21–23, 25]. In line with these findings, we found that systemic application of IL-33 restricted primary melanoma tumor growth in both B16-F1 (Fig. 1b–d) and B16-F10 melanoma (Fig. 1e–g). As an alarmin, IL-33 is a key initiator of acute local inflammation, thus participating at several points in shaping innate and adaptive immune responses [30]. In pathological conditions, with loss of epithelial integrity such as tumors, IL-33 promotes anti-tumor Th1 immune response [5]. It was argued that IL-33 had a significant role in cancer immunosurveillance against primary tumors, which failed during the metastatic transition [19].
Further, transgenic expression of IL-33 in the lungs inhibits growth and metastasis of B16 melanoma by increasing the cytotoxic capacity of CD8+ T cells and NK cells [24]. Local application of IL-33 inhibits the number and size of B16 melanoma metastases in the lungs, through activation of tumoricidal eosinophils [25]. It had been suggested that IL-33 acts ambiguously in different pathological conditions, but the role of IL-33 needs to be elaborated in response to tumors [31]. Intranasal administration of IL-33 promoted the growth of pulmonary metastases of low metastatic B16-F1 melanoma variant (Fig. 1k). Therefore, IL-33 may contribute to both pro- and anti-melanoma mechanisms, possibly depending on the inherent characteristics of tumor cells and tumor stage.
To our knowledge, this is the first report to show that the systemic application of IL-33, as the most convenient approach for tumor therapy, promotes B16-F1 metastatic growth in the lungs (Fig. 2e, f). This correlates with the downregulatory effects of IL-33 in mammary carcinoma and inflammatory diseases [7–10].
As previously reported, the deletion of ST2 enhances anti-tumor immune response, resulting in a suppression of mouse breast cancer growth and metastases [8]. Here, we showed that IL-33 increased ST2 mRNA expression in B16-F1 cells in vitro (Fig. 2g), suggesting possible direct effects of IL-33 on melanoma cells. Also, IL-33 increased the expression of ST2 on both tumor and immune cells in metastases (Fig. 2k–m).
Elevated serum levels of IL-33 in gastric cancer patients were found to correlate with an advanced stage of the disease and poor prognosis [12]. Accordingly, melanoma patients with lymph node metastases at stage III had significantly higher concentration of IL-33 in comparison with patients without metastases (stage I) or healthy individuals (Fig. 2n).
Intranasal or intraperitoneal administration of IL-33, as well as over-expression of IL-33 in transgenic mice, has been associated with eosinophilic inflammation and airways hyper-responsiveness [32, 33]. It has been reported that eosinophils can play a significant role in melanoma anti-tumor immune response [25]. After the injection of B16-F1 cells with lower metastatic potential [27], IL-33 stimulated a significant accumulation of the eosinophils in the lungs (Fig. 3a, b), but the growth of metastases was not affected (Fig. 2e, f).
IL-33 may promote as well as suppress the anti-tumor response of NK cells [8, 9, 21, 24, 25]. Here, we showed that IL-33 did not significantly affect the percentage of PD-1 and CTLA-4 positive NK cells, while it decreased the percentage of NK cells expressing inhibitory KLRG-1 receptor in melanoma-bearing mice (Fig. 3f–i). KLRG-1 is a co-inhibitory receptor of NK cells and antigen-experienced human T cells [34], involved in impaired anti-tumor immunity of memory T cells in the tumor microenvironment [35]. In addition, IL-33 markedly increased the frequency of IFN-γ positive NK cells (Fig. 3j, k). Although there is abundant evidence of anti-tumor effects of IFN-γ, it appears that IFN-γ induces expression of PD-L1 on melanoma cells and downregulates melanoma antigens subsequently suppressing CD8+ T cell activation [36, 37]. IL-33 stimulates the expression of PD-1 on CD8+ T cells (Fig. 5g, i), indicating that IL-33 mediates the production of IFN-γ by NK cells that suppresses cytotoxicity of CD8+ T cells through enhanced expression of PD-L1 on melanoma cells. In addition, lower expression of KLRG-1 is inversely associated with the enhanced production of IFN-γ by NK cells [38].
CD8+ T cells are prime mediators of tumor immunosurveillance in patients with melanoma [39]. A recent study revealed that IL-33 upregulated IFN-γ, TNF-β, CD107a and granzyme B while it reduced the expression of IL-10 among CD8+ T cells in vitro [23]. We reported significantly lower cytolytic activity of CD8+ T cells derived from the lungs of melanoma-bearing mice treated with IL-33 (Fig. 4). Also, IL-33 reduced the percentage of CD8+ T cells expressing TNF-α and IFN-γ, while enhancing the frequency of IL-10 positive CD8+ T cells in metastatic melanoma (Fig. 5c–f), thus indicating their non-cytotoxic or Th2-like phenotype [40].
Further, IL-33 significantly increased the percentage of PD-1+ exhausting, as well as CD8+ T cells expressing inhibitory CTLA-4 receptor (Fig. 5g, i, k, l). The therapeutic application of anti-CTLA-4 and anti-PD-1 antibodies has already resulted in significant improvements in disease outcomes of various human cancers, especially melanoma [41]. Ligation of these checkpoint receptors at the tumor site leads to T cell exhaustion and downregulation of effector functions and their blockade can restore T cell activation and function leading to improved anti-tumor immunity [42]. IL-33 increased the percentage of KLRG1+CD8+ T cells in the lungs of the melanoma-bearing mice (Fig. 5j, l). Our results showed that IL-33 suppressed adaptive immune response through changes in the functional characteristics of CD8+ T cells in metastatic lungs.
Complementation of IL-33 expression in murine metastatic lung carcinoma cells (A9) upregulates expression and functionality of MHC molecules, thus increasing tumor cell immunogenicity and recognition by CD8+ T cells [19]. In addition to the suppressive effect on CD8+ T cells, our results showed that IL-33 downregulated MHC I and TAP1 mRNA expression in B16-F1 cells (Fig. 5m, n), consequently reducing their immunogenicity that can lead to evasion of immune response and progression of metastatic disease.
Recruitment of MDSCs and regulatory T cells within the tumor microenvironment is an important mechanism that contributed to the failure of anti-tumor immunity [43, 44]. We have previously shown that systemic administration of IL-33 promoted the accumulation of immunosuppressive cells in 4T1 mammary tumors [9]. Herein, IL-33 increased the percentage of MDSCs (Fig. 6a, b), thus contributing to immunosuppressive microenvironment favorable for melanoma metastatic growth. Wen et al. [43] reported that stromal IL-33 was highly expressed in the advanced stage of head and neck squamous cell carcinoma and positively correlated with poor prognosis and Treg cells. Accordingly, systemic administration of IL-33 increased the percentage of FoxP3+CD4+ regulatory T cells in melanoma-bearing mice (Fig. 6f). We have also shown that IL-33 significantly increased the frequency of CD4+IL-10+ as well as CD4+FoxP3+CTLA-4+ Tregs (Fig. 6d, e, g, h). IL-10 produced by Tregs is expected to be a major obstacle to CD8+ T cells mediated tumor lysis [45]. IL-33 treatment led to a significant increase in the expression of FoxP3, IL-10 and TGF-β mRNA in the lungs (Fig. 6i–k), further indicating immunosuppressive metastatic microenvironment.
It appears that the effect of IL-33 on melanoma metastases depends on the metastatic capacity of the melanoma cell line. Here, we showed that IL-33 promoted growth of pulmonary metastases in B16-F1 low metastatic melanoma through the suppression of acquired anti-melanoma immunity. IL-33 reduced the cytotoxic capacity of CD8+ T cells and stimulated the accumulation of immunosuppressive cells in tumor microenvironment. These effects of systemic IL-33 application question its usage in metastatic melanoma therapy in humans.
Acknowledgements
The authors thank Professor Milan Knezevic for great help in pathohistological analyses and Aleksandar Arsenijevic, Aleksandar Ilic and Dusan Tomasevic for excellent technical assistance.
Abbreviations
- AJCC
American Joint Committee on Cancer
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- KLRG1
Killer cell lectin-like receptor subfamily G member 1
- TAP1
Transporter associated with antigen processing 1
Author contributions
AJ performed most of the experiments, analyzed the data and drafted the manuscript. GDR contributed to the design and interpretation of the experiments, analyzed the data, generated figures and revised the manuscript. JP contributed to the design and interpretation of the experiments, assisted and/or performed some of the experiments, analyzed the data and revised the manuscript. IJ, MM and DV assisted and/or performed some of the experiments and analyzed the data. IS oversaw the consent and contributed to the collection of human samples. NA and MLL coordinated the design and execution of the experiments, data analysis, and manuscript drafting and revision. All authors approved the final version of the manuscript.
Funding
This work was funded by grants from the Ministry of Education, Science and Technological Development, Serbia (ON 175071, ON 175069 and ON 175103), a bilateral project with People’s Republic of China (06/2018) and by the Faculty of Medical Sciences of the University of Kragujevac, Serbia (MP 02/14).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Cell line authentication
The murine melanoma B16-F1 and B16-F10 cell lines, purchased from American Type Culture Collection (ATCC, Manassas, USA), were routinely cultured. Cell culture was performed under standardized protocols to ensure that phenotypically similar cells are implanted during each experiment. Viability of cells was determined by trypan blue exclusion and only cell suspensions with > 95% viable cells were used.
Ethical approval and ethical standards
All animal care procedures and experimental protocols were performed in accordance with institutional and the official guidelines of EU Directive 2010/63/EU, at the Faculty of Medical Sciences, University of Kragujevac, Serbia. The experiments were approved by the Animal Ethics Board of the Faculty of Medical Sciences, University of Kragujevac, Serbia (01-2588, 17/03/2014). Consented melanoma patients were recruited from the Clinics of Dermatovenerology and Plastic Surgery, Military Medical Academy, Belgrade, Serbia, while healthy donors (with no prior history of cancer) were recruited on periodical systematic examinations. The study was approved by the local Research Ethics Committee, Military Medical Academy (11-03/2014).
Animal source
C57BL/6 mice were bred in animal breeding facilities of the Faculty of Medical Sciences, University of Kragujevac, Serbia.
Informed consent
Written informed consent was obtained prior to sample collection from all individual participants included in the study in accordance with the Declaration of Helsinki.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Miodrag L. Lukic, Email: miodrag.lukic@medf.kg.ac.rs
Gordana D. Radosavljevic, Email: perun.gr@gmail.com
References
- 1.Hsu MY, Meier F, Herlyn M. Melanoma development and progression: a conspiracy between tumor and host. Differentiation. 2002;70(9–10):522–536. doi: 10.1046/j.1432-0436.2002.700906.x. [DOI] [PubMed] [Google Scholar]
- 2.Sandru A, Voinea S, Panaitescu E, Blidaru A. Survival rates of patients with metastatic malignant melanoma. J Med Life. 2014;7(4):572–576. [PMC free article] [PubMed] [Google Scholar]
- 3.Dummer R, Hauschild A, Lindenblatt N, Pentheroudakis G, Keilholz U. Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v126–v132. doi: 10.1093/annonc/mdv297. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411(6835):380–384. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 5.Molofsky AB, Savage AK, Locksley RM. Interleukin-33 in tissue homeostasis, injury, and inflammation. Immunity. 2015;42(6):1005–1019. doi: 10.1016/j.immuni.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liew FY, Girard JP, Turnquist HR. Interleukin-33 in health and disease. Nat Rev Immunol. 2016;16(11):676–689. doi: 10.1038/nri.2016.95. [DOI] [PubMed] [Google Scholar]
- 7.Milovanovic M, Volarevic V, Radosavljevic G, Jovanovic I, Pejnovic N, Arsenijevic N, Lukic ML. IL-33/ST2 axis in inflammation and immunopathology. Immunol Res. 2012;52(1–2):89–99. doi: 10.1007/s12026-012-8283-9. [DOI] [PubMed] [Google Scholar]
- 8.Jovanovic I, Radosavljevic G, Mitrovic M, Juranic VL, McKenzie AN, Arsenijevic N, Jonjic S, Lukic ML. ST2 deletion enhances innate and acquired immunity to murine mammary carcinoma. Eur J Immunol. 2011;41(7):1902–1912. doi: 10.1002/eji.201141417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jovanovic IP, Pejnovic NN, Radosavljevic GD, Pantic JM, Milovanovic MZ, Arsenijevic NN, Lukic ML. Interleukin-33/ST2 axis promotes breast cancer growth and metastases by facilitating intratumoral accumulation of immunosuppressive and innate lymphoid cells. Int J Cancer. 2014;134(7):1669–1682. doi: 10.1002/ijc.28481. [DOI] [PubMed] [Google Scholar]
- 10.Milosavljevic MZ, Jovanovic IP, Pejnovic NN, Mitrovic SL, Arsenijevic NN, Simovic Markovic BJ, Lukic ML. Deletion of IL-33R attenuates VEGF expression and enhances necrosis in mammary carcinoma. Oncotarget. 2016;7(14):18106–18115. doi: 10.18632/oncotarget.7635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmieder A, Multhoff G, Radons J. Interleukin-33 acts as a pro-inflammatory cytokine and modulates its receptor gene expression in highly metastatic human pancreatic carcinoma cells. Cytokine. 2012;60(2):514–521. doi: 10.1016/j.cyto.2012.06.286. [DOI] [PubMed] [Google Scholar]
- 12.Sun P, Ben Q, Tu S, Dong W, Qi X, Wu Y. Serum interleukin-33 levels in patients with gastric cancer. Dig Dis Sci. 2011;56(12):3596–3601. doi: 10.1007/s10620-011-1760-5. [DOI] [PubMed] [Google Scholar]
- 13.Cui G, Qi H, Gundersen MD, Yang H, Christiansen I, Sorbye SW, Goll R, Florholmen J. Dynamics of the IL-33/ST2 network in the progression of human colorectal adenoma to sporadic colorectal cancer. Cancer Immunol Immunother. 2015;64(2):181–190. doi: 10.1007/s00262-014-1624-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu X, Zhu L, Lu X, Bian H, Wu X, Yang W, Qin Q. IL-33/ST2 pathway contributes to metastasis of human colorectal cancer. Biochem Biophys Res Commun. 2014;453(3):486–492. doi: 10.1016/j.bbrc.2014.09.106. [DOI] [PubMed] [Google Scholar]
- 15.Wang C, Chen Z, Bu X, Han Y, Shan S, Ren T, Song W. IL-33 signaling fuels outgrowth and metastasis of human lung cancer. Biochem Biophys Res Commun. 2016;479(3):461–468. doi: 10.1016/j.bbrc.2016.09.081. [DOI] [PubMed] [Google Scholar]
- 16.Wang K, Shan S, Yang Z, Gu X, Wang Y, Wang C, Ren T. IL-33 blockade suppresses tumor growth of human lung cancer through direct and indirect pathways in a preclinical model. Oncotarget. 2017;8(40):68571–68582. doi: 10.18632/oncotarget.19786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brunner SM, Rubner C, Kesselring R, Martin M, Griesshammer E, Ruemmele P, Stempfl T, Teufel A, Schlitt HJ, Fichtner-Feigl S. Tumor-infiltrating, interleukin-33-producing effector-memory CD8(+) T cells in resected hepatocellular carcinoma prolong patient survival. Hepatology. 2015;61(6):1957–1967. doi: 10.1002/hep.27728. [DOI] [PubMed] [Google Scholar]
- 18.Yang M, Feng Y, Yue C, Xu B, Chen L, Jiang J, Lu B, Zhu Y. Lower expression level of IL-33 is associated with poor prognosis of pulmonary adenocarcinoma. PLoS ONE. 2018;13(3):e0193428. doi: 10.1371/journal.pone.0193428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saranchova I, Han J, Huang H, Fenninger F, Choi KB, Munro L, Pfeifer C, Welch I, Wyatt AW, Fazli L, Gleave ME, Jefferies WA. Discovery of a metastatic immune escape mechanism initiated by the loss of expression of the tumour biomarker interleukin-33. Sci Rep. 2016;6:30555. doi: 10.1038/srep30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim J, Kim W, Moon UJ, Kim HJ, Choi HJ, Sin JI, Park NH, Cho HR, Kwon B. Intratumorally establishing type 2 innate lymphoid cells blocks tumor growth. J Immunol. 2016;196(5):2410–2423. doi: 10.4049/jimmunol.1501730. [DOI] [PubMed] [Google Scholar]
- 21.Gao X, Wang X, Yang Q, Zhao X, Wen W, Li G, Lu J, Qin W, Qi Y, Xie F, Jiang J, Wu C, Zhang X, Chen X, Turnquist H, Zhu Y, Lu B. Tumoral expression of IL-33 inhibits tumor growth and modifies the tumor microenvironment through CD8+ T and NK cells. J Immunol. 2015;194(1):438–445. doi: 10.4049/jimmunol.1401344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dominguez D, Ye C, Geng Z, Chen S, Fan J, Qin L, Long A, Wang L, Zhang Z, Zhang Y, Fang D, Kuzel TM, Zhang B. Exogenous IL-33 restores dendritic cell activation and maturation in established cancer. J Immunol. 2017;198(3):1365–1375. doi: 10.4049/jimmunol.1501399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X, Lv Q, Feng Y, Gu Y, Xia R, Ma J, He H, Zhu Y. Interleukin-33, a potential cytokine expressed in tumor microenvironment involves in antitumor immunotherapy through facilitates CD8+ T Cells. J Interferon Cytokine Res. 2018;38(11):491–499. doi: 10.1089/jir.2018.0069. [DOI] [PubMed] [Google Scholar]
- 24.Gao K, Li X, Zhang L, Bai L, Dong W, Gao K, Shi G, Xia X, Wu L, Zhang L. Transgenic expression of IL-33 activates CD8(+) T cells and NK cells and inhibits tumor growth and metastasis in mice. Cancer Lett. 2013;335(2):463–471. doi: 10.1016/j.canlet.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Lucarini V, Ziccheddu G, Macchia I, La Sorsa V, Peschiaroli F, Buccione C, Sistigu A, Sanchez M, Andreone S, D’Urso MT, Spada M, Macchia D, Afferni C, Mattei F, Schiavoni G. IL-33 restricts tumor growth and inhibits pulmonary metastasis in melanoma-bearing mice through eosinophils. Oncoimmunology. 2017;6(6):e1317420. doi: 10.1080/2162402X.2017.1317420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radosavljevic G, Jovanovic I, Majstorovic I, Mitrovic M, Lisnic VJ, Arsenijevic N, Jonjic S, Lukic ML. Deletion of galectin-3 in the host attenuates metastasis of murine melanoma by modulating tumor adhesion and NK cell activity. Clin Exp Metastasis. 2011;28(5):451–462. doi: 10.1007/s10585-011-9383-y. [DOI] [PubMed] [Google Scholar]
- 27.Fidler IJ. Selection of successive tumor lines for metastasis. Nature New Biol. 1973;242(118):148–149. doi: 10.1038/newbio242148a0. [DOI] [PubMed] [Google Scholar]
- 28.Petersen RP, Hanish S, Haney JC, Miller CC, 3rd, Burfeind WR, Jr, Tyler DS, Seigler HF, Wolfe W, D’Amico TA, Harpole DH., Jr Improved survival with pulmonary metastasectomy: an analysis of 1720 patients with pulmonary metastatic melanoma. J Thorac Cardiovasc Surg. 2007;133(1):104–110. doi: 10.1016/j.jtcvs.2006.08.065. [DOI] [PubMed] [Google Scholar]
- 29.Dao DT, Vuong JT, Anez-Bustillos L, Pan A, Mitchell PD, Fell GL, Baker MA, Bielenberg DR, Puder M. Intranasal delivery of VEGF enhances compensatory lung growth in mice. PLoS ONE. 2018;13(6):e0198700. doi: 10.1371/journal.pone.0198700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin NT, Martin MU. Interleukin 33 is a guardian of barriers and a local alarmin. Nat Immunol. 2016;17(2):122–131. doi: 10.1038/ni.3370. [DOI] [PubMed] [Google Scholar]
- 31.Liew FY. IL-33: a Janus cytokine. Ann Rheum Dis. 2012;71(Suppl 2):i101–i104. doi: 10.1136/annrheumdis-2011-200589. [DOI] [PubMed] [Google Scholar]
- 32.Oboki K, Nakae S, Matsumoto K, Saito H. IL-33 and airway inflammation. Allergy Asthma Immunol Res. 2011;3(2):81–88. doi: 10.4168/aair.2011.3.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhiguang X, Wei C, Steven R, Wei D, Wei Z, Rong M, Zhanguo L, Lianfeng Z. Over-expression of IL-33 leads to spontaneous pulmonary inflammation in mIL-33 transgenic mice. Immunol Lett. 2010;131(2):159–165. doi: 10.1016/j.imlet.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Ibegbu CC, Xu YX, Harris W, Maggio D, Miller JD, Kourtis AP. Expression of killer cell lectin-like receptor G1 on antigen-specific human CD8+ T lymphocytes during active, latent, and resolved infection and its relation with CD57. J Immunol. 2005;174(10):6088–6094. doi: 10.4049/jimmunol.174.10.6088. [DOI] [PubMed] [Google Scholar]
- 35.Li L, Wan S, Tao K, Wang G, Zhao E. KLRG1 restricts memory T cell antitumor immunity. Oncotarget. 2016;7(38):61670–61678. doi: 10.18632/oncotarget.11430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Furuta J, Inozume T, Harada K, Shimada S. CD271 on melanoma cell is an IFN-γ-inducible immunosuppressive factor that mediates downregulation of melanoma antigens. J Invest Dermatol. 2014;134(5):1369–1377. doi: 10.1038/jid.2013.490. [DOI] [PubMed] [Google Scholar]
- 37.Lin L, Rayman P, Pavicic PG, Jr, Tannenbaum C, Hamilton T, Montero A, Ko J, Gastman B, Finke J, Ernstoff M, Diaz-Montero CM. Ex vivo conditioning with IL-12 protects tumor-infiltrating CD8+ T cells from negative regulation by local IFN-γ. Cancer Immunol Immunother. 2019;68(3):395–405. doi: 10.1007/s00262-018-2280-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang JM, Cheng YQ, Shi L, Ying RS, Wu XY, Li GY, Moorman JP, Yao ZQ. KLRG1 negatively regulates natural killer cell functions through the Akt pathway in individuals with chronic hepatitis C virus infection. J Virol. 2013;87(21):11626–11636. doi: 10.1128/JVI.01515-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paczesny S, Banchereau J, Wittkowski KM, Saracino G, Fay J, Palucka AK. Expansion of melanoma-specific cytolytic CD8+ T cell precursors in patients with metastatic melanoma vaccinated with CD34+ progenitor-derived dendritic cells. J Exp Med. 2004;199(11):1503–1511. doi: 10.1084/jem.20032118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le Gros G, Erard F. Non-cytotoxic, IL-4, IL-5, IL-10 producing CD8+ T cells: their activation and effector functions. Curr Opin Immunol. 1994;6(3):453–457. doi: 10.1016/0952-7915(94)90127-9. [DOI] [PubMed] [Google Scholar]
- 41.Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front Oncol. 2018;8:86. doi: 10.3389/fonc.2018.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu H, Weber A, Morse J, Kodumudi K, Scott E, Mullinax J, Sarnaik AA, Pilon-Thomas S. Cell mediated immunity after combination therapy with intralesional PV-10 and blockade of the PD-1/PD-L1 pathway in a murine melanoma model. PLoS ONE. 2018;13(4):e0196033. doi: 10.1371/journal.pone.0196033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wen YH, Lin HQ, Li H, Zhao Y, Lui VWY, Chen L, Wu XM, Sun W, Wen WP. Stromal interleukin-33 promotes regulatory T cell-mediated immunosuppression in head and neck squamous cell carcinoma and correlates with poor prognosis. Cancer Immunol Immunother. 2019;68(2):221–232. doi: 10.1007/s00262-018-2265-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiao P, Wan X, Cui B, Liu Y, Qiu C, Rong J, Zheng M, Song Y, Chen L, He J, Tan Q, Wang X, Shao X, Liu Y, Cao X, Wang Q. Interleukin 33 in tumor microenvironment is crucial for the accumulation and function of myeloid-derived suppressor cells. Oncoimmunology. 2015;5(1):e1063772. doi: 10.1080/2162402X.2015.1063772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dennis KL, Blatner NR, Gounari F, Khazaie K. Current status of interleukin-10 and regulatory T cells in cancer. Curr Opin Oncol. 2013;25(6):637–645. doi: 10.1097/CCO.0000000000000006. [DOI] [PMC free article] [PubMed] [Google Scholar]