Abstract
Objective
Mesenchymal stem cells (MSCs), one of the most important stromal cells in the tumor microenvironment, play a major role in the immunomodulation and development of tumors. In contrast to immunomodulatory effects of bone marrow-derived MSCs, resident MSCs were not well studied in tumor. The aim of this study was to compare the immunomodulatory properties and protein secretion profiles of MSCs isolated from breast tumor (T-MSC) and normal breast adipose tissue (N-MSC).
Materials and methods
T-MSCs and N-MSCs were isolated by the explant culture method and characterized, and their immunomodulatory function was assessed on peripheral blood lymphocytes (PBLs) by evaluating the effects of MSC conditioned media on the proliferation and induction of some cytokines and regulatory T cells (Tregs) by BrdU assay, ELISA, and flow cytometry. In addition, we compared the secretion of indoleamine 2,3-dioxygenase (IDO), vascular endothelial growth factor (VEGF), matrix metallopeptidase (MMP)-2, MMP-9, and Galectin-1.
Results
T-MSCs showed a higher secretion of transforming growth factor beta (TGF-β), prostaglandin E2 (PGE2), IDO, and VEGF and lower secretion of MMP-2 and MMP-9 compared with N-MSCs. However, no significant difference was found in the secretion of interferon gamma (IFN-γ), interleukin 10 (IL10), IL4, IL17, and Galectin-1 in T-MSCs and N-MSCs. The immunomodulatory effect of soluble factors on PBLs showed that T-MSCs, in contrast to N-MSCs, stimulate PBL proliferation. Importantly, the ability of T-MSCs to induce IL10, TGF-β, IFN-γ, and PGE2 was higher than that of N-MSCs. In addition, T-MSCs and N-MSCs exhibited no significant difference in Treg induction.
Conclusion
MSCs educated in stage II breast cancer and normal breast adipose tissue, although sharing a similar morphology and immunophenotype, exhibited a clearly different profile in some immunomodulatory functions and protein secretions.
Keywords: Mesenchymal stem cells (MSCs), Breast cancer, Immunomodulatory
Introduction
Breast cancer is the most commonly diagnosed cancer in women worldwide, whose high mortality rate has made it a global public health concern [1]. A wide variety of researches have been carried out so far to find the roles of the immune system in the breast cancer development and progression; one of the most important reasons of cancer development and progression is the shift of immune response from pro-inflammatory to anti-inflammatory cytokines during the progression of breast cancer [2, 3].
Stromal cells, mainly including cells involved in inflammatory responses such as macrophages, lymphocytes, vascular endothelial cells, mesenchymal stem cells (MSCs), and tumor-associated fibroblasts, appear to play a key role in breast cancer microenvironment. Such stromal cells were demonstrated to greatly influence cancer cell behavior in tumor microenvironment [4]. MSCs are multipotent non-hematopoietic progenitor cells capable of differentiating into multiple mesodermal cell lineages. MSCs, significant stromal cells in tumor environment, have the ability to migrate to tumor sites and play different roles in cancer progression [5, 6]. In fact, tumor cells mediate the homing of circular and adjacent MSCs into tumor microenvironment through secretion of different factors including inflammatory cytokines. After their homing, MSCs can produce a wide variety of cytokines and growth factors capable of positively affecting tumor progression and metastasis [7, 8]. There are various mechanisms by which MSCs play a supporting role in tumor progression, mostly relying on the immunomodulatory characteristics of MSCs [7, 9]. MSCs exert their effects through a cell-to-cell contact and cell-independent fashion using the production of some agents, including prostaglandin E2 (PGE2), indoleamine 2,3-dioxygenase (IDO), TGF-β, Galectin, etc. [10–12]. This finally leads to the differentiation of regulatory T cells (Tregs) and type 2 immune responses [13, 14].
However, despite the presence of supporting data demonstrating their effects on tumor progression, there are some studies showing the proliferation inhibitory and apoptotic roles of MSCs in tumor cells. Different effects of MSCs on tumor growth are dependent on a variety of factors, including the origin and type of MSCs as well as tumor models [15, 16]. To address the issues underlying this controversy, there is a critical need to further investigate the behaviors of MSCs in tumor microenvironments.
Inflammatory agents in tumor microenvironment help MSCs home in and be educated. A wide variety of receptors present on the surface of MSCs result in their increased plasticity to acquire various cell fates and functions based on their tumor microenvironment [17].
There is little information about whether MSCs are located in stage II breast tumor tissue, how these cells are educated in normal and tumor conditions, and how they participate in the immunomodulation of breast cancer. The present study compared immunomodulatory characteristics and potential secretion of MSCs educated in normal breast adipose tissues (N-MSCs) of healthy individuals and breast tumor tissues (T-MSCs) of patients with breast cancer.
Materials and methods
Sample collection
Eleven tumor samples were collected from patients with stage II breast cancer who received no therapies such as chemotherapy and radiotherapy. Patients who had autoimmune diseases or received immunosuppressive regimens for other reasons were excluded from this study.
All patients, with the mean age of 43.4 ± 4.6, were selected from Golestan province of Iran. After surgery, the samples were kept in the sterile Dulbecco’s Modified Eagle’s medium (DMEM) containing penicillin–streptomycin antibiotics and subsequently transferred to the laboratory at 4–6 °C. The normal breast adipose tissues were collected from 11 healthy women, with the mean age of 40.2 ± 5.6, under the breast aesthetic surgery. The healthy women were selected from Golestan province of Iran and screened for autoimmune diseases, such as lupus, or those who received immunosuppressive treatment for other reasons were excluded from this study.
Similar conditions, as mentioned for tumor samples, were conducted for transferring the samples to the laboratory. Written informed consent was obtained from the patients participating in this investigation. The protocol was approved by the Ethics Committee of Tehran University of Medical Sciences (TUMS), and all procedures were performed according to ethical committee approval.
MSCs isolation
T-MSCs were isolated through a modified explant culture method according to our previous study [18]. Briefly, after breast tumor tissues were surgically removed, freshly prepared primary specimens were transferred to the laboratory within 30 min in phosphate-buffered saline (PBS; Sigma-Aldrich). After washing by PBS, the tumor tissues were cut into 1–3 mm pieces, and each cut piece was cultured in a 24-well plate containing 500 μL of DMEM culture medium (GIBCO, USA) supplemented with 4.5 g/mL glucose, 10% heat-inactivated fetal bovine serum (FBS; GIBCO, USA), and 1% penicillin/streptomycin (Biosera, UK). After incubation in 37 °C and 5% CO2 for 7–10 days, wells containing cells with homogenous fibroblast-like morphologies were considered to be a positive well. Subsequently, the explant tissues in positive wells were discarded and the outgrown cells were cultured in freshly prepared media for additional 5–7 days until the formation of a confluent monolayer (P0). A similar experiment was conducted on normal healthy breast adipose tissues in cosmetic mammoplasty surgery as explained previously.
Surgically resected adipose tissue (approximately 5 g) was washed by PBS to remove excess blood. The fat was transferred into a Petri dish and subsequently minced into approximately 5-mm fragments. The fragments of tissue were evenly distributed on the surface of a tissue culture dish (nearly 1 g adipose tissue per 100 mm dish). Afterward, the explants were plated in DMEM supplemented with 10% FBS, 2 mM glutamine, and 1% penicillin/streptomycin. The dishes were incubated at 37 °C with 5% humidified CO2. On day 5–7 after plating, the explants tissue was discarded, and the outgrown cells were seeded in freshly prepared media for additional 5–7 days until the formation of a confluent monolayer; these initial cells were referred to be passage P0.
MSC characterization
Flow cytometry analysis
For this purpose, the cells at passage 3 were harvested by 1% trypsin–EDTA solution, centrifuged at 300 × g for 5 min, and washed twice with PBS containing 2% FBS. Afterward, the cell phenotypes were determined by antihuman antibodies against several cell surface markers, including CD73, CD44, CD29, CD105, CD90, HLADR, CD45, CD34, CD133, CD31, and CD11b (all obtained from Dako). Cells that were stained with FITC- or PE-conjugated mouse IgG (BD Biosciences, USA) were considered to be a negative control. The cells were then washed three times with PBS after a 40-min incubation at room temperature. Approximately 10,000 events were counted by BD FACS Calibur instrument and the obtained data were analyzed by FlowJo software.
Mesodermal lineage differentiation
MSCs were analyzed at passage 3 for their potential to differentiate into osteocyte and adipocyte lineages.
Adipogenic differentiation
The induction of adipogenic differentiation was carried out in the confluent culture of T-MSCs and N-MSCs (cells cultured in 12-well plates at 5000 cells/cm2) in complete medium supplemented with 0.5 mM of 3-isobutyl-1-methylxanthine, 200 of mM indomethacin, 1 mM of dexamethasone, and 10 mg/mL of insulin (all purchased from Sigma-Aldrich, USA) in a humidified 37 °C incubator with 5% CO2. After 18 days, adipogenic differentiation was evaluated by staining the lipid droplets using oil red O (Sigma-Aldrich, USA).
Osteogenic differentiation
The confluent cells were harvested and cultured in 12-well plates at a density of 5000 cell/cm2 in complete medium supplemented with 10 mM of b-glycerophosphate, 0.1 mM of dexamethasone, and 0.2 mM of ascorbic acid (all bought from Sigma-Aldrich) at 37 °C in a humidified incubator with 5% CO2. After 18 days, mineralization was determined by staining using alizarin red S (Sigma-Aldrich, USA).
Comparison of T-MSC and N-MSC cell proliferation
The MSC proliferation curves were constructed every day using cell counting at a regular time. T-MSCs and N-MSCs were cultured at passage 3 in DMEM at an initial density of 5000 per well during the logarithmic growth phase in 24-well plates. The number of adherent cells per well was counted triplicate on days 1–8 of culture.
Preparation of MSC conditioned media
MSCs were plated at passage 2 in a 75-cm2 tissue culture flask for the preparation of T-MSC and N-MSC conditioned media (CM). MSCs, when reaching 90% confluency, were seeded in serum-free DMEM for 48 h. Afterward, supernatant was prepared, filtered using a 0.22-mm membrane, and then stored at − 80 °C. To evaluate the immunomodulatory function of CM on the immune cells, peripheral blood lymphocytes (PBLs) were cultured in 25% or 50% CM mixed with freshly prepared PBL culture media and the plates were incubated for 3 days in a humidified 37 °C incubator with 5% CO2. The proliferation and cytokine production of PBLs were measured in the presence and absence of 5 µg/mL phytohemagglutinin (PHA).
Peripheral blood lymphocyte (PBL) isolation
PBLs were obtained from five normal healthy donors. In brief, 20 ml of peripheral blood samples was diluted with an equal volume of PBS, layered onto a Histopaque (specific gravity 1.077; Sigma-Aldrich, USA), and centrifuged at 600 × g for 20 min. Peripheral blood mononuclear cells (PBMCs) were collected from the interphase and washed with PBS. Afterward, the PBMCs were cultured for 2 h in a humidified incubator at 37 °C with 5% CO2 to remove monocytes from mononuclear cells. Following the incubation time, the flout cells (PBLs) were collected, and cell viability was assessed by trypan blue exclusion.
PBL proliferation assay
PBLs (1 × 105 cells/100 µL) were cultured triplicate in 96-well round-bottom plates (BD, Falcon) in the presence of 25% and 50% CM derived from T-MSCs and N-MSCs for 72 h. PBLs proliferation was measured by a cell proliferation BrdU ELISA kit (Roche Diagnostics, Germany) based on the manufacturer’s instructions. Briefly, 10 µl/well BrdU labeling solution was added to the wells after 72 h of incubation and the cells were reincubated for further 2 h at 37 °C. After removal of labeling medium and cell fixation, 100 µl/well anti-BrdU-POD was added to the wells and incubated. After washing, 100 l/well substrate solution was added to the wells and incubated in room temperature. The absorbance of the samples was measured in an ELISA reader at 370 nm.
Measurement of cytokine and PGE2 production
TGF-β, IL-10, IL-17, IFN-γ, IL-4, and PGE2 levels were assessed by the ELISA kit (ebiosciences) using cell culture supernatants from T-MSCs, N-MSCs, and cultured PBLs in the presence of T-MSC-CM and N-MSC-CM. The procedures were conducted based on the manufacturer’s protocols. A density of 106 PBLs per well was cultured in 24-well plates in the presence of MSC-CM, PHA, or complete media (as a control), in a final volume of 1 mL. RPMI-1640 supplemented with 10% heat-inactivated FBS, 100 mg/mL of streptomycin, and 100 U/mL of penicillin was used for cell culture. The plates were incubated for 48 h in a humidified atmosphere at 37 °C with 5% CO2, and the resultant supernatants were collected after centrifugation. For the measurement of cytokine production by PBLs in the presence of CM, MCS-CM was applied as a blank to subtract background absorbance of the MCS-produced cytokines.
Induction of regulatory T cells (Tregs)
MSC-CM was evaluated for its capability to induce the generation of CD4+ CD25high FoxP3+ T cells. PBLs were cultured for 7 days in 12-well plates with T-MSC-CM and N-MSC-CM. On day 7, PBLs were harvested, counted, and analyzed using flow cytometry. PBLs (1 × 106) were washed twice with PBS and stained with Percp-conjugated mouse antihuman CD4 and FITC-conjugated mouse antihuman CD25 (BD Biosciences, USA) to measure CD25 and CD4 expression, respectively. Approximately, 400 µL of 1% cell fix was added for intracellular staining, incubated at 4 °C for 5 min, and washed with 1 mL of freshly prepared ice-cold PBS. Afterward, 500 µL of 0.2% saponin was added, incubated for 10 min at 4 °C, and centrifuged. At the same time, the cells were stained with 5 µl of PE-conjugated mouse antihuman Foxp3 (BD Biosciences, USA) antibodies. Following a 30-min incubation on ice, PBLs were washed with PBS twice, and the cells were stained with FITC-, PE-, or Percp-labeled mouse IgG (BD Biosciences) as negative controls. Roughly 20,000 events were collected on a three-color Becton–Dickinson FACS Vantage instrument, and the data were analyzed by FlowJo software.
Indoleamine 2,3-dioxygenase (IDO) activity determination
The biological activity of IDO was characterized using measurement of the kynurenine level in culture supernatants derived from N-MSCs and T-MSCs. For this purpose, 100 mL of supernatants was mixed with 50 mL of 30% trichloroacetic acid, gently vortexed, and centrifuged at 8000 g for 5 min. Afterward, 75 mL of the supernatant was added to an equal volume of Ehrlich reagent (100 mg of p-dimethylbenzaldehyde in 5 mL of glacial acetic acid) in 96-well plates, followed by measurement of the absorbance at 490 nm.
Comparison of VEGF, MMP-9, MMP-2, and Galectin-1 secretions by T-MSCs and N-MSCs
Vascular endothelial growth factor (VEGF), matrix metallopeptidase 2 (MMP-2), MMP-9, and Galectin-1 were assessed by an ELISA kit (ebiosciences) using cell culture supernatants derived from T-MSCs and N-MSCs. The procedures were carried out based on the manufacturer’s instructions.
Statistical analysis
Statistical analyses were conducted by t test by the SigmaStat program (ver. 3.0; SigmaStat, USA) and ANOVA test in SPSS statics 19 software (SPSS Inc., Chicago, IL). The data, resulting from a mean of at least three independent experiments, are presented as the mean ± SEM. p values less than 0.05 were considered to be statistically significant.
Results
MSC isolation and characterization
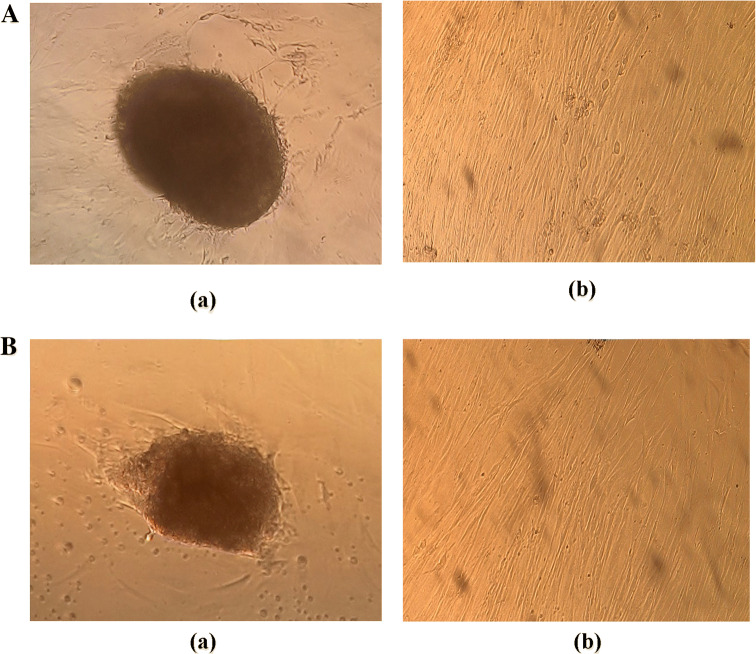
MSCs were isolated and cultured as described above. The explants revealed fibroblastic-like outgrowths 3–5 days after culture (Fig. 1A (a) and B (a)). The isolated MSCs, as depicted in Fig. 1A (b) and B (b), showed a spindle shape and homogenous population in culture. Cells at passage 3 were used in characterization protocols using flow cytometry analysis and adipocytic, osteocytic differentiation tests. Table 1 indicates results from flow cytometric analysis of cell surface markers derived from both patients and normal-derived MSCs. The results demonstrated that T-MSCs had the ability to express the same surface antigens as N-MSCs, being positive for CD73, CD44, CD29, CD105, and CD90, but negative for CD45, CD11b, CD34, CD133, CD31, and HLA–DR. We found no significant differences in the percentage of specific cell surface markers by MSCs isolated from stage II breast tumor and normal breast adipose tissues.
Fig. 1.
Isolation of MSCs from breast tumor tissues and normal breast adipose tissues by explant culture. A (a). Migration of fibroblastic cells from the tumor tissue explant on day 3. A (b). Explant-derived cells on day 9. B (a) Migration of fibroblastic cells from the adipose tissue explant on day 5. B (b) Explant-derived cells on day 14. Scale bars = 100 mm
Table 1.
Quantification of cell surface markers by flow cytometry
| Marker | T-MSC | N-MSC |
|---|---|---|
| CD73 | 97.6 ± 1.6 | 93.7 ± 2.2 |
| CD44 | 90.4 ± 2 | 83.7 ± 2.6 |
| CD29 | 88.3 ± 2.4 | 90.5 ± 3.1 |
| CD105 | 82.7 ± 4.3 | 81.8 ± 2.3 |
| CD90 | 90.3 ± 1.1 | 94.7 ± 3.2 |
| CD45 | 3.8 ± 0.9 | 2.3 ± 0.7 |
| CD34 | 1.7 ± 0.4 | 1.2 ± 0.6 |
| CD11b | 1.8 ± 0.6 | 1.4 ± 0.8 |
| CD133 | 2.1 ± 0.7 | 1.1 ± 0.5 |
| CD31 | 0.9 ± 0.3 | 1.4 ± 0.4 |
| HLA-DR | 5.7 ± 1.4 | 2.4 ± 0.9 |
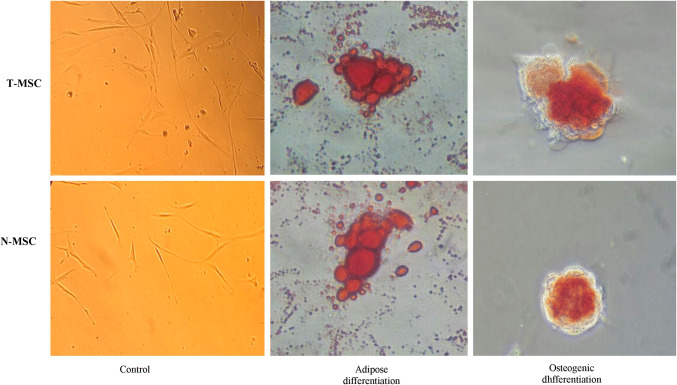
T-MSCs and N-MSCs underwent differentiation when induced by specific factors. Three days after induction, morphological changes were found in the adipogenesis experiments. Adipocytes began to morphologically appear, and the accumulation of lipid vacuole was obviously detected on day 16, in which their numbers increased over time, finally leading to Oil Red O staining of lipid vacuoles. On day 16, around 20% of the cells seemed to be adipocytes. Osteogenic activity was detected first on day 25 after the treatment, and mineral deposits were found after 7 days. Mineralization proceeded gradually and showed massive mineral deposits after 25 days; Alizarin red staining was carried out, and extracellular calcium (Ca2 +) deposits became red. No morphological changes were found in primary fibroblast-like cells in negative controls containing wells with DMEM (no osteogenic induction), and no Ca deposit was detected after staining (Fig. 2).
Fig. 2.
Differentiation potential of T-MSCs and N-MSC: differentiation into osteocytes after induction culture was assessed by Alizarin Red S staining for calcium mineralization and adipogenic differentiation was determined by Oil Red O staining for lipid vacuoles. Control cultures in normal growth medium were also stained and were negative. Scale bars = 100 mm
Proliferation curve of T-MSCs and N-MSCs
Results from proliferation curves of T-MSCs and N-MSCs revealed that T-MSCs have a higher proliferation potential than N-MSCs in the same culture conditions 4 days after culture (P < 0.05) (Fig. 3). Curve equation evaluations calculated the doubling time of N-MSCs and T-MSCs as 2.9 and 1.6 days, respectively.
Fig. 3.
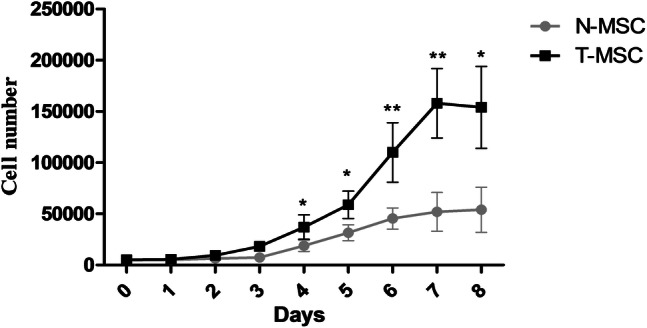
Growth curves of mesenchymal stem cells (MSCs) from breast tumor tissues (T-MSC) and normal breast adipose tissues (N-MSC). The growth curves were used to compare the cell growth characteristics between T-MSCs and N-MSCs. Data presented as mean ± SEM of three independent experiments. *P < 0.05, **P < 0.01
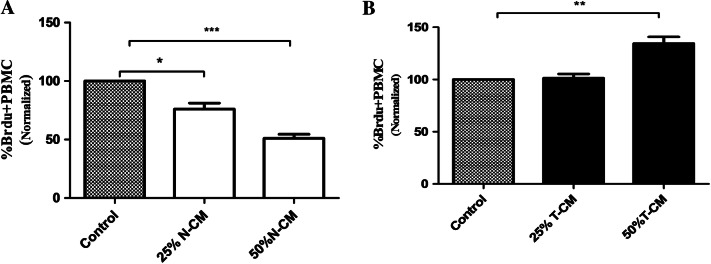
Effects of T-MSC and N-MSC soluble factors on PBL proliferation
Supernatant obtained from N-MSC and T-MSC cultures was added to PBLs stimulated with PHA to determine whether soluble factors secreted by T-MSCs and N-MSCs are associated with the immunomodulatory effect. For the BrdU assay, PBLs cultured in T-MSC-CM (50%) demonstrated increased proliferation of PBLs, showing to be statistically significant in comparison with that of the control group (P < 0.05) (Fig. 4A). Unlike T-MSC-CM, N-MSC-CM (25% and 50%) significantly (P < 0.05) suppressed PBL proliferation in comparison with the control group (Fig. 4B).
Fig. 4.
N-MSC conditioned media (N-CM) and T-MSC conditioned media (T-CM) display different immune-suppressive functions. T-CM co-incubated with stimulated PBLs unlike N-CM increased lymphocyte proliferation. Data presented as mean ± SEM of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001. BrdU 5-bromo-29-deoxyuridine, MSC mesenchymal stem cell
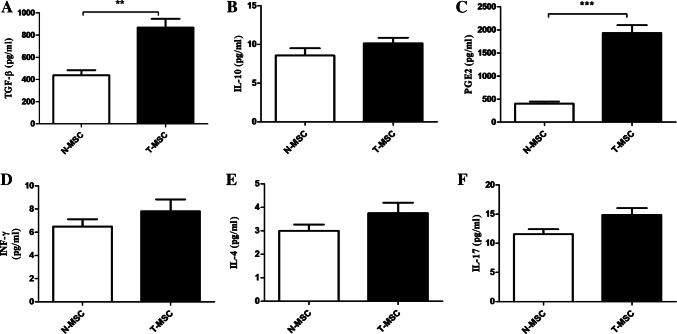
Cytokine production by N-MSCs and T-MSCs
Results from TGF-β, IFN-γ, IL-4, IL-17, IL-10, and PGE2 production demonstrated that N-MSCs and T-MSCs could consistently secrete TGF-β and PGE2, but not IL10, IL17, IFN-γ, and IL-4 in culture medium (Fig. 5). Our result revealed that T-MSCs were able to significantly produce (P < 0.05) a higher level of TGF-β and PGE2 than N-MSCs. In contrast, statistical analysis showed no differences between IL-17, IL-10, IL4, and IFN-γ production (P > 0.05).
Fig. 5.
TGF-b (A), IL-10 (B), PGE2 (C), IFN-γ (D), IL-4 (E), and IL17 production by T-MSCs and N-MSC after three passages. Data are expressed as the mean ± SEM of three independent experiments. **P < 0.01, ***P < 0.001
Effects of N-MSC and T-MSC soluble factors on cytokine production by PBLs
We analyzed the ability of N-MSC-CM and T-MSC-CM to modulate TGF-β, IFN-γ, IL-4, IL-17, IL-10, and PGE2 production by PBLs. Figure 6 A–L indicates that PBLs, when cultured with N-MSC-CM and T-MSC-CM, resulted in a significant increase (P < 0.05) in the production of TGF-β, IL-4, PGE2, and IL-10. On the other hand, T-MSC and N-MSC soluble factors also led to increased IL-17 and IFN-γ secretion in stimulated and unstimulated PBLs. However, this effect was not statically significant. Our findings clearly demonstrated that T-MSC-CM has a significantly higher probability (P < 0.05) in the production of IL-10, TGF-β, and PGE2 when compared with N-MSC-CM in stimulated and unstimulated PBLs. Nevertheless, no significant differences were found in the induction of IL4 production in PBLs between T-MSCs and N-MSCs.
Fig. 6.

The effect of T-MSCs conditioned media (T-CM) and N-MSC conditioned media (N-CM) on PBLs cytokine production. PBLs were incubated for 3 days with MSCs conditioned media in the absence (A, C, E, G, I, K) and presence (B, D, F, H, J, L) of PHA as stimulator. Untreated PBLs were used as control. Data are expressed as the mean ± SEM of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001
Induction of regulatory T cells by soluble factors of T-MSCs and N-MSCs
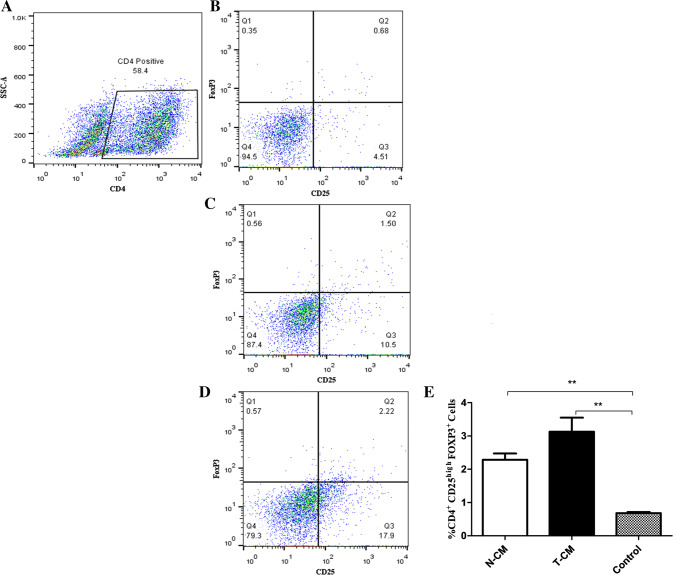
Treg formation was investigated in co-cultures of PBLs and MSC-CM. Seven days after co-culture, PBLs cultured in the presence of T-MSC-CM (Fig. 7C) and N-MSC-CM (Fig. 7D) both contained a population of CD4+CD25highFoxP3+ T cells. PBLs cultured in the presence of MSC-CM exhibited a significantly higher percentage (P < 0.05) of Tregs during 1 week when compared with those cultured in the absence of MSC-CM (Fig. 7E). As depicted in Fig. 7E, supernatant from T-MSCs exhibited stronger effects for the increase in Treg population compared with that from N-MSCs. However, the two groups showed no statistically significant differences.
Fig. 7.
Flow cytometry analysis to explore the expressions of CD4, CD25, and Foxp3 in PBLs treated with N-MSC conditioned media (N-CM) and T-MSCs conditioned media (T-CM). (A) CD4 + Cells population in PBLs, (B) untreated PBLs, (C) treated with N-CM, (D) treated with T-CM, (E) the percentage of CD4+CD25highFoxP3+ T cells is significantly higher in the population that was cultured with N-CM and T-CM compared to PBLs that were cultured without MSC-CM (control); however, the two groups showed no statistical differences. Data are expressed as the mean ± SEM of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001
Comparison of VEGF, MMP-9, MMP-2, Galectin-1, and IDO activity in T-MSCs and N-MSCs
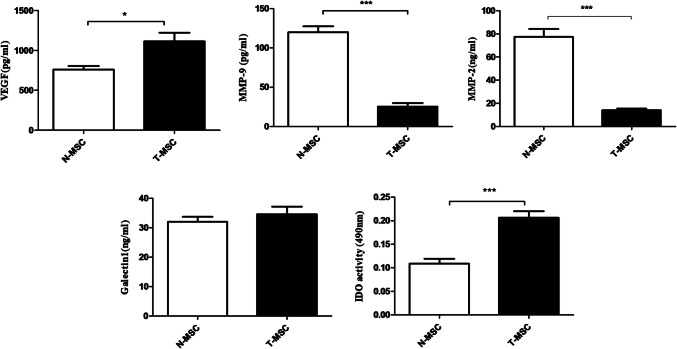
T-MSCs were found to produce significantly higher levels (P < 0.05) of VEGF and IDO as compared with N-MSCs. On the other hand, T-MSCs produced significantly lower levels (P < 0.05) of MMP-2 and MMP-9 as compared with N-MSCs. Nonetheless, T-MSCs and N-MSCs showed no significant differences in the secretion of Galectin-1 (Fig 8).
Fig. 8.
Comparison of VEGF, MMP-9, MMP-2, Galectin-1, and IDO production by T-MSCs and N-MSC after three passages. Data are expressed as the mean ± SD of three independent experiments. *P < 0.05, ***P < 0.001
Discussion
MSCs derived from solid tumors, in contrast to cancer-associated MSCs obtained from the bone marrow of hematological malignancies [19], have not been investigated in depth, and their role in breast cancer progression remains poorly documented. A comprehensive understanding of their role in human breast tumor microenvironment helps scientists elucidate their potential targets for the treatment of breast cancer. There are limited data on the immunological properties of MSCs present in the microenvironment of breast tumor. As previously documented, MSCs derived from various tissues, although sharing some similarities, have functional differences including surface markers, as well as cytokine and enzyme productions [17, 18]. Therefore, the present study hypothesized that MSCs isolated from breast tumor tissue and normal breast adipose tissue are different in their immunomodulatory functions and protein secretion.
Our results revealed that canonical MSCs could be successfully isolated and identified in primary breast tumor and adipose tissue by the explant culture method. In this study, MSCs derived from stage II human breast tumor and normal breast adipose tissue were demonstrated to have a similar morphology, immunophenotype, and a multi-lineage differentiation potential (osteoblast and adipocyte lineage differentiation) under suitable conditions. T-MSCs grew more rapidly than N-MSCs, and both demonstrated fibroblastic morphologies. Our results showed that T-MSCs and N-MSCs were homogeneously positive for the mesenchymal cell markers, including CD29, CD44, CD73, CD105, and CD90, but negative for CD34, CD45, CD11b, CD31, CD133, and HLA–DR. Moreover, we found that T-MSCs and N-MSCs have different effects on the proliferation of the PBLs in vitro. We choose CM from T-MSCs and N-MSCs to culture PBLs rather than PBL/MSC co-culture. Cell co-culture studies seem to be infrequently unreliable because of potential artificial growths of one cell type over the other induced by the culture environment rather than by actual anticancer effects [20]. To rule out this possibility, the present study focused exactly on the role of the MSC-CM rather than of direct cells. MSCs are believed to inhibit T lymphocyte proliferative responses to stimulation by mitogens [21]. The present study investigated this inhibitory effect in N-MSCs, in confirmation of previous studies, showing a significant suppression (P < 0.05) of PBL proliferation in the presence of 25% and 50% of N-MSC-CM. T-MSC-CM, in contrast to N-MSC-CM, not only lacked the ability to suppress PBL proliferation but also significantly stimulate PBL proliferation in a 50% concentration. In a similar study, soluble agents from MSCs isolated from breast tumor increased the proliferation of MCF-7 cells in two proportions 10% and 20% [22]. As a result, these findings showed that T-MSC-CM increases the growth of MCF-7 cells and PBLs in vitro.
Of note, PBLs proliferation increased in the presence of high levels of TGF-β, IL10, IDO, Galectin-1, and PGE2, all suppressing PBLs proliferation, showing that additional mechanisms and mediators may be involved in the control of PBLs proliferation by T-MSCs. Interestingly, Lavini-Ramos et al. showed that MMP9 and MMP2 play a critical role in human adipose MSC immunoregulatory mechanisms and have high suppression potency. They showed that the MMP2/9 inhibitor significantly restores T cell proliferations that are suppressed by adipose-derived MSC-CM. In addition, the MMP inhibitor was combined with IDO, CD73, and HLAG inhibitors to assess their synergic action; however, no further decrease or additional effects were found in adipose MSC suppressive activity [23]. In this regard, results from our study also showed that the secretion of MMP-2 and MMP-9 in T-MSCs is significantly lower than normal adipose MSCs. On the other hand, due to the role of MMP-2 and MMP-9 secreted from MSCs in the inhibition of T cell proliferation as well as the lower levels of MMP-2 and MMP-9 in T-MSC-CM as compared with N-MSCs, it may be a possible reason why solution agents from T-MSCs failed to inhibit PBLs proliferation in contrast to N-MSCs. However, further investigations are required to explain the essential mechanism of increased effects on the growth of PBLs by MSCs isolated from stage II breast tumor tissue.
There are a wide variety of secreted mediators attributed to immunomodulatory properties of MSCs, including TGF-β, PGE2, and NO that are the main suppressors produced by MSCs in murine models and IDO in human beings [24]. Consequently, this study compared the production of some cytokines and mediators secreted by MSCs isolated from breast tumor and normal breast adipose tissue. In agreement with previous studies, our findings demonstrated the production of PGE2 and TGF-β by MSCs isolated from breast tumor and normal breast adipose tissue. Our results revealed that T-MSCs produce significantly higher levels (P < 0.05) of PGE2 and TGF-β when compared with N-MSCs. Nonetheless, a significant increased level of PGE2 was detected in T-MSCs, which, accompanied with TGF-β, could have additional effects. It demonstrated that high levels of PGE2 in cancer patients make them higher invasiveness [25]. TGF-β, when added to the environment, could result in Treg induction and increased IL10 production [26]. A variety of studies investigated the effect of neutralizing antibodies on TGF-β, hepatocyte growth factor (HGF), an inhibitor of PGE-2, or an inhibitor of IDO production, demonstrating that they reverse the inhibition of T cell proliferation by MSCs [27, 28]. The cytokine profile of MSCs was reported to be the same as that reported for bone marrow MSCs and MSCs isolated from different tissues. Nonetheless, a variety of studies demonstrated some discrepancies, highlighting organ-specific differences in the immunomodulatory effects of MSCs [29]. Furthermore, other factors, including HGF, HLA-G, and leukemia inhibitory factor (LIF), are related to the immunomodulatory effects of MSCs [30]. In this study, we did not evaluate production of these soluble factors in T-MSCs and N-MSCs.
We also analyzed the ability of T-MSCs and N-MSCs to modulate TGF-β, IFN-γ, IL-4, IL-17, IL-10, and PGE2 by PBLs. Results from in vitro study showed increased production of TGF-β, IFN-γ, IL-4, IL-10, and PGE2 by unstimulated and stimulated PBLs in response to T-MSC-CM and N-MSC-CM. A great number of studies revealed the role of three types of CD4 helper T lymphocytes (Th), including Th1, Th2, and Th17, in the immune response against various tumors which are characterized by their distinct cytokine profiles [31]. Recent studies have elucidated the in vitro and in vivo potential of MSCs to suppress immune responses through a shift in the Th1/Th17 to Th2 cell balance, demonstrating a shift from a pro-inflammatory state (IFN-γ production) to anti-inflammatory states (IL-4 production) [32]. Nevertheless, our study demonstrated increased production of the Th2 cytokine profile (IL4 and IL10) and TGF-β in PBLs by T-MSC-CM and N-MSC-CM. It is noteworthy that these MSCs not only produce IL4 and IL10 but also induce the production of these cytokines in PBLs. However, no statistically significant difference was observed in the induction of IL4, (in contrast to IL10 and TGF-β) in PBLs by T-MSC-CM and N-MSC-CM.
Results from IFN-γ assessment showed that both adipose and tumor MCS-CM increased IFN-γ in PBLs. Interestingly, T-MSC-CM exhibited higher potency as compared with N-MSC-CM. Although surprisingly, there are some studies whose results were similar to those obtained in this study. The inhibitory effect of MSCs on the secretion of IFN-γ seems to depend on cell contact. The regulation of IFN-γ is complicated and presumably dictated by at least two mechanisms, depending on whether or not MSCs are in direct contact with activated PBLs [33]. In a study, soluble factors of MSCs isolated from human amniotic membrane led to decreased secretion of IL17 from PBLs [34]. However, other studies demonstrated that MSCs are able to induce differentiation toward TH17 under inflammatory conditions (the presence of IL-6 in the environment); otherwise, differentiation to Tregs is favored when MSCs produce IL-10 and TGF-β [35]. Results from this study showed that soluble agents from MSCs derived from normal breast adipose and breast tumor, unlike amniotic membrane, lack the ability to reduce this cytokine in PBLs. The justification for this difference may be tissue sources from which the cells were isolated and show different modulation on the PBLs.
Inducible Tregs are dominant Treg subsets in pathologic situations, including cancers, developed in response to distinctive microenvironmental stimuli and regulate different immunological responses [36]. The induction of Tregs is another important approach by which MSCs exert their immunomodulatory functions. Based on some previous studies, soluble factors are involved, while other studies indicated the requirement of cell–cell contact [37, 38]. Based on these studies, we hypothesized that the immunoregulatory effects of T-MSCs and N-MSCs may be different in the induction of Tregs in PBLs. Our findings demonstrated the percentage of CD4+CD25highFoxp3+ Cells seems to be upregulated in PBLs cultured with the supernatants of MSCs derived from stage II breast tumor and normal breast adipose tissue. However, in comparison, although soluble agents from T-MSCs had a higher mean in Treg induction, their difference with N-MSCs showed no statistical significance, indicating that education of MSCs in the stage II breast tumor for the induction of Treg did not have great differences with education of normal breast adipose.
The VEGF family is important mediators of angiogenesis, and it is well known that the process plays a central role in tumor progression [39]. Furthermore, VEGF not only plays a role in immunity and inflammation, but also has a responsibility for inflammatory cell recruitment and co-stimulatory molecule expression on recruited and resident mononuclear cells [40]. In addition, VEGF has an indirect immunosuppressive function on lymphocyte activation and proliferation through increased IDO secretion from dendritic cells [41]. Based on these reports, we compared VEGF, as an immunomodulatory factor, in the T-MSCs and N-MSCs. Results from this study showed that T-MSCs and N-MSCs have the ability to secrete VEGF, but, importantly, T-MSCs secrete a higher rate of this factor. This difference in the secretion level of VEGF is likely due to the presence of the inflammatory environment in the tumor microenvironment. IDO is a famous immune suppression factor which is not expressed constitutively in MSCs. IDO prevents T cell proliferation and increases immune tolerance by tryptophan depletion. Results from our study demonstrated that the activity of IDO in MSCs isolated from the tumor was greater than that isolated from adipose MSCs. This means that the regulation of the immune system in the tumor environment may have differences with non-cancerous conditions.
Galectin-1 is a 14.5-kDa prototype member of the galectin family, which is known as a negative regulator of immune responses. Galectin-1 with anti-proliferative effects on activated T cells causes them to undergo apoptosis in a variety of settings [42]. Furthermore, Galectin-1 plays a supportive effect on the survival of naive T cells without promoting cell proliferation and prevents the secretion of typical cytokines, including Th1 and Th17 cells, while promoting Th2-type cytokine secretion [43]. Boja et al. showed Galactin-1 secretion by MSCs isolated from human bone marrow and determined their inhibitory effects on T lymphocytes [44]. Results from this study showed that T-MSCs and normal adipose breast MSCs secrete this factor. However, although T-MSCs secreted a higher rate of this factor, there was no significant difference with N-MSCs. These results indicated that the microenvironment of stage II breast cancer had no clear effect on the increased expression of Galactin-1 in T-MSCs.
However, there are some controversies regarding protein secretion and immunomodulatory function of MSCs. The present study speculated that this may be associated with MSC source, individual variations in the physiological immune status of individuals, differences in culture and experimental methods, the type, site, and stage of tumor, or a combination of these factors.
Conclusion
Our study demonstrated that MSCs educated in normal breast adipose and stage II breast tumor have significant differences in immunomodulatory properties and secretion of some cytokines and mediators. Actually, soluble agents secreted from MSCs educated in stage II breast tumor tissue, in contrast to MSCs educated in normal breast adipose tissue, do not shift immune responses merely to inhibitory responses. In fact, T-MSCs, as compared with N-MSCs, although leading to increased levels of inhibitory mediators such as PGE2, TGF-β, and IL10 in PBLs, increase PBL proliferation. On the other hand, MSCs educated in stage II breast cancer and breast adipose tissue showed no significant differences in the polarization of Treg and induction of IL4 by soluble factors in PBLs.
Acknowledgements
The authors would like to thank those normal individuals and breast cancer patients for their kind participation in this project. This work was supported by a grant from Tehran University of Medical Sciences and Health Services (Grant Number: 30069).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Alireza Razavi, Email: razavial@tums.ac.ir.
Seyed Mahmoud Hashemi, Email: smmhashemi@sbmu.ac.ir.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Bates JP, Derakhshandeh R, Jones L, Webb TJ. Mechanisms of immune evasion in breast cancer. BMC Cancer. 2018;18(1):556. doi: 10.1186/s12885-018-4441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdollahpour-Alitappeh M, Lotfinia M, Bagheri N, Sineh Sepehr K, Habibi-Anbouhi M, Kobarfard F, et al. Trastuzumab-monomethyl auristatin E conjugate exhibits potent cytotoxic activity in vitro against HER2-positive human breast cancer. J Cell Physiol. 2019;234(3):2693–2704. doi: 10.1002/jcp.27085. [DOI] [PubMed] [Google Scholar]
- 4.Bahrami A, Hassanian SM, Khazaei M, Hasanzadeh M, Shahidsales S, Maftouh M, et al. The therapeutic potential of targeting tumor microenvironment in breast cancer: rational strategies and recent progress. J Cell Biochem. 2018;119(1):111–122. doi: 10.1002/jcb.26183. [DOI] [PubMed] [Google Scholar]
- 5.D’souza N, Burns JS, Grisendi G, Candini O, Veronesi E, Piccinno S, et al. (2012) MSC and tumors: homing, differentiation, and secretion influence therapeutic potential. In: Mesenchymal stem cells-basics and clinical application II. Springer, pp 209–66 [DOI] [PubMed]
- 6.Van Pham P, Vu NB (2018) Mesenchymal stem cells as vectors for cancer therapy. In: Stem cells for cancer and genetic disease treatment. Springer, pp 13–27
- 7.Poggi A, Varesano S, Zocchi MR. How to hit mesenchymal stromal cells and make the tumor microenvironment immunostimulant rather than immunosuppressive. Front Immunol. 2018;9:262. doi: 10.3389/fimmu.2018.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Melzer C, von der Ohe J, Hass R. Enhanced metastatic capacity of breast cancer cells after interaction and hybrid formation with mesenchymal stroma/stem cells (MSC) Cell Commun Signal. 2018;16(1):2. doi: 10.1186/s12964-018-0215-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Razmkhah M, Abtahi S, Ghaderi A. Mesenchymal stem cells, immune cells and tumor cells crosstalk: a sinister triangle in the tumor microenvironment. Curr Stem Cell Res Ther. 2019;14(1):43–51. doi: 10.2174/1574888X13666180816114809. [DOI] [PubMed] [Google Scholar]
- 10.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8(7):523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sato K, Ozaki K, Oh I, Meguro A, Hatanaka K, Nagai T, et al. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood. 2007;109(1):228–234. doi: 10.1182/blood-2006-02-002246. [DOI] [PubMed] [Google Scholar]
- 12.Fakhimi M, Talei AR, Ghaderi A, Habibagahi M, Razmkhah M. Helios, CD73 and CD39 induction in regulatory T cells exposed to adipose derived mesenchymal stem cells. Cell J. 2020;22(2):236–244. doi: 10.22074/cellj.2020.6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vasilev G, Ivanova M, Ivanova-Todorova E, Tumangelova-Yuzeir K, Krasimirova E, Stoilov R, et al. Secretory factors produced by adipose mesenchymal stem cells downregulate Th17 and increase Treg cells in peripheral blood mononuclear cells from rheumatoid arthritis patients. Rheumatol Int. 2019;39(5):819–826. doi: 10.1007/s00296-019-04296-7. [DOI] [PubMed] [Google Scholar]
- 14.Kadle RL, Abdou SA, Villarreal-Ponce AP, Soares MA, Sultan DL, David JA, et al. Microenvironmental cues enhance mesenchymal stem cell-mediated immunomodulation and regulatory T-cell expansion. PLoS ONE. 2018;13(3):e0193178. doi: 10.1371/journal.pone.0193178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He N, Kong Y, Lei X, Liu Y, Wang J, Xu C, et al. MSCs inhibit tumor progression and enhance radiosensitivity of breast cancer cells by down-regulating Stat3 signaling pathway. Cell Death Dis. 2018;9(10):1026. doi: 10.1038/s41419-018-0949-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.François S, Usunier B, Forgue-Lafitte ME, L’Homme B, Benderitter M, Douay L, et al. Mesenchymal stem cell administration attenuates colon cancer progression by modulating the immune component within the colorectal tumor microenvironment. Stem Cells Transl Med. 2019;8(3):285–300. doi: 10.1002/sctm.18-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li P, Gong Z, Shultz LD, Ren G. Mesenchymal stem cells: from regeneration to cancer. Pharmacol Ther. 2019;200(2019):42–54. doi: 10.1016/j.pharmthera.2019.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sineh Sepehr K, Razavi A, Saeidi M, Mossahebi-Mohammadi M, Abdollahpour-Alitappeh M, Hashemi SM. Development of a novel explant culture method for the isolation of mesenchymal stem cells from human breast tumor. J Immun Immunochem. 2018;39(2):207–217. doi: 10.1080/15321819.2018.1447487. [DOI] [PubMed] [Google Scholar]
- 19.Falconi G, Fabiani E, Criscuolo M, Fianchi L, Buccisano F, Maurillo L, et al. Expression profile of bone marrow mesenchymal stromal cells isolated from patients with therapy-related myeloid neoplasms. Leuk Res. 2017;55:S116–S117. doi: 10.1016/S0145-2126(17)30307-7. [DOI] [Google Scholar]
- 20.Gauthaman K, Yee FC, Cheyyatraivendran S, Biswas A, Choolani M, Bongso A. Human umbilical cord Wharton’s jelly stem cell (hWJSC) extracts inhibit cancer cell growth in vitro. J Cell Biochem. 2012;113(6):2027–2039. doi: 10.1002/jcb.24073. [DOI] [PubMed] [Google Scholar]
- 21.Geyh S, Rodriguez-Paredes M, Jäger P, Khandanpour C, Cadeddu R, Gutekunst J, et al. Functional inhibition of mesenchymal stromal cells in acute myeloid leukemia. Leukemia. 2016;30(3):683. doi: 10.1038/leu.2015.325. [DOI] [PubMed] [Google Scholar]
- 22.Zhang C, Zhai W, Xie Y, Chen Q, Zhu W, Sun X. Mesenchymal stem cells derived from breast cancer tissue promote the proliferation and migration of the MCF-7 cell line in vitro. Oncol Lett. 2013;6(6):1577–1582. doi: 10.3892/ol.2013.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lavini-Ramos C, Silva HM, Soares-Schanoski A, Monteiro SM, Ferreira LRP, Pacanaro AP, et al. MMP9 integrates multiple immunoregulatory pathways that discriminate high suppressive activity of human mesenchymal stem cells. Sci Rep. 2017;7(1):874. doi: 10.1038/s41598-017-00923-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ling W, Zhang J, Yuan Z, Ren G, Zhang L, Chen X, et al. Mesenchymal stem cells use IDO to regulate immunity in tumor microenvironment. Can Res. 2014;74(5):1576–1587. doi: 10.1158/0008-5472.CAN-13-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vo BT, Morton D, Jr, Komaragiri S, Millena AC, Leath C, Khan SA. TGF-β effects on prostate cancer cell migration and invasion are mediated by PGE2 through activation of PI3K/AKT/mTOR pathway. Endocrinology. 2013;154(5):1768–1779. doi: 10.1210/en.2012-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baratelli F, Lee JM, Hazra S, Lin Y, Walser TC, Schaue D, et al. PGE2 contributes to TGF-β induced T regulatory cell function in human non-small cell lung cancer. Am J Transl Res. 2010;2(4):356. [PMC free article] [PubMed] [Google Scholar]
- 27.Fontaine MJ, Shih H, Schäfer R, Pittenger MF. Unraveling the mesenchymal stromal cells’ paracrine immunomodulatory effects. Transfus Med Rev. 2016;30(1):37–43. doi: 10.1016/j.tmrv.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 28.Ho MS, Mei SH, Stewart DJ. The immunomodulatory and therapeutic effects of mesenchymal stromal cells for acute lung injury and sepsis. J Cell Physiol. 2015;230(11):2606–2617. doi: 10.1002/jcp.25028. [DOI] [PubMed] [Google Scholar]
- 29.Kyurkchiev D, Bochev I, Ivanova-Todorova E, Mourdjeva M, Oreshkova T, Belemezova K, et al. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J Stem Cells. 2014;6(5):552. doi: 10.4252/wjsc.v6.i5.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Volarevic V, Gazdic M, Markovic BS, Jovicic N, Djonov V, Arsenijevic N. Mesenchymal stem cell-derived factors: immuno-modulatory effects and therapeutic potential. Bio Factors. 2017;43(5):633–644. doi: 10.1002/biof.1374. [DOI] [PubMed] [Google Scholar]
- 31.Ramadan A, Griesenauer B, Adom D, Kapur R, Hanenberg H, Liu C, et al. Specifically differentiated T cell subset promotes tumor immunity over fatal immunity. J Exp Med. 2017;214(12):3577–3596. doi: 10.1084/jem.20170041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duffy MM, Ritter T, Ceredig R, Griffin MD. Mesenchymal stem cell effects on T-cell effector pathways. Stem Cell Res Therapy. 2011;2(4):34. doi: 10.1186/scrt75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clark KC, Fierro FA, Ko EM, Walker NJ, Arzi B, Tepper CG, et al. Human and feline adipose-derived mesenchymal stem cells have comparable phenotype, immunomodulatory functions, and transcriptome. Stem Cell Res Therapy. 2017;8(1):69. doi: 10.1186/s13287-017-0528-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang JW, Koo HC, Hwang SY, Kang SK, Ra JC, Lee MH, et al. Immunomodulatory effects of human amniotic membrane-derived mesenchymal stem cells. J Vet Sci. 2012;13(1):23–31. doi: 10.4142/jvs.2012.13.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Svobodova E, Krulova M, Zajicova A, Pokorna K, Prochazkova J, Trosan P, et al. The role of mouse mesenchymal stem cells in differentiation of naive T-cells into anti-inflammatory regulatory T-cell or proinflammatory helper T-cell 17 population. Stem Cells Develop. 2011;21(6):901–910. doi: 10.1089/scd.2011.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whiteside TL. Induced regulatory T cells in inhibitory microenvironments created by cancer. Exp Opin Biol Therapy. 2014;14(10):1411–1425. doi: 10.1517/14712598.2014.927432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patel SA, Dave MA, Bliss SA, Giec-Ujda AB, Bryan M, Pliner LF, et al. Treg/Th17 polarization by distinct subsets of breast cancer cells is dictated by the interaction with mesenchymal stem cells. J Cancer Stem Cell Res. 2014;2014(2):1–21. doi: 10.14343/JCSCR.2014.2e1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martínez-Peinado P, Pascual-García S, Roche E, Sempere-Ortells JM. Differences of clonogenic mesenchymal stem cells on immunomodulation of lymphocyte subsets. J Immunol Res. 2018;2018:1–11. doi: 10.1155/2018/7232717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mashreghi M, Azarpara H, Bazaz MR, Jafari A, Masoudifar A, Mirzaei H, et al. Angiogenesis biomarkers and their targeting ligands as potential targets for tumor angiogenesis. J Cell Physiol. 2018;233(4):2949–2965. doi: 10.1002/jcp.26049. [DOI] [PubMed] [Google Scholar]
- 40.Kim Y-S, Hong S-W, Choi J-P, Shin T-S, Moon H-G, Choi E-J, et al. Vascular endothelial growth factor is a key mediator in the development of T cell priming and its polarization to type 1 and type 17 T helper cells in the airways. J Immunol. 2009;183(8):5113–5120. doi: 10.4049/jimmunol.0901566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marti LC, Pavon L, Severino P, Sibov T, Guilhen D, Moreira-Filho CA. Vascular endothelial growth factor-A enhances indoleamine 2, 3-dioxygenase expression by dendritic cells and subsequently impacts lymphocyte proliferation. Memórias do Instituto Oswaldo Cruz. 2014;109(1):70–79. doi: 10.1590/0074-0276130252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deák M, Hornung Á, Novák J, Demydenko D, Szabó E, Czibula Á, et al. Novel role for galectin-1 in T-cells under physiological and pathological conditions. Immunobiology. 2015;220(4):483–489. doi: 10.1016/j.imbio.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 43.García M, Jurado F, San Segundo D, López-Hoyos M, Iruzubieta P, Llerena S, et al. (2015) In: Transplantation proceedings of Galectin-1 in stable liver transplant recipients. Elsevier [DOI] [PubMed]
- 44.Fajka-Boja R, Urbán VS, Szebeni GJ, Czibula Á, Blaskó A, Kriston-Pál É, et al. Galectin-1 is a local but not systemic immunomodulatory factor in mesenchymal stromal cells. Cytotherapy. 2016;18(3):360–370. doi: 10.1016/j.jcyt.2015.12.004. [DOI] [PubMed] [Google Scholar]