Abstract
The efficacy of immune checkpoint inhibitors (ICIs) in elderly and poor performance status (PS) patients is controversial, because clinical evidence is limited. This study aimed to find a predictive biomarker for the efficacy of anti-programmed cell death 1 (PD-1) antibodies in these patient populations. We retrospectively reviewed medical records of advanced non-small-cell lung cancer (NSCLC) patients who were ≥ 75 years of age or classified as PS 2 and received anti-PD-1 antibody treatment between December 2015 and May 2018. We evaluated the association between the efficacy of the anti-PD-1 antibody in these patients and the clinical variables thought to affect ICI efficacy. A total of 235 patients with advanced NSCLC were treated with anti-PD-1 antibodies, among whom 31 patients were ≥ 75 years of age and 22 were PS 2. A Cox proportional hazard model showed that only high levels of serum vascular endothelial growth factor (VEGF) were significantly associated with a shorter progression-free survival in patients aged ≥ 75 years and those with PS 2. Among these cohorts, the overall response rate to anti-PD-1 treatment tended to be lower when serum VEGF was high compared to patients with low serum VEGF. Our results demonstrate that serum VEGF concentration may be a negative predictive biomarker in elderly and poor PS advanced NSCLC patients receiving anti-PD-1 antibody treatment. This finding may help identify patients who will not benefit from anti-PD-1 antibody therapy.
Electronic supplementary material
The online version of this article (10.1007/s00262-020-02539-2) contains supplementary material, which is available to authorized users.
Keywords: Anti-PD-1 antibody, Elderly, Non-small-cell lung cancer, Performance status, Vascular endothelial growth factor
Introduction
Successful immune checkpoint inhibitor (ICI) therapy provides longer progression-free survival (PFS) and overall survival (OS) in non-small-cell lung cancer (NSCLC) patients and has changed the treatment paradigm for advanced NSCLC [1, 2]. However, these beneficial effects are limited to some patients; approximately 30–40% of NSCLC patients administered ICI treatment show disease progression during the first three months of treatment [1–3]. Immune reactivation by ICIs may be dependent on the biological characteristics of tumor cells, as well as the tumor immune microenvironment. Staining of programmed cell death-ligand 1 (PD-L1) in tumor cells and tumor mutation burden can indicate that some biological characteristics of tumor cells may have use as biomarkers for ICI efficacy [4, 5]. Tumor infiltration lymphocytes could be a biomarker reflecting the immunological characteristics of patients [6], but these immunological characteristics could vary depending on the treatment program, age, and general physical condition of the patient. Therefore, a dynamic biomarker that reflects the immunological characteristics at the start of a treatment is needed. In NSCLC, it is assumed that increased age and poor general physical condition diminish immune cell activity and may reduce ICI efficacy [7, 8]. Some prior clinical studies reported that the efficacy of anti-PD-1 treatment in elderly patients was less than that in non-elderly patients [1, 2]. Conversely, at least one clinical trial found that anti-PD-1 treatment efficacy in the elderly was similar to that in other patients [3]. Moreover, in a retrospective study, PS was found to be an independent predictor of PFS in patients who received anti-PD-1 therapy [9]. Comprehensive data supporting the use of ICIs for the treatment of advanced NSCLC patients are lacking for elderly and PS 2 populations; therefore, we sought to identify a marker that predicts anti-PD-1 antibody treatment efficacy in these subpopulations of NSCLC patients.
Recently, ICI therapy used in combination with other agents, including chemotherapy, other ICIs, and molecular targeted drugs, was developed and expected to have additive or synergetic effects on the immune response. Among these agents, vascular endothelial growth factor (VEGF)/VEGF receptor inhibitors are promising partners for combination with ICIs. VEGF plays a crucial role in promoting tumor angiogenesis, which is essential for cancer proliferation, migration, and metastasis. In addition, clinical and preclinical findings have shown that VEGF is immunosuppressive [10]. VEGF decreases the activity of effector T cells and the differentiation and maturation of dendritic cells [11–14], while enhancing the activation of regulatory T cells (Treg) and myeloid-derived suppressor cells [15]. Therefore, VEGF is associated not only with tumor angiogenesis but also with the tumor immune microenvironment, which may affect anti-PD-1 antibody efficacy. Indeed, some clinical trials showed favorable antitumor activity of anti-PD-1 antibody in combination with VEGF/VEGF receptor inhibitors in many kinds of cancers [16, 17]. Therefore, the role of VEGF in the tumor immune microenvironment has received attention.
According to recent reports [18, 19], many biomarkers of ICI efficacy have been identified; however, a biomarker for underrepresented populations, such as elderly or poor PS patients, is limited. The purpose of this study was to identify a biomarker for anti-PD-1 antibody efficacy in elderly and poor PS patients.
Materials and methods
We retrospectively reviewed the medical records of 235 advanced NSCLC patients who had received anti-PD-1 antibody therapy (e.g., nivolumab and pembrolizumab) as a first-to third-line treatment at the National Cancer Center Tokyo Japan between December 2015 and May 2018, with the follow-up period ending December 31, 2018. Among this patient population, we identified 31 elderly patients who were ≥ 75 years of age, and 22 patients with poor PS, who had ECOG PS 2 (no patients with PS ≥ 3 received anti-PD-1 therapy). Patients were excluded from our study if we could not obtain their serum samples for analysis. Serum samples were collected before administering anti-PD-1 antibody treatment and then stored at − 20 °C until further processing at the National Cancer Center Biobank (Tokyo, Japan). We reviewed medical records for patient characteristics; laboratory findings, such as programmed death-ligand 1 (PD-L1) tumor proportion score (22C3), white blood cell (WBC) count, lymphocyte count, levels of albumen, lactate dehydrogenase (LDH), C-reactive protein (CRP), and serum vascular endothelial growth factor (VEGF); and prognosis. The appropriate cutoff point for serum VEGF was determined by receiver operating characteristic (ROC) curve analysis. The cutoff points for other blood tests were defined by the median value for all patients. The study was conducted in accordance with the provisions of the Declaration of Helsinki and was approved by the Ethics Committee of the National Cancer Center Hospital (2018–268).
Median overall survival (OS), progression-free survival (PFS), and time to treatment failure (TTF) were estimated using the Kaplan–Meier method. A ROC curve was used to determine the optimal cutoff value of VEGF for predicting early progression over three months after anti-PD-1 therapy. The tumor’s response to treatment was objectively assessed using the response evaluation criteria in solid tumors (RECIST v1.1). Univariate analysis was performed using a Cox proportional hazard model with sex, smoking status, histology, PD-L1 tumor proportion score (22C3), treatment line, white blood cells, lymphocyte count, albumin, lactate dehydrogenase, C-reactive protein, and serum VEGF levels as covariates. These covariates were adopted based on the results of recent trials that suggested these variables might affect PD-1/PD-L1 checkpoint inhibitor efficacy [2, 4, 9, 20–26]. Because of the small sample size of the study population, we did not conduct a multivariate analysis. All P values are based on a two-sided hypothesis, and values less than 0.05 were considered statistically significant. All statistical analyses were performed using the JMP Pro software, v13.0.0 (SAS Institute, Cary, NC).
Results
Elderly patients
A total of 31 elderly patients (i.e., patients ≥ 75 years of age) were reviewed in this study (Supplementary Fig. 1a) and all patient characteristics are summarized in Table 1. Of the 31 patients, one patient (3.2%) was ECOG PS ≥ 2, 22 patients (71%) were male, nine (30%) were never-smokers, 15 (58%) were PD-L1 ≥ 50%, and nine patients (29%) were treated as first-line anticancer treatment.
Table 1.
Baseline characteristics at the onset of anti-PD-1 antibody treatment
| Elderly patients, n (%) | PS2 patients, n (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| All, n (%) | Low VEGF | High VEGF | P | All, n (%) | Low VEGF | High VEGF | P | |
| Patients | 31 | 16 | 15 | 22 | 12 | 10 | ||
| Age | ||||||||
| Median (range) | 77 (75–84) | 78 (75–84) | 76 (75–83) | 66 (30–77) | 57 (34–73) | 60 (30–77) | ||
| ≥ 75 y | 31 (100) | 16 (100) | 15 (100) | 1 (4.5) | 0 (0) | 1 (10) | 0.45 | |
| ECOG PS | ||||||||
| ≥ 2 | 1 (3.2) | 0 (0) | 1 (6.7) | 0.48 | 22 (100) | 12 (100) | 10 (100) | |
| Gender | ||||||||
| Male | 22 (71) | 10 (63) | 12 (80) | 0.43 | 13 (59) | 8 (67) | 5 (50) | 0.67 |
| Smoking status | ||||||||
| Never-smoker | 9 (29) | 5 (33) | 4 (27) | 1.00 | 4 (18) | 3 (25) | 1 (10) | 0.59 |
| Histology | 0.22 | 0.62 | ||||||
| Squamous | 7 (23) | 2 (13) | 5 (33) | 5 (23) | 2 (17) | 3 (30) | ||
| Non-squamous | 24 (77) | 14 (88) | 10 (67) | 17 (77) | 10 (83) | 7 (70) | ||
| EGFR mutated | 6 (26) | 4 (29) | 2 (22) | 1.00 | 2 (14) | 1 (11) | 1 (20) | 1.00 |
| PD-L1 22C3 TPS | 0.69 | 0.36 | ||||||
| < 50% | 11 (42) | 5 (36) | 6 (50) | 7 (44) | 2 (29) | 5 (56) | ||
| ≥ 50% | 15 (58) | 9 (64) | 6 (50) | 9 (56) | 5 (71) | 4 (44) | ||
| Treatment line | 0.43 | 1.00 | ||||||
| 1 | 9 (29) | 6 (38) | 3 (20) | 4 (18) | 2 (17) | 2 (20) | ||
| 2–3 | 22 (71) | 10 (63) | 12 (80) | 18 (82) | 10 (83) | 8 (80) | ||
VEGF vascular endothelial growth factor, y years of age, ECOG PS Eastern Cooperative Oncology Group performance status, EGFR epidermal growth factor receptor, PD-L1 programmed death-ligand 1, TPS tumor proportion score
The median PFS for the patients ≥ 75 years of age was 6.9 mo (95% confidence interval (CI): 1.9–not reached (NR)). The overall response rate (ORR) was 29%, and the disease control rate (DCR) was 55%.
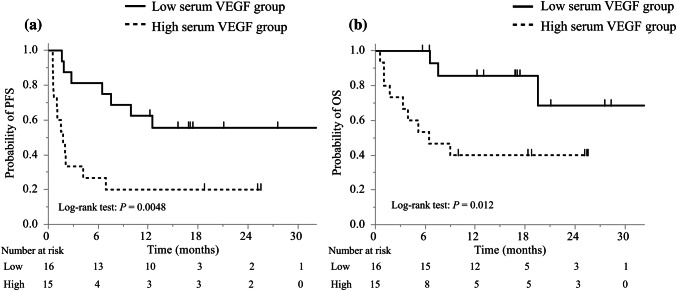
The best discriminative cutoff point of VEGF for early progression within three months was observed at 367 pg/mL through ROC analysis in elderly patients; the area under the ROC curve (AUC) was 0.74 (Supplementary Fig. 2a). The elderly patients were divided into low and high serum VEGF groups, and their characteristics are summarized in Table 1. There were no significant differences in baseline characteristics between the two groups, including the PD-L1 status. Univariate analysis showed that only high levels of serum VEGF were significantly associated with a shorter PFS (hazard ratio (HR): 3.6; 95% CI: 1.4–9.3; P = 0.008; Table 2a). The ORR was lower in the high serum VEGF group than in the low serum VEGF group (ORR: 13% vs. 44%; Table 3). The DCR was significantly lower in the high serum VEGF group than in the low serum VEGF group (33% vs. 75%, P = 0.032), and the median PFS was 1.8 months (95% CI: 0.6–4.2) in the high serum VEGF group and NR (95% CI: 6.4–NR) in the low serum VEGF group (Fig. 1a). The median TTF of the patients in the high serum VEGF group was significantly shorter than that of the patients in the low serum VEGF group (1.6 months vs. 10.7 months, respectively; HR: 2.4; 95% CI: 1.1-5.2; Log-rank test: P = 0.028) (Supplementary Fig. 3a). The median OS of the patients in the high serum VEGF group was significantly shorter than that of the patients in the low serum VEGF group (6.4 months vs. NR, respectively; HR: 4.6; 95% CI: 1.2–17.1; Log-rank test: P = 0.012) (Fig. 1b).
Table 2.
Cox proportional hazard model of PFS in (a) elderly and (b) poor ECOG PS 2 patients
| Univariate analysis | ||
|---|---|---|
| HR (95% CI) | P | |
| (a) | ||
| Gender (male/female) | 1.1 (0.42–3.4) | 0.86 |
| Smoking status (never/current or former) | 2.2 (0.80–5.7) | 0.12 |
| Histology (Sq/Non-Sq) | 1.6 (0.53–4.3) | 0.37 |
| PD-L1 status (< 50%/≥ 50%) | 2.8 (0.95–8.6) | 0.062 |
| Treatment line (2–3/1) | 1.9 (0.70–6.8) | 0.21 |
| WBC (≥ median/< median) | 1.4 (0.55–3.5) | 0.50 |
| Lymphocyte count (≥ median/< median) | 1.1 (0.44–2.8) | 0.85 |
| Albumin (≥ median/< median) | 1.0 (0.39–2.9) | 1.00 |
| LDH (≥ median/< median) | 1.6 (0.63–4.0) | 0.34 |
| CRP (≥ median/< median) | 1.4 (0.57–3.6) | 0.45 |
| VEGF (≥ 367 pg/mL/< 367 pg/mL) | 3.6 (1.4–9.3) | 0.008 |
| (b) | ||
| Gender (male/female) | 1.5 (0.60–4.2) | 0.38 |
| Smoking status (never/current or former) | 0.54 (0.12–1.7) | 0.31 |
| Histology (Sq/Non-Sq) | 1.5 (0.42–4.5) | 0.48 |
| PD-L1 status (< 50%/≥ 50%) | 2.2 (0.67–7.9) | 0.19 |
| Treatment line (2–3/1) | 1.1 (0.36–4.8) | 0.88 |
| WBC (≥ median/< median) | 1.2 (0.44–3.1) | 0.73 |
| Lymphocyte count (≥ median/< median) | 1.7 (0.64–4.6) | 0.29 |
| Albumin (≥ median/< median) | 0.54 (0.21–1.4) | 0.21 |
| LDH (≥ median/< median) | 0.88 (0.33–2.2) | 0.78 |
| CRP (≥ median/< median) | 2.3 (0.87–6.1) | 0.094 |
| VEGF (≥ 454 pg/mL/< 454 pg/mL) | 4.6 (1.5–14.3) | 0.009 |
HR hazard ratio, CI confidence interval, Sq squamous cell carcinoma, PD-L1 programmed death-ligand 1, WBC white blood cell, LDH lactate dehydrogenase, CRP C-reactive protein, VEGF vascular endothelial growth factor
Table 3.
Patient response to the anti-PD-1 antibody
| Elderly patients | ECOG PS2 patients | |||||
|---|---|---|---|---|---|---|
| Low VEGF | High VEGF | P | Low VEGF | High VEGF | P | |
| Patients | 16 | 15 | 12 | 10 | ||
| Best overall response, n (%) | ||||||
| Complete response | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Partial response | 7 (44) | 2 (13) | 2 (17) | 0 (0) | ||
| Stable disease | 5 (31) | 3 (20) | 3 (25) | 0 (0) | ||
| Progressive disease | 3 (19) | 9 (60) | 6 (50) | 9 (90) | ||
| Not evaluable | 1 (6) | 1 (7) | 1 (8) | 1 (10) | ||
| ORR, % (95% CI) | 44 (22–66) | 13 (0–32) | 0.11 | 17 (0–38) | 0 (0–18) | 0.48 |
| DCR, % (95% CI) | 75 (55–95) | 33 (12–55) | 0.032 | 42 (17–66) | 0 (0–17) | 0.040 |
ECOG PS Eastern Cooperative Oncology Group Performance Status, VEGF vascular endothelial growth factor, ORR objective response rate, DCR disease control rate
Fig. 1.
Kaplan–Meier curves of a PFS and b OS of elderly patients. PFS progression-free survival, OS overall survival, VEGF vascular endothelial growth factor
ECOG PS 2 patients
A total of 22 ECOG PS 2 patients were reviewed in this study (Supplementary Fig. 1b) and patient characteristics are summarized in Table 1. Of these 22 patients, one patient (4.5%) was ≥ 75 years old, 13 patients (59%) were male, four patients (18%) were never-smokers, nine patients (56%) were PD-L1 ≥ 50%, and four patients (18%) were treated as first-line anticancer treatment.
The median PFS of the PS 2 patients was 2.1 months (95% CI: 0.73–5.96), the ORR was 9.1%, and the DCR was 23%.
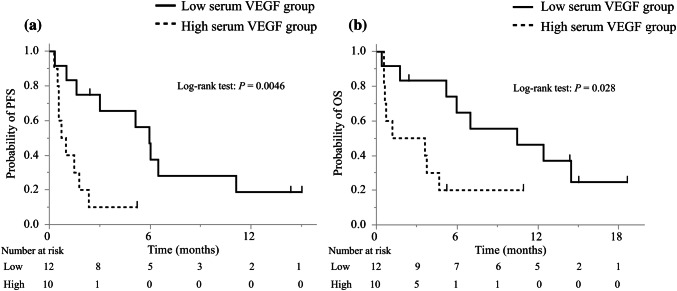
The best discriminative cutoff point of VEGF for early progression within three months was observed at 454 pg/mL through ROC analysis in PS 2 patients. The area under the ROC curve (AUC) was 0.68 (Supplementary Fig. 2b). The PS 2 patients were also divided into low and high serum VEGF groups; however there were no significant differences in baseline characteristics, including the PD-L1 status, between the two groups (Table 1). The univariate analysis showed that only high levels of serum VEGF were significantly associated with a shorter PFS (HR: 4.6; 95% CI, 1.5–14.3; P = 0.009; Table 2b). The ORR was lower in the high serum VEGF group than in the low serum VEGF group (0% vs. 17%; Table 3), and the DCR was significantly lower in the high serum VEGF group than in the low serum VEGF group (0% vs. 42%; P = 0.040). No patient in the high serum VEGF group showed tumor shrinkage. The median PFS was 0.86 months (95% CI: 0.30–1.8) in the high serum VEGF group and 6.0 months (95% CI: 1.0–11.1) in the low serum VEGF group (Fig. 2a). The median TTF of the patients in the high serum VEGF group was significantly shorter than that of the patients in the low serum VEGF group (0.85 months vs. 2.4 months; HR: 3.4; 95% CI: 1.2-9.7; P = 0.011; Supplementary Fig. 3b). The median OS of the patients in the high serum VEGF group was significant lower than that of the patients in the low serum VEGF group (2.4 months vs. 10.5 months, respectively; HR: 3.3; 95% CI: 1.1–10.0; P = 0.028; Fig. 2b).
Fig. 2.
Kaplan–Meier curves of a PFS and b OS of ECOG PS 2 patients. PFS progression-free survival, OS overall survival, VEGF vascular endothelial growth factor, ECOG PS Eastern Cooperative Oncology Group performance status
Discussion
This study demonstrated that the efficacy of anti-PD-1 antibody treatment was inversely associated with the serum concentrations of VEGF in patients ≥ 75 years of age and in ECOG PS 2 patients. Our results support the contention that the serum VEGF may be of value as a negative predictive biomarker anti-PD-1 antibody treatment efficacy in patients within these demographics.
The potential impact of aging on the outcome of patients treated with ICIs remains controversial based on data from the CheckMate017, CheckMate057, and Keynote189 trials [1, 2, 27]. The OS of the nivolumab treatment group was significantly longer than that of the docetaxel group; however, no significant differences were found between these groups in patients ≥ 75 years old. These results suggest that elderly patients may have a different immune system response to anti-PD-1 antibodies than younger patients. Previous research has reported that aging impacts specific signaling pathways in human cancer cells and alters immune checkpoints that reshape the tumor microenvironment [28]. Therefore, it has been hypothesized that changes in the tumor microenvironment in elderly patients are due to their less functional immune cells. Because the tumor microenvironment differs between elderly and non-elderly patients, predictive markers for anti-PD-1 antibody efficacy should be considered for each age group separately. Our study demonstrated that PFS in the high serum VEGF group was significantly shorter than that in the low serum VEGF group among elderly patients, and hence, serum VEGF may be a predictive biomarker for patients ≥ 75 years of age.
CheckMate171 was a phase II nivolumab monotherapy for pretreated advanced NSCLC patients, including ECOG PS 2 patients who had a median OS that was shorter in patients with ECOG PS 2 than in the overall study group (5.4 months vs. 9.9 months, respectively) [29]. Additionally, CheckMate153, a phase III/IV nivolumab trial, demonstrated that the 6-month OS was lower in ECOG PS 2 patients than in ECOG PS0-1 patients (31.5% vs. 62.7%), indicating that the anti-PD-1 antibody was less effective in ECOG PS 2 patients [7]. Similarly, our study showed that ORR and the median PFS in patients with ECOG PS 2 were 9.1% and 2.1 months, respectively. Therefore, it is important to select patients who benefit from ICIs, especially in ECOG PS 2 patients. We showed that among the ECOG PS 2 patients, PFS was significantly shorter in the high serum VEGF group than in the low serum VEGF group. Moreover, tumor shrinkage did not occur in the high serum VEGF group, suggesting that serum VEGF may be a predictive biomarker for ECOG PS 2 patients.
The serum VEGF level in patients with NSCLC is a prognostic marker. Some studies have also shown that VEGF is negatively correlated with patient survival [30–32]; however, the predictive value of VEGF level as a biomarker for ICI efficacy is unknown. A previous study found that high serum VEGF was associated with worse clinical outcomes in melanoma patients receiving anti-CTL antigen 4 antibody treatment, indicating that the disease control rate was significantly reduced in patients with high serum VEGF levels compared to patients with lower serum VEGF levels (23% vs. 41%) [24]. Our study demonstrated that the level of serum VEGF is a predictive biomarker for the efficacy of anti-PD-1 antibody in advanced NSCLC patients aged ≥ 75 years and in ECOG PS 2 patients. Indeed, the ORR and DCR were lower in high serum VEGF group than in low serum VEGF group (aged ≥ 75 years ORR, 19% vs. 40%; DCR, 38% vs. 73%; PS 2 ORR, 0% vs. 18%; DCR, 0% vs. 45%).
The present study has several limitations. First, it was based on the retrospective review of medical records and stored serum samples. There was a potential for selection bias, because our study was conducted at a single institution and we could only include patients for whom a serum sample was stored. Second, our sample size was very small; therefore, our study may lack the statistical power necessary to reliably and confidently determine all factors associated with treatment efficacy. Finally, we included only a subpopulation of advanced NSCLC patients in our study. However, despite these limitations, this is the first report to demonstrate an association between serum VEGF level and the efficacy of anti-PD-1 antibody treatment in elderly and ECOG PS 2 patients with advanced NSCLC.
In conclusion, serum VEGF levels may be a useful negative predictive biomarker for the efficacy of anti-PD-1 antibodies in advanced NSCLC patients who are ≥ 75 years old, and in those with ECOG PS 2. These results emphasize a potential role for VEGF in the immune microenvironment and the possible synergies that involve combinations of immune checkpoint inhibitors and anti-VEGF/VEGFR inhibitors in these patients. To address the unmet medical needs of NSCLC patients aged ≥ 75 years and ECOG PS 2 patients, additional prospective studies are needed to determine the efficacy of treatment with immune checkpoint inhibitors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1 Patient selection for (a) elderly and (b) ECOG PS 2 cohorts. ECOG PS, Eastern Cooperative Oncology Group performance status; NSCLC, non-small cell lung cancer; PD-1, programmed cell death 1 (TIFF 996 kb)
Supplementary Fig. 2 Receiver operating characteristic curves for serum VEGF based on progression for three months. VEGF, vascular endothelial growth factor; ECOG PS, Eastern Cooperative Oncology Group performance status (TIFF 1794 kb)
Supplementary Fig. 3 Kaplan–Meier curves of TTF for (a) elderly and (b) ECOG PS 2 patients. TTF, time to treatment failure; ECOG PS, Eastern Cooperative Oncology Group performance status; VEGF, vascular endothelial growth factor (TIFF 1794 kb)
Abbreviations
- CI
Confidence interval
- DCR
Disease control rate
- ECOG
Eastern Cooperative Oncology Group
- HR
Hazard ratio
- ICI
Immune checkpoint inhibitor
- NR
Not reached
- NSCLC
Non-small-cell lung cancer
- ORR
Overall response rate
- OS
Overall survival
- PD-1
Programmed cell death 1
- PD-L1
Programmed death-ligand 1
- PFS
Progression-free survival
- PS
Performance status
- VEGF
Vascular endothelial growth factor
Author contributions
RS contributed to the collection of clinical data, data quality control, statistical data analysis, interpretation of results, and writing of the manuscript. SM interpreted the results and participated in writing the manuscript. YS, YM, TY, YG, SK, HH, YF, Nobuyuki Y, Noboru Y, and YO contributed to manuscript writing and editing. All authors have approved the manuscript’s final version.
Funding
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Compliance with ethical standards
Conflict of interest
Shuji Murakami has served on speakers’ bureaus for Taiho Pharmaceutical and Ono Pharmaceutical. Yasushi Goto has held consulting/advisory roles for Taiho Pharmaceutical; served on speakers’ bureaus for Taiho Pharmaceutical, Ono Pharmaceutical, Bristol-Myers Squibb, and Merck Sharp and Dohme (MSD); and received research funding from Taiho Pharmaceutical, Bristol-Myers Squibb, and Ono Pharmaceutical. Shintaro Kanda has received research funding from Ono Pharmaceutical and honoraria from Ono Pharmaceutical and Bristol-Myers Squibb. Hidehito Horinouchi has received research funding from MSD, Bristol-Myers Squibb, Ono Pharmaceutical, and Taiho Pharmaceutical. Yutaka Fujiwara has received research funding from MSD and served on speakers’ bureaus for MSD, Taiho Pharmaceutical, Bristol-Myers Squibb, and Ono Pharmaceutical. Nobuyuki Yamamoto has held consulting/advisory roles for Taiho Pharmaceutical; served on speakers’ bureaus for Ono Pharmaceutical, Bristol-Myers Squibb, and MSD; and received honoraria from Ono Pharmaceutical and MSD. Noboru Yamamoto has received research funding from Taiho Pharmaceutical, Bristol-Myers Squibb, and Ono Pharmaceutical; and served on speakers’ bureaus for Bristol-Myers Squibb and Ono Pharmaceutical. Yuichiro Ohe has received research funding from Taiho Pharmaceutical and MSD and honoraria from Taiho Pharmaceutical and MSD. All remaining authors declare no conflicts of interest.
Ethical approval
This study was approved by the Institutional Review Board of the National Cancer Center Hospital, Tokyo, Japan, and has been performed in accordance with the ethical standards described in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards (No. 2018–268).
Informed consent
Informed consent was not obtained from each patient, because this retrospective analysis of existing data did not require any interaction with patients and did not intervene in their treatment.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/nejmoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/nejmoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herbst RS, Baas P, Kim D-W, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. The Lancet. 2016;387:1540–1550. doi: 10.1016/s0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 4.Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. doi: 10.1056/nejmoa1606774. [DOI] [PubMed] [Google Scholar]
- 5.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. The Lancet. 2017;389:255–265. doi: 10.1016/s0140-6736(16)32517-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spigel D, Schwartzberg L, Waterhouse D, et al. P3.02c-026 is nivolumab safe and effective in elderly and PS2 patients with non-small cell lung cancer (NSCLC)? Results of CHECKMAte 153. J Thoracic Oncol. 2017;12:S1287–S1288. doi: 10.1016/j.jtho.2016.11.1821. [DOI] [Google Scholar]
- 8.Gandara DR, Kowanetz M, Mok TSK, et al. 1295OBlood-based biomarkers for cancer immunotherapy: tumor mutational burden in blood (bTMB) is associated with improved atezolizumab (atezo) efficacy in 2L + NSCLC (POPLAR and OAK) Ann Oncol. 2017 doi: 10.1093/annonc/mdx380. [DOI] [Google Scholar]
- 9.Taniguchi Y, Tamiya A, Isa SI, et al. Predictive factors for poor progression-free survival in patients with non-small cell lung cancer treated with nivolumab. Anticancer Res. 2017;37:5857–5862. doi: 10.21873/anticanres.12030. [DOI] [PubMed] [Google Scholar]
- 10.Feng PH, Chen KY, Huang YC, et al. Bevacizumab reduces S100A9-positive MDSCs linked to intracranial control in patients with EGFR-mutant lung adenocarcinoma. J Thorac Oncol. 2018;13:958–967. doi: 10.1016/j.jtho.2018.03.032. [DOI] [PubMed] [Google Scholar]
- 11.Ziogas AC, Gavalas NG, Tsiatas M, et al. VEGF directly suppresses activation of T cells from ovarian cancer patients and healthy individuals via VEGF receptor Type 2. Int J Cancer. 2012;130:857–864. doi: 10.1002/ijc.26094. [DOI] [PubMed] [Google Scholar]
- 12.Voron T, Marcheteau E, Pernot S, Colussi O, Tartour E, Taieb J, Terme M. Control of the immune response by pro-angiogenic factors. Front Oncol. 2014;4:70. doi: 10.3389/fonc.2014.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oyama T, Ran S, Ishida T, Nadaf S, Kerr L, Carbone DP, Gabrilovich DI. Vascular endothelial growth factor affects dendritic cell maturation through the inhibition of nuclear factor-kappa B activation in hemopoietic progenitor cells. J Immunol. 1998;160:1224–1232. [PubMed] [Google Scholar]
- 14.Gabrilovich DI, Chen HL, Girgis KR, Cunningham HT, Meny GM, Nadaf S, Kavanaugh D, Carbone DP. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med. 1996;2:1096–1103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 15.Nefedova Y, Huang M, Kusmartsev S, Bhattacharya R, Cheng P, Salup R, Jove R, Gabrilovich D. Hyperactivation of STAT3 is involved in abnormal differentiation of dendritic cells in cancer. J Immunol. 2003;172:464–474. doi: 10.4049/jimmunol.172.1.464. [DOI] [PubMed] [Google Scholar]
- 16.Makker V, Rasco D, Vogelzang NJ, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer: an interim analysis of a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019;20:711–718. doi: 10.1016/s1470-2045(19)30020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herbst RS, Arkenau H-T, Santana-Davila R, et al. Ramucirumab plus pembrolizumab in patients with previously treated advanced non-small-cell lung cancer, gastro-oesophageal cancer, or urothelial carcinomas (JVDF): a multicohort, non-randomised, open-label, phase 1a/b trial. Lancet Oncol. 2019;20:1109–1123. doi: 10.1016/s1470-2045(19)30458-9. [DOI] [PubMed] [Google Scholar]
- 18.Tamiya M, Tamiya A, Inoue T, et al. Metastatic site as a predictor of nivolumab efficacy in patients with advanced non-small cell lung cancer: a retrospective multicenter trial. PLoS ONE. 2018;13:e0192227. doi: 10.1371/journal.pone.0192227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shibaki R, Murakami S, Shinno Y, et al. Malignant pleural effusion as a predictor of the efficacy of anti-PD-1 antibody in patients with non-small cell lung cancer. Thorac Cancer. 2019 doi: 10.1111/1759-7714.13004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wallis CJD, Butaney M, Satkunasivam R, Freedland SJ, Patel SP, Hamid O, Pal SK, Klaassen Z. Association of patient sex with efficacy of immune checkpoint inhibitors and overall survival in advanced cancers: a systematic review and meta-analysis. JAMA Oncol. 2019 doi: 10.1001/jamaoncol.2018.5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdel-Rahman O. Smoking and EGFR status may predict outcomes of advanced NSCLC treated with PD-(L)1 inhibitors beyond first line: a meta-analysis. Clin Respir J. 2018;12:1809–1819. doi: 10.1111/crj.12742. [DOI] [PubMed] [Google Scholar]
- 22.Buder-Bakhaya K, Hassel JC. Biomarkers for clinical benefit of immune checkpoint inhibitor treatment-a review from the melanoma perspective and beyond. Front Immunol. 2018;9:1474. doi: 10.3389/fimmu.2018.01474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/nejmoa1501824. [DOI] [PubMed] [Google Scholar]
- 24.Yuan J, Zhou J, Dong Z, et al. Pretreatment serum VEGF is associated with clinical response and overall survival in advanced melanoma patients treated with ipilimumab. Cancer Immunol Res. 2014;2:127–132. doi: 10.1158/2326-6066.cir-13-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inoue T, Tamiya M, Tamiya A, et al. Analysis of early death in Japanese patients with advanced non-small-cell lung cancer treated with nivolumab. Clin Lung Cancer. 2018;19:e171–e176. doi: 10.1016/j.cllc.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Shiroyama T, Suzuki H, Tamiya M, et al. Pretreatment advanced lung cancer inflammation index (ALI) for predicting early progression in nivolumab-treated patients with advanced non-small cell lung cancer. Cancer Med. 2018;7:13–20. doi: 10.1002/cam4.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–2092. doi: 10.1056/nejmoa1801005. [DOI] [PubMed] [Google Scholar]
- 28.Wu Y, Wei J, Chen X, Qin Y, Mao R, Song J, Fan Y. Comprehensive transcriptome profiling in elderly cancer patients reveals aging-altered immune cells and immune checkpoints. Int J Cancer. 2019;144:1657–1663. doi: 10.1002/ijc.31875. [DOI] [PubMed] [Google Scholar]
- 29.Popat S, Ardizzoni A, Ciuleanu T, et al. Nivolumab in previously treated patients with metastatic squamous NSCLC: results of a European single-arm, phase 2 trial (CheckMate 171) including patients aged 70 years and with poor performance status. Ann Oncol. 2017;28:10. [Google Scholar]
- 30.Lin Q, Guo L, Lin G, Chen Z, Chen T, Lin J, Zhang B, Gu X. Clinical and prognostic significance of OPN and VEGF expression in patients with non-small-cell lung cancer. Cancer Epidemiol. 2015;39:539–544. doi: 10.1016/j.canep.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 31.Naikoo NA, Rasool R, Shah S, Ahangar AG, Siddiqi MA, Shah ZA. Upregulation of vascular endothelial growth factor (VEGF), its role in progression and prognosis of non-small cell lung carcinoma. Cancer Genet. 2017;216–217:67–73. doi: 10.1016/j.cancergen.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Farhat FS, Tfayli A, Fakhruddin N, Mahfouz R, Otrock ZK, Alameddine RS, Awada AH, Shamseddine A. Expression, prognostic and predictive impact of VEGF and bFGF in non-small cell lung cancer. Crit Rev Oncol. 2012;84:149–160. doi: 10.1016/j.critrevonc.2012.02.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1 Patient selection for (a) elderly and (b) ECOG PS 2 cohorts. ECOG PS, Eastern Cooperative Oncology Group performance status; NSCLC, non-small cell lung cancer; PD-1, programmed cell death 1 (TIFF 996 kb)
Supplementary Fig. 2 Receiver operating characteristic curves for serum VEGF based on progression for three months. VEGF, vascular endothelial growth factor; ECOG PS, Eastern Cooperative Oncology Group performance status (TIFF 1794 kb)
Supplementary Fig. 3 Kaplan–Meier curves of TTF for (a) elderly and (b) ECOG PS 2 patients. TTF, time to treatment failure; ECOG PS, Eastern Cooperative Oncology Group performance status; VEGF, vascular endothelial growth factor (TIFF 1794 kb)