Abstract
Immune checkpoint inhibitor (ICI)-related pneumonitis is a relatively rare but clinically serious and potentially life-threatening adverse event. The majority of cases can be managed by drug discontinuation, with the administration of corticosteroids added in severe cases. However, worsening of pneumonitis can develop in a subset of patients despite treatment with high doses of corticosteroids. We herein report a case of steroid-refractory ICI-related pneumonitis in a recurrent non-small cell lung cancer (NSCLC) patient treated with pembrolizumab that was successfully improved by triple combination therapy (high-dose corticosteroids, tacrolimus, and cyclophosphamide). After 3 weeks of initial pembrolizumab administration, the patient was diagnosed with ICI-related pneumonitis. Chest computed tomography (CT) showed patchy distributed bilateral consolidation and ground-glass opacities (GGOs) with traction bronchiectasis and bronchiolectasis resembling the diffuse alveolar damage (DAD) radiographic pattern. Although methylprednisolone pulse therapy was initiated, worsening of respiratory failure resulted in the patient being transferred to the intensive care unit. Because of an insufficient therapeutic response to high-dose corticosteroids, tacrolimus and cyclophosphamide pulse therapy were additively performed as triple combination therapy according to the treatment strategy for pulmonary complications of clinically amyopathic dermatomyositis (CADM). In response to this triple combination therapy, the patient’s respiratory condition gradually improved, and chest CT showed the marked amelioration of pulmonary opacities. This is the first report suggesting the efficacy of triple combination therapy (high-dose corticosteroids, tacrolimus, and cyclophosphamide) for steroid-refractory ICI-related pneumonitis complicated with respiratory failure.
Keywords: Immune checkpoint inhibitor, Pembrolizumab, Pneumonitis, Steroid-refractory, Non-small cell lung cancer, Triple combination therapy
Introduction
Immune checkpoint inhibitors (ICIs), including cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programmed cell death 1 (PD-1) or its ligand programmed cell death ligand 1 (PD-L1) inhibitors, have been developed as effective treatment modalities for a variety of malignancies [1]. For example, PD-1 and PD-L1 inhibitors have shown clinical efficacy in the treatment of non-small cell lung cancer (NSCLC) [2].
However, as they exert their anti-tumor activity, ICIs can induce inflammatory side effects associated with an enhanced immune system, which are generally termed immune-related adverse events (irAEs) [3, 4]. irAEs can involve different organs throughout the body [5–7]. Among irAEs, pneumonitis is a relatively rare but clinically important and potentially life-threatening complication [1, 2, 8, 9]. Most irAEs, including pneumonitis, can be managed by drug discontinuation, with the additional administration of corticosteroids in severe cases to suppress lymphocyte activation [4, 10]. However, worsening of pneumonitis can occur in a subset of patients despite treatment with high doses of corticosteroids [8]. Infliximab, an antibody against tumor necrosis factor alpha (TNF-α), has been generally recommended in cases of steroid-refractory ICI-related pneumonitis [1, 3, 4, 10, 11]. However, the selection and usage of additional immunosuppressive agents remains controversial in such cases.
Interstitial lung disease (ILD) is a representative complication in polymyositis (PM) and dermatomyositis (DM). PM/DM-associated ILD can be steroid-refractory during the treatment course, and immunosuppressive agents, including calcineurin inhibitors and cyclophosphamide, are required to achieve remission. Cases with clinically amyopathic dermatomyositis (CADM) or anti-CADM 140/anti-melanoma differentiation-associated gene 5 (MDA 5) antibody are particularly prone to developing rapidly-progressive ILD with a poor prognosis [12]. The potential effectiveness of an intensive treatment protocol of triple combination therapy comprised of high-dose corticosteroids, calcineurin inhibitors (such as ciclosporin and tacrolimus) and cyclophosphamide has been reported in such severe cases [13, 14].
We herein report a case of steroid-refractory ICI-related pneumonitis caused by pembrolizumab (PD-1 inhibitor) in a recurrent NSCLC patient that was successfully managed by treatment with triple combination therapy.
Case report
We encountered a 59-year-old man with recurrent NSCLC (ex-smoker, smoking index: 70 pack-years). The patient was clinically diagnosed with stage IIIB adenocarcinoma of the right middle lobe (cT1bN3M0) without an epidermal growth factor receptor (EGFR) mutation or anaplastic lymphoma kinase (ALK)/c-ros oncogene 1 (ROS1) rearrangement. The percentage of tumor cells with membranous PD-L1 staining (tumor proportion score) was 1%.
The patient was treated by four cycles of chemotherapy comprising cisplatin plus vinorelbine with concurrent radiation therapy (66 Gy/33 fractions), resulting in complete remission. He developed focal radiation pneumonitis accompanied by subsequent fibrosis of the right lung without necessitating corticosteroid therapy. Treatment was completed, and he received regular follow-up examinations. However, after 2 years of chemoradiotherapy, relapse was demonstrated by multiple lung metastases. The patient was then treated with four cycles of chemotherapy comprising cisplatin plus pemetrexed. After additional 27 cycles of maintenance treatment with pemetrexed, progressive disease was noted by the development of carcinomatous pericarditis. Intrapericardial instillation of bleomycin following pericardial drainage was performed, and pembrolizumab monotherapy (200 mg every 3 weeks) was selected as the third-line treatment.
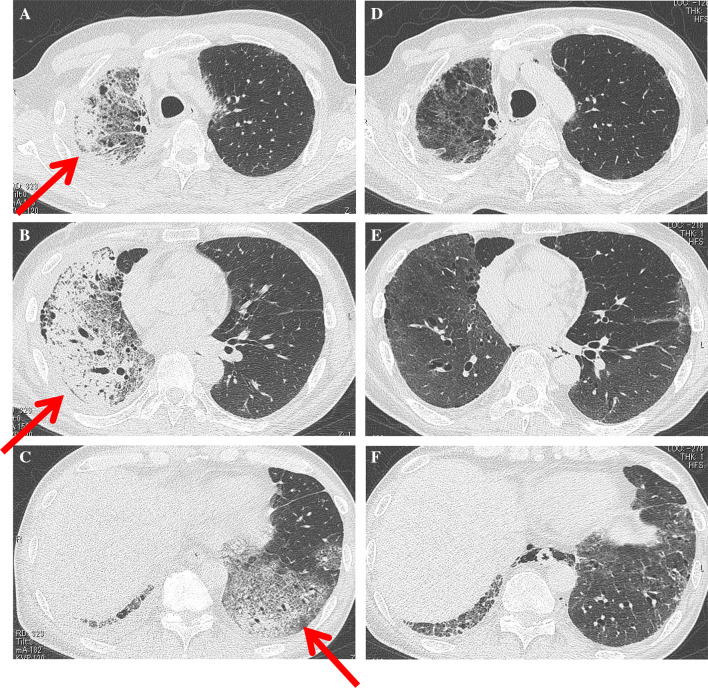
After 3 weeks of the initial pembrolizumab administration, the patient suffered from increasing dyspnea and was admitted to the hospital to start oxygen therapy. Chest computed tomography (CT) on admission showed non-segmentally distributed extensive consolidations and ground-glass opacities (GGOs) with subpleural predominance in the whole right lung and the lower lobe of the left lung. Traction bronchiectasis and bronchiolectasis with volume loss suggesting the diffuse alveolar damage (DAD) radiographic pattern were also demonstrated (Fig. 1a–c). The left ventricular ejection fraction examined by ultrasound cardiography was more than 60%, and the plasma level of B-type natriuretic peptide (BNP) was 123.5 pg/mL, which was almost equal to his baseline value. Therefore, involvement of congested heart failure was excluded.
Fig. 1.
a–c Chest CT scan on admission (after 3 weeks of initial pembrolizumab administration) showing non-segmentally extended consolidations and GGOs with a predominant subpleural distribution in the whole lobes of the right lung and the lower lobe of the left lung (arrows). Traction bronchiectasis and bronchiolectasis with volume loss were also demonstrated. d–f Chest CT scan after triple combination therapy showing the marked amelioration of consolidations and GGOs in the bilateral lungs
Bronchoalveolar lavage (BAL) was performed from right B8. The total cell count was 2.6 × 105/mL, and the percentages of neutrophils, eosinophils, and lymphocytes were 13%, 4%, and 72%, respectively. Microbiological examination findings were all negative, and a cytology study showed a few atypical cells (no obvious lung infiltration of malignant cells). Given the absence of causative agents for infection in the BAL fluid evaluation, synthetic antibiotics were not introduced. Based on radiological and BAL examination findings, the patient was diagnosed with ICI-related pneumonitis, and pembrolizumab was stopped. Because of respiratory failure (PaO2/FiO2 = 220), methylprednisolone pulse therapy (1000 mg daily for 3 days) was selected with subsequent maintenance prednisolone (80 mg daily). However, his respiratory condition deteriorated on the 7th hospital day, and chest radiography showed extensive consolidations and GGOs in almost the entire area of both lungs (Fig. 2a, b). Both Krebs von den Lungen-6 (KL-6) and surfactant protein-D (SP-D) are produced by type II alveolar epithelial cells, and their serum levels have been recognized to reflect alveolar epithelial cell injury, so they are widely used as biomarkers for pulmonary fibrosis in the clinical setting, especially in Japan. The serum levels of KL-6 and SP-D gradually elevated, until KL-6 peaked at 1898 U/mL (normal range: 0–499 U/mL) and SP-D at 1170 ng/mL (normal range: 0–109 ng/mL), respectively.
Fig. 2.
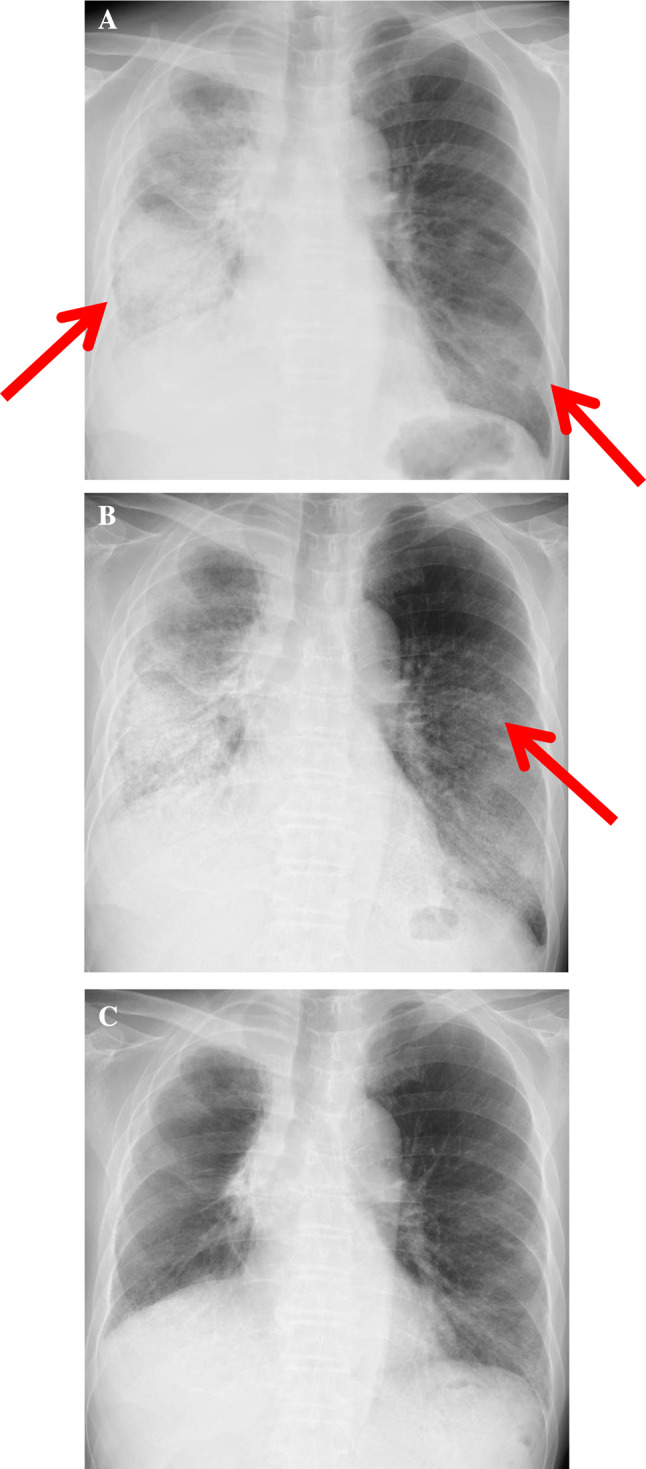
a Chest radiography on admission (after 3 weeks of the initial pembrolizumab administration) showing extensive consolidations and GGOs in the right whole pulmonary fields and the left lower pulmonary field (arrows). b Chest radiography in the ICU (after methylprednisolone pulse therapy) showing the expansion of GGOs in the left middle pulmonary field (arrow). c Chest radiography after triple combination therapy showing the improvement of the pulmonary opacities in the bilateral lungs
Due to the deterioration of his respiratory failure (PaO2/FiO2 = 68), the patient was transferred to the intensive care unit (ICU), and high-flow nasal cannula (HFNC) oxygen therapy was initiated. At this point, the patient was considered to be suffering from grade 4 pneumonitis, defined as life-threatening respiratory compromise; urgent intervention indicated according to Common Toxicity Criteria for Adverse Events (CTCAE) version 4.0 [15]. Because of an inadequate therapeutic response to high-dose corticosteroids, additional immunosuppressants were considered. Although infliximab is usually recommended in cases of steroid-refractory ICI-related pneumonitis, we considered that more intensive immunosuppressive treatment was needed because the patient has developed rapidly-progressive respiratory failure under the treatment with high-dose corticosteroids. Therefore, tacrolimus (2 mg/day, target trough level of 5–10 ng/mL) and cyclophosphamide pulse therapy (500 mg for 1 day) were initiated as triple combination therapy, according to the treatment strategy for CADM-ILD, in addition to a second course of methylprednisolone pulse therapy. In response to the triple combination therapy, his respiratory failure gradually improved, and chest radiography also showed the marked amelioration of pulmonary opacities (Fig. 2c).
Ten days after the administration of triple combination therapy, the patient was withdrawn from HFNC oxygen therapy and returned to the general ward. He received only one dose of cyclophosphamide pulse therapy. The prednisolone dose was tapered to 25 mg daily over 6 weeks, and tacrolimus was continued. Follow-up chest CT showed marked improvement of the consolidations and GGOs in the bilateral lungs (Fig. 1d–f). The serum levels of KL-6 and SP-D gradually decreased (KL-6: 1341 U/mL and SP-D: 183 ng/mL). The sizes of the multiple lung metastases were unchanged, and the response to pembrolizumab was defined as stable disease. Because his respiratory condition had not fully recovered, he was discharged with home oxygen therapy.
Discussion
To our knowledge, this is the first report demonstrating the potential effectiveness of triple combination therapy (high-dose corticosteroids, tacrolimus, and cyclophosphamide) on steroid-refractory ICI-related pneumonitis.
A wide variation in the overall incidence of ICI-related pneumonitis has been described in previous reports. In a recent meta-analysis including 4496 patients from 20 PD-1 inhibitor trials (nivolumab or pembrolizumab; 12 melanoma studies, five NSCLC studies, and three renal cell carcinoma studies), Nishino et al. [16] estimated the overall incidence of pneumonitis during PD-1 inhibitor monotherapy to be 2.7% (95% confidence interval [CI], 1.9–3.6%) for all-grades and 0.8% (95% CI, 0.4–1.2%) for grade ≥ 3 pneumonitis. Multivariable analyses demonstrated higher odds of pneumonitis in NSCLC for all-grade (odds ratio, 1.43; 95% CI, 1.08–1.89; p = 0.005) and grade ≥ 3 pneumonitis (odds ratio, 2.85; 95% CI, 1.60–5.08; p < 0.001) than melanoma. Nishino et al. [9] also reported that among 170 patients treated in ten different trials of nivolumab, 20 patients (ten melanoma, six lymphoma, and four lung cancer) developed pneumonitis (11.8%). The largest series of ICI-related pneumonitis survey reported by Delaunay et al. [17] noted that the estimate of overall incidence was 3.5%, which is consistent with the findings of recent meta-analyses [2, 16]. In the data shown by Naidoo et al. [8], any-grade pneumonitis developed in approximately 5% (43 of 915) of patients treated with anti–PD-1/PD-L1 monoclonal antibodies, and grade ≥ 3 pneumonitis developed in 1% (12 of 915). However, Suresh et al. [18] retrospectively examined 205 patients with NSCLC including both trial-enrolled and non-trial-enrolled patients and demonstrated a higher incidence of ICI pneumonitis (19%). Cho et al. [19] also retrospectively analyzed 167 NSCLC patients treated with an ICI. The incidences of all-grade and grade 3–4 pneumonitis were 13.2% and 4.2%, respectively. These findings suggest that ICI-related pneumonitis may have a higher incidence in real-world settings than those observed in clinical trials.
A wide variation in the timing of developing ICI-related pneumonitis has also been reported in previous papers. The median times from ICI therapy initiation to pneumonitis were 2.8 months (range, 0.3–19.2 months) as reported by Naidoo et al. [8], 2.6 months (range, 0.5–11.5 months) as reported by Nishino et al. [9], and 2.3 months (range, 0.2–27.4 months) as reported by Delaunay et al. [17]. In our case, the time to the onset of pneumonitis was 3 weeks from the initiation of pembrolizumab, which is earlier than the median times in previous reports. However, Delaunay et al. [17] reported that there was no association between the median time to the onset and the severity of pneumonitis: 2.8 months for grades 1–2 and 2.2 months for grades 3–5 (p = 0.32).
ICI-related pneumonitis is also characterized by a very wide range of clinical and radiological presentations [10]. The most common radiographic pattern of ICI-related pneumonitis was reported to be cryptogenic organizing pneumonia (COP) [9, 10, 19], followed by hypersensitivity pneumonia (HP) and nonspecific interstitial pneumonia (NSIP) [17]. These radiographic patterns are associated with the toxicity grades of pneumonitis, and the acute interstitial pneumonia (AIP)/DAD pattern had the highest grade, followed by COP, whereas NSIP and HP had lower grades [9]. The radiographic pattern was shown to be consistent with the AIP/DAD pattern, suggesting a high disease severity of ICI-related pneumonitis in the present case, which was supported by the inadequate therapeutic response to high-dose corticosteroids.
In the setting of progressive lung involvement during ICI treatment for lung cancer, it is critically important to achieve a precise diagnosis in order to decide on a subsequent treatment plan. A bronchoscopic examination of the BAL and a lung biopsy can be useful for diagnosing ICI-related pneumonitis by eliminating other causes, such as respiratory infections or malignant lung infiltration [1, 8, 10]. We performed bronchoscopy, and the diagnosis of ICI-related pneumonitis was supported by the findings of markedly increased percentages of lymphocytes in the BAL reflecting the participation of lymphocyte-mediated exaggerated immunological reactions. Although no lung biopsy was performed due to the patient’s respiratory failure, cytology and a bacteriological examination in BAL were able to exclude the involvement of respiratory infections and tumor progression in this case.
In general, irAEs, including pneumonitis, can be sufficiently managed by the discontinuation or delayed administration of the ICI or by short-term treatment with oral corticosteroids [3, 4, 8–10]. Indeed, Naidoo et al. [8] reported that 72% (31 of 43) of cases were grade 1–2, and pneumonitis improved/resolved in 86% (37 of 43) of cases, including in 97% (30 of 31) of grade 1–2 cases and 58% (7 of 12) of grade ≥ 3 cases with drug discontinuation and/or administration of corticosteroids. Delaunay et al. [17] reported the recovery or improvement in 68.3% of patients by drug discontinuation with corticosteroid administration in particularly severe cases; however, ICI-related pneumonitis can be associated with a poor prognosis, and 9.4% of those patients died as a direct result of this complication. Cho et al. [19] reported that the mortality rate of pneumonitis was 18.2% and concluded that ICIs should be used with caution when treating NSCLC patients complicated with chronic lung disease. Indeed, among cases of ICI-related pneumonitis with a variety of malignancies, pneumonitis-related death was mainly observed in patients with NSCLC [16]. This increased incidence and severity of pneumonitis in NSCLC may be due to an increased susceptibility because of frequent tobacco exposure and/or underlying chronic respiratory diseases (chronic obstructive pulmonary disease, pulmonary fibrosis and tumoral involvement) [10]. Although our patient had not suffered from autoimmune diseases and no specifically positive autoantibody was detected, chest CT revealed a preexisting mild reticular shadow with subpleural predominance in the bilateral lower lobes without clinically evident ILD. In addition, our patient had received thoracic radiation therapy and developed focal radiation pneumonitis accompanied by subsequent fibrosis of the right lung without necessitating corticosteroid therapy, which was also a possible risk factor for the development of ICI-related pneumonitis [20]. Furthermore, our patient had also received intrapericardial instillation of bleomycin before starting pembrolizumab monotherapy, and bleomycin is a representative profibrotic chemotherapeutic agent. Accordingly, it is plausible that these preexisting fibrotic lesions and history of bleomycin treatment may have been associated with not only the development but also the treatment refractoriness of his ICI-related pneumonitis.
In cases with worsening clinical and radiological findings after treatment with high-dose corticosteroids, additional immunosuppressants should be considered for treating ICI-related pneumonitis, including infliximab, cyclophosphamide, intravenous immunoglobulin, and mycophenolate mofetil [1]. Infliximab, an antibody against TNF-α, is used to manage Crohn’s disease and ulcerative colitis and has also shown efficacy in patients with moderate-to-severe colitis induced by ICIs. It has been suggested that infliximab may also be effective in patients with ICI-related pneumonitis [9, 11, 18, 21]. Accordingly, in treatment algorithms for irAEs, infliximab is usually recommended in cases of steroid-refractory ICI-related pneumonitis [3]. However, among 14 published cases of steroid-refractory ICI-related pneumonitis treated with infliximab, 8 showed deterioration. Those patients ultimately died during the treatment course (Table 1), and the cause of death tended to be infection [8, 9, 11, 18, 21]. Awareness of this potential serious complication from immunosuppression is necessary during infliximab treatment. Accordingly, the treatment of infliximab may be inappropriate in some cases of steroid-refractory ICI-related pneumonitis, although the usefulness of other immunosuppressant remains unclear.
Table 1.
Overview of published cases with steroid-refractory ICI-related pneumonitis treated with infliximab
| Publication | Number of patients | Treatment | Outcome |
|---|---|---|---|
| Nishino et al. [11] | 2 | Corticosteroids with infliximab | One patient improved and one patient died |
| Naidoo et al. [8] | 5 | Corticosteroids, three patients with infliximab and two patients with infliximab plus cyclophosphamide | All five patients died |
| Nishino et al. [9] | 3 | Corticosteroids with infliximab | Two patients improved and one patient died |
| Ortega Sanchez et al. [21] | 1 | Corticosteroids plus mycophenolate mofetil with infliximab | Improved |
| Suresh et al. [18] | 3 | Corticosteroids, two patients with infliximab and one patient with infliximab plus intravenous immunoglobulin | Two patients improved and one patient died |
There have been several reports describing the use of immunosuppressant combinations including cyclophosphamide. Naidoo et al. [8] reported that cases of steroid-refractory ICI-related pneumonitis required additional immunosuppression (both infliximab plus cyclophosphamide). High doses of corticosteroids resulted in an inadequate therapeutic response in the present case, so we considered triple combination immunosuppression treatment for CADM-ILD, which is composed of high doses of corticosteroids, calcineurin inhibitors (such as ciclosporin and tacrolimus), and cyclophosphamide. CADM with positive anti-MDA 5 antibody often develops treatment-resistant and rapidly-progressive ILD [22], which is life-threatening and recognized as one of the most severe complications among autoimmune disorders [12]. In CADM-ILD, 80% of fatal cases die within 90 days of the initial presentation due to refractory ILD [23]. Triple combination therapy is considered effective for CADM with rapidly-progressive ILD [12]. Although chest CT revealed a pattern resembling a DAD pattern, the BAL fluid showed a markedly increased percentage of lymphocytes (72%) in our case, indicating the existence of an ICI-mediated immune process for the development of refractory lung injury. CADM-ILD is mediated by the immune system and can present with a radiographical DAD pattern in severe cases. Accordingly, we considered it reasonable to select this intensive immunosuppressant combination therapy for our patient, and marked improvement was observed. We speculate that triple combination therapy may be a promising therapeutic option for steroid-refractory ICI-related pneumonitis, in cases with not only NSIP and COP patterns known to be associated with immune-mediated lung injury but also a pattern resembling a DAD pattern with a high lymphocyte count in the BAL fluid.
Several limitations associated with the present study warrant mention. First, this is a case report, so the accumulation of more cases is necessary to support the usefulness of triple combination therapy. Second, preexisting fibrotic lesions and prior bleomycin treatment may have affected not only the treatment refractoriness but also the effectiveness of triple combination therapy in this case. Third, it is important to compare the safety and effectiveness between infliximab and triple combination therapy for steroid-refractory ICI-related pneumonitis in a future study.
In conclusion, triple combination therapy (high-dose corticosteroids, tacrolimus, and cyclophosphamide) might be an effective therapeutic strategy for steroid-refractory ICI-related pneumonitis. However, further investigations are needed in order to clarify the mechanisms of ICI-related pneumonitis and establish an optimal management approach.
Abbreviations
- AIP
Acute interstitial pneumonia
- ALK
Anaplastic lymphoma kinase
- BAL
Bronchoalveolar lavage
- BNP
B-type natriuretic peptide
- CADM
Clinically amyopathic dermatomyositis
- CI
Confidence interval
- COP
Cryptogenic organizing pneumonia
- CT
Computed tomography
- CTCAE
Common Toxicity Criteria for Adverse Events
- CTLA-4
Cytotoxic T-lymphocyte antigen 4
- DAD
Diffuse alveolar damage
- DM
Dermatomyositis
- EGFR
Epidermal growth factor receptor
- GGO
Ground-glass opacities
- HFNC
High-flow nasal cannula
- HP
Hypersensitivity pneumonia
- ICI
Immune checkpoint inhibitor
- ICU
Intensive-care unit
- ILD
Interstitial lung disease
- irAE
Immune-related adverse event
- KL-6
Krebs von den Lungen-6
- MDA 5
Melanoma differentiation-associated gene 5
- NSCLC
Non-small cell lung cancer
- NSIP
Nonspecific interstitial pneumonia
- PD-1
Programmed cell death 1
- PD-L1
Programmed cell death ligand 1
- PM
Polymyositis
- ROS1
C-ros oncogene 1
- SP-D
Surfactant protein-D
- TNF-α
Tumor necrosis factor alpha
Author contributions
HU and SM treated the patient. HU and JA wrote the case report. All authors made substantial contributions to data interpretation, discussion, manuscript preparation, review and revision. All authors read and approved the final manuscript.
Funding
This study was not supported by any funding.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Written informed consent was obtained from the patient for publication of this case report.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chuzi S, Tavora F, Cruz M, Costa R, Chae YK, Carneiro BA, Giles FJ. Clinical features, diagnostic challenges, and management strategies in checkpoint inhibitor-related pneumonitis. Cancer Manag Res. 2017;9:207–213. doi: 10.2147/CMAR.S136818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khunger M, Rakshit S, Pasupuleti V, Hernandez AV, Mazzone P, Stevenson J, Pennell NA, Velcheti V. Incidence of pneumonitis with use of programmed death 1 and programmed death-ligand 1 inhibitors in non-small cell lung cancer: a systematic review and meta-analysis of trials. Chest. 2017;152:271–281. doi: 10.1016/j.chest.2017.04.177. [DOI] [PubMed] [Google Scholar]
- 3.Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378:158–168. doi: 10.1056/NEJMra1703481. [DOI] [PubMed] [Google Scholar]
- 4.Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncol. 2016;2:1346–1353. doi: 10.1001/jamaoncol.2016.1051. [DOI] [PubMed] [Google Scholar]
- 5.Michot JM, Bigenwald C, Champiat S, Collins M, Carbonnel F, Postel-Vinay S, Berdelou A, Varga A, Bahleda R, Hollebecque A, Massard C, Fuerea A, Ribrag V, Gazzah A, Armand JP, Amellal N, Angevin E, Noel N, Boutros C, Mateus C, Robert C, Soria JC, Marabelle A, Lambotte O. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139–148. doi: 10.1016/j.ejca.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 6.Tirumani SH, Ramaiya NH, Keraliya A, Bailey ND, Ott PA, Hodi FS, Nishino M. Radiographic profiling of immune-related adverse events in advanced melanoma patients treated with ipilimumab. Cancer Immunol Res. 2015;3:1185–1192. doi: 10.1158/2326-6066.CIR-15-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishino M, Tirumani SH, Ramaiya NH, Hodi FS. Cancer immunotherapy and immune-related response assessment: the role of radiologists in the new arena of cancer treatment. Eur J Radiol. 2015;84:1259–1268. doi: 10.1016/j.ejrad.2015.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naidoo J, Wang X, Woo KM, Iyriboz T, Halpenny D, Cunningham J, Chaft JE, Segal NH, Callahan MK, Lesokhin AM, Rosenberg J, Voss MH, Rudin CM, Rizvi H, Hou X, Rodriguez K, Albano M, Gordon R-A, Leduc C, Rekhtman N, Harris B, Menzies AM, Guminski AD, Carlino MS, Kong BY, Wolchok JD, Postow MA, Long GV, Hellmann MD. Pneumonitis in patients treated with anti–programmed death-1/programmed death ligand 1 therapy. J Clin Oncol. 2016;35:709–717. doi: 10.1200/JCO.2016.68.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishino M, Ramaiya NH, Awad MM, Sholl LM, Maattala JA, Taibi M, Hatabu H, Ott PA, Armand PF, Stephen Hodi F. PD-1 inhibitor-related pneumonitis in advanced cancer patients: radiographic patterns and clinical course. Clin Cancer Res. 2016;22:6051–6060. doi: 10.1158/1078-0432.CCR-16-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montani D, Seferian A, Parent F, Humbert M. Immune checkpoint inhibitor-associated interstitial lung diseases: some progress but still many issues. Eur Respir J. 2017;50:1701319. doi: 10.1183/13993003.01319-2017. [DOI] [PubMed] [Google Scholar]
- 11.Nishino M, Sholl LM, Stephen Hodi F. Anti–PD-1–related pneumonitis during cancer immunotherapy. N Engl J Med. 2015;373:288–290. doi: 10.1056/NEJMc1505197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurita T, Yasuda S, Amengual O, Atsumi T. The efficacy of calcineurin inhibitors for the treatment of interstitial lung disease associated with polymyositis/dermatomyositis. Lupus. 2015;24:3–9. doi: 10.1177/0961203314554849. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka F, Origuchi T, Migita K, Tominaga M, Kawakami A, Kawabe Y, Eguchi K. Successful combined therapy of cyclophosphamide and cyclosporine for acute exacerbated interstitial pneumonia associated with dermatomyositis. Intern Med. 2000;39:428–430. doi: 10.2169/internalmedicine.39.428. [DOI] [PubMed] [Google Scholar]
- 14.Kameda H, Nagasawa H, Ogawa H, Sekiguchi N, Takei H, Tokuhira M, Amano K, Takeuchi T. Combination therapy with corticosteroids, cyclosporine A, and intravenous pulse cyclophosphamide for acute/subacute interstitial pneumonia in patients with dermatomyositis. J Rheumatol. 2005;32:1719–1726. [PubMed] [Google Scholar]
- 15.Cancer Therapy Evaluation Program, National Cancer Institute (2009) Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. (v4.03: 14 Jun 2010). https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40. Accessed 28 May 2009
- 16.Nishino M, Giobbie-Hurder A, Hatabu H, Ramaiya NH, Hodi FS. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: a systematic review and meta-analysis. JAMA Oncol. 2016;2:1607–1616. doi: 10.1001/jamaoncol.2016.2453. [DOI] [PubMed] [Google Scholar]
- 17.Delaunay M, Cadranel J, Lusque A, Meyer N, Gounant V, Moro-Sibilot D, Michot JM, Raimbourg J, Girard N, Guisier F, Planchard D, Metivier AC, Tomasini P, Dansin E, Pérol M, Campana M, Gautschi O, Früh M, Fumet JD, Audigier-Valette C, Couraud S, Dalle S, Leccia MT, Jaffro M, Collot S, Prévot G, Milia J, Mazieres J. Immune-checkpoint inhibitors associated with interstitial lung disease in cancer patients. Eur Respir J. 2017;50:1700050. doi: 10.1183/13993003.00050-2017. [DOI] [PubMed] [Google Scholar]
- 18.Suresh K, Voong KR, Shankar B, Forde PM, Ettinger DS, Marrone KA, Kelly RJ, Hann CL, Levy B, Feliciano JL, Brahmer JR, Feller-Kopman D, Lerner AD, Lee H, Yarmus L, D'Alessio F, Hales RK, Lin CT, Psoter KJ, Danoff SK, Naidoo J. Pneumonitis in non-small cell lung cancer patients receiving immune checkpoint immunotherapy: incidence and risk factors. J Thorac Oncol. 2018;13:1930–1939. doi: 10.1016/j.jtho.2018.08.2035. [DOI] [PubMed] [Google Scholar]
- 19.Cho JY, Kim J, Lee JS, Kim YJ, Kim SH, Lee YJ, Cho YJ, Yoon HI, Lee JH, Lee CT, Park JS. Characteristics, incidence, and risk factors of immune checkpoint inhibitor-related pneumonitis in patients with non-small cell lung cancer. Lung Cancer. 2018;125:150–156. doi: 10.1016/j.lungcan.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 20.Cadranel J, Canellas A, Matton L, Darrason M, Parrot A, Naccache JM, Lavolé A, Ruppert AM, Fallet V. Pulmonary complications of immune checkpoint inhibitors in patients with nonsmall cell lung cancer. Eur Respir Rev. 2019;28:190058. doi: 10.1183/16000617.0058-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanchez GO, Jahn K, Savic S, Zippelius A, Läubli H. Treatment of mycophenolate-resistant immune-related organizing pneumonia with infliximab. J Immunother Cancer. 2018;6:85. doi: 10.1186/s40425-018-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukae H, Ishimoto H, Sakamoto N, Hara S, Kakugawa T, Nakayama S, Ishimatsu Y, Kawakami A, Eguchi K, Kohno S. Clinical differences between interstitial lung disease associated with clinically amyopathic dermatomyositis and classic dermatomyositis. Chest. 2009;136:1341–1347. doi: 10.1378/chest.08-2740. [DOI] [PubMed] [Google Scholar]
- 23.Ikeda S, Arita M, Misaki K, Mishima S, Takaiwa T, Nishiyama A, Ito A, Furuta K, Yokoyama T, Tokioka F, Noyama M, Yoshioka H, Ishida T. Incidence and impact of interstitial lung disease and malignancy in patients with polymyositis, dermatomyositis, and clinically amyopathic dermatomyositis: a retrospective cohort study. Springerplus. 2015;4:240. doi: 10.1186/s40064-015-1013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]