Abstract
Introduction
The importance of immune tumor microenvironment in the prognosis of patients with head and neck squamous carcinomas (HNSCC) is increasingly recognized. We analyzed the prognostic relevance of PD-L1 and PD-1 expressions in relation to the infiltration by CD8+ and FOXP3+ tumor-infiltrating lymphocytes (TILs).
Methods
Samples from 372 surgically treated HPV-negative HNSCC patients were evaluated by immunohistochemistry for PD-L1 expression [both tumor proportion score (TPS) and combined proportion score (CPS)], PD-1 expression in immune cells, and density of infiltrating CD8+ and FOXP3+ TILs. PD-L1 expression and CD8+ TIL density were combined to establish the type of tumor microenvironment.
Results
29.5% cases exhibited PD-L1 TPS positivity (≥ 1%), whereas PD-L1 CPS positivity (≥ 1%) was observed in 40% cases. 47.5% cases showed positive PD-1 expression (≥ 1%). PD-L1 and PD-1 positivity correlated with a high density of both CD8+ and FOXP3+ TILs. In univariate analysis, PD-L1 TPS positivity (P = 0.026), PD-L1 CPS positivity (P = 0.004), high density of CD8+ TIL (P = 0.001), and high density of FOXP3+ TIL (P = 0.004) were associated with a better disease-specific survival (DSS). However, in multivariate analysis, only high density of CD8+ TIL was associated with a better DSS (P = 0.002). The type of tumor microenvironment correlated with DSS (P = .008), with the better DSS observed in cases with type I (PD-L1 CPS positivity and high density of CD8+ TIL).
Conclusions
High infiltration by CD8+ TIL is associated with better survival outcomes. Positive PD-L1 expression correlates with a high infiltration by TILs, explaining its association with better prognosis.
Electronic supplementary material
The online version of this article (10.1007/s00262-020-02604-w) contains supplementary material, which is available to authorized users.
Keywords: Head and neck cancer, PD-L1, Tumor-infiltrating lymphocytes, Prognosis
Introduction
It is now recognized that the immune system plays a key role in the development and progression of cancers [1], including the head and neck squamous cell carcinomas (HNSCC). Different mechanisms of tumor immune evasion have been described in HNSCC [2], and various immune checkpoints are exploited by the immune cells in the surrounding tumor microenvironment to induce the exhaustion of effector T cells [2]. One of these mechanisms of evasion from host immune responses is the tumor expression of programmed cell death ligand 1 (PD-L1) that interacts with programmed cell death protein (PD-1) expressed on cytotoxic T lymphocytes, thereby reducing T cell activation and proliferation [3]. Immunotherapy has emerged as a promising treatment strategy to redirect host immune responses and match tumor adaptability. Immune checkpoint blockade, particularly by using antibodies directed against PD-1/PD-L1 pathway members, has demonstrated efficacy in the treatment of several neoplasms [3, 4]. In 2016, the US Food and Drug Administration (FDA) approved the anti-PD-1 monoclonal antibodies nivolumab and pembrolizumab for the treatment of patients with recurrent/metastatic (R/M) HNSCC refractory to platinum-based therapy, and in 2019, the FDA approved pembrolizumab for the first-line treatment of patients with unresectable R/M HNSCC [5]. PD-L1 expression has been proposed as a predictive biomarker for checkpoint inhibitors, although it has shown moderate predictive value across multiple solid tumors [6]. In HNSCC, tumor PD-L1 expression is generally correlated with improved efficacy to anti-PD-1/PD-L1 blockers, and the predictive value increases when considering both PD-L1 expression and the levels of tumor-infiltrating immune cells. However, the predictive value of PD-L1 expression has limitations, as some PD-L1-negative patients were also found to respond to treatment with these immunotherapeutic agents [7, 8].
Besides the potential role of PD-L1 as a predictive biomarker for HNSCC response to anti-PD-1/PD-L1 treatment, the prognostic relevance in this tumor is still controversial. Two recent meta-analyses failed to show a clear impact of PD-L1 expression on the prognosis of HNSCC patients [9, 10]. The heterogeneity between studies was significant. These conflicting results may have been caused by methodological differences in the immunohistochemical staining for PD-L1, including the specific antibodies used and/or in the cutoff values used in each study. Moreover, the heterogeneous tumor locations, staging, treatment regimens, as well as the HPV status of the HNSCC patients enrolled in these studies may have also contributed to these varying results. In our view, a more refined and careful dissection of the immune microenvironments associated with PD-L1 expression may be helpful to better define its prognostic role in HNSCC. Previous studies on tumor-infiltrating lymphocytes (TILs) have shown that increased infiltration by CD8+ T cells or regulatory T cells (Treg) are associated with favorable outcomes [11]. Unfortunately, up to now, the interactions between PD-L1, PD-1, and the different subgroups of TILs, as well as the impact of these interactions on clinical outcomes, have not been well documented in HNSCC.
This prompted us to investigate the expression of PD-L1 and PD-1 in a large homogeneous series of 372 resected HPV-negative HNSCC and to establish associations with clinicopathologic features, presence of TILs in the tumor microenvironment, and clinical outcomes.
Methods
Patients and tissue specimens
Surgical tissue specimens from 372 patients with HPV-negative HNSCC who underwent resection of their tumors at the Hospital Universitario Central de Asturias between 1990 and 2009 were retrospectively collected. Sample use and experimental procedures were performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients. Formalin-fixed paraffin-embedded (FFPE) tissue samples and data from donors were provided by the Principado de Asturias BioBank (PT17/0015/0023), integrated in the Spanish National Biobanks Network, and histological diagnosis was confirmed by an experienced pathologist. Samples were processed following standard operating procedures with the appropriate approval of the Regional Ethical Committee from Principado de Asturias for the project PI16/00280 (approval number: 70/16; date: 5 May 2016).
The characteristics of the studied cases are shown in Table 1. All patients had a single primary tumor, microscopically clear surgical margins, and received no treatment prior to surgery. Only fourteen patients were women, and the mean age was 58.6 years (range 30–86 years). The stage of the tumors was determined according to the TNM system of the International Union Against Cancer (7th Edition). Two hundred thirty-two (62%) of 372 patients received postoperative radiotherapy. Patients were followed up for a minimum of 36 months. The mean follow-up for the whole series was 34.6 months (median 21.5 months). Recurrence was defined as tumor relapse in the five first years after treatment at any site: local recurrence, regional recurrence, and/or distant metastasis. Information on HPV status was available for all patients. HPV status was analyzed using p16 immunohistochemistry, high-risk HPV DNA detection by in situ hybridization, and genotyping by GP5+/6+-PCR, as previously reported [12, 13].
Table 1.
Clinicopathologic characteristics of the patients studied
| Characteristic | No. cases (%) |
|---|---|
| Age, mean (range) | 58.6 (30–86 years) |
| Tobacco | |
| Non-smokers | 12 (3.5) |
| Moderate (1–50 packs-year) | 207 (55.5) |
| Heavy (> 50 packs-year) | 153 (41) |
| Alcohol | |
| Non-drinkers | 37 (10) |
| Drinkers | 335 (90) |
| Location | |
| Oropharynx | 241 (65) |
| Hypopharynx | 64 (17) |
| Larynx | 67 (18) |
| pT classification | |
| T1 | 38 (10) |
| T2 | 77 (21) |
| T3 | 125 (34) |
| T4 | 132 (35) |
| pN classification | |
| N0 | 103 (28) |
| N1 | 46 (12) |
| N2 | 183 (49) |
| N3 | 40 (11) |
| Stage | |
| I | 20 (5) |
| II | 24 (6) |
| III | 64 (17) |
| IV | 264 (71) |
| Degree of differentiation | |
| Well-differentiated | 147 (39) |
| Moderately differentiated | 148 (40) |
| Poorly differentiated | 77 (21) |
| Total | 372 |
Tissue microarray (TMA) construction
Three morphologically representative areas were selected from each individual tumor paraffin block. Subsequently, three 1-mm cylinders were taken to construct TMA blocks, as described previously [12], containing a total of 372 HNSCC (135 tonsillar, 106 base of tongue, 64 hypopharyngeal, and 67 laryngeal carcinomas). In addition, each TMA included three cores of normal squamous epithelium as an internal negative control. This normal epithelium was obtained from adult male, non-smokers and non-drinkers, patients that were operated from tonsillectomy and patients operated from benign vocal cord lesions.
Immunohistochemical study
The TMA blocks were cut into 3-µm sections and dried on Flex IHC microscope slides (Dako, Santa Clara, CA). The sections were deparaffinized with standard xylene and hydrated through graded alcohols into water. Antigen retrieval was performed using EnVision Flex Target Retrieval solution, high pH (Dako). Staining was done at room temperature on an automatic staining workstation (Dako Autostainer Plus) using the following primary antibodies: anti-PD-L1 antibody (clone E1L3N; Cell Signaling Technology, Danvers, MA) at 1:200 dilution, anti-PD-1 (clone EH33, Cell Signaling Technology) at 1:200 dilution, anti-CD8 (clone SP16, Neomarkers, Freemont, CA) at 1:400 dilution, or anti-FOXP3 (clone 236A/E7, Abcam, Cambridge, UK) at 1:400 dilution. Detection was performed with EnVision Flex + Visualization System (Dako) for 30 min at room temperature, and immunostaining was developed using diaminobenzidine. Sections were washed in water, counterstained with hematoxylin, and dehydrated before being mounted in DPX mounting medium.
Positive controls were placenta samples for PD-L1 expression, and normal tonsil samples for PD-1, CD8, and FOXP3 expression.
All slides were reviewed by two expert authors. For tumor PD-L1 expression, only the membrane staining of tumor cells was evaluated and expression was scored as: negative PD-L1 expression (< 1% stained cells), low PD-L1 expression (≥ 1– 10%), intermediate PD-L1 expression (11–50%), or high PD-L1 expression (> 50%). Tumor proportion score (TPS) positivity was defined as ≥ 1% stained tumor cells based on current recommendations [5]. Combined proportion score (CPS), the number of PD-L1-positive cells (tumor, lymphocytes, and macrophages) in relation to total tumor cells, was also considered positive if ≥ 1%.
PD-1 expression was evaluated in stromal immune cells and was considered positive when ≥ 1% of the cells were stained.
Tumor-infiltrating lymphocytes (TILs) were defined as lymphocytes within the tumor, while the peritumoral area was not included in this assessment. Quantification of TILs staining was performed automatically using ImageJ software for CD8 (marker of cytotoxic T lymphocytes) and manually for FOXP3 (marker of T-regulatory lymphocytes), counting the cells in the tumor areas of each core within the TMAs (i.e., 3 cores per tumor case). The tumoral areas quantified in each core were measured, and the average total number of cells positive for each marker in the 3 cores was expressed in density per mm2. For statistical purposes the median value was used as the cutoff to define high and low density for both CD8+ and FOXP3+ TILs.
All scoring was conducted blinded to clinical outcomes.
Statistical analysis
Chi-squared and Fisher’s exact tests were used for comparison between categorical variables. For time-to-event analysis, Kaplan–Meier curves were plotted. Cox proportional hazards models were utilized for univariate and multivariate analyses. The hazard ratios (HR) with 95% confidence interval (CI) and P values were reported. All tests were two-sided. P values of ≤ 0.05 were considered statistically significant.
Results
Analysis of PD-L1 and PD-1 expression and TIL infiltration in HNSCC tissue specimens
PD-L1 immunostaining was successfully evaluated in 349 of 372 cases; 103 (29.5%) of 349 cases exhibited positive PD-L1 expression in tumor cells (Fig. 1a). From these cases, 66 (19%) were scored as low expressors (1–10% stained tumor cells), 18 (5%) as intermediate (11–50% stained tumor cells), and 19 cases (5.5%) as showing high expression (> 50% stained tumor cells) (Supplementary figure 1). Stromal PD-L1 expression was detected in 92 cases (26%); of these, 37 cases (11% of total cases) were negative in the tumor cells, but there was a significant correlation between PD-L1 expression in tumor cells and in immune cells (Spearman’s Rho coefficient = 0.373, P < 0.001). Then, a PD-L1 combined proportion score (CPS) ≥ 1% was observed in 140 cases (40%).
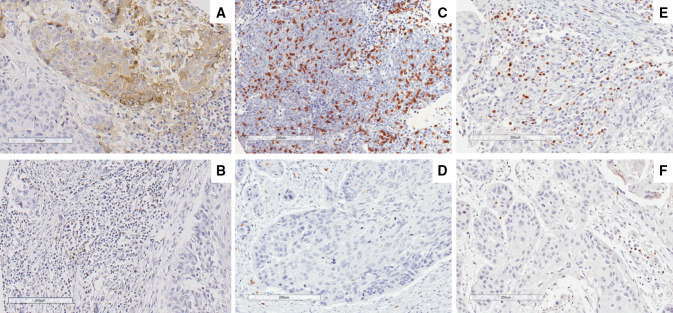
Fig. 1.
Representative examples of positive PD-L1 expression in tumor and stromal immune cells (a), PD-1 expression in immune cells (b), high and low density of CD8+ TIL (c, d) and high and low density of FOXP3+ TIL (e, f). Original magnification X200
PD-1 immunostaining was evaluated in 319 out of 372 HNSCC; 152 (47.5%) of the 319 cases exhibited positive PD-1 expression in stromal immune cells (Fig. 1b). PD-1 expression significantly correlated with both PD-L1 TPS (Spearman’s Rho coefficient = 0.373, P < 0.001) and PD-L1 CPS (Spearman’s Rho coefficient = 0.288, P < 0.001).
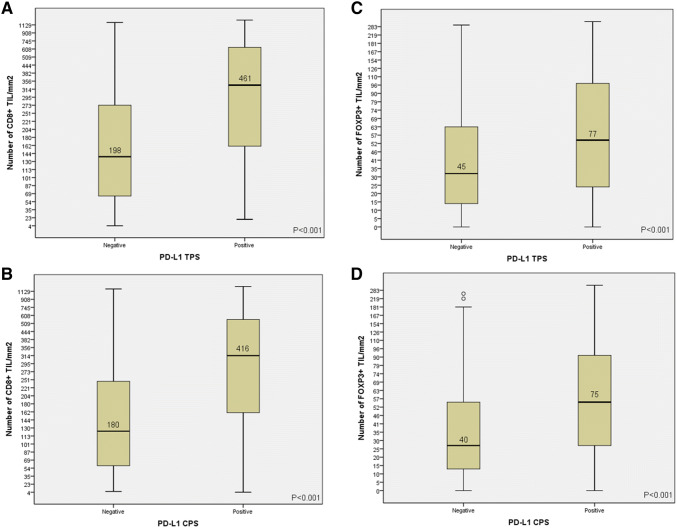
The number of CD8+ TIL was evaluated in 337 HNSCC, showing a mean number of 275 CD8+ TIL (median = 182, range 4–2670) per mm2. The number of FOXP3+ TIL was evaluated in 338 cases and were less numerous than CD8+ TIL, with a mean number of 54 (median = 37, range 0–321) per mm2. The mean ratio between CD8+ TIL and FOXP3+ TIL was 9.4 (median 5.1, range 0.1–129). Representative examples of high and low density of TILs are shown in Fig. 1c–f. There was a strong positive correlation between the number of CD8+ and FOXP3+ TILs (Spearman’s Rho coefficient = 0.473, P < 0.001). The number of infiltrating CD8+ and FOXP3+ TILs was also found to correlate with PD-L1 expression and was significantly higher in cases with a positive PD-L1 TPS and with a positive PD-L1 CPS (Fig. 2). Similarly, CD8+ and FOXP3+ TILs' infiltration was also correlated with positive PD-1 expression (Spearman’s Rho coefficient = 0.316, P < 0.001, and Spearman’s Rho coefficient = 0.239, P < 0.001, respectively).
Fig. 2.
Correlations between PD-L1 tumor proportion score (TPS) and composite proportion score (CPS) and the density of CD8+ (a, b) and FOXP3+ TILs (c, d). Box plots with the median (black line) and the mean values are shown
PD-L1 CPS and CD8+ TIL density were combined to categorize the tumor microenvironment in four subtypes, as described by Teng et al. [14]: PD-L1 CPS-positive/high CD8+ TIL (type I, adaptive immune resistance), which was observed in 67/335 cases (20%); PD-L1 CPS-negative/low CD8+ TIL (type II, immunological ignorance), observed in 143/335 cases (43%); PD-L1 CPS-positive/low CD8+ TIL (type III, intrinsic induction), observed in 30/335 cases (9%); PD-L1 CPS-negative/high CD8+ TIL (type IV, immune tolerance), observed in 95/335 cases (28%).
Correlations of PD-L1 and PD-1 expression and TIL infiltration with clinicopathologic parameters
Positive PD-L1 TPS and positive PD-L1 CPS were significantly less frequent in pT4 (P = 0.033 and P = 0.04, respectively) and in advanced stage IV tumors (P = 0.017 and P = 0.029, respectively). Positive PD-L1 CPS was more frequent in tumors without recurrence (P = 0.043) (Table 2). In parallel to our observation with PD-L1, high CD8+ TIL infiltration was also significantly more frequent in early (I–III) stages (P = 0.03). High FOXP3+ TIL infiltration was more frequently observed in laryngeal carcinomas (P = 0.002) as compared to the other locations (tonsillar, base of tongue, or hypopharyngeal). High CD8+ and high FOXP3+ TILs' densities were more frequent in nonrecurrent tumors, although the differences did not reach statistical significance. No other correlations with pathological characteristics were observed.
Table 2.
Correlations of PD-L1 and PD-1 expression and TILs' densities with pathological parameters
| Characteristic | Positive PD-L1 TPS (%) | P | Positive PD-L1 CPS (%) | P | Positive PD-1 score (%) | P | CD8+ above median (%) | P | FOXP3+ above median (%) | P |
|---|---|---|---|---|---|---|---|---|---|---|
| Location | ||||||||||
| Oropharynx | 69/231 (30) | .94 | 89/231 (38) | .46 | 107/222 (48) | .56 | 110/225 (49) | .84 | 96/225 (43) | .002 |
| Hypopharynx | 18/60 (30) | 28/59 (47) | 23/55 (42) | 29/57 (51) | 32/57 (56) | |||||
| Larynx | 16/58 (28) | 23/58 (40) | 22/42 (54) | 25/55 (45) | 38/56 (68) | |||||
| pT Classification | ||||||||||
| T1-T2 | 34/102 (33) | .033 | 46/102 (45) | .04 | 40/91(44) | .77 | 50/96 (52) | .45 | 56/97 (58) | .44 |
| T3 | 42/120 (35) | 54/119 (45) | 53/110 (48) | 59/116 (51) | 59/116 (51) | |||||
| T4 | 26/127 (20) | 39/124 (31) | 56/115 (49) | 54/122 (44) | 50/122 (41) | |||||
| pN Classification | ||||||||||
| N0 | 31/90 (34) | .28 | 40/90 (44) | .38 | 36/78 (46) | .79 | 47/87 (54) | .26 | 44/88 (50) | .9 |
| N1–3 | 72/259 (28) | 100/258 (39) | 116/241 (48) | 117/250 (47) | 122/250 (49) | |||||
| Stage | ||||||||||
| I–II | 13/35 (37) | .017 | 16/35 (46) | .029 | 13/28 (46) | .79 | 19/32 (59) | .03 | 20/33 (61) | .15 |
| III | 26/61 (43) | 33/61 (54) | 25/57 (44) | 36/59 (61) | 33/59 (56) | |||||
| IV | 64/253 (25) | 91/252 (36) | 114/234 (49) | 109/246 (44) | 113/246 (46) | |||||
| Degree of differentiation | ||||||||||
| Well-differentiated | 43/137 (31) | .47 | 54/138 (39) | .7 | 55/125 (44) | .35 | 65/134 (48) | .25 | 58/135 (43) | .18 |
| Moderately differentiated | 36/139 (26) | 54/139 (39) | 67/128 (52) | 61/138 (44) | 75/140 (54) | |||||
| Poorly differentiated | 24/73 (33) | 32/72 (42) | 31/70 (44) | 40/71 (56) | 37/71(52) | |||||
| Recurrence | ||||||||||
| No | 45/133 (34) | .18 | 63/133 (47) | .043 | 58/117 (50) | .64 | 69/127 (54) | .11 | 71/128 (56) | .07 |
| Yes | 58/216 (27) | 67/215 (36) | 94/202 (46) | 95/210 (45) | 95/210 (45) | |||||
| Total | 103/349 (29.5) | 130/348 (37) | 152/319 (47.5) | 164/337 (49) | 166/338 (49) |
Correlations of PD-L1 and PD-1 expressions and TIL infiltration with patient survival
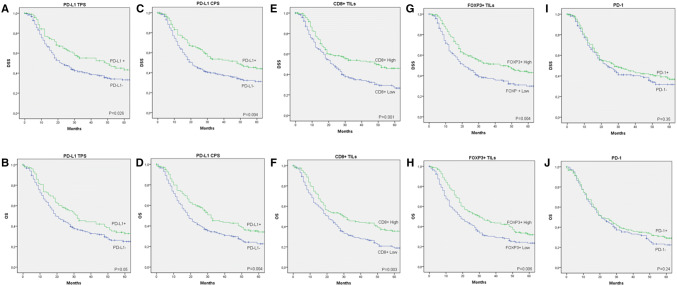
Patients harboring positive PD-L1 TPS or CPS tumors showed a significantly improved disease-specific survival (DSS) and overall survival (OS) (Fig. 3a–d). Although PD-L1 positive score was associated with better survival, no significant differences in survival rates were observed among the different levels of tumor PD-L1 expression (Supplementary figure 2). Positive PD-L1 expression in immune stromal cells was also associated with a better survival (P = 0.011 for DSS and P = 0.009 for OS). Patients with high CD8+ TIL infiltration or high FOXP3+ TIL infiltration also showed significantly better DSS and OS (Fig. 3e–h). The ratio between CD8+ and FOXP3+ TILs (using the median ratio as cutoff point) had no influence on patient’s prognosis (P = 0.84 for DSS and P = 0.78 for OS). Moreover, high CD8+ TIL infiltration was associated with better prognosis irrespective of high FOXP3+ TIL levels (P = 0.009 for DSS and P = 0.007 for OS; Supplementary figure 3). Positive PD-1 expression did not correlate with DSS nor with OS (Fig. 3i, j).
Fig. 3.
Disease-specific survival (DSS) and overall survival (OS) curves categorized according to PD-L1 tumor proportion score (TPS; a, b), PD-L1 composite proportion score (CPS; c, d), CD8+ TIL density (e, f), FOXP3+ TIL density (g, h), and PD-1 expression (i, j)
According to the tumor microenvironment type, the subgroup of HNSCC patients classified as type I (PD-L1 CPS+/high CD8+) showed the highest 5-year DSS (53%), followed by those classified as type IV (48%), type III (30%), and the worst DSS found in type II subgroup (26%) (P = 0.008; Supplementary figure 4). According to these results, patients with high CD8+ TIL infiltration clearly exhibited a better prognosis, irrespective of PD-L1 expression status; however, among the patients with low CD8+ TIL density, those with positive PD-L1 expression showed a better prognosis.
Multivariate analysis, including tumor localization, T classification, N classification, degree of differentiation, PD-L1 CPS, PD-1 expression, CD8+ TIL, and FOXP3+ TIL, showed that the parameters independently associated with a worse DSS were T4 classification (HR = 1.56, 95% CI = 1.16–2.10, P = 0.003), N+ classification (HR = 2.27, 95% CI = 1.51–3.42, P < 0.001), and low CD8+ TIL density (HR = 1.6, 95% CI = 1.19–2.16, P = 0.002). Accordingly, the same parameters were also found to independently associate with a worse OS: T4 classification (HR = 1.61, 95% CI = 1.24–2.10, P < 0.001), N+ classification (HR = 1.84, 95% CI = 1.33–2.56, P < 0.001), and low CD8+ TIL density (HR = 1.45, 95% CI = 1.12–1.89, P = 0.005).
The impact of the immunoprofiles on disease course was also examined separately in each anatomic site (Table 3). Positive PD-L1 CPS was associated with a better DSS and OS in all tumor locations, although the differences were only statistically significant in the oropharynx, probably due to the lower number of cases from other sites. High CD8+ TIL density was associated with a better DSS and OS in all locations, being the differences statistically significant in oropharynx and hypopharynx. High FOXP3+ TIL density was also associated with better DSS and OS in all sites, but the differences were not statistically significant. By contrast, positive PD-1 expression was associated with a better DSS and OS in oropharynx (although significance was not reached), but with a worse DSS and OS in hypopharynx and larynx, although the differences were only significant for the hypopharyngeal subgroup.
Table 3.
Five-year disease-specific survival (DSS) and overall survival (OS) according PD-L1 expression, PD-1 expression, CD8+ TIL, and FOXP3+ TIL
| Oropharynx | Hypopharynx | Larynx | ||||
|---|---|---|---|---|---|---|
| DSS | OS | DSS | OS | DSS | OS | |
| PD-L1 CPS | ||||||
| Positive | 40% | 29% | 35% | 27% | 65% | 58% |
| Negative | 34% | 22% | 18% | 16% | 41% | 36% |
| HR (95% CI) | 0.67 (0.46–0.97) | 0.71 (0.52–0.98) | 0.87 (0.46–1.6) | 0.81 (0.45–1.45) | 0.44 (0.19–1.02) | 0.49 (0.23–1.04) |
| P | 0.035 | 0.038 | 0.66 | 0.48 | 0.056 | 0.06 |
| PD-1 expression | ||||||
| Positive | 39% | 29% | 18% | 16% | 39% | 36% |
| Negative | 31% | 20% | 25% | 21% | 51% | 45% |
| HR (95% CI) | 0.72 (0.5–1.02) | 0.75 (0.55–1.02) | 1.89 (1.02–3.5) | 1.59 (0.87–2.87) | 1.2 (0.5–2.85) | 1.15 (0.5–2.63) |
| P | 0.07 | 0.07 | 0.04 | 0.13 | 0.68 | 0.73 |
| CD8+ TIL | ||||||
| High | 44% | 31% | 43% | 34% | 59% | 54% |
| Low | 28% | 17% | 10% | 10% | 42% | 33% |
| HR (95% CI) | 0.62 (0.43–0.88) | 0.67 (0.49–0.91) | 0.49 (0.26–0.92) | 0.57 (0.31–1.04) | 0.74 (0.34–1.62) | 0.72 (0.35–1.58) |
| P | 0.008 | 0.011 | 0.027 | 0.06 | 0.46 | 0.38 |
| FOXP3+ TIL | ||||||
| High | 40% | 26% | 35% | 32% | 57% | 45% |
| Low | 32% | 24% | 14% | 11% | 42% | 40% |
| HR (95% CI) | 0.72 (0.5–1.02) | 0.78 (0.57–1.05) | 0.63 (0.34–1.16) | 0.63 (0.35–1.11) | 0.64 (0.29–1.42) | 0.87 (0.41–1.84) |
| P | 0.068 | 0.11 | 0.14 | 0.11 | 0.28 | 0.72 |
Discussion
The interaction between tumor and host immune systems is a continuous process, the so-called immune editing, which includes tumor elimination, equilibrium state, and escape [15]. PD-L1 expression by tumor cells has been identified as one mechanism to escape the immune surveillance [2], and several reports have shown PD-L1 expression to be a negative prognostic factor in some cancer types, while some others found an association of PD-L1 expression with better outcomes [16, 17]. Similarly, controversial data have been reported in the context of HNSCC, with two meta-analysis studies that failed to demonstrate a clear association of PD-L1 expression with prognosis [9, 10]. Thus, varying results were obtained regarding the impact of PD-L1 expression with both positive and negative effects in the different studies included in these meta-analyses. These discrepancies could be attributed to methodological differences, such as the use of different antibodies, various cutoff values of PD-L1 positivity, varying scoring criteria (including or not the immune cells), and the inclusion in a single study cohort of patients with different HPV status and treatments. In addition, the varying prognostic relevance could also point to the high level of complexity and heterogeneity involved in tumor–stroma communication and interactions between tumor PD-L1 expression and other stromal immune cells within the tumor microenvironment. Taking all this into consideration, we have selected a large cohort of homogeneously treated HNSCC HPV-negative patients; the analysis of PD-L1 expression was performed considering both tumor and immune stromal cells using the cutoff recommended by recent guidelines [5] and also alongside assessing the relationship between PD-L1 expression, PD-1 expression, and the presence of infiltrating cytotoxic and regulatory TILs in the tumor microenvironment. Furthermore, we assessed the impact on HNSCC prognosis of combined PD-L1 expression and TILs infiltration by classifying the tumors into four types (I–IV) according to the immune tumor microenvironment, as reported for other cancers [14].
We observed that positive PD-L1 expression (in either tumor cells, immune cells, or combined expression in both tumor and immune cells) was associated with prolonged survival rates among resected HNSCC. Notably, this association was observed in all locations, although it was only significant in the oropharynx, probably due to a lower sample size of the hypopharyngeal and laryngeal cases. In marked contrast, PD-1 positivity in immune cells was not associated with survival. A recent study also analyzed the expression of PD-L1 on tumor cells and PD-1 on TILs in a series of surgically treated HNSCCs [18]. These authors showed that PD-L1 expression in the whole series did not correlate with survival, although a trend to a better prognosis was observed for positive cases, as we have found in our study. In marked contrast, PD-L1 expression was associated with a worse prognosis in the oral carcinoma subgroup, suggesting an influence of the tumor site in the prognostic significance of PD-L1. High levels of PD-1-positive TILs were associated with a better prognosis in the previously mentioned study, although this may probably reflect the overall high levels of TILs infiltration. In relation to this, high density of TILs, both CD8+ and FOXP3+, was also associated with better survival rates in our study. Nevertheless, multivariate analysis revealed that the only immunological parameter significantly associated with better DSS and OS was the high density of CD8+ TIL. CD8+ T cells are predominantly cytotoxic T cells, crucial components of the cellular immune system that are pivotal for cell-mediated antitumor immune responses. High infiltration of CD8+ T cells has been consistently found to associate with favorable outcomes in HNSCC, as demonstrated in a recent meta-analysis [11]. Indeed, almost every study that assessed the prognostic value of infiltrating CD8+ TIL described an association between high CD8+ T cells and a better outcome for HNSCC patients, irrespective of HPV status [11]. PD-L1 positivity (both in tumor and stromal cells) and high density of FOXP3+ TIL were strongly correlated with high density of CD8+ TIL infiltration. This could be plausible explanation for the associations of these three parameters with better survival, as observed in the univariate analyses. Our findings are consistent with various studies on HNSCCs [19, 20] and other malignancies [21–23] showing the association of PD-L1 expression with increased TILs and improved survival. PD-L1 expression may, therefore, represent a surrogate marker of endogenous antitumor immune response, thereby explaining its unexpected association with good prognosis in some tumors.
PD-L1 is generally not expressed in normal tissues but inflammatory cytokines, particularly IFNγ, can upregulate its expression in various cell types, including tumor cells. This indicates that tumors upregulate PD-L1 in response to IFNγ released by TILs as an adaptive immune-resistance mechanism to suppress local effector T-cell function, suggesting that immunosurveillance exists even in advanced cancers [3, 14]. However, PD-L1 can also be constitutively expressed on tumors without TILs through intrinsic oncogenic pathways [24]. Based on combined PD-L1 expression and TILs density, a classification of tumors into four groups has been proposed [14]. The type I is characterized by PD-L1 positivity with high TILs driving adaptive immune resistance; patients within this type are most likely to benefit from PD-1/PD-L1 blockade immunotherapy. Type II tumors have PD-L1-negative expression with no TILs indicating immune ignorance. Type III tumors show PD-L1-positive expression with no TILs indicating induction by intrinsic oncogenic pathways. And type IV is characterized by negative PD-L1 expression with high TILs indicating immune tolerance, and the suppressed immunity is supposed to be dominated by other suppressors rather than the PD-1/PD-L1 axis. In human melanoma (where the data are most mature), type I (38%) and type II (41%) tumors predominate, with the former having considerably the best prognosis [14]. Good analogous frequencies of tumor type using this classification are not yet available for most other cancers, but Teng et al. [14] argue that type I cancer microenvironments are not expected to be as prevalent as observed in melanoma. Accordingly, only 20% of our patients were classified as type I tumors, data comparable with those of two other studies in HNSCC [19, 20], which showed a 14–20% of type I tumors and also more similar to the 24% and 19% described in lung squamous cell carcinoma and adenocarcinoma, respectively [25].
High infiltration of CD8+ T cells reflects immune response and has been associated with better prognosis [11]. Therefore, types I and IV tumors are predicted to have the better prognosis, while the types II and III a poorer prognosis. Indeed, in our study, the subset of patients classified as type I and IV tumors exhibited significantly better DSS and OS than patients with types II and III tumors. The best survival was observed in type I tumors, while the worst in type II. Similar results were observed in the meta-analysis of Yang et al. [10] when combining PD-L1 expression with the density of CD8+ TIL in three studies in which these data were available [19, 20, 26]. Importantly, our work is in complete agreement with two of these studies [19, 20], which also showed a positive correlation between PD-L1 expression and CD8+ TIL, and a better prognosis for positive PD-L1 expression, high CD8+ TIL, and type I tumor microenvironment. Interestingly, these two studies, as ours, included tumors only [20] or predominantly [19] located in the oropharynx and in one of them [19] receiving surgical treatment. It should be noted that, in contrast with our study, these two studies also included a significant proportion of HPV-positive patients (19% and 37%), and none included laryngeal tumors. The other study [26] also reported a positive correlation between positive PD-L1 expression and CD8+ TIL, although neither positive PD-L1 expression nor type I tumor microenvironment were associated with better prognosis. Noteworthily, this study included hypopharyngeal tumors treated with induction chemotherapy. Another recent study on hypopharyngeal carcinomas surgically treated also found a positive correlation of PD-L1 expression and CD8+ TIL and a better prognosis for high CD8+ TIL and type I tumor microenvironment, but not for PD-L1 expression [27]. Then, again, it seems that the location of the tumor and type of treatment could influence the prognostic significance of these parameters.
According to our findings, high tumor-infiltrating FOXP3+ TILs also predict a better clinical outcome. This has also been previously described in HNSCC [11, 27] and other tumors [28]. Nevertheless, it seems quite unexpected that the presence of immune-suppressing T-cells in the tumor microenvironment was found to associate with a better survival. We observed a close correlation between infiltrating CD8+ and FOXP3+ T cells, and both were consistently found to associate with improved outcomes in univariate analyses. Nonetheless, the prognostic significance of FOXP3+ TIL was lost in multivariate analysis suggesting that its association with a better prognosis could be indirect, due to the correlation with CD8+ TIL infiltration. Other studies also suggested that the number of FOXP3+ TIL could simply reflect the total amount of T cells present within the tumor [27, 29]. Then, our hypothesis is that the beneficial effect of CD8+ TILs outweighs the immunosuppressive effect of the FOXP3+ TILs. To investigate this potential phenomenon, the CD8/FOXP3 ratio could be used. A high ratio, which reflects a predominance of CD8+ TIL, has been associated with better clinical outcomes in different tumor types [30]. Few previous studies assessed this ratio in HNSCCs, showing also an association with better outcome [31, 32]. By contrast, we did not observe evidences of association between the CD8/FOXP3 ratio and the survival in our cohort of HNSCC patients. Therefore, the prognostic value of the CD8/FOXP3 ratio in HNSCC would need to be further validated in additional studies.
PD-1 expression on T cells is known to perform an inhibitory function against effector T cells, and PD-1 is highly upregulated in exhausted T cells, suggesting a negative prognostic significance of PD-1 expression. By contrast, positive PD-1 expression was not associated with poorer survival outcomes in our series of resected HNSCC patients. In fact, other studies on HNSCCs have found that high PD-1 expression on immune cells correlated with better survival [18, 33, 34]. To explain the better prognosis of high PD-1 expression, these authors suggest that the increase in PD-1 expression could be the result of T cell receptor activation, and PD-1 might remain upregulated in the context of persistent antigen-specific immune stimulation.
The present study brings together a number of unique characteristics such as the selection of cases to preclude the influence of various uncontrolled variables (HPV status, tumor site, different treatment regimens), a large sample size, and the joint evaluation of PD-L1 and PD-1 expression and TIL infiltration of both cytotoxic CD8+ and regulatory FOXP3+ T cells. Nevertheless, this study has also several limitations. First, there are potential biases due to the retrospective nature of our study. Second, we used tissue microarrays, and since PD-L1/PD-1 expression and TILs infiltration pattern are heterogeneous, our scoring could not reflect the situation on the entire tumor. However, we observed that the expression was highly concordant in the three representative tissue cores selected and evaluated from each tumor. Even in the case of analysis of diagnostic sections, this heterogeneity could also influence the scoring. Third, the study was performed in a single center. And, fourth, it would help to the robustness of our study to perform a parallel analysis in a second independent cohort as validation group for the hypothesis generated.
In conclusion, high TILs infiltration, in particular by CD8+ T cells, was found to associate with better survival outcomes in HPV-negative surgically treated HNSCC patients. Positive PD-L1 expression was also associated with a better prognosis, although this association seems to be due to the correlation of PD-L1 positivity with high density of CD8+ TIL. The combination of high CD8+ TIL infiltration and positive PD-L1 expression (type I tumor microenvironment or adaptive immune resistance) confers a favorable prognosis to HNSCC patients, thus clearly indicating that this is the most favorable immune microenvironment to mediate effective host immune responses that can restrain tumor growth. Thus, the subset of patients with type I tumor immune microenvironment is the most likely to benefit from PD-1/PD-L1 disrupting therapies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was supported by Grants from the Plan Nacional de I+D+I 2013–2016 [ISCIII (PI16/00280 and PI19/00560 to JMGP and PI19/00098 to LMM), CIBERONC (CB16/12/00390 to JPR and CB16/12/00443 to LMM)], the Instituto de Investigación Sanitaria del Principado de Asturias (ISPA), Fundación Merck Salud (17-CC-008 to JPR), Ayudas a Grupos PCTI Principado de Asturias (IDI2018/155 to JPR), and the FEDER Funding Program from the European Union. RGD is recipient of a Severo Ochoa predoctoral fellowship (BP19-063) from the Principado de Asturias, and NRI is recipient of a FPU predoctoral fellowship (FPU17/01985) from the Spanish Ministry of Education. We want to particularly acknowledge for its collaboration the Principado de Asturias BioBank (PT17/0015/0023), financed jointly by Servicio de Salud del Principado de Asturias, Instituto de Salud Carlos III, and Fundación Bancaria Cajastur and integrated in the Spanish National Biobanks Network.
Author contributions
JPR and JMGP were involved in the conceptualization; MSC, RGD, NRI, EA, JA, and IG contributed to methodology; JPR, JMGP, FLÁ, and LMM helped in formal analysis and investigation; JPR was involved in writing—original draft preparation; JMGP, FLÁ, and LMM contributed to writing—review and editing; JPR, JMGP, and LMM helped in funding acquisition; JPR and LMM contributed to resources; JPR, JMGP, and LMM helped in the supervision. All authors read and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee [Regional Ethical Committee from Principado de Asturias for the project PI16/00280 (approval number: 70/16; date: 5 May 2016)] and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Juana M. García-Pedrero, Email: juanagp.finba@gmail.com
Juan P. Rodrigo, Email: jprodrigo@uniovi.es
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Ferris RL. Immunology and immunotherapy of head and neck cancer. J Clin Oncol. 2015;33:3293–3304. doi: 10.1200/JCO.2015.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gong J, Chehrazi-Raffle A, Reddi S, Salgia R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer. 2018;6:8. doi: 10.1186/s40425-018-0316-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen EEW, Bell RB, Bifulco CB, Burtness B, Gillison ML, Harrington KJ, Le QT, Lee NY, Leidner R, Lewis RL, Licitra L, Mehanna H, Mell LK, Raben A, Sikora AG, Uppaluri R, Whitworth F, Zandberg DP, Ferris RL. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC) J Immunother Cancer. 2019;7:184. doi: 10.1186/s40425-019-0662-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carbognin L, Pilotto S, Milella M, Vaccaro V, Brunelli M, Caliò A, Cuppone F, Sperduti I, Giannarelli D, Chilosi M, Bronte V, Scarpa A, Bria E, Tortora G. Differential activity of Nivolumab, Pembrolizumab and MPDL3280A according to the tumor expression of programmed death-Ligand-1 (PD-L1): sensitivity analysis of trials in melanoma, lung and genitourinary cancers. PLoS ONE. 2015;10:e0130142. doi: 10.1371/journal.pone.0130142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehra R, Seiwert TY, Gupta S, Weiss J, Gluck I, Eder JP, Burtness B, Tahara M, Keam B, Kang H, Muro K, Geva R, Chung HC, Lin CC, Aurora-Garg D, Ray A, Pathiraja K, Cheng J, Chow LQM, Haddad R. Efficacy and safety of pembrolizumab in recurrent/metastatic head and neck squamous cell carcinoma: pooled analyses after long-term follow-up in KEYNOTE-012. Br J Cancer. 2018;119:153–159. doi: 10.1038/s41416-018-0131-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferris RL, Blumenschein G, Jr, Fayette J, Guigay J, Colevas AD, Licitra L, Harrington KJ, Kasper S, Vokes EE, Even C, Worden F, Saba NF, Docampo LCI, Haddad R, Rordorf T, Kiyota N, Tahara M, Lynch M, Jayaprakash V, Li L, Gillison ML. Nivolumab vs investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol. 2018;81:45–51. doi: 10.1016/j.oraloncology.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J, Wang P, Xu Y. Prognostic value of programmed cell death ligand 1 expression in patients with head and neck cancer: a systematic review and meta-analysis. PLoS ONE. 2017;12:e0179536. doi: 10.1371/journal.pone.0179536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang WF, Wong MCM, Thomson PJ, Li KY, Su YX. The prognostic role of PD-L1 expression for survival in head and neck squamous cell carcinoma: a systematic review and meta-analysis. Oral Oncol. 2018;86:81–90. doi: 10.1016/j.oraloncology.2018.09.016. [DOI] [PubMed] [Google Scholar]
- 11.de Ruiter EJ, Ooft ML, Devriese LA, Willems SM. The prognostic role of tumor infiltrating T-lymphocytes in squamous cell carcinoma of the head and neck: a systematic review and meta-analysis. Oncoimmunology. 2017;6:e1356148. doi: 10.1080/2162402X.2017.1356148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodrigo JP, Heideman DA, García-Pedrero JM, Fresno MF, Brakenhoff RH, Díaz Molina JP, Snijders PJ, Hermsen MA. Time trends in the prevalence of HPV in oropharyngeal squamous cell carcinomas in northern Spain (1990–2009) Int J Cancer. 2014;134:487–492. doi: 10.1002/ijc.28355. [DOI] [PubMed] [Google Scholar]
- 13.Rodrigo JP, Hermsen MA, Fresno MF, Brakenhoff RH, García-Velasco F, Snijders PJ, Heideman DA, García-Pedrero JM. Prevalence of human papillomavirus in laryngeal and hypopharyngeal squamous cell carcinomas in northern Spain. Cancer Epidemiol. 2015;39:37–41. doi: 10.1016/j.canep.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Teng MW, Ngiow SF, Ribas A, Smyth MJ. Classifying cancers based on T-cell infiltration and PD-L1. Cancer Res. 2015;75:2139–2145. doi: 10.1158/0008-5472.CAN-15-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 16.Wu P, Wu D, Li L, Chai Y, Huang J. PD-L1 and survival in solid tumors: a meta-analysis. PLoS ONE. 2015;10:e0131403. doi: 10.1371/journal.pone.0131403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Q, Liu F, Liu L. Prognostic significance of PD-L1 in solid tumor: an updated meta-analysis. Medicine (Baltimore) 2017;96:e6369. doi: 10.1097/MD.0000000000006369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schneider S, Kadletz L, Wiebringhaus R, Kenner L, Selzer E, Füreder T, Rajky O, Berghoff AS, Preusser M, Heiduschka G. PD-1 and PD-L1 expression in HNSCC primary cancer and related lymph node metastasis—impact on clinical outcome. Histopathology. 2018;73:573–584. doi: 10.1111/his.13646. [DOI] [PubMed] [Google Scholar]
- 19.Balermpas P, Rödel F, Krause M, Linge A, Lohaus F, Baumann M, Tinhofer I, Budach V, Sak A, Stuschke M, Gkika E, Grosu AL, Abdollahi A, Debus J, Stangl S, Ganswindt U, Belka C, Pigorsch S, Multhoff G, Combs SE, Welz S, Zips D, Lim SY, Rödel C, Fokas E, DKTK-ROG The PD-1/PD-L1 axis and human papilloma virus in patients with head and neck cancer after adjuvant chemoradiotherapy: a multicentre study of the German Cancer Consortium Radiation Oncology Group (DKTK-ROG) Int J Cancer. 2017;141:594–603. doi: 10.1002/ijc.30770. [DOI] [PubMed] [Google Scholar]
- 20.De Meulenaere A, Vermassen T, Aspeslagh S, Deron P, Duprez F, Laukens D, Van Dorpe J, Ferdinande L, Rottey S. Tumor PD-L1 status and CD8+ tumor-infiltrating T cells: markers of improved prognosis in oropharyngeal cancer. Oncotarget. 2017;8:80443–80452. doi: 10.18632/oncotarget.19045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N, Honjo T, Fujii S. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci USA. 2007;104:3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lipson EJ, Vincent JG, Loyo M, Kagohara LT, Luber BS, Wang H, Xu H, Nayar SK, Wang TS, Sidransky D, Anders RA, Topalian SL, Taube JM. PD-L1 expression in the Merkel cell carcinoma microenvironment: association with inflammation, Merkel cell polyomavirus and overall survival. Cancer Immunol Res. 2013;1:54–63. doi: 10.1158/2326-6066.CIR-13-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vilain RE, Menzies AM, Wilmott JS, Kakavand H, Madore J, Guminski A, Liniker E, Kong BY, Cooper AJ, Howle JR, Saw RPM, Jakrot V, Lo S, Thompson JF, Carlino MS, Kefford RF, Long GV, Scolyer RA. Dynamic changes in PD-L1 expression and immune infiltrates early during treatment predict response to PD-1 blockade in Melanoma. Clin Cancer Res. 2017;23:5024–5033. doi: 10.1158/1078-0432.CCR-16-0698. [DOI] [PubMed] [Google Scholar]
- 24.Concha-Benavente F, Srivastava RM, Trivedi S, Lei Y, Chandran U, Seethala RR, Freeman GJ, Ferris RL. Identification of the cell-intrinsic and -extrinsic pathways downstream of EGFR and IFN-gamma that induce PD-L1 expression in head and neck cancer. Cancer Res. 2016;76:1031–1043. doi: 10.1158/0008-5472.CAN-15-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parra ER, Behrens C, Rodriguez-Canales J, Lin H, Mino B, Blando J, Zhang J, Gibbons DL, Heymach JV, Sepesi B, Swisher SG, Weissferdt A, Kalhor N, Izzo J, Kadara H, Moran C, Lee JJ, Wistuba II. Image analysis-based assessment of PD-L1 and tumor-associated immune cells density supports distinct intratumoral microenvironment groups in non-small cell lung carcinoma patients. Clin Cancer Res. 2016;22:6278–6289. doi: 10.1158/1078-0432.CCR-15-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ono T, Azuma K, Kawahara A, Sasada T, Hattori S, Sato F, Shin B, Chitose SI, Akiba J, Hirohito U. Association between PD-L1 expression combined with tumor-infiltrating lymphocytes and the prognosis of patients with advanced hypopharyngeal squamous cell carcinoma. Oncotarget. 2017;8:92699–92714. doi: 10.18632/oncotarget.21564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu C, Tian S, Lin L, Zhang J, Ding H. Prognostic and clinicopathological significance of PD-L1 and tumor infiltrating lymphocytes in hypopharyngeal squamous cell carcinoma. Oral Oncol. 2020;102:104560. doi: 10.1016/j.oraloncology.2019.104560. [DOI] [PubMed] [Google Scholar]
- 28.Shang B, Liu Y, Jiang SJ, Liu Y. Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: a systematic review and meta-analysis. Sci Rep. 2015;5:15179. doi: 10.1038/srep15179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park K, Cho KJ, Lee M, Yoon DH, Kim SB. Importance of FOXP3 in prognosis and its relationship with p16 in tonsillar squamous cell carcinoma. Anticancer Res. 2013;33:5667–5673. [PubMed] [Google Scholar]
- 30.Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 2011;105:93–103. doi: 10.1038/bjc.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nasman A, Romanitan M, Nordfors C, Grun N, Johansson H, Hammarstedt L, Marklund L, Munck-Wikland E, Dalianis T, Ramqvist T. Tumor infiltrating CD8C and Foxp3C lymphocytes correlate to clinical outcome and human papillomavirus (HPV) status in tonsillar cancer. PLoS ONE. 2012;7(6):e38711. doi: 10.1371/journal.pone.0038711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen WY, Wu CT, Wang CW, Lan KH, Liang HK, Huang BS, Chang YL, Kuo SH, Cheng AL. Prognostic significance of tumor-infiltrating lymphocytes in patients with operable tongue cancer. Radiat Oncol. 2018;13:157. doi: 10.1186/s13014-018-1099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim HR, Ha SJ, Hong MH, Heo SJ, Koh YW, Choi EC, Kim EK, Pyo KH, Jung I, Seo D, Choi J, Cho BC, Yoon SO. PD-L1 expression on immune cells, but not on tumor cells, is a favorable prognostic factor for head and neck cancer patients. Sci Rep. 2016;6:36956. doi: 10.1038/srep36956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Badoual C, Hans S, Merillon N, Van Ryswick C, Ravel P, Benhamouda N, Levionnois E, Nizard M, Si-Mohamed A, Besnier N, Gey A, Rotem-Yehudar R, Pere H, Tran T, Guerin CL, Chauvat A, Dransart E, Alanio C, Albert S, Barry B, Sandoval F, Quintin-Colonna F, Bruneval P, Fridman WH, Lemoine FM, Oudard S, Johannes L, Olive D, Brasnu D, Tartour E. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res. 2013;73:128–138. doi: 10.1158/0008-5472.CAN-12-2606. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.