Abstract
Tumor-associated antigens (TAAs) have been tested in various clinical trials in cancer treatment but the patterns of specific T cell response to personalized TAA immunization remains to be fully understood. We report antigen-specific T cell responses in patients immunized with dendritic cell vaccines pulsed with personalized TAA panels. Tumor samples from patients were first analyzed to identify overexpressed TAAs. Autologous DCs were then transfected with pre-manufactured mRNAs encoding the full-length TAAs, overexpressed in the patients' tumors. Patients with glioblastoma multiforme (GBM) or advanced lung cancer received DC vaccines transfected with personalized TAA panels, in combination with low-dose cyclophosphamide, poly I:C, imiquimod and anti-PD-1 antibody. Antigen-specific T cell responses were measured. Safety and efficacy were evaluated. A total of ten patients were treated with DC vaccines transfected with personalized TAA panels containing 3–13 different TAAs. Among the seven patients tested for anti-TAA T cell responses, most of the TAAs induced antigen-specific CD4+ and/or CD8+ T cell responses, regardless of their expression levels in the tumor tissues. No Grade III/IV adverse events were observed among these patients. Furthermore, the treated patients were associated with favorable overall survival when compared to patients who received standard treatment in the same institution. Personalized TAA immunization-induced-specific CD4+ and CD8+ T cell responses without obvious autoimmune adverse events and was associated with favorable overall survival. These results support further studies on DC immunization with personalized TAA panels for combined immunotherapeutic regimens in solid tumor patients.
Trial registration ClinicalTrials.gov, NCT02709616 (March, 2016), NCT02808364 (June 2016), NCT02808416 (June, 2016).
Electronic supplementary material
The online version of this article (10.1007/s00262-020-02496-w) contains supplementary material, which is available to authorized users.
Keywords: Tumor-associated antigen, Non-small cell lung cancer, Glioblastoma multiforme, DC vaccine, Personalization, CD4+ T cell response, CD8+ T cell response
Introduction
Dendritic cell (DC) vaccine against tumor antigens is a promising cancer immunotherapeutic approach [1, 2]. Clinical studies have used different types of tumor antigens loaded into DCs to treat cancer patients. Autologous DCs pulsed with tumor cell lysates were tested in many clinical trials and produced some promising efficacy results [3–6]. A major advantage of tumor cell lysates as tumor antigens is that they contain both tumor-associated antigens (TAA) and tumor specific-antigens (TSA, neoantigens), however, procurement of sufficient amount of tumor tissues in advanced stage cancer patients is difficult to achieve. To circumvent this hurdle, DC vaccines loaded with more defined TAA in the form of synthesized peptides, DNAs or mRNAs were tested in clinical studies. Immunization with autologous DCs transfected with mRNA encoding full-length prostate-specific antigen (PSA) induced PSA-specific T cell responses and significant PSA decrease in prostate cancer patients [7]. Furthermore, DCs transfected with mRNAs encoding four full-length TAAs were combined with anti-CTLA4 antibody ipilimumab to treat advanced melanoma. The combination was safe and resulted in a 51% 6-month disease control rate and 38% overall tumor response [8].
Recently, neoantigen-based cancer vaccines have received intense attention [9, 10]. Clinical studies show that neoantigens delivered through DCs or other means induce potent antigen-specific T cell response and favorable clinical responses in melanoma and GBM patients [11–15]. Although very promising, two major issues need to be resolved before its wide application: low occurrence rate of neoantigens among total tumor mutations and lengthy time taken to identify and apply to cancer patients. These two issues can be resolved by combining neoantigen and TAA treatment. In a recent clinical trial, Hilf et al. first immunized GBM patients with TAA-based cancer vaccines and then followed with neoantigen-based cancer vaccines [14]. This group of patients had a median overall survival of 29.0 months, compared favorably to 14.6 months by standard treatment [16]. Together, the above clinical studies suggest that continued development of cancer vaccines based on TAAs and neoantigens is needed to improve treatment efficacy.
Several clinically significant questions regarding TAA-based cancer vaccine remain largely unknown. Given that TAAs are overexpressed at different levels in tumor tissues, which TAAs should be chosen for patient immunization in personalized treatment? Are TAA overexpression levels correlated with TAA-based vaccine-induced antigen-specific T cell responses? Does the number of TAAs used in immunization impact each antigen’s capability to induce antigen-specific T cell responses? We conducted clinical trials to test personalized TAA-based DC vaccines in lung cancer with brain metastasis and GBM in a specialized brain disease hospital, Guangdong 999 Brain Hospital. To prepare for the trials, we first assayed the expression pattern of ~ 100 reported TAAs and several known immunosuppressive factors in the tumor microenvironment (TME) in preserved tumor samples. We pre-manufactured GMP quality mRNA for 87 full-length TAAs that appeared in our assays. Upon identification of overexpressed TAAs in individual patient’s samples, the patients received 3–16 infusions of DCs transfected with 3–13 different TAAs. To maximize induction of anti-TAA-specific T cell responses, the patients also received low-dose cyclophosphamide for regulatory T cell depletion [17, 18], poly I:C for enhancing anti-tumor immunity [14, 19–21] and imiquimod for increasing DC maturation and function [22–24]. In addition, four lung cancer patients also received anti-PD-1 Nivolumab treatment. To our surprise, most of the immunized TAAs induced antigen-specific CD4+ and/or CD8+ T cell responses regardless of their expression levels and the number of immunized TAAs. The patients are associated with favorable survival with the combined treatment. These results suggest that further development of personalized TAA-based cancer vaccines is warranted for combination regimen in cancer immunotherapy.
Materials and methods
Clinical trial design
Personalized TAA-based DC vaccine trials of solid tumors (newly diagnosed GBM NCT02709616; recurrent GBM NCT02808364; lung cancer with brain metastasis NCT02808416) were established in Jinan University Affiliated Guangdong 999 Brain Hospital. These were open-label single center and one arm phase I trials. The treatment protocols were approved by the hospital's ethical committee. All trial treatments were strictly adhered to the guidelines of the Declaration of Helsinki and had written consent from the patients. Patients were recruited in March, 2016 to October, 2017 and the follow-up was up to June, 2019. A total of ten patients including five non-small cell lung cancer (NSCLC) with brain metastasis and five GBM patients were recruited to the study (Table 1). The survival time of 13 lung cancer and 28 GBM patients (Table S1) who received standard treatment by the same physician team during the last 3 years was used as a comparison. The primary objective of the trials was to test the safety of personalized TAA-based DC vaccine trials with the secondary objective to investigate CD4+ and CD8+ T cell responses to TAA-based DC vaccines. To enhance antigen-specific T cell induction to TAAs, low-dose cyclophosphamide for regulatory T cell depletion and poly I:C as well as imiquimod as immune adjuvants were incorporated into the trial design. Moreover, immune checkpoint inhibitor anti-PD-1 was concurrently used in four lung cancer patients as prescribed by physicians. The patients were evaluated every 3 months for treatment response and disease progression using RECIST1.1 for lung cancer patients and iRANO criteria for GBM. Adverse events were evaluated and reported using CTCAE v4.
Table 1.
Patient characteristics and treatment
| Patient | Disease/stage | Age | Gender | Organ metastasis | Mutation | Prior lines of treatments |
|---|---|---|---|---|---|---|
| BT001 | NSCLC/IV | 45 | M | Lung, bone, brain | EGFR Exon 19 deletion | RT/targeted |
| BT045 | NSCLC/IV | 45 | F | Lung, bone, liver, brain | No mutation | RT |
| BT051 | NSCLC/IV | 50 | M | Lung, lymph node, brain, Liver, pancreas, adrenal gland | No mutation | RT/Chemo |
| BT077 | NSCLC/IV | 59 | M | Lung, brain | ALK + | RT/targeted |
| BT079 | NSCLC/IV | 68 | F | Lung, brain | ALK + | RT/chemo/targeted |
| MGMT expression by IHC | Concurrent treatment | |||||
|---|---|---|---|---|---|---|
| BT030 | GBM/Primary | 45 | M | + | Surgery/Chemo/RT | |
| BT044 | GBM/Primary | 18 | M | + + + | Surgery/Chemo/RT | |
| BT063 | GBM/Primary | 37 | M | - | Surgery/Chemo/RT | |
| BT056 | GBM/Recurrent | 55 | F | + | Surgery | |
| BT057 | GBM/Recurrent | 28 | M | + + + | Surgery |
MGMTO6-methylguanine-DNA methyltransferase, ALK anaplastic lymphoma kinase, RT radiotherapy
Measurement of mRNA expression of TAA and TME immunosuppressive factors
Total RNAs were extracted from tumor samples, obtained by resection or biopsy, and reverse transcribed. The cDNAs were analyzed by quantitative polymerase chain reaction (qPCR) for the expression of TAAs and TME immunosuppressive factors. All reactions were performed in triplicates. The primers for the tested TAA genes are listed in Table S2. The mRNA expression levels of TAAs and TME immunosuppressive factors in the tumor samples were compared with those in either para-tumor biopsies or pooled samples of normal tissues and shown as mean fold changes.
Personalized DC vaccine generation and therapy
Peripheral blood mononuclear cells (PBMCs) were obtained via leukapheresis and further purified by density gradient centrifugation. PBMCs were cryopreserved and thawed for each DC vaccine preparation a week before the vaccination. PBMCs were suspended for 2 h at 2 × 108 in 30 ml AIM-V media (invitrogen) in T-175 cell culture flasks. After 2 h incubation in a 37 °C, 5% CO2 humidified incubator, adherent cells were harvested and cultured in 30 ml AIM-V containing 800 IU/ml GM-CSF and 500 IU/ml IL-4 to induce immature DC (iDC). On day 6, iDCs were harvested by enzyme-free cell stripper buffer (Gibco), divided into equal portions, transfected with the individual TAA-mRNAs and pooled after maturation. For each DC infusion, 3–7 different TAAs were used. iDCs suspended in PBS buffer at 5 × 106/100 μl were mixed with TAA-mRNA at 5 μg/106 cells and pulsed using a Gene Pulser Xcell Electroporation System (Bio-Rad) in a 4-mm cuvette. The TAA-transfected iDCs were matured in AIM-V contained 800 IU/ml GM-CSF, 500 IU/ml IL-4, 160 ng/ml IL-6, 5 ng/ml IL-1β, 5 ng/ml TNF-α and 1 µg/ml PGE2 for 18 h and pooled for injection. All cytokines were purchased from R&D Systems. PGE2 was purchased from Sigma. The TAA-transfected mature DCs (mDCs) were analyzed for quality assurance and achieved high maturity (Table S3). All patients received a low-dose cyclophosphamide (200 mg/m2) treatment 24 h before each vaccination to deplete regulatory T cells [17, 18]. The TAA-specific DC vaccines were infused back to patients by intradermal injection and intravenous infusion at 1:5 ratio. Immune adjuvants such as imiquimod was applied on the skin for 5 days, beginning 2 days before vaccination, and poly I:C at 50 μg/kg was injected intramuscularly every 2 days, beginning at the day of vaccination, for 2 weeks after each DC vaccination. The personalized TAA-specific autologous DC vaccination can be achieved within 7 days from the time of tumor biopsy or resection (day 1). Autologous DC culture was initiated on day 1. A high throughput qPCR assay to identify overexpressed TAAs was performed on days 2–4. All mRNAs encoding full-length TAAs were pre-manufactured in a GMP facility. Patient-specific TAAs were chosen to electroporate into iDC on day 6. The TAA-transfected iDC was induced to undergo maturation for 16–20 h and mDCs were injected into patients on day 7. Patients received DC vaccines at an interval of 2–4 weeks.
TAA constructs and GMP quality mRNA production
Psp73-gp96ss-TAA-LAMP1-A64 plasmids were constructed as described previously [25]. Full-length TAAs were fused with a LAMP1 domain to enhance MHC class II antigen presentation. Plasmids were all linearized by digestion with SpeI-HF (NEB) and used as templates in in vitro transcription reactions. The in vitro transcription was performed with T7 RNA polymerase (mMESSAGE mMACHINE T7 Transcription kit; Ambion) according to the manufacturer’s instructions. The transcribed RNA was purified after TURBO DNase (Ambion) digestion on RNeasy columns (Qiagen) according to the manufacturer’s instructions. RNA quality was verified by the Agilent 2100 Bioanalyzer, and the levels of endotoxin contamination were below 0.25 EU/μg. RNA concentration was measured by Nanodrop. Pre-manufactured TAA-mRNAs were confirmed for their protein expression after transfection into 293 T cells or human DCs by western Blot analysis. The manufactured TAA-mRNAs were stored at − 80 °C in aliquots. For a fast turnaround and timely treatment, we have generated pre-manufactured GMP quality mRNAs encoding 87 different full-length TAAs.
Detection of TAA-specific T cells
PBMCs collected at different times plated at a rate of 5 × 105 cells/well in 48-well plates were co-cultured with autologous TAA-pulsed-mDC at 10:1 ratio in the presence of 10 ng/ml IL-7 (R&D). On day 3, IL-2 (R&D) was supplemented into the media at a concentration of 100 IU/ml and fresh IL-2 and IL-7 were then supplied every 3 days. PBMCs were cultured in RPMI-1640 medium supplemented with non-essential amino acids, l-glutamine, HEPES, β-Mercaptoethanol, sodium pyruvate, penicillin/streptomycin (Gibco), and 10% FBS (Gibco). On day 12, non-adherent T cells were collected as effector T cells and restimulated with TAA-mDCs for 6 h in RPMI-1640 with 10% FBS and Golgistop (BD). TAA-specific T cells were determined using intracellular staining of IFN-γ and TNF-α. The in vitro stimulated T cells were stained with LIVE/DEAD-AmCyan, and a panel of cell surface markers including CD3-FITC, CD4-PE/Cy7, and CD8-PerCP, followed by fixation/permeabilization and then stained with PE-anti-IFN-γ and APC-anti-TNF-α.
Statistical analysis
Data were shown as mean ± standard error of the mean (SEM). Statistical analyses were performed with Prism 7.0 (GraphPad Software Inc, USA) software. A p value of < 0.05 was considered statistically significant.
Results
Heterogeneous expression of TAAs and TME immunosuppressive factors in lung cancer tumor samples
Tumor heterogeneity represents a tremendous challenge for precision immunotherapy. To prepare for personalized TAA-pulsed DC vaccine-based immunotherapy, we first analyzed the expression pattern of TAAs and TME immunosuppressive factors in tumor samples from lung lesions of NSCLC patients. Biopsy samples from advanced stage cancer patients are often limited in size. For practical reasons, we used a high throughput qPCR assay to quantify the mRNA expression of these genes. The expression levels of the tested genes were compared with those in para-tumor lung tissues from 21 lung cancer patients who did not receive immunotherapy. TAAs had a highly variable expression pattern. Among the shown TAAs, XAGE-1b was highly expressed in six tumor samples while Trag-3 was only overexpressed in one tumor sample (Fig. S1A). Tumors from patients S2 and A11 showed overexpression of most of the TAAs while tumors from majority of the patients showed overexpression of only a few TAAs (Fig. S1A). Among the seven tested TME immunosuppressive factors, TDO1 was the most upregulated in primary lung cancer lesions (Fig. S1B). We then assayed TAAs and TME immunosuppressive factors in eight NSCLC brain metastatic samples. Several TAAs, including MUC1, IGF2BP3 and Stat3, were highly upregulated in these brain metastatic samples (Fig. S1C). Interestingly, IDO1 was the most overexpressed immunosuppressive factor in the lung cancer brain metastatic samples (Fig. S1D). These data are consistent with critical roles of Stat3 and IDO1 in promoting tumor proliferation and metastasis [26, 27]. Additional analysis on the expression of TAAs and TME suppressive factors in GBM tumor samples was performed and also showed expression variability (described elsewhere [28]). Together, these results demonstrate high degree of expression heterogeneity of TAAs and TME immunosuppressive factors in individual cancer patients and suggest that tumor vaccination requires personalization to better target the specific TAAs. Based on the analysis, we prepared 87 pre-manufactured GMP quality full-length mRNA TAAs for rapid application of DC vaccine immunization.
Patient characteristics and treatment
Ten patients (5 NSCLC and 5 GBM) were enrolled for this study and treated with DC vaccines transfected with personalized TAA-mRNA panels. All five NSCLC patients were at stage IV with brain and other organ metastasis and received prior RT, chemotherapy and targeted therapy (Table 1). The status of the five lung cancer patients was PD at the time of enrollment. During the study, these five patients received either DC vaccines alone (BT001) or combination therapy with anti-PD-1 (Nivolumab) during the course of treatment (BT045, BT051, BT077 and BT079) (Table 2). Among the five treated GBM patients, three were newly diagnosed and two were recurrent (Table 1). The GBM patients also received concurrent standard treatments (Table 1). The ten patients received 3–16 DC vaccine infusions (Table 2). The selection of TAAs for treatment was based on the following criteria: (1) the TAA expression was at least > twofold of that expressed in para-tumor tissues or pooled samples of normal tissues. (2) There were published reports demonstrating identification of anti-TAA-specific CD4+ or CD8+ T cells in cancer patients. (3) Low relative expression of the TAAs in normal human tissues as reported in the Gencode site (www.gencodegenes.org). The number of individual TAAs used for patient immunization was between 3 and 13 (Tables 2 and 3). For each TAA, the number of immunizations delivered through DC vaccines ranged from 3 to 7 times (Table 3). In addition, cyclophosphamide, poly I:C and imiquimod were incorporated into the treatment regimen as described in the clinical trial design.
Table 2.
Immunotherapy and clinical outcomes
| Patient | Number of DC infusion | Number of immunized | Combination therapy | OS (month) | Response | Adverse effect times | |
|---|---|---|---|---|---|---|---|
| I/II | III/IV | ||||||
| NSCLC | |||||||
| BT001 | 8 | 10 | – | 27 | PR | 2 (skin rash) | 0 |
| BT045 | 6 | 12 | Anti-PD-1 (100 mg × 4) | 8 | PR | 1 (fever) | 0 |
| BT051 | 16 | 13 | Anti-PD-1 (100 mg × 9) | 17 | PR | 0 | 0 |
| BT077 | 8 | 9 | Anti-PD-1 (100 mg × 6) | 15 | PR | 0 | 0 |
| BT079 | 12 | 9 | Anti-PD-1 (100 mg × 8) | 89 | PR | 0 | 0 |
| GBM | |||||||
| BT030 | 6 | 3 | – | 27 | 1 (fever) | 0 | |
| BT044 | 8 | 8 | – | 8 | 0 | 0 | |
| BT063 | 4 | 3 | – | > 30 | 0 | 0 | |
| BT056 | 5 | 8 | – | 19 | 0 | 0 | |
| BT057 | 3 | 3 | –– | 11 | 0 | 0 | |
Anti-PD-1 administration: single dose × number of infusions
Table 3.
TAA-specific CD4+ and CD8+ T cell responses in NSCLC and GBM patients
| Patient | Immunized TAAs | Expression level | Number of immunization | CD4-IFN-γ | CD4-TNF-α | CD8-IFN-γ | CD8-TNF-α | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ABLR (%) | Fold increase | ABLR (%) | Fold increase | ABLR (%) | Fold increase | ABLR (%) | Fold increase | ||||
| BT001 | MAGE12 | 3.1 | 4 | 0.2 | > 50 | 0 | 0 | 0 | 0 | 0.3 | > 50 |
| HTERT | 3.2 | 4 | 0 | 0 | 1.4 | > 50 | 1.0 | 0.3 | 0.6 | 0.4 | |
| NY-SAR-35 | 3.4 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| HER2 | 3.8 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 5T4 | 4.0 | 4 | 0 | 0 | 0.8 | > 50 | 0 | 0 | 1.1 | > 50 | |
| NY-BR-1 | 4.3 | 4 | 0 | 0 | 0 | 0 | 0.6 | > 50 | 0 | 0 | |
| CTAG2 | 4.5 | 4 | 0.2 | > 50 | 0 | 0 | 1.8 | > 50 | 0 | 0 | |
| LY6K | 4.6 | 4 | 0 | 0 | 0 | 0 | 3.8 | > 50 | 3.0 | > 50 | |
| SOX11 | 5.9 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| KIF20A | 15.6 | 4 | 0 | 0 | 0 | 0 | 3.2 | > 50 | 1.5 | > 50 | |
| BT051 | IGF2BP3 | 3.1 | 6 | 1.1 | > 50 | 0.1 | 1 | 0.7 | > 50 | 0 | 0 |
| SPANXB1 | 4.1 | 4 | 1.2 | > 50 | 0.1 | 2.8 | 1.2 | > 50 | 0.1 | > 50 | |
| SOX11 | 5.7 | 7 | 0.8 | > 50 | 0.3 | > 50 | 0.5 | > 50 | 0 | 0 | |
| SURVIVIN | 6.1 | 4 | 0 | 0 | 0 | 0 | 0.2 | > 50 | 0 | 0 | |
| LCK | 8.1 | 6 | 0.2 | 2 | 0.1 | 1 | 0.3 | 1.5 | 0 | 0 | |
| NY-BR-1 | 10.7 | 7 | 0.5 | 5 | 0 | 0 | 0 | 0 | 0.3 | > 50 | |
| ART4 | 17.4 | 6 | 0.3 | 3 | 0 | 0 | 0.1 | 0.2 | 0 | 0 | |
| PCNA | 21.4 | 5 | 0.4 | 2.5 | 0.1 | 0.5 | 0.8 | > 50 | 0.1 | > 50 | |
| RPSA | 32 | 5 | 1.8 | > 50 | 0.6 | > 50 | 1.5 | > 50 | 0.1 | > 50 | |
| SOX10 | 94.4 | 7 | 1.1 | > 50 | 0.1 | > 50 | 0 | 0 | 0.03 | > 50 | |
| CDC45L | 167.7 | 4 | 1.0 | > 50 | 0 | 0 | 1.2 | > 50 | 0.3 | > 50 | |
| TRP2 | 519.1 | 7 | 0 | 0 | 0.6 | > 50 | 1.1 | > 50 | 0.01 | > 50 | |
| FABP7 | 7968.0 | 5 | 0.6 | > 50 | 0.5 | > 50 | 1.0 | > 50 | 0.1 | > 50 | |
| BT077 | SURVIVIN | 9.9 | 4 | 0.8 | 18 | 0.7 | > 50 | 5.3 | 0.8 | 1.8 | 6 |
| MELK | 15.3 | 4 | 1.2 | 0.7 | 1.5 | > 50 | 9.8 | 0.8 | 3.9 | 13 | |
| CEA | 30.7 | 4 | 0 | 0 | 0 | 0 | 0.7 | > 50 | 0 | 0 | |
| NY-ESO-1 | 288.0 | 4 | 0.1 | > 50 | 0.3 | > 50 | 0 | 0 | 0.6 | > 50 | |
| CDC45L | 359.5 | 4 | 0.6 | > 50 | 0.2 | > 50 | 6.6 | > 50 | 0.1 | > 50 | |
| CDCA1 | 404.5 | 4 | 0.4 | > 50 | 0.1 | > 50 | 3.7 | > 50 | 0.3 | > 50 | |
| SP17 | 430.5 | 4 | 0.7 | 7 | 0.2 | 2 | 3.9 | 39 | 0.3 | > 50 | |
| FABP7 | 8306.4 | 4 | 0 | 0 | 0.1 | 1 | 0 | 0 | 0.1 | > 50 | |
| CHI3L2 | 400,099.7 | 4 | 2.0 | 11 | 1.8 | > 50 | 4.3 | 0.7 | 4.3 | 43 | |
| BT079 | FABP7 | 229.1 | 6 | 0 | 0 | 0 | 0 | 0.3 | 1.5 | 0 | 0 |
| 5T4 | 230.7 | 6 | 0.3 | 0.8 | 0 | 0 | 0 | 0 | 0 | 0 | |
| CDCA1 | 268.7 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| CEA | 328.6 | 6 | 2.9 | 4.1 | 0.9 | 1.8 | 6.7 | 11.2 | 2.0 | > 50 | |
| SP17 | 385.3 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| AIM2 | 445.1 | 6 | 1.2 | > 50 | 0.2 | 0.7 | 10.0 | 14.3 | 3.1 | > 50 | |
| NY-ESO-1 | 4420.5 | 6 | 0 | 0 | 0.2 | > 50 | 1.0 | > 50 | 0 | 0 | |
| CHI3L2 | 19,349.4 | 6 | 2.9 | 9.7 | 1.5 | 5 | 6.9 | 34.5 | 6.2 | > 50 | |
| XAGE1B | 318,293.9 | 6 | 3.7 | 5.3 | 0.7 | 2.3 | 2.1 | 3.5 | 1.5 | > 50 | |
| BT030 | CD133 | 5.7 | 6 | 0.3 | > 50 | 0.2 | > 50 | 0.1 | > 50 | 0 | 0 |
| SOX11 | 19.9 | 6 | 1.0 | > 50 | 1.4 | > 50 | 0.3 | > 50 | 0.2 | > 50 | |
| LRRC8A | 26.7 | 6 | 0 | 0 | 0.5 | > 50 | 0 | 0 | 0.1 | 1 | |
| BT063 | NLGN4X | 3.3 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| IL13RA2 | 4.7 | 4 | 0.4 | > 50 | 0.1 | > 50 | 0.2 | > 50 | 0 | 0 | |
| CD133 | 5.6 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| BT057 | IL13RA2 | 18.2 | 3 | 0.9 | 0.6 | 1.3 | 0.8 | 0.9 | 1 | 0 | 0 |
| LRRC8A | 129.2 | 3 | 1.5 | 0.9 | 1.7 | 1 | 1.8 | 1.4 | 0.3 | 1.5 | |
| SURVIVIN | 193.9 | 3 | 4.2 | 2.8 | 3.6 | 2.6 | 0 | 0 | 0 | 0 | |
(1) Expression level indicates TAA-mRNA expression level in personalized tumor samples quantified by qPCR and compared to those in para-tumor tissues. (2) ABLR (Above Base Line Response) (%) indicates the enhanced antigen-specific T cell responses after immunization. It was calculated by deducting the percent of TAA-specific T cell responses prior to immunization from the percent after immunization as follows: (TAApost%-MOCKpost%)-(TAApre%-MOCKpre%). (3) Fold increase indicates the fold change over T cell responses prior to immunization as calculated [(TAApost%-MOCKpost%)-(TAApre%-MOCKpre%)]/(TAApre%-MOCKpre%). A positive TAA response is defined as ≥ 0.5 fold (a 50% increase over prior immunization value) and marked by bold A > 50-fold increase represents a case that TAA-specific T cells were not detectable in patients before immunization and readily detectable after immunization
Dynamic changes of TAA expression in different lesions during disease progression
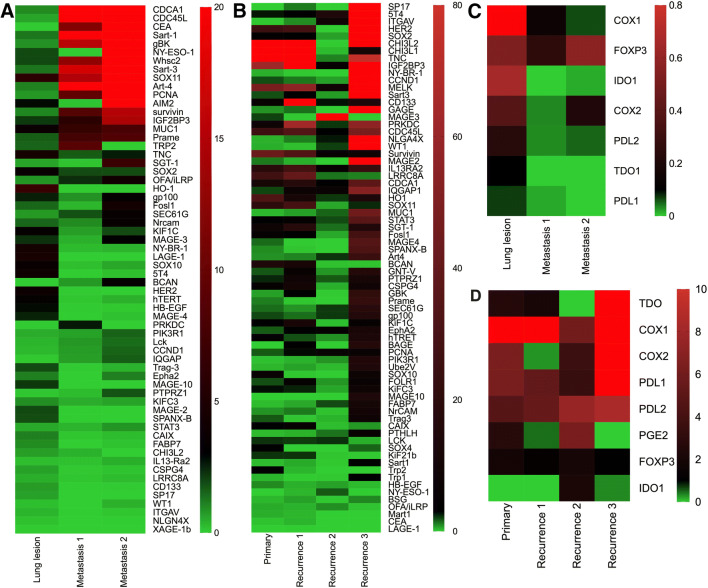
Two patients (BT001 and BT030) underwent multiple biopsies during the course of their disease progression. As disease progressed, more TAAs were upregulated (Fig. 1a, b). In lung cancer patient BT001, 17 TAAs from CDCA1 to Prame as well as SGT-1 as listed were strongly upregulated in vertebral metastasis site 2 when compared to his primary lung lesion (Fig. 1a). Moreover, ~ 80% of the tested TAAs in GBM patient BT030 were strongly upregulated by recurrence in three samples (Fig. 1b). The expression of TME immunosuppressive factors also exhibited dynamic patterns. The overall expression of these factors was downregulated in lung cancer vertebral lesions from patient BT001 while upregulated in recurrent GBM lesions from patient BT030 (Fig. 1c, d). These results suggest that personalized TAA-based immunization needs to be evolved to match the TAA changes during disease progression in long-term treatment. It should be noted that the TAAs used for treating these two patients were chosen based on their expression levels in the primary lesions.
Fig. 1.
Heatmap of mRNA expression of TAAs and TME immunosuppressive factors in serial tumor samples from patients BT001 and BT030. The mRNAs of the indicated TAA and TME immunosuppressive factors in tumor samples from lung and brain were assayed by qPCR in triplicates. Lung para-tumor tissue from biopsy was used as the control for comparison for patient BT001 and pooled normal brain tissues were used as controls for comparison for patient BT030. The mRNA expression levels of the measured genes were expressed as mean fold changes. a Expression of TAAs in tumor samples from patient BT001. Three tumor samples are from lung (Lung lesion), vertebral metastasis (metastasis 1 and 2) at day 376, 682, 701 after diagnosis. b Expression of TAAs in tumor samples from patient BT030. Four tumor samples are from newly diagnosed GBM and recurrences at day 0, 326, 453, 584 after diagnosis. c Expression of TME immunosuppressive factors in tumor samples from patient BT001 as in (a). d Expression of TME immunosuppressive factors in tumor samples from patient BT030 as in (b). Color bar and number represent fold changes
TAA-specific T cell responses
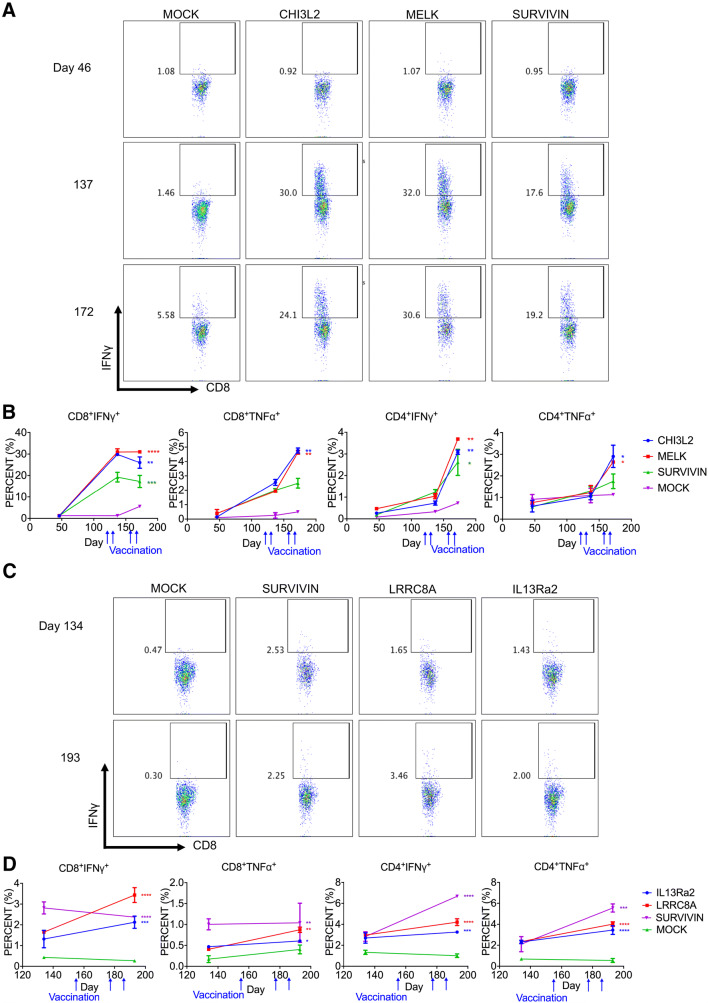
To examine TAA-specific T cell responses, we stimulated peripheral T cells taken at different time points during the in vitro treatment with mRNA TAA-pulsed autologous DCs and assayed intracellular production of IFN-γ and TNF-α for antigen-specific CD4+ and CD8+ T cells. Antigen-specific CD4+ or CD8+ T cell responses were induced for most of the TAAs regardless of their expression levels in tumors (Table 3 and Fig. 2). Among the tested seven patients, antigen-specific CD4+ or CD8+ T cell responses were induced to all immunized TAAs in four patients (BT051 13 TAAs, BT077 9 TAAs, BT030 3 TAAs, BT057 3 TAAs). In the other three patients, patient BT001 had responses to 7 of 10 TAAs while patient BT079 had responses to 7 of 9 TAAs. Patient BT063 had antigen-specific T cell responses to 1 of 3 TAAs. Interestingly, Survivin, a commonly identified TAA, induced three different types of responses: antigen-specific CD8+ T cell response in patient BT051 (6.1-fold overexpression), antigen-specific CD4+ T cell response in patient BT057 (193.9-fold overexpression), and both CD4+ and CD8+ T cell response in patient BT077 (9.9-fold overexpression) (Table 3). Although overexpressed at similar levels, several TAAs induced antigen-specific responses in one patient but not the other patient: CD133 (BT030/Yes; BT063/No), and Sox11 (BT030/Yes; BT001/No). Furthermore, antigen-specific T cell responses were enhanced as the number of immunizations increased (Fig. 2B). Furthermore, there appeared to be some correlation between the percent of the immunized TAAs that induced antigen-specific T cell responses and clinical outcomes (Table S4). Together, these results demonstrated that personalized TAA immunization-induced antigen-specific T cell responses to a majority of the TAAs and T cells’ response to specific TAA varied among patients.
Fig. 2.
TAA-specific T cell responses from patients BT077 (NSCLC) and BT057 (GBM). PBMCs collected at the indicated time were stimulated with autologous DCs transfected with the corresponding TAA-mRNAs for 12 days and restimulated for 6 h. T cells were stained for intracellular expression of TNFα and IFNγ. Mock represents DCs without antigen transfection as background for T cell stimulation. a FACS profiles of antigen-specific CD8+ T cell responses after 2- or 4-doses of TAA-pulsed DC vaccination in patient BT077. b Kinetics of antigen-specific CD4+ and CD8+ T cell responses against TAAs after DC immunization in patient BT077. c FACS profiles of CD8+ T cell responses after the 3 immunizations of TAA-pulsed DC cells in patient BT057. d Kinetics of antigen-specific CD4+ and CD8+ T cell responses against TAAs after DC immunization in patient BT057. Mock represents mock-transfected DCs as a control. Two-way ANOVA with Bonferroni test: ***P < 0.001, **P < 0.01, *P < 0.05
Treatment responses
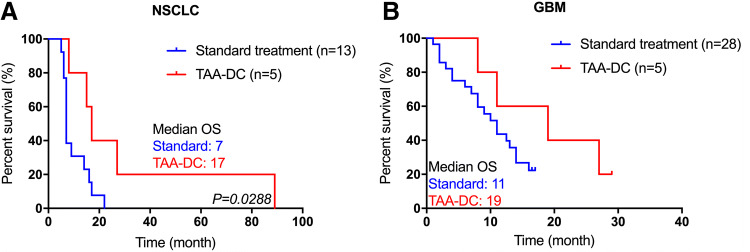
Immunization with overexpressed TAAs may induce autoimmune responses by cross-reactive T cells. All adverse events were Grade I/II consisting of local skin reactions and flu-like low fevers without treatment (Table 2). No Grade III/IV adverse events occurred during the course of the treatment. All five NSCLC patients assessed at 3 months after the initiation of immunotherapy demonstrated PR. Four of the five patients also received combination therapy with anti-PD-1. The overall survival time ranged from 8 to 89 months since the diagnosis of their diseases (Table 2). The median survival time was 17 months for the lung cancer patients and 19 months for the GBM patients (Fig. 3). Over the course of the trial, 13 lung cancer patients with brain metastasis and 28 patients with GBM received standard treatment (RT/chemo/targeted/surgery) by the same medical team (Table S1). In the NSCLC control group, anti-PD-1 mAb treatment was not used in their standard treatment because it was yet to be approved in the Chinese market. The median survival time for these two groups of patients was 7 and 11 months, respectively, (Fig. 3). Patients who received immunotherapy had favorable outcome compared to patients who received standard therapy at the same institution. These preliminary results need to be confirmed in large randomized trials.
Fig. 3.
Overall survival of patients in NSCLC with brain metastases and GBM. Kaplan–Meier curves showing survival for patients with TAA-mDC vaccination or standard treatment. a Lung cancer with brain metastases patients. b GBM patients. P value (P) was calculated using Cox regression analysis
Discussion
In this report, we conducted clinical trials to investigate antigen-specific T cell responses to DC vaccines transfected with personalized TAA panels in solid tumor patients. To prepare for the clinical trials, we analyzed some previously preserved tumor samples from NSCLC and GBM patients, identified overexpressed TAAs and manufactured GMP quality mRNAs encoding full-length TAAs. Although a few previous clinical trials using TAA-based cancer vaccination showed some clinical efficacies, a majority of the studies reported suboptimal clinical results [29, 30], suggesting the need to further develop and optimize TAA-based tumor vaccines.
Upon assaying TAA expression levels in tumor samples from patients, we used several criteria to select TAA for personalized immunization including a > twofold overexpression compared to para-tumor tissues, known capability to induce antigen-specific T cells in patients and relative low expression levels in normal tissues. To further enhance the possibility of antigen-specific T cell induction, we also incorporated low-dose cyclophosphamide treatment for regulatory T cell depletion [17, 18] and poly I:C for enhancing anti-tumor immunity [14, 19–21]. In addition, four lung cancer patients also received anti-PD-1 Nivolumab treatment. The patients received 3–13 different TAAs and 3–16 DC vaccine infusions. We made the following observations. (1) Antigen-specific T cell responses do not appear to be influenced by the expression levels of TAAs. The immunized TAAs had expression levels ranging from 400,099.7-fold to 3.1-fold overexpression. Nevertheless, anti-TAA-specific CD4+ and/or CD8+ T cells were induced in all patients and to most of the immunized TAAs. The immunization-induced enhanced anti-TAA-specific CD4+ and CD8+ T cell responses ranged from 0.1 to 10.0%. Also 0.1% appeared to be a small increase in percentage, in many cases it represented a change from a non-detectable change (0.0%) to in the induction of antigen-specific T cells. (2). A large TAA panel used in a single patient can still induce antigen-specific T cell responses to each of the TAAs. In the four patients (BT001, BT051, BT077, BT079) who received 9–13 different TAAs, immunization-induced antigen-specific T cell responses to most or all of the TAAs in the panel were detected, indicating these immunized TAAs did not interfere each other’s host responses. This highly efficient induction of antigen-specific T cell responses to most of the immunized TAAs is likely due to a combination of multiple immune activating agents: Treg depletion, polyI:C adjuvant activity and the use of anti-PD-1 in lung cancer patients.
Our preliminary data show a favorable objective response for the NSCLC patients and overall survival for both NSCLC and GBM patients when compared to those who received standard treatment by the same physician team. However, it should be considered that the sample size is too small to draw any firm conclusion. In addition, the favorable response in the NSCLC patients receiving TAA-mRNA DCs could be at least partially from anti-PD-1 antibody as the patients in the control group did not receive anti-PD-1. Therefore, large scale clinical trials are needed to validate this approach. Nevertheless, recent clinical studies suggest that combined use of TAA-based tumor vaccines with other treatment modalities produce impressive clinical results. The study by Hilf et al. using combined TAA- and neoantigen-based tumor vaccines in newly diagnosed GBM patients yielded a 29.0 months median overall survival in 15 treated patients [14]. In contrast, immunization with neoantigen alone in eight GBM patients produced a 16.8 months median overall survival [15]. Furthermore, autologous DCs pulsed with mRNA encoding full-length TAAs induce potent anti-tumor T cell responses and exhibit strong clinical therapeutic efficacies [8, 31, 32] and when combined with immune checkpoint blockade antibody anti-CTLA-4, TAA-mRNA pulsed DC vaccines demonstrate significant treatment efficacy in melanoma patients [8]. Thus, our preliminary clinical study suggests that personalized cellular vaccine using full-length TAAs can be combined with other strategies.
Conclusion
This study demonstrates that TAA expression pattern is highly heterogeneous among different patients and personalized TAA-specific autologous DC vaccination can be rapidly applied to patient treatment. Furthermore, TAA-specific T cell responses to most of the immunized antigens can be induced. Our results suggest that further development of personalized TAA tumor vaccine is warranted.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank David Boczkowski and Smita K. Nair at Duke University Medical Center for providing plasmids.
Abbreviations
- DCs
Dendritic cells
- GBM
Glioblastoma multiforme
- GMP
Good manufacturing practice
- iDC
Immature DC
- NSCLC
Non-small cell lung cancer
- ORR
Objective response rate
- OS
Overall survival
- PBMCs
Peripheral blood mononuclear cells
- PR
Partial response
- qPCR
Quantitative polymerase chain reaction
- SEM
Standard error of the mean
- TAAs
Tumor-associated antigens
- TME
Tumor microenvironment
Author contributions
HXL, JZ and YWH conceived, designed, or planned the study. QTW and SNS performed DC vaccine work and YN coordinated the trials. TL, RJH, YYF, and MZ carried patient treatment and care. JJ, ZL, JQL, YPX, XHY, HZ, BBZ, and SYL performed and/or directed some of the assays. QTW, SNS, JJ and YWH analyzed the data. QTW, SNS, JJ, JZ, YN, SYL and YWH interpreted the results. QTW and YWH drafted the manuscript with contributions from all authors. QTW, SNS, JJ and YWH critically reviewed or revised the manuscript. All authors reviewed the interim drafts and the final version of the manuscript and agreed with its content and submission. All authors had access to all the relevant study data and related analyses and vouch for the completeness and accuracy of the presented data. All authors agree to be accountable for all aspects of the work and will ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors read and approved the final manuscript.
Funding
The study was supported by Beijing Tricision Biotherapeutics Inc and Guangzhou Trinomab Inc. The project is also in part supported by the Guangdong Innovative and Entrepreneurial Research Team Program (No 2013Y113), Zhuhai Innovative and Entrepreneurial Research Team Program (No ZH01110405160015PWC), and National Basic Research Program of China (No 2015CB553706) to HX Liao at Jinan University. The funder provided support in the form of salaries for authors [S.-N. S., J.J., Y.-P. X., S.-Y. L.] and associated clinical trial costs, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Compliance with ethical standards
Conflict of interest
You-Wen He is a co-founder of Beijing Tricision Biotherapeutics. Huaxin Liao is a co-founder of Guangzhou Trinomab Inc. Shi-You Li is a co-founder of Beijing Tricision Biotherapeutics Inc. Sheng-Nan Sun and Jun Jiang are full-time employees of Beijing Tricision Biotherapeutics Inc. Yun-Peng Xiao is full-time employee of Guangzhou Trinomab Biotechnology Inc.
Ethical approval and ethical standards
Written informed consent was obtained from all patients before any of the study procedures were conducted. This study was approved by Jinan University Affiliated Guangdong 999 Brain Hospital Ethical Committee. All treatment procedures were performed in accordance with the ethical standards of the institutional and/or national research regulations and with the Helsinki declaration ethical standards. The study protocols are 1. “Personalized cellular vaccine for advanced solid tumors with brain metastases” (ID#:2015006). 2. “Personalized cellular vaccine for newly diagnosed glioblastoma multiforme” (ID#:2015001). 3. “Personalized cellular vaccine for recurrent glioblastoma multiforme” (ID#:2016007).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qian-Ting Wang, Ying Nie and Sheng-Nan Sun contributed equally to this study.
Contributor Information
Hua-Xin Liao, Email: larryhliao@163.com.
Jian Zhang, Email: tzhangjian@126.com.
You-Wen He, Email: youwen.he@duke.edu.
References
- 1.Saxena M, Bhardwaj N. Re-emergence of dendritic cell vaccines for cancer treatment. Trends Cancer. 2018;4:119–137. doi: 10.1016/j.trecan.2017.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santos PM, Butterfield LH. Dendritic cell-based cancer vaccines. J Immunol. 2018;200:443–449. doi: 10.4049/jimmunol.1701024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 4.Baek S, Kim CS, Kim SB, Kim YM, Kwon SW, Kim Y, Kim H, Lee H. Combination therapy of renal cell carcinoma or breast cancer patients with dendritic cell vaccine and IL-2: results from a phase I/II trial. J Transl Med. 2011;9:178. doi: 10.1186/1479-5876-9-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanyi JL, Bobisse S, Ophir E, et al. Personalized cancer vaccine effectively mobilizes antitumor T cell immunity in ovarian cancer. Sci Transl Med. 2018 doi: 10.1126/scitranslmed.aao5931. [DOI] [PubMed] [Google Scholar]
- 6.Liau LM, Ashkan K, Tran DD, et al. First results on survival from a large Phase 3 clinical trial of an autologous dendritic cell vaccine in newly diagnosed glioblastoma. J Transl Med. 2018;16:142. doi: 10.1186/s12967-018-1507-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heiser A, Coleman D, Dannull J, et al. Autologous dendritic cells transfected with prostate-specific antigen RNA stimulate CTL responses against metastatic prostate tumors. J Clin Invest. 2002;109:409–417. doi: 10.1172/JCI14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilgenhof S, Corthals J, Heirman C, van Baren N, Lucas S, Kvistborg P, Thielemans K, Neyns B. Phase II study of autologous monocyte-derived mRNA electroporated dendritic cells (TriMixDC-MEL) plus ipilimumab in patients with pretreated advanced melanoma. J Clin Oncol. 2016;34:1330–1338. doi: 10.1200/JCO.2015.63.4121. [DOI] [PubMed] [Google Scholar]
- 9.Wirth TC, Kuhnel F. Neoantigen targeting-dawn of a new era in cancer immunotherapy? Front Immunol. 2017;8:1848. doi: 10.3389/fimmu.2017.01848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vormehr M, Tureci O, Sahin U. Harnessing tumor mutations for truly individualized cancer vaccines. Annu Rev Med. 2019;70:395–407. doi: 10.1146/annurev-med-042617-101816. [DOI] [PubMed] [Google Scholar]
- 11.Carreno BM, Magrini V, Becker-Hapak M, et al. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science. 2015;348:803–808. doi: 10.1126/science.aaa3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sahin U, Derhovanessian E, Miller M, et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 2017;547:222–226. doi: 10.1038/nature23003. [DOI] [PubMed] [Google Scholar]
- 13.Ott PA, Hu Z, Keskin DB, et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017;547:217–221. doi: 10.1038/nature22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hilf N, Kuttruff-Coqui S, Frenzel K, et al. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature. 2019;565:240–245. doi: 10.1038/s41586-018-0810-y. [DOI] [PubMed] [Google Scholar]
- 15.Keskin DB, Anandappa AJ, Sun J, et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature. 2019;565:234–239. doi: 10.1038/s41586-018-0792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 17.Walter S, Weinschenk T, Stenzl A, et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med. 2012;18:1254–1261. doi: 10.1038/nm.2883. [DOI] [PubMed] [Google Scholar]
- 18.Emens LA, Asquith JM, Leatherman JM, et al. Timed sequential treatment with cyclophosphamide, doxorubicin, and an allogeneic granulocyte-macrophage colony-stimulating factor-secreting breast tumor vaccine: a chemotherapy dose-ranging factorial study of safety and immune activation. J Clin Oncol. 2009;27:5911–5918. doi: 10.1200/JCO.2009.23.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okada H, Kalinski P, Ueda R, et al. Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with {alpha}-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J Clin Oncol. 2011;29:330–336. doi: 10.1200/JCO.2010.30.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morse MA, Chapman R, Powderly J, et al. Phase I study utilizing a novel antigen-presenting cell-targeted vaccine with toll-like receptor stimulation to induce immunity to self-antigens in cancer patients. Clin Cancer Res. 2011;17:4844–4853. doi: 10.1158/1078-0432.CCR-11-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez-Ruiz ME, Perez-Gracia JL, Rodriguez I, et al. Combined immunotherapy encompassing intratumoral poly-ICLC, dendritic-cell vaccination and radiotherapy in advanced cancer patients. Ann Oncol. 2018;29:1312–1319. doi: 10.1093/annonc/mdy089. [DOI] [PubMed] [Google Scholar]
- 22.Nair S, McLaughlin C, Weizer A, Su Z, Boczkowski D, Dannull J, Vieweg J, Gilboa E. Injection of immature dendritic cells into adjuvant-treated skin obviates the need for ex vivo maturation. J Immunol. 2003;171:6275–6282. doi: 10.4049/jimmunol.171.11.6275. [DOI] [PubMed] [Google Scholar]
- 23.Prins RM, Craft N, Bruhn KW, Khan-Farooqi H, Koya RC, Stripecke R, Miller JF, Liau LM. The TLR-7 agonist, imiquimod, enhances dendritic cell survival and promotes tumor antigen-specific T cell priming: relation to central nervous system antitumor immunity. J Immunol. 2006;176:157–164. doi: 10.4049/jimmunol.176.1.157. [DOI] [PubMed] [Google Scholar]
- 24.Adams S, O'Neill DW, Nonaka D, et al. Immunization of malignant melanoma patients with full-length NY-ESO-1 protein using TLR7 agonist imiquimod as vaccine adjuvant. J Immunol. 2008;181:776–784. doi: 10.4049/jimmunol.181.1.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su Z, Vieweg J, Weizer AZ, et al. Enhanced induction of telomerase-specific CD4(+) T cells using dendritic cells transfected with RNA encoding a chimeric gene product. Cancer Res. 2002;62:5041–5048. [PubMed] [Google Scholar]
- 26.Johnson DE, O'Keefe RA, Grandis JR. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol. 2018;15:234–248. doi: 10.1038/nrclinonc.2018.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu CP, Fu SF, Chen X, Ye J, Ye Y, Kong LD, Zhu Z. The clinicopathological and prognostic significance of IDO1 expression in human solid tumors: evidence from a systematic review and meta-analysis. Cell Physiol Biochem. 2018;49:134–143. doi: 10.1159/000492849. [DOI] [PubMed] [Google Scholar]
- 28.Li J, Wang Q, Nie Y, et al. A multi-element expression score is a prognostic factor in glioblastoma multiforme. Cancer Manag Res. 2019;11:8977–8989. doi: 10.2147/CMAR.S228174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Butterfield LH. Lessons learned from cancer vaccine trials and target antigen choice. Cancer Immunol Immunother. 2016;65:805–812. doi: 10.1007/s00262-016-1801-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pilla L, Ferrone S, Maccalli C. Methods for improving the immunogenicity and efficacy of cancer vaccines. Expert Opin Biol Ther. 2018;18:765–784. doi: 10.1080/14712598.2018.1485649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Batich KA, Reap EA, Archer GE, et al. Long-term survival in glioblastoma with cytomegalovirus pp65-targeted vaccination. Clin Cancer Res. 2017;23:1898–1909. doi: 10.1158/1078-0432.CCR-16-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitchell DA, Batich KA, Gunn MD, et al. Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature. 2015;519:366–369. doi: 10.1038/nature14320. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.