Abstract
Background
Hepatitis C virus (HCV) interferes with activation of innate and adaptive immune responses. Theoretically, the efficacy and toxicity of immune checkpoint inhibitors in cancer patients infected with HCV may differ. Nevertheless, HCV was an exclusion criterion in most checkpoint inhibitor trials. We evaluated the efficacy and safety of nivolumab in metastatic renal cell carcinoma (mRCC) patients with or without chronic HCV infection.
Methods
In a matched cohort study, data were collected from 174 patients, retrospectively. All patients had clear-cell mRCC, chronic HCV infection (case study group), no evidence of other malignancy or cirrhosis, and had received nivolumab (3 mg/kg every 2 weeks) until disease progression or unacceptable toxicity. Quantitation of HCV RNA in plasma samples was performed before and during treatment with nivolumab with the automated HCV test (Hoffmann-La Roche, Switzerland). The primary endpoint was overall survival (OS). Secondary endpoints included progression-free survival (PFS), objective response rate (ORR), and rate of grade 3–4 adverse events in study and control cohorts.
Results
A total of 44 matched patients were included. Groups were well balanced. HCV-infected patients had significantly longer OS and PFS. Median OS was 27.5 (95% CI 25.3–29.7) and 21.7 (20.3–23.1) in study and control groups, respectively (P = 0.005). Median PFS was 7.5 (5.7–9.3) and 4.9 (4–5.8) (P = 0.013). Despite no differences in ORR between groups (27% vs. 23%, P = 0.7), patients with HCV had significantly more durable responses (P = 0.01). Nivolumab was well tolerated in all HCV-positive patients. No unexpected toxicity was observed. Assessment of viral load during nivolumab therapy was available in 14 of 22 (64%) patients with HCV. Nivolumab did not significantly impact HCV concentration (mean change 210 IU/ml, P = 0.82) in the absence of antiviral therapy.
Conclusions
The efficacy and safety profiles observed in this study support the administration of nivolumab in mRCC patients infected with HCV and warrant further investigation.
Keywords: Metastatic renal cell carcinoma, Chronic hepatitis C, Nivolumab
Introduction
Immune checkpoints are inhibitory molecules expressed on immune cells that activate immunosuppressive signaling pathways [1]. These molecules are crucial for maintaining self-tolerance and for modulating the length and magnitude of effector immune responses in peripheral tissues, in order to minimize collateral tissue damage [2]. In recent years, immune checkpoint inhibitors targeting programmed cell death receptor-1 (PD-1; nivolumab, pembrolizumab), programmed cell death-ligand 1 (PD-L1; atezolizumab, durvalumab, avelumab) or cytotoxic T-lymphocyte-associated protein 4 (CTLA-4; ipilimumab) have presented an alternative revolutionary therapeutic approach for patients with different types of cancer including renal cell carcinoma (RCC) [3].
Hepatitis C virus (HCV) interferes with the activation of innate and adaptive immune responses in patients with acute and chronic infections. The key players in immune responses induced by HCV are interferons I and III, interferon stimulated genes, NK cells, T cells, and antibody-type responses [4]. It is noteworthy, that patients with acute HCV infection always had unregulated interferon and interferon stimulated genes [5]. On the other hand, chronic HCV infection is correlated with focused CD4+ and CD8+ T cell responses [6].
Theoretically, the efficacy and toxicity of immune checkpoint inhibitors in cancer patients infected with HCV may differ. Nevertheless, HCV infection has been an exclusion criterion for most checkpoint inhibitor trials [7]. We evaluated the efficacy and safety of nivolumab, anti-PD1 checkpoint inhibitor in metastatic RCC patients with or without chronic HCV infection.
Materials and methods
Study design and patient selection
The current study had a retrospective matched cohort design based on a review of medical records of metastatic clear-cell RCC patients aged ≥ 18 years at the time of diagnosis with (study cohort) or without (control cohort) chronic HCV infection. Patients were selected to match the age, sex, International Metastatic RCC Database Consortium (IMDC) risk criteria, number of metastatic sites, number of treatment lines, and previous nephrectomy. All patients were treated with nivolumab (3 mg/kg, once every 2 weeks) in the second-, third- or fourth-line setting.
Oncologists treating RCC patients from six regions of the Russian Federation, Belarus, and Kazakhstan participated in this study. Relevant medical record data of eligible patients were retrospectively analyzed by the participating physicians using a protected, online-based data collection form. Patient data were depersonalized and anonymous. The study was conducted according to the Declaration of Helsinki. The study protocol was approved by the principal investigators and the Kidney Cancer Research Bureau independent ethics committee. All patients provided their written informed consent.
Clinical outcomes and statistical analysis
Variables of demographic and clinical features were described for each patient. The primary endpoint was overall survival (OS) in the study and control cohorts. Secondary endpoints included objective response rate, according to RECIST 1.1 criteria, evaluated by investigators, progression-free survival, and rate of 3–4 grade adverse events according to Common Terminology Criteria for Adverse Events (CTCAE) v4.03 criteria. Disease progression was evaluated by radiology and clinical investigation. Markers of progression also included therapy change and death. Shift to subsequent treatment was defined as a shift due to disease progression or toxicity. Quantitation of HCV RNA in plasma samples was performed at 2 weeks before and every 24 weeks during treatment with nivolumab. The plasma specimens were tested with the automated HCV test (Hoffmann-La Roche, Switzerland). Briefly, HCV RNA was derived from a 2 ml plasma sample in the COBAS AmpliPrep system as stated by the manufacturer’s instructions and transferred to the COBAS TaqMan 48 system for reverse transcription polymerase chain reaction. The linear range of the HCV concentration was 15–100,000,000 IU/ml.
Descriptive statistics (mean, median, and proportion) were used to summarize baseline patient characteristics and treatment features. Survival times were calculated from the date of therapy initiation to the date of death (OS). Survival was analyzed using the Kaplan–Meier method, with statistical significance of survival differences assessed using a non-parametric log-rank test. The statistical significance of descriptive differences in study variables and clinical outcomes between these groups was assessed using t tests and Chi-square tests, as appropriate, with corresponding P values reported. The multivariate regression analysis was conducted to evaluate correlation of HCV RNA concentrations and OS. All statistical analyses were carried out using IBM SPSS Statistics Base v22.0 (SPSS, Inc., Chicago, IL, USA).
Results
Patient characteristics
We screened 174 metastatic RCC patients treated with nivolumab. Forty-four (25%) patients were included in the study (n = 22) and control (n = 22) matched cohorts. Mean number of enrolled patients in one region was 7.3 (range, 1–9). Groups were well balanced (Table 1). Mean age at diagnosis of metastatic RCC was 62 and 63 years in the study and control cohorts, respectively. Seventy-seven percent of patients had favorable and intermediate risk according to the IMDC, which did not vary by line (second- or later-line) of therapy initiation. No significant differences in age (< 60 >), number of metastatic sites (1 vs. ≥ 2), and gender (male vs. female) between study and control cohorts were found (all P > 0.2). Among the 22 patients for whom HCV was documented, 1 (4.6%) patient had brain metastases. No patient in the control cohort had brain metastasis at or prior to the treatment initiation.
Table 1.
Patient and treatment characteristics
| HCV-infected patients (N = 22) | HCV-negative patients (N = 22) | |
|---|---|---|
| Age (years), mean (range) | 62 (48–74) | 63 (41–79) |
| Male, N (%) | 16 (73) | 16 (73) |
| IMDC poor risk factors, N (%) | ||
| 0 | 6 (27) | 6 (27) |
| 1–2 | 11 (50) | 11 (50) |
| ≥ 3 | 5 (23) | 5 (23) |
| Metastatic sites, N (%) | ||
| 1 | 4 (18) | 5 (23) |
| ≥ 2 | 18 (82) | 17 (77) |
| Number of treatment lines before nivolumab, N (%) | ||
| 1 | 8 (36) | 8 (36) |
| 2 | 10 (46) | 13 (59) |
| 3 | 4 (18) | 1 (5) |
| Previous nephrectomy, N (%) | 20 (91) | 20 (91) |
A majority of patients (> 80%) were recorded as having nephrectomy. More than half of patients received sunitinib in the first-line setting, and targeted therapy was the most common cancer-directed treatment modality observed before nivolumab initiation. Eight patients (36%) initiated nivolumab as a second-line therapy in both arms. There were no relevant differences in a number of treatment lines before nivolumab (< 2 >) between two cohorts (P > 0.7).
Disease progression following initial clinical response was the most common reason for nivolumab discontinuation (95.5%; 21 patients in each cohort). Treatment-related toxicities or side effects were cited as a reason for final nivolumab discontinuation in 4.5% of patients.
Assessment of viral load during nivolumab therapy was available in 14 of 22 (64%) patients with HCV. Median time of HCV quantitation from initiation of nivolumab therapy was 7.9 months (range, 3.5–18). Two (9%) HCV-positive patients received antiviral therapy (ledipasvir/sofosbuvir) at 4 weeks and 5 weeks after starting nivolumab treatment.
Efficacy and safety
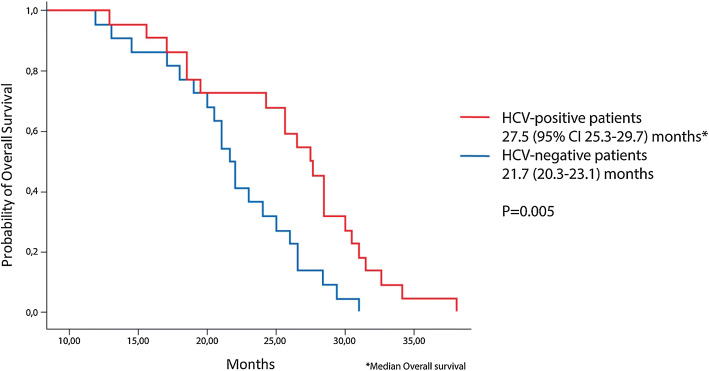
Median follow-up was 20.5 months (range, 15–26). At the time of the last follow-up, 26 of the 44 patients included in the analysis had died, while 15 were still alive. Median OS from the start of treatment was 27.5 months (95% CI, 25.3–29.7) in the study cohort and 21.7 months (95% CI, 20.3–23.1) in the control cohort (P = 0.005). Survival curves are shown in Fig. 1. OS time was similar in HCV-positive patients initiating nivolumab as a second- and later-line therapy (median, 28.2 and 25.7 months, P = 0.31). The 15-month OS rate was 94% and 86% in study and control cohorts, respectively. There were no significant differences in OS depending on HCV RNA concentrations. It can be assumed that this is due to initially high levels of viral load in all patients.
Fig. 1.
Kaplan–Meier curves for overall survival
Disease progression on nivolumab was documented in 5 of 44 (11%) enrolled patients. Despite the absence of significant differences in objective response rate between cohorts (27% vs. 23%, P = 0.7), patients with HCV had significantly more durable responses (P = 0.01). Three patients (50%) in the study cohort and 1 (5%) patient in the control cohort had an ongoing response for 10 months or longer. All responses were partial. The median progression-free survival was 7.5 months (95% CI, 5.7 to 9.3) and 4.9 months (95% CI, 4 to 5.8) in the study and control cohorts, correspondingly (P = 0.013). Grade 3–4 adverse events were observed in 5 (23%) HCV-infected patients and in 3 (14%) patients without HCV. No treatment-related deaths occurred. One or more dose interruptions due to toxic effects of nivolumab were observed in 5 (23%) patients infected with HCV. The most common any grade adverse events associated with nivolumab in these patients were fatigue (45%), rash (27%), and elevation of AST/ALT (23%). All 5 patients with elevation of AST/ALT had no changes in viral load. Exposure to nivolumab was not associated with increased risk for hepatocellular carcinoma and cirrhosis (N = 0).
Nivolumab did not significantly impact HCV RNA concentration (mean change was 210 IU/ml, P = 0.82) in patients that did not receive antiviral therapy. Patients treated with the combination of ledipasvir and sofosbuvir had an undetectable viral load for at least 6 months. A clinical or laboratory relevant drug–drug interactions were not detected.
Discussion
Treatment decisions in special populations are always challenging, first of all because of the lacking evidence of risk–benefit ratio in these patients from clinical data generated throughout the development of a new drug. Therefore, real-world approaches are usually used to support further investigation of novel treatment options in various patient groups not included in clinical trials.
According to the World Health Organization (WHO), in 2015, globally, an estimated 71 million people (1% of the population) were living with chronic HCV infection [8]. Approximately 2.4 million people (1% of all adults) in the USA are living with HCV infection [9]. In a recent SWOG multicenter prospective cohort study of over 3000 patients with newly diagnosed cancer, 2.4% had HCV infection, suggesting that the overall prevalence in patients with cancer is similar to that in the general population [10]. Although some studies suggest that the incidence of HCV infection decreased, since the number of new HCV infections (1.75 million) exceeds the number of those who die and those who are cured (1.24 million in total), the global prevalence may continue to grow [8]. It can be assumed that with an increase in the number of HCV infection cases in the world, the number of cancer patients with HCV infection will also increase. Thus, evidence-based recommendations for the treatment of this patient population are required.
Here, we report the results of a retrospective matched cohort study of efficacy and tolerability of nivolumab therapy in HCV-infected patients with metastatic RCC. These patients are usually excluded from clinical trials due to concerns primarily regarding safety of checkpoint inhibition in chronic viral infections with potentially increased rate of immune-related adverse events, or immune reconstitution inflammatory syndrome like phenomenon, leading to increased risk of serious infections.
Nivolumab is a PD-1 blocking antibody indicated for the treatment of patients with advanced RCC and other tumor types. Currently, limited evidence on the efficacy and safety of PD-1 inhibitors in patients with HCV is available, mostly from case reports [11, 12] and group analyses. As reported in a retrospective case series of seven patients with metastatic melanoma or non-small-cell lung cancer and concurrent chronic or past hepatitis B virus/HCV infection, the efficacy of anti-PD-1 therapy was similar to the published clinical trials data, the treatment was well tolerated [13]. In a phase I/II trial of nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040), treatment efficacy was comparable, and toxicity profiles were acceptable in both HCV-infected and non-infected patient groups [14]. Similar results were demonstrated in the KEYNOTE-240 phase III trial which evaluated pembrolizumab in previously treated patients with advanced hepatocellular carcinoma. In 64 HCV-infected patients out of a total of 413 patients enrolled in the study, the efficacy was comparable with uninfected population, with no reported cases of hepatitis C viral flares [15].
In cancer and in chronic infections, T cells are constantly exposed to antigen and inflammatory signals, which eventually lead to T cell exhaustion. Altered T cell function is associated with poor tumor or infection control, and by modulating certain pathways (e.g., targeting PD-1), this function and immune responses can be effectively restored. Moreover, epitope escape in the chronic phase of infection may be driven by exhausted T cells [16]. Although PD-1 blockade demonstrated HCV-specific T cells functionality restoration in vitro [17], it is not clear yet whether exhausted T cells can be reactivated by PD-1 blockade in patients with chronic HCV infection. In a randomized, double-blind, placebo-controlled assessment of a fully human monoclonal antibody to PD-1 in patients with chronic HCV infection, 11% of patients who received treatment and one placebo patient achieved the primary study endpoint of the HCV RNA reduction [18]. Interestingly, in a CheckMate 040 study, PD-1 blockade demonstrated limited antiviral activity in some HCV-infected patients with transient reductions in HCV RNA [14]. This limited evidence brings additional rationale to treat HCV-infected cancer patients with checkpoint inhibitors to unlock the immune potential for disease control.
Here, we demonstrated a beneficial efficacy of nivolumab in HCV-infected patients with metastatic RCC compared to non-infected matched patients. Due to similarities of immune alterations in cancer and chronic HCV infection, it may be a mutually beneficial therapeutic strategy for disease control. Survival benefit in HCV-infected patients may be due to synergistic therapeutic effects for both diseases, and/or additional immune potentiation in patients with chronic viral infection. More durable responses may also be associated with improved T cell memory function. These hypotheses need additional investigation in both non-clinical and clinical settings. In our study, no assessment of HCV infection was planned; therefore, we cannot evaluate the antiviral potential of the treatment. Nivolumab was well tolerated in all patients with chronic HCV infection with no unexpected toxicity observed. The increase in the level of ALT/AST in the blood was more likely associated with nivolumab treatment because these patients had a stable viral load. Our data support the conclusions of previous clinical studies and reports that nivolumab can be safely used in HCV-infected patients. However, these patients should be treated with caution, and further investigation is needed to evaluate treatment safety in special clinical situations, for example if immunosuppressive therapy is required for immune-related adverse events management.
A limitation of this study is in its retrospective design, as well as in a limited number of patients, and a lack of complete data on viral load changes during the treatment with nivolumab. Further randomized controlled trials would allow making more robust conclusions on potential benefits for HCV-infected patients with metastatic RCC treated with checkpoint inhibitors.
In conclusion, the efficacy and safety profiles observed in this study support the administration of nivolumab in metastatic RCC patients with chronic HCV infection and warrant further investigation.
Acknowledgements
We are thankful to Anton Barchuk, Ekaterina Kozlova, and Irina Chebykina for excellent research support and technical assistance.
Author contributions
Ilya Tsimafeyeu and Kristina Zakurdaeva contributed to conception and design. Ilya Tsimafeyeu, Rustem Gafanov, Svetlana Protsenko, Anna Semenova, Ani Oganesyan, Nurzhan Nurgaliyev, Sergei Krasny, Anastasia Bondarenko, and Sufia Safina were involved in provision of study materials or patients. Ilya Tsimafeyeu, Rustem Gafanov, Svetlana Protsenko, Anna Semenova, Ani Oganesyan, Nurzhan Nurgaliyev, Sergei Krasny, Anastasia Bondarenko, Sufia Safina, and Kristina Zakurdaeva contributed to collection and assembly of data. Ilya Tsimafeyeu, Anastasia Bondarenko, Sufia Safina, and Kristina Zakurdaeva contributed to data analysis and interpretation. Ilya Tsimafeyeu and Kristina Zakurdaeva were involved in manuscript writing. All authors final approved the manuscript. All authors contributed to accountable for all aspects of the work.
Compliance with ethical standards
Conflict of interest
We declare that the authors have no competing interests, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Baumeister SH, Freeman GJ, Dranoff G, Sharpe AH. Coinhibitory pathways in immunotherapy for cancer. Annu Rev Immunol. 2016;34:539–573. doi: 10.1146/annurev-immunol-032414-112049. [DOI] [PubMed] [Google Scholar]
- 2.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsimafeyeu I, Zolotareva T, Varlamov S, Zukov R, et al. Five-year survival of patients with metastatic renal cell carcinoma in the Russian Federation: results from the RENSUR5 registry. Clin Genitourin Cancer. 2017;15(6):e1069–e1072. doi: 10.1016/j.clgc.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 4.Wykes MN, Lewin SR. Immune checkpoint blockade in infectious diseases. Nat Rev Immunol. 2018;18(2):91–104. doi: 10.1038/nri.2017.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaplan DE. Immunopathogenesis of hepatitis C virus infection. Gastroenterol Clin. 2015;44:735–760. doi: 10.1016/j.gtc.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wedemeyer H, He XS, Nascimbeni M, Davis AR, et al. Impaired effector function of hepatitis C virus-specific CD8 + T cells in chronic hepatitis C virus infection. J Immunol. 2002;169(6):3447–3458. doi: 10.4049/jimmunol.169.6.3447. [DOI] [PubMed] [Google Scholar]
- 7.Johnson DB, Sullivan RJ, Menzies AM. Immune checkpoint inhibitors in challenging populations. Cancer. 2017;123(11):1904–1911. doi: 10.1002/cncr.30642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization . Global hepatitis report 2017. Geneva: World Health Organization; 2017. [Google Scholar]
- 9.Hofmeister MG, Rosenthal EM, Barker LK, et al. Estimating prevalence of hepatitis C virus infection in the United States, 2013–2016. Hepatology. 2019;69(3):1020–1031. doi: 10.1002/hep.30297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramsey SD, Unger JM, Baker LH, et al. Prevalence of hepatitis B virus, hepatitis C virus, and HIV infection among patients with newly diagnosed cancer from academic and community oncology practices. JAMA Oncol. 2019;5(4):497–505. doi: 10.1001/jamaoncol.2018.6437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tagliamento M, Grossi F, Paolino S, et al. Nivolumab treatment in advanced lung cancer patient with chronic active hepatitis C and systemic lupus erythematosus. Immunotherapy. 2019;11(10):873–879. doi: 10.2217/imt-2019-0025. [DOI] [PubMed] [Google Scholar]
- 12.Davar D, Wilson M, Pruckner C, et al. PD-1 blockade in advanced melanoma in patients with hepatitis C and/or HIV. Case Rep Oncol Med. 2015;2015:737389. doi: 10.1155/2015/737389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kothapalli A, Khattak MA. Safety and efficacy of anti-PD-1 therapy for metastatic melanoma and non-small-cell lung cancer in patients with viral hepatitis: a case series. Melanoma Res. 2018;28(2):155–158. doi: 10.1097/CMR.0000000000000434. [DOI] [PubMed] [Google Scholar]
- 14.Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finn RS, Ryoo B-Y, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38(3):193–202. doi: 10.1200/JCO.19.01307. [DOI] [PubMed] [Google Scholar]
- 16.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15(8):486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salem ML, El-Badawy A. Programmed death-1/programmed death-L1 signaling pathway and its blockade in hepatitis C virus immunotherapy. World J Hepatol. 2015;7:2449–2458. doi: 10.4254/wjh.v7.i23.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gardiner D, Lalezari J, Lawitz E, DiMicco M, Ghalib R, et al. A randomized, double-blind, placebo-controlled assessment of BMS-936558, a fully human monoclonal antibody to programmed death-1 (PD-1), in patients with chronic hepatitis C virus infection. PLoS One. 2013;8:e63818. doi: 10.1371/journal.pone.0063818. [DOI] [PMC free article] [PubMed] [Google Scholar]