Abstract
Little is known about the clinical significance of the peripheral blood CD4+ CD25+ FOXP3+ regulatory T cells (Tregs) and T helper-17 (Th17) cells in lymphoma patients. In this study, the prognostic and clinical significance of peripheral blood Tregs and Th17 cells were evaluated in lymphoma patients during different phases. The frequency of Tregs and Th17 lymphocytes was measured by flow cytometry method in 47 classical Hodgkin’s lymphoma (cHL) and 48 diffuse large B cell lymphoma (DLBCL) patients. Our results showed that the frequency of Tregs and absolute Treg count was significantly reduced in relapsed patients compared to patients at the remission phase, as well as with newly diagnosed untreated patients in both groups. Patients who reached complete remission had elevated frequency of CD4+ FOXP3+ lymphocytes, Tregs, absolute Treg count, Treg/CD4 and Treg/Th17 ratio in the cHL group and CD4+ CD25+ cells in DLBCL group. The frequency of Tregs, absolute Treg count and Treg/Th17 ratio in cHL patients and CD4+ FOXP3+ and CD4+ CD25+ cells in DLBCL patients positively associated with survival rate. Moreover, the percentage of Tregs and absolute Treg count positively correlated with white blood cell, platelet count and ESR level in cHL patients and with white blood cell count in DLBCL patients. The initial number of Tregs/Th17 cells and also the Treg/Th17 ratio was not associated with changes in disease-free survival (DFS) in both groups. Therefore, higher frequency of peripheral blood Tregs and Treg/Th17 ratio might be associated with a favorable outcome in lymphoma patients, better response to chemotherapy and lower rate of relapse.
Keywords: Regulatory T cells (Tregs), FOXP3, T helper 17 (Th-17), Lymphoma, Prognosis
Introduction
Lymphoma is a heterogeneous group of hematologic malignancies, originated from abnormality of the immune cells in lymphatic tissue, which is classified into two groups of Hodgkin’s lymphoma (HL) and non-Hodgkin’s lymphoma (NHL) with the most common subtypes, classical Hodgkin’s lymphoma (cHL) and diffuse large B cell lymphoma (DLBCL) [1, 2]. The role of immune system in lymphoma is indispensable and its dysfunction has shown to contribute to the pathogenesis and progression of the disease [3, 4].
CD4+ CD25+ FOXP3+ naturally occurring regulatory T cells (Tregs) are a subgroup of specialized CD4+ T cells accounting for 1–4% of circulating CD4+ lymphocyte in humans. They have a central role in the regulation of immune response by suppressing the activation of other T cell subsets, and thus maintaining immune system homeostasis [5]. Recent studies described that the number of peripheral or intratumoral Tregs inversely correlates with the clinical outcomes in epithelial carcinomas, such as hepatocellular carcinoma, ovarian and breast cancer [6–8]. However, this scenario is somehow different for hematologic malignancies like lymphoma in which the cells of the immune system are affected themselves and become malignant. In this respect, it has been shown that the role of Tregs in lymphoid malignancies seems to be context-dependent and controversial; hence, it might be associated with either poor or favorable prognosis, depending on the tumor type [5, 9–18].
The T helper 17 (Th17) cells are another group of CD4+ T cells characterized by the production of inflammatory cytokines IL-17A and IL-17F, and play a major role in triggering an inflammatory response [19]. The role of Th17 cells in the prognosis of solid tumors is controversial [20–23]. Also, few data are available about the association of the peripheral blood Th17 and prognosis of lymphoma patients [24, 25].
Therefore, in the present study, we analyzed the frequency of peripheral blood Tregs and Th17 cells in lymphoma patients including patients with cHL and NHL with DLBCL subtype at different phases of the disease to assess whether the change in the number of circulatory Tregs/Th17 was associated with prognosis and clinical outcome of these patients.
Patients and methods
Patient selection criteria
This study was performed on a total 95 lymphoma patients; 47 cHL patients including 10 newly diagnosed (ND), 24 in remission and 13 in relapsed phase of the disease and 48 NHL patients with DLBCL subtype containing 11 newly diagnosed (ND), 30 at remission and 7 patients in relapsed phase who had referred to our hematology and oncology center affiliated with Shiraz University of Medical Sciences during April 2018 to November 2019.
The diagnosis was confirmed by immunohistochemistry. At presentation, all staging workup including bone marrow aspiration and biopsy and also laboratory tests including complete blood count, hemoglobin level (Hb), liver function test, blood urea and creatinine, erythrocyte sedimentation rate (ESR) and lactate dehydrogenase (LDH) level were examined for all patients. Patients who had previously received chemotherapy and/or corticosteroids, also HIV positive patients and all patients with underlying comorbid diseases were excluded from the study. The staging was performed based on the Ann Arbor system.
Patients were treated according to the type of lymphoma, as newly diagnosed cHL patients received 6 cycles of ABVD regimen [doxorubicin 25 mg/m2 iv (days 1 and 15), bleomycin 10 IU/m2 iv (days 1 and 15), vinblastine 6 mg/m2 iv (days 1 and 15), dacarbazine 375 mg/m2 iv (days 1 and 15)] and 3 cycles of ESHAP salvage chemotherapy [etoposide 40 mg/m2 iv over 1 h (days 1–4), methylprednisolone 500 mg iv bolus over 15 min (days 1–5), cisplatin 25 mg/m2 iv continuous infusion over 24 h (Days 1–4) and high-dose cytarabine 2000 mg/m2 iv over 2 h (day 5) were used for relapsed patients. The treatment protocol for newly diagnosed DLBCL patients was six cycles of R-CHOP [rituximab 375 mg/m2 iv (Day 0), cyclophosphamide 750 mg/m2 iv (Day 1), hydroxydoxorubicin 50 mg/m2 iv (Day 1), vincristine 1.4 mg/m2 iv (max 2 mg/day) (Day 1), prednisolone 40 mg/m2 oral (Days 1–5)] and also R-ESHAP salvage chemotherapy [rituximab 375 mg/m2 iv (Day 0), etoposide 40 mg/m2 oral over 1 h (Days 1–4), methylprednisolone 500 mg iv over 15 min (Days 1–5), cisplatin 25 mg/m2 iv continuous infusion over 24 h (Days 1–4) and high-dose cytarabine 2000 mg/m2 iv over 2 h (day 5) for patients in relapsed or refractory disease phase. After three cycles and also at the end of the chemotherapy, patients were assessed for response to treatment. The International Prognostic Index (IPI) was determined for each patient based on the prognostic factors containing age, stage, extranodal involvement, LDH level and also ECOG performance status (PS).
Sample collection
Five-milliliter of peripheral blood was collected in Ethylenediaminetetraacetic acid (EDTA)-containing tubes from all patients. The peripheral blood mononuclear cells (PBMCs) were isolated, using Ficoll-hypaque (Inno-Train, Germany) density gradient centrifugation and cells were washed twice with phosphate-buffered saline (PBS) (1×) buffer prior to flow cytometry staining.
Immunofluorescence staining of Tregs
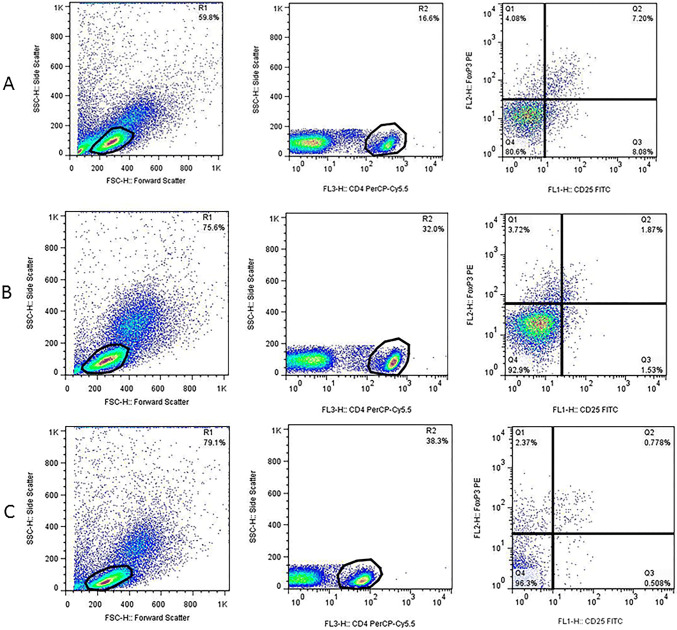
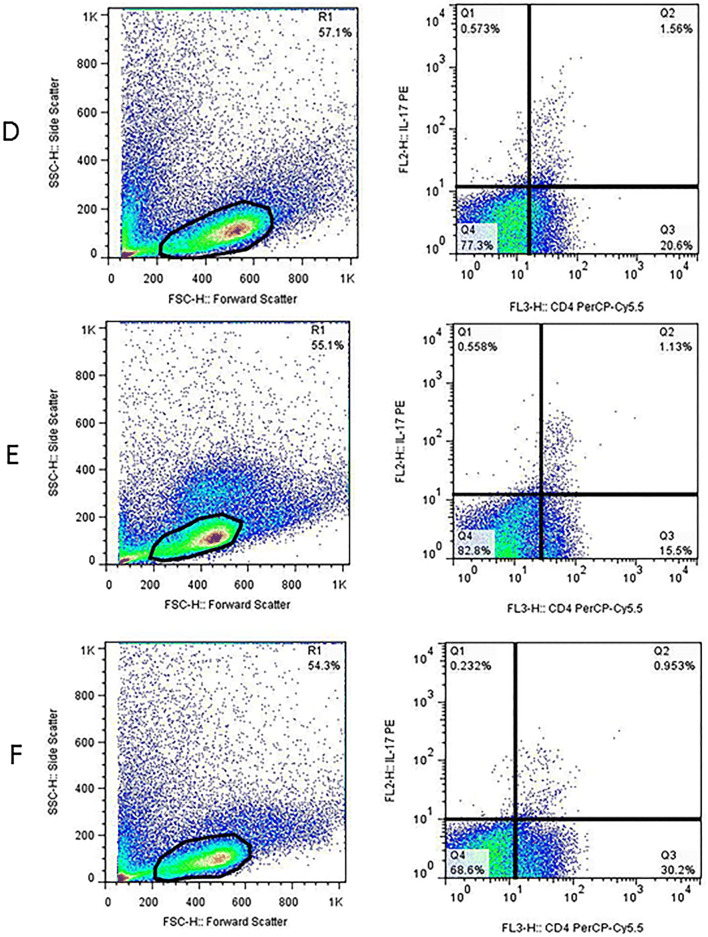
After washing, the cell pellets were resuspended in staining buffer (5% FBS in PBS 1×) and adjusted to 5 × 105 cells per 100 µl staining buffer. For Treg staining, the following procedure was used. Cells were first stained for surface markers, using PerCP-Cy™5.5 mouse anti-human CD4 (Clone SK3) (BD Biosciences, USA) and FITC mouse anti-human CD25 (Clone M-A251) (BD Biosciences, USA) antibodies and incubated at room temperature for 20 min in the dark. After washing, cells were fixed and permeabilized according to the manufacturer’s instruction, using human FOXP3 buffer set (BD Biosciences, USA) followed by cell washing. For intracellular staining of FOXP3 protein, cells were stained with PE mouse anti-human FOXP3 antibody (Clone 259D/C7) (BD Biosciences, USA) and incubated at room temperature for 20 min in the dark. After washing step, cell pellets were resuspended in 200–300 µl staining buffer and analyzed by FACSCalibure flow cytometer (BD Biosciences, USA) and FlowJo software. The lymphocyte population was first defined based on the Forward versus Side scatter. The percentage of Tregs was calculated by the selection of CD4+ T cells in the lymphocyte population followed by an enumeration of the CD25+ FOXP3+ cells within the CD4+ gate. Finally, the frequency of Tregs was compared between each patient subgroups (Fig. 1).
Fig. 1.
Flow cytometric analysis of Treg (a–c) and Th17 (d–f) frequency in lymphoma patients at different disease phases; For Treg quantification, CD4+ cells (R2) were first selected in lymphocyte population (R1) followed by gating of CD25+ FOXP3+ cells (Q2 quadrant) within CD4+ lymphocytes. For Th17 enumeration, the CD4+ IL-17+ cells (Q2 quadrant) were gated in lymphocyte population (R1). a/d, b/e and c/f are representative of a newly diagnosed (ND), patient at remission and a relapsed/refractory patient, respectively
Intracellular cytokine staining of Th17 cells
The frequency of Th17 cells was determined by intracellular staining for IL-17 cytokine. Briefly, 5 × 105 cells were resuspended in 1 ml RPMI1640 supplemented with 10% FBS, and stimulated with 50 ng/ml PMA (Phorbol 12-myristate 13-acetate) (Sigma, Germany) and 1 μg/ml Inomycin (Sigma, Germany) for 4 h in 5% CO2-containing incubator at 37 °C. The protein transport was blocked, using Monensin solution (BD Biosciences, USA). After that cells were collected, washed twice and dissolved in 100 µl staining buffer. To detect Th17 lymphocytes, cells were first stained for surface marker CD4, using PerCP-Cy™5.5 mouse anti-human CD4 (Clone SK3) (BD Biosciences, USA) antibody and incubate at 4 °C for 30 min in the dark, followed by fixation/permeabilization, using cytofix/cytoperm buffer (BD Biosciences, USA) according to the manufacture’s instruction. The presence of IL-17 cytokine was detected by incubation with PE mouse anti-human IL-17 cytokine (Clone N49-653) (BD Biosciences, USA) at 4 °C for 30 min in the dark. After washing the cells twice with perm/wash buffer, the cell pellet was dissolved in 200–300 µl staining buffer and analyzed by FACSCalibure flow cytometer (BD Biosciences), using FlowJo software. The percentage of Th17 was calculated by selecting CD4+ IL-17+ T cells within the lymphocyte population. Finally, the frequency of Th17 cells was compared between each patient groups (Fig. 1).
Statistical analysis
Data were analyzed, using SPSS (Statistical Package for the Social Sciences) version 22.0. Quantitative variables are described as mean ± standard deviation (SD) and median. Nonparametric Mann–Whitney U test was used to compare the two groups. For comparing more than two groups, Kruskal–Wallis test was used. Pearson’s correlation test was performed to evaluate the association of quantitative variables. The duration of survival was calculated from the date of entry into the study. The survival curve was plotted, using the Kaplan–Mayer method and log-rank test. p value < 0.05 was considered to be statistically significant.
Results
This study included 47 cHL patients with mean age 38.68 ± 13.08 range (23–83) years and 48 DLBCL with mean age 50.14 ± 14.9 range (23–82) years. The male/female ratio was 31/16 in cHL and 24/24 in DLBCL patients. The laboratory and clinical characteristics of patients are summarized in Table 1.
Table 1.
The laboratory and clinical characteristics of lymphoma patients
| Variable | cHL patients (n = 47) | NHL patients (n = 48) |
|---|---|---|
| Age (years) | ||
| Mean ± SD | 38.68 ± 13.08 | 50.14 ± 14.9 |
| Range (years) | 23–83 | 23–82 |
| Sex | ||
| Male (%) | 31 (65.9) | 24 (50) |
| Female (%) | 16 (34.1) | 24 (50) |
| Laboratory characteristics | Mean ± SD | Mean ± SD |
|---|---|---|
| WBC count (× 103/mL) | 6.36 ± 2.81 | 5.84 ± 2.14 |
| Platelet count (× 103/mL) | 228 ± 91.14 | 225 ± 74.35 |
| Lymphocyte (%) | 34 ± 12.25 | 36.41 ± 11.51 |
| Neutrophil (%) | 58.75 ± 12.25 | 66.52 ± 13.4 |
| Hb level (g/dL) | 12.85 ± 2.71 | 12.54 ± 2.13 |
| ESR (mm/h) | 27.89 ± 4.91 | 25.56 ± 4.71 |
| LDH (U/L) | 323.92 ± 127.06 | 317.5 ± 126.34 |
| BUN (mg/dL) | 14.53 ± 4.49 | 15.65 ± 5.86 |
| Creatinine (mg/dL) | 0.94 ± 0.17 | 0.9 ± 0.21 |
| Clinical parameters | Number (%) | |
|---|---|---|
| Disease status | ||
| New case | 10 (21.3) | 11 (22.9) |
| Remission | 24 (51.1) | 30 (62.5) |
| Relapse | 13 (27.6) | 7 (14.6) |
| B symptom | ||
| Yes | 28 (59.6) | 28 (58.3) |
| No | 19 (40.4) | 20 (41.7) |
| Nodal involvement | ||
| Nodal involvement | 42 (89.4) | 30 (62.5) |
| Extranodal involvement | 0 (0) | 5 (10.4) |
| Nodal + extranodal involvement | 5 (10.6) | 13 (27.1) |
| Stage | ||
| I–II | 21 (44.7) | 28 (58.3) |
| III–IV and othersa | 26 (55.3) | 20 (41.7) |
| Performance status (ECOG) | ||
| < 2 | 27 (57.4) | 20 (41.7) |
| ≥ 2 | 20 (42.6) | 28 (58.3) |
| IPI (score) | ||
| Low (0–1) | 20 (42.6) | 18 (37.5) |
| Intermediate (2, 3) | 23 (48.9) | 25 (52.1) |
| High (3, 4) | 4 (8.5) | 5 (10.4) |
| Disease outcome | ||||||
|---|---|---|---|---|---|---|
| Initial status of patients | CR | Relapse | Death | CR | Relapse | Death |
| New case | 10 | 0 | 0 | 11 | 0 | 0 |
| Remission | 21 | 2 | 1 | 26 | 3 | 1 |
| Relapse | 9 | 2 | 2 | 3 | 2 | 2 |
| Total | 40 | 4 | 3 | 40 | 5 | 3 |
WBC white blood cells, Hb hemoglobin, ESR erythrocyte sedimentation rate, LDH lactate dehydrogenase, BUN blood urea nitrogen, IPI score international prognostic index score, CR complete remission
aPatients with only extranodal involvement
Treg/Th17 frequency in cHL patients at different disease phase
The frequency of Tregs and Th17 lymphocytes was compared between cHL patients at different disease phases (Table 2) and the mean fluorescent intensity (MFI) of FOXP3 and IL-17 was considered as their mean expression level. Accordingly, our results showed that the frequency of CD25+, CD4+ CD25+, CD4+ FOXP3+ lymphocytes, FOXP3 MFI and Treg/CD4 ratio was significantly higher in ND cHL patients compared to patients at remission phase (*P < 0.05). Also, the proportion of Tregs and absolute Treg number was significantly higher in ND patients compared to patients at remission (median 3.08% vs. 0.78%, *P < 0.001 and 58.15% vs 17.16%, *P < 0.001, respectively).
Table 2.
Flow cytometry analysis of the cHL and NHL patients at different disease status
| cHL patients (n = 47) | NHL patients (n = 48) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable (% in lymphocyte gate) | ND (n = 10) |
Remission (n = 24) | Relapse (n = 13) | P1 | P2 | P3 | ND (n = 11) | Remission (n = 30) | Relapse (n = 7) | P1 | P2 | P3 |
| CD4+ cells |
34.15 (16.6–42.1) |
33.15 (8.72–56.7) |
26.5 (9.63–44.8) |
0.897 | 0.132 | 0.186 |
31.2 (22.7–47) |
26.9 (10.7–51.6) |
16.3 (2.82–30.8) |
0.424 | *0.015 | *0.035 |
| CD25+ cells |
3.94 (1.67–5.94) |
1.75 (1–2.93) |
1.81 (0.86–3.38) |
< 0.001* | 0.479 | < 0.001* |
2.27 (1–5.28) |
1.82 (1.16–3.86) |
1.72 (1.36–5.16) |
0.441 | 0.747 | 0.791 |
| FOXP3+ cells |
2.4 (1.46–5.24) |
1.98 (1.04–3.76) |
1.61 (1.11–2.41) |
0.101 | 0.011* | 0.003* |
2.63 (1.01–4.26) |
2.16 (0.96–4.47) |
1.86 (0.99–3.49) |
0.315 | 0.185 | 0.126 |
| FOXP3 MFI |
13.55 (2.38–28.9) |
5.07 (3.17–17.2) |
5.17 (3.13–28.8) |
0.004* | 0.387 | 0.186 |
5.25 (1.93–35.3) |
5.92 (2.35–17) |
5.6 (3.9–18) |
0.851 | 0.925 | 1 |
| CD4+ CD25+ cells |
2.75 (0.19–3.34) |
0.57 (0.16–1.31) |
0.29 (0.13–2.6) |
< 0.001* | 0.169 | < 0.001* |
1.41 (0.99–3.37) |
0.62 (0.18–2.62) |
0.25 (0.05–0.97) |
< 0.001* | 0.003* | < 0.001* |
| CD4+ FOXP3+ cells |
1.82 (0.63–4.35) |
0.86 (0.31–2.68) |
0.48 (0.24–1.5) |
0.009* | 0.036* | < 0.001* |
1.63 (0.58–3.31) |
1.23 (0.33–3.38) |
0.73 (0.25–1.04) |
0.204 | 0.04* | 0.015* |
| CD4+ CD25+ FOXP3+ Tregs (%) |
3.08 (1.87–7.43) |
0.78 (0.42–1.72) |
0.33 (0.04–1.33) |
< 0.001* | 0.007* | < 0.001* |
2.57 (1.55–3.27) |
1.11 (0.34–4.48) |
0.29 (0.15–0.95) |
< 0.001* | 0.001* | < 0.001* |
| Absolute Treg number (cells/µL) |
58.15 (32.22–191.79) |
17.16 (6.01–39.42) |
6.6 (0.55–41.55) |
< 0.001* | 0.035* | < 0.001* |
54.79 (27.9–91.8) |
18.01 (7.4–79.25) |
14 (2.03–24.82) |
< 0.001* | 0.159 | < 0.001* |
| Treg/CD4+ ratio |
0.087 (0.05–0.43) |
0.027 (0.01–0.17) |
0.018 (0.001–0.04) |
< 0.001* | 0.095 | < 0.001* |
0.079 (0.02–1.59) |
0.046 (0.01–0.2) |
0.02 (0.01–0.023) |
0.01* | 0.1 | 0.02* |
| IL-17+ cells |
1.86 (1.36–2.72) |
1.87 (0.66–2.7) |
1.74 (1.2–2.88) |
0.953 | 0.608 | 0.602 |
2.06 (0.86–3.36) |
1.76 (0.8–4.65) |
1.97 (0.57–3.1) |
0.359 | 0.686 | 0.904 |
| IL-17 MFI |
2.16 (1.74–2.53) |
1.83 (1.4–2.55) |
1.85 (1.39–2.97) |
0.032* | 0.814 | 0.06 |
2.28 (1.28–4.55) |
2 (1.46–2.99) |
2.24 (1.37–3.18) |
0.287 | 0.582 | 0.86 |
| Th17 lymphocyte |
1.1 (0.57–1.77) |
1.16 (0.35–1.97) |
1.08 (0.54–1.86) |
0.538 | 0.561 | 0.794 |
1.27 (0.71–1.55) |
1.15 (0.48–1.88) |
1.06 (0.12–1.47) |
0.739 | 0.293 | 0.246 |
| Treg/Th17 ratio |
4.88 (1.06–9.36) |
0.69 (0.31–2.7) |
0.46 (0.03–0.93) |
< 0.001* | 0.005* | < 0.001* |
1.9 (1.57–3.9) |
1.05 (0.22–5.6) |
0.51 (0.16–5.42) |
< 0.001* | 0.023* | 0.011* |
* indicates P < 0.05
Data are shown as median % (min–max); P1 = P value between ND and patients at remission phase, P2 = P value between patients at remission and relapsed phase, P3 = P value between ND and patients at relapsed phase
MFI mean fluorescent intensity
On the other hand, comparison of the patients at the remission phase and those with relapsed/refractory disease showed that the frequency of FOXP3+ cells and CD4+ FOXP3+ T cells was significantly higher in patients at remission phase compared to those with relapsed/refractory disease (*P < 0.05). It is worth mentioning that patients at remission had significantly more Tregs and absolute Treg number compared to those at the relapse phase (median 0.78% vs. 0.33%; *P = 0.007 and 17.16% vs. 6.6%; *P = 0.035, respectively). No significant difference was observed between patients at the remission phase and those with relapsed/refractory disease regarding CD25+, CD4+ CD25+, CD25+ FOXP3+ lymphocytes, FOXP3 MFI and Treg/CD4 ratio (P > 0.05).
In addition, the number of CD25+, FOXP3+, CD4+ CD25+, CD4+ FOXP3+ cells and Treg/CD4 ratio was significantly higher in ND patients compared to those with relapsed/refractory disease (*P < 0.05). Importantly, ND patients had a significantly higher proportion of Tregs and also absolute Treg count than those with relapsed/refractory form of the disease (median 3.08% vs. 0.33%; *P < 0.001 and 58.15% vs. 6.6%; *P < 0.001, respectively).
The percentage of IL-17 cells, MFI of IL-17 and frequency of Th17 cells were compared within different cHL groups. The results revealed that there was a significant increase in MFI of IL-17 in ND patients compared to patients at the remission phase (2.16% vs. 1.83%; *P = 0.032). The percentage of IL-17+ cells, frequency of Th17 lymphocytes and MFI of IL-17 was not different between ND patients compared to those at remission and also patients with relapsed/refractory disease (P > 0.05).
The Treg/Th17 ratio was significantly decreased in relapsed/refractory cHL patients compared to those at remission phase as well as ND patients (*P = 0.005 and *P < 0.001, respectively).
Treg/Th17 frequency in DLBCL patients at different disease phase
The proportion of Tregs was evaluated in DLBCL patients at different disease phases (Table 2). Our results showed that like cHL patients, ND DLBCL patients had a significantly elevated number of CD4+ CD25+ lymphocytes as well as Treg/CD4 ratio compared to patients at remission phase (*P < .0.05). The frequency of Tregs and absolute Treg number was also higher in ND DLBCL patients compared to patients at remission (median 2.57% vs. 1.11%; *P < 0.001 and 54.79% vs.18.01%; *P < 0.001, respectively).
On the other hand, DLBCL patients at remission state had significantly more percentage of CD4+, CD4+ CD25+ and CD4+ FOXP3+ lymphocytes compared to those with relapsed/refractory form of the disease (*P < 0.05). Similar to cHL patients, DLBCL patients at the remission phase had higher number of Tregs compared to those at the relapse phase (median 1.11% vs. 0.29%; *P < 0.001).
The number of CD4+, CD4+ CD25+, CD4+ FOXP3+ lymphocytes and Treg/CD4 ratio was significantly higher in ND DLBCL patients compared to patients with relapsed/refractory form of the disease (*P < 0.05). Additionally, the level of Tregs and absolute Treg count was significantly greater in ND DLBCL patients than those with relapsed/refractory form of the disease (median 2.57% vs. 0.29%; *P < 0.001 and 54.79% vs. 14%; *P < 0.001, respectively).
Flow cytometry analysis of the Th17 cells showed that like cHL patients, DLBCL patients with relapsed/refractory form of the disease had a lower Treg/Th17 ratio compared to those at remission and also ND ones (*P = 0.023 and *P = 0.011, respectively).
Study of Treg/Th17 frequency and clinical and laboratory features
The relationship between Treg and Th17 number and laboratory data including white blood cells (WBC) and platelet count, serum Hb, LDH and ESR level was evaluated. Also, the frequency of Treg and Th17 lymphocytes was assessed regarding the main clinical features like nodal/extranodal involvement, disease stage, B symptom, performance status, and IPI score.
In case of cHL patients, the analysis revealed a positive correlation between Treg number and WBC count (r = 0.343 and *P = 0.018), platelet count (r = 0.677 and *P < 0.001) and ESR level (r = 0.318 and *P = 0.029). In addition, the absolute number of Tregs positively correlated with WBC (r = 0.615 and *P < 0.001) and platelet count (r = 0.646 and *P < 0.001). A positive association was observed between the number of CD4+ CD25+ and CD4+ FOXP3+ T cells with WBC (r = 0.389;*P = 0.007 and r = 0.332; *P = 0.023, respectively) and platelet count (r = 0.699; *P < 0.001 and r = 0.528; *P < 0.001, respectively).
In case of DLBCL patients, no significant association was observed between MFI of FOXP3 and the number of CD4+ CD25+ and CD4+ FOXP3+ T cells and laboratory parameters (P > 0.05). Similar to cHL patients, a significant correlation was observed between Treg frequency and also absolute Treg number with WBC count (r = 0.324; *P = 0.024 and r = 0.518; *P < 0.001, respectively).
There was no significant correlation between IL-17 MFI and the percentage of Th17 lymphocytes and laboratory parameters, such as WBC and platelet count, serum Hb, LDH and ESR level in both groups of patients (P > 0.05).
Evaluating the clinical features of lymphoma patients showed that the distribution of Tregs and Th17 lymphocytes was not different regarding the main clinical characteristics of patients including B symptom, nodal/extranodal involvement and the disease stages in both cHL and DLBCL patients (P < 0.05).
However, the MFI of FOXP3 was significantly higher in cHL patients with nodal involvement than those with nodal + extranodal involvement (median 5.99% vs. 3.82%; *P = 0.02).
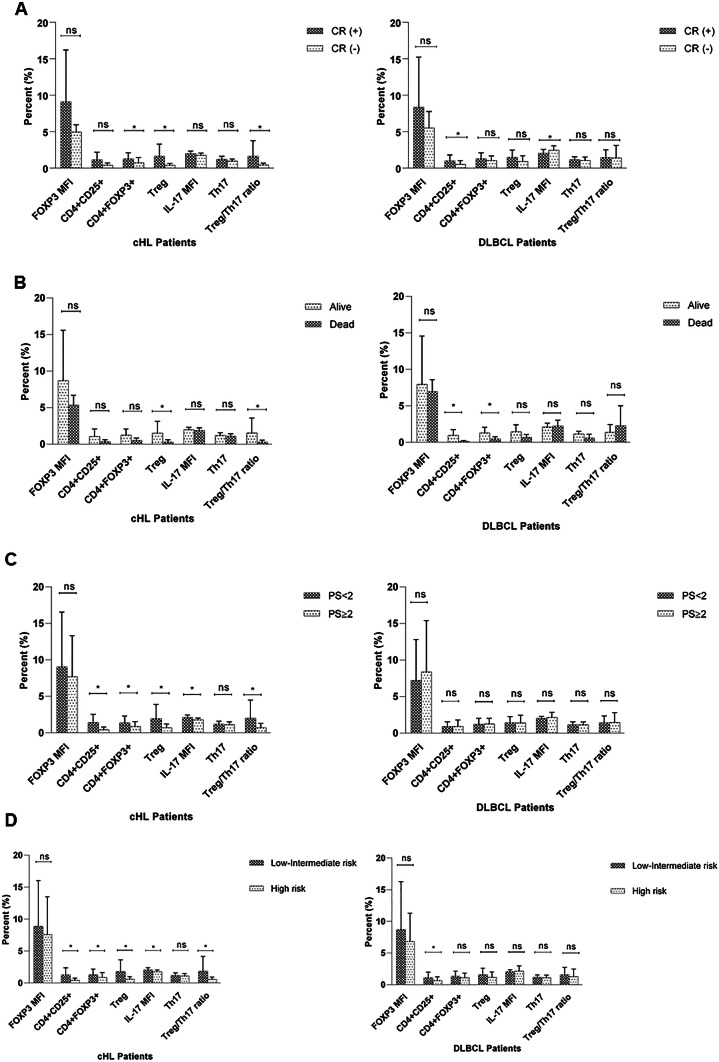
Patients were divided into two groups based on the performance status (ECOG) (< 2 and ≥ 2) and IPI score (low-intermediate and high risk) (Fig. 2). Accordingly, in case of cHL patients, the percentage of CD4+ CD25+, CD4+ FOXP3+, IL-17 MFI, Treg/Th17 ratio, as well as frequency of Treg lymphocytes and absolute Treg count was significantly higher in patients with PS < 2 compared to those with PS ≥ 2 (*P = 0.003, *P = 0.016, *P = 0.003, *P = 0.009, *P = 0.003 and *P = 0.003, respectively) (Fig. 2). cHL patients within low-intermediate risk group had significantly higher proportion of CD4+ CD25+, CD4+ FOXP3+, IL-17 MFI, Treg/Th17 and Treg/CD4 ratio as well as Treg lymphocytes and total Treg count compared to the high-risk group (*P = 0.016, *P = 0.035, *P = 0.029, *P = 0.007, *P = 0.034 *P = 0.004 and *P = 0.001, respectively) (Fig. 2). However, no significant change was observed about the frequency of these cells regarding performance status and IPI risk grouping in DLBCL patients (P > 0.05).
Fig. 2.
The relationship between frequency of Treg and Th17 lymphocytes and complete remission (CR) (a), survival (b), Performance status (PS) (c) and IPI score (d), respectively, in cHL and DLBCL patients. CR (+) represents patients who achieved complete remission and CR (−) represents patients who did not achieve complete remission, ns not significant and *P < 0.05
Association between initial Treg/Th17 number and response to therapy and survival rate
Patients were examined every 2–3 weeks during chemotherapy and also every 3 months for those patients with controlled disease, and then, they were followed up from 6 to 18 months. At the end of the study, 40 (85.1%) achieved complete remission (CR), and 7 (14.9%) did not, among them, 3 (6.4%) had expired in cHL group (Table 1). Whereas in DLBCL patients, 40 (83.3%) entered CR and 8 (16.7%) did not, among them, 3 (6.3%) had died (Table 1).
After that, the association between initial Treg and Th17 number with response to therapy and probability of disease-free survival (DFS) was assessed in both cHL and DLBCL patients. Evaluation of initial Treg and Th17 number in cHL patients with different response to chemotherapy showed that patients who achieved CR had significantly higher proportion of CD4+ FOXP3+, Tregs, absolute Treg number, Treg/Th17 and Treg/CD4 ratio than those who did not (median 0.98% vs. 0.4%; *P = 0.033, 1.13% vs. 0.3%; *P = 0.002, 24.69% vs. 6.01%; *P < 0.001, 0.77% vs. 0.52%; *P = 0.008 and 0.034% vs. 0.016%; *P = 0.015, respectively) (Fig. 2). In DLBCL patients, the frequency of CD4+ CD25+ T cells was higher in patients who had undergone CR than those who did not, which was near the significance level (median 0.76% vs. 0.33%; P = 0.05) (Fig. 2). Interestingly, the MFI of IL-17 was increased in DLBCL patients who did not achieve complete remission (median 2.01% vs. 2.55%, *P = 0.024, respectively).
Patients were also studied for the possible relationship between Treg and Th17 numbers and survival rate. The median percentage of Tregs, absolute Treg count and Treg/Th17 ratio was significantly higher in patients who were still alive than those who died (1% vs. 0.22%; *P = 0.025, 18.66% vs. 3.3%; *P = 0.006; 0.75% vs. 0.3%; *P = 0.023, respectively) in cHL patients. However, in DLBCL patients, those who were alive had significantly increased level of CD4+ CD25+ and CD4+ FOXP3+ lymphocytes than those who died (0.75% vs. 0.13%; *P = 0.001 and 1.2% vs. 0.38%; *P = 0.027, respectively).
The relationship between initial Treg and Th17 numbers and remission duration was investigated. For this purpose, patients were categorized into two groups based on the median value for Treg and Th17 (group 1 ≤ median and group 2 > median, median cut-off = 0.9 and 1.09 for cHL patients and median cut-off = 1.19 and 1.15 for DLBCL patients, respectively) and survival curve was plotted, using Kaplan-Mayer survival analysis. Our results showed that the initial number of Tregs, CD4+ CD25+, CD4+ FOXP3+, MFI of FOXP3, Th17 cells and also Treg/Th17 ratio was not associated with the change in DFS in both cHL and DLBCL patients (P > 0.05).
Discussion
The role of intratumoral Treg and Th17 cells in hematologic malignancies, such as lymphoma is up for debate [12, 13, 17, 18]. However, little data is available on the clinical significance of the peripheral blood Treg/Th17 number in lymphoma patients like HL and NHL.
In this study, we evaluated the prognostic significance of the peripheral blood Treg and Th17 lymphocytes in lymphoma patients including cHL and DLBCL patients at different disease phases and also their association with laboratory and clinical parameters. Our results showed that the level of Tregs and also absolute Treg number was significantly diminished in relapsed patients compared to newly diagnosed as well as patients at the remission phase in both cHL and DLBCL groups.
In a study by Gunduz et al. [26] on 21 ND cHL and 40 ND DLBCL patients, the frequency of peripheral blood Tregs was higher in patients than the control group, although the difference was not statistically significant. However, they were unable to evaluate the association of Tregs with the prognosis of cHL and NHL patients, since all of their patients achieved CR. On the other hand, Chang et al. [14] reported that higher frequency of peripheral blood Tregs (marked as CD4+ CD25+ cells) was associated with poor prognosis of patients with DLBCL (n = 77).
Data on the role of Th17 lymphocytes and the prognosis of lymphoma patients are limited. In a study by Lu et al. on 26 mixed B-NHL patients (including 17 ND and 6 relapsed DLBCL), 10 ND cHL and 31 healthy controls showed that the frequency of peripheral blood Th17 lymphocytes were significantly reduced in B-NHL patients compared to healthy individuals. Following one or two cycles of chemotherapy, peripheral blood Th17 lymphocytes was normalized, but it was elevated in relapsed patients in comparison to untreated ND patients or normal individuals [24]. No significant change was observed in the frequency of Th17 lymphocytes regarding the disease phase in cHL patients [24]. Conversely, Hus et al. reported on 29 ND DLBCL patients the opposite role of Treg and Th17 cells in B-NHL prognosis as patients with worse prognosis had a lower percentage of Th17 and increased Treg number compared to those with the controlled disease [25]. However, in our study, the level of Th17 lymphocytes did not significantly change between patients at different disease phases in both groups.
The logic for these discrepancies is unclear; however, it might be due to differences in ethnic groups, study population (a mixed or specific subtype of lymphoma), number of patients, and staining protocol used for Tregs and Th17 enumeration. It is also noteworthy that most of these studies had evaluated Treg and/or Th17 lymphocytes in untreated ND patients and/or patients at remission, but they did not evaluate patients at the relapse phase, which was done by our group. Another difference was that we did not use a control group in our study. However, to determine the normal range of Tregs and Th17 lymphocytes as well as to verify the accuracy of our staining protocol, the frequency of Tregs and Th17 lymphocytes was measured in six healthy individuals with no history of cancer or inflammatory diseases. Consequently, the median (range) of Treg and Th17 numbers among them was 1.81 (1.43–4.2) and 0.57 (0.48–1.26), respectively.
According to the results, it seems that in cHL and DLBCL patients, the number of Tregs was significantly higher at the beginning of the diagnosis, which was normalized following the first line of chemotherapy regiment, but decreased significantly in patients with relapse/refractory disease. Therefore, the high level of peripheral blood Tregs can be considered as a favorable prognostic factor for prediction of the disease outcome like response to treatment and relapse occurrence in cHL and DLBCL patients. However, this assumption has to be clarified in a larger population, especially those who have relapsed/refractory disease. In this regard, longer follow-up and assessment of Tregs in those patients who have relapsed during their follow-up can be highly informative. Moreover, a possible explanation for the significant reduction of Treg population in the peripheral blood of relapsed patients might be due to the fact that Tregs perhaps preferentially migrate, and thus accumulate highly in the tumor microenvironment of these patients. Consequently, the circulatory Treg level is decreased in these patients. This hypothesis has to be verified by exploring Tregs in the tumor milieu and assessment of its correlation with peripheral Treg count in lymphoma patients at different disease phases.
Analysis of Treg/Th17 frequency and laboratory and clinical parameters of lymphoma patients revealed that the level of Tregs and absolute Treg count positively correlated with WBC, platelet count and ESR level in cHL patients as well as with WBC count in DLBCL patients. Also, the percentage of Tregs and Treg/Th17 ratio positively associated with achieving complete remission (CR) and survival rate and inversely associated with PS and IPI score in cHL patients.
In line with our findings, Gunduz et al. showed that Treg level in the peripheral blood of ND cHL and DLBCL patients can be associated with poor prognostic factors in these patients since it positively associated with IPS, CRP, LDH and had a negative correlation with albumin, absolute lymphocyte count in cHL patients. In a study by Glowala-Kosinska et al. [16] in 27 ND DLBCL and 17 healthy controls, it was shown that the higher initial number of Tregs was positively associated with increased CR rate and the probability of event-free survival (EFS). Consistent with our results, in a previous study by Dehghani et al. [15] on 45 ND NHL patients (mixed population), a positive correlation was observed between the frequency of CD4+ CD25high Tregs and CR and patients’ survival. However, we did not observe any association between the initial number of Tregs/Th17 cells and also Treg/Th17 ratio and change in disease-free survival (DFS) in both cHL and DLBCL patients. Lack of this association is most probably due to the limited duration of our follow-up and failure to follow up all patients equally (patients did not enter the study at the same time).
The clinical significance of the Tregs in lymphoma patients still remain obscure. Owing to the critical role of Tregs in hematologic malignancies, Wang et al. [9] proposed four types of Tregs in malignant lymphoma patients. These four groups are as follows: (a) suppressor Tregs; (b) malignant Tregs; (c) direct tumor-killing Tregs; and (d) incompetent Tregs [9]. When Tregs acts as either suppressor or malignant Tregs, natural anti-tumor cytotoxicity of the immune system is suppressed; thus, reduced numbers of Tregs are associated with a favorable prognosis. Conversely, when Tregs serve as tumor-killing or incompetent Tregs, anti-tumor cytotoxicity of the immune system is enhanced; therefore, it can be assumed that increased numbers of Tregs are associated with a good prognosis. However, the underlying mechanism that determines the activation of suppressor/malignant or tumor-killing/incompetent Tregs in lymphoma patients is not clearly defined, yet. Most notably, it was shown that in normal conditions, activated Tregs have the capacity to suppress activation and differentiation of normal B cells [27]. Considering this, it is proposed that malignant B cells can be targeted in the same way by these Tregs [10]. Interestingly, Lindqvist et al. showed that both CD4+ FOXP3− and CD4+ FOXP3+ Tregs from patients with B cell malignancy express cytolytic markers like CD107a and FasL, which are capable of killing autologous leukemic B cells in vitro by a granzyme-mediated mechanism and the induction of apoptosis [10, 28]. In support of this hypothesis, Grygorowicz et al. [29] described that human regulatory T cells can suppress the proliferation of B lymphoma cells. Accordingly, it is possible that Tregs with cytotoxic function is directly involved in the pathogenesis of B cell lymphoma by regulating malignant B cell growth and survival; hence, their increase might be beneficial for lymphoma patients, which is different from what has been generally accepted for solid tumors. As to whether these cytotoxic Tregs can have the same function in B cell lymphoma, such as DLBCL and non-B cell lymphoma like cHL, has to be clarified, using in vitro and in vivo experiments.
Conclusion
To the best of our knowledge, this is a first report on Treg and Th17 cells in lymphoma patients at the different phases of the disease. According to the results, the higher frequency of peripheral blood Tregs, elevated level of absolute Treg count and Treg/Th17 ratio is associated with favorable prognosis, including better response to chemotherapy and improved survival in cHL patients as well as a lower rate of relapse in both cHL and DLBCL patients. Longer follow-up along with study of the larger population, especially ND and patients with relapsed/refractory disease is recommended. Also, the evaluation of the functional properties of Tregs in these patients can provide valuable data to explore the clinical significance of Tregs and Th17 cells in the outcome of lymphoma patients. Particularly, assessment of the intratumoral Tregs and its correlation with peripheral blood Tregs is also warranted.
Acknowledgement
The authors wish to thank Mr. H. Argasi at the Research Consultation Center (RCC) of Shiraz University of Medical Sciences for his invaluable assistance in editing this manuscript.
Author contributions
MD contributed to study design, analysis and interpretation of data and writing the paper, MK contributed to performing the research and critically revision of the ms, HG and MR contributed to analysis and interpretation of data, NA contributed to study design, analysis and interpretation of data, writing paper and performing the research.
Funding
This study was financially supported by a grant provided by Shiraz University of Medical Sciences (Grant number 95-01-32-12334).
Compliance with ethical standards
Conflict of interest
The authors declared that they have no conflict of interest.
Ethical approval
This study was performed according to the ethical standards of the local Ethics Committee of Shiraz University of Medical Sciences and in compliance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shankland KR, Armitage JO, Hancock BW. Non-hodgkin lymphoma. The Lancet. 2012;380:848–857. doi: 10.1016/S0140-6736(12)60605-9. [DOI] [PubMed] [Google Scholar]
- 2.Dehghani M, Haddadi S, Vojdani R. Signs, symptoms and complications of non-Hodgkin’s lymphoma according to grade and stage in South Iran. Asian Pac J Cancer Prev. 2015;16:3551–3557. doi: 10.7314/APJCP.2015.16.8.3551. [DOI] [PubMed] [Google Scholar]
- 3.Kumar D, Xu ML. Microenvironment cell contribution to lymphoma immunity. Front Oncol. 2018;8:288. doi: 10.3389/fonc.2018.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Charette M, Houot R. Hide or defend, the two strategies of lymphoma immune evasion: potential implications for immunotherapy. Haematologica. 2018;103:1256–1268. doi: 10.3324/haematol.2017.184192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D’Arena G, Vitale C, Coscia M, et al. Regulatory T cells and their prognostic relevance in hematologic malignancies. J Immunol Res. 2017;2017:1832968. doi: 10.1155/2017/1832968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kobayashi N, Hiraoka N, Yamagami W, Ojima H, Kanai Y, Kosuge T, Nakajima A, Hirohashi S. FOXP3+ regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin Cancer Res. 2007;13:902–911. doi: 10.1158/1078-0432.CCR-06-2363. [DOI] [PubMed] [Google Scholar]
- 7.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 8.Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, Harris AL, Banham AH. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006;24:5373–5380. doi: 10.1200/JCO.2006.05.9584. [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Ke X-Y. The four types of Tregs in malignant lymphomas. J Hematol Oncol. 2011;4:50. doi: 10.1186/1756-8722-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lindqvist CA, Loskog AS. T regulatory cells in B-cell malignancy–tumour support or kiss of death? Immunology. 2012;135:255–260. doi: 10.1111/j.1365-2567.2011.03539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koenecke C, Ukena SN, Ganser A, Franzke A. Regulatory T cells as therapeutic target in Hodgkin’s lymphoma. Expert Opin Ther Targets. 2008;12:769–782. doi: 10.1517/14728222.12.6.769. [DOI] [PubMed] [Google Scholar]
- 12.Carreras J, Lopez-Guillermo A, Fox BC, Colomo L, Martinez A, Roncador G, Montserrat E, Campo E, Banham AH. High numbers of tumor-infiltrating FOXP3-positive regulatory T cells are associated with improved overall survival in follicular lymphoma. Blood. 2006;108:2957–2964. doi: 10.1182/blood-2006-04-018218. [DOI] [PubMed] [Google Scholar]
- 13.Carreras J, Lopez-Guillermo A, Roncador G, et al. High numbers of tumor-infiltrating programmed cell death 1–positive regulatory lymphocytes are associated with improved overall survival in follicular lymphoma. J Clin Oncol. 2009;27:1470–1476. doi: 10.1200/JCO.2008.18.0513. [DOI] [PubMed] [Google Scholar]
- 14.Chang C, Wu S-Y, Kang Y-W, Lin K-P, Chen T-Y, Medeiros LJ, Chang K-C. High levels of regulatory T cells in blood are a poor prognostic factor in patients with diffuse large B-cell lymphoma. Am J Clin Pathol. 2015;144:935–944. doi: 10.1309/AJCPUJGMVV6ZF4GG. [DOI] [PubMed] [Google Scholar]
- 15.Dehghani M, Sharifpour S, Amirghofran Z, Zare HR. Prognostic significance of T cell subsets in peripheral blood of B cell non-Hodgkin’s lymphoma patients. Med Oncol. 2012;29:2364–2371. doi: 10.1007/s12032-012-0176-1. [DOI] [PubMed] [Google Scholar]
- 16.Głowala-Kosińska M, Chwieduk A, Nieckula J, Saduś-Wojciechowska M, Grosicki S, Rusin A, Nowara E, Giebel S. Association of circulating regulatory T cell number with the incidence and prognosis of diffuse large B-cell lymphoma. Eur J Haematol. 2013;91:122–128. doi: 10.1111/ejh.12144. [DOI] [PubMed] [Google Scholar]
- 17.Lee N-R, Song E-K, Jang KY, Choi HN, Moon WS, Kwon K, Lee J-H, Yim C-Y, Kwak J-Y. Prognostic impact of tumor infiltrating FOXP3 positive regulatory T cells in diffuse large B-cell lymphoma at diagnosis. Leuk Lymphoma. 2008;49:247–256. doi: 10.1080/10428190701824536. [DOI] [PubMed] [Google Scholar]
- 18.Tzankov A, Meier C, Hirschmann P, Went P, Pileri SA, Dirnhofer S. Correlation of high numbers of intratumoral FOXP3+ regulatory T cells with improved survival in germinal center-like diffuse large B-cell lymphoma, follicular lymphoma and classical Hodgkin’s lymphoma. Haematologica. 2008;93:193–200. doi: 10.3324/haematol.11702. [DOI] [PubMed] [Google Scholar]
- 19.Annunziato F, Cosmi L, Santarlasci V, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Ma D, Zhang Y, Tian Y, Wang X, Qiao Y, Cui B. The imbalance of Th17/Treg in patients with uterine cervical cancer. Clin Chim Acta. 2011;412:894–900. doi: 10.1016/j.cca.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 21.Kryczek I, Wei S, Zou L, Altuwaijri S, Szeliga W, Kolls J, Chang A, Zou W. Cutting edge: Th17 and regulatory T cell dynamics and the regulation by IL-2 in the tumor microenvironment. J Immunol. 2007;178:6730–6733. doi: 10.4049/jimmunol.178.11.6730. [DOI] [PubMed] [Google Scholar]
- 22.Kryczek I, Banerjee M, Cheng P, et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114:1141–1149. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sfanos KS, Bruno TC, Maris CH, Xu L, Thoburn CJ, DeMarzo AM, Meeker AK, Isaacs WB, Drake CG. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin Cancer Res. 2008;14:3254–3261. doi: 10.1158/1078-0432.CCR-07-5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu T, Yu S, Liu Y, Yin C, Ye J, Liu Z, Ma D, Ji C. Aberrant circulating Th17 cells in patients with B-cell Non-Hodgkin’s Lymphoma. PLoS ONE. 2016;11:e0148044. doi: 10.1371/journal.pone.0148044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hus I, Bojarska-Junak A, Kamińska M, Dobrzyńska-Rutkowska A, Szatan K, Szymczyk A, Kukiełka-Budny B, Szczepanek D, Roliński J. Imbalance in circulatory iNKT, Th17 and T regulatory cell frequencies in patients with B-cell non-Hodgkin’s lymphoma. Oncol Lett. 2017;14:7957–7964. doi: 10.3892/ol.2017.7232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gunduz E, Sermet S, Musmul A. Peripheral blood regulatory T cell levels are correlated with some poor prognostic markers in newly diagnosed lymphoma patients. Cytometry Part B Clin Cytom. 2016;90:449–454. doi: 10.1002/cyto.b.21330. [DOI] [PubMed] [Google Scholar]
- 27.Lim HW, Hillsamer P, Banham AH, Kim CH. Cutting edge: direct suppression of B cells by CD4+ CD25+ regulatory T cells. J Immunol. 2005;175:4180–4183. doi: 10.4049/jimmunol.175.7.4180. [DOI] [PubMed] [Google Scholar]
- 28.Lindqvist CA, Christiansson LH, Thörn I, et al. Both CD4+ FoxP3+ and CD4+ FoxP3− T cells from patients with B-cell malignancy express cytolytic markers and kill autologous leukaemic B cells in vitro. Immunology. 2011;133:296–306. doi: 10.1111/j.1365-2567.2011.03439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grygorowicz MA, Biernacka M, Bujko M, et al. Human regulatory T cells suppress proliferation of B lymphoma cells. Leuk Lymphoma. 2016;57:1903–1920. doi: 10.3109/10428194.2015.1121260. [DOI] [PubMed] [Google Scholar]