Abstract
Background and objective
Activation of the immune checkpoints and expression of chemokines and chemokine receptors have been reported to promote HCC progression. This study aimed to assess the differential expression of Tim-3, PD-1, and CCR5 on peripheral blood lymphocytes from patients with HCV-related HCC and correlate their expression with the treatment outcomes.
Patients and methods
The study incorporated 40 patients with chronic HCV-related HCC and 40 healthy controls. Patients were radiologically assessed for hepatic focal lesions and portal vein thrombosis. Response to HCC treatment and overall survival (OS) outcomes were determined. The expression of Tim-3, PD-1, and CCR5 among CD19+, CD4+, and CD8+ lymphocytes was assessed by flow cytometry.
Results
Higher frequencies of CD4+ and CD8+ cells expressing each of Tim-3 and PD-1 and PD-1+CD19+ cells were observed in the HCV-related HCC patients in comparison with controls. The highest expression of Tim-3 and PD-1 was by the CD8+ cells. Strong relations were detected among PD-1+CD19+, PD-1+CD4+ and PD-1+CD8+ cells. Elevated levels of PD-1+ lymphocytes were significantly associated with poor treatment response and shorter OS.
Conclusion
Modulation of the expression of immune checkpoints as Tim-3 and PD-1, and of CCR5 on T cells is somehow related to HCC. CD8+ T cells expressing PD-1 were the most relevant to HCC prognosis (OS and treatment response) and could represent a promising target for immune therapy against HCC. Future studies need to focus on exploring PD-1+ B cells and Tim-3+CD4+ cells, which seem to play a significant role in the pathogenesis of HCC.
Electronic supplementary material
The online version of this article (10.1007/s00262-019-02465-y) contains supplementary material, which is available to authorized users.
Keywords: HCC, HCV, Tim-3, PD-1, CCR5, Survival
Introduction
Hepatocellular carcinoma (HCC) is the most common of all primary liver cancers [2]. It is the sixth most prominent cancer globally and the third most common cause of cancer-related deaths. HCC is associated with poor prognosis, because it is usually multifocal at the time of discovery, with metastasis, a high recurrence rate, and few treatment options [3, 4]. The risk for its development is considerably greater in patients with hepatitis B virus (HBV) or hepatitis C virus (HCV)-related cirrhosis [5–7].
The coordinated immune responses of regulatory, helper, and cytotoxic T lymphocytes have a significant impact on the progression of chronic liver disease to HCC [8–10]. Cytotoxic T cells in particular have an essential role in getting rid of infected or malignant liver cells [11]. According to recent research, B cells have shown diverse roles in carcinogenesis and tumor progression [12, 13]. While some have shown that B cells have tumor-promoting functions [14–16], others concluded that B cells have antitumor protective features [17, 18].
Various immune responses, including alterations in immune cell functions, cytokine level, and the expression of immune receptors or ligands, have been reported to promote HCC progression [19–22]. Chemokines and their receptors are critically involved in the pathogenesis of HCC through enhancement of inflammation, angiogenesis, effects on immune cells, and direct effects on tumor invasion, growth and survival [23]. A close relation was observed between the CCL5–CCR5 (chemokine ligand 5—chemokine receptor 5) axis and liver chronic inflammation induced by different pathogens that ultimately led to the development of HCC [24].
Some tumors evade the immune response by expressing inhibitory ligands, which bind co-inhibitory receptors (immune checkpoints) expressed on tumor-specific immune cells and cause immune suppression [25]. PD-1 (programmed cell death protein-1) and Tim-3 (T-cell immunoglobulin and mucin-domain containing-3) are the most studied immune checkpoint receptors [26]. PD-L1 and PD-L2 are the two known ligands for PD-1. Activation of PD-1/PD-L1 signaling is crucial for peripheral T-cell tolerance through down-regulation of effector T-cell-mediated immune responses to guard against immune-mediated tissue damage [27]. Once engaged by its ligands, PD-1 reduces T-cell cytokine responses with substantial effects on IFN-γ, TNF-α, and IL-2 production [27, 28]. PD-1 signaling also affects T-cell differentiation and survival directly by inhibiting the early activation events [29]. It inhibits T-cell activation kinases through SHP2 phosphatase activity and other signaling pathways [30].
Engagement of TIM-3 and PD-1 molecules is associated with exhausted T cells with reduced numbers, functions, and subsequently suppressed immune responses to tumors [25, 31] and chronic viral infections [32]. Their expression by T cells has prognostic implication for a diversity of tumors making them potential targets for cancer immunotherapy [25, 33]. Previous studies have reported higher expression levels of both PD-1 and Tim-3 by CD4+ and CD8+ T cells in patients with HBV and HCV infection [34–36] and HCC patients [37–39] compared with healthy subjects.
Understanding the complexity of interactions between the different immune cells expressing these immune checkpoints, chemokines, and chemokine receptors is of utmost importance in tailoring newer immune-therapeutics against HCC. Thereby, this study aimed to assess the differential expression of Tim-3, PD-1, and CCR5 by peripheral blood T and B lymphocytes from HCV patients with HCC and correlate their expression with the treatment response and survival outcomes in these patients.
Materials and methods
Forty HCV-related HCC patients and 40 age- and sex-matched healthy controls were incorporated in this case–control study. Patients were recruited from AL-Rajhi Liver Center, South Egypt Cancer Institute, Assiut University Hospital. We excluded from the study patients concurrently infected with HIV, hepatitis A, B, D viruses, or schistosoma. Patients having alcoholic or drug-induced liver disease or treated with immune-modulating drugs were also excluded from this study.
Diagnosis of HCV infection and liver cirrhosis was based on the clinical, biochemical, and ultrasonographic findings. The Child–Pugh score was employed to estimate the severity of cirrhosis; it is also considered a useful marker for global liver function, primarily for the selection of HCC patients for different lines of treatment [40]. Besides, Access 2 (Beckman Coulter, USA) was used to evaluate serum levels of alpha-fetoprotein (AFP).
All patients were radiologically evaluated via triphasic computed tomography (CT) with contrast and magnetic resonance imaging (MRI). HCC diagnosis was based on LI-RADS and AASLD criteria that include early arterial hyperenhancement and delayed venous phase washout appearance and capsule appearance. The number of hepatic focal lesions, portal vein thrombosis, and liver volume, including the Future Liver Remnant (FLR), were also evaluated. The likelihood that the disease is confined to the liver, the size/location of the tumor and the patient’s hepatic function were assessed to determine whether the patient's condition permit resection or not.
Patients with early-stage disease (solitary HCC nodule or up to 3 nodules ≤ 3 cm, Child–Pugh A or B) were identified and evaluated for curative-intent therapies such as resection and radiofrequency ablation (RFA), percutaneous ethanol injection, and trans-arterial chemo-embolization (TACE) by a multidisciplinary team. Systemic treatment was a palliative-intent therapy given for patients with sufficient hepatic reserve and performance status (PS) but no longer eligible for surgical or liver-directed therapies and included:
1- Sorafenib: The first-line standard of care based upon the 3-month median survival benefit demonstrated in the SHARP trial that was used in patients with Child–Pugh A hepatic reserve, with Eastern Cooperative Oncology Group Performance Status (ECOG PS) grades 0–2.
2- Capecitabine (Xeloda) given mainly on a metronomic base that meant to give Xeloda one tablet, 1–3 times daily, continuously without drug-free period according to the tolerance of the patient.
3- Supportive treatments (liver support drugs, platelet transfusion, diuretics, etc.).
All patients were regularly followed-up after treatment and every 3 months with clinical examination, liver function tests, AFP, and triphasic CT abdomen to evaluate their responses, manage toxicities developed, and to determine the overall survival. Our patients were followed up for a range of 2–30 months with a mean follow-up period of 10.7 ± 6.2 months. Clinical and laboratory features of the study subjects are shown in Table 1.
Table 1.
Clinical and laboratory characteristics of the study subjects
| Characteristics | HCV–HCC patients (n = 40) |
Controls (n = 40) |
|---|---|---|
| Age (years) | 63.7 ± 1 | 58 ± 2 |
| Sex | ||
| Males | 36 (90%) | 34 (85%) |
| Females | 4 (10%) | 6 (15%) |
| ALT (U/L) | 45.5 ± 7 | 10.3 ± 0.3 |
| AST (U/L) | 94 ± 14 | 11 ± 0.5 |
| Albumin (gm/L) | 30.7 ± 1 | 40.3 ± 0.1 |
| Total protein (gm/L) | 69.9 ± 2 | 70 ± 3 |
| Total bilirubin (mg/dL) | 23.5 ± 2 | 0.8 ± 0.04 |
| Prothrombin time (seconds) | 13.9 ± 0.3 | 12 ± 0.3 |
| INR | 1.2 ± 0.03 | 1 ± 0.01 |
| Hemoglobin (g/dl) | 10.9 ± 0.4 | 13.2 ± 0.3 |
| WBCs (109/L) | 7.2 ± 0.5 | 8.2 ± 0.3 |
| Platelet count | 162.2 ± 14 | 254.5 ± 11 |
| Liver cirrhosis | 40 (100%) | – |
| Child–Pugh score | 7.1 ± 0.3 | – |
| Ascites | ||
| No | 23 (57.5%) | – |
| Mild | 3 (7.5%) | |
| Moderate | 14 (35%) | |
| Hepatic encephalopathy | 8 (20%) | – |
| Splenomegaly | 20 (50%) | – |
| HFL | ||
| 1 | 14 (35%) | – |
| 2 | 8 (20%) | |
| 3 | 18 (45%) | |
| Portal vein thrombosis | 31 (77.5%) | – |
| Alpha-fetoprotein (ng/ml) | 12,661.8 ± 11,036.6 | – |
| Patients on Daclatasvir /sofosbuvir treatment regimen | 9 (22.5%) | – |
| Deaths | 6 (15%) | – |
HCV–HCC hepatitis C related hepatocellular carcinoma, ALT alanine aminotransferase, AST aspartate aminotransferase, INR international normalized ratio, MCV mean corpuscular volume, MCHC mean corpuscular hemoglobin concentration, WBCs white blood cells, HFL hepatic focal lesions
Data expressed as mean ± SE or number (percentage), accordingly
Flow cytometric detection of lymphocytes and their expression of TIM-3, PD-1, and CCR5
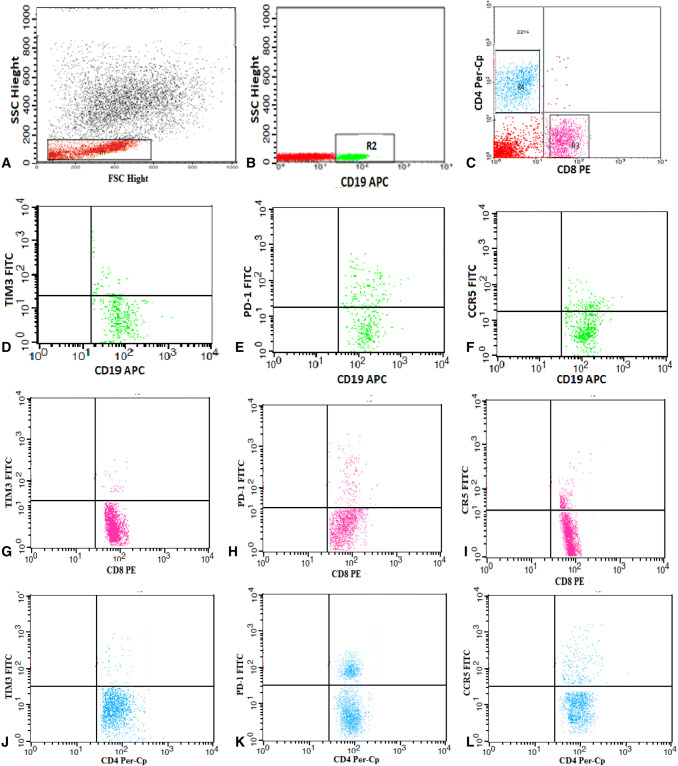
Peripheral blood mononuclear cells (PBMCs) were isolated from peripheral blood using Ficoll density gradient centrifugation (Biochrom GmbH, Germany). About 2 × 106 cells were stained with 5 µl fluoroisothiocyanate (FITC) conjugated Tim-3, phycoerythrin (PE) conjugated CD8, peridinium-chlorophyll-protein (Per-CP)-conjugated CD4 and allophycocyanin (APC) conjugated CD19 in one tube. Also, the second tube included 5 µl FITC-conjugated PD-1, PE-conjugated CD8, Per-CP-conjugated CD4, and APC-conjugated CD19. And, the third tube included 5 µl FITC-conjugated CCR5, PE-conjugated CD8, Per-CP-conjugated CD4, and APC-conjugated CD19. All monoclonal antibodies were purchased from Becton Dickinson (BD Bioscience, CA, USA) except Tim-3 and PD-1 from BioLegend (GmbH, Germany). Incubation for 20 min at 4 °C was performed in the dark, and then washing with phosphate buffer saline (PBS) was done. An isotype-matched anti-human IgG negative control was used with each sample. The analysis was accomplished by FACSCalibur flow cytometry with Cell Quest software (BD Biosciences, USA). The lymphocyte population was defined according to their forward and side scatters. The region (R1) was drawn to select the lymphocyte population to assess the expression of CD19, CD8, and CD4. The CD19+, CD8+, and CD4+ lymphocyte subsets were then gated by drawing regions (R2, R3, and R4, respectively). The expression of Tim-3, PD-1, and CCR5 was assessed among the three lymphocyte subsets and was reported as percentages in each cell population (Fig. 1).
Fig. 1.
Flow cytometric detection of lymphocytes and their expression of TIM-3, PD-1, and CCR5. a Forward and side scatter histogram was used to define the lymphocyte population. b, c The expression of CD19, CD8 and CD4 was assessed on the lymphocytes, and then, the CD19+, CD8+, and CD4+ lymphocyte subsets were gated for further analysis of TIM-3, PD-1, and CCR5. d–f The expression of Tim-3, PD-1, and CCR5 on CD19. g–i The expression of Tim-3, PD-1, and CCR5 on CD8. j–l The expression of Tim-3, PD-1, and CCR5 on CD4
Statistics
Descriptive data in this study were in the form of mean, median, range, standard error, and percentages. The Mann–Whitney U test and independent t test were used to compare two groups, while the one-way ANOVA test to compare more than two groups of quantitative variables. A p value < 0.05 was considered significant. The Pearson correlation was employed to assess the relations of immune checkpoints and chemokine receptors with the different treatment outcomes. The overall survival (OS) was defined as the interval from the diagnosis to the date of death, or the last follow up and was estimated by the Kaplan–Meier estimator. All of our results were analyzed using SPSS ver. 24.
Results
Comparison of the mean percentages of CD19+, CD4+, and CD8+ cells expressing Tim-3, PD-1, and CCR5 among groups
The mean percentages of CD4+ and CD8+cells expressing CCR5 were significantly lower in patients compared with the healthy control group (p < 0.0001). On the contrary, a noticeable higher frequency of CD4+ and CD8+ cells expressing each of Tim-3 and PD-1 and also of PD-1+CD19+ cells was observed in patients in comparison with controls (p < 0.0001). The results are summarized in Table 2.
Table 2.
Mean percentages of CD19+, CD4+ andCD8+ cells expressing Tim-3, PD-1, and CCR5 among groups
| Cells | HCV–HCC patients (n = 40) |
Controls (n = 40) |
p value |
|---|---|---|---|
| Tim-3+ CD19+ (%) | 1.3 ± 0.01 | 1.2 ± 0.01 | 0.2 |
| Tim-3+ CD4+ (%) | 8.9 ± 0.5 | 4 ± 0.3 | < 0.0001 |
| Tim-3+ CD8+ (%) | 10.4 ± 1 | 5.5 ± 0.2 | < 0.0001 |
| PD-1+CD19+ (%) | 23 ± 0.5 | 17.2 ± 0.5 | < 0.0001 |
| PD-1+CD4+ (%) | 20.3 ± 0.4 | 15.2 ± 0.4 | < 0.0001 |
| PD-1+CD8+ (%) | 30.9 ± 1 | 21.1 ± 0.4 | < 0.0001 |
| CCR5+CD19+ (%) | 10.6 ± 0.4 | 11.5 ± 0.5 | 0.1 |
| CCR5+CD4+ (%) | 10.6 ± 0.6 | 15.1 ± 0.9 | < 0.0001 |
| CCR5+CD8+ (%) | 20 ± 0.3 | 22.5 ± 0.5 | < 0.0001 |
n number, HCV–HCC hepatitis C related hepatocellular carcinoma
Results expressed as mean percentages ± SE
Independent T Test, bold p values indicate significance (< 0.05)
Differences in the expression of Tim-3, PD-1, and CCR5 between the CD19+, CD4+, and CD8+ cells in the study patients
Differences in the expression of Tim-3, PD-1, and CCR5 between the different lymphocytes subsets are illustrated in “Supplementary Fig. 1”. The highest expression of Tim-3, PD-1, and CCR5 was detected among the CD8+ cells compared with CD4+ T cells and B cells (p < 0.0001). The expression of CCR5 was more or less equal by both CD19+ and CD4+ cells (p value = 0.96). The expression of Tim-3 by both CD8+and CD4+cells was markedly higher than that by CD19+cells (p < 0.0001). PD-1 expression by CD8+cells was higher than that by CD19+ cells followed by CD4+cells (p < 0.0001).
Correlations among CD19+, CD4+, and CD8+ cells expressing Tim-3, PD-1 and CCR5, and their relations with the other laboratory parameters
Several relations were observed among the studied cells, as shown in Table 3. A perfect correlation was found between CD19+ and CD4+ cells expressing PD-1. Moreover, CD19+ and CD4+ cells expressing PD-1 showed moderate positive correlations with Tim-3+CD4+ and PD-1+CD8+ cells and a moderate negative correlation with CCR5+CD4+ cells. A moderate positive correlation was also observed between CD4+ and CD8+ cells expressing Tim-3.
Table 3.
Correlations among CD19+, CD4+ and CD8+ cells expressing Tim-3, PD-1, and CCR5
| Cells | Tim-3+ CD19+ (%) | Tim-3+ CD4+ (%) | Tim-3+ CD8+ (%) | PD-1+CD19+ (%) | PD-1+CD4+ (%) | PD-1+CD8+ (%) | CCR5+CD19+ (%) | CCR5+CD4+ (%) | CCR5+CD8+ (%) |
|---|---|---|---|---|---|---|---|---|---|
| Tim-3+ CD19+ (%) | – |
r = 0.17 p value = 0.06 |
r = 0.06 p value = 0.3 |
r = 0.2 p value = 0.03 |
r = 0.2 p value = 0.03 |
r = − 0.02 p value = 0.4 |
r = − 0.1 p value = 0.2 |
r = − 0.2 p value = 0.01 |
r = 0.2 p value = 0.04 |
| Tim-3+ CD4+ (%) |
r = 0.17 p value = 0.06 |
– |
r = 0.7 p value = < 0.0001 |
r = 0.6 p value < 0.0001 |
r = 0.6 p value < 0.0001 |
r = 0.4 p value < 0.0001 |
r = − 0.04 p value = 0.4 |
r = − 0.2 p value = 0.02 |
r = − 0.4 p value < 0.0001 |
| Tim-3+ CD8+ (%) |
r = 0.06 p value = 0.3 |
r = 0.7 p < 0.0001 |
– |
r = 0.3 p value = 0.004 |
r = 0.3 p value = 0.004 |
r = 0.3 p value = 0.01 |
r = 0.17 p value = 0.07 |
r = − 0.1 p value 0.2 |
r = − 0.3 p value = 0.004 |
| PD-1+CD19+ (%) |
r = 0.2 p value = 0.03 |
r = 0.6 p value < 0.0001 |
r = 0.3 p value = 0.004 |
– |
r = 1 p < 0.0001 |
r = 0.6 p value < 0.0001 |
r = − 0.2 p value = 0.04 |
r = − 0.5 p value < 0.0001 |
r = − 0.2 p value = 0.02 |
| PD-1+CD4+ (%) |
r = 0.2 p value = 0.03 |
r = 0.6 p value < 0.0001 |
r = 0.3 p value = 0.004 |
r = 1 p < 0.0001 |
– |
r = 0.6 p value < 0.0001 |
r = − 0.2 p value = 0.04 |
r = − 0.5 p value < 0.0001 |
r = − 0.2 p value = 0.02 |
| PD-1+CD8+ (%) |
r = − 0.02 p value = 0.4 |
r = 0.4 p value < 0.0001 |
r = 0.3 p value = 0.01 |
r = 0.6 p value < 0.0001 |
r = 0.6 p value < 0.0001 |
– |
r = − 0.3 p value = 0.007 |
r = − 0.4 p value = 0.001 |
r = − 0.2 p value = 0.02 |
| CCR5+CD19+ (%) |
r = − 0.1 p value = 0.2 |
r = − 0.04 p value = 0.4 |
r = 0.17 p value = 0.07 |
r = − 0.2 p value = 0.04 |
r = − 0.2 p value = 0.04 |
r = − 0.3 p value = 0.007 |
– |
r = − 0.06 p value = 0.3 |
r = − 0.2 p value = 0.02 |
| CCR5+CD4+(%) |
r = − 0.2 p value = 0.01 |
r = − 0.2 p value = 0.02 |
r = − 0.1 p value 0.2 |
r = − 0.5 p value < 0.0001 |
r = − 0.5 p value < 0.0001 |
r = − 0.4 p value = 0.01 |
r = − 0.06 p value = 0.3 |
– |
r = − 0.08 p value = 0.2 |
| CCR5+CD8+ (%) |
r = 0.2 p value = 0.04 |
r = − 0.4 p value < 0.0001 |
r = − 0.3 p value = 0.004 |
r = − 0.2 p value = 0.02 |
r = − 0.2 p value = 0.02 |
r = − 0.2 p value = 0.02 |
r = − 0.2 p value = 0.02 |
r = 0.08 p value = 0.2 |
– |
Pearson correlation, r correlation coefficient, bold r and p values indicate significance (p < 0.05)
Moreover, tested cells have only shown a few significant correlations with most of the evaluated laboratory parameters. Tim-3+CD4+ cells had a positive correlation with the level of total bilirubin (r = 0.3, p value = 0.03) and a negative correlation with albumin (r = − 0.3, p value = 0.049). The level of CCR5+CD8+ cells was negatively correlated with the platelet count (r = − 0.3, p value = 0.02). None of the cells expressing the tested markers showed significant correlations with AFP or the number of hepatic focal lesions.
Comparison of the levels of CD19+, CD4+, and CD8+ cells expressing Tim-3, PD-1, and CCR5 between patients on Daclatasvir/ sofosbuvir treatment regimen and patients not receiving treatment for HCV
Only the mean percentages of both CD4+ and CD8+ cells expressing Tim-3 were significantly higher in non-treated patients compared with those who were on a treatment regimen, as shown in Table 4.
Table 4.
Comparison of the levels of CD19+, CD4+ and CD8+ cells expressing Tim-3, PD-1, and CCR5 (A) between HCV-related HCC patients on Daclatasvir/ sofosbuvir treatment regimen and patients not receiving treatment for HCV and (B) between patients with different HCC treatment responses
| Response | Tim-3- CD19 |
Tim-3- CD4 |
Tim-3- CD8 |
PD-1- CD19 |
PD-1- CD4 |
PD-1- CD8 |
CCR5- CD19 |
CCR5- CD4 |
CCR5- CD8 |
|---|---|---|---|---|---|---|---|---|---|
| A) Daclatasvir/ sofosbuvir treatment* | |||||||||
|
Non HCV treated (n = 31) |
1.3 ± 0.01 | 9.6 ± 0.6 | 11.4 ± 1.3 | 23.2 ± 0.6 | 20.5 ± 0.5 | 30.7 ± 1 | 10.5 ± 0.5 | 10.9 ± 0.6 | 20 ± 0.3 |
|
HCV treated (n = 9) |
1.3 ± 0.04 | 6.6 ± 0.8 | 7 ± 1 | 22.4 ± 1 | 19.7 ± 0.9 | 31.8 ± 1.9 | 10.8 ± 0.5 | 9.4 ± 1 | 20 ± 0.8 |
| p value | 0.6 | 0.02 | 0.009 | 0.5 | 0.5 | 0.6 | 0.8 | 0.4 | 0.7 |
| B) HCC treatment response** | |||||||||
| CR (n = 13) | 1.2 ± 0.1 | 5 ± 2 | 6.4 ± 0.5 | 16.7 ± 0.3 | 14.7 ± 0.2 | 20 ± 0.01 | 12.3 ± 3 | 14.1 ± 2 | 21.1 ± 0.4 |
| PR (n = 10) | 1.2 ± 0.03 | 6.6 ± 1 | 7.6 ± 1 | 19.9 ± 2 | 17.5 ± 2 | 23.3 ± 2 | 10.4 ± 1 | 14.3 ± 2 | 19.97 ± 1 |
| SD (n = 10) | 1.2 ± 0.03 | 6.4 ± 2 | 8.9 ± 2 | 20.6 ± 0.3 | 18.1 ± 0.3 | 27 ± 1 | 10.3 ± 1 | 10.8 ± 1 | 21.3 ± 0.6 |
| PD (n = 7) | 1.2 ± 0.3 | 7.8 ± 1 | 8.98 ± 0.8 | 23.4 ± 1 | 20.6 ± 3 | 35.96 ± 1 | 9.6 ± 0.7 | 9.95 ± 2 | 20.5 ± 0.4 |
| p value | 0.7 | 0.4 | 0.6 | 0.003 | 0.003 | 0.001 | 0.2 | 0.1 | 0.7 |
HCC hepatocellular carcinoma, CR complete response, PR partial response, SD stable disease, PD progressive disease n number
Results expressed as mean percentages ± SE
*Mann Whitney U test and
**One-way ANOVA test for significance, bold p values indicate significance (< 0.05)
Relations of the levels of CD19+, CD4+and CD8+ cells expressing Tim-3, PD-1 and CCR5 with the different patterns of response to HCC treatment
The percentages of expression of Tim-3 and CCR5 did not have a significant impact on the response to HCC treatment, while there was a considerable increase in the proportions of cells expressing PD-1 with the worsening of the response to treatment as presented in Table 4.
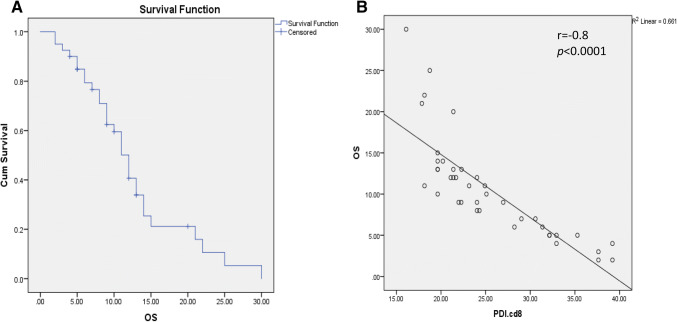
Overall survival in HCV-related HCC patients
The median overall survival of the whole study sample was 12 months ± 0.9, with a confidence interval from 10.2 to 13.8 (Fig. 2). Our results elucidated several associations between OS and the different immune cells. A positive correlation was observed between OS and PD-1+ CD4+/PD-1+CD8+ ratio (r = 0.4, p = 0.02). Conversely, OS showed negative correlations with each of CD19+, CD4+ and CD8+ lymphocytes expressing PD1 (r = − 0.6, p < 0.0001, r = − 0.6, p < 0.0001and r = − 0.8, p < 0.0001, respectively) (Fig. 2). Also, OS was inversely associated with Tim-3+CD4+ cells (r = − 0.4, p = 0.01).
Fig. 2.
Overall survival curve of the whole study population (a) and its correlation with PD-1+CD8+ cells (b)
Discussion
The significance of T cells in combating tumors has become extensively investigated and well known. Meanwhile, the likely role of B cells in the immune response to tumors is less well studied [41]. Tumors are rich in B cells [42], but the abundant subsets and their biologic role in tumor pathogenesis are poorly understood [43].
The expression and function of CCR5 on T cells are well established [44, 45]. Aberrant CCR5 expression can be detected in different kinds of tumors. Higher CCR5 expression by B cells was found in lymphoma patients [46]. Agreeing with a previous study [47], CCR5 expression by both peripheral CD4+ and CD8+T lymphocytes was significantly lower in our HCC patients compared with the healthy control; this was more evident in CD4+ T lymphocytes. Liu et al. reported an increased T-cell expression of this chemokine receptor in the tumor tissue, proposing that they might be involved in directing the migration of T cells to the tumor area [48] and in optimizing helper-dependent CD8 + T cell priming [49]. In contrast, no difference was observed in the level of CCR5 expression by B cells between our HCC patients and the healthy subjects.
Several studies [34–36] have shown higher expression levels of both PD-1 and Tim-3 by CD4+ and CD8+ T cells in patients with HBV and HCV infection both in the liver and in peripheral blood compared with healthy subjects. Others have reported the elevated frequency of CD4+ and CD8+ T cells expressing PD-1 and Tim-3 in HCC patients [37–39]. Even though Li and colleagues [50] stated that the expression levels of Tim-3 by CD8+ T cells was related to a poor prognosis for tumor progression, they noted that the frequency of cells expressing Tim-3 did not show significant correlations with AFP or tumor multiplicity.
In agreement with the previous studies, the levels of CD4+ and CD8+ T cells expressing PD-1 and Tim-3 were higher in our HCC patients compared with the healthy control group and were not showing any correlations with either AFP or the number of hepatic focal lesions. The highest expression of Tim-3 and PD-1 was also detected among the CD8+ cells indicating the marked exhaustion of these cells in HCC patients. Moreover, a moderate correlation was found between CD4 and CD8 cells expressing PD-1. Similar to a prior study [34], there was a strong correlation between the expression of Tim-3 by CD4+ cells and that by CD8+ T cells.
The role offered by PD-1 expression on B cells still was not well studied [51]. A recent study [43] has identified a PD-1 expressing B cell subset whose frequency correlates with the disease stage and is associated with early recurrence of HCC. In the same way, our results indicated that the level of expression of PD-1 by B cells increases significantly in HCC patients having HCV compared with the control. PD-1 expression level by B cells was even more than that by CD4+ T cells. It is worth mentioning that B cells expressing PD-1 showed a perfect correlation with CD4+PD-1+ cells and moderate correlations with CD4+TIM-3+ and CD8+PD-1+ cells, which probably proposes that B cells expressing PD-1, CD4 and CD8 cells jointly participate in the immune dysfunction occurring in HCV patients with HCC. Furthermore, no abnormal expression of Tim-3 was detected among B cells.
In our study, CD4+ cells expressing CCR5 were inversely related to each of CD4+PD-1+ and CD19+PD-1+ cells. This was somewhat in line with an earlier study [52], which reported a down-regulation of some molecules affecting T-cell trafficking to and/or retention in the tumor following the co-inhibition of the PD-1 and Tim-3 signaling pathways.
Sofosbuvir/daclatasvir combination of direct-acting antiviral drugs (DAA) is one of the favored regimens in the WHO guidelines for HCV treatment with cure rates above 95% [53–55]. It is of utmost importance to understand how DAA treatment restores the immune fitness in the liver, the number, and function of exhausted lymphocytes and the expression of inhibitory molecules on liver cells [56, 57]. In our patients, a marked reduction of the levels of CD4 and CD8 T cells expressing Tim-3 was found in the patients on Daclatasvir/sofosbuvir treatment regimen compared with those not receiving treatment for HCV. The previous finding suggests a means by which these drugs can enhance the dampened immune response to HCC.
Previous studies have elicited that a higher expression of Tim-3 [37, 58, 59] and PD-1 [38] was significantly correlated with shorter OS and a more advanced tumor stage in cancer patients. Tim-3 and PD-1 cause T-cell exhaustion which enhances HCC growth [60] with a more advanced tumor stage and shorter survival. Similarly, our results showed that elevated levels of Tim-3 CD4+ cells and PD-1+ lymphocytes were correlated significantly with a shorter OS. In addition, lower proportions of PD-1+ lymphocytes were associated with a better response to treatment in our patients.
In the current study, we analyzed the differential expression of Tim-3, PD-1, and CCR5 by peripheral blood T and B lymphocytes from HCV patients with HCC and correlated their levels with the treatment response and survival outcomes. Our results suggest that, of all the tested markers, PD-1 was the most relevant to HCC prognosis. This was verified by the better OS and improved response to HCC treatments associated with the lower levels of cells expressing PD-1, especially CD8+ cells. No doubt that the significantly higher levels of T cells expressing Tim-3 point to their likely importance in HCC pathogenesis. However, it seems that they may be indirectly affecting tumor progression. Even though a better OS was somewhat related to the lower levels of CD4+ expressing Tim-3, the lower levels of these cells were not significantly associated with improved response to treatment. Tim-3+CD4+ cells were the only cells showing a significant positive correlation with bilirubin and a negative correlation with albumin. Together with Tim-3+CD8+ cells, they were the only cells displaying decreased levels in patients receiving HCV treatment. B cells expressing PD-1 also seem to have an essential role in HCC. This is supported by the strong relations detected between the percentage of these cells and PD-1+CD4+ and PD-1+CD8+ cells. Thus, future studies should focus on a more thorough exploration of this subset of B cells.
The study was limited by the small sample size and lack of gene expression work. Thereby, reliable conclusions regarding the interrelationship between the expression levels of these molecules and the relation between their levels and achieving sustained treatment response were challenging to be drawn. Further studies with more detailed immunological characterization are needed to confirm the impact of HCC treatments on different B- and T-cell subsets expressing these molecules.
Conclusion
Modulation of the expression of immune checkpoints as Tim-3 and PD-1, and of CCR5 on T cells is somehow related to HCC. CD8+ T cells expressing PD-1 were the most relevant to HCC prognosis (OS and treatment response) and could represent a promising target for immune therapy against HCC. Future studies need to focus on exploring PD-1+ B cells and Tim-3+CD4+ cells, which seem to play a significant role in the pathogenesis of HCC.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- APC
Allophycocyanin
- CCL5-CCR5
Chemokine ligand 5-chemokine receptor 5
- DAA
Direct-acting antiviral drugs
- FITC
Fluoroisothiocyanate
- FLR
Future liver remnant
- HCC
Hepatocellular carcinoma
- OS
Overall survival
- PBMCs
Peripheral blood mononuclear cells
- PBS
Phosphate buffer saline
- PD-1
Programmed cell death protein-1
- PE
Phycoerythrin
- Per-CP
Peridinium-chlorophyll-protein
- PS
Performance status
- RFA
Radiofrequency ablation
- TACE
Trans-arterial chemo-embolization
- Tim-3
T-cell immunoglobulin and mucin-domain containing-3
Author contributions
AMZ, HFH, AR and OE-B conceived and designed the experiments. AR, ASE, EAH, HF and AS recruited patients, carried out the clinical investigations and collected patients’ clinical data. AMZ performed the experiments. AMZ, HFH and OE-B shared in the analysis of the flow cytometry data. OE-B. performed the statistical analysis. OE-B and AMZ accomplished the interpretation of results and wrote the initial draft. All authors participated in critical review and revision of the final manuscript, and HFH managed the submission of the manuscript.
Compliance with ethical standards
Conflict of interest
All authors declare that they have no conflict of interests.
Ethical approval
The research was done according to the ethical guidelines of the Declaration of Helsinki 1975 and was accepted by the local ethics committee of the Faculty of Medicine, Assiut University, Assiut, Egypt (IRB no: 17300300).
Informed consent
Written informed consent was obtained from all participants before inclusion in the study. Participants consented for the use of their specimens and data for research and publication.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rayan A, Zahran A, El-Badawy O, Soliman A. P-056 Differential expression of CCR5, TIM3 and PD-1 on peripheral blood T and B lymphocytes from hepatitis C patients with hepatocellular carcinoma and their impact on treatment outcomes. Ann Oncol. 2019;30((Supplement_4)):mdz155.055. [Google Scholar]
- 2.Nordenstedt H, White DL, El-Serag HB. The changing pattern of epidemiology in hepatocellular carcinoma. Dig Liver Dis. 2010;42(Suppl 3):S206–214. doi: 10.1016/s1590-8658(10)60507-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laursen L. A preventable cancer. Nature. 2014;516(7529):S2–3. doi: 10.1038/516S2a. [DOI] [PubMed] [Google Scholar]
- 4.Zahran AM, Abdel-Rahim MH, Refaat A, Sayed M, Othman MM, Khalak LMR, Hetta HF. Circulating hematopoietic stem cells, endothelial progenitor cells and cancer stem cells in hepatocellular carcinoma patients: contribution to diagnosis and prognosis. Acta Oncol. 2020;59(1):33–39. doi: 10.1080/0284186X.2019.1657940. [DOI] [PubMed] [Google Scholar]
- 5.Chaturvedi VK, Singh A, Dubey SK, Hetta HF, John J, Singh M. Molecular mechanistic insight of hepatitis B virus mediated hepatocellular carcinoma. Microb Pathog. 2019;128:184–194. doi: 10.1016/j.micpath.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Anzola M. Hepatocellular carcinoma: role of hepatitis B and hepatitis C viruses proteins in hepatocarcinogenesis. J Viral Hepatitis. 2004;11(5):383–393. doi: 10.1111/j.1365-2893.2004.00521.x. [DOI] [PubMed] [Google Scholar]
- 7.Hetta HF, Mekky MA, Khalil NK, Mohamed WA, El-Feky MA, Ahmed SH, Daef EA, Medhat A, Nassar MI, Sherman KE. Extra-hepatic infection of hepatitis C virus in the colon tissue and its relationship with hepatitis C virus pathogenesis. J Med Microbiol. 2016;65(8):703. doi: 10.1099/jmm.0.000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zahran AM, Nafady-Hego H, Mansor SG, Abbas WA, Abdel-Malek MO, Mekky MA, Hetta HF. Increased frequency and FOXP3 expression of human CD8+ CD25High+ T lymphocytes and its relation to CD4 regulatory T cells in patients with hepatocellular carcinoma. Hum Immunol. 2019;80:510–516. doi: 10.1016/j.humimm.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 9.Hassan EA, Ahmed EH, Nafee AM, El-Gafary N, Hetta HF, El-Mokhtar MA. Regulatory T cells, IL10 and IL6 in HCV related hepatocellular carcinoma after transarterial chemoembolization (TACE) Egypt J Immunol. 2019;26:69–78. [PubMed] [Google Scholar]
- 10.Zahran AM, Ashmawy AM, Rayan A, Elkady A, Elsherbiny NM, Hetta HF. Frequency and implications of natural killer and natural killer T cells in hepatocellular carcinoma. Egypt J Immunol. 2018;25:45. [PubMed] [Google Scholar]
- 11.Elsegood CL, Tirnitz-Parker JE, Olynyk JK, Yeoh GC. Immune checkpoint inhibition: prospects for prevention and therapy of hepatocellular carcinoma. Clin Transl Immunol. 2017;6(11):e161. doi: 10.1038/cti.2017.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarvaria A, Madrigal JA, Saudemont A. B cell regulation in cancer and anti-tumor immunity. Cell Mol Immunol. 2017;14(8):662. doi: 10.1038/cmi.2017.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hetta H, Elkady A, Tohamy T, Badary M. Regulatory B cells: key players in hepatocellular carcinoma progression. Gastroenterol Hepatol Open Access. 2016;5(2):00136. doi: 10.15406/ghoa.2016.05.00136. [DOI] [Google Scholar]
- 14.Fremd C, Schuetz F, Sohn C, Beckhove P, Domschke C. B cell-regulated immune responses in tumor models and cancer patients. Oncoimmunology. 2013;2(7):e25443. doi: 10.4161/onci.25443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inoue S, Leitner WW, Golding B, Scott D. Inhibitory effects of B cells on antitumor immunity. Can Res. 2006;66(15):7741–7747. doi: 10.1158/0008-5472.can-05-3766. [DOI] [PubMed] [Google Scholar]
- 16.Schioppa T, Moore R, Thompson RG, Rosser EC, Kulbe H, Nedospasov S, Mauri C, Coussens LM, Balkwill FR. B regulatory cells and the tumor-promoting actions of TNF-alpha during squamous carcinogenesis. Proc Natl Acad Sci USA. 2011;108(26):10662–10667. doi: 10.1073/pnas.1100994108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DiLillo DJ, Yanaba K, Tedder TF. B cells are required for optimal CD4+ and CD8+ T cell tumor immunity: therapeutic B cell depletion enhances B16 melanoma growth in mice. J Immunol (Baltimore, Md : 1950) 2010;184(7):4006–4016. doi: 10.4049/jimmunol.0903009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Q, Teitz-Tennenbaum S, Donald EJ, Li M, Chang AE. In vivo sensitized and in vitro activated B cells mediate tumor regression in cancer adoptive immunotherapy. J Immunol (Baltimore, Md : 1950) 2009;183(5):3195–3203. doi: 10.4049/jimmunol.0803773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raufi A, Tirona MT. Prospect of the use of checkpoint inhibitors in hepatocellular cancer treatments. Cancer Manag Res. 2017;9:19–27. doi: 10.2147/cmar.s111673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hetta HF, Zahran AM, Mansor SG, Abdel-Malek MO, Mekky MA, Abbas WA. Frequency and implications of myeloid-derived suppressor cells and lymphocyte subsets in Egyptian patients with hepatitis C virus-related hepatocellular carcinoma. J Med Virol. 2019;91(7):1319–1328. doi: 10.1002/jmv.25428. [DOI] [PubMed] [Google Scholar]
- 21.Zahran AM, Zahran ZAM, El-Badawy O, Abdel-Rahim MH, Ali WA, Rayan A, El-Masry MA, Abozaid MA, Hetta HF. Prognostic impact of toll-like receptors 2 and 4 expression on monocytes in Egyptian patients with hepatocellular carcinoma. Immunol Res. 2019;67(2–3):157–165. doi: 10.1007/s12026-019-09075-x. [DOI] [PubMed] [Google Scholar]
- 22.Hetta HF, Khairy H, Ismail S. Circulating IL17A and IFN-gamma serum levels in cirrhotic hepatitis C virus infected patients with autoimmune thyroiditis. Int J Curr Microbiol Appl Sci. 2017;6(3):1972–1983. doi: 10.20546/ijcmas.2017.603.225. [DOI] [Google Scholar]
- 23.Liang CM, Chen L, Hu H, Ma HY, Gao LL, Qin J, Zhong CP. Chemokines and their receptors play important roles in the development of hepatocellular carcinoma. World J Hepatol. 2015;7(10):1390–1402. doi: 10.4254/wjh.v7.i10.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nahon P, Sutton A, Rufat P, Simon C, Trinchet JC, Gattegno L, Beaugrand M, Charnaux N. Chemokine system polymorphisms, survival and hepatocellular carcinoma occurrence in patients with hepatitis C virus-related cirrhosis. World J Gastroenterol. 2008;14(5):713–719. doi: 10.3748/wjg.14.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Das M, Zhu C, Kuchroo VK. Tim-3 and its role in regulating anti-tumor immunity. Immunol Rev. 2017;276(1):97–111. doi: 10.1111/imr.12520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hato T, Goyal L, Greten TF, Duda DG, Zhu AX. Immune checkpoint blockade in hepatocellular carcinoma: current progress and future directions. Hepatology (Baltimore, MD) 2014;60(5):1776–1782. doi: 10.1002/hep.27246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen J, Jiang CC, Jin L, Zhang XD. Regulation of PD-L1: a novel role of pro-survival signalling in cancer. Ann Oncol. 2016;27(3):409–416. doi: 10.1093/annonc/mdv615. [DOI] [PubMed] [Google Scholar]
- 29.Carter L, Fouser LA, Jussif J, Fitz L, Deng B, Wood CR, Collins M, Honjo T, Freeman GJ, Carreno BM. PD-1:PD-L inhibitory pathway affects both CD4(+) and CD8(+) T cells and is overcome by IL-2. Eur J Immunol. 2002;32(3):634–643. doi: 10.1002/1521-4141(200203)32:3<634::aid-immu634>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 30.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blank C, Gajewski TF, Mackensen A. Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy. Cancer Immunol Immunother. 2005;54(4):307–314. doi: 10.1007/s00262-004-0593-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y. Immunomodulatory effects of Tim-3 and PD-1 on chronic hepatitis B virus infection. Infect Int. 2018;7(1):6–11. doi: 10.2478/ii-2018-0012. [DOI] [Google Scholar]
- 33.Missale G, Cariani E. Time for hepatocellular carcinoma immunotherapy: insights for successful clinical applications in this challenging tumor. Hepatoma Res. 2018;4:22. doi: 10.20517/2394-5079.2018.72. [DOI] [Google Scholar]
- 34.Golden-Mason L, Palmer BE, Kassam N, Townshend-Bulson L, Livingston S, McMahon BJ, Castelblanco N, Kuchroo V, Gretch DR, Rosen HR. Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4+ and CD8+ T cells. J Virol. 2009;83(18):9122–9130. doi: 10.1128/JVI.00639-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu W, Shi Y, Li J, Chen F, Chen Z, Zheng M. Tim-3 expression on peripheral T cell subsets correlates with disease progression in hepatitis B infection. Virol J. 2011;8(1):113. doi: 10.1186/1743-422X-8-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmidt J, Blum HE, Thimme R. T-cell responses in hepatitis B and C virus infection: similarities and differences. Emerg Microbes Infect. 2013;2(3):e15. doi: 10.1038/emi.2013.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li H, Wu K, Tao K, Chen L, Zheng Q, Lu X, Liu J, Shi L, Liu C, Wang G, Zou W. Tim-3/galectin-9 signaling pathway mediates T-cell dysfunction and predicts poor prognosis in patients with hepatitis B virus-associated hepatocellular carcinoma. Hepatology (Baltimore, MD) 2012;56(4):1342–1351. doi: 10.1002/hep.25777. [DOI] [PubMed] [Google Scholar]
- 38.Shi F, Shi M, Zeng Z, Qi RZ, Liu ZW, Zhang JY, Yang YP, Tien P, Wang FS. PD-1 and PD-L1 upregulation promotes CD8(+) T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients. Int J Cancer. 2011;128(4):887–896. doi: 10.1002/ijc.25397. [DOI] [PubMed] [Google Scholar]
- 39.He G, Zhang H, Zhou J, Wang B, Chen Y, Kong Y, Xie X, Wang X, Fei R, Wei L. Peritumoural neutrophils negatively regulate adaptive immunity via the PD-L1/PD-1 signalling pathway in hepatocellular carcinoma. J Exp Clin Cancer Res. 2015;34(1):141. doi: 10.1186/s13046-015-0256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60(8):646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 41.Tsou P, Katayama H, Ostrin EJ, Hanash SM. The emerging role of B cells in tumor immunity. Can Res. 2016;76(19):5597–5601. doi: 10.1158/0008-5472.can-16-0431. [DOI] [PubMed] [Google Scholar]
- 42.Nelson BH. CD20+ B cells: the other tumor-infiltrating lymphocytes. J Immunol. 2010;185(9):4977–4982. doi: 10.4049/jimmunol.1001323. [DOI] [PubMed] [Google Scholar]
- 43.Xiao X, Lao XM, Chen MM, Liu RX, Wei Y, Ouyang FZ, Chen DP, Zhao XY, Zhao Q, Li XF, Liu CL, Zheng L, Kuang DM. PD-1hi Identifies a novel regulatory B-cell population in human hepatoma that promotes disease progression. Cancer Discov. 2016;6(5):546–559. doi: 10.1158/2159-8290.cd-15-1408. [DOI] [PubMed] [Google Scholar]
- 44.Fukada K, Sobao Y, Tomiyama H, Oka S, Takiguchi M. Functional expression of the chemokine receptor CCR5 on virus epitope-specific memory and effector CD8+ T cells. J Immunol. 2002;168(5):2225–2232. doi: 10.4049/jimmunol.168.5.2225. [DOI] [PubMed] [Google Scholar]
- 45.Luther SA, Cyster JG. Chemokines as regulators of T cell differentiation. Nat Immunol. 2001;2(2):102. doi: 10.1038/84205. [DOI] [PubMed] [Google Scholar]
- 46.Butrym A, Kryczek I, Dlubek D, Jaskula E, Lange A, Jurczyszyn A, Mazur G. High expression of CC chemokine receptor 5 (CCR5) promotes disease progression in patients with B-cell non-Hodgkin lymphomas. Curr Probl Cancer. 2018;42(2):268–275. doi: 10.1016/j.currproblcancer.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 47.Liu Y, Poon RT, Feng X, Yu WC, Luk JM, Fan ST. Reduced expression of chemokine receptors on peripheral blood lymphocytes in patients with hepatocellular carcinoma. Am J Gastroenterol. 2004;99(6):1111–1121. doi: 10.1111/j.1572-0241.2004.30265.x. [DOI] [PubMed] [Google Scholar]
- 48.Liu Y, Poon RT, Hughes J, Feng X, Yu WC, Fan ST. Chemokine receptors support infiltration of lymphocyte subpopulations in human hepatocellular carcinoma. Clin Immunol. 2005;114(2):174–182. doi: 10.1016/j.clim.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 49.Gonzalez-Martin A, Mira E, Manes S. CCR5 in cancer immunotherapy: More than an “attractive” receptor for T cells. Oncoimmunology. 2012;1(1):106–108. doi: 10.4161/onci.1.1.17995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu YY, Wang Y, Walsh TR, Yi LX, Zhang R, Spencer J, Doi Y, Tian G, Dong B, Huang X, Yu LF, Gu D, Ren H, Chen X, Lv L, He D, Zhou H, Liang Z, Liu JH, Shen J. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis. 2016;16(2):161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 51.Ren Z, Peng H, Fu Y-X. PD-1 shapes B cells as evildoers in the tumor microenvironment. Cancer Discov. 2016;6(5):477–478. doi: 10.1158/2159-8290.CD-16-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sakuishi K, Ngiow SF, Sullivan JM, Teng MW, Kuchroo VK, Smyth MJ, Anderson AC. TIM3(+)FOXP3(+) regulatory T cells are tissue-specific promoters of T-cell dysfunction in cancer. Oncoimmunology. 2013;2(4):e23849. doi: 10.4161/onci.23849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.World Health Organization . Global hepatitis report 2017. Washington: World Health Organization; 2017. [Google Scholar]
- 54.Mekky MA, Abdel-Malek MO, Osman HA, Abdel-Aziz EM, Hashim A-KA, Hetta HF, Morsy KH. Efficacy of ombitasvir/paritaprevir/ritonavir/ribavirin in management of HCV genotype 4 and end-stage kidney disease. Clin Res Hepatol Gastroenterol. 2019;43(1):82–87. doi: 10.1016/j.clinre.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 55.Mekky MA, Sayed HI, Abdelmalek MO, Saleh MA, Osman OA, Osman HA, Morsy KH, Hetta HF. Prevalence and predictors of occult hepatitis C virus infection among Egyptian patients who achieved sustained virologic response to sofosbuvir/daclatasvir therapy: a multi-center study. Infect Drug Resist. 2019;12:273. doi: 10.2147/IDR.S181638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mazouz S, Boisvert M, Shoukry NH, Lamarre D. Reversing immune dysfunction and liver damage after direct-acting antiviral treatment for hepatitis C. Can Liver J. 2018;1(2):78–105. doi: 10.3138/canlivj.1.2.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Abdel-Hameed EA, Rouster SD, Ji H, Ulm A, Hetta HF, Anwar N, Sherman KE, Shata MTM. Evaluating the role of cellular immune responses in the emergence of HCV NS3 resistance mutations during protease inhibitor therapy. Viral Immunol. 2016;29(4):252–258. doi: 10.1089/vim.2015.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Y, Cai P, Liang T, Wang L, Hu L. TIM-3 is a potential prognostic marker for patients with solid tumors: a systematic review and meta-analysis. Oncotarget. 2017;8(19):31705. doi: 10.18632/oncotarget.15954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zahran AM, Saleh M, Mostafa F, Sayed MM, Rayan A, Ali AM, Hetta HF. Up-regulation of regulatory T cells, CD200 and TIM3 expression in cytogenetically normal acute myeloid leukemia. Cancer Biomarkers. 2018;22(3):587–595. doi: 10.3233/CBM-181368. [DOI] [PubMed] [Google Scholar]
- 60.Ma C, Kesarwala AH, Eggert T, Medina-Echeverz J, Kleiner DE, Jin P, Stroncek DF, Terabe M, Kapoor V, ElGindi M. NAFLD causes selective CD4+ T lymphocyte loss and promotes hepatocarcinogenesis. Nature. 2016;531(7593):253. doi: 10.1038/nature16969. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.