Abstract
Objective
To explore the programmed death-ligand 1 (PD-L1) expression in varied subtypes of pituitary neuroendocrine tumors with assessment of their clinical behavior at diagnosis and follow-up.
Methods
We conducted a retrospective monocentric study, including all patients operated in the Academic Hospital of Angers (France) for a pituitary neuroendocrine tumor between 2012 and 2018. PDL-1 immunostaining was performed using a European Conformity—In Vitro Diagnostic-labeled anti-PDL1 antibody (clone 22C3). PD-L1 immunostaining was evaluated as the percentage of tumor cells showing positive membrane staining, into four grades: grade 0 = < 1%, grade 1 = 1 to 5%, grade 2 = 6 to 49% and grade 3 = ≥ 50%. PD-L1 expression was compared with tumor features (secretion, proliferation, invasion) and outcome.
Results
The study included 139 pituitary neuroendocrine tumors, including 84 (60%) nonfunctioning adenomas. Twenty-five pituitary neuroendocrine tumors were PD-L1 positive (18%), including 3 grade 3, 8 grade 2 and 14 grade 1. PD-L1 expression was not different between functioning and nonfunctioning adenomas (p = 0.26). Among 16 tumors with proliferative markers (Ki-67 ≥ 3% and p53 positive), only one was PD-L1 positive.
Conclusion
In our series, PD-L1 was expressed in a rather small proportion of PitNET (18%), and this immune marker was not associated with any biological characteristic or behavior of the pituitary tumors. Thus, PD-L1 staining may be necessary before considering PD-L1 blockage in pituitary neuroendocrine tumors, in case of therapeutic impasse.
Keywords: PD-L1, Pituitary neuroendocrine tumor, Immunotherapy, Clone 22C3, Immunohistochemistry
Introduction
Pituitary neuroendocrine tumors (PitNET) account for 16.5% of all intracranial tumors, being the second most frequent tumor type after meningioma [1]. They are mostly well controlled after surgery. In the postoperative follow-up, about 30% of the patients have tumor progression or persistent secretion, especially in the presence of residual tumor [2]. In addition to surgery, medical therapies and radiotherapy are alternative resources to control secretion and/or tumor progression [3]. However, few patients experience an aggressive evolution and are resistant to standard management [4]. Recently, a new prognostic clinicopathological classification (HYPOPRONOS) based on invasion and proliferation markers was proposed to predict the risk of recurrence of pituitary tumors [5]. Despite advances in prognostic classification, there is no reliable pathological marker which predicts PitNET outcome.
Immunotherapy with inhibitors of T-cell checkpoints has shown durable efficacy in a variety of malignancies including melanoma, lung cancer or urothelial carcinoma [6–8]. The tumoral expression of programmed cell death-ligand 1 (PD-L1) is recognized as a predictor of the response to therapy targeting programmed cell death-1 (PD1)/PD-L1 immune checkpoint in lung carcinoma [9]. In the literature, one case of ACTH-secreting pituitary carcinoma treated with the association of the anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA4) ipilimumab and the anti-PD-1 nivolumab showed a marked response [10]. Moreover, immunotherapy (mainly tissue association against PD1 and CTLA4) has been associated with treatment-related hypophysitis into 15% of patients, suggesting that the T-cell checkpoints may be operating in the pituitary gland [11–14]. More interestingly, it has been reported that PitNET may express PD-L1 transcript and/or protein [15–19]. However, the associations between PD-L1 expression and hormonal secretion, invasion and proliferation in PitNET are controversial [15–17, 19] and the association between expression and tumor behavior has been studied in only two studies [17, 18]. Moreover, in all these studies, immunostaining was performed with antibodies suitable for research but not validated as companion tests for diagnostic procedure. The objectives of this study were first to assess PD-L1 expression in a large retrospective monocentric cohort of PitNET with an anti-PD-L1 clone that has been validated to predict response to pembrolizumab and second to correlate PD-L1 expression with secretion, proliferation, invasion and outcomes of the tumors.
Materials and methods
Patients
We retrospectively analyzed clinical data of patients operated for PitNET (primary ± recurrent surgery) in the Academic Hospital of Angers (France) between 2012 and 2018. We excluded patients with no available tissue sample and patients with primary surgery before 2012. The medical records included patients’ clinical history, imaging data, laboratory tests and pathology results. The diagnosis and classification of PitNET were based on clinical presentation, plasma hormone levels, MRI features (tumor size and cavernous sinus invasion) and histopathological features (immune subtype, Ki-67 index, mitotic count and p53 positivity) [3]. HYPOPRONOS classification was used for PitNET grading [5]. Tumor recurrence or progression was defined as previously described [5]. Briefly, patients in complete remission showed no evidence of disease during follow-up (normal plasma hormone levels and no visible radiological tumor remnant). Patients with postoperatively active disease (increased plasma hormone levels) but controlled by medical treatment and patients with stabled residual tumor were also considered controlled. Recurrence was defined as an increase in plasma hormone levels after previous remission in functioning PitNET or reappearance of tumor mass in nonfunctioning PitNET. Tumor progression was defined as evidence of regrowth of residual tumor on MRI and/or an increase in plasma hormone levels.
Written informed consent has been obtained from each patient after full explanation of the purpose of all procedures used. The retrospective data collection was registered to the Commission nationale de l’informatique et des libertés (CNIL) (CNIL2017-051), according to French laws. The agreement of ethics committee was not necessary because we used unused equipment for diagnostic or therapeutic purposes, according to French laws.
Immunohistochemistry
Formalin-fixed paraffin-embedded tumor specimens were cut into 4-μm-thick sections. Immunohistochemistry was performed on a Bond-III automated immune stainer (Leica Microsystem, Nussloch, Germany). After heat antigen retrieval during 20 min in ER2 pH 9 buffer, sections were incubated at room temperature for 20 min with the primary mouse monoclonal anti-PDL-1 antibody (clone 22C3 Agilent/Dako, Santa Clara, USA) at a 1:50 dilution and were revealed with the Leica Bond Refine detection kit for 10 min. The specimens were then counterstained with hematoxylin. Each immunohistochemistry run included tonsil tissues as positive control.
Twenty-seven cases with sufficient tissue, accounting for 23 positive and 4 negative cases with clone 22C3, were further tested with the primary rabbit monoclonal anti-PD-L1 antibody (clone QR1, Quartett Biochemicals, Berlin, Germany) at a 1:100 dilution.
Both PDL-1 antibodies were European Conformity—In Vitro Diagnostic (CE-IVD)-marked reagents.
In addition, p53 immunohistochemistry (clone D07, ready-to-use antibody, Leica Microsystem, Nussloch, Germany) was performed for 98 cases and Ki-67 (Mib1, dilution 1:100, Agilent/Dako, Santa Clara, USA,) for 6 cases, corresponding to patients for which information about p53 or Ki-67 was lacking in the initial pathology report.
PDL-1 positivity was defined by the presence of partial or complete linear membrane staining on tumor cell irrespective of the intensity of the staining. PD-L1 expression was determined by the tumor proportion score (TPS) defined as the percentage of viable tumor cells showing positive membrane staining among all tumor cells. For statistical purposes, cases were finally classified into four grades: grade 0 = TPS < 1%, grade 1 = TPS 1 to 5%, grade 2 = TPS 6 to 49% and grade 3 = TPS ≥ 50%. The Ki-67 proliferative index was determined as the percentage of labeled nuclei in the whole specimen and considered high if ≥ 3% [20, 21]. P53 expression was considered positive in tumors with more than 10% of stained cells, irrespective of the intensity of the nuclear staining [5].
Mitotic count
Mitotic count was performed by one observer with the aid of an ocular grid in 10 high-power fields (HPF) in hotspot areas. The mean count in 10 HPF was considered as a proliferation marker if > 2 mitoses.
Statistical analysis
The statistical association between expression of PD-L1 and clinicopathological factors was evaluated by Chi-squared test or Fisher’s exact probability test as appropriate for the comparison of categorical variables. For quantitative variables, a Mann–Whitney test or an unpaired t test was used for comparing two groups and Kruskal–Wallis was used for multiple comparisons. Univariate progression-free survival analysis was performed using a Cox regression model. A p value < 0.05 was considered as statistically significant.
Statistical analysis was performed with Prism (version 6, GraphPad Software Inc., San Diego, USA).
Results
PD-L1 expression and clinical and hormonal characteristics in PitNET
Among the 157 patients retrospectively included in this study, 9 patients were excluded because of unavailable pituitary tissue and 9 because primary surgery was performed before 2012. One hundred and thirty-nine samples from patients with pituitary tumors were analyzed (133 at initial surgery and in addition 6 at initial and repeated surgeries). The patient cohort comprised 61 (44%) women. Patients were 55 ± 17 years old. The mean follow-up was 3.3 ± 2.2 years. After the initial surgery, 4 (4%) patients received radiotherapy and 6 (5%) underwent a surgical revision. The 139 primary tumors consisted in 84 (60%) nonfunctioning adenomas, 20 (15%) adrenocorticotropic (ACTH)-PitNET, 19 (14%) growth hormone (GH)-PitNET, 9 (6%) prolactin and growth hormone (PRL-GH) PitNET and 7 (5%) prolactin hormone (PRL)-PitNET. Other characteristics are detailed in Table 1.
Table 1.
Relationship between PD-L1 expression, clinical and histopathological characteristics
| Characteristics | Patients (n = 139) | Tumor PD-L1 expression | ||||
|---|---|---|---|---|---|---|
| Grade 0 (n = 114) |
Grade 1 (n = 14) |
Grade 2 (n = 8) |
Grade 3 (n = 3) |
p value | ||
| Age (years), mean ± SD | 55 ± 17 | 56 ± 17 | 57 ± 14 | 41 ± 17 | 47 ± 9.3 | 0.39 |
| Sex, female, n (%) | 61 (44%) | 52 (85%) | 5 (8%) | 3 (5%) | 1 (2%) | 0.38 |
| Tumor size, n (%) | 0.15 | |||||
| Microadenoma | 25 (18%) | 23 (92%) | 1 (4%) | 0 (0%) | 1 (4%) | |
| Macroadenoma | 114 (82%) | 91 (80%) | 13 (11%) | 8 (7%) | 2 (2%) | |
| Functioning PitNET, n (%) | ||||||
| Nonfunctioning | 84 (60%) | 69 (83%) | 7 (8%) | 6 (7%) | 2 (2%) | n.a |
| Functioning | ||||||
| PRL adenomas | 7 (5%) | 7 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| GH adenomas | 19 (14%) | 14 (73%) | 2 (11%) | 2 (11%) | 1 (5%) | |
| PRL + GH adenomas | 9 (6%) | 7 (78%) | 2 (22%) | 0 (0%) | 0 (0%) | |
| ACTH adenomas | 20 (15%) | 17 (85%) | 3 (15%) | 0 (0%) | 0 (0%) | |
| Among functioning PitNET | ||||||
| IGF1 (xULN) | 2.03 ± 0.86 | 2.09 ± 0.90 | 1.29 ± 0.07 | 1.70 ± 0.0 | 2.99 ± 0.0 | 0.87 |
| GH/OGTT (mUI/L) | 17.95 ± 24.93 | 19.59 ± 26.94 | 7.00 ± 0.00 | – | 10.80 ± 0.0 | n.a |
| PRL (ng/ml) | 464.4 ± 810.8 | 463.9 ± 843.9 | 471.8 ± 0.00 | – | – | n.a |
| Cortisol/ONDST (µg/L) | 94.78 ± 73.80 | 102.6 ± 74.78 | 32.0 ± 0.00 | – | – | n.a |
| 24 h UFC (xULN) | 7.70 ± 9.87 | 6.77 ± 8.95 | 13.76 ± 17.85 | – | – | 0.93 |
PitNET pituitary neuroendocrine tumor, PRL prolactin, GH growth hormone, OGTT oral glucose tolerance test, ONDST overnight dexamethasone suppression test, UFC urinary-free cortisol, ULN upper normal limit, n.a not applicable. p value indicates the results of comparison between negative PDL1 tumors (grade 0) versus positive PDL1 tumors (grade 1 to 3)
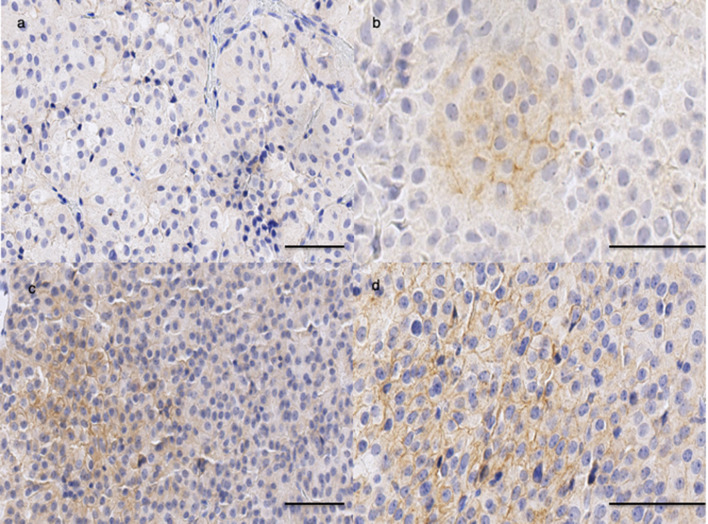
Twenty-five PitNET were PD-L1 positive (18%) with clone 22C3, including 3 grade 3, 8 grade 2 and 14 grade 1, as illustrated in Fig. 1. No PitNET showed PD-L1-positive lymphocytes or macrophages in the stroma.
Fig. 1.
PD-L1 protein expression in pituitary tumors (clone 22C3). Positive expression of PD-L1 in pituitary adenoma: grade 1 (a), grade 2 (b), grade 3 (c); bar = 50 μm
For further statistical analysis, two groups were compared: negative PDL1 tumors (grade 0) versus positive PDL1 tumors (grades 1 to 3). PD-L1 expression was not different between functioning and nonfunctioning PitNET (Table 1). For ACTH-PitNET, GH-PitNET, PRL-PitNET and GH-PRL-PitNET, there was no association between the level of initial secretion and PD-L1 expression (Table 1).
PD-L1 expression was not associated with aggressive criteria in PitNET
The proportion of positive PD-L1 tumors did not differ between PitNET with or without sinus cavernous and/or sphenoid sinus invasion (p = 0.82) (Table 2).
Table 2.
Association between tumor behavior and PD-L1 expression
| Characteristics | Patients (n = 139) | Tumor PD-L1 expression | ||||
|---|---|---|---|---|---|---|
| Grade 0 (n = 114) | Grade 1 (n = 14) |
Grade 2 (n = 8) |
Grade 3 (n = 3) |
p value | ||
| Invasiona | 0.82 | |||||
| No | 89 (64%) | 73 (82%) | 8 (9%) | 6 (7%) | 2 (3%) | |
| Yes | 49 (36%) | 41 (84%) | 5 (10%) | 2 (4%) | 1 (2%) | |
| Ki-67 | 0.40 | |||||
| < 3% | 125 (90%) | 101 (81%) | 14 (11%) | 7 (6%) | 3 (2%) | |
| ≥ 3% | 14 (10%) | 13 (93%) | 0 (0%) | 1 (7%) | 0 (0%) | |
| p53 Expression | 0.37 | |||||
| Negative | 136 (98%) | 112 (82%) | 13 (10%) | 8 (6%) | 3 (2%) | |
| Positive | 3 (2%) | 2 (67%) | 1 (33%) | 0 (0%) | 0 (0%) | |
| Mitotic count | 0.52 | |||||
| ≤ 2/10 HPF | 125 (90%) | 104 (83%) | 12 (10%) | 7 (6%) | 2 (1%) | |
| > 2/10 HPF | 14 (10%) | 10 (71%) | 2 (14%) | 1 (7.5%) | 1 (7.5%) | |
| Proliferationb | n.a | |||||
| No | 136 (98%) | 111 (82%) | 14 (10%) | 8 (6%) | 3 (2%) | |
| Yes | 3 (2%) | 3 (100%) | 0 (0%) | 0 (%) | 0 (0%) | |
| Gradec | n.a | |||||
| 1a | 88 (64%) | 72 (82%) | 9 (10%) | 5 (6%) | 2 (2%) | |
| 1b | 1 (1%) | 1 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| 2a | 47 (34%) | 39 (83%) | 5 (11%) | 2 (4%) | 1 (2%) | |
| 2b | 2 (1%) | 2 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | |
HPF high-power field, n.a not applicable. p value indicates results of comparison between negative PDL1 tumors (grade 0) versus positive PDL1 tumors (grade 1 to 3)
aInvasion is defined as MRI signs of cavernous and/or sphenoid sinus invasion. Missing data for one patient
bProliferation was considered based on the presence of at least two of the following three criteria: > 2 mitoses per 10 high-power field, Ki-67 ≥ 3%, p53 positive
cStratification of the tumors into four grades: grade 1a, noninvasive; grade 1b, noninvasive and proliferative; grade 2a, invasive; grade 2b, invasive and proliferative; missing data for one patients
Tumors with Ki-67 ≥ 3% (n = 14) or p53 positivity (n = 3) were PD-L1 negative, except one. In addition, the mitotic count did not significantly differ between the two groups (p = 0.52). PD-L1 expression was thus not correlated with proliferation (Table 2).
Stratification of the tumors into the recent HYPOPRONOS prognostic classification [5] revealed no differences according to PD-L1 expression (p = 0.87).
Granulation pattern may be considered for the prediction of treatment outcomes, especially in GH adenomas [22]. Among PRL, GH and PRL-GH adenomas, 17 were sparsely granulated and 18 were densely granulated. PD-L1 expression did not differ between sparsely or densely granulated adenomas (p = 0.49).
PD-L1 expression and PitNET recurrence or progression
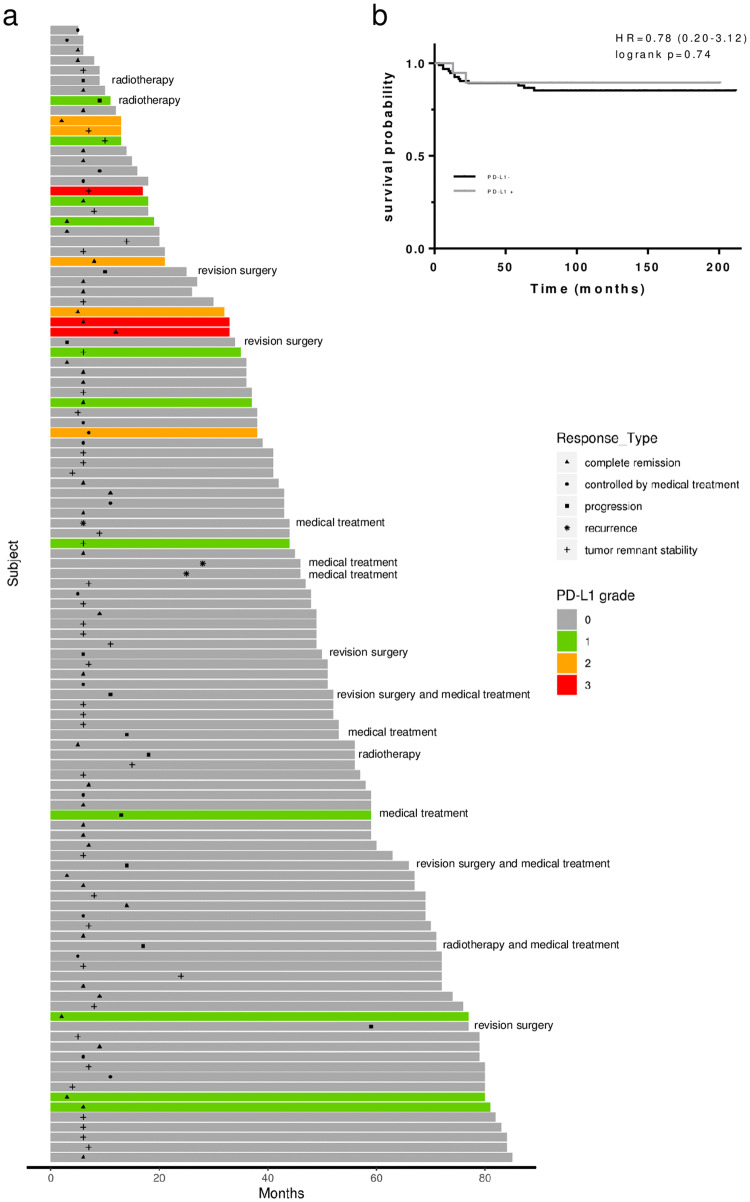
From the initial cohort, follow-up data were available from 113 patients. The median length of follow-up was 3.8 (2.0–5.5) years. Among these, 4 (4%) underwent adjuvant radiation therapy, including 1 PD-L1-positive PitNET (grade 1). Six (5%) patients underwent revision surgery. None of them exhibited PD-L1 expression (Fig. 2a). PD-L1 expression did not differ according to postoperative evolution: complete remission, remnant stability, disease controlled by medical treatment, revision neurosurgery or radiotherapy (p = 0.25). The Kaplan–Meier progression-free survival curve obtained for PD-L1 different grades was not statistically different (log-rank test, p = 0.74, Fig. 2b).
Fig. 2.
Progression-free survival in PitNET. a Swimmer plot of 113 patients after neurosurgery for PitNET. For each patient, the bar shows the length of follow-up (in months), classified by the PD-L1 grade (0, gray; 1, green; 2, orange; 3, red). Graph also includes temporal indicators for the disease evolution, classified by complete remission (triangle), controlled by medical treatment (round), tumor-remnant stability (cross) or tumor progression (square) or recurrence (asterisk). In case of progression or recurrence, the additional treatment was specified at the end of the bar. b Progression-free survival analysis of PD-L1 in PitNET patients. Kaplan–Meier analysis performed according to the expression status of PD-L1 (PD-L1 negative, dark curve, vs. PD-L1 positive, gray curve). Difference between progression-free survival curves was realized with log-rank test (p = 0.74). Time 0 month corresponded to the day of surgery
Lastly, we compared PD-L1 expression in primary and recurrent samples for 6 patients. Interestingly, no patient with surgical revision expressed PDL1 in both the initial tumor and the recurrence.
Comparison of anti-PD-L1 antibody clones in PitNET
We compared our two laboratory-developed PD-L1 assays, in all tumors classified as positive with the 22C3 clone (except 2 with insufficient material) as well as 4 negative tumors. The global concordance between the two tests was very good with 23 out of 23 positive tumors and 4 out of 4 negative tumors with both antibodies. However, the staining intensity was stronger with the QR1 clone and more tumors were classified in the grade 2 or 3 categories with this antibody than with the 22C3 clone (Table 3). Nevertheless, the results obtained with the 22C3 clone regarding secretion profile, proliferation, invasion or patient outcomes were not different with the QR1 clone, without association with any tumor features.
Table 3.
Comparison of immunostaining between clone 22C3 and clone QR1 in 27 tumors
| Assay | Grade 0 | Grade 1 | Grade 2 | Grade 3 |
|---|---|---|---|---|
| 22C3 | 4 | 13 | 7 | 3 |
| QR1 | 4 | 4 | 8 | 11 |
Discussion
Our results indicated that PD-L1 was expressed in a rather small proportion of PitNET (18%). This immune marker did not seem to be associated with any biological characteristics or behavior of the pituitary tumors in our series.
Only four previous series have explored PD-L1 expression in PitNET, and their results are barely comparable within each other [15–18]. Mei et al. reported variable levels of PD-L1 transcript and protein expression in 48 pituitary adenomas, assessed as a mean intensity of immune-positive staining using image software interpretation [15]. In this study, the expression of PD-L1 protein was variable, with a tenfold difference in protein level. In another series of 48 tumors, Salomon et al. using multiplexed immunohistochemistry found high levels of PD-L1 in different tumor types, but, similarly as Mei et al. without any precision concerning the subcellular topography of the staining nor the exact number of negative cases [16]. The coincidence between PD-L1 immunostaining and the immune infiltrates might suggest either that PD-L1 was expressed by lymphocytes rather than pituitary cells or that lymphocytic infiltration induced PD-L1 expression on pituitary cells, as previously described [15, 16]. The larger series reported by Wang et al. reported a 36.6% rate of PD-L1-positive PitNET among 191 cases, but the positivity was defined as cytoplasmic or membranous staining in over 5% of tumor cells. More recently, Sato et al. assessed PD-L1 immunodetection in 27 PitNET with the same scoring categories as we used and found that 63% of PitNET had PD-L1 immunostaining > 5% (grade 2-3) [18]. However, in this study, as in all previously published studies, immunohistochemistry was not performed with a CE-IVD-labeled anti-PDL1 antibody. In fact, PD-L1 immunodetection concordance between assays may be poor, as previously described [23]. Moreover, performing immunohistochemistry with clones that have been validated in clinical trials of cancer immunotherapy may be a better option than using research-only-marked clones, inasmuch a response to immunotherapy is the final goal. Due to a lack of consensus regarding assessment criteria, there are different difficulties in the assessment of PD-L1 expression in PitNET. Previous studies used different antibody clones and the cut-off value varied from 1 to 5% cut-offs, when available [17–19]. We initially analyzed PitNET samples according to four grades as previously published [18]. Since there was no difference between the different grades, individually or in pooled groups, it would be simpler to divide patients into two groups according to the presence or absence of PD-L1 as in lung cancer [9].
Another explanation for the relatively low level of PD-L1-positive tumors in our series is the inconstant and weak lymphocytic infiltration that we observed whereas a concomitant presence of lymphocyte population has been described as important for PD-L1 expression [24]. It has also been described that radiotherapy attracts T cells into tumors that secondarily induce an immune response [25]. Thus, a post-radiotherapy lymphocytic infiltration might induce PD-L1 expression in PitNET, as described in sarcomas [26]. Unfortunately, there was no information about radiotherapy in previous series and none of the PD-L1-positive tumor reported here was irradiated.
PD-L1 expression was not different between the functioning and nonfunctioning groups which was inconsistent with some previous reports but consistent with one study [15–17]. Moreover, we did not find any association between intensity of hormonal secretion, the granulation and PD-L1 expression.
We explored the prognostic impact of PD-L1 expression in PitNET according to HYPOPRONOS classification. Invasion, defined as cavernous and/or sphenoid sinus invasion (CSI) on MRI, was not more frequent in the PD-L1-positive group. Sato et al. recently reported a trend to higher, though not significant PD-L1 expression in PitNET with cavernous sinus invasion (8/17 CSI + versus 1/10 CSI- with PD-L1 > 5%) [18]. Regarding the proliferative activity, we found that only one of 17 tumors with high Ki-67 (≥ 3%) and/or positive p53 expression was PD-L1 positive. No significant differences could be established for any proliferation criteria, and this may be due to the small number of PD-L1-positive cases. When we classified the tumors according to HYPOPRONOS grade, PD-L1-positive tumors were never classified in grade 1b or 2b, suggesting that PD-L1 expression seemed to be associated with less proliferation. Divergent results were previously observed about Ki-67 and PD-L1 expression, thus leaving this question open [15, 17].
We also compared the evolution of PD-L1 expression in 6 patients with recurrence. Neither primary tumors displayed PD-L1 expression nor the relapse tissue, which is inconsistent with the results of Mei et al. [15]. Therefore, PitNET progression in our study was not accompanied by PD-L1 expression. However, PitNET are benign tumor with sometimes a local aggressive behavior, unlike carcinoma, which is malignant tumor.
The description in the literature of the spectacular response of one case of ACTH-PitNET carcinoma with ipilimumab and nivolumab treatment is puzzling in regard to cumulated data concerning PD-L1 in PitNET. However, no PD-L1 expression was demonstrated in the liver metastasis, which may suggest that ipilimumab (anti CTLA-4) rather than nivolumab (anti-PD-L1) was responsible for tumor regression [10].
We also tested positive cases (along with 4 negative cases) with the QR1 clone, previously used in quality tests for laboratory-developed assays in lung carcinomas [27]. QR1 immunostaining revealed more PitNET with grades 2 and 3, but the correlations with HYPOPRONOS or with PitNET secretion were not different than those observed with the 22C3 clone.
The strengths of this study were, first, the use of PD-L1 immunostaining with the 22C3 clone approved for theranostic evaluation and, second, a large panel of PitNET of different histological types, whereas previous studies have been limited by smaller samples, or were restricted to some subtypes of adenomas. The limitation is the absence of pituitary carcinoma in our series.
In conclusion, our results showed that most PitNET do not exhibit PD-L1 expression. This study raises the need of PD-L1 evaluation in PitNET before considering PD-L1 blockage, in case of therapeutic impasse. The question of using this immunotherapy after radiotherapy is still open since post-radiotherapy lymphocytic infiltration may induce PD-L1 expression.
Acknowledgements
We thank Pr Patrick Saulnier for support with statistical analysis and Rym Ben Boubaker for her help in graph realization. We thank Fanny Rabin, François Lhériau and Lydie Denechaud for their technical skill in performing immunohistochemistry.
Abbreviations
- ACTH
Adrenocorticotropic hormone
- CTLA4
Cytotoxic T-lymphocyte-associated protein 4
- CE-IVD
European Conformity—In Vitro Diagnostic
- GH
Growth hormone
- HPF
High-power fields
- PitNET
Pituitary neuroendocrine tumors
- PD-L1
Programmed cell death-ligand 1
- PRL
Prolactin
- TPS
Tumor proportion score
Authors’ contribution statement
VS acquired clinical data, performed the statistical analysis, interpreted the results and drafted the manuscript; AC acquired pathology data, interpreted the results and drafted the manuscript; M-CR and CB interpreted the results and critically reviewed or revised the manuscript; PM and PR critically reviewed or revised the manuscript. All authors read and approved the final version of this manuscript.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Compliance with ethical standards
Conflict of interest
All authors declare no financial or other potential conflict of interest.
Research involving human participants/ethical approval retrospective study
Written informed consent was obtained from each patient. The retrospective data collection was registered to the Commission nationale de l’informatique et des libertés (CNIL) (CNIL no. 2017-051), according to French laws. Institutional review board was not necessary, according to French laws.
Footnotes
PRECIS: PD-L1 expression was assessed in a large cohort of pituitary neuroendocrine tumors using in vitro diagnostic antibody (clone 22C3). A small number of PD-L1 (18%) expressed PD-L1, and this marker was not associated with any tumor features.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ostrom QT, Gittleman H, Truitt G, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011–2015. Neuro-Oncol. 2018 doi: 10.1093/neuonc/noy131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tampourlou M, Ntali G, Ahmed S, et al. Outcome of nonfunctioning pituitary adenomas that regrow after primary treatment: a study from two large UK centers. J Clin Endocrinol Metab. 2017;102:1889–1897. doi: 10.1210/jc.2016-4061. [DOI] [PubMed] [Google Scholar]
- 3.Molitch ME. Diagnosis and treatment of pituitary adenomas: a review. JAMA. 2017;317:516–524. doi: 10.1001/jama.2016.19699. [DOI] [PubMed] [Google Scholar]
- 4.Raverot G, Burman P, McCormack A, et al. European society of endocrinology clinical practice guidelines for the management of aggressive pituitary tumours and carcinomas. Eur J Endocrinol. 2018;178:G1–G24. doi: 10.1530/EJE-17-0796. [DOI] [PubMed] [Google Scholar]
- 5.Raverot G, Dantony E, Beauvy J, et al. Risk of recurrence in pituitary neuroendocrine tumors: a prospective study using a five-tiered classification. J Clin Endocrinol Metab. 2017;102:3368–3374. doi: 10.1210/jc.2017-00773. [DOI] [PubMed] [Google Scholar]
- 6.Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376:1015–1026. doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1–positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 8.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 9.Herbst RS, Baas P, Kim D-W, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 10.Lin AL, Jonsson P, Tabar V, et al. Marked response of a hypermutated ACTH-secreting pituitary carcinoma to ipilimumab and nivolumab. J Clin Endocrinol Metab. 2018;103:3925–3930. doi: 10.1210/jc.2018-01347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eigentler TK, Hassel JC, Berking C, et al. Diagnosis, monitoring and management of immune-related adverse drug reactions of anti-PD-1 antibody therapy. Cancer Treat Rev. 2016;45:7–18. doi: 10.1016/j.ctrv.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Faje A. Immunotherapy and hypophysitis: clinical presentation, treatment, and biologic insights. Pituitary. 2016;19:82–92. doi: 10.1007/s11102-015-0671-4. [DOI] [PubMed] [Google Scholar]
- 13.Solinas C, Porcu M, De Silva P, et al. Cancer immunotherapy-associated hypophysitis. Semin Oncol. 2018;45:181–186. doi: 10.1053/j.seminoncol.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Torino F, Barnabei A, Paragliola RM, et al. Endocrine side-effects of anti-cancer drugs: mAbs and pituitary dysfunction: clinical evidence and pathogenic hypotheses. Eur J Endocrinol. 2013;169:R153–R164. doi: 10.1530/EJE-13-0434. [DOI] [PubMed] [Google Scholar]
- 15.Mei Y, Bi WL, Greenwald NF, et al. Increased expression of programmed death ligand 1 (PD-L1) in human pituitary tumors. Oncotarget. 2016;7:76565–76576. doi: 10.18632/oncotarget.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salomon MP, Wang X, Marzese DM, et al. The epigenomic landscape of pituitary adenomas reveals specific alterations and differentiates among acromegaly, Cushing’s disease and endocrine-inactive subtypes. Clin Cancer Res. 2018;24:4126–4136. doi: 10.1158/1078-0432.CCR-17-2206. [DOI] [PubMed] [Google Scholar]
- 17.Wang P, Wang T, Yang Y, et al. The expression profile of PD-L1 and CD8 + lymphocyte in pituitary adenomas indicating for immunotherapy. J Neurooncol. 2018;139:89–95. doi: 10.1007/s11060-018-2844-2. [DOI] [PubMed] [Google Scholar]
- 18.Sato M, Tamura R, Tamura H, et al. Analysis of tumor angiogenesis and immune microenvironment in non-functional pituitary endocrine tumors. J Clin Med. 2019 doi: 10.3390/jcm8050695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kemeny HR, Elsamadicy AA, Farber SH, et al. Targeting PD-L1 initiates effective anti-tumor immunity in a murine model of Cushing’s Disease. Clin Cancer Res. 2019 doi: 10.1158/1078-0432.CCR-18-3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gejman R, Swearingen B, Hedley-Whyte ET. Role of Ki-67 proliferation index and p53 expression in predicting progression of pituitary adenomas. Hum Pathol. 2008;39:758–766. doi: 10.1016/j.humpath.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Lopes MBS. The 2017 World Health Organization classification of tumors of the pituitary gland: a summary. Acta Neuropathol. 2017;134:521–535. doi: 10.1007/s00401-017-1769-8. [DOI] [PubMed] [Google Scholar]
- 22.Saeger W, Honegger J, Theodoropoulou M, et al. Clinical impact of the current WHO classification of pituitary adenomas. Endocr Pathol. 2016;27:104–114. doi: 10.1007/s12022-016-9418-7. [DOI] [PubMed] [Google Scholar]
- 23.Büttner R, Gosney JR, Skov BG, et al. Programmed death-ligand 1 immunohistochemistry testing: a review of analytical assays and clinical implementation in non-small-cell lung cancer. J Clin Oncol. 2017;35:3867–3876. doi: 10.1200/JCO.2017.74.7642. [DOI] [PubMed] [Google Scholar]
- 24.Taube JM, Anders RA, Young GD, et al. Colocalization of inflammatory response with B7-H1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012 doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teng MWL, Ngiow SF, Ribas A, Smyth MJ. Classifying cancers based on T cell infiltration and PD-L1. Cancer Res. 2015;75:2139–2145. doi: 10.1158/0008-5472.CAN-15-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel KR, Martinez A, Stahl JM, et al. Increase in PD-L1 expression after pre-operative radiotherapy for soft tissue sarcoma. Oncoimmunology. 2018 doi: 10.1080/2162402X.2018.1442168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scheel AH, Baenfer G, Baretton G, et al. Interlaboratory concordance of PD-L1 immunohistochemistry for non-small-cell lung cancer. Histopathology. 2018;72:449–459. doi: 10.1111/his.13375. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.