Abstract
We discussed the potentialities of tumor mutation burden (TMB) as a predictive marker for immunotherapy in breast cancer, also highlighting the limits that have hindered its introduction in the clinical practice. Although some studies have demonstrated the possibility to select patients more responsive to immune-checkpoint inhibitors by evaluating TMB, some issues emerged regarding the complexity of the methodologies for its determination, the costs of the analysis, and the necessity to improve the TMB determination with that of neoantigen identification.
Keywords: TMB, Breast cancer, PD-L1, Immune-checkpoint inhibitors
Editorial
Immune-checkpoint inhibitors (ICIs) represent a new treatment strategy for a variety of cancers. However, not all patients respond to anti-programmed cell death protein-1 (PD-1) or programmed cell death protein-ligand 1 (PD-L1) therapies; therefore, it is important to identify biomarkers that can predict clinical response.
Tumor mutation burden (TMB) emerged as a prominent independent biomarker for prediction of response to PD-1/PD-L1 pathway inhibitors in several cancer types [1]. While TMB has been widely investigated in lung cancer, few studies have been conducted on Breast Cancer (BC) and the significance of TMB in this tumor type remains unclear. TMB is the measure of the number of somatic/acquired mutations within a tumor genome, defined as the total number of non-synonymous point mutations per coding area of a tumor genome (Mut/Mb) [2], and it is very heterogeneous among different tumor types. It is well known that tumors with high TMB produce a larger number of neoantigens, making them more immunogenic [1]. However, not all mutations are able to generate neoantigens, since a wide series of biological processes are necessary, from the presence of a DNA mutation to the effective generation of a neoantigen and its recognition by the immune cells.
Previous studies have demonstrated that TMB could be a potential predictive biomarker for ICIs, but difficulties on its detection arose [1, 3]. TMB is a highly dynamic entity and some authors demonstrated that it is very heterogeneous and can change even between primary tumor tissue and metastasis [3]. Moreover, it can be difficult to be evaluated in small tumors.
Several studies have highlighted the possibility to evaluate TMB in liquid biopsy on free-circulating DNA with promising, but conflicting results [4]. To establish the role of TMB on tissue and liquid biopsies, further studies are needed. Another factor that can explain the different results obtained is the wide disparity in the platforms and cut-off used and proposed to evaluate TMB [4]. The main question is how many genes have to be analyzed for “a perfect” TMB. Different cut offs, in terms of number of mutations, have been proposed (e.g., 10, 16, 20 Mut/MB) to reach the best accuracy in identifying an ICI responsive patient. The lack of standardization in terms of which and how many genes have to be considered affects the interpretation of the results. Moreover, the tests are often company-sponsored and strictly dependent on the platform present in the lab. The choice of the test and the type of platform used are conditioned by the country where the patient lives. Among the economic problems, one of the reasons why TMB is not routinely performed is the lack of reimbursement of the test. The gene panel assay FoundationOne CDx for profiling solid tumors for molecular alterations has been approved by the US Food and Drug Administration (FDA). Nowadays, this assay is available in clinical practice, but many other tests are available for research use only. The assays differ for the number of genes and the types of mutations analyzed, sequencing system, enrichment approach and bioinformatic pipeline.
Given the complexity of the immune response and tumor biology, some authors have tried to compare and combine TMB with other biomarkers, to improve the accuracy in patient selection for immunotherapies. Recently, the treatment of triple-negative BC patients has changed, due to the results obtained in IMpassion130 clinical trial in terms of survival using the combination of Nab-Paclitaxel plus Atezolizumab [5, 6]. In this study, to select ICIs responders, PD-L1 was identified as the best biomarker, even if it has some limitations.
Only about 10% of BCs show PD‐L1 expression, measured either on tumor cells or on TILs, with higher rates in TNBC. Contrary to other tumor types, PD‐L1 expression on tumor cells is not a valid predictive biomarker of ICI efficacy in BC. PD‐L1 in IMpassion130 trials was assessed on infiltrating immune cells and measured as the fraction of positive immune area in relation to the whole tumor area, considering as positive tumors with expression on ≥ 1% [6, 7].
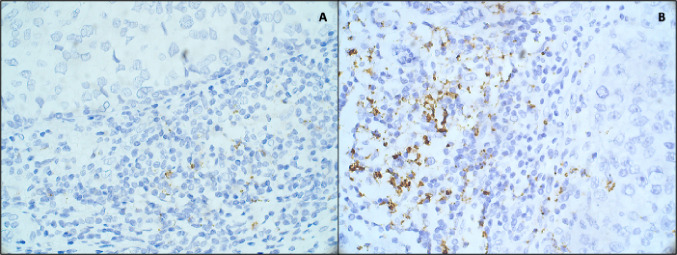
In the IMpassion130 study, the anti-PD-L1 antibody clone SP142 (Roche Ventana) was used. As we observed in our experience on different BC subtypes, the SP142 clone was able to identify not only PD-L1-positive tumor cells, but also PD-L1-positive immune cells (Fig. 1).
Fig. 1.
Immunohistochemical staining for PD-L1 expression at ×40 magnification, using Ventana Platform and Ventana antibody (clone SP142) of a Luminal BC and b triple-negative BC tissues, showing a low and a high PD-L1 expression in immune cells, respectively.
The main question is whether TMB could be used instead or together with PD-L1 in the clinic to better select ICIs responders. It has been demonstrated that TMB does not correlate with PD-L1 expression, since these biomarkers identify different populations of patients, even if some authors reported that both biomarkers had similar predictive value [8].
The role of TMB in BC, particularly in relation to PD-L1 expression, remains unclear. Some authors demonstrated that high TMB was observed with a low frequency (3/62 cases, 4.8%) in BC [9]. TMB levels were positively associated with tumor-infiltrating lymphocytes (TILs), and a significantly higher TMB was observed in breast carcinomas with DNA-damage repair (DDR) gene mutation(s) [9]. These data indicate the importance of DDR proteins in maintaining DNA integrity and immune reaction and breast carcinoma, indicating that patients with mutations in DDR genes may benefit from immunotherapy [9].
In another study, a durable complete response with ICIs in BC patients with high TMB and APOBEC signature was seen [10]. The study highlights that some patients may still experience response to ICIs despite having low levels of TILs and PD-L1 negativity [10]. Probably, the incorporation of both TMB and PD-L1 expression into multivariable predictive models should give a higher predictive power [8].
Despite the promising results, TMB is still not conventionally used in the clinical practice in BC, mostly due to low reproducibility of its results among different laboratories, even if several efforts have been done for TMB harmonization. Methodological standardization of TMB assay must be obtained before it can be fully usable in clinical practice. In addition, its detection in liquid biopsy compared to its evaluation on the tissue needs to be better explored.
Author contributions
SB performed the study conception and design. Material preparation, data collection and analysis were performed by SB, SR, FL, MMT, MP and RM. The first draft of the manuscript was written by SB and SR. All authors read and approved the final manuscript.
Funding
None.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Consent for publication
All authors consent to the publication of the manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chan TA, Yarchoan M, Jaffee E, et al. Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann Oncol. 2019;30(1):44–56. doi: 10.1093/annonc/mdy495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meléndez B, Van Campenhout C, Rorive S, et al. Methods of measurement for tumor mutational burden in tumor tissue. Transl Lung Cancer Res. 2018;7:661–667. doi: 10.21037/tlcr.2018.08.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedlaender A, Nouspikel T, Christinat Y, et al. Tissue-plasma TMB comparison and plasma TMB monitoring in patients with metastatic non-small cell lung cancer receiving immune checkpoint inhibitors. Front Oncol. 2020;10:142. doi: 10.3389/fonc.2020.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ou S-HI, Nagasaka M, Zhu VW. Liquid biopsy to identify actionable genomic alterations. Am Soc Clin Oncol Educ B. 2018 doi: 10.1200/EDBK_199765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Narayan P, Wahby S, Gao JJ, et al. FDA approval summary: atezolizumab plus paclitaxel protein-bound for the treatment of patients with advanced or metastatic TNBC whose tumors express PD-L1. Clin cancer Res Off J Am Assoc Cancer Res. 2020;26:2284–2289. doi: 10.1158/1078-0432.CCR-19-3545. [DOI] [PubMed] [Google Scholar]
- 6.Schmid P, Adams S, Rugo HS, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379:2108–2121. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 7.Palleschi M, Maltoni R, Sarti S, et al. Immunotherapy: the end of the “dark age” for metastatic triple-negative breast cancer? Breast J. 2020 doi: 10.1111/tbj.13662. [DOI] [PubMed] [Google Scholar]
- 8.Rizvi H, Sanchez-Vega F, La K, et al. Molecular determinants of response to anti-programmed cell death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in patients with non–small-cell lung cancer profiled with targeted next-generation sequencing. J Clin Oncol. 2018;36:633–641. doi: 10.1200/JCO.2017.75.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mei P, Freitag CE, Wei L, et al. High tumor mutation burden is associated with DNA damage repair gene mutation in breast carcinomas. Diagn Pathol. 2020;15:50. doi: 10.1186/s13000-020-00971-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chumsri S, Sokol ES, Soyano-Muller AE, et al. Durable complete response with immune checkpoint inhibitor in breast cancer with high tumor mutational burden and APOBEC signature. J Natl Compr Canc Netw. 2020 doi: 10.6004/jnccn.2020.7543. [DOI] [PubMed] [Google Scholar]