Abstract
Purpose
Tumor microenvironment is important in the progression of clear cell renal cell carcinoma (ccRCC), and its prognostic value is still unclear. Recent reports demonstrated tumor-infiltrating CD39+CD8+ T cells are abundant, but their function remains obscure. We aim to assess clinical value of CD39+CD8+ T cells and seek a potential therapeutic target in ccRCC.
Experimental design
We immunohistochemically evaluated clinical value of CD39+CD8+ T cells in a retrospective Zhongshan Hospital cohort of 243 ccRCC patients. Fresh tumor samples (n = 48), non-tumor tissues and peripheral blood for flow cytometry analyses were collected to analyze immune cell functions from Zhongshan Hospital. The survival benefit of tyrosine kinase inhibitors (TKIs) in this subpopulation was evaluated. Kaplan–Meier analysis and COX regression model were applied for survival analyses. Bioinformatics analysis performed in TCGA KIRC cohort and the scRNA-seq cohort.
Results
We found that accumulation of CD39+CD8+ T cells indicated poor prognosis (p < 0.0001) and indicated therapeutic benefit of TKIs therapy (p = 0.015). CD39+CD8+ T cells showed decreased TNF-α and IFN-γ with elevated PD-1 and TIM-3 expression. Further analysis of tumor-infiltrating immune cell landscape in the ccRCC revealed the positive correlation between CD39+CD8+ T cells and Tregs (p = 0.037) and M2-polarized macrophages (p < 0.0001). Finally, inhibition of CD39 partially restores the anti-tumor function of CD8+ T cells.
Conclusions
High CD39+CD8+ T cells indicated poor prognosis in ccRCC, due to impaired anti-tumor function of CD39+CD8+ T cells and indicated therapeutic benefit of TKIs therapy.
Graphic abstract
Electronic supplementary material
The online version of this article (10.1007/s00262-020-02563-2) contains supplementary material, which is available to authorized users.
Keywords: Clear cell renal cell carcinoma, CD39, CD8+ T cell, Prognosis, Tyrosine kinase inhibitors
Introduction
Renal cell carcinoma is a common urologic tumor and represents 2–3% of all malignancies in adults worldwide annually [1, 2]. Clear cell renal cell carcinoma (ccRCC) is the most common histological subtype of RCC, accounting for nearly 70–80% of all renal cell carcinoma [3]. Surgical resection is the main treatment for localized renal cancer, and postoperative patients have a relative good prognosis [4]. However, one-third of the patients with recurrences or metastases after surgeries display poor prognosis [5]. Tyrosine kinase inhibitors (TKIs) such as sorafenib and sunitinib have been approved for the treatment of advanced ccRCC in the first-line therapy [6]. However, only small proportion of patients have respond to TKI therapy [7].
Besides TKIs, the result of CheckMate 214 research has shown the promising efficacy of immune checkpoint inhibitors (ICIs) for patients with advanced renal cell carcinoma [8], which means the focus of cancer therapy has transformed from the tumor itself to the tumor microenvironment (TME), to overcome the dysfunction or exhaustion of T cells which were suppressed by the tumor cells [9]. Indeed, many researches have demonstrated that the CD8+ T cells are enriched in the TME of ccRCC, but its association with clinical prognosis and responses are controversial [10, 11]. In the TME, CD8+ T cells can be shifted from the effector state to the dysfunction state. Two distinct features of exhausted CD8+ T cells are the decrease in effector cytokine and sustained expression of multiple inhibitory receptors. Evan N demonstrated that CD39−CD8+ T cells are bystanders in human tumor infiltration and the frequencies of CD39+CD8+ T cells correlated with clinical parameters in lung tumors, showing that CD39+CD8+ T cells include more tumor reactive T cells [12]. The defined state of CD8+ T cells may be the key to figure out the relationship between targeted therapy and prognosis in ccRCC.
The ectonucleotidase CD39 is expressed on several components of TME [13], especially effector T cells, Tregs and myeloid cells. CD39 is now emerging as an attractive therapeutic target to promote anti-tumor immune responses [14]. The functionality of CD39+CD8+ T cells has not been resolved. Besides, no study focused on the correlation between the CD39+CD8+ T cells and the prognosis and response to TKIs in ccRCC patients.
Here, we use flow cytometry and immunohistochemistry to demonstrate that CD39 is highly expressed on the CD8+ T cells and displays an exhausted function. Then, POM-1, an inhibitor of CD39, is used to restore the function of CD8+ T cells. Hence, considering the new immunotherapeutic strategies, our results suggest that CD39 could be regarded as a new immune checkpoint to recover the immune response against tumor in ccRCC.
Materials and methods
Patients and clinical database
Two cohorts of ccRCC patients were enrolled in the study. One cohort was composed of 243 non-metastatic ccRCC patients (age range 15–83 years, median 55) who received partial or radical nephrectomy at the department of Urology, Zhongshan Hospital, Fudan University, from January 2005 to June 2007. Patient inclusion criteria involve informed consent, ccRCC and no history of other malignancies. The other cohort, including 85 patients (age range 24–78 years, median 60) with metastatic ccRCC, and treated with sunitinib or sorafenib, screened at the Department of Urology, Zhongshan Hospital, Fudan University, between March 2005 and June 2014. All patients were followed up every 3 months, and the last follow-up was on April 30, 2017. Patient inclusion criteria contain: (1) informed consent, (2) diagnosis of metastatic ccRCC, (3) sunitinib or sorafenib as first-line systemic therapy. Following exclusion criteria were conformed in two cohorts: (1) lack of tissue sample, (2) tumor necrosis area greater than 80%, (3) received prior systemic therapy. Patients and their families were fully informed before the operation that the surgical samples would be used only for scientific research and the operation would not be affected. Clinicopathological characteristics of two cohorts are exhibited in Supplementary Tables 1 and 2.
Baseline demographic, clinical data were collected retrospectively from medical records. Histologic subtypes were determined according to the 2004 WHO criteria [15]. Metastases were diagnosed following radiographic. Initial tumor node metastasis stages were reassigned based on the 2010 AJCC TNM classification [16]. Retrospective information on treatment, response and survival was obtained from medical records and follow-up. Disease progression was defined according to the RECIST1.1 criteria [17]. The tumor stage, size, grade and necrosis (SSIGN) score remains a validated prognostic tool to predict survival of clear cell renal cell carcinoma patients [18]. The mRNA-seq data for the ccRCC were downloaded from https://science.sciencemag.org/content/361/6402/594/tab-figures-data [19]. The bioinformatics analyses of mRNA expression of 530 ccRCC patients were described in supplementary methods.
Flow cytometry
In order to observe the distribution of CD39+CD8+ T cells in the tissues, we collected peripheral blood and resected specimen from first ten renal clear cell carcinomas patients in stage I and II. Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized venous blood by lysing solution (BD Biosciences). Fresh tumor and non-tumor tissues (at least 2 cm away from the tumor site) were minced and digested with collagenase IV to prepare single cell suspensions. Then, single cells and PBMCs were stained with appropriate monoclonal antibodies for 30 min at 4 degree centigrade. Intracellular protein was performed with Fixation/Permeabilization Solution Kit (BD Biosciences). Cell suspensions and PBMC were stained with fluorochrome-labeled antibodies. The details of flow cytometry are provided in Supplementary Table 4. Flow cytometry was performed with a BD FACScelesta, and cells were analyzed using FlowJo software (Tree Star).
Immunohistochemistry (IHC) and immunofluorescence (IF)
Tissue microarray construction and the IHC protocol have been described previously [20]. Double staining was performed on human sample using a Double IHC Kit (ZSGB-BIO DS-0005) according to the provided factory instructions. The details of immunohistochemistry are provided in Supplementary Table 3. Immunohistochemistry sections were scanned by Olympus CDD camera, Nikon eclipse Ti-s microscope (200× magnification) and NIS-Elements F3.2 software. Two genitourinary pathologists, masked to the follow-up data, count the number of positive staining cells at 200× magnification, and the average number was used as the final number. The representative pictures for immunohistochemistry are shown in Supplementary Fig. 2.
For IF staining, the slides were incubated with the antibodies at 4 °C overnight. Then, samples were incubated with species-appropriate rabbit/mouse secondary antibodies coupled to AlexaFluor dyes (555, 488, Invitrogen) for 2 h at room temperature. DAPI (ab104139) was used to mount cover slips. The sections were analyzed through Leica DMi8 microsystems.
In vitro CD39 inhibition assay
Fresh renal cancer tissues were washed two times with RPMI-1640 containing 1% fetal bovine serum before being minced. Then, the specimens were collected in RPMI-1640 medium containing 1 mg/ml collagenase IV and incubated for 3 h at 37 °C under continuous rotation. The cell suspensions were then filtered through a 40-µm cell strainer (BD) and collected. Then, the cells were cultured with POM-1 (100 µmol/L) or vehicle for 24 h under 37 °C and 5% CO2. After overnight culture, the cells were subjected to phenotypic analysis by flow cytometry as above.
Statistics
The differences in mean ± SD and correlation analysis were evaluated with parametric (Student’s t test, paired t test and Pearson’s test) or nonparametric (Mann–Whitney U test and Spearman’s test) tests. Kaplan–Meier method and log rank test were used to demonstrate survival curves between different groups. Overall survival (OS) and recurrence-free survival (RFS) were calculated from the date of surgery to time of death and recurrence, respectively. We used the median (ratio of CD39+CD8+/CD8+=0.382) as the cut-off. Chi square test and Fisher’s exact test were used to assess correlations between CD39 expression and patient characteristics. Time-dependent ROC curve was performed by adding the weighted value of the CD39 to the SSIGN score. All analyses were two-sided and performed at a significance level of 5% (p < 0.05) using SPSS version 21.0, R 3.6.1 and graphpad8.0. The bioinformatics analysis of scRNA-seq and TCGA was described in supplementary methods.
Results
Accumulation of CD39+CD8+ T cells in renal cell carcinoma associated with disease progression
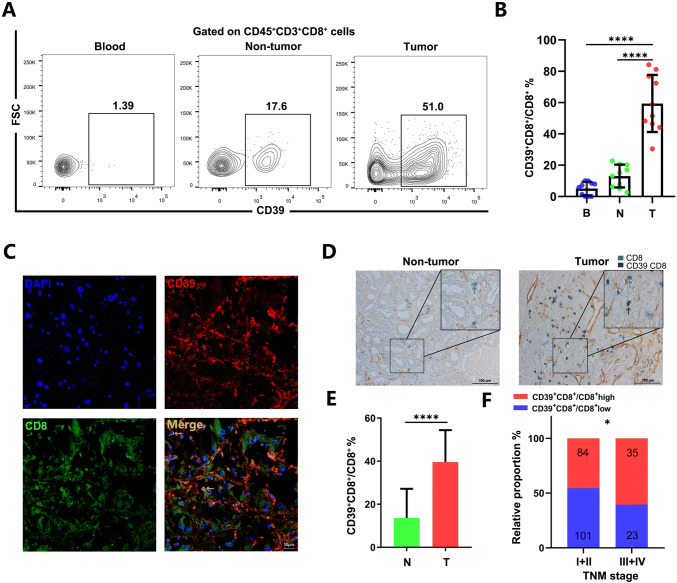
Previous studies reported the role of the CD39+CD8+ T cells was important to regulate immune response against tumors [9]. To explore the role of CD39, we examined the expression of CD39+CD8+ T cells from tumors, non-tumor tissue and PBMCs taken from ten ccRCC patients. Compared with PBMCs and non-tumor tissues, the frequency of the CD39+CD8+ T cells was higher in tumor (p < 0.0001, Fig. 1a, b). To test our findings in a non-metastatic ccRCC cohort (n = 243) with a different method, we stained fixed sections for CD8 and CD39. We found more CD39+CD8+ T cells in tumors compared with non-tumor tissues (p < 0.0001, Fig. 1c–e). Besides, we observed significantly elevated percentage of CD39+CD8+ T cells within the advanced TNM stage in ccRCC (p = 0.0470 Fig. 1f).
Fig. 1.
Accumulation of CD39+CD8+ T cells in renal cell carcinoma associated with disease progression. a, b The representative flow cytometry and statistics analysis of CD39 expression on CD8+ T cells in matched PBMCs, non-tumor tissues and tumor from patients with ccRCC (n = 10). ✱✱✱✱p < 0.0001 by paired t test. c Representative images of the immunofluorescence staining with CD39 (red), CD8 (green),DAPI (blue) and Merge (double positive) on ccRCC tissues. Scans were imaged at 200 magnification. d, e The percentage of CD39 expressed on CD8+ T cells is more in tumor than in non-tumor tissues. ✱✱✱✱p < 0.0001 by Student’ s t test. f The percentage of CD39+CD8+ T cells was elevated as the TNM stage progressed. ✱p < 0.05 by Chi square test. B = blood, N = non-tumor, T = tumor
Accumulation of CD39+CD8+ T cells predicts poor patient prognosis in ccRCC
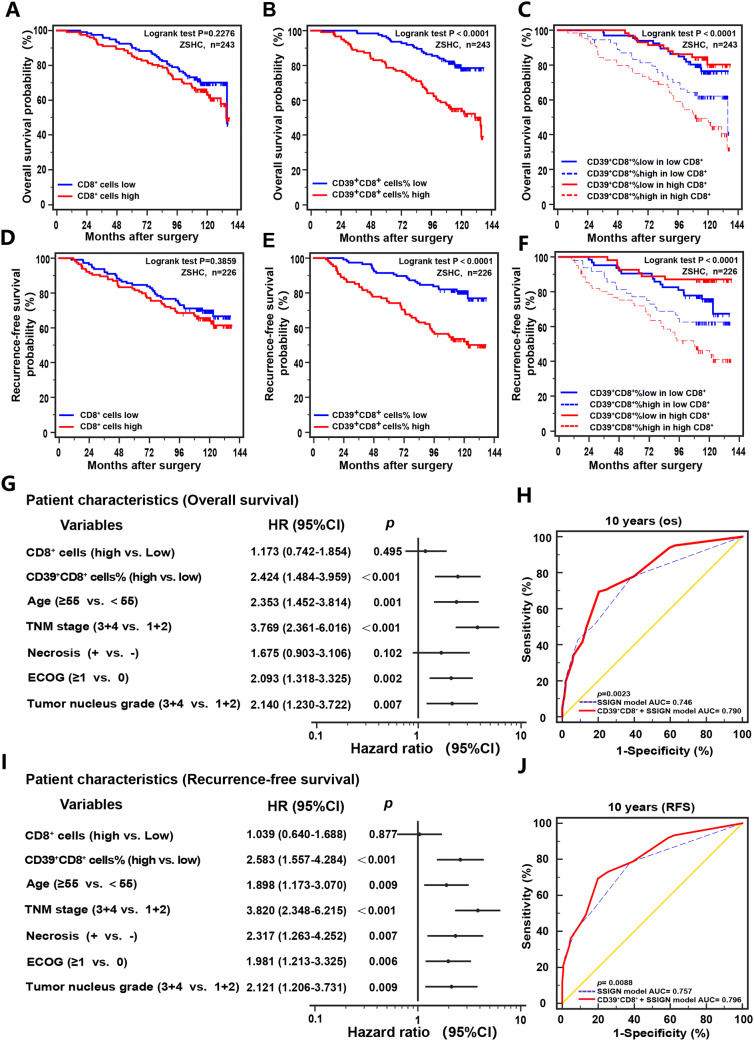
Although there are lots of CD8+ T cells infiltrated with ccRCC, its function to the anti-tumor immune response is not confirmed. The Kaplan–Meier curve for the 243 non-metastatic ccRCC patients shows that the overall survival (p = 0.2276) and recurrence-free survival (p = 0.3859) are not different between CD8+ T cells low and high (Fig. 2a, d). Interestingly, we found association between CD39+CD8+ T cells and prognosis. Accumulation of CD39+CD8+T cells was correlated with worse survival in both OS (p < 0.0001) and RFS (p < 0.0001, Fig. 2b, e). In high CD8+ T cells infiltration patients, the 10-year OS was estimated at 49.3% compared with 80.3% in patients with CD39+CD8+ cells low proportion subgroup (Fig. 2c), so did 10-year RFS (Fig. 2f). Multivariate analysis showed that besides ages and tumor invasion, the ratio of CD39+CD8+ T cells was an independent risk factor for OS and RFS (Fig. 2g, i). Besides, the combination of CD39+CD8+ T cells with SSIGN score showed a better prognostic value than SSIGN score alone (Fig. 2h, j).
Fig. 2.
CD39+CD8+ T cells predict sunitinib therapeutic response. Kaplan–Meier curve of overall survival (OS) and recurrence-free survival (RFS) of 243 non-metastatic ccRCC patients according to the CD8+ T cells expression (a, d), and percentage of CD39+CD8+ cells (b, e) and to the combination of both markers (c, f). Log-rank tests were used to derive p values for comparisons between groups. Multivariate Cox analysis identified the independent prognostic factors for OS and RFS in ccRCC patients (g, i). ROC analysis for predictive accuracy of SSIGN alone and combined with CD39 expression level for 10-year OS (h) and RFS (j). HR, hazard radio; CI, confidence interval
The proliferation of CD39+CD8+ T cells is repressed along with loss of function
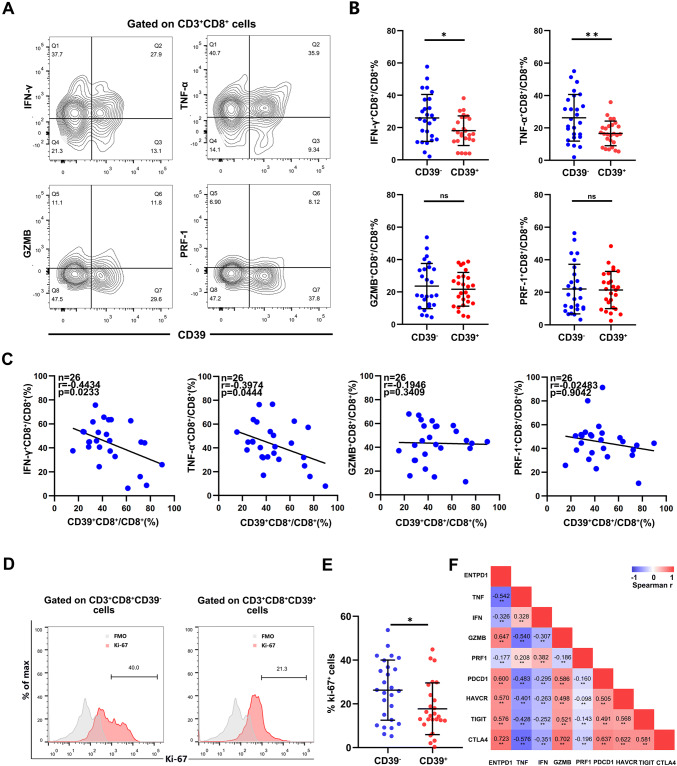
Next, we analyzed the function of CD39+CD8+ T cells infiltration in fresh renal cancer tissues. The proportion of IFN-γ+CD8+ T cell (p = 0.0254) and TNF-α+CD8+ T cell (p = 0.0031) was reduced in CD39+CD8+ T cells (Fig. 3a, b). Besides, our measurement of the CD39+CD8+ T cells infiltration was negatively correlated with IFN-γ+CD8+ T cell (Spearman’s r = − 0.4434 p = 0.0233) and TNF-α+CD8+ T cell (Spearman’s r = − 0.3974 p = 0.0444 Fig. 3c). Furthermore, the proliferation of CD39+CD8+ T cells was damaged (p = 0.0469 Fig. 3d, e). We find the correlation between expression of ENTPD-1 with effector and exhausted markers through scRNA-seq (Fig. 3f). Collectively, these findings show that the cytotoxic function of CD39+CD8+ T cells was impaired in the anti-tumor immune response.
Fig. 3.
The proliferation of CD39+CD8+ T cells is repressed along with loss of function. a, b Representative flow cytometry plots for TNF-α, IFN-γ, GZMB and PRF-1 in CD39− and CD39+ cells, with quantification below for 26 patients. c Correlation assessed by Pearson’s correlation analysis and linear regression analysis between percentage of CD39+CD8+ T cells and the frequencies of four effector markers in ccRCC patients based on the results of flow cytometry. d, e Representative FACS figures and statistics analysis of Ki-67+ cells frequencies in ccRCC tissue from patients with CD39− and CD39+. f Correlation between expression of ENTPD-1 and effector/exhausted markers through scRNA-seq. Bar plots show mean ± SD in panel B. ns: no significance, ✱p < 0.05; ✱✱p < 0.01 by paired t test and Spearman’s test
PD-1 and Tim-3 are increased in the CD39+CD8+ T cells, and POM-1 can restore partial function of CD39+CD8+ T cells
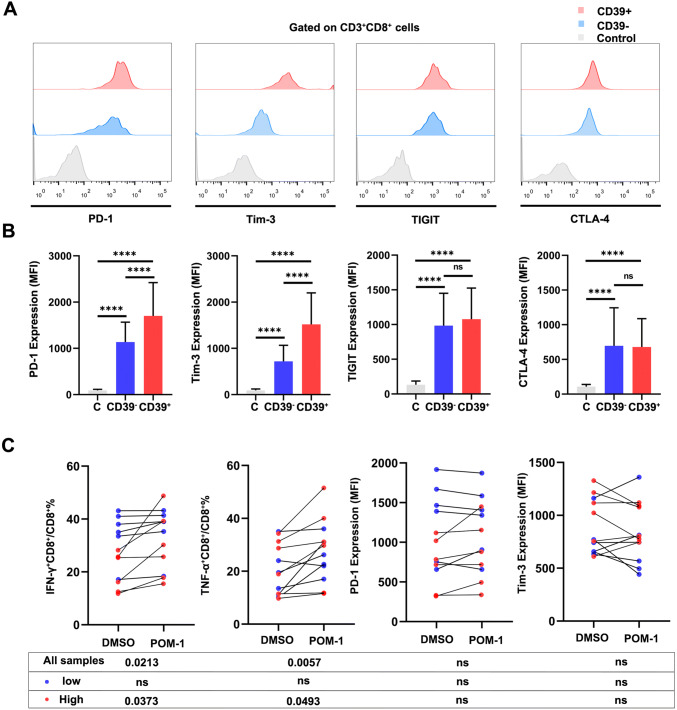
We further evaluated the immune checkpoint, including PD-1, Tim-3, CTLA-4 and TIGIT. Among CD8+ T cells, CD39+CD8+ T cells exhibited higher expression of PD-1 (p < 0.0001) and Tim-3 (p < 0.0001, Fig. 4a, b). Given the potential impact of CD39+CD8+ T cells on ccRCC, we next examined the therapeutic impact of targeting CD39 using POM-1. The single cells from ccRCC tumor were cultured with POM-1 (100 µmol/L) or vehicle for 24 h. Then, we examined the function and immune checkpoint through flow cytometry. The proportion of IFN-γ+ (p = 0.0213) and TNF-α+ cells (p = 0.0057) was recovered after POM-1 therapy, especially on the high percentage of CD39+CD8+ T cells. However, POM-1 did not change expression of the immune checkpoint, including PD-1 and Tim-3 (Fig. 4c). These data warrant that the CD39 target therapy can enhance anti-tumor response through CD39+CD8+ T cells.
Fig. 4.
PD-1 and Tim-3 are increased in the CD39+CD8+ T cells, and POM-1 can recover partial function of CD39+CD8+ T cells. a, b Expression of immune checkpoint in CD39−CD8+ and CD39+CD8+ T cells (n = 26, FMO was used for the control). c Frequencies of TNF-α+CD8+ T cells and IFN-γ+CD8+ T cells from patients (n = 12) after treated POM-1 or DMSO. Expression of PD-1 and Tim-3 from patients (n = 12) after treated POM-1 or DMSO. Bar plots show mean ± SD. ns: no significance, ✱p < 0.05; ✱✱p < 0.01; ✱✱✱p < 0.001 and ✱✱✱✱p < 0.0001 by paired t test. Low: percentage of CD39+CD8+ T cells low; high: percentage of CD39+CD8+ T cells high
Infiltration of CD39+CD8+ T cells in the tumor microenvironment is accompanied by Tregs and M2
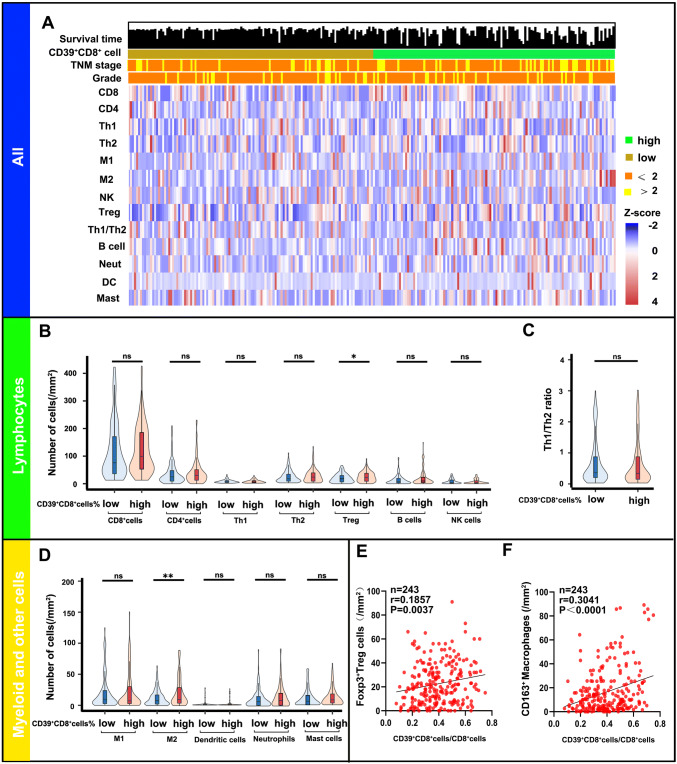
Given the impaired function of the CD39+CD8+ T cells, we want to know the correlation between the entire immune landscape and CD39+CD8+ cells in ccRCC. As shown in Fig. 5a, we employed 12 types of immune cells to define the immune landscape in cohort 1 (non-metastatic ccRCC, n = 243). High percentage of CD39+CD8+ T cells infiltration possessed more pro-tumor cells (Tregs p = 0.0187 and M2 p = 0.0075), while numbers of other immune cells infiltration were not different between the percentage of CD39+CD8+ cells low and high (Fig. 5b–d). Besides, high percentage of CD39+CD8+ T cells was associated with high proportion of Tregs (Spearman’s r = 0.1857 p = 0.0037) and M2 (Spearman’s r = 0.3041 p < 0.0001 Fig. 5e, f).
Fig. 5.
Infiltration of CD39+CD8+ T cells in the tumor microenvironment is accompanied by Tregs and M2. a Heat map displaying scaled expression of various immune cell types between high/low percentage of CD39+CD8+ T cells. b–f Comparison of the various immune cell types between high/low percentage of CD39+CD8+ T cells. ns: no significance, ✱p < 0.05 and ✱✱p < 0.01 by Mann–Whitney U test; B cell = B cells; CD4 = CD4+ T cells; Th1 = type 1 T helper cells; Th2 = type 2 T helper cells; CD8 = CD8+ T cells; DC = dendritic cells; M1 = M1 macrophages; M2 = M2 macrophages; Mast = mast cells; Neut = neutrophils; NK = nature killer cells; Treg = regulatory T cells
CD39+CD8+ T cells predict sunitinib therapeutic response
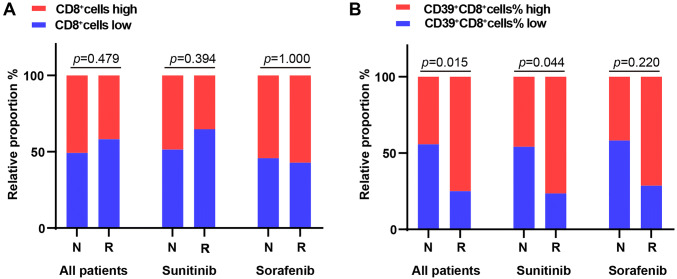
In the other cohort, including 85 patients with metastatic ccRCC, and treated with sunitinib or sorafenib, we classified two categories: regression/response (n = 24, including complete response and partial response) and progression/non-response (n = 61, including stable disease and progressive disease). We noticed that the number of CD8+ T cells infiltration was not correlated with the response rate of TKI (Chi square test p = 0.4790, Fig. 6a). However, the patients with the high percentage of CD39+CD8+ cells had higher response rate compared to the patients with the lower percentage of CD39+CD8+ cells (Chi square test p = 0.0150) and this benefit only existed in sunitinib (Fisher’s exact test p = 0.0440, Fig. 6b). In short, these results above show that CD39+CD8+ T cells are associated with poor prognosis and tumor progression, but have better response in TKI cohort.
Fig. 6.
CD39+CD8+ T cells predict sunitinib therapeutic response. a, b TKI treatment responses (n = 85) in the group of CD8+ and the percentage of CD39+CD8+ cells
Discussion
Accumulating evidence suggests that TME, including cancer cells and immune cells, supports tumor growth and evasion [21, 22]. CD39, as a ectoenzyme, is of great importance on the physiology and pathophysiology in the kidney [23]. In this study, we discovered the suppressive function of CD39+CD8+ T cells in ccRCC. The results of our study showed that the frequencies of CD39+CD8+ T cells were more in the ccRCC tissues than in normal tissue and PBMCs and positively correlated with TNM stage. Previous studies reported that expression of CD39 is both increased by hypoxic conditions and TGF-β [23, 24], providing a reasonable explanation that expression of CD39 may result as a consequence of the integration of different signals in the ccRCC.
For a long time, CD8+ T cells were believed to play a positive role in different solid tumors [25]. However, a study by Nakano et al. reported that CD8+ T cells do not indicate the anti-tumor immunity in renal cancer [10], same as in lung cancer [26]. In our study, we discovered that the number of CD8+ T cells was not associated with patients prognosis in ccRCC, but low tumor infiltrating CD8+CD39+ T cells, as an independent factor, predicted better OS (Fig. 2b) in ccRCC patients, which showed that the state of CD8+ T cells, in addition to distribution and number of CD8+ T cells, was significant for induction of tumor immunity [27]. The mechanism of CD39+CD8+ T cells leading to tumor progression is still obscure. Our analysis showed that anti-tumor function of CD39+CD8+ T cells was impaired, secreting less TNF-α and INF-γ (Fig. 3b), consistent with previous studies [28]. Besides, immune checkpoint (PD-1 and Tim-3) was accumulated in the CD39+CD8+ T cells. The decreased cytotoxicity and increased suppressive molecules could contribute to tumor growth and evasion, which may result in the poor outcome of ccRCC patients (Supplementary Fig. 1). Additionally, we found suppressive immune components were increasing within the CD39+CD8+T cells infiltration in the TME (Fig. 5). High percentage of CD39+CD8+ T cells infiltration group possessed more pro-tumor cells (Tregs and M2), while effector cells (NK, M1, DC and CD4+ T cells) showed no differences between high and low groups. M2 and Tregs, as mainly anti-inflammatory cells, can be promoted via A2BR signaling [23]. So present results revealed CD39+CD8+ T cells may be a new prognostic biomarker for postoperative patients with ccRCC.
In the past decades, TKIs, mainly targeting vascular endothelial growth factor receptor (VEGFR), like sunitinib and sorafenib were generally used to treat metastatic ccRCC patients, but only small proportion of patients responded to the drugs [7]. The new predictive marker was needed to avert treating advanced renal cancer patients with no benefit of TKIs. Hypoxia can induce the expression of VEGF with a HIF-1α dependent manner, binding to the hypoxia response element in the VEGF promoter to activate its transcription [29]. Besides, Doctor Beldi demonstrated that targeted disruption of CD39 would reduce angiogenesis in partial hepatectomy and VEGF receptor transactivation failed in CD39-null endothelial cells [30]. So in our metastatic ccRCC cohort, results showed that patients with high CD39+CD8+ T cells had better response to TKI therapy in metastatic ccRCC (Chi square test p = 0.015), which has not been reported in previous studies. In our study, we used anti-VEGFA antibody (ab28775) to explore the relationship between CD39+CD8+ T cells and VEGFA expression in 85 patients with metastatic ccRCC. We found that VEGFA expressions were higher in high CD39+CD8+ T cells group (p < 0.05, Supplementary Fig. 3a, b). Besides, high percentage of CD39+CD8+ T cells was associated with high proportion of VEGFA+ cells (Spearman’s r = 0.3646 p = 0.0006, Supplementary Fig. 3c). Therefore, we hypothesized that highly infiltrated CD39+CD8+ T cells in renal cell carcinoma indicated not only the immunosuppressive microenvironment, but also indicated activation of the VEGF pathway, which leads to ccRCC patients with high CD39+CD8+ T cells showing therapeutic benefit of TKIs therapy.
Recently, ICIs have shown promise in advanced renal cancer. An open label, multicenter, phase III trial compared nivolumab plus ipilimumab versus sunitinib in advanced renal cancer patients showing that objective response rates were significantly higher of the former [8]. However, the response rates to anti-CTLA4 were only 13% in advanced renal cancer patients. So we need to find some new immune checkpoints to increase the methods of the therapies. Some agents were developed to block the adenosine axis and showed significantly important anti-tumor activity [31]. As shown above, we found anti-tumor function of CD39+CD8+ T cells was impaired. After employment of POM-1, the effector function of CD39+CD8+ T cells is partially restored without changing the immune checkpoint. Accordingly, our research provided evidence that CD39+CD8+ T cells induced a suppressive immune microenvironment and could provide a new immune checkpoint target in ccRCC.
The major limitations of the study are its single institution study composed of one ethnicity, and we do not know the detailed mechanism of CD8+CD39+ T cells in vivo. The association between TME and CD39+CD8+ T cells is also needed to be explored. Because of the affordability and clinical efficacy, sorafenib as a second-line TKIs in patients with metastatic RCC today had been used as first-line treatment in some patients in the cohort in the early years. Moreover, the effect and safety of combined CD39 inhibitor and ICIs therapy needed to be studied in further clinical trials.
Conclusion
Our study showed that high percentage of CD39+CD8+ T cells was a poor prognostic factor in ccRCC patients. However, it indicated better response to TKI treatment in metastatic ccRCC. Moreover, CD39 inhibitors may drive anti-tumor response and provide potential therapeutic effect in ccRCC patients. Future studies and prospective validations are still needed to better understand the molecular mechanisms.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the financial support from grants from National Natural Science Foundation of China (81671628, 31770851, 81702496, 81702497, 81702805, 81772696, 81871306, 81872082, 81902556, 81902563, 81902898, 81974393), National Key R&D Program of China (2017YFC0114303), Shanghai Municipal Natural Science Foundation (16ZR1406500, 17ZR1405100, 19ZR1431800), Guide Project of Science and Technology Commission of Shanghai Municipality (17411963100), Shanghai Sailing Program (18YF1404500, 19YF1407900, 19YF1427200), Shanghai Municipal Commission of Health and Family Planning Program (20174Y0042, 201840168, 20184Y0151), Fudan University Shanghai Cancer Center for Outstanding Youth Scholars Foundation (YJYQ201802) and Shanghai Cancer Research Charity Center. All these study sponsors have no roles in the study design, collection, analysis and interpretation of data.
Author contributions
YQ, YX, ZL and YQ were involved in acquisition of data, analysis and interpretation of data, statistical analysis and drafting of the manuscript; YQ, YC, QZ, HZ, JW, YC, QB, YW, YZ, LX, LC, YK, WZ and BD contributed to technical and material support; LL, JG and JX were involved in study concept and design, analysis and interpretation of data, drafting of the manuscript, funding and study supervision. All authors read and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Ethical standard
This study was approved by the Clinical Research Ethics Committee of Zhongshan Hospital, Fudan University, with the approval number B2015-030. Informed consent was obtained from each patient included in this study. Our study followed the Helsinki declaration.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yu Qi, Yu Xia, Zhiyuan Lin, and Yang Qu authors have contributed equally to this work.
Contributor Information
Li Liu, Email: liu.li@zs-hospital.sh.cn.
Jianming Guo, Email: guo.jianming@zs-hospital.sh.cn.
Jiejie Xu, Email: jjxufdu@fudan.edu.cn.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Schodel J, Grampp S, Maher ER, et al. Hypoxia, hypoxia-inducible transcription factors, and renal cancer. Eur Urol. 2016;69(4):646–657. doi: 10.1016/j.eururo.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen HT, Mcgovern FJ. Renal-cell carcinoma. N Engl J Med. 2005;353:2477–2490. doi: 10.1056/NEJMra043172. [DOI] [PubMed] [Google Scholar]
- 4.Scherr AJ, Lima JPS, Sasse EC, Lima CS, Sasse AD. Adjuvant therapy for locally advanced renal cell cancer: a systematic review with meta-analysis. BMC Cancer. 2011;11(1):115. doi: 10.1186/1471-2407-11-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta K, Miller JD, Li JZ, et al. Epidemiologic and socioeconomic burden of metastatic renal cell carcinoma (mRCC): a literature review. Cancer Treat Rev. 2008;34(3):193–205. doi: 10.1016/j.ctrv.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Rj M, Te Hutson PT. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 7.Rini BI, Atkins MB. Resistance to targeted therapy in renal-cell carcinoma. Lancet Oncol. 2009;10(10):992–1000. doi: 10.1016/S1470-2045(09)70240-2. [DOI] [PubMed] [Google Scholar]
- 8.Motzer RJ, Tannir NM, Mcdermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277–1290. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sade-Feldman M, Yizhak K, Bjorgaard SL, et al. Defining T cell states associated with response to checkpoint immunotherapy in melanoma. Cell. 2018;175(4):998–1013. doi: 10.1016/j.cell.2018.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakano Osamu, Sato Makoto, Naito Yoshitaka, et al. Proliferative activity of intratumoral CD8+ T-lymphocytes as a prognostic factor in human renal cell carcinoma. Cancer Res. 2001;61(13):5132–5136. [PubMed] [Google Scholar]
- 11.Yao J, Xi W, Zhu Y, et al. Checkpoint molecule PD-1-assisted CD8(+) T lymphocyte count in tumor microenvironment predicts overall survival of patients with metastatic renal cell carcinoma treated with tyrosine kinase inhibitors. Cancer Manag Res. 2018;10:3419–3431. doi: 10.2147/CMAR.S172039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simoni Y, Becht E, Fehlings M, et al. Bystander CD8(+) T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature. 2018;557(7706):575–579. doi: 10.1038/s41586-018-0130-2. [DOI] [PubMed] [Google Scholar]
- 13.Zhang H, Vijayan D, Li XY, et al. The role of NK cells and CD39 in the immunological control of tumor metastases. Oncoimmunology. 2019;8(6):e1593809. doi: 10.1080/2162402X.2019.1593809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bastid J, Regairaz A, Bonnefoy N, et al. Inhibition of CD39 enzymatic function at the surface of tumor cells alleviates their immunosuppressive activity. Cancer Immunol Res. 2015;3(3):254–265. doi: 10.1158/2326-6066.CIR-14-0018. [DOI] [PubMed] [Google Scholar]
- 15.Delahunt B, Srigley JR, Montironi R, Egevad L. Advances in renal neoplasia: recommendations from the 2012 international society of urological pathology consensus conference. Urology. 2014;83(5):969–974. doi: 10.1016/j.urology.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 17.Eisenhauer EA, Therasse P, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 18.Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score. J Urol. 2002;168(6):2395–2400. doi: 10.1016/S0022-5347(05)64153-5. [DOI] [PubMed] [Google Scholar]
- 19.Young MD, Mitchell TJ, Vieira Braga FA, et al. Single-cell transcriptomes from human kidneys reveal the cellular identity of renal tumors. Science. 2018;361(6402):594–599. doi: 10.1126/science.aat1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J, Liu L, Qu Y, et al. Prognostic value of SETD2 expression in patients with metastatic renal cell carcinoma treated with tyrosine kinase inhibitors. J Urol. 2016;196(5):1363–1370. doi: 10.1016/j.juro.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 21.Brummelman J, Mazza EMC, Alvisi G, et al. High-dimensional single cell analysis identifies stem-like cytotoxic CD8(+) T cells infiltrating human tumors. J Exp Med. 2018;215(10):2520–2535. doi: 10.1084/jem.20180684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finn OJ. Cancer vaccines: between the idea and the reality. Nat Rev Immunol. 2003;3(8):630–641. doi: 10.1038/nri1150. [DOI] [PubMed] [Google Scholar]
- 23.Kishore BK, Robson SC, Dwyer KM. CD39-adenosinergic axis in renal pathophysiology and therapeutics. Purinergic Signal. 2018;14(2):109–120. doi: 10.1007/s11302-017-9596-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, Wang L, Chen X, et al. CD39/CD73 upregulation on myeloid-derived suppressor cells via TGF-beta-mTOR-HIF-1 signaling in patients with non-small cell lung cancer. Oncoimmunology. 2017;6(6):e1320011. doi: 10.1080/2162402X.2017.1320011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsumoto H, Thike AA, Li H, et al. Increased CD4 and CD8-positive T cell infiltrate signifies good prognosis in a subset of triple-negative breast cancer. Breast Cancer Res Treat. 2016;156(2):237–247. doi: 10.1007/s10549-016-3743-x. [DOI] [PubMed] [Google Scholar]
- 26.Mori M, Ohtani H, Naito Y, et al. Infiltration of CD8+ T cells in non-small cell lung cancer is associated with dedifferentiation of cancer cells, but not with prognosis. Tohoku J Exp Med. 2000;191:113–118. doi: 10.1620/tjem.191.113. [DOI] [PubMed] [Google Scholar]
- 27.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 28.Gourdin N, Bossennec M, Rodriguez C, et al. Autocrine adenosine regulates tumor polyfunctional CD73(+)CD4(+) effector T cells devoid of immune checkpoints. Cancer Res. 2018;78(13):3604–3618. doi: 10.1158/0008-5472.CAN-17-2405. [DOI] [PubMed] [Google Scholar]
- 29.Weijts BG, Bakker WJ, Cornelissen PW, et al. E2F7 and E2F8 promote angiogenesis through transcriptional activation of VEGFA in cooperation with HIF1. EMBO J. 2012;31(19):3871–3884. doi: 10.1038/emboj.2012.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beldi G, Wu Y, Sun X, et al. Regulated catalysis of extracellular nucleotides by vascular CD39/ENTPD1 is required for liver regeneration. Gastroenterology. 2008;135(5):1751–1760. doi: 10.1053/j.gastro.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vigano S, Alatzoglou D, Irving M, et al. Targeting adenosine in cancer immunotherapy to enhance T-cell function. Front Immunol. 2019;10:925. doi: 10.3389/fimmu.2019.00925. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.