Abstract
With dichotomous etiology and pathogenesis, intestinal type and diffuse type gastric cancers vary in their clinical and molecular features to the point of representing distinct entities. However, the differences of tumor-infiltrating immune cells within the two types of gastric cancer have not been well researched. This study was aimed to evaluate the functional impact of Lauren classification on immune contexture in gastric cancer patients. Tumor tissues of gastric cancer patients from Zhongshan Hospital and gastric cancer data from The Cancer Genome Atlas (TCGA) cohort were analyzed. By immunohistochemistry and flow cytometry, we found that intratumoral CD8+ T cells were more abundant but less functional in diffuse type as compared with those in intestinal type tumor tissues. Survival analysis indicated that CD8+ T cells yielded favorable prognosis only in intestinal type patients other than diffuse type cancer patients. Moreover, such diffuse type-associated CD8+ T cell dysfunction was featured by elevated expression of immunosuppressive factors including interleukin-10 (IL-10), transforming growth factor-β1 (TGF-β1) and indoleamine 2,3-dioxygenase 1 (IDO1). In summary, we found that the density, prognostic significance and functional status of intratumoral CD8+ T cells varied with Lauren subtypes in gastric cancer. These results further indicated Lauren classification might be a potential therapeutic marker, and should be considered in therapeutic decisions, especially immunotherapeutic eligibility.
Electronic supplementary material
The online version of this article (10.1007/s00262-020-02550-7) contains supplementary material, which is available to authorized users.
Keywords: CD8+ T cells, Gastric cancer, Lauren classification, Prognosis
Introduction
Gastric cancer is a complex, heterogenous entity that encompasses tumors with varying histological types, molecular profiles and biological behaviors. Lauren [1] described two distinct histological types of gastric cancer: intestinal type and diffuse type, based on the tissue structure. Besides the morphological differences, the two types of gastric cancer also differ in their molecular features, which consequently contribute to distinct therapeutic response to chemotherapy regimens within the two subtypes of gastric cancer [2–4]. Furthermore, the Lauren classification has already been used to indicate trastuzumab application according to NCCN guidelines [5].
In the past 10 years, tumor immune microenvironment has drawn increasing attention because of the promising clinical trial data of immunotherapy [6]. To our best knowledge, previous studies which referred to heterogeneity between intestinal type and diffuse type gastric cancers were predominantly concentrated on tumor cells. Few studies investigated the difference in tumor immune microenvironment of the two types of gastric cancer [7]. Therefore, with the coming of immunotherapy era, uncovering the heterogeneity in immune microenvironment between the two Lauren subtypes of gastric cancer might shed light on the identification of patients who may respond to immunotherapy.
A central role in immune protection against cancer is orchestrated by T-lymphocytes, particularly CD8+ T cells [8]. Their infiltration in tumor cell nests is usually associated with a favorable prognosis and may predict therapeutic response to immune checkpoint inhibitors [9–12]. A very recent study by Pernot S has paid attention to the differential infiltration of CD8+ T cells within the two Lauren subtypes of gastric cancer [13]. However, the functional differences between intestinal type-associated CD8+ T cells and diffuse type-associated CD8+ T cells were neglected in Pernot S’ study. Thus, we analyzed both the number and the functional status of intratumoral CD8+ T cells in larger cohorts in our study. It was shown that CD8+ T cells were more abundant but less functional in diffuse type gastric cancer. Furthermore, we found elevated expression of immunosuppressive factors including interleukin-10 (IL-10), transforming growth factor-β1 (TGF-β1) and indoleamine 2,3-dioxygenase 1 (IDO1) could depict the diffuse type-associated CD8+ T cell dysfunction in gastric cancer.
Materials and methods
Patients
Initially, the study recruited 496 patients, but only 468 patients had comprehensive information about clinicopathological data and survival outcomes for complete analysis. After immunohistochemistry, specimens of 12 patients were lost. Consequently, 456 patients were included as the Zhongshan Tissue Microarray (TMA) cohort. A total of 235 gastric cancer patients with Lauren subtype identification, survival data and complete RNA-seq information from The Cancer Genome Atlas (TCGA) database were enrolled as TCGA cohort. Fresh tumor tissue samples were obtained from 63 gastric cancer patients during surgery at Zhongshan Hospital from April to November 2018 (Zhongshan FCM cohort). The clinical characteristics of all gastric cancer patients are summarized in Table S1. Due to the small number and the similarity with diffuse type gastric cancer in clinical and prognostic aspects [14], the Lauren classification mixed type gastric cancer was combined into the diffuse type in our analysis as previous studies [15, 16]. Overall survival was calculated from the date of gastrectomy to the date of death or the last follow-up. The median follow-up time was 33.3 and 43.5 months in TCGA cohort and Zhongshan TMA cohort, respectively. Written informed consent was obtained from each patient included, and the protocol of all study cohorts was approved by the institutional review board and ethics committee of Zhongshan Hospital, Fudan University.
Assay methods
The protocol of immunohistochemistry and used antibodies are shown in Table S2. After immunostaining, sections were scanned at high-power magnification (× 200) and three randomly selected fields were captured by NIS-Elements F3.2 software. The semiquantitative H-score system described previously was used for IL-10, TGF-β1 and IDO1 level evaluation [17]. Density of CD8+ and granzyme B (GZMB)+ T cells was counted as the number of positive staining cells per high magnification view (× 200). CD8+ and GZMB+ T cell counting, IL-10, TGF-β1 and IDO1 expression for each field were evaluated by two pathologists who were blinded to the clinical data. In case of disagreement, the pictures were reviewed and a consensus was reached by the two observers. Finally, three fields were averaged as the ultimate score of each sample. 45/HPF and − 0.54 were determined as the cutoff values to discriminate high and low CD8+ T cell infiltration subgroups in Zhongshan TMA and TCGA cohort by minimum P method with the use of X-tile software (Version 3.6.1, Yale University).
For flow cytometry, CD8+ T cell number was detected in all 63 patients by flow cytometry, but functional phenotype of CD8+ T cells was detected only in 43 patients, of whom the formalin-fixed paraffin-embedded tissues were retrospectively collected for immunohistochemistry. Paired normal gastric mucosa tissues were obtained from 16 patients for flow cytometry analysis. The protocol for flow cytometry and antibodies used are shown in Table S3. Flow cytometry results were analyzed by FlowJo software (TreeStar).
The RNA-seq data for TCGA gastric cancer cohort were downloaded from the UCSC Xena at Jan. 2018 (https://xenabrowser.net/datapages/). To estimate CD8+ T cell infiltration level in the TCGA cohort, the average expression level of predefined CD8+ T cell gene set was determined utilizing normalization value to the mean and standard deviation among samples [18].
Statistical analysis
Survival analyses were performed by Kaplan–Meier method, log-rank test and Cox hazards regression model. Univariate and multivariate linear regression models of log-transformed CD8+ T cell numbers (per high magnification view and per 105 cells in Zhongshan TMA cohort and Zhongshan FCM cohort, respectively) versus clinicopathological variables were fitted. Student’s t test and Spearman’s correlation test were used for differential and correlation analysis. For all tests in this study, a P value of < 0.05 was considered statistically significant. All analyses were conducted using GraphPad Prism (Version 6.00) and IBM SPSS Statistics (version 21) software.
Results
Intratumoral CD8+ T cells exhibit more abundant manner in diffuse type gastric cancer patients
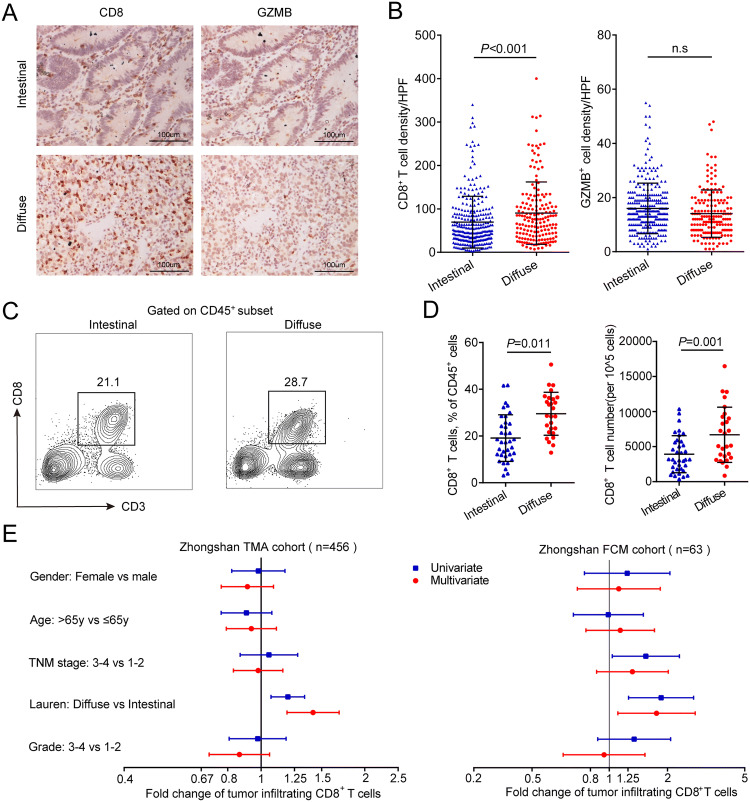
By means of immunohistochemistry, we detected the expression of CD8 and GZMB, which is regarded as a functional tissue marker of CD8+ T cells, in sequential slides of Zhongshan TMA cohort. We found that the density of intratumoral CD8+ T cells was higher in diffuse type than in intestinal type gastric cancer (P < 0.001, Fig. 1a, b). Interestingly, there was no significant difference of intratumoral GZMB+ cell density between the two subtypes of gastric cancer (Fig. 1a, b). We further conducted flow cytometry analysis, and the results also showed that diffuse type gastric cancer tissues contained higher proportion of CD8+ T cells among CD45+ leukocytes and higher total CD8+ T cell number, compared with intestinal type gastric cancer tissues (P = 0.011 and P = 0.001, Fig. 1c, d). Multivariate linear regression model was constructed to evaluate whether Lauren classification could still discriminate CD8+ T cell infiltration when adjusted by other clinicopathological variables. As we hypothesized, there was still significant difference in intratumoral CD8+ T cell infiltration between intestinal and diffuse types of gastric cancer in multivariate linear regression model (Fig. 1e). We then evaluated the expression of predefined CD8+ T cell signature genes in TCGA cohort and found higher expression of CD8+ T cell signature genes in diffuse type than in intestinal type tumors (P < 0.001, Figure S1A and B). Considering the distinct pathogenesis of gastric cancer with different Lauren subtypes, we investigated the CD8+ T cell infiltration pattern in peritumoral gastric mucosa. However, no significant difference was found between CD8+ T cells from peritumoral tissues of patients with intestinal type and diffuse type tumors (Figure S2A and B). Altogether, these data demonstrated that intratumoral CD8+ T cells are more abundant in diffuse type than that in intestinal type gastric cancer.
Fig. 1.
Distribution of tumor-infiltrating CD8+ T cells by Lauren classification. a Representative immunohistochemistry images of CD8 and GZMB staining in gastric cancer tissues with different Lauren subtypes (200× magnification). b Density of CD8+ and GZMB+ T cell infiltration in intestinal type (n = 286) and diffuse type (n = 170) gastric cancer patients from Zhongshan TMA cohort. c Representative images from flow cytometry showing CD8+ T cells proportions in intestinal type and diffuse type gastric cancer. d Proportions among all CD45+ leukocytes and absolute numbers of tumor-infiltrating CD8+ T cells in intestinal type (n = 36) and diffuse type (n = 27) gastric cancer patients from Zhongshan FCM cohort. e Univariate and multivariate associations between tumor-infiltrating CD8+ T cells and clinicopathological variables in Zhongshan TMA cohort and FCM cohort. Data are represented as mean ± SD, and P values are calculated using unpaired two-tailed t test. GZMB granzyme B, TMA tissue microarray, FCM flow cytometry
Intratumoral CD8+ T cells yield favorable prognostic value in intestinal type gastric cancer patients
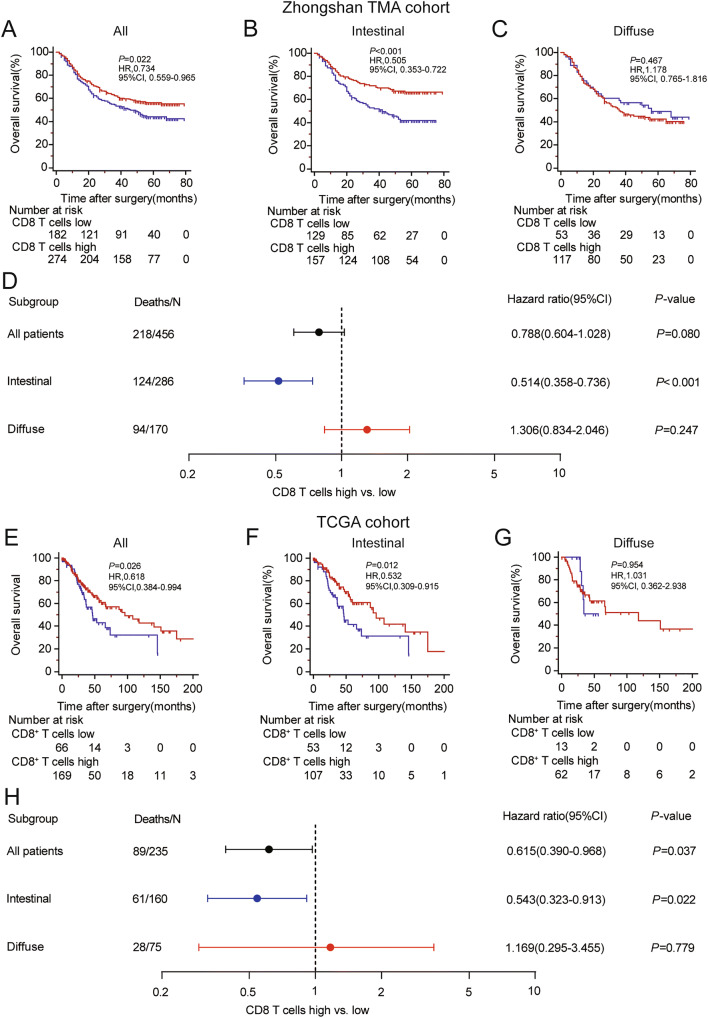
CD8+ T cells are recognized as the main effector cells in anti-tumor immunity, and increased CD8+ T cell infiltration predicts better prognosis in many solid cancers such as hepatocellular carcinoma [19], ovarian cancer [20], non-small cell lung cancer [21] and breast cancer [22]. However, previous studies on gastric cancer indicated that CD8+ T cell infiltration could not stratify overall survival and even indicated a poor prognosis [23, 24]. Since the Lauren classification might have a potential impact on CD8+ T cells, we consequently investigated whether the prognostic value of CD8+ T cells would vary with Lauren classification in gastric cancer. We evaluated the prognostic value of CD8+T cells in Zhongshan TMA cohort and TCGA cohort. Patients with higher density of intratumoral CD8+ T cells have a slightly improved prognosis than those with lower density of intratumoral CD8+ T cells in both Zhongshan TMA cohort (P = 0.022, HR = 0.734, 95%CI 0.559–0.965, Fig. 2a) and TCGA cohort (P = 0.026, HR = 0.618, 95%CI 0.384–0.994, Fig. 2e). However, when subgroup analysis was performed, CD8+ T cell infiltration could only indicate better prognosis in intestinal type patients (P < 0.001, HR = 0.505, 95%CI 0.353–0.722 and P = 0.012, HR = 0.532, 95%CI 0.309–0.915, respectively, Fig. 2b, f), rather than diffuse type patients (P = 0.467, HR = 1.178, 95%CI 0.765–1.816 and P = 0.954, HR = 1.031, 95%CI 0.362–2.938, respectively, Fig. 2c, g). To further validate different prognostic values of CD8+ T cells between these two subtypes of gastric cancer, multivariate Cox regression model including gender, age, TNM stage, tumor grade and CD8+ T cell infiltration was constructed in patients with different Lauren subtypes. Consistently, CD8+ T cell infiltration could still predict better prognosis in intestinal type patients (P < 0.001, HR = 0.514, 95%CI 0.358–0.736 and P = 0.022, HR = 0.543, 95%CI 0.323–0.913, Fig. 2d, h), rather than diffuse type patients (P = 0.247 HR = 1.306, 95%CI 0.834–2.046 and P = 0.779 HR = 1.169, 95%CI 0.295–3.455, Fig. 2d, h) in the two independent cohort. The full Cox multivariate analysis is shown in Table S4. These data suggested that intratumoral CD8+ T cells yielded favorable prognostic value merely in intestinal type gastric cancer patients.
Fig. 2.
Prognostic value of CD8+ T cell infiltration for gastric cancer patients with different Lauren subtypes. a–c Kaplan–Meier curves of overall survivals stratified by CD8+ T cell infiltration for all patients (a), intestinal type patients (b) and diffuse type patients (c) in the Zhongshan TMA cohort. Blue curves, CD8+T cells low infiltration; red curves, CD8+ T cells high infiltration. d Forest plot of CD8+ T cell infiltration on survival analysis adjusted for TNM stage, age, gender and tumor grade by multivariate Cox regression model in Zhongshan TMA cohort. e–g Kaplan–Meier curves of overall survivals stratified by CD8+ T cell infiltration for all patients (e), intestinal type patients (f) and diffuse type patients (g) in the TCGA cohort. Blue curves, CD8+T cells low infiltration; red curves, CD8+ T cells high infiltration. h Forest plot for CD8+ T cell infiltration on survival analysis adjusted for TNM stage, age, gender and tumor grade by multivariate Cox regression model in the TCGA cohort. TNM tumor nodes metastasis, TMA tissue microarray, TCGA the cancer genome atlas
Intratumoral CD8+ T cells perform dysfunctional phenotype in diffuse type gastric cancer patients
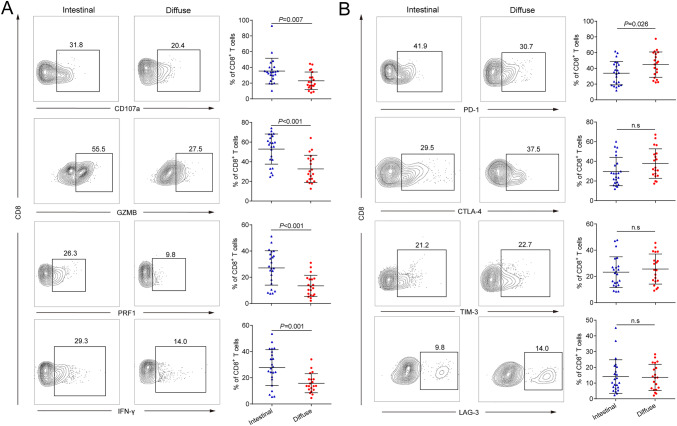
Considering the discrepant prognostic value of CD8+ T cells in patients with different Lauren subtypes, we therefore assessed whether phenotypic and functional properties of CD8+ T cells also varied with Lauren classification by flow cytometry. As we hypothesized, flow cytometry showed visibly reduced expression of effector molecules including CD107a, granzyme B (GZMB), perforin (PRF1) and interferon gamma (IFN-γ) on intratumoral CD8+ T cells from diffuse type gastric cancer (P = 0.007, P < 0.001, P < 0.001 and P = 0.001, respectively; Fig. 3a). Meanwhile, we detected the expression of immune checkpoint molecules including programmed cell death-1 (PD-1), cytotoxic T-lymphocyte associated protein-4 (CTLA-4), T cell immunoglobulin domain and mucin domain-3 (TIM-3) and lymphocyte activation gene-3 (LAG-3), which were generally considered as T cell dysfunctional markers in tumor tissues. Interestingly, we only observed significantly higher expression of PD-1 on CD8+ T cells (P = 0.026, Fig. 3b) from diffuse type gastric cancer. As to CTLA-4, TIM-3 and LAG-3 expression, no significant difference was found between CD8+ T cells from the two subtypes of gastric cancer (Fig. 3b). Analyses for normal gastric mucosa showed the phenotypical difference was only observed in intratumoral CD8+ T cells but not in CD8+ T cells from normal gastric mucosa (Figure S2D and E). Conclusively, these data revealed that intratumoral CD8+ T cells performed dysfunctional phenotype in diffuse type gastric cancer patients.
Fig. 3.
Phenotypic and functional properties of CD8+ T cells by Lauren subtypes. a Flow cytometrical analysis of effector molecules CD107a, GZMB, PRF1 and IFN-γ expression on CD8+ T cells from intestinal (n = 23) and diffuse type (n = 20) gastric cancer tissues. b Flow cytometrical analysis of effector molecules PD-1, CTLA-4, TIM-3 and LAG-3 expression on CD8+ T cells from intestinal (n = 23) and diffuse type (n = 20) gastric cancer tissues. Data are represented as mean ± SD and P values are calculated using unpaired two-tailed t test. GZMB granzyme B, IFN-γ interferon gamma, PRF1 perforin, PD-1 programmed cell death 1, CTLA-4 cytotoxic T-lymphocyte associated protein 4, TIM-3 T cell immunoglobulin domain and mucin domain 3, LAG-3 lymphocyte activation gene-3, n.s no significance
Intratumoral CD8+ T cell dysfunction correlates with lavish immunosuppressive factors in diffuse type gastric cancer patients
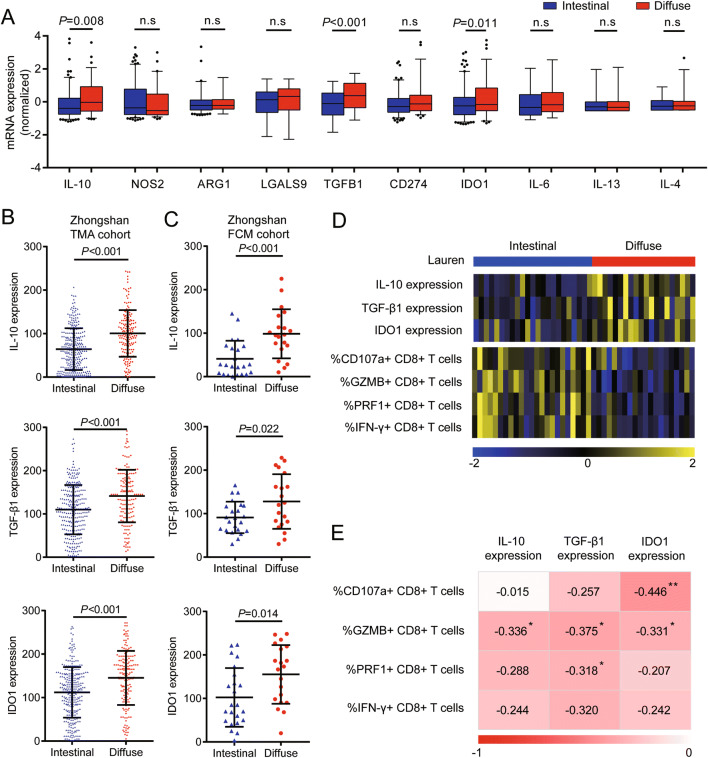
Besides immune checkpoint molecules, some immunoregulatory cytokines produced in the tumor microenvironment also contribute to T cell dysfunction. Thus, we tended to investigate whether these two subtypes of gastric cancer express different immunosuppressive factors. We analyzed the expression of factors well known for their immunosuppressive function [25] in TCGA tumor mRNA expression data. Notably, we found elevated expression of IL-10, TGF-β1 and IDO1 (Fig. 4a) in diffuse type gastric cancer. This finding was verified by immunohistochemistry in Zhongshan TMA cohort (Fig. 4b and Figure S3). To further validate the association between CD8+ T cell dysfunction and expression of immunosuppressive factors, we retrospectively collected formalin-fixed paraffin-embedded tissues of the 43 patients with CD8+ T cell function data from Zhongshan FCM cohort and detected the expression of immunosuppressive factors including IL-10, TGF-β1 and IDO1. Consistent with the results in the Zhongshan TMA cohort, diffuse type gastric cancer showed higher expression level of IL-10, TGF-β1 and IDO1 in the FCM cohort (Fig. 4c). Then, we analyzed whether suppressive factor expression was correlated with CD8+ T cell dysfunction in Zhongshan FCM cohort. A 2D heatmap showed generally higher expression of immunosuppressive factors and lower expression of effector molecules on CD8+ T cells in diffuse type versus intestinal type tumors (Fig. 4d). Correlation analysis showed that the expression of effector molecules on CD8+ T cells was negatively correlated with the expression level of immunosuppressive factors including IL-10, TGF-β1 and IDO1 in gastric cancer (Fig. 4e). In conclusion, these results indicated that intratumoral CD8+ T cell dysfunction might be correlated with lavish immunosuppressive factors in diffuse type gastric cancer patients.
Fig. 4.
CD8+ T cell dysfunction in diffuse type gastric cancer correlates with the lavish expression of immunosuppressive factors. a mRNA expression of immunosuppressive factors by different Lauren subtypes of gastric cancer in the TCGA cohort (n = 235). b Expression of IL-10, TGF-β1 and IDO1 in gastric cancer tissues with different Lauren subtypes detected by immunohistochemistry in Zhongshan TMA cohort (n = 456). c Expression of IL-10, TGF-β1 and IDO1 in gastric cancer tissues with different Lauren subtypes detected by immunohistochemistry in Zhongshan FCM cohort (n = 43). d A 2D heatmap showing different expressions of immunosuppressive factors and CD8+ T cell functional status in Zhongshan FCM cohort (n = 43). Colors show row-normalized z-score of immunosuppressive factor expression scores or cell populations percentages. e Association between expression level of immunosuppressive factors and functional status of tumor-infiltrating CD8+ T cells. Data are represented as mean ± SD and P values are calculated using unpaired two-tailed t test. IL-10 interleukin-10, TGF-β1 transforming growth factor-β1, TMA tissue microarray, FCM flow cytometry, n.s no significance
Discussion
It has been reported that immune infiltration and its prognostic significance might be distinguished by histological types in many tumors like breast cancer, non-small cell lung cancer and ovarian cancer [26–28]. Although the Lauren classification system dates back to 1965, it is still widely accepted and has been proved to be one of the most meaningful classifications by histology for gastric cancer. Thus, we conducted this study to test the hypothesis that Lauren classification might signify distinct immune environment signature in gastric cancer.
In this study, a higher number of CD8+ T cells was found in diffuse type tumors, compared with intestinal type tumors. Interestingly, our results seemed opposite to Pernot S’, which found more CD8+ T cell infiltration in intestinal type of gastric cancer [13]. We speculated that this might be caused by the heterogeneity of patients, because the patients in Pernot S’ study were all metastasis cases, which only played a small part of our study. Remarkably, a different picture emerged when we focused on functional status instead of only counting their numbers. In this regard, the correlations were inverse, providing evidence for better CD8+ T cell function in intestinal type gastric cancer, with significantly higher fractions of CD8+ T cells expressing CD107a, GZMB, IFN-γ and PRF1. Indeed, our findings are essentially consistent with the conclusion of studies on epithelial–mesenchymal transition (EMT) and anti-tumor immune response. EMT process, a biological program featured by loss of cell adhesion and increased invasive behavior, is seen as a hallmark of diffuse type gastric cancer [29, 30]. Tumors with a higher level of EMT usually exhibit more T cell infiltration in other solid tumors [31, 32]. However, despite the positive correlation of EMT score and T cell infiltration, tumors with high EMT score often exhibit immunosuppressive microenvironment featured by T cell dysfunction [33, 34]. Consequently, it’s logical that intratumoral CD8+ T cells are more abundant but less functional in diffuse type gastric cancer, which usually exhibits higher degree of epithelial mesenchymal transformation.
Immunosuppressive factors like IL-10, TGF-β1 and IDO1 have been shown to induce EMT [35–37], which gives possible explanations to CD8+ T cell dysfunction in diffuse type tumors with higher mesenchymal transition level. Furthermore, in mesenchymal type tumors, these immunosuppressive factors may attenuate response to immune checkpoint inhibitors (ICPIs), regardless of T cell infiltration level [33, 38], while in two recent clinical trials, diffuse type gastric cancer really tended to show lower response rate to immune checkpoint inhibitors despite increased CD8+ T cell infiltration [39, 40]. Therefore, Lauren classification may add significant information to identification of gastric cancer patients who may benefit from ICPIs, with easier clinical application. Additionally, combination with targeting IL-10, TGF-β1 or IDO1 may be potentially effective to improve the effects of ICPIs in diffuse type gastric cancer in view of the aberrant expression of these immunosuppressive factors in this type of tumors.
Several limitations should be addressed in this study. We concentrated on CD8+ T cells in the present study. However, considering the intricate interactions between immune contextures, future work will be required to provide comprehensive examination of the immune landscape among Lauren subtypes using the high-dimensional analytical tools like cytometry by time of flight and next-generation sequencing. Also, the underlying mechanism how Lauren classification shapes the immune microenvironment should be uncovered by studies in the future.
In summary, our findings indicated that intratumoral CD8+ T cells were more abundant but less functional in diffuse type gastric cancer as compared with that in intestinal type. We showed for the first time that CD8+ T cell infiltration in diffuse type gastric cancer might not be associated with better survival. Increased CD8+ T cell infiltration was found accompanied by elevated expression of IL-10, TGF-β1 and IDO1 in diffuse type gastric cancer, which may contribute to impaired CD8+ T cell function in this type of tumors. These results indicate that ICPIs may be more effective in intestinal type gastric cancer rather than diffuse type disease. Importantly, we identified possible targets that were overexpressed in diffuse type gastric cancer and could provide the foundation for rational immune therapy combination strategies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
Not applicable.
Author contributions
In this study, RCL, HZ, YFC and XL involved in acquisition of data, analysis and interpretation of data and drafting of the manuscript; RCL took part in the statistical analysis; YFC, YYQ, HL, JTW, KY, LLC, CL, HYH, HL, ZBS, JQ and WJZ took part in technical and material support; YHS and JJX participated in study concept and design, analysis and interpretation of data, drafting of the manuscript, obtained funding and study supervision. All authors read and approved the final manuscript.
Funding
This study was funded by Grants from National Natural Science Foundation of China (81671628, 81672324, 31770851, 81871306, 81871926, 81871930, 81902402, 81902901, 81972219), Shanghai Municipal Natural Science Foundation (18ZR1432900) and Shanghai Sailing Program (17YF1402200, 18YF1404600, 19YF1407500). All these study sponsors have no roles in the study design, in the collection, analysis and interpretation of data.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ruochen Li, Heng Zhang, Yifan Cao, and Xin Liu have contributed equally to this work.
Contributor Information
Yihong Sun, Email: sun.yihong@zs-hospital.sh.cn.
Jiejie Xu, Email: jjxufdu@fudan.edu.cn.
References
- 1.Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. an attempt at a histological classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 2.Jimenez Fonseca P, Carmona-Bayonas A, Hernandez R, et al. Lauren subtypes of advanced gastric cancer influence survival and response to chemotherapy: real-world data from the AGAMENON National Cancer Registry. Br J Cancer. 2017;117:775–782. doi: 10.1038/bjc.2017.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma J, Shen H, Kapesa L, Zeng S. Lauren classification and individualized chemotherapy in gastric cancer. Oncol Lett. 2016;11:2959–2964. doi: 10.3892/ol.2016.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng X, Yu S, Wang Y, et al. The role of oxaliplatin in the adjuvant setting of different Lauren's type of gastric adenocarcinoma after D2 gastrectomy: a real-world study. Gastric Cancer. 2018 doi: 10.1007/s10120-018-0895-x. [DOI] [PubMed] [Google Scholar]
- 5.Network NCC (2017) (NCCN) Clinical practice guidelines in oncology. Gastric Cancer, Version 1.2017
- 6.Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16:275–287. doi: 10.1038/nrc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang M, Busuttil RA, Pattison S, Neeson PJ, Boussioutas A. Immunological battlefield in gastric cancer and role of immunotherapies. World J Gastroenterol. 2016;22:6373–6384. doi: 10.3748/wjg.v22.i28.6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Speiser DE, Ho PC, Verdeil G. Regulatory circuits of T cell function in cancer. Nat Rev Immunol. 2016;16:599–611. doi: 10.1038/nri.2016.80. [DOI] [PubMed] [Google Scholar]
- 9.Sharma P, Shen Y, Wen S, et al. CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc Natl Acad Sci USA. 2007;104:3967–3972. doi: 10.1073/pnas.0611618104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hegde PS, Karanikas V, Evers S. The where, the when, and the how of immune monitoring for cancer immunotherapies in the era of checkpoint inhibition. Clin Cancer Res. 2016;22:1865–1874. doi: 10.1158/1078-0432.CCR-15-1507. [DOI] [PubMed] [Google Scholar]
- 11.Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daud AI, Loo K, Pauli ML, et al. Tumor immune profiling predicts response to anti-PD-1 therapy in human melanoma. J Clin Investig. 2016;126:3447–3452. doi: 10.1172/JCI87324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pernot S, Terme M, Radosevic-Robin N, et al. Infiltrating and peripheral immune cell analysis in advanced gastric cancer according to the Lauren classification and its prognostic significance. Gastric Cancer. 2019 doi: 10.1007/s10120-019-00983-3. [DOI] [PubMed] [Google Scholar]
- 14.Chen YC, Fang WL, Wang RF, et al. Clinicopathological variation of Lauren classification in gastric cancer. Pathol Oncol Res POR. 2016;22:197–202. doi: 10.1007/s12253-015-9996-6. [DOI] [PubMed] [Google Scholar]
- 15.Huang SC, Ng KF, Yeh TS, Cheng CT, Lin JS, Liu YJ, Chuang HC, Chen TC. Subtraction of Epstein–Barr virus and microsatellite instability genotypes from the Lauren histotypes: Combined molecular and histologic subtyping with clinicopathological and prognostic significance validated in a cohort of 1,248 cases. Int J Cancer. 2019 doi: 10.1002/ijc.32215. [DOI] [PubMed] [Google Scholar]
- 16.Lee JH, Chang KK, Yoon C, Tang LH, Strong VE, Yoon SS. Lauren histologic type is the most important factor associated with pattern of recurrence following resection of gastric adenocarcinoma. Ann Surg. 2018;267:105–113. doi: 10.1097/SLA.0000000000002040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Specht E, Kaemmerer D, Sanger J, Wirtz RM, Schulz S, Lupp A. Comparison of immunoreactive score, HER2/neu score and H score for the immunohistochemical evaluation of somatostatin receptors in bronchopulmonary neuroendocrine neoplasms. Histopathology. 2015;67:368–377. doi: 10.1111/his.12662. [DOI] [PubMed] [Google Scholar]
- 18.Davoli T, Uno H, Wooten EC, Elledge SJ. Tumor aneuploidy correlates with markers of immune evasion and with reduced response to immunotherapy. Science. 2017 doi: 10.1126/science.aaf8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS, Xu Y, Li YW, Tang ZY. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25:2586–2593. doi: 10.1200/jco.2006.09.4565. [DOI] [PubMed] [Google Scholar]
- 20.Hamanishi J, Mandai M, Iwasaki M, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci USA. 2007;104:3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donnem T, Hald SM, Paulsen EE, et al. Stromal CD8+ T-cell density—a promising supplement to TNM staging in non-small cell lung cancer. Clin Cancer Res. 2015;21:2635–2643. doi: 10.1158/1078-0432.ccr-14-1905. [DOI] [PubMed] [Google Scholar]
- 22.Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, Ellis IO, Green AR. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011;29:1949–1955. doi: 10.1200/jco.2010.30.5037. [DOI] [PubMed] [Google Scholar]
- 23.Thompson ED, Zahurak M, Murphy A, et al. Patterns of PD-L1 expression and CD8 T cell infiltration in gastric adenocarcinomas and associated immune stroma. Gut. 2017;66:794–801. doi: 10.1136/gutjnl-2015-310839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wen T, Wang Z, Li Y, et al. A four-factor immunoscore system that predicts clinical outcome for stage II/III gastric cancer. Cancer Immunol Res. 2017;5:524–534. doi: 10.1158/2326-6066.cir-16-0381. [DOI] [PubMed] [Google Scholar]
- 25.Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321–330. doi: 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]
- 26.Goode EL, Block MS, Kalli KR, et al. Dose-response association of CD8+ tumor-infiltrating lymphocytes and survival time in high-grade serous ovarian cancer. JAMA Oncol. 2017;3:e173290. doi: 10.1001/jamaoncol.2017.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lizotte PH, Ivanova EV, Awad MM, Jones RE, Keogh L, Liu H, Dries R, Almonte C. Multiparametric profiling of non-small-cell lung cancers reveals distinct immunophenotypes. JCI Insight. 2016;1:e89014. doi: 10.1172/jci.insight.89014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Desmedt C, Salgado R, Fornili M, et al. Immune infiltration in invasive lobular breast cancer. J Natl Cancer Inst. 2018;110:768–776. doi: 10.1093/jnci/djx268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cristescu R, Lee J, Nebozhyn M, et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med. 2015;21:449–456. doi: 10.1038/nm.3850. [DOI] [PubMed] [Google Scholar]
- 30.Oh SC, Sohn BH, Cheong JH, et al. Clinical and genomic landscape of gastric cancer with a mesenchymal phenotype. Nat Commun. 2018;9:1777. doi: 10.1038/s41467-018-04179-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lou Y, Diao L, Cuentas ER, et al. Epithelial–mesenchymal transition is associated with a distinct tumor microenvironment including elevation of inflammatory signals and multiple immune checkpoints in lung adenocarcinoma. Clin Cancer Res. 2016;22:3630–3642. doi: 10.1158/1078-0432.CCR-15-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mak MP, Tong P, Diao L, et al. A patient-derived, pan-cancer EMT signature identifies global molecular alterations and immune target enrichment following epithelial-to-mesenchymal transition. Clin Cancer Res. 2016;22:609–620. doi: 10.1158/1078-0432.CCR-15-0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang L, Saci A, Szabo PM, et al. EMT- and stroma-related gene expression and resistance to PD-1 blockade in urothelial cancer. Nat Commun. 2018;9:3503. doi: 10.1038/s41467-018-05992-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen L, Gibbons DL, Goswami S, et al. Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat Commun. 2014;5:5241. doi: 10.1038/ncomms6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu CY, Xu JY, Shi XY, Huang W, Ruan TY, Xie P, Ding JL. M2-polarized tumor-associated macrophages promoted epithelial-mesenchymal transition in pancreatic cancer cells, partially through TLR4/IL-10 signaling pathway. Lab Investig. 2013;93:844–54. doi: 10.1038/labinvest.2013.69. [DOI] [PubMed] [Google Scholar]
- 36.Zhu L, Fu X, Chen X, Han X, Dong P. M2 macrophages induce EMT through the TGF-beta/Smad2 signaling pathway. Cell Biol Int. 2017;41:960–968. doi: 10.1002/cbin.10788. [DOI] [PubMed] [Google Scholar]
- 37.Kolijn K, Verhoef EI, Smid M, Bottcher R, Jenster GW, Debets R, van Leenders G. Epithelial–mesenchymal transition in human prostate cancer demonstrates enhanced immune evasion marked by IDO1 expression. Can Res. 2018;78:4671–4679. doi: 10.1158/0008-5472.CAN-17-3752. [DOI] [PubMed] [Google Scholar]
- 38.Mariathasan S, Turley SJ, Nickles D, et al. TGFbeta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 2018;554:544–548. doi: 10.1038/nature25501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461–2471. doi: 10.1016/s0140-6736(17)31827-5. [DOI] [PubMed] [Google Scholar]
- 40.Shitara K, Ozguroglu M, Bang YJ, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. 2018;392:123–133. doi: 10.1016/s0140-6736(18)31257-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.