Abstract
Purpose
The goal of this study is to identify the pathological findings and expression of immune checkpoint marker (PD-1, PD-L1, and CTLA-4) in the tumor microenvironment of both primary and chemoreduced retinoblastoma and correlate them with clinicopathological parameters and patient outcome.
Methods
Total of 262 prospective cases was included prospectively in which 144 cases underwent primary enucleation and 118 cases received chemotherapy/radiotherapy before enucleation (chemoreduced retinoblastoma). Immunohistochemistry, qRT-PCR and western blotting were performed to evaluate the expression pattern of immune checkpoint markers in primary and chemoreduced retinoblastoma.
Results
Tumor microenvironment were different for both primary and chemoreduced retinoblastoma. Expression of PD-1 was found in 29/144 (20.13%) and 48/118 (40.67%) in primary and chemoreduced retinoblastoma, respectively, whereas PD-L1 was expressed in 46/144 (31.94%) and 22/118 (18.64%) in cases of primary and chemoreduced retinoblastoma, respectively. Expression pattern of CTLA-4 protein was similar in both groups of retinoblastoma. On multivariate analysis, massive choroidal invasion, bilaterality and PD-L1 expression (p = 0.034) were found to be statistically significant factors in primary retinoblastoma, whereas PD-1 expression (p = 0.015) and foamy macrophages were significant factors in chemoreduced retinoblastoma. Overall survival was reduced in cases of PD-L1 (80.76%) expressed primary retinoblastoma, and PD-1 (63.28%) expressed chemoreduced retinoblastoma.
Conclusions
This is the first of its kind study predicting a relevant role of the immune checkpoint markers in both groups of primary and chemoreduced retinoblastoma with prognostic significance. Differential expression of these markers in both group of retinoblastoma is a novel finding and might be an interesting and beneficial target for chemoresistant tumors.
Electronic supplementary material
The online version of this article (10.1007/s00262-020-02529-4) contains supplementary material, which is available to authorized users.
Keywords: Cancer immunotherapy, Histopathology, Immune checkpoint markers, Chemotherapy, Retinoblastoma
Introduction
Retinoblastoma (Rb) is the most common intraocular malignancy of childhood caused by mutation of RB1 genes [1]. Advancement in the management of retinoblastoma has improved the prognosis, yet there is a need for further study for therapies for the chemoresistant cases [2]. Histopathological high-risk factors of retinoblastoma are associated with a higher risk of orbital recurrence and distant metastasis [3].
Currently, chemotherapy is the most commonly used modalities in the treatment of retinoblastoma. Chemotherapy may be administrated systemically, subconjunctivally, intra-arterially or intravitreally [4]. It has become the standard of care for the management of orbital exenteration cases. The most significant concern arises after chemoreduction is tumor unresponsiveness and tumor recurrence [5].
Over the past few decades, the paradigm of cancer treatment has experienced remarkable advancements in treatments, as they are not only targeting the neoplastic cells but also target the dynamic tumor microenvironment [6]. Changes in the tumor microenvironment and the protein communication between primary retinoblastoma and chemoreduced retinoblastomas are still unexplored. Therefore, it is essential to understand the contribution of immune checkpoint markers in the tumor microenvironment (TME) of retinoblastoma.
The TME comprises malignant cells and non-malignant cells such as cytokines, growth factors, extracellular proteins, endothelial cells, fibroblasts, and inflammatory cells [7]. New immunotherapeutic strategies hold great potential for targeting the tumor microenvironment, as they are more likely to participate in tumor progression, and metastasis [8]. Despite evolution in chemotherapeutic agents and the delivery of these agents, a greater understanding of the pathophysiology of retinoblastoma is needed to develop novel targeted treatments [9]. Non-malignant cells are genetically more stable than tumor cells, and therefore, targeting the tumor microenvironment are less prone to cause adaptive mutation and metastasis [10]. Understanding the functional changes of the TME may offer important consideration in ongoing research in primary and chemoreduced retinoblastoma.
Use of immune checkpoint inhibitors has improved overall survival in the treatment of many different solid tumors [11]. Recently, immunotherapy is used as a novel therapeutic strategy in various kinds of tumors over the last decade. A large number of studies focused on immune-checkpoints, and their ligands are known as programmed cell death 1 (PD-1), and programmed cell death-ligand 1 (PD-L1) along with Cytotoxic T-Lymphocyte-Associated Antigen-4 (CTLA-4) reveal promising results and antitumor efficacy for advanced tumors [12]. Many documents have indicated that the lack of immunologic control is a hallmark of cancer [13]. PD-1 and its ligand PD-L1 play a crucial role in tumor immune escape and the formation of TME, closely related to tumor generation and development [14].
PD-1 is expressed on the surface of activated immune cell types such as T cells, B cells, and myeloid cells. It has two ligands, PD-L1 (B7-H1) and PD-L2 (B7-DC) and these are cell-surface glycoproteins belonging to the B7 family, which is mainly expressed in placenta, tonsil, and retina [15]. PD-L1 majorly expressed in hematopoietic cells such as dendritic, myeloid, T and B cells, non-hematopoietic cells including endothelial, epithelial and tumor cells [16].
Immunohistochemistry (IHC) is a broadly accepted method for evaluating the PD-1/PD-L1 expression in cancer biology. Detection of PD-L1 protein expression by IHC has widely used as a predictive biomarker assay for anti–PD-1/PD-L1 and CTLA-4 therapies in tumors [17]. PD-L1, the ligand for PD-1, is highly expressed in several cancers, and hence, the role of PD-1 in cancer immune evasion is well established [18]. Expression of PD-L1 is found in numerous tumor types, such as melanoma, glioblastoma, and cancers in lung, kidney, head and neck, stomach, colon, pancreas, breast, cervix, cervical, and ovarian cancer [19]. PD-L1 is also expressed in hematologic malignancies, such as multiple myeloma, lymphoma, and various leukemia types and are associated with worse prognosis [20].
Previous studies in cancers showed changes in the pattern of tumor microenvironment of primary and chemo-treated tumors. The difference in the histopathological findings along with the expression of immune markers in the tumor microenvironment of primary retinoblastoma (Group I) and chemoreduced retinoblastoma (Group II) cases has not been studied so far. Therefore, our study aims to evaluate the expression pattern of PD-1 and its ligand PD-L1 and CTLA-4 protein in both groups of patients and to determine their significance with clinical, pathological features and prognostic outcome.
Material and methods
Study design
This study was the prospective study conducted at the All India Institute of Medical Sciences, New Delhi, India, after approval of the institutional ethics committee. All procedures conformed to the Declaration of Helsinki for research involving human subjects. Before sample collection, written informed consent obtained from the guardians of all patients. Total of 262 prospective cases included in which 144 cases underwent primary enucleation and 118 cases received chemotherapy/radiotherapy before enucleation (chemoreduced retinoblastomas) during the 24 months (October 2016–September 2018). Formalin-fixed paraffin-embedded blocks were used for the hematoxylin and eosins (H&E) slides along with the immunohistochemical study. In addition to this, fresh tumor tissues and control tissues were collected for mRNA and western blotting study. Fresh tumor tissues were fixed in RNAlater (Sigma-Aldrich) to avoid degradation of RNA and were then stored at − 80 °C until further use.
Clinicopathological details
Clinical and demographic data such as sex, age at presentation, laterality, and grouping were obtained for all the patients from medical records. H&E slides were reviewed for differentiation, necrosis, calcification and high-risk factors. Retinoblastomas were classified as poorly differentiated retinoblastoma (PDRB) and well-differentiated retinoblastoma (WDRB). Invasion of tumor cells was assessed on the basis of high-risk histopathological factors such as massive choroidal invasion, anterior chamber, iris, and ciliary body, optic nerve, scleral and extrascleral invasion (Supplementary Fig. 1a–f). Factors such as macrophages, glial cells, fibrosis, gliosis, giant cells, and the presence of cholesterol clefts were also reviewed in chemoreduced retinoblastoma (Supplementary Fig. 1g–l). Pathological tumor staging was determined according to the 8th edition of the American Joint Committee on Cancer (AJCC) staging criteria [21]. Follow-up was obtained for a period of 6–30 months in our study.
Immunohistochemistry
Formalin-fixed paraffin-embedded tissues (FFPE) were collected and subjected to immunohistochemistry. Immunohistochemical staining using the avidin–biotin indirect method was performed in 4–5 µm thicknesses of tissue blocks fixed on poly-l-lysine-coated slides. Briefly, deparaffinization of tissue sections was done in graded xylene and rehydrated in ethanol. The microwave oven method was done for heat-induced antigen retrieval in citrate buffer solution (pH 6.0) for 20 min at 100 °C. Sections were allowed to cool down at room temperature and subsequently blocked in blocking buffer containing 1.5% H2O2 in methanol for 30 min for activation of endogenous peroxidase. Slides were washed and then incubated with corresponding primary antibodies [Anti-PD-1, Clone: D4W2J (Cell Signaling); Anti-PD-L1, Clone: E1L3N (Cell Signaling); Anti-CTLA-4, Clone: F-8 (Santa Cruz Biotechnology)] at a dilution of 1:200 for each antibody overnight at 4 °C. After three times of washing, slides were incubated with the HRP-conjugated secondary antibody (Ultravision Quanto Detection system, Thermo Fisher Scientific, Fremont, CA, USA) for 20 min in the dark at room temperature. Immunoreactivity was visualized by using 3′, 3′-diaminobenzidine (DAB) peroxidase substrate for 2–3 min and counterstained with hematoxylin. Finally, the slides were mounted in distyrene-plasticizer-xylene (DPX) and examined under light microscopy. Normal retina as a positive control, corresponding positive controls for antibody, and negative controls were run for each set of experiments.
Assessment of immunohistochemical staining
The samples were independently scored by two authors (LS and MKS) under the supervision of the experienced pathologist (SK) who established a semiquantitative score for each marker. Expression pattern of PD-1, PD-L1 and CTLA-4 analysis was scored by assessment of the proportion and intensity of the stained tumor cells and adjacent stromal cells. Positively stained cells were counted in ten randomly selected fields under the light microscope (40× magnifications). Staining intensity was graded as negative (0), weak (1+), moderate (2+) or intense (3+), and the percentage of the stained cells was classified as ≤ 10% (grade 1), 11–50% (grade 2) or > 50% (grade 3), and the percentage positivity and staining intensity scores were multiplied to create a single IHC score. The maximum score obtained in this study was 9, and the minimum was 0. Protein expression was considered to be positive when the IHC score was ≥ 4 and negative or reduced when the IHC score was < 4.
Western blotting
NE-PER Nuclear and Cytoplasmic Extraction Reagents kit (Pierce, Rockford, IL, USA) was used to extract the cytoplasmic proteins from 10 fresh tissues of primary and chemoreduced retinoblastoma each. Protein concentrations were determined with the Bio-Rad protein assay (Bio-Rad, Hercules, CA, USA). 25ug of total reduced protein was loaded into each lane of 12% gel and run at 100 V in sodium dodecyl sulfate (SDS) running buffer at room temperature. After SDS-PAGE, protein bands were transferred onto a nitrocellulose membrane (MDI Membrane Technologies, California, USA) at 90 V for one hour. It was then blocked in 5% of BSA in TBS containing 0.05% Tween 20 for one hour at room temperature. The blots were incubated overnight with primary antibodies against anti-PD-1, anti-PD-L1 and anti-CTLA-4 protein at 4◦C. Following incubation with primary antibodies, membrane was washed thrice-using TBST buffer (150 mM NaCl, 10 mM Tris–HCl (pH 7.6), 0.1% Tween-20 (Sigma-Aldrich) and incubated at 4◦C with HRP-conjugated secondary antibody (Cell Signaling Technology, Danvers, MA) for one hour. After secondary incubation, the membrane was rewashed thrice with TBST, and detection of protein bands was visualized using the ECL Plus detection kit (GE Healthcare). Β-Actin was used as an internal control (1:5000; Sigma).
RNA isolation and qRT-PCR
Total RNA was extracted from 24 fresh tumor tissues and ten normal retina as control samples using RNA isolation kit (Purelink RNA Kit, Ambion). mRNA levels of PD-1, PD-L1, and CTLA-4 genes were determined by qPCR using the SYBR Green (Applied Biosystems, USA) on ABI Step One instrument (Applied Biosystems, USA) from both groups of retinoblastoma. The RNA was then reversely transcribed into cDNA using verso kit following manufacturer’s instructions. The qRT-PCR primer sequence used were PD-1 (sense) CGTGGCCTATCCACTCCTCA; PD-1 (anti-sense) ATCCCTTGTCCCAGCCACTC; PD-L1 (sense) AAATGGAACCTGGCGAAAGC; PD-L1 (anti-sense) GATGAGCCCCTCAGGCATTT; CTLA-4 (sense) TGGCTTGCCTTGGATTTCAGC; CTLA-4 (anti-sense) ACACACAAAGCTGGCGATGC. The PCR conditions were as follows: 95 °C for 10 min, followed by 35 cycles of 95 °C for 30 s, 60 °C (PD-1)/57 °C (PD-L1)/62 °C (CTLA-4) for 30 s and 72 °C for 30 s. Each PCR reaction was followed by continuous melt curve analysis. Data were normalized using reference gene, and calculation for the fold change was done according to the mathematical model R = 2-CT, where CT = CT of selected genes–CT of the reference gene, and CT = CT test– CT control. All qRT-PCR reactions were performed in triplicate, and the data are presented as the mean ± SD.
Statistical analysis
Level of significance for all tests was considered as p < 0.05, and all tests were two-sided to evaluate the statistical association of PD-1, PD-L1 and CTLA-4 protein expression with clinicopathological parameters and patient outcome. All statistical analyses were performed using Stata 9 software (StataCorp LP, College Station, TX, USA). Overall survival (OS) of the patient was estimated with a positive or negative expression of protein markers using the log-rank test. Hazard ratios and their 95% confidence intervals (CI) were noted for each marker. Univariate and multivariate analyses for prognostic significance of clinicopathological features were performed using the Cox proportional hazard survival along with the Kaplan–Meier method.
Results
Baseline demographic, clinical and histopathological features
A total of 262 cases have been included in which 144 cases were primary enucleated retinoblastomas, and 118 cases were chemoreduced enucleated retinoblastoma eyes. Supplementary Table 1 summarizes all the demographic details and clinicopathological characteristics of primary retinoblastoma (Group I) and chemoreduced retinoblastoma (Group II).
Primary retinoblastoma (Group I; n = 144)
There was male preponderance (57.6%) with an age range from 2 months to 8 years. At the time of presentation, 121 cases had unilateral retinoblastomas. According to AJCC classification, there were 52.8% cases, which belong to T3a–T4b pathological stage. On histopathological evaluation, poorly differentiated retinoblastomas (PDRB) were present in 125 cases. Necrosis and calcification were found in 66 and 64 cases, respectively. The presence of various histopathological high-risk factors such as massive choroidal/scleral/iris and ciliary body, retrolaminar, and optic nerve cut end invasion was identified in 78 (54.2%) tumors. Optic nerve invasion (post-laminar invasion and cut end of resected margin) was the most frequent histopathological high-risk factor found in 64/144 (44.4%) case. Seven patients died during the follow-up period due to progressive disease.
Chemoreduced retinoblastoma (Group II; n = 118)
There were 74 males and 44 females in this group. Sixteen patients had phthisical eyeball with grossly distorted ocular structures and thickened coats of the eye. Presence of a poorly differentiated tumor was found in 87.3% (103/118). A massive choroidal invasion was found in 19.5% of cases. Extensive necrosis and calcification were present in 40 and 73 cases, respectively. There was a presence of marked fibrosis in 11.1% (13/118) and gliosis in 10.2% cases. Foamy macrophages were present in 21.2% (25/118) cases. Ten patients died during follow-up due to disease.
Tumor expression of immune markers in primary retinoblastoma (Group I)
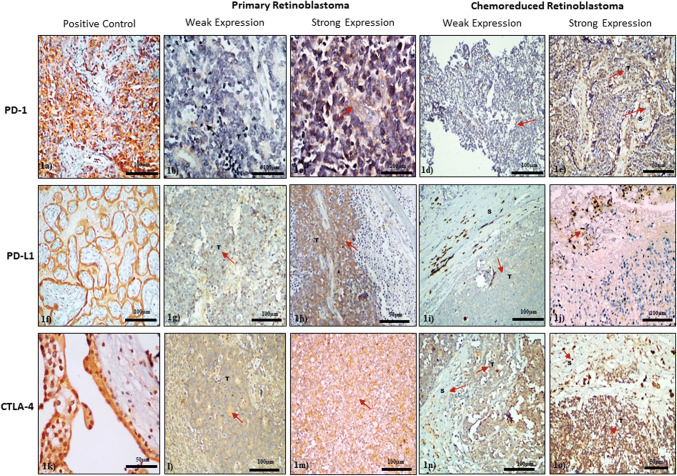
Immunoreactivity of PD-1, PD-L1, and CTLA-4 was detected in the cytoplasm of tumor cells. PD-1 was found only in 20.13% (29/144) cases, whereas PD-L1 and CTLA-4 were present in 31.9% (46/118) and 36.8% (53/118) cases, respectively (Fig. 1). Table 1 provides the correlation of these markers with clinicopathological parameters. PD-1 and PD-L1 expression were significantly associated with higher pathological staging and age (> 2 years). Massive choroidal invasion and tumor invasion (> 1 HRFs) significantly correlated with PD-1, PD-L1, and CTLA-4 expression. Significant association of CTLA-4 expression was found with higher tumor staging, choroidal invasion, and optic nerve invasion.
Fig. 1.
Representative images of PD-1, PD-L1 and CTLA-4 protein expression in primary and chemoreduced retinoblastoma tumor by immunohistochemistry: a Tonsil control for PD-1 expression; b, c Weak and strong expression of PD-1 in tumor cells primary retinoblastoma; d, e Weak and strong expression of PD-1 in stromal and tumor cells of chemoreduced retinoblastoma; f Late placenta control for PD-L1 expression; g, h Weak and strong expression of PD-L1 in tumor cells of primary retinoblastoma; i, j Weak and strong expression of PD-L1 in tumor and stromal cells of chemoreduced retinoblastoma; k Human early placenta control for CTLA-4 expression; l, m Weak and strong expression of CTLA-4 in primary retinoblastoma tumor cells; n, o Weak and strong expression of CTLA-4 in tumor and stromal cells of chemoreduced retinoblastoma
Table 1.
Correlations between PD-1, PD-L1 and CTLA-4 Proteins with clinicopathological parameters in primary retinoblastoma Cases
| Pathological parameters N: 144 | PD-1 | PD-L1 | CTLA-4 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Negative N: 115 | Positive N: 29 | p value | Negative N: 98 | Positive N: 46 | p value* | Negative N: 91 | Positive N: 53 | p value* | |
| Sex | 0.848 | ||||||||
| Male (83) | 46 | 15 | 0.256 | 69 | 14 | 0.254 | 53 | 30 | |
| Female (61) | 69 | 14 | 46 | 15 | 38 | 23 | |||
| Age | 0.847 | ||||||||
| < 2 years (80) | 69 | 11 | 0.033 | 69 | 11 | 0.033 | 50 | 30 | |
| > 2 years (64) | 46 | 18 | 46 | 18 | 41 | 23 | |||
| Laterality | 0.245 | ||||||||
| Unilateral (121) | 95 | 26 | 0.355 | 95 | 26 | 0.355 | 74 | 47 | |
| Bilateral (23) | 20 | 3 | 20 | 3 | 17 | 6 | |||
| Grouping | 0.038 | ||||||||
| Group D-A (10) | 7 | 3 | 0.421 | 6 | 4 | 0.726 | 3 | 7 | |
| Group E (134) | 108 | 26 | 92 | 42 | 88 | 46 | |||
| Staging | 0.001 | ||||||||
| T1N0M0 (68) | 64 | 4 | 0.001 | 52 | 16 | 0.041 | 56 | 12 | |
| T2aN0M0–T4bN0M0 (76) | 51 | 25 | 46 | 30 | 35 | 41 | |||
| Differentiation | 0.612 | ||||||||
| WDRB(19) | 16 | 3 | 0.612 | 14 | 5 | 0.572 | 13 | 6 | |
| PDRB (125) | 99 | 26 | 84 | 41 | 78 | 47 | |||
| Necrosis | 0.919 | ||||||||
| No (78) | 78 | 0 | 0.001 | 56 | 22 | 0.295 | 49 | 29 | |
| Yes (66) | 37 | 29 | 42 | 24 | 42 | 24 | |||
| Calcification | 0.589 | ||||||||
| No (80) | 63 | 17 | 0.710 | 55 | 25 | 0.842 | 49 | 31 | |
| Yes (64) | 52 | 12 | 43 | 21 | 42 | 24 | |||
| Massive choroidal invasion | 0.001 | ||||||||
| No (103) | 91 | 16 | 0.008 | 82 | 25 | 0.001 | 85 | 22 | |
| Yes (37) | 24 | 13 | 16 | 21 | 6 | 31 | |||
| AC invasion | 0.228 | ||||||||
| No (135) | 110 | 25 | 0.060 | 94 | 41 | 0.117 | 87 | 48 | |
| Yes (9) | 5 | 4 | 4 | 5 | 4 | 5 | |||
| Scleral invasion | 0.715 | ||||||||
| No (132) | 108 | 24 | 0.052 | 91 | 41 | 0.451 | 84 | 48 | |
| Yes (12) | 7 | 5 | 7 | 5 | 7 | 5 | |||
| Iris & CB invasion | 0.182 | ||||||||
| No (131) | 107 | 24 | 0.084 | 92 | 39 | 0.076 | 85 | 46 | |
| Yes (13) | 8 | 5 | 6 | 7 | 6 | 7 | |||
| Resected margin of ON invasion | 0.010 | ||||||||
| No (80) | 71 | 9 | 0.003 | 59 | 21 | 0.101 | 58 | 22 | |
| Yes (64) | 44 | 20 | 39 | 25 | 33 | 31 | |||
| Tumor invasion/HRFs | 0.001 | ||||||||
| No (66) | 66 | 1 | 0.001 | 53 | 13 | 0.004 | 55 | 12 | |
| Yes (78) | 49 | 28 | 45 | 33 | 36 | 41 | |||
* Bold signifies statistical significant value
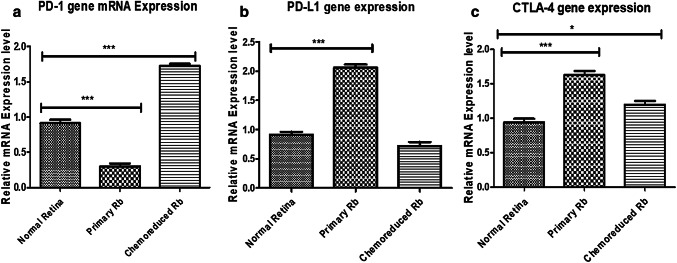
Similar to IHC results, PD-1 was found in 10% (1/10), whereas PD-L1 and CTLA-4 were present in 70% (7/10) and 10% (1/10) cases by western blotting (Supplementary Fig. 2a-e). In this study, we also found significant upregulation of PD-L1 (3.1-fold) and CTLA-4 (1.9-fold) gene and downregulation for PD-1 (0.2-fold) gene in tumor tissues as compared to the normal retina (control). The qRT-PCR results confirmed that the relative mRNA expression of all three transcripts was in concurrence with immunohistochemical findings. Among these, PD-L1 showed the highest differential expression level in primary retinoblastoma (Fig. 2).
Fig. 2.
Differential mRNA expression analysis of a PD-1, b PD-L1 and c CTLA-4 gene expression in normal retina, primary retinoblastoma and chemoreduced retinoblastoma tumor samples by qRT-PCR
Pattern of immune markers expression in chemoreduced retinoblastoma (Group II)
Due to the changes in the tumor microenvironment, the expression pattern of PD-1, PD-L1 and CTLA-4 proteins was different in chemoreduced retinoblastoma as compared to primary retinoblastoma (Fig. 1). Positivity of PD-1 was increased (40.6%; 48/118) in this group, whereas the expression of PD-L1 was decreased and found only in 18.6% (22/118) cases. Expression of CTLA-4 protein was found in 32.2% (38/118). Expression of PD-1 was significantly associated with scleral/extrascleral invasion, choroidal invasion, cholesterol clefts, and foamy macrophages. Choroidal invasion, scleral invasion, and pathological staging were significantly associated with PD-L1 and CTLA-4 expression (Table 2).
Table 2.
Correlations between PD-1, PD-L1 and CTLA-4 Proteins with clinicopathological parameters in chemoreduced retinoblastoma Cases
| Pathological parameters N: 118 | PD-1 | PD-L1 | CTLA-4 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Negative N: 70 | Positive N: 48 | p value* | Negative N: 96 | Positive N: 22 | p value* | Negative N: 80 | Positive N: 38 | p value* | |
| Sex | 0.735 | ||||||||
| Female (44) | 30 | 14 | 0.131 | 59 | 15 | 0.557 | 51 | 23 | |
| Male (74) | 40 | 34 | 37 | 7 | 29 | 15 | |||
| Age | 0.360 | ||||||||
| < 2 years (60) | 39 | 21 | 0.202 | 48 | 12 | 0.700 | 43 | 17 | |
| > 2 years (58) | 31 | 27 | 48 | 10 | 37 | 21 | |||
| Laterality | 0.842 | ||||||||
| Unilateral (73) | 44 | 29 | 0.789 | 61 | 12 | 0.433 | 49 | 24 | |
| Bilateral (45) | 26 | 19 | 35 | 10 | 31 | 14 | |||
| Grouping | 0.145 | ||||||||
| Group E (109) | 65 | 44 | 1.000 | 90 | 19 | 0.365 | 76 | 33 | |
| Group D-A (9) | 5 | 4 | 6 | 3 | 4 | 5 | |||
| Staging | 0.001 | ||||||||
| T1–T2b(82) | 52 | 30 | 0.172 | 71 | 11 | 0.028 | 64 | 18 | |
| T3a–T4(36) | 18 | 18 | 25 | 11 | 16 | 20 | |||
| Differentiation | 0.279 | ||||||||
| WDRB (15) | 12 | 3 | 0.081 | 15 | 0 | 0.047 | 12 | 3 | |
| PDRB (103) | 58 | 45 | 81 | 22 | 68 | 35 | |||
| Necrosis | 0.086 | ||||||||
| No (78) | 47 | 31 | 0.773 | 64 | 14 | 0.787 | 57 | 21 | |
| Yes (40) | 23 | 17 | 32 | 8 | 23 | 17 | |||
| Calcification | 0.545 | ||||||||
| No (45) | 27 | 18 | 0.906 | 36 | 9 | 0.767 | 32 | 13 | |
| Yes (73) | 43 | 30 | 60 | 13 | 48 | 25 | |||
| Massive choroidal invasion | 0.001 | ||||||||
| No (95) | 61 | 34 | 0.032 | 82 | 13 | 0.007 | 73 | 22 | |
| Yes (23) | 9 | 14 | 14 | 9 | 7 | 16 | |||
| AC invasion | 0.703 | ||||||||
| No (113) | 67 | 46 | 0.975 | 94 | 19 | 0.015 | 77 | 36 | |
| Yes (5) | 3 | 2 | 2 | 3 | 3 | 2 | |||
| Scleral invasion | 0.001 | ||||||||
| No (94) | 62 | 32 | 0.004 | 81 | 13 | 0.008 | 73 | 21 | |
| Yes (24) | 8 | 16 | 15 | 9 | 7 | 17 | |||
| Iris invasion | 0.952 | ||||||||
| No (112) | 69 | 43 | 0.061 | 92 | 20 | 0.343 | 76 | 36 | |
| Yes (6) | 1 | 5 | 4 | 2 | 4 | 2 | |||
| Resected margin of ON invasion | 0.109 | ||||||||
| No (94) | 59 | 35 | 0.132 | 77 | 17 | 0.758 | 67 | 27 | |
| Yes (24) | 11 | 13 | 19 | 5 | 13 | 11 | |||
| Phthisical eye | 0.579 | ||||||||
| No (102) | 63 | 39 | 0.173 | 83 | 19 | 1.000 | 68 | 34 | |
| Yes (16) | 7 | 9 | 13 | 3 | 12 | 4 | |||
| Foamy macrophages | 0.159 | ||||||||
| No (93) | 66 | 27 | 0.001 | 75 | 18 | 0.703 | 66 | 27 | |
| Yes (25) | 4 | 21 | 21 | 4 | 14 | 11 | |||
| Retinal gliosis | 0.930 | ||||||||
| No (106) | 64 | 42 | 0.488 | 87 | 19 | 0.551 | 63 | 43 | |
| Yes (12) | 6 | 6 | 9 | 3 | 7 | 5 | |||
| Fibrosis | 0.609 | ||||||||
| No (105) | 63 | 42 | 0.670 | 86 | 19 | 0.664 | 62 | 43 | |
| Yes (13) | 7 | 6 | 10 | 3 | 8 | 5 | |||
| Cholesterol clefts | 0.505 | ||||||||
| No (109) | 68 | 41 | 0.018 | 90 | 19 | 0.238 | 65 | 36 | |
| Yes (9) | 2 | 7 | 6 | 3 | 5 | 2 | |||
| Extrascleral invasion | 0.061 | ||||||||
| No (108) | 68 | 40 | 0.019 | 89 | 19 | 0.343 | 76 | 32 | |
| Yes (10) | 2 | 8 | 7 | 3 | 4 | 6 | |||
| CB invasion | 0.361 | ||||||||
| No (111) | 67 | 44 | 0.361 | 90 | 21 | 0.760 | 75 | 36 | |
| Yes (7) | 3 | 4 | 6 | 1 | 5 | 2 | |||
* Bold signifies statistical significant value
We observed PD-1 expression in 50% (5/10), whereas PD-L1 and CTLA-4 were expressed in 40% (4/10) and 20% (2/10) cases by western blotting, respectively (Supplementary Fig. 2f–j). While comparing with normal retina (control), expression of PD-L1 (0.7-fold) was found to be less in comparison to PD-1 (1.7-fold), and CTLA-4 (1.3-fold) gene in tumor tissues by qRT-PCR (Fig. 2). These results confirmed that the relative mRNA expression of all three transcripts (PD-1, PD-L1, and CTLA-4) was in concomitance with immunohistochemical findings.
Association of clinicopathological factors and immune parameters with patients survival in Group I
Table 3 shows the prognostic significance of all the clinicopathological features of primary and chemoreduced retinoblastoma by Cox’s proportional hazards survival along with the Kaplan Meier analysis. In univariate analysis of Group I, a higher level of bilaterality, choroidal invasion, scleral invasion, and anterior chamber invasion had a significant effect on poor prognosis and worse survival in primary retinoblastoma patients. The outcome of immune markers was poor as the hazard ratio (HR) for relapse in patients with PD-L1, and CTLA-4 expression was 5.9 (95% CI 1.16–30.42) and 4.5 (95% CI 0.82–21.54), respectively, by univariate analysis. In multivariate analysis, choroidal invasion and bilaterality were the most important poor prognostic indicators of retinoblastoma. Expression of PD-L1 was found to be an independent prognostic marker as evidence of 12.988 HR (95% CI 0.92–34.23). Kaplan–Meier curves for relapse and log-rank test comparisons also showed that choroidal involvement (p = 0.011), scleral invasion (p < 0.001), iris and ciliary body invasion (p = 0.042) and anterior chamber (p = 0.014) were associated with the risk of relapse. The HR for death in patients with PD-L1 expressing tumors was 5.93 (95% CI 1.17–30.02; p = 0.016) and OS was 80.76% (95% CI 0.08–0.92; p = 0.016) (supplementary Fig. 3a–e) (supplementary Table 2).
Table 3.
Univariate and multivariate analysis of clinicopathological features of primary and chemoreduced retinoblastoma by Cox’s Proportional Hazards Survival along with the probabilities for Kaplan–Meier Analysis
| Parameters | Primary retinoblastoma (Group I; n = 144) | Chemoreduced retinoblastoma (Group II; n = 118) | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| Hazard ratio’s | p value* | Hazard ratio’s | p value* | Hazard ratio’s | p value* | Hazard ratio’s | p value* | |
| Sex | 0.612 (0.12–3.13) | 0.545 | – | – | 1.267 (0.359–4.47) | 0.716 | – | – |
| Age | 1.563 (0.35–6.94) | 0.556 | – | – | 0.747 0.21–2.63) | 0.649 | – | – |
| Laterality | 4.514 (1.02–20.05) | 0.048 | 6.233 (1.29–28.75) | 0.021 | 0.884 (0.23–3.45) | 0.863 | – | – |
| Staging | 1.967 (0.38–10.07) | 0.400 | – | – | 0.902 (0.23–3.46) | 0.880 | – | – |
| Differentiation | – | 0.212 | – | – | – | 0.118 | – | – |
| Necrosis | 3.045 (0.59–15.68) | 0.185 | – | – | 2.085 (0.56–7.67) | 0.269 | – | – |
| Calcification | 3.611 (0.70–18.53) | 0.103 | – | – | 2.646 (0.53–13.06) | 0.232 | – | – |
| Massive choroidal invasion | 6.441 (1.25–32.95) | 0.026 | 10.336 (1.32–80.91) | 0.026 | 5 (1.31–19.07) | 0.018 | 1.427 (0.39–5.14) | 0.587 |
| Scleral invasion | 17.558 (3.94–78.18) | 0.001 | 4.869 (0.86– 28.73) | 0.106 | 0.887 (0.18–4.16) | 0.880 | ||
| Extrascleral infiltration | – | – | – | – | 11.333 (2.50–51.27) | 0.002 | 4.700 (0.76– 29.06) | 0.096 |
| Iris and ciliary body invasion | 4.679 (0.91–24.06) | 0.066 | – | – | 5.15 (.85–30.86) | 0.073 | – | – |
| Anterior chamber invasion | 6.037 (1.17–30.95) | 0.032 | 1.332 (0.25–6.83) | 0.731 | 2.888 (0.29–28.66) | 0.365 | – | – |
| Presence of macrophages | – | – | – | – | 21.411 (4.17– 109.68) | 0.001 | 15.629 (2.47–98.80) | 0.003 |
| Gliosis | – | – | – | – | 1.249 (0.15–9.83) | 0.838 | – | – |
| Fibrosis | – | – | – | – | – | 0.118 | – | – |
| Presence of cholesterol clefts | – | – | – | – | 1.388 (0.15–12.38) | 0.769 | – | – |
| Resected margin of ON invasion | 0.432 (0.08–2.21) | 0.294 | – | – | 1.779 (0.46–6.85) | 0.404 | – | – |
| Invasion of > 1 HRFs | 2.161 (0.42–11.05) | 0.335 | – | – | 0.577 (0.14–2.35) | 0.444 | – | |
| PD–1 | 2.000 (0.39–10.24) | 0.408 | – | – | 6.805 (1.37–33.61) | 0.019 | 9.295 (2.68–32.16) | 0.001 |
| PD-L1 | 5.946 (1.16–30.42) | 0.022 | 12.988 (0.92–34.23) | 0.001 | 0.460 (0.05–3.83) | 0.808 | – | – |
| CTLA-4 | 4.513 (0.82–21.54) | 0.048 | – | – | 0.893 (0.21–3.66) | 0.876 | ||
* Bold signifies statistical significant value
Association of clinicopathological factors and immune parameters with patients survival in Group II
In univariate analysis, choroidal invasion, extrascleral invasion, and foamy macrophages were significantly associated factors in which foamy macrophages was found to be an independent prognostic factor by multivariate analysis in chemoreduced retinoblastoma. Among all the immune markers, PD-1 was significantly associated with decreased overall survival as the hazard ratio was 6.8 (95% CI 1.375–33.611; p = 0.019) by univariate analysis. By multivariate analysis, the HR for death in patients with PD-1 expressing tumors was 9.3 (95% CI 2.68–32.16; p value = 0.001), and overall survival was 63.28% (95% CI 0.32–0.82; p value = 0.003) by Kaplan–Meier analysis (supplementary Fig. 3f–i) (supplementary Table 3). No significant association was found between increased or decreased expression of PD-L1 and CTLA-4 protein.
Association of immune markers with overall survival by log-rank test
Supplementary Table 4 summarized the log-rank test for equality of survival functions of immune markers in primary and chemoreduced retinoblastoma patients. The overall survival was decreased to 83.33% in primary retinoblastoma patients having PD-1, PD-L1, and CTLA-4 expressing tumors. When both PD-L1 and CTLA-4 expressed together in the tumor, survival reduced to 77.92% in which three deaths occurred in primary retinoblastoma. On the other hand, in chemoreduced patients, the overall survival decreased to 50%. Most of the death (5/10) occurred in patients who showed only PD-1 expressing tumors where overall survival was 65.38%.
Discussion
Primary retinoblastoma remains a challenge for the oncologist to salvage the eye and restore vision. Systemic chemotherapy may be administered in chemoreduced retinoblastoma as treatment modalities, but many cases are chemoresistant, and so require enucleation [22]. Pathology differs for both primary and chemoreduced retinoblastoma as primary retinoblastoma presents with a large amount of viable tumor, whereas chemoreduced retinoblastoma showed disarray of ocular structure, less viable tumor, scleral thickening, cholesterol clefts, retinal gliosis, fibrosis and foamy macrophages suggestive of the tumor microenvironment [23].
Extensive experiments have been performed to understand the molecular biology of the Rb gene, but the induction and expression of tumor-specific T cells against retinoblastoma have not been conducted [24]. This lack of information prompts the researcher to find out the role of immunotherapy as a potential marker in the tumor microenvironment of primary and chemoreduced retinoblastoma. Recently, immunotherapy is the emerging field in cancer biology, but the role of immunotherapy is not known in retinoblastoma as yet. In our study, we observed the differences in the expression pattern of PD-1, PD-L1 and CTLA-4 protein in both groups of retinoblastomas.
Increased expression of PD-L1 (46/144) and decreased expression of PD-1 (29/144) were seen in primary retinoblastoma. The overall survival rate was statistically significant in PD-L1 expressing tumors (89.13%; p value = 0.015) as compared to PD-1 expression (93.10%; p value = 0.394). On the other hand, the inverse pattern was observed in chemoreduced retinoblastoma where expression of PD-1 (48/118) was increased, and PD-L1 was decreased (22/118). Statistical correlation was found with an overall survival rate in PD-1 expressing chemoreduced patients (63.28%; p value = 0.003). Expression of CTLA-4 protein showed a similar pattern in both primary and chemoreduced retinoblastoma, but no significant correlation was found with patient outcome. Although the exact mechanism of PD-1 expression in tumor cells is unclear, there is an evidence that intrinsic expression of PD-1 promotes tumor growth independently of adaptive immunity in tumor cell lines by involving multiple factors such as gene copy number alterations, epigenetic modifications, and tumor microenvironment [25–28]. Therefore, our results might be due to the existence of retinal tumor microenvironment that contributes PD-1/PD-L1 pathway significantly to tumor progression in both the groups of retinoblastoma [29].
Some studies showed necrosis as a poor prognostic factor in the tumor microenvironment in most of the solid tumors [30]. Similarly, Chong et al. [31] showed extensive necrosis to be a poor prognostic factor in both primary and chemoreduced retinoblastoma. This is due to the inadequate nutrient supply to tumor cells as a consequence of the imbalance between tumor growth and angiogenesis, the host’s cytotoxic immune response to the tumor and downregulation of programmed (apoptotic) cell death by the tumor itself [32]. In our study, necrosis was found in 45.8% and 33.9% of cases of primary and chemoreduced retinoblastoma, respectively. Both Groups I and Group II showed PD-1 expression mostly in necrotic tumors compared to PD-L1 expression (p value = 0.001). Our results are in line with Thompson et al. [33] stating that immune-checkpoints markers were associated with necrotic tumors of clear cell carcinoma. This might be because necrosis plays an important role in activating tumor-associated cells such as dendritic cells, leukocytes, and endothelial cells [34].
It was suggested that macrophage alteration in tumor microenvironment contributes to retinoblastoma tumorigenesis that ultimately results in tumor development [35]. The presence of macrophages correlates with poor prognosis in human cancers [36]. On histopathology, macrophages are typically not seen in primary retinoblastoma, but they are more frequently observed in chemoreduced retinoblastoma [37]. This might be due to the activation of macrophages by chemotherapeutic agents, which was also found in breast cancer and leukemia [38, 39]. In our study, foamy macrophages were statistically significant with an expression of PD-1 protein (p < 0.001) and the overall survival was reduced by 85.71% in Group II. This supports the hypothesis by Radhakrishnan et al. [23] that foamy macrophages are suggestive of the inflammatory response in chemoreduced cases.
Only a few studies showed expression of immune markers in patients of solid tumor such as epithelial ovarian cancer [40], thymic epithelial tumors [41], high-grade serous ovarian cancer [42], non-small cell lung cancer [43], cervical cancer [44], and breast cancer [45] with and without chemotherapy. PD-1 and PD-L1 expression vary in pre- and post-chemotherapy patients of non-small cell lung cancer where positivity of PD-L1 reduced from 75 to 37.5% after NACT [43]. The mean PD-L1 score was increased from 42 to 93 in tumor cells, whereas PD-1 positivity increased from 33 to 100% in tumor-infiltrating immune cells in thymic carcinoma after chemotherapy, without any significant difference in progression-free survival [41]. Similar to these results, expression of PD-1 and PD-L1, but not CTLA-4, was altered in primary and chemoreduced retinoblastoma along with the overall survival of patients. Overall survival was decreased in patients expressing both PD-1 and CTLA-4 protein in chemoreduced retinoblastoma (77.92%) as compared to primary retinoblastoma (100%). This might support that therapies against PD-1 along with CTLA-4 might be useful in chemoreduced retinoblastoma rather than therapy alone. These results are in line with melanoma and non-small cell lung cancer (NSCLC) patients for the use of dual anti-CTLA4 and PD-1/PD-L1 blockade immunotherapy [46].
Usui et al. [24] showed the expression of PD-L1 in IFN-gamma-treated Y-79 human retinoblastoma cell line that might contribute to the suppression of T cells. There are studies, which confirmed the expression of spleen tyrosine kinase (SYK) on the cell membrane, which facilitates cytotoxic immune cells and recognizes dendritic cells (DCs) in human retinoblastoma Y-79 cell line. They suggested that the effect of SYK-targeted-DC-mediated CTLs as a potential immunotherapy target against retinoblastoma [47]. It is thus increasingly clear that T-lymphocytes expressing PD-1 and its ligand PD-L1 on antigen presenting cells might play a role in retinoblastoma. This is a combined approach of immunotherapy with conventional modalities can become an attractive therapeutic target for treating retinoblastoma patients. These results provide valuable data for future studies of the PD-1/PD-L1 pathway in retinoblastoma tumor and their association with clinicopathological parameters and patient response. This study might help in predicting the role of immune markers for selecting patients for better therapeutic strategies. Follow-up of all patients treated with chemoreduction needs to be warranted.
To conclude, this is the first and largest study in the literature to identify the expression of immune markers in both groups of human retinoblastoma tissue samples, and the expression of PD-1/PD-L1 dynamic changes in patients with chemoreduction. These preliminary results provide potential data for new treatment strategies using immune checkpoint inhibitors in combination with chemotherapy. Further in-vitro/in-vivo studies of these proteins might be needed to determine their precise role for clinical trials in human retinoblastoma patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We are very grateful to Mr. Pankaj Kumar for his excellent technical assistance.
Abbreviations
- AJCC
American Joint Committee on Cancer
- CTLA-4
Cytotoxic T-lymphocyte-associated antigen-4
- DAB
3,3′-Diaminobenzidine
- IHC
Immunohistochemistry
- PD-1
Programmed death-1
- PD-L1
Programmed death-ligand 1
- qRT-PCR
Quantitative real-time polymerase chain reaction
- Rb
Retinoblastoma
- SDS–PAGE
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- TILs
Tumor-infiltrating lymphocytes
Author contributions
LS and MR were responsible for the conception and design of the work; MKS contributed the acquisition, analysis, and interpretation of data for the work; SB helped in the follow-up of the patients; RM and NL recruited the patients and provided the tissue samples; SS helped in reviewing the histopathology slides; All authors reviewed and approved the final version of the manuscript.
Funding
The work was supported by Department for Science and Technology (DST), Govt. of India for providing National Post-Doctoral fellowship (N-PDF) to Dr. Lata Singh and conducting this research (NPDF/2016/000903).
Compliance with ethical standards
Conflict of interest
The author(s) have no proprietary or commercial interest in any materials discussed in this article.
Ethical approval
Ethical approval was obtained from Institute’s Ethical Committee, All India Institute of Medical Sciences (Ref. No. IEC-424/RP-6/2016) and carried out in accordance with the Declaration of Helsinki principles.
Informed consent
Written consent was obtained from their legal guardians of all the patients for collection of tissue samples prior to the surgery.
Participants
This research involved the human participants.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lata Singh and Seema Kashyap have equally contributed.
References
- 1.Singh L, Kashyap S. Update on pathology of retinoblastoma. Int J Ophthalmol. 2018;11(12):2011–2016. doi: 10.18240/ijo.2018.12.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chawla B, Singh R. Recent advances and challenges in the management of retinoblastoma. Indian J Ophthalmol. 2017;65(2):133–139. doi: 10.4103/ijo.IJO_883_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghassemi F, Khodabande A. Risk definition and management strategies in retinoblastoma: current perspectives. Clin ophthalmol. 2015;9:985–994. doi: 10.2147/OPTH.S59828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yanık Ö, Gündüz K, Yavuz K, et al. Chemotherapy in retinoblastoma: current approaches. Turk J Ophthalmol. 2015;45(6):259–267. doi: 10.4274/tjo.06888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gombos DS, Hungerford J, Abramson DH, et al. Secondary acute myelogenous leukemia in patients with retinoblastoma: is chemotherapy a factor? Ophthalmology. 2007;114(7):1378–1383. doi: 10.1016/j.ophtha.2007.03.074. [DOI] [PubMed] [Google Scholar]
- 6.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat med. 2013;19(11):1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen F, Zhuang X, Lin L, et al. New horizons in tumor microenvironment biology: challenges and opportunities. BMC Med. 2015;13(1):45. doi: 10.1186/s12916-015-0278-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang M, Zhao J, Zhang L, et al. Role of tumor microenvironment in tumorigenesis. J Cancer. 2017;8(5):761–773. doi: 10.7150/jca.17648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sachdeva UM, O’Brien JM. Understanding pRb: toward the necessary development of targeted treatments for retinoblastoma. J Clin Invest. 2012;122(2):425–434. doi: 10.1172/JCI57114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang H, Declerck YA. Targeting the tumor microenvironment: from understanding pathways to effective clinical trials. Cancer Res. 2013;73(16):4695–4777. doi: 10.1158/0008-5472.CAN-13-0661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shih K, Arkenau HT, Infante JR. Clinical impact of checkpoint inhibitors as novel cancer therapies. Drugs. 2014;74(17):1993–2013. doi: 10.1007/s40265-014-0305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and Anti-CTLA-4 Therapies in cancer: mechanisms of action, efficacy, and limitations. Front Oncol. 2018;8:86. doi: 10.3389/fonc.2018.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Henick BS, Herbst RS, Goldberg SB. The PD-1 pathway as a therapeutic target to overcome immune escape mechanisms in cancer. Expert Opin Ther Targets. 2014;18(12):1407–1420. doi: 10.1517/14728222.2014.955794. [DOI] [PubMed] [Google Scholar]
- 15.Yang W, Li H, Chen PW, et al. PD-L1 expression on human ocular cells and its possible role in regulating immune-mediated ocular inflammation. Invest Ophthalmol Vis Sci. 2009;50(1):273–280. doi: 10.1167/iovs.08-2397. [DOI] [PubMed] [Google Scholar]
- 16.Alsaab HO, Sau S, Alzhrani R, et al. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol. 2017;8:561. doi: 10.3389/fphar.2017.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Udall M, Rizzo M, Kenny J, et al. PD-L1 diagnostic tests: a systematic literature review of scoring algorithms and test-validation metrics. Diagn Pathol. 2018;13(1):12. doi: 10.1186/s13000-018-0689-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Juneja VR, McGuire KA, Manguso RT, et al. PD-L1 on tumor cells is sufficient for immune evasion in immunogenic tumors and inhibits CD8 T cell cytotoxicity. J Exp Med. 2017;214(4):895–904. doi: 10.1084/jem.20160801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Q, Liu F, Liu L. Prognostic significance of PD-L1 in solid tumor: an updated meta-analysis. Medicine. 2017;96(18):e6369. doi: 10.1097/MD.0000000000006369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pianko MJ, Liu Y, Bagchi S, et al. Immune checkpoint blockade for hematologic malignancies: a review. Stem Cell Investig. 2017;4:32. doi: 10.21037/sci.2017.03.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mallipatna A, Gallie BL, Chévez-Barrios P. Retinoblastoma. In: Amin MB, Edge SB, Greene FL, editors. AJCC Cancer Staging Manual. 8. New York, NY: Springer; 2017. pp. 819–831. [Google Scholar]
- 22.Di Nicolantonio F, Neale M, Onadim Z et al (2003) The chemosensitivity profile of retinoblastoma. In: Chemosensitivity testing in oncology. Springer, Berlin, pp 73–80 [DOI] [PubMed]
- 23.Radhakrishnan V, Kashyap S, Pushker N, et al. Outcome, pathologic findings, and compliance in orbital retinoblastoma (international retinoblastoma staging system stage III) treated with neoadjuvant chemotherapy: a prospective study. Ophthalmology. 2012;119(7):1470–1477. doi: 10.1016/j.ophtha.2012.01.029. [DOI] [PubMed] [Google Scholar]
- 24.Usui Y, Okunuki Y, Hattori T, et al. Expression of costimulatory molecules on human retinoblastoma cells Y-79: functional expression of CD40 and B7H1. Invest Ophthalmol Vis Sci. 2006;47(10):4607–4613. doi: 10.1167/iovs.06-0181. [DOI] [PubMed] [Google Scholar]
- 25.Li H, Li X, Liu S, et al. Programmed cell death-1 (PD-1) checkpoint blockade in combination with a mammalian target of rapamycin inhibitor restrains hepatocellular carcinoma growth induced by hepatoma cell-intrinsic PD-1. Hepatology. 2017;66(6):1920–1933. doi: 10.1002/hep.29360. [DOI] [PubMed] [Google Scholar]
- 26.Kleffel S, Posch C, Barthel SR, et al. Melanoma cell-intrinsic PD-1 receptor functions promote tumor growth. Cell. 2015;162(6):1242–1256. doi: 10.1016/j.cell.2015.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Du S, McCall N, Park K, et al. Blockade of tumor-expressed PD-1 promotes lung cancer growth. Oncoimmunology. 2018;7(4):e1408747. doi: 10.1080/2162402X.2017.1408747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yao H, Wang H, Li C, et al. Cancer cell-intrinsic PD-1 and implications in combinatorial immunotherapy. Front Immunol. 2018;9:1774. doi: 10.3389/fimmu.2018.01774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raguraman R, Parameswaran S, Kanwar JR, et al. Evidence of tumour microenvironment and stromal cellular components in retinoblastoma. Ocul Oncol Pathol. 2019;5(2):85–93. doi: 10.1159/000488709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lotfi R, Kaltenmeier C, Lotze MT, et al. Until death do us part: necrosis and oxidation promote the tumor microenvironment. Transfus Med Hemother. 2016;43(2):120–132. doi: 10.1159/000444941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chong EM, Coffee RE, Chintagumpala M, et al. Extensively necrotic retinoblastoma is associated with high-risk prognostic factors. Arch Pathol Lab Med. 2006;130(11):1669–1672. doi: 10.5858/2006-130-1669-ENRIAW. [DOI] [PubMed] [Google Scholar]
- 32.Lotfi R, Schrezenmeier H, Lotze MT. Immunotherapy for cancer: promoting innate immunity. Front Biosci. 2009;14:818–832. doi: 10.2741/3280. [DOI] [PubMed] [Google Scholar]
- 33.Thompson RH, Kuntz SM, Leibovich BC, et al. Tumor B7–H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66(7):3381–3385. doi: 10.1158/0008-5472.CAN-05-4303. [DOI] [PubMed] [Google Scholar]
- 34.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat immunol. 2013;14(10):1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Richards DM, Hettinger J, Feuerer M. Monocytes and macrophages in cancer: development and functions. Cancer Microenviron. 2013;6(2):179–191. doi: 10.1007/s12307-012-0123-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Palma M, Lewis CE. Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell. 2013;23(3):277–286. doi: 10.1016/j.ccr.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 37.Demirci H, Eagle RC, Shields CL, et al. Histopathologic findings in eyes with retinoblastoma treated only with chemoreduction. Arch Ophthalmol. 2003;121(8):1125–1131. doi: 10.1001/archopht.121.8.1125. [DOI] [PubMed] [Google Scholar]
- 38.Pollard JW. Macrophages define the invasive microenvironment in breast cancer. J Leukoc Biol. 2008;84(3):623–630. doi: 10.1189/jlb.1107762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Long M, Beckwith K, Do P, et al. Ibrutinib treatment improves T cell number and function in CLL patients. J Clin Invest. 2017;127(8):3052–3064. doi: 10.1172/JCI89756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mesnage SJ, Auguste A, Genestie C, et al. Neoadjuvant chemotherapy (NACT) increases immune infiltration and programmed death-ligand 1 (PD-L1) expression in epithelial ovarian cancer (EOC) Ann of Oncol. 2016;28(3):651–657. doi: 10.1093/annonc/mdw625. [DOI] [PubMed] [Google Scholar]
- 41.Katsuya Y, Horinouchi H, Asao T, et al. Expression of programmed death 1 (PD-1) and its ligand (PD-L1) in thymic epithelial tumors: impact on treatment efficacy and alteration in expression after chemotherapy. Lung Cancer. 2016;99:4–10. doi: 10.1016/j.lungcan.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 42.Kim HS, Kim JY, Lee YJ, et al. Expression of programmed cell death ligand 1 and immune checkpoint markers in residual tumors after neoadjuvant chemotherapy for advanced high-grade serous ovarian cancer. Gynecol oncol. 2018;151(3):414–421. doi: 10.1016/j.ygyno.2018.08.023. [DOI] [PubMed] [Google Scholar]
- 43.Sheng J, Fang W, Yu J, et al. Expression of programmed death ligand-1 on tumor cells varies pre and post chemotherapy in non-small cell lung cancer. Sci Rep. 2016;6:20090. doi: 10.1038/srep20090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meng Y, Liang H, Hu J, et al. PD-L1 Expression correlates with tumor infiltrating lymphocytes and response to neoadjuvant chemotherapy in cervical cancer. J Cancer. 2018;9(16):2938–2945. doi: 10.7150/jca.22532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wimberly H, Brown JR, Schalper K, et al. PD-L1 expression correlates with tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy in breast cancer. Cancer Immunol Res. 2015;3(4):326–332. doi: 10.1158/2326-6066.CIR-14-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chae YK, Arya A, Iams W, et al. Current landscape and future of dual anti-CTLA4 and PD-1/PD-L1 blockade immunotherapy in cancer; lessons learned from clinical trials with melanoma and non-small cell lung cancer (NSCLC) J Immunother Cancer. 2018;6(1):39. doi: 10.1186/s40425-018-0349-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen X, Kunda PE, Lin J, et al. SYK-targeted dendritic cell-mediated cytotoxic T lymphocytes enhance the effect of immunotherapy on retinoblastoma. J Cancer Res Clin Oncol. 2018;144(4):675–684. doi: 10.1007/s00432-018-2584-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singh L, Singh MK, Rizvi MA, Pushker N, Sen S, Kashyap S. Differential expression patterns of immune checkpoint markers in tumour-stromal microenvironment of primary and chemoreduced retinoblastoma. Ann Oncol. 2019;30(11):447003. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.