Abstract
Natural killer (NK) cells, a predominant innate lymphocyte subset, mediates eradicating malignant cells. Purinergic signaling by ectonucleotidase CD39 can suppress T-cell response in caner. However, the role of CD39 in NK cells has not been fully elucidated. Here, we characterized CD39 expression on NK cells and its clinical relevance in esophageal squamous cell carcinoma (ESCC). Peripheral blood and tissue samples were collected from 36 ESCC patients. We observed that the proportion of NK cells significantly decreased but CD39 was obviously up-regulated on NK cells from cancerous tissues compared to paired peripheral blood in ESCC patients. Furthermore, tumor-infiltrating NK cells with high CD39 expression exhibited a phenotype of functional impairment. In vitro, conditioned media of ESCC cell lines could induce CD39 expression on peripheral NK cells from healthy donors. IL-6 was identified as the major cytokine produced by ESCC cell lines and also elevated in both tumor tissues and blood serum from ESCC patients. Recombinant IL-6 significantly induced surface CD39 expression in human NK cells, while IL-6-receptor antagonist tocilizumab prevented this effect. Finally, tumor-infiltrating CD39+ NK cells were correlated with poor prognosis in ESCC patients. Thus, tumor-derived IL-6 might impair NK cell functions through induction of CD39 expression. CD39+ NK cells may serve as a potential prognostic biomarker for ESCC patients.
Electronic supplementary material
The online version of this article (10.1007/s00262-020-02629-1) contains supplementary material, which is available to authorized users.
Keywords: Tumor microenvironment, NK cells, CD39, Esophageal squamous cell carcinoma
Introduction
Esophageal cancer is one of the major causes of cancer-related deaths worldwide that cause more than half a million death each year [1]. Esophageal squamous cell carcinoma (ESCC) accounts for 90% of the esophageal cancer cases in Asian countries. Conventional treatments and targeted drugs gain little progress in the treatment of ESCC during the past two decades. Recent studies highlight the importance of tumor microenvironment in the progression of disease through inhibition of anti-tumor immune responses. Clinical results with immune checkpoint inhibitors have shown encouraging responses for the treatment of advanced ESCC [2, 3]. However, only a small group of cancer patients may benefit from PD-1/PD-L1 immune-checkpoint blockade [4]. Thus, further investigation is needed to reverse the immune suppression and achieve immune normalization in tumor microenvironment.
Natural killer (NK) cells are important lymphocytes of the innate immune system that constitute the first line of host defense against infectious pathogens and cancer [5]. NK cells play an important role in tumor immunity via secreting cytokines such as IFN-γ and killing tumor cells directly. However, cancer cells may avoid the immune defense of NK cells by various mechanisms. Previous studies suggested that NK cells within tumor microenvironment often have severe functional defects and associated with deteriorating disease conditions [6]. NK cells elicit anti-tumor activity based on the balance between activating and inhibitory cell surface receptors. Blocking the inhibitory receptor NKG2A exhibited significant anti-tumor activity by promoting NK and T cell functions in both mice and humans [7].
CD39 is a cell surface ectonucleotidase that hydrolyzes extracellular ATP and plays a key role in modulation of the purinergic signaling [8]. The production of the purine nucleoside adenosine is up-regulated and can heavily suppress T cell activity in tumor microenvironment [9]. CD39 is expressed on tumor-filtrating T cells, myeloid cells, some NK cells as well as cancer cells of different tumor types [10]. High expression of CD39 was identified to mark exhausted CD8+ T cells from tumor tissues and blockade of CD39 signaling restores T cell functions in vitro and in vivo [11]. NK cell numbers and functions were increased in CD39-deficient mice [12]. However, the role of CD39 expression on human NK cells in tumor microenvironment remains largely unknown.
In the present study, we investigated the expression and the role of CD39 on NK cells in ESCC patients. Our results characterized the frequency and function of CD39+ NK cells in tumor microenvironment. Mechanistically, IL-6 secreted by esophageal cancer cells induced the expression of CD39 in NK cells. Importantly, CD39+ NK cells are associated with poor overall survival and recurrence free survival in ESCC patients. Our data here predict CD39 as a potential target which might strengthen NK cell-mediated anti-tumor immunity.
Materials and methods
Human samples
Fresh tumor tissues and paired peripheral blood were obtained from 36 patients with pathologically confirmed ESCC (Supplementary Table 1), who underwent esophagectomy at the First Affiliated Hospital of Zhengzhou University (Zhengzhou, China). Peripheral blood was also collected from 10 healthy donors.
Isolation of lymphocytes from peripheral blood and tumor tissues
Peripheral blood was collected and analyzed within 6 h. Peripheral blood mononuclear cells (PBMC) were prepared by density gradient centrifugation using Ficoll-Hypaque (Haoyang, China). To prepare tumor infiltrating lymphocytes (TIL), tumors were minced and mechanically dissociated with the GentleMACS Tumor Dissociation Kit (Miltenyi Biotec) according to the manufacturer's instructions.
Flow cytometry analysis
The cells were re-suspended in PBS with 20% FBS for 15 min at 4 °C to block Fc receptors. Surface staining was performed with specified fluorochrome—conjugated antibodies for 30 min at 4 °C in the dark. For cytokine detection, the cells were stimulated with Phorbol 12-myristate 13-acetate (PMA) and ionomycin for 6 h in the presence of the protein transport inhibitor brefeldin A (BioLegend). Intracellular staining for cytokines was carried out after surface staining. The cells were fixed with 4% paraformaldehyde for 30 min at 4 °C and permeabilized with the permeabilization buffer, followed by staining with specific antibodies against human cytokines. To exclude dead cells, the samples were stained with 7-AAD viability staining solution (BioLegend). All samples were acquired and analyzed using a FACS Canto II cytometer (BD Biosciences). Details of the antibodies were listed in Supplementary Table 2.
Cell culture and treatment
Two ESCC cell lines TE-1 and TE-7 were maintained under standard cell culture techniques. PBMC from healthy donor were cultured in RPMI-1640 medium (Sigma) supplemented with 10% FBS (HyClone), 200 IU/mL recombinant human IL-2 (SL PHARM, China), 100 units/mL penicillin and 100 μg/mL streptomycin (Solarbio, China) for 48 h. All cultures were maintained in a humidified incubator with 5% CO2 at 37 °C. To induce CD39 expression, cells were cultured in conditioned media collected from ESCC cell lines, recombinant human IL-6 (20 ng/mL, Peprotech) with control antibody or tocilizumab (40 ng/mL, Roche), respectively.
Multiplex cytokine profiling assay
The supernatants of ESCC cell lines were collected after 24 h of culture. The concentration of cytokines was evaluated using a bead-based LEGENDplex kit (BioLegend) including 13 human cytokines according to the manufacturer's instructions. The levels of cytokines were measured and analyzed using a FACS Canto II cytometer (BD Biosciences).
Enzyme-linked immunosorbent assay (ELISA)
IL-6 was measured in serum from ESCC patients or conditioned media from ESCC cell lines using the ELISA kit (BioLegend) following the manufacturer’s instructions.
Quantitative real-time PCR
Total RNA was extracted from tissues using RNAiso Plus (TakaRa, Japan) according to the manufacturer's instructions. The concentration of total RNA was quantified by a NanoDrop spectrophotometer (Thermo Fisher Scientific), and cDNA was synthesized using PrimeScript RT reagent Kit with gDNA Eraser (TakaRa). qPCR was performed using specific primers and SYBR Green qPCR Master Mix (Roche). Data were collected and analyzed using an Mx3005P instrument (Agilent Technologies).
Statistical analysis
Statistical analysis was performed in all the experiments by using GraphPad Prism 7 software. The paired or unpaired Student’s t test was used to determine statistical significance. Survival curves were drawn using the Kaplan–Meier method. Progression free survival and overall survival with tumor-infiltrating CD39+ NK cells were analyzed using the log-rank (Mantel-Cox) test. Data are presented as the mean ± standard deviation from at least three independent experiments. A p values < 0.05 was considered significant. * p < 0.05, ** p < 0.01 and *** p < 0.001.
Results
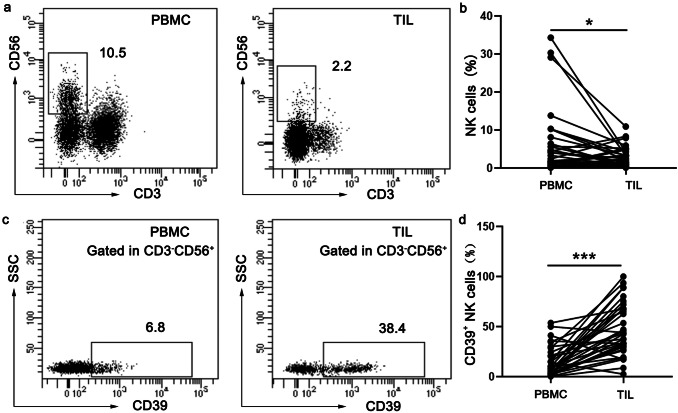
CD39 is up-regulated on tumor infiltrating NK cells in ESCC patients
The interaction between tumor cells and the surrounding immune cells forms a complex and variable tumor microenvironment to determine tumor progression and clinical outcomes of cancer patients [13]. Numerous studies have shown the exhaustion of effector T cells in tumor microenvironment, but the status of tumor-infiltrating NK cells is not very clear in esophageal cancer. In this study, we characterized NK cells from PBMC and paired TIL in 36 ESCC patients by using flow cytometry (Fig. 1a). The frequency of NK cells was significantly decreased in TIL compared with matched PBMC (Fig. 1b). We further analyzed the surface expression of CD39 on NK cells (Fig. 1c). Compared with NK cells from PBMC in ESCC patients, NK cells from tumor microenvironment expressed significantly higher levels of surface CD39 (Fig. 1d). We further examined several well-defined exhausted markers (PD-1, TIGIT and Tim-3) along with CD39 on tumor-infiltrating NK cells from ESCC patients. ESCC patients lacked the co-expression of other inhibitory receptors in the majority of CD39+ NK cells (Supplementary Figure 1). Following CD39 activity, CD73 orchestrates a crucial homeostatic balance of extracellular adenosine levels [8]. We also examined co-expression of CD39 and CD73 on tumor-infiltrating NK cells from ESCC patients. Unlike CD39, CD73 was expressed on a very small population of NK cells in ESCC patients (Supplementary Figure 2).
Fig. 1.
CD39 is up-regulated on tumor infiltrating NK cells from esophageal cancer patients. a Representative FACS plots of NK (CD3−CD56+) cells in paired PBMC and TIL from a patient with esophageal cancer. b The percentages of total NK cells were quantified in paired PBMC and TIL from esophageal cancer patients (n = 36). c Representative FACS plots of CD39+ NK cells in paired PBMC and TIL from a patient with esophageal cancer. d The percentages of CD39+ NK cells in paired PBMC and TIL from patients with esophageal cancer (n = 36). *p < 0.05; ***p < 0.001
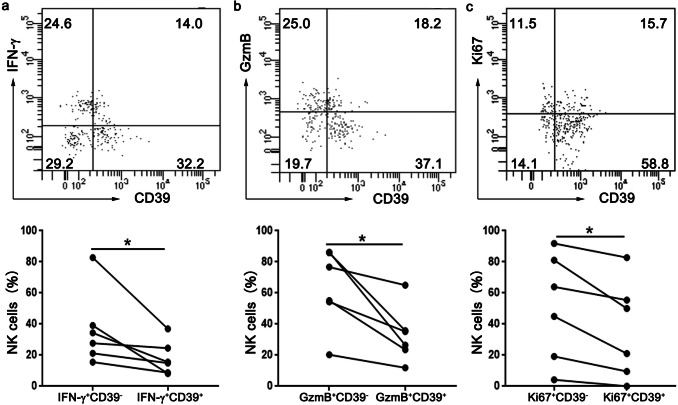
CD39+ NK cells are functional comprised in ESCC patients
Next we investigated the functions of CD39+ NK cells and CD39− NK cells freshly isolated from tumor tissues of ESCC patients. Using flow cytometry, we observed weaker production of IFN-γ (Fig. 2a) and granzyme B (Fig. 2b) in CD39+ NK cells after stimulation with PMA and ionomycin. Furthermore, CD39+ NK cells also showed low frequencies of proliferating (Ki-67+) marker (Fig. 2c). These results strongly suggest that CD39+ NK cells were functionally impaired as shown by defects in cytokines production and proliferation.
Fig. 2.
CD39+ NK cells are functionally suppressed in TIL from esophageal cancer patients. Assessment of intracellular levels of IFN-γ (a), GzmB (b) and Ki67 (c) on CD39+ NK cells and CD39− NK cells in TIL from esophageal cancer patients using flow cytometry. Top panel: representative FACS plots, bottom panel: statistical analysis. *p < 0.05
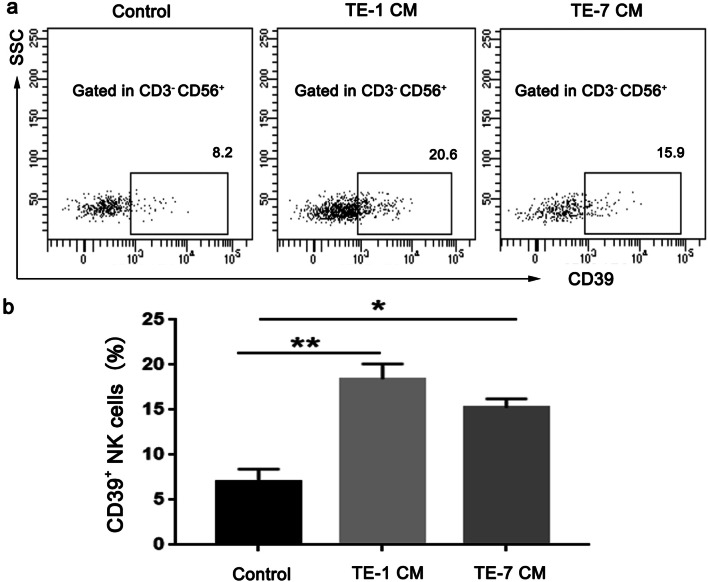
ESCC cancer cells induce the expression of CD39 on NK cells
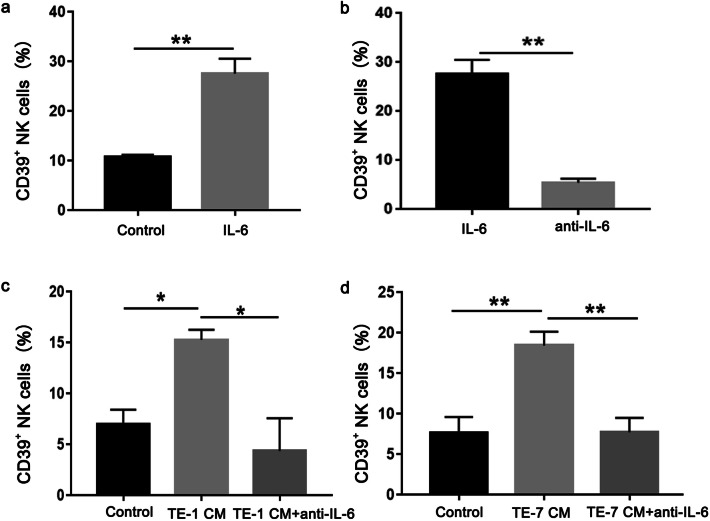
To investigate if ESCC cancer cells could induce CD39 expression on NK cells, PBMC isolated form healthy donors were cultured in vitro in the presence of conditioned medium from two ESCC cell lines, TE-1 and TE-7 (Fig. 3a). PBMC in the presence of conditioned media from TE-1 and TE-7 cells showed an increased population of CD39+ NK cells compared control PBMC (Fig. 3b).
Fig. 3.
Esophageal cancer cells induce CD 39 expression on human NK cells in vitro. PBMCs from healthy donors were cultured with control or conditioned media (CM) from ESCC cell lines TE-1 (a) and TE-7 (b) for 48 h, respectively. Representatives of three independent experiments. The surface CD39 expression on NK cells was measured by flow cytometry. Left panel: representative FACS plots, right panel: statistical analysis (n = 6–7). *p < 0.05; **p < 0.01
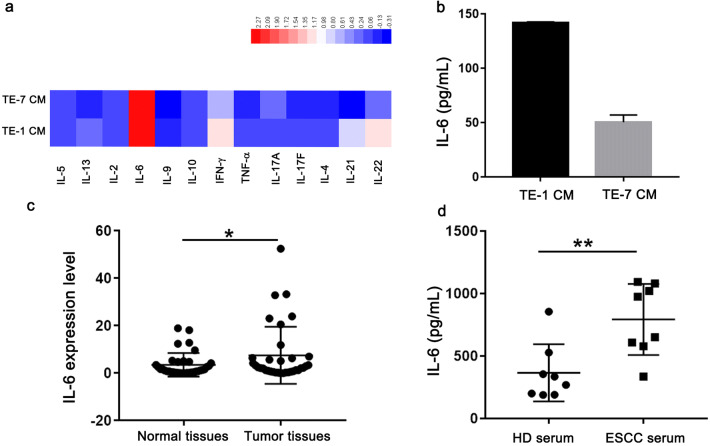
ESCC cancer cells and tissues produce significant levels of IL-6
A panel of secreted cytokines was measured by cytometric bead array in the conditioned media from ESCC cancer cells after 48 h of culture. High level of IL-6 was observed in the supernatant from TE-1 and TE-7 cells (Fig. 4a). The amount of IL-6 in the conditioned media from ESCC cancer cells was further verified by ELISA assay (Fig. 4b). Next, we evaluated IL-6 levels in normal tissues and ESCC tumors as determined by RT-qPCR. As expected, the level of IL-6 was elevated in tumor tissues than that from adjacent norm tissues from 35 ESCC patients (Fig. 4c). Finally, the levels of IL-6 were investigated by ELISA in serum from 8 ESCC patients and 8 healthy donors. Elevated levels of IL-6 have been found in ESCC patients as compared to healthy controls (Fig. 4d).
Fig. 4.
IL-6 is produced by esophageal cancer cells and elevated in esophageal cancer patients. a The levels of cytokines in conditioned media (CM) from esophageal cancer cell lines were analyzed using a multiplex cytokine profiling assay. b The concentrations of IL-6 in CM from TE-1 and TE-7 cells were determined by ELISA assay. Representatives of three independent experiments. c Relative mRNA levels of IL-6 expression in tumor tissues and adjacent normal tissues from esophageal cancer patients (n = 35) were measured by qRT-PCR assay. d The levels of IL-6 in serum from healthy donors (n = 8) or ESCC patients (n = 8) were measured by ELISA assay. *p < 0.05; **p < 0.01
IL-6 is required for ESCC cells-induced CD39 expression on NK cells
As the concentration of IL-6 is most pronounced in cytokines secreted by ESCC cell lines, we next investigated the effects of recombinant human IL-6 protein on NK cells. The results showed that exogenous IL-6 leaded to an increase of CD39 expression on NK cells (Fig. 5a). Tocilizumab is a humanized anti-IL-6 receptor antibody, which significantly inhibited the IL-6 induced CD39 expression on NK cells (Fig. 5b). Furthermore, we found that tocilizumab completely blocked CD39 expression induced by the conditioned media from ESCC cell lines TE-1 (Fig. 5c) and TE-7 (Fig. 5d). These results suggested that the conditioned media from ESCC cell lines enhances the CD39 expression on NK cells via IL-6 secretion.
Fig. 5.
Esophageal cancer cells induce CD39 expression on human NK cells via secretion of IL-6. a PBMC from healthy donors were cultured with recombinant human IL-6 for 48 h. b Anti-IL-6 receptor antibody was added into the culture system with PBMC and IL-6. c and d PBMC were cultured with conditioned media (CM) from TE-1 and TE-7 cells in the presence or absence of anti-IL-6 receptor antibody for 48 h. The surface CD39 expression on NK cells was analyzed by flow cytometry. Representatives of three independent experiments. *p < 0.05; **p < 0.01
The frequency of CD39+ NK cells significantly correlates with the poor prognosis of ESCC patients
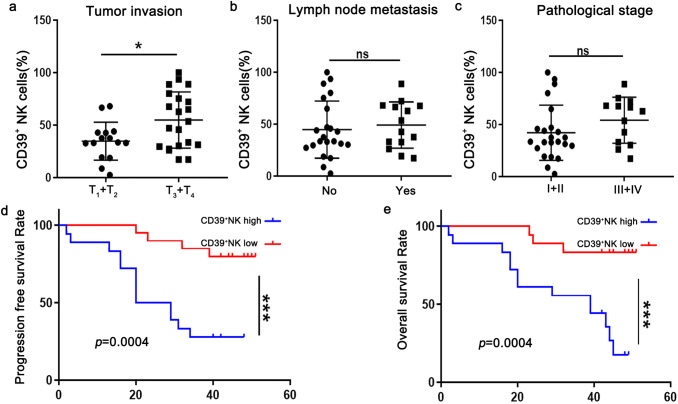
To explore the clinical relevance, we finally analyzed the proportion of CD39+ NK cells in relation to clinical characteristics and survival in 36 ESCC patients. The high invasion (T3 + T4) group had significantly high frequency of CD39+ NK cells than the low (T1 + T2) invasion group (Fig. 6a). However, the frequency of CD39+ NK cells did not correlated with other pathologic parameters, such as lymph node metastasis (Fig. 6b) and TNM staging (Fig. 6c) in ESCC samples in our study. Notably, we found that ESCC patients with high CD39+ NK cells experienced a significant decrease in progress free survival (Fig. 6d) and over survival (Fig. 6e). These data highlight the possibility of CD39+ NK cells to predict poor clinical outcomes in ESCC patients.
Fig. 6.
Tumor-infiltrating CD39+ NK cells are significantly associated with poor prognosis for ESCC patients. The frequency of tumor-infiltrating CD39+ NK cells were analyzed according to tumor invasion (a) lymph node metastasis (b) and pathological stage (c) in 36 ESCC patients. The Kaplan–Meier curves show the progression free survival (d) and overall survival (e) of ESCC patients. *p < 0.05; ***p < 0.001; ns, not significant
Discussion
Dysfunction of tumor infiltrating NK cells is thought to be a key determinant of tumor immune escape and reversal of NK-cell dysfunction has displayed great potential in tumor therapy. T cell exhaustion has been intensively studied in different types of cancers, however little is known about the exhaustion of NK cells. In this study, we characterize CD39 expression on NK cells in esophageal cancer patients, and uncover a possible mechanisms that induces the expression of CD39 on NK cell via IL-6 produced by tumor cells. Furthermore, we demonstrate the potential role of CD39+ NK cells to predict clinical prognosis of ESCC patients.
Numerous studies have shown that CD39 is up-regulated on regulatory T cells and acts as a suppressive molecule to inhibit T cell functions [14, 15]. Despite the expression of CD39 in a variety of immune cells in the tumor microenvironment, the expression and function of CD39 is still largely unknown in NK cells. Here, we found a substantial increase of CD39 expression in NK cells isolated from ESCC tissues, which is consistent with a recent report in non-small-cell lung cancer [16]. Furthermore, we identified that CD39+ NK cells isolated from ESCC tissues display defective functions. These findings support CD39 as an immune checkpoint that limits NK cell functions in ESCC tissues.
NK cells play a crucial role in eradicating cancer cells, and dysfunction of NK cells has been recognized as a key mechanism of tumor immune escape [17]. Different inhibitory receptors or immune checkpoints have been reported on NK cells in physiological or pathological conditions. Several studies reported that PD-1 is an exhaustion maker for NK cells and PD-1 blockade can increase the antitumor activity of NK cells [18, 19]. Tigit, Tim-3 and CD96 have also been reported as inhibitory molecules or exhaustion markers on NK cells in cancers, and blocking signaling can restore the function of NK cells [20–22]. In this study, we focus on the expression and function of CD39 on NK cells in esophageal cancer tissues. NK cells express A2A receptor, a high affinity subtype of adenosine receptors expressed on lymphocytes [23]. Extracellular adenosine generated by the ectonucleotidases CD39 has been considered as an important mechanism that inhibits the activities of NK cells within the tumor microenvironment [24]. However, whether surface CD39 expression restricts NK cell functions through adenosine/A2A receptor signaling in ESCC tumors is yet to be determined. A recent study showed that CD73 expression defines an inducible population of NK cells with immunoregulatory properties in human breast tumors [25]. In this study, we observed significant expression of CD39 but not CD73 on tumor-filtrating NK cells from ESCC patients. This discrepancy in CD39 and CD73 expression on tumor-infiltrating NK cells may be related with different tissue origins.
It has been well documented that chronic inflammation is involved in cancer development and progression. Different pro-inflammatory cytokines, such as IL-6, IL-27, TGF-β and IL-2, have been reported to induce CD39 expression on T cells [26]. However, the mechanisms involved in CD39 expression of NK cells in tumor microenvironment were unclear. Here, we demonstrated that esophageal cancer cells could induce CD39 expression on NK cells via secretion of IL-6. IL-6 is a multifunctional cytokine that plays important roles in both pro-inflammatory and anti-inflammatory responses in many kinds of inflammatory diseases and cancers. Chronic activation of IL-6 is able to promote tumor cell proliferation and inhibit cell death [27]. In addition, a substantial body of evidence suggests that IL-6 serves as an extrinsic factor to regulate immune responses in cancers [28, 29]. Thus, targeting IL-6 signaling pathways provides a promising therapeutic strategy for the treatment of cancer patients [30, 31]. Our results showed that IL-6 expression was elevated in ESCC tissues and positively associated with CD39 expression, supporting the role of IL-6 in CD39+ NK cells. Further studies are required to dissect the molecular mechanisms by which IL-6 regulates CD39 expression on NK cells in ESCC tumor. IL-6 is an immunosuppressive cytokine within the tumor microenvironment, and can act on both tumor cells and tumor-infiltrating immune cells to activate JAK/STAT3 signaling [31]. IL-6-mediated activation of Stat3 promoted CD39 and CD73 expression by binding to their promoter during Th17 cell differentiation. Furthermore, IL-6-induced Th17 cells could increase tumor growth in a CD39-dependent manner [26]. It has also been reported that IL-27 controls CD39 expression in conventional dendritic cells via STAT3 [32]. Thus, it is possible that the activation of STAT3 signaling constitutes a common mechanism for CD39 expression triggered by IL-6 to mediate tumor immune evasion.
There are accumulating evidences for the low density of NK cells in various types of cancers, such as melanoma, lung cancer, breast cancer, renal cell cancer and gastrointestinal tumor [33, 34]. Previous studies described that the frequency of circulating NK cells was significantly higher in early stage compared to advanced stage in breast cancer patients [35]. NK cells play a key role of in tumorigenesis and metastasis, highlighting the potential approaches to prevent metastatic disease by harnessing NK cells [36]. However, few studies have reported the prognostic value of NK cell subsets, especially in esophageal cancer. In this study, we found that ESCC patients with higher frequency of tumor infiltrating CD39+ NK cells showed the worse prognosis for both progression-free survival and overall survival. These data support that the dysfunction of NK cells may contribute to the disease progression in esophageal cancer patients. It would be of great interest to identify whether CD39+ NK cells can serve as a predictive biomarker for the outcome of esophageal cancer patients.
Conclusion
Collectively, our results show that CD39 is overexpressed on NK cells from esophageal cancer patients. Furthermore, CD39+ NK cells display an exhausted phenotype, and IL-6 can induce CD39 expression. More importantly, the frequency of CD39+ NK cells correlates with the poor prognosis. The data presented in this study suggest that CD39 act as an exhaustive marker on NK cells from patients with esophageal cancer. Our findings highlight the potential of CD39 as a promising therapeutic target to restore the functions of NK cells.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (81773045). We thank our laboratory members for their fruitful discussion.
Abbreviations
- CM
Conditioned media
- ESCC
Esophageal squamous cell carcinoma
- NK
Natural killer
- PBMC
Peripheral blood mononuclear cells
- PD-1
Programmed death 1
- PD-L1
Programmed death ligand 1
- PMA
Phorbol 12-myristate 13-acetate
- TIGIT
T cell immunoglobulin and ITIM domain
- TIL
Tumor infiltrating lymphocytes
- Tim-3
T cell immunoglobulin and mucin domain-containing protein 3
Author contributions
LH, and YZ conceived and designed the study. YJZ, YL, BT and QTZ conducted experiments and data analysis. WD, MXG, SZ and YQ performed sample collection and analysis. LH and YJZ wrote the manuscript. All the authors approved the manuscript.
Compliance with ethical standards
Conflict of interest
Authors declare no conflicts of interest for this article.
Ethical approval
The study has been approved by the Institutional Ethical Committee of the First Affiliated Hospital of Zhengzhou University, China (no. 2016-LW-52), and performed in accordance with the ethical standards as laid down in the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yujia Zheng and Yu Li contributed equally to this work.
Contributor Information
Yi Zhang, Email: yizhang@zzu.edu.cn.
Lan Huang, Email: lanhuang@zzu.edu.cn.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Kudo T, Hamamoto Y, Kato K, et al. Nivolumab treatment for oesophageal squamous-cell carcinoma: an open-label, multicentre, phase 2 trial. Lancet Oncol. 2017;18:631–639. doi: 10.1016/S1470-2045(17)30181-X. [DOI] [PubMed] [Google Scholar]
- 3.Kato K, Cho BC, Takahashi M, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:1506–1517. doi: 10.1016/S1470-2045(19)30626-6. [DOI] [PubMed] [Google Scholar]
- 4.Huang J, Xu B, Mo H, et al. Safety, activity, and biomarkers of SHR-1210, an Anti-PD-1 antibody, for patients with advanced esophageal carcinoma. Clin Cancer Res. 2018;24:1296–1304. doi: 10.1158/1078-0432.CCR-17-2439. [DOI] [PubMed] [Google Scholar]
- 5.Morvan MG, Lanier LL. NK cells and cancer: you can teach innate cells new tricks. Nat Rev Cancer. 2016;16:7–19. doi: 10.1038/nrc.2015.5. [DOI] [PubMed] [Google Scholar]
- 6.Platonova S, Cherfils-Vicini J, Damotte D, et al. Profound coordinated alterations of intratumoral NK cell phenotype and function in lung carcinoma. Cancer Res. 2011;71:5412–5422. doi: 10.1158/0008-5472.CAN-10-4179. [DOI] [PubMed] [Google Scholar]
- 7.Andre P, Denis C, Soulas C, et al. Anti-NKG2A mAb is a checkpoint inhibitor that promotes anti-tumor immunity by unleashing both T and NK cells. Cell. 2018;175(1731–43):e13. doi: 10.1016/j.cell.2018.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allard B, Longhi MS, Robson SC, Stagg J. The ectonucleotidases CD39 and CD73: novel checkpoint inhibitor targets. Immunol Rev. 2017;276:121–144. doi: 10.1111/imr.12528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canale FP, Ramello MC, Nunez N, et al. CD39 expression defines cell exhaustion in tumor-infiltrating CD8(+) T cells. Cancer Res. 2018;78:115–128. doi: 10.1158/0008-5472.CAN-16-2684. [DOI] [PubMed] [Google Scholar]
- 10.Bonnefoy N, Bastid J, Alberici G, Bensussan A, Eliaou JF. CD39: a complementary target to immune checkpoints to counteract tumor-mediated immunosuppression. Oncoimmunology. 2015;4:e1003015. doi: 10.1080/2162402X.2014.1003015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kashyap AS, Thelemann T, Klar R, et al. Antisense oligonucleotide targeting CD39 improves anti-tumor T cell immunity. J Immunother Cancer. 2019;7:67. doi: 10.1186/s40425-019-0545-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang H, Vijayan D, Li XY, Robson SC, Geetha N, Teng MWL, Smyth MJ. The role of NK cells and CD39 in the immunological control of tumor metastases. Oncoimmunology. 2019;8:e1593809. doi: 10.1080/2162402X.2019.1593809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McAllister SS, Weinberg RA. The tumour-induced systemic environment as a critical regulator of cancer progression and metastasis. Nat Cell Biol. 2014;16:717–727. doi: 10.1038/ncb3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hilchey SP, Kobie JJ, Cochran MR, et al. Human follicular lymphoma CD39+-infiltrating T cells contribute to adenosine-mediated T cell hyporesponsiveness. J Immunol (Baltimore, Md.: 1950) 2009;183:6157–6166. doi: 10.4049/jimmunol.0900475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta PK, Godec J, Wolski D, et al. CD39 expression identifies terminally exhausted CD8+ T cells. PLoS Pathog. 2015;11:e1005177. doi: 10.1371/journal.ppat.1005177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tondell A, Wahl SGF, Sponaas AM, Sorhaug S, Borset M, Haug M. Ectonucleotidase CD39 and checkpoint signalling receptor programmed death 1 are highly elevated in intratumoral immune cells in non-small-cell lung cancer. Transl Oncol. 2019;13:17–24. doi: 10.1016/j.tranon.2019.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guillerey C, Huntington ND, Smyth MJ. Targeting natural killer cells in cancer immunotherapy. Nat Immunol. 2016;17:1025–1036. doi: 10.1038/ni.3518. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Cheng Y, Xu Y, et al. Increased expression of programmed cell death protein 1 on NK cells inhibits NK-cell-mediated anti-tumor function and indicates poor prognosis in digestive cancers. Oncogene. 2017;36:6143–6153. doi: 10.1038/onc.2017.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Concha-Benavente F, Kansy B, Moskovitz J, Moy J, Chandran U, Ferris RL. PD-L1 mediates dysfunction in activated PD-1(+) NK cells in head and neck cancer patients. Cancer Immunol Res. 2018;6:1548–1560. doi: 10.1158/2326-6066.CIR-18-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.da Silva IP, Gallois A, Jimenez-Baranda S, Khan S, Anderson AC, Kuchroo VK, Osman I, Bhardwaj N. Reversal of NK-cell exhaustion in advanced melanoma by Tim-3 blockade. Cancer Immunol Res. 2014;2:410–422. doi: 10.1158/2326-6066.CIR-13-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Q, Bi J, Zheng X, et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat Immunol. 2018;19:723–732. doi: 10.1038/s41590-018-0132-0. [DOI] [PubMed] [Google Scholar]
- 22.Sun H, Huang Q, Huang M, et al. Human CD96 correlates to natural killer cell exhaustion and predicts the prognosis of human hepatocellular carcinoma. Hepatology. 2019;70:168–183. doi: 10.1002/hep.30347. [DOI] [PubMed] [Google Scholar]
- 23.Ohta A. A metabolic immune checkpoint: adenosine in tumor microenvironment. Front Immunol. 2016;7:109. doi: 10.3389/fimmu.2016.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chambers AM, Wang J, Lupo KB, Yu H, Atallah Lanman NM, Matosevic S. Adenosinergic signaling alters natural killer cell functional responses. Front Immunol. 2018;9:2533. doi: 10.3389/fimmu.2018.02533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neo SY, Yang Y, Record J, et al. CD73 immune checkpoint defines regulatory NK cells within the tumor microenvironment. J Clin Investig. 2020;130:1185–1198. doi: 10.1172/jci128895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chalmin F, Mignot G, Bruchard M, et al. Stat3 and Gfi-1 transcription factors control Th17 cell immunosuppressive activity via the regulation of ectonucleotidase expression. Immunity. 2012;36:362–373. doi: 10.1016/j.immuni.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 27.Kang S, Tanaka T, Narazaki M, Kishimoto T. Targeting interleukin-6 signaling in clinic. Immunity. 2019;50:1007–1023. doi: 10.1016/j.immuni.2019.03.026. [DOI] [PubMed] [Google Scholar]
- 28.Tsukamoto H, Senju S, Matsumura K, Swain SL, Nishimura Y. IL-6-mediated environmental conditioning of defective Th1 differentiation dampens antitumour immune responses in old age. Nat Commun. 2015;6:6702. doi: 10.1038/ncomms7702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flint TR, Janowitz T, Connell CM, Roberts EW, Denton AE, Coll AP, Jodrell DI, Fearon DT. Tumor-induced IL-6 reprograms host metabolism to suppress anti-tumor immunity. Cell Metab. 2016;24:672–684. doi: 10.1016/j.cmet.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goumas FA, Holmer R, Egberts JH, et al. Inhibition of IL-6 signaling significantly reduces primary tumor growth and recurrences in orthotopic xenograft models of pancreatic cancer. Int J Cancer. 2015;137:1035–1046. doi: 10.1002/ijc.29445. [DOI] [PubMed] [Google Scholar]
- 31.Johnson DE, O'Keefe RA, Grandis JR. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clinic Oncol. 2018;15:234–248. doi: 10.1038/nrclinonc.2018.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mascanfroni ID, Yeste A, Vieira SM, et al. IL-27 acts on DCs to suppress the T cell response and autoimmunity by inducing expression of the immunoregulatory molecule CD39. Nat Immunol. 2013;14:1054–1063. doi: 10.1038/ni.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fregni G, Messaoudene M, Fourmentraux-Neves E, et al. Phenotypic and functional characteristics of blood natural killer cells from melanoma patients at different clinical stages. PLoS ONE. 2013;8:e76928. doi: 10.1371/journal.pone.0076928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mamessier E, Sylvain A, Thibult ML, et al. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J Clin Invest. 2011;121:3609–3622. doi: 10.1172/JCI45816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mamessier E, Pradel LC, Thibult ML, et al. Peripheral blood NK cells from breast cancer patients are tumor-induced composite subsets. J Immunol (Baltimore, Md.: 1950). 2013;190:2424–2436. doi: 10.4049/jimmunol.1200140. [DOI] [PubMed] [Google Scholar]
- 36.Lopez-Soto A, Gonzalez S, Smyth MJ, Galluzzi L. Control of metastasis by NK cells. Cancer Cell. 2017;32:135–154. doi: 10.1016/j.ccell.2017.06.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.