Abstract
Background:
Identifying hypertension (HTN) early is crucial in preventing and lowering the long-term risk of heart disease, yet HTN in children often goes undiagnosed. An electronic health record linked, web-based clinical decision support (CDS) called PedsBP can help address this care gap and has been previously shown to increase recognition of HTN by primary care clinicians.
Objectives:
To adapt the PedsBP tool for use in a mostly rural health system and then to evaluate the effectiveness of PedsBP for repeat of hypertensive level blood pressure (BP) measurements and HTN recognition among youth 6–17 years of age in primary care settings, comparing low-intensity and high-intensity implementation approaches.
Methods and design:
PedsBP was evaluated through a pragmatic, clinic-randomized trial. The tool was piloted in 2 primary care clinics and modified prior to the full trial. Forty community-based, primary care clinics (or clusters of clinics) were randomly allocated in a 1:1:1 ratio to usual care, low-intensity implementation (CDS only), or high-intensity implementation (CDS plus in-person training, monthly use reports, and ongoing communication between study staff and clinics). Accrual of eligible patients started on August 1, 2022 and will continue for 18 months. Primary outcomes include repeating hypertensive level BP measurements at office visits and clinical recognition of HTN. Secondary outcomes include lifestyle counseling, dietician referral, and BP at follow-up.
Conclusion:
This report focuses on the design and feasibility of adapting and implementing PedsBP in a rural primary care setting. The trial and analysis are ongoing with main results expected in mid-2024.
Keywords: Children, Adolescents, Decision support, Blood pressure, Hypertension
1. Introduction
Hypertension (HTN) in youth often tracks into adulthood, contributing to adult cardiovascular morbidity and mortality [1–3]. National guidelines for the diagnosis and treatment of HTN in children and adolescents were last updated in 2017, with varying definitions for HTN by age [4]. To date, most children and adolescents with elevated blood pressure (BP) or HTN are undiagnosed or inadequately treated [5,6].
The 2017 American Academy of Pediatrics Guidelines recommend that for otherwise healthy children, BP should be measured annually while youth with obesity or other HTN risk factors should have BP measured at every clinical encounter [4]. Early identification of HTN may help children and families adopt lifestyle changes needed to reduce their risk for long-term cardiovascular sequelae. The 2017 Guidelines recommend that for the management of hypertensive level BP and HTN in youth, timing of repeat BP measurements and indications for treatment (lifestyle intervention, antihypertensive medication, or both) should be based on (a) the level of BP elevation, (b) whether the elevation is incident or persistent, and (c) whether the adolescent is overweight. For children and adolescents 6 years and older with new onset HTN who are overweight or obese, have a family history of HTN and do not have signs of a secondary cause for HTN, the guidelines indicate that an extensive work-up is not needed [4].
Despite potential benefits of early identification, HTN in children and adolescents is often unrecognized [5–7]. The need for practical and sustainable approaches to implementing guidelines has been identified as a research gap [8]. Low rates of recognition and low adherence to pediatric BP guidelines are likely due to barriers in knowledge, attitudes, and behaviors [9]. Providers may also not be familiar with clinical criteria for HTN in youth. Competing demands at primary care visits are common and may limit time to address hypertensive level BP. In addition, the classification of HTN is complex and time consuming [7]. Cutoffs for systolic and diastolic BP ≥95th percentile for children ≤12 years vary by sex, age, and height percentile. Without the help of algorithmic clinical decision support (CDS), BP values across multiple visits must be interpreted using a series of complex matrixed tables, making it difficult to review in a busy primary care setting.
We previously developed, piloted, and refined the CDS in a two-year cluster-randomized trial, conducted in 20 primary care clinics within HealthPartners (HP) [10]. Data from the first year of the trial showed that compared to those having visits at usual care clinics, patients with hypertensive level BP recorded at visits to CDS intervention clinics were more likely to have their hypertensive level BP remeasured during the visit and were more likely to be diagnosed with hypertensive level BP [11]. Results from the full trial showed that of 31,579 study-eligible patients 10–17 years of age with one or more BP measures over a two-year period, 1.8% met clinical criteria for HTN. Within 6 months of meeting criteria for incident HTN, 54.9% of patients seen at CDS clinics versus 21.3% of patients attending usual care clinics had their HTN clinically recognized [10]. Using only diagnosis codes to identify recognition, the rates were 47% and 14%, respectively. The previous trial also showed that HTN recognition was strongly associated with lifestyle referral or provider counseling regarding exercise and weight loss [10]. A formal cost analysis showed no significant increase in medical expenditures among patients with incident HTN in the clinics where the CDS was available [12].
In summary, lack of familiarity with HTN definitions, low clinician buy-in for HTN guidelines, time pressures in providing comprehensive care for children and adolescents, complexities of the current BP tables, and the need to review several previous BP measurements to diagnose HTN all contribute to under-recognition [13,14]. CDS, integrated within an existing electronic health record (EHR) platform and delivered at the point of care is ideally suited to address barriers to HTN recognition and management [15]. Automated calculations of BP percentiles for current and prior visits can reduce the time needed to identify an incident or hypertensive level BP. Computer prompts regarding BP classification, recommended evaluations, and timing for follow-up can assist providers unfamiliar with pediatric BP guidelines. The PedsBP tool was designed to address barriers in recognizing HTN and has shown that it increases repeat BP measurement after a first hypertensive level BP and significantly improved recognition. Compared to urban youth, children living in rural regions have higher rates of obesity and thus, are at increased risk for HTN and future cardiovascular disease [16]. Yet, youth in rural regions also experience reduced access to pediatricians and pediatric subspecialists [17–19]. Thus, expanding PedsBP CDS for use in a large, primarily rural health system is a logical next step.
2. Clinical trial design and methods
2.1. Overview
PedsBP is a pragmatic, 3-arm cluster randomized trial in which 40 clinics are randomized in a 1:1:1 ratio to either usual care (UC, N = 13), low-intensity implementation (N = 14), or high-intensity implementation (N = 13) at a largely rural health system. Study eligible patients are allocated to the study arm assigned to the clinic where they had an index visit. An index visit is the first clinic visit at which the first recorded BP is elevated, or the patient newly meets criteria for HTN. The overall study design and implementation process, including enrollment and randomization allocation is shown in Fig. 1.
Fig. 1.
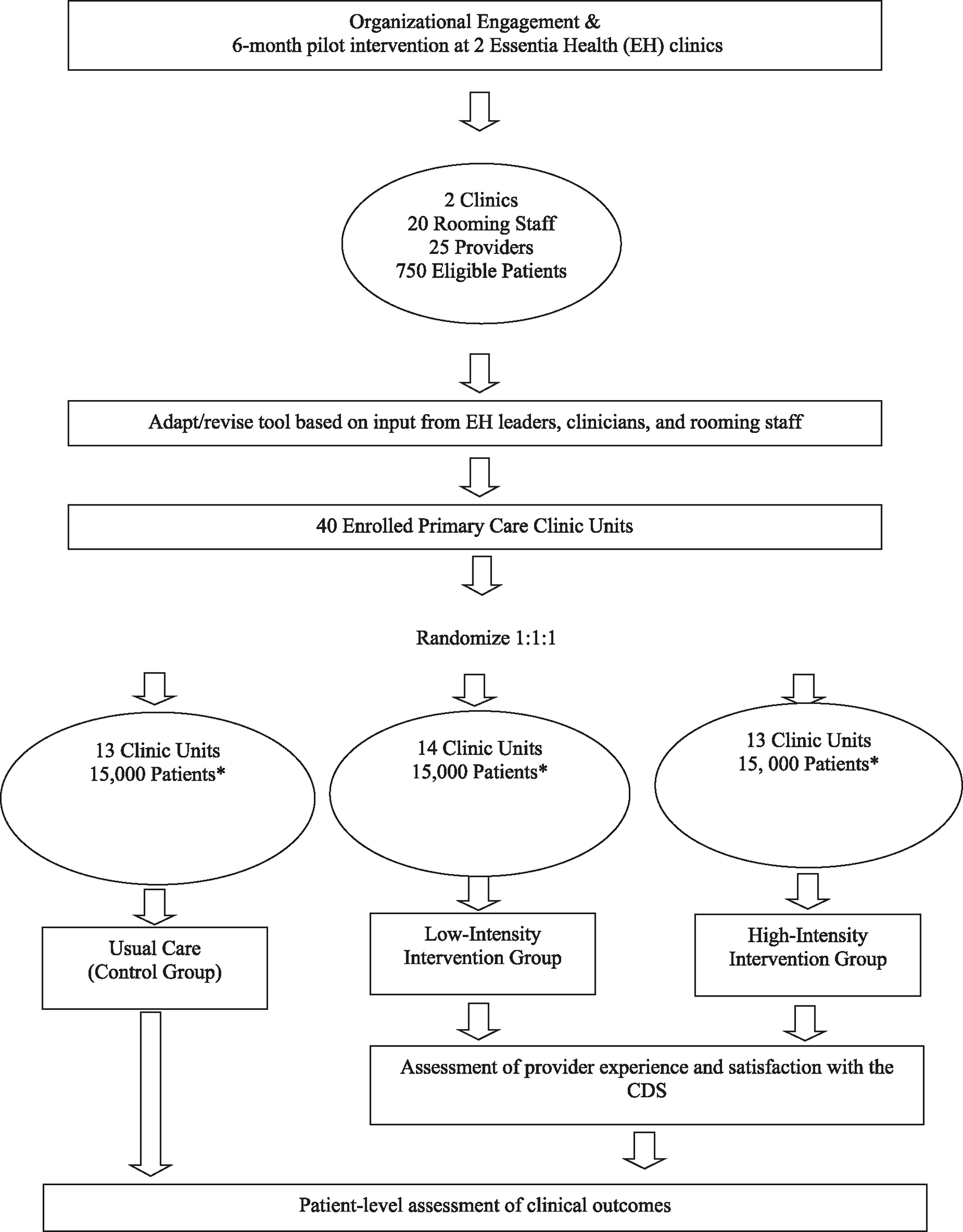
Cluster randomized trial implementation process and design.
*Patient enrollment numbers are estimated.
The study aims are: Aim 1: Among youth 6–17 years of age with a hypertensive level BP measurement, evaluate the effectiveness of the adapted PedsBP for repeat BP measurement. Aim 2: Among youth 6–17 years of age meeting criteria for HTN, to evaluate the effectiveness of the adapted PedsBP for improving HTN recognition. Specific hypotheses are described in more detail below.
2.2. Study setting
The study is being conducted at Essentia Health (EH), a large integrated health system serving the areas of Minnesota, North Dakota, and Wisconsin. EH has over 2100 physicians and advanced practitioners across 14 hospitals and 73 clinics. We identified 44 primary care clinics with the largest number of visits among youth 6–17 years of age. There were three pediatric-only clinics. Two sets of 3 small clinics each were pooled as 2 clinic units due to their small patient volume, shared providers, and geographic proximity, resulting in a total of 40 clinic units for randomization. Additionally, 2 primary care clinics served as pilot sites for PedsBP.
2.3. Eligibility and exclusion criteria
The PedsBP uses automated algorithms to assess eligibility at the beginning of each primary care encounter at the point when BP is entered into the EHR (Fig. 2 illustrates the data flow between the EHR and the CDS). Patients are eligible for the study if they meet the following criteria at a primary care visit: first recorded BP is elevated, or the patient newly meets criteria for HTN.
Fig. 2.
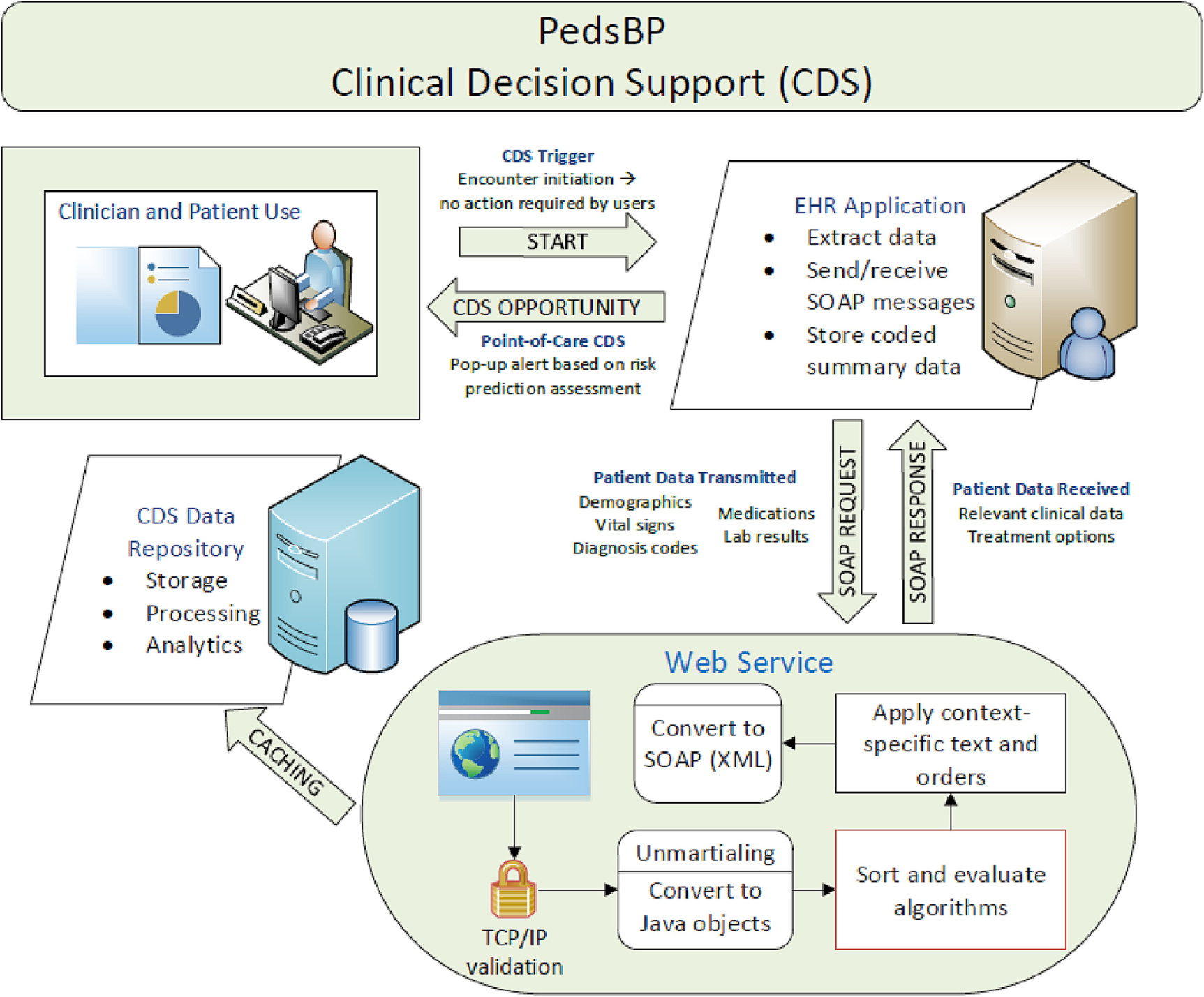
Illustration of flow of data between electronic health record (EHR) and web service.
Age 6–17, inclusive at the time of the visit
BP measured and entered in the vital sign section during an ambulatory visit in a randomized primary care clinic
Not pregnant or within the period of 12 weeks postpartum
To be included in the study analysis, patients must also meet the additional criteria below.
Have at least one visit in a randomized clinic during the intervention period where they meet eligibility criteria for PedsBP
No previous HTN diagnosis prior to the visit
Not taking antihypertensive medication
Did not opt out of research
Additionally, primary care providers (PCPs) are eligible to receive CDS prompts if they: (a) practice at a randomized primary care clinic, (b) are a pediatric or family medicine care provider (pediatrician, family physician, nurse practitioner or physician assistant), and (c) provide ongoing clinical care for children and adolescents.
2.4. Recruitment
Patients 6–17 years of age with a BP recorded at participating EH primary care clinics are eligible and automatically enrolled on the basis of data recorded in the EHR and captured by the PedsBP CDS.
2.5. Group randomization
Covariate-based constrained randomization was used to enhance study arm balance on key factors during randomization of the 40 clinic units in this cluster-randomized trial [20]. Clinic-level attributes used as balance factors were obtained via EHR data and ascertained for care in clinics in the 12 months prior to randomization and included count of patients, percentage of patients with Medicaid coverage, and urban/rural status. Three high-volume pediatric clinics were randomized separately. Randomization was conducted with the CCR macro for use within SAS, and was conducted one month prior to implementation of the low- and high-intensity strategies in clinics [21]. Fourteen clinic units received the PedsBP intervention using a low-intensity strategy, 13 received it with a high-intensity strategy, and 13 were assigned to Usual Care (UC).
3. PedsBP CDS intervention
PedsBP was initially developed and tested in a single healthcare system. The goal of the current study is to adapt the existing CDS to align with local preferences and workflows to improve recognition of HTN in a rural setting, and to assess different intervention strategies on uptake and primary outcomes.
3.1. Intervention description
PedsBP is an algorithmic web-based decision support tool that is seamlessly integrated in the HER [10,22]. It consists of seven key features: (i) a best practice advisory (BPA) regarding the need for height data to classify the BP by percentile, (ii) a BPA to repeat any BP that is ≥95th percentile or ≥ 130/80 mmHg, (iii) classification of current and prior BPs in the last two years and identification of BPs in stage 1 HTN and stage 2 HTN range, (iv) review of previous hypertension diagnoses and BPs in order to classify a hypertensive level BP at the current or index visit as a first or second hypertensive level BP, or as meeting criteria for HTN, (v) review of medications and diagnoses that may affect BP, (vi) tailored CDS based on HTN category and previous diagnoses, (vii) graphical representation of current and prior BP data by age and BP percentile (Fig. 3). These features are described in more detail below.
Fig. 3.
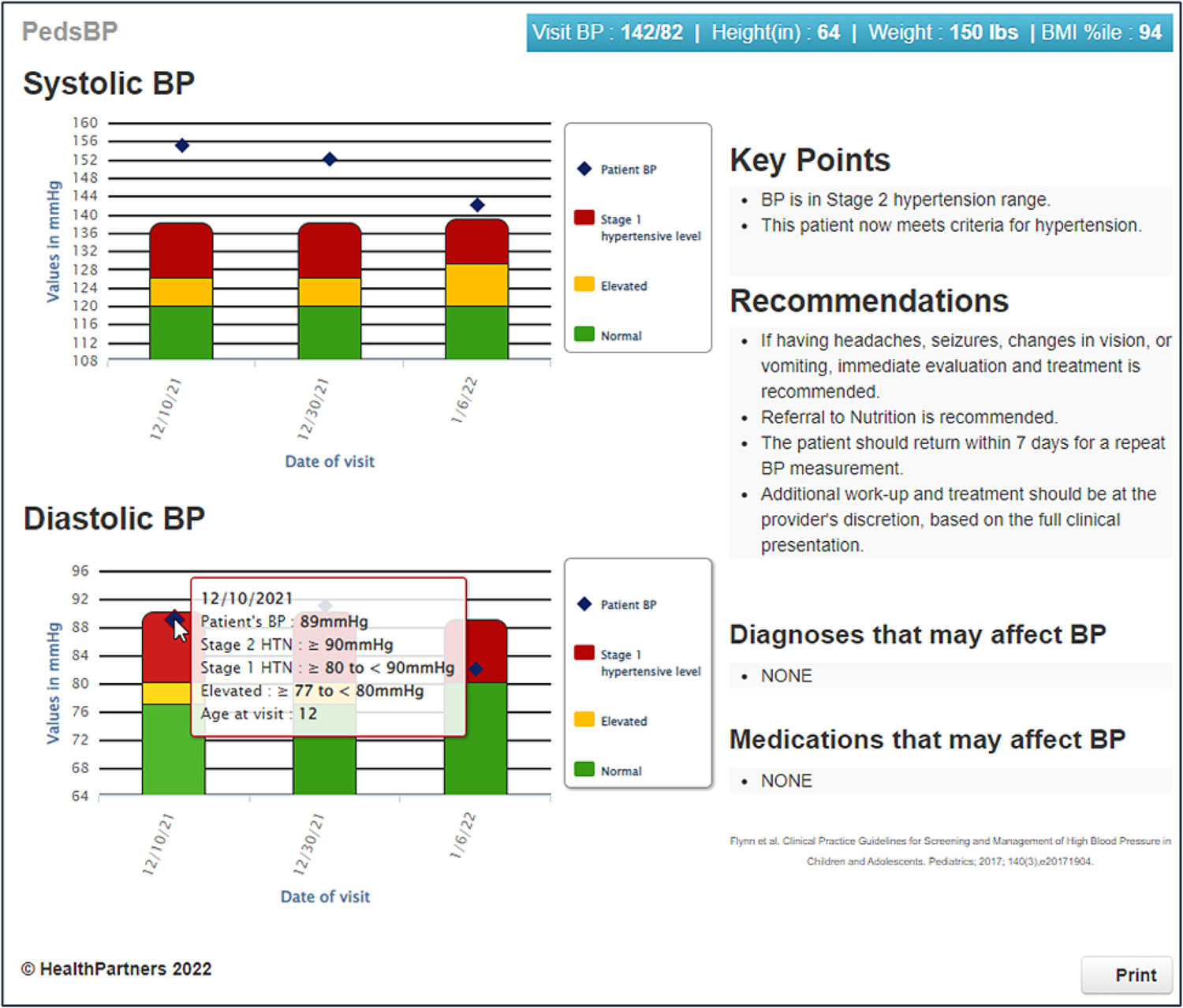
PedsBP display example.
The first component of the CDS is a BPA to ensure a current height measurement for children 12 and under (needed to compute BP percentiles): (a) If a height is entered at the visit, it will be used, (b) if no height is entered when entering a BP, the closest antecedent height recorded within 1 year is used to calculate a BP percentile, assuming a constant height percentile, (c) if there is no height available for the current visit or the prior 12 months, a BPA displays requesting a height be measured and recorded in the EHR. As height measurement is standard in pediatric care, this BPA is estimated to fire in <3% of visits.
The second component of the CDS, a BPA to repeat a hypertensive level BP, is triggered for BPs ≥95th percentile in children 6–12 years of age or ≥ 130/80 mmHg in youth 13–17 years of age when the BP is entered in the vital signs section of the EHR, and the vital signs box is closed.
In the third component, the CDS uses the lowest SBP for that visit to assign BP percentiles and classify BPs as normotensive, hypertensive level BP, stage 1 HTN, or stage 2 HTN. If the initial hypertensive level BP is not repeated, the BP percentile will be calculated for the available measurements. Exact BP percentiles are calculated consistent with the 2017 guidelines [4].
In the fourth component of the CDS, for patients with BP ≥95th percentile (6–12 years of age) or ≥ 130/80 mmHg (13–17 years of age), the CDS reviews data pulled from the EHR including prior HTN diagnoses, prior BPs and heights. The current visit and previous BPs and diagnosis data are used to categorize hypertensive level BPs as incident or persistent, and to determine whether a patient meets clinical criteria for new onset stage 1 or stage 2 HTN, or pre-existing HTN.
In the fifth component of the CDS, current medications (e.g., oral steroids or stimulants that increase BP or diuretics that decrease BP) and prior diagnoses that may affect BP are displayed. In addition, current body mass index (BMI) and BMI percentile are calculated. For patients with BMI ≥95th percentile with new onset HTN, obesity is noted in the CDS as a potential cause for the hypertension.
In the sixth component of the CDS, providers receive tailored CDS for BPs ≥95th percentile or ≥ 130/80 mmHg. The CDS recommendations are specific, based on the magnitude of BP elevation (stage 1 or stage 2 HTN) and whether the BP is incident or persistent. If the patient has a prior diagnosis of HTN, the CDS provides tailored feedback regarding whether the BP is at goal (<95th percentile) or possibly requires initiation or a change in medication.
In the seventh component of the CDS, current and previous systolic BPs and diastolic BPs, within the last two years are graphed and displayed, with hypertensive level BP, stage 1 HTN, and stage 2 HTN BP cutoffs specified. This feature allows providers to visualize patient-level variability and trends in BP over time. The display is color coded such that BPs that are elevated are in red, those in the prehypertensive range are yellow and normal BPs are in green. Scrolling over the bar graph displays the exact BP and BP cutoff for each BP category. The CDS also displays the CDS recommendations, a summary of any medications (e.g., stimulants, steroids, or antihypertensives) and prior diagnoses (e.g., cardiac, renal or endocrine disorders) that may be affecting current BP. Weight and BMI percentiles are also displayed in the blue bar at the top of the CDS. In addition, the CDS can be printed for families, to assist with shared decision making. An example of the CDS interface is shown in Fig. 3.
3.2. High vs. low intensity training approaches
A key focus of this study is to compare implementation strategies as healthcare systems often underestimate training needs of clinic staff when changing workflows or implementing CDS. The study uses principles of implementation outlined by Powell et al. to assess whether a high-intensity (i.e., high cost) implementation approach has benefits above and beyond a low-intensity (i.e., low cost) approach and to what extent [23].
Rooming staff are routinely trained on measuring BP as part of the health system clinical protocols, thus processes around BP measurement were not included in study training. All three study arms followed the same standardized approach to BP measurement. Training for clinics in both the high-intensity and low-intensity intervention arms was developed using the organization’s standard online training platform (Saba Software, Inc.) and incorporated general training, study-specific training, and mechanisms for competency assessment. Within primary care clinics randomized to receive PedsBP, rooming teams were oriented to the PedsBP alerts and received basic education on HTN. PCPs were trained virtually at section meetings, in person and on assigned Saba e-learning on how to use the PedsBP CDS. All staff were instructed and encouraged to let the study team know of any issues or questions. Table 1 details the differences in training approaches for low- and high-intensity arms. 206 of 301 (68.4%) staff in high-intensity clinics and 139 of 202 (59.9%) staff in low-intensity clinics were trained as of December 1, 2022.
Table 1.
Training approaches and completion for low- and high-intensity arms.
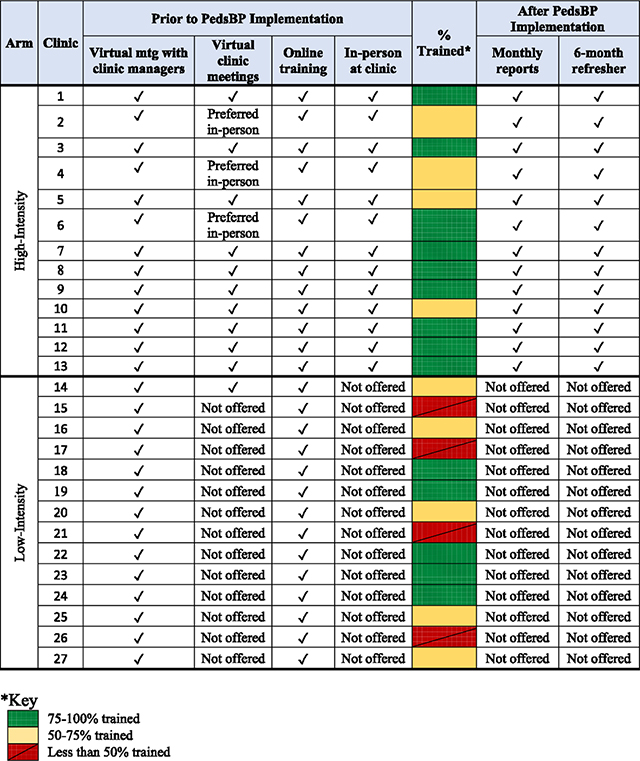
|
3.3. High intensity
The 13 clinics randomized to the high-intensity arm completed the Saba training with the addition of reminders from study staff to complete the online training. Additional training was conducted via video conference with clinic managers to explain training expectations, via in-person clinic lunch training session, and video conferencing at section meetings. This hands-on approach to training allows for better relationship building, creates opportunities for clinic staff to ask questions if something is unclear, and in general, gives more meaning to the study if users understand the who, what, why, and where that a more personal touch provides. The study team also presented information about the study at high-intensity clinic departmental section meetings, which heightened the profile of the study with clinic leadership. The high-intensity clinics also receive monthly use-reports via email showing the number of encounters with a hypertensive level BP, number with recheck (2nd) BP, percentage of recheck BPs that were elevated, and number of times the PedsBP CDS was opened. Additionally, the monthly report includes counts with 1st BP elevated and the number that were rechecked by staff were sent via email. Benchmarks were set based on the highest performing clinics metrics over the first 3 months after study implementation.
3.4. Low-intensity
The 14 low-intensity intervention clinics received education and training following standard practice for delivering any updates to primary care at EH (online Saba training), which included a short web-based instructional video demonstrating provider and rooming staff roles in the PedsBP CDS. The study team met via video conference with the clinic managers to explain training expectations. No planned presentations were made virtually at section meetings or in-person training unless specifically requested. No further training or additional contact with the low-intensity clinic staff is planned for the duration of the study.
3.5. Usual care
PCPs practicing in the usual care arm clinics do not have access to the PedsBP CDS tool. There are no other pediatric HTN CDS tools currently implemented at EH clinics other than the CDS for this project however, the foundation EHR system alerts when a BP is elevated with a red exclamation mark in the vital signs flowsheet.
4. Study variables and outcomes
4.1. Primary outcomes
The primary outcomes for the study are: (a) repeat BP measurement of an initial hypertensive level BP (≥95th percentile for ages 6–12 or ≥ 130/80 mmHg for ages 13–17), at the same clinic visit (H1,H2) based on automated BP data from the CDS, and (b) clinical recognition of HTN within 6 months of meeting criteria for HTN (H3, H4). Clinical recognition will include a new diagnosis of HTN (ICD-10: I10) or hypertensive level BP (ICD-10: R03) or adding HTN or hypertensive level BP to the problem list. Our definitions allow for clinical recognition to occur within six months of meeting criteria, acknowledging that patients may meet criteria for HTN but have more pressing needs at their index visit. We also understand that providers may prefer to not label patients as having HTN based on a current and two prior clinic-based BPs. Instead, providers may diagnose hypertensive level BP and recommend additional follow-up for BP measurement or ambulatory BP monitoring, prior to diagnosing HTN.
Secondary outcomes will describe management within six months of meeting criteria for HTN including: diet and exercise counseling, dietitian referrals, subspecialty referrals (cardiology, endocrinology and nephrology), initiation of antihypertensive medications and receipt of diagnostic imaging (echocardiogram or renal ultrasound). Demographic variables include age, race, sex, ethnicity, and patient insurance.
4.2. Safety monitoring
A Data Safety Monitor will review safety data at 6 and 12 months following the start of the trial. Safety data will include recruitment counts, hospitalization for any cause, death, stroke or transient ischemic attacks, acute renal failure, malignant HTN, including flags for hypertensive level BP > 180/120 mmHg and BP > 160/100 mmHg, echocardiogram, renal ultrasound or any new order for anti-hypertensive medications.
4.3. Statistical analysis
Aim 1 hypotheses posit that patients with a hypertensive level BP seen at clinics with low- or high-intensity PedsBP CDS will be more likely to have their BP re-measured as compared to patients seen at usual care clinics (H1), and that repeat measurement will be more likely for patients seen at high-intensity rather than low-intensity clinics (H2). Aim 2 hypotheses posit that patients who newly meet HTN criteria at clinics with low- or high-intensity PedsBP CDS will be more likely to have their HTN recognized within 6 months as compared to patients seen at usual care clinics (H3), and that recognition of HTN will be more likely for patients seen at high-intensity rather than low-intensity clinics (H4).
For all outcomes, the primary predictor is the study arm to which a clinic is randomized: high-intensity PedsBP, low-intensity PedsBP, or usual care. Generalized linear mixed-model regression with a logit link, binomial error distribution, and random clinic intercept will test the effect of the interventions on binary endpoints measured once following intervention implementation. Models will include study arm contrasts, stratification factors used in study arm randomization, and patient-level covariates to be pre-specified in the statistical analysis plan and likely to include age, sex, SBP percentile of the hypertensive level BP (H1, H2), BMI percentile at the index visit (H3, H4). Study arm contrasts will test differences in endpoints for CDS (low-intensity or high-intensity) vs. usual care and high-intensity vs. low-intensity.
4.4. Sample size
Aim 1. Based on preliminary data from study clinics yielding an expected 5262 patients, this study has 99% power for the H1 comparison of patients at low- plus high-intensity clinics with usual care clinics (alpha = 0.05, two-sided test, ICC = 0.03) to detect an absolute difference in BP re-measurement of 15% between the usual care vs. low- plus high-intensity arms. The initial CDS trial had a clinic-level ICC for BP remeasurement of 0.03 and BP re-measurement of 28% in usual care and 47% in the CDS arm. With an expected 3552 patients, this study has 80% power for the H2 comparison of low-intensity vs. high-intensity clinics, with alpha = 0.05, two-sided tests, ICC = 0.03, to detect a minimum absolute detectable difference in BP re-measurement of 10% between the low-intensity and high intensity arms.
Aim 2. Based on the prior trial [10], along with observational studies of pediatric HTN [6,24,25] we anticipate 1% of patients aged 6–12 and 2% of patients aged 13–17 will newly meet HTN criteria at a visit over 18 months, yielding a sample size of 530 patients. The original study found that 21% of patients newly meeting HTN criteria were recognized over 6 months in usual care and 55% in the CDS arm [10], and the clinic-level ICC for HTN recognition was 0.035. With 530 patients (13/clinic) and 40 clinics, this study has 92% power (alpha = 0.05, two-sided test, ICC = 0.035) for H3 to detect an absolute difference in HTN recognition of 17% between the usual care (20%) and low- plus high-intensity arms (37%), and a minimum absolute detectable difference of 14% (20% usual care vs. 34% low- plus high intensity arms) at 80% power. For H4 the minimum absolute detectable difference at 80% power for HTN recognition among 359 patients (13/clinic) is 17% between low-intensity (30%) and high-intensity (47%) arms. Power computations account for the design effect due to clinic randomization and unequal counts of clinics and were conducted with PASS 2019.
4.5. Ethical and regulatory considerations
The study design and procedures were approved by the EH Institutional Review Board. The trial has been registered on ct.gov (NCT05126082). A waiver of patient consent was requested and approved by the IRB. The study is being monitored by an independent medical monitor. The intervention offers point-of-care CDS to PCPs and suggests appropriate care options. Suggestions provided to PCPs are based on national recommendations and were further vetted by clinical leaders at EH prior to implementation. Alerts are designed to support clinicians’ decision making, not to override clinical judgment. As a minimal risk, pragmatic trial, we do not propose interim analyses or stopping rules. We will collect data from the EHR or the CDS to identify potential rare adverse events related to untreated or undertreated HTN, such as malignant HTN or stroke. In addition, using automated methods we will monitor for hospitalizations within 6 months following exposure to the CDS, with additional clinical review regarding any potential for the CDS to have impacted subsequent events.
5. Discussion
The implementation of the electronic PedsBP CDS across the 40 CDS intervention clinics aims to increase pediatric BP remeasurement and HTN recognition in a primarily rural health system. In addition, this three-arm trial will evaluate whether a high-intensity approach to implementation is more effective than a low-intensity approach.
Even when an EHR-linked CDS is effective in the setting where it was initially developed, implementation in a new environment, such as a new health system, is prone to challenges [26]. We applied learnings from the initial development and implementation of PedsBP at HP to guide implementation at EH, ensuring that the CDS would be delivered to the right person, through the right channel, and at the right time following the CDS 5 Rights framework [27]. We solicited input from EH clinical and operations leaders, and from providers and rooming staff during the pilot, in order to adapt the CDS, accommodating differences in workflow, EHR configuration, clinic culture, and patient preferences. We also engaged an expert in human factors to optimize the CDS display, ensuring usability. Adoption and effectiveness in the new setting may also be impacted by differences implementation strategies. Approaches can vary in intensity from simple online webinars or email updates regarding changes to the EHR, to comprehensive in-person training with audit-feedback of use rates. While high-intensity implementation approaches including on-site training may increase adoption, these may also be challenging to implement in rural health systems where clinics span a geographic region of over 400 miles, thus significantly increasing training costs. This research represents a unique opportunity to improve health in an at-risk, rural population and to evaluate the effectiveness of differing approaches to CDS implementation.
6. Conclusion
The adoption of an established electronic PedsBP CDS in a large rural health system is feasible. Leadership and stakeholder engagement is key. The impact of high-intensity compared to low-intensity or usual care implementation will be studied and help inform future dissemination projects.
Acknowledgments
The authors would like to acknowledge Dr. Joseph Konstan on their assistance with the project. This work is supported in part by the Agency for Healthcare Research and Quality (AHRQ R18HS027402).
Footnotes
Declaration of Competing Interest
The authors do not have any disclosures or conflicts of interest related to the study. This work is supported in part by the Agency for Healthcare Research and Quality (AHRQ R18HS027402).
Statement
Implementation of a clinical decision support tool to increase identification of pediatric hypertension.
Data availability
Data will be made available on request.
References
- [1].Chen X, Wang Y, Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis, Circulation. 117 (25) (2008) 3171–3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Urbina EM, Mendizabal B, Becker RC, et al. , Association of blood pressure level with left ventricular mass in adolescents, Hypertension. 74 (3) (2019) 590–596. [DOI] [PubMed] [Google Scholar]
- [3].Oikonen M, Nuotio J, Magnussen CG, et al. , Repeated blood pressure measurements in childhood in prediction of hypertension in adulthood, Hypertension. 67 (1) (2016) 41–47. [DOI] [PubMed] [Google Scholar]
- [4].Flynn JT, Kaelber DC, Baker-Smith CM, et al. , Clinical practice guideline for screening and management of high blood pressure in children and adolescents, Pediatrics. 140 (3) (2017). [DOI] [PubMed] [Google Scholar]
- [5].Hansen ML, Gunn PW, Kaelber DC, Underdiagnosis of hypertension in children and adolescents, J. Am. Med. Assoc. 298 (8) (2007) 874–879. [DOI] [PubMed] [Google Scholar]
- [6].Kaelber DC, Liu W, Ross M, et al. , Diagnosis and medication treatment of pediatric hypertension: a retrospective cohort study, Pediatrics. 138 (6) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Brady TM, Solomon BS, Neu AM, Siberry GK, Parekh RS, Patient-, provider-, and clinic-level predictors of unrecognized elevated blood pressure in children, Pediatrics. 125 (6) (2010) e1286–e1293. [DOI] [PubMed] [Google Scholar]
- [8].Taylor-Zapata P, Baker-Smith CM, Burckart G, et al. , Research gaps in primary pediatric hypertension, Pediatrics. 143 (5) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cabana MD, Rand CS, Powe NR, et al. , Why don’t physicians follow clinical practice guidelines? A framework for improvement, J. Am. Med. Assoc. 282 (15) (1999) 1458–1465. [DOI] [PubMed] [Google Scholar]
- [10].Kharbanda EO, Asche SE, Sinaiko AR, et al. , Clinical decision support for recognition and management of hypertension: a randomized trial, Pediatrics. 141 (2) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kharbanda EO, Asche SE, Sinaiko A, et al. , Evaluation of an electronic clinical decision support tool for incident elevated BP in adolescents, Acad. Pediatr 18 (1) (2018) 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Dehmer SP, Sinaiko AR, Trower NK, et al. , Clinical decision support for recognizing and managing hypertensive blood pressure in youth: no significant impact on medical costs, Acad. Pediatr 20 (6) (2020) 848–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].McKee MD, Rubin SE, Campos G, O’Sullivan LF, Challenges of providing confidential care to adolescents in urban primary care: clinician perspectives, Ann. Fam. Med 9 (1) (2011) 37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Henry-Reid LM, O’Connor KG, Klein JD, Cooper E, Flynn P, Futterman DC, Current pediatrician practices in identifying high-risk behaviors of adolescents, Pediatrics. 125 (4) (2010) e741–e747. [DOI] [PubMed] [Google Scholar]
- [15].Keyworth C, Hart J, Armitage CJ, Tully MP, What maximizes the effectiveness and implementation of technology-based interventions to support healthcare professional practice? A systematic literature review, BMC Med. Inform. Decis Mak 18 (1) (2018) 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Johnson JA 3rd, Johnson AM, Urban-rural differences in childhood and adolescent obesity in the United States: a systematic review and meta-analysis, Child. Obes 11 (3) (2015) 233–241. [DOI] [PubMed] [Google Scholar]
- [17].Shipman SA, Lan J, Chang CH, Goodman DC, Geographic maldistribution of primary care for children, Pediatrics. 127 (1) (2011) 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mayer ML, Disparities in geographic access to pediatric subspecialty care, Matern. Child Health J 12 (5) (2008) 624–632. [DOI] [PubMed] [Google Scholar]
- [19].Rosenblatt RA, A view from the periphery - health care in rural America, N. Engl. J. Med. 351 (11) (2004) 1049–1051. [DOI] [PubMed] [Google Scholar]
- [20].Moulton LH, Covariate-based constrained randomization of group-randomized trials, Clin. Trials 1 (3) (2004) 297–305. [DOI] [PubMed] [Google Scholar]
- [21].Greene EJ, A SAS macro for covariate-constrained randomization of general cluster-randomized and Unstratified designs, J. Stat. Softw. (CS1) (2017) 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kharbanda EO, Nordin JD, Sinaiko AR, et al. , TeenBP: development and piloting of an EHR-linked clinical decision support system to improve recognition of hypertension in adolescents, EGEMS (Wash DC). 3 (2) (2015) 1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Powell BJ, Fernandez ME, Williams NJ, et al. , Enhancing the impact of implementation strategies in healthcare: a research agenda, Front. Public Health 7 (2019) 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lo JC, Sinaiko A, Chandra M, et al. , Prehypertension and hypertension in community-based pediatric practice, Pediatrics. 131 (2) (2013) e415–e424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Koebnick C, Black MH, Wu J, et al. , The prevalence of primary pediatric prehypertension and hypertension in a real-world managed care system, J. Clin. Hypertens (Greenwich). 15 (11) (2013) 784–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].O’Sullivan D, Fraccaro P, Carson E, Weller P, Decision time for clinical decision support systems, Clin. Med. (Lond.) 14 (4) (2014) 338–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Condren M, Carter J, Mushtaq N, et al. , The impact of new guidelines on the prevalence of hypertension in children: a cross-sectional evaluation, J. Clin. Hypertens (Greenwich). 21 (4) (2019) 510–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.


