Abstract
Colorectal cancer (CRC) patients with metastatic lesions have low 5-year survival rates. During metastasis, cancer cells often obtain unique characteristics such as epithelial–mesenchymal transition (EMT). Vimentin a biomarker contributes to EMT by changing cell shape and motility. Since abnormal phosphorylation is a hallmark of malignancy, targeting phosphorylated vimentin is a feasible approach for the treatment of metastatic tumors while sparing non-tumor cells. Recent evidence has revealed that both CD8 cytotoxic T lymphocytes (CTLs) and also CD4 helper T lymphocytes (HTLs) can distinguish post-translationally modified antigens from normal antigens. Here, we showed that the expression of phosphorylated vimentin was upregulated in metastatic sites of CRC. We also showed that a chemotherapeutic reagent augmented the expression of phosphorylated vimentin. The novel phosphorylated helper peptide epitopes from vimentin could elicit a sufficient T cell response. Notably, precursor lymphocytes that specifically reacted to these phosphorylated vimentin-derived peptides were detected in CRC patients. These results suggest that immunotherapy targeting phosphorylated vimentin could be promising for metastatic CRC patients.
Electronic supplementary material
The online version of this article (10.1007/s00262-020-02524-9) contains supplementary material, which is available to authorized users.
Keywords: CD4 T cell, Vimentin, Post-translational modification, Phosphorylation, Epitope, Colorectal cancer
Introduction
Colorectal cancer (CRC) is the third most common cancer and the third leading cause of cancer-related deaths worldwide. The treatment of metastatic CRC patients still remains challenging [1]. Although CRC patients having mismatch-repair deficiency can receive a clinical benefit from immune checkpoint blockade, the frequency of mismatch-repair deficiency is only around 20% in CRC, indicating that novel therapeutic breakthroughs are necessary to cure the majority of patients [2, 3].
Since metastatic tumors possess different features such as epithelial-to-mesenchymal transition (EMT) compared to primary lesions, it is rational to target these characteristics to treat patients with metastatic CRC. Among EMT-related proteins, the expression and function of vimentin have been examined in detail [4]. Several groups have reported that the expression of vimentin is related to the poor prognosis of CRC patients [5–8]. In addition, the phosphorylation of vimentin enhances the growth of metastatic tumors [9]. Since phosphorylation is a tightly regulated post-translational cytosolic event, excessive phosphorylation of cellular proteins is a characteristic feature of malignant conversion [10, 11]. We recently focused on post-translationally modified antigens that might be more immunogenic than wild-type antigens [12, 13] and reported that human CD4 helper T lymphocytes (HTLs) can specifically recognize phosphorylated p53 [12]. Thus, phosphorylated vimentin might be a suitable target to overcome metastatic CRC.
Based on the success of immune checkpoint inhibitors, immunotherapy is considered as an effective armamentarium to treat cancer. Among immunotherapies, peptide vaccines are an active immunotherapy to stimulate antigen-specific T cells. Although the selection of peptides, immune adjuvants, and the route of administration should be improved to enhance antitumor responses [14], peptide vaccine therapy has benefits such as cost-effectiveness, simplicity of synthesis, and a general acceptance in clinical practice [15]. Tumor antigen-specific CD8 cytotoxic T lymphocytes (CTLs) tend to reduce their cytotoxic activity without the aid of CD4 helper T lymphocytes (HTLs) [16]. As we and other researchers have reported, peptide-reactive HTLs play a pivotal role not only in helping CTLs, but also in exhibiting direct toxicity toward tumor cells [13, 17]. Therefore, the identification of HTL epitopes from tumor-associated antigens (TAAs) is a key factor in developing an effective peptide vaccine against tumors.
In the present study, we identified phospho-peptide epitopes from vimentin that could elicit an effective HTL response. The treatment of tumor cells with a chemotherapeutic agent enhanced the expression of phosphorylated vimentin and augmented the ability of HTL responses against tumor cells. Moreover, we identified precursor T cells specific for phosphorylated vimentin in CRC patients, suggesting that phosphorylated epitopes from vimentin have sufficient immunogenicity. The combination of our vimentin-derived phosphorylated peptide vaccine with chemotherapy could be a novel approach to treat CRC patients.
Materials and methods
Cell lines
We used mouse fibroblast cell lines (L-cells) that were transfected with plasmids expressing individual human HLA-DR molecules (DR4, DR8, DR9, DR14, and DR53), CRC cell lines SW480 (DR1/13) and SW620 (DR1/13), lung squamous cell carcinoma (SCC) cell lines Calu-1 (DR7/14, -53) and Jurkat (T cell lymphoma, a cell line not expressing HLA-DR), gingival SCC cell line Sa-3 (DR9/10, -53), lung large cell carcinoma cell line Lu65 (DR4/15, -53), and renal cell carcinoma cell line SW839 (DR1/9, -53). All cell lines were maintained as recommended by the supplier.
Immunohistochemistry
Immunohistochemistry was performed as previously described [12]. Polyclonal rabbit antihuman phospho-vimentin S39 (1:200, Cell Signaling Technology (CST), #13614, Denver, CO) and rabbit antihuman phospho-vimentin S72 (1:200, Abcam, ab52944, Cambridge, MA) served as primary antibodies. Cases with more than 5% of phospho-vimentin-stained cells were regarded as positive. Anti-HLA-DR (TAL.1B5, 1:100, DAKO, Glostrup, Denmark) mAb was used to stain HLA-DR. FFPE sections were stained with VENTANA BenchMark GX (Roche Diagnostics, Rotkreuz, Switzerland) utilizing a VENTANA ultraView Universal DAB Detection Kit (Roche Diagnostics) and treated with Cell Conditioning 1 buffer (Roche Diagnostics) for antigen retrieval. HLA-DR expression in specimens with 10% tumor cell membranous staining was considered “positive” as previously reported [18]. Representative data were obtained by a BZ-X710 microscope (Keyence, Tokyo, Japan).
Western blot analyses
Expression of phosphorylated vimentin was evaluated by western blotting after the treatment of tumor cell lines with or without 1 µM doxorubicin for 24 h as previously described [12]. Antibodies used to identify specific protein expression were as follows: rabbit antihuman wt vimentin mAb (1:1,000, CST, #5741), rabbit antihuman phospho-vimentin S39 mAb (1:1,000, CST, #13614), rabbit antihuman phospho-vimentin S72 mAb (1:10,000, Abcam, EP1070Y), rabbit antihuman phospho-vimentin S83 mAb (1:1,000, CST, #3878), and mouse anti-β-actin (1: 1,000, Santa Cruz, sc-47778, C4, Santa Cruz, CA).
Synthetic peptides
We designed peptide epitopes from the previously described sequences [19]. Synthetic peptides were purchased from GenScript (Tokyo, Japan). Wild-type (wt) vimentin28-49 (LRSYVTTSTRTYSLGSALRPSTS), wt-vimentin63-90 (TRSSAVRLRSSVPGVRLLQDSVDFSLAD), vimentin28-49/phospho-S39, vimentin63-90/phospho-S72, vimentin63-90/phospho-S83, and vimentin63-90/phospho-S72 and S83 (referred to as p-VimS39, p-VimS72, p-VimS83, and p-VimS72/S83) peptides were used throughout this study. The tetanus toxoid (TT830-843; QYIKANSKFIGITE) peptide was used as a positive control that could bind to multiple HLA class II molecules [20].
In vitro induction of phosphorylated vimentin peptide-reactive CD4 helper T cells
The technique utilized for the induction of peptide-reactive HTL cell lines from peripheral blood mononuclear cells (PBMCs) of healthy volunteers (volunteer 1: HLA-DR4/9, DR53; volunteer 2: HLA-DR9/14, 53; and volunteer 3: HLA-DR4/8, 53) has been previously described in detail [21].
Briefly, we purified HTLs using CD4 MACS microbeads (Miltenyi Biotec, Auburn, CA). Then, we repeatedly stimulated these HTLs with peptide-pulsed autologous dendritic cells (DCs) or γ-irradiated autologous PBMCs and peptides (3 µg/mL). The HTL responses or the cytotoxic activity to phosphorylated vimentin peptide-loaded autologous PBMCs was examined by measuring the levels of cytokines (IFN-γ and GM-CSF ELISA kits, BD Pharmingen, Granzyme B ELISA, MABTECH, Stockholm, Sweden). An analysis of direct recognition of tumor cells by phosphorylated vimentin-reactive HTLs was performed as previously reported [22]. To increase phosphorylation of vimentin, the tumor cells were preincubated with or without 1 µM doxorubicin (CST) during the last 24 h of IFN-γ treatment.
Measurement of peptide-specific responses in CRC patients
PBMCs were collected from fresh heparinized whole blood of CRC patients by Lymphoprep™ (Axis-Shield PoC AS, Oslo, Norway) centrifugation and co-cultured with the peptides (10 µg/mL) as described previously [18, 23]. The PBMCs were stimulated for 2 weeks (1 stimulus/week). The production of IFN-γ was evaluated 7 days after the second peptide stimulation.
Statistical analysis
The data were analyzed using Student’s t test. p < 0.05 indicates statistical significance. Error bars in the data indicate the standard error of the mean (SEM). GraphPad Prism 6 was used for analyses.
Results
Expression of phosphorylated vimentin in primary- and metastatic-site specimens of colorectal cancer
We first examined the expression of phosphorylated vimentin in surgical specimens of primary CRC and their metastatic sites by immunohistochemistry. Twenty-two CRC patients in the Department of Surgery at Asahikawa Medical University who later developed distant metastases were included. The clinical factors of these CRC patients are described in Table 1. Phospho-VimS39 and phospho-VimS72 were expressed in primary tumor specimens of 12/22 (55%) and 15/22 (68%) patients and in metastatic tumor specimens of 15/22 (68%) and 20/22 (91%) patients, respectively. Phospho-vimentin expression was positive in the cytoplasm. Representative data are shown in Supplementary Fig. 1A–C. Phospho-vimentin was not expressed in healthy colon tissue (Supplementary Fig. 3). In addition to CRC, phospho-vimentin is also expressed in head and neck cancer, suggesting that the phosphorylation of vimentin is a common feature of malignant diseases. These results showed that multiple serine residues of vimentin were simultaneously phosphorylated in CRC, indicating the potential of phosphorylated vimentin as an immunotherapeutic target. Because MHC class II was expressed in 60% of patients whose primary tumor expressed HLA-DR (Supplementary Fig. 2), patients with metastatic CRC are candidates for CD4-based immunotherapy.
Table 1.
Clinical features and phospho-vimentin and HLA-DR expression of CRC patients
| Case no. | Age | Sex | T | N | M | Stage | Primary tumor | Metastatic tumor | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p-v S39 | p-v S72 | HLA-DR | p-v S39 | p-v S72 | HLA-DR | |||||||
| 1 | 77 | F | 3 | 2a | 1a | IVA | – | – | – | Cytoplasm | Cytoplasm | – |
| 2 | 53 | M | 2 | 2a | 1a | IVA | – | – | – | – | – | – |
| 3 | 59 | F | 4a | 2a | 1a | IVA | – | – | – | Cytoplasm | Cytoplasm | – |
| 4 | 44 | F | 2 | 2a | 1a | IVA | Cytoplasm | Cytoplasm | + | – | Cytoplasm | + |
| 5 | 68 | M | 2 | 0 | 0 | I | – | – | + | – | – | – |
| 6 | 70 | M | 3 | 0 | 1a | IVA | Cytoplasm | Cytoplasm | + | – | Cytoplasm | + |
| 7 | 54 | F | 4a | 2b | 0 | IIIC | Cytoplasm | – | – | – | Cytoplasm | – |
| 8 | 78 | F | 3 | 2a | 1a | IVA | Cytoplasm | Cytoplasm | + | – | cytoplasm | – |
| 9 | 66 | M | 3 | 1b | 1a | IVA | – | – | – | – | Cytoplasm | – |
| 10 | 50 | F | 4a | 1b | 1a | IVA | – | – | + | – | Cytoplasm | + |
| 11 | 48 | F | 3 | 0 | 0 | IIA | – | Cytoplasm | – | Cytoplasm | Cytoplasm | – |
| 12 | 71 | M | 3 | 0 | 1a | IVA | Cytoplasm | Cytoplasm | – | – | Cytoplasm | – |
| 13 | 54 | F | 3 | 1b | 1b | IVB | – | Cytoplasm | + | – | Cytoplasm | – |
| 14 | 65 | F | 3 | 0 | 1a | IVA | Cytoplasm | Cytoplasm | – | Cytoplasm | Cytoplasm | – |
| 15 | 67 | M | 3 | 1b | 1a | IVA | – | Cytoplasm | + | Cytoplasm | Cytoplasm | – |
| 16 | 52 | M | 3 | 1c | 0 | IIIB | Nucleus | Cytoplasm | – | Nucleus | Cytoplasm | – |
| 17 | 60 | M | 3 | 0 | 1a | IVA | Cytoplasm | Cytoplasm | – | Cytoplasm | Cytoplasm | – |
| 18 | 77 | F | 3 | 1a | 0 | IIIB | Cytoplasm | Cytoplasm | – | Cytoplasm | Cytoplasm | – |
| 19 | 65 | F | 3 | 2a | 1a | IVA | – | Cytoplasm | + | Cytoplasm | Cytoplasm | + |
| 20 | 66 | M | 4a | 1b | 1b | IVB | Cytoplasm | Cytoplasm | + | Cytoplasm | Cytoplasm | + |
| 21 | 65 | F | 3 | 2a | 1a | IVA | Cytoplasm | Cytoplasm | – | Cytoplasm | Cytoplasm | – |
| 22 | 60 | F | 3 | 0 | 1a | IVA | Cytoplasm | Cytoplasm | + | Cytoplasm | Cytoplasm | + |
The case is regarded positive when more than 5% of cells were stained and the location information was described
Expression of phosphorylated vimentin in tumor cell lines
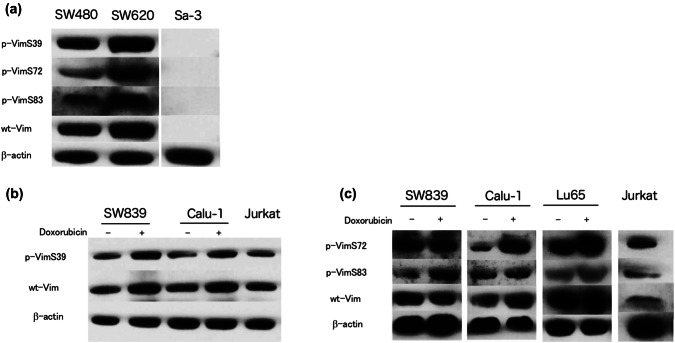
We next assessed the expression of phosphorylated vimentin in tumor cells. Western blot analysis revealed that phosphorylated vimentin S39 and S72 were expressed in several tumor cell lines including CRC cell lines (Fig. 1a–c). Sa3 (head and neck squamous cell cancer) cells expressed neither wt (wild-type, non-phosphorylated) nor phosphorylated vimentin. Since chemotherapy is a standard strategy to treat metastatic CRC, we used chemotherapeutic reagents (doxorubicin, paclitaxel, and 5-FU) to determine whether such reagents could enhance the expression of vimentin. As shown in Fig. 1b, c, doxorubicin augmented the expression of both wt and phosphorylated vimentin. Neither paclitaxel nor 5-FU affected the expression of wt or phosphorylated vimentin (data not shown). Although doxorubicin is not included in the standard chemotherapy regimen for CRC, it is applied for liver metastasis of CRC patients [24]. Moreover, doxorubicin is combined with a checkpoint inhibitor for CRC in the form of cancer photochemotherapy, indicating that doxorubicin might be useful to induce phosphorylated vimentin peptide epitopes in a clinical setting [25].
Fig. 1.
Expression of wild-type (wt) vimentin protein and phosphorylated vimentin in tumor cells. a The expressions of wt vimentin protein and phosphorylated vimentin (phosphorylated Ser39, Ser72, or Ser83) in tumor cell lines was examined by western blotting. b, c The tumor cell lines were treated with or without doxorubicin (1 µM) for 24 h before assessment. SW480 and SW620: colon cancer. Calu-1: lung squamous cell carcinoma. Sa-3: head and neck squamous cell carcinoma. Lu65: lung adenocarcinoma. SW839: renal cell carcinoma. Jurkat: T cell lymphoma
In vitro induction of CD4 T cells that react to phosphorylated vimentin
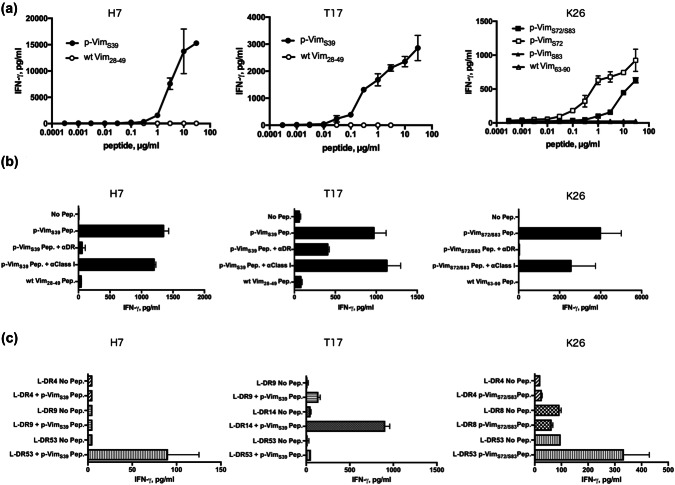
To use vimentin as a peptide vaccine to treat CRC, we next sought to find the phosphorylated epitope peptide from its amino acid sequence. The citrullinated peptide epitope, cit-vimentin28-49, has been previously reported as a naturally processed epitope recognized by human HTLs [19]. Since vimentin28-49 contains a phosphorylated serine 39 residue, we investigated the immunogenicity of vimentin28-49/phospho-S39 peptide. In addition, we selected three other peptides, vimentin63-90/phospho-S72, vimentin63-90/phospho-S83, and vimentin63-90/phospho-S72 and S83, which possess phosphorylated serine 72 and serine 83 residues. We investigated whether the four peptides can elicit the phosphorylated peptide-specific HTL response from human PBMCs. As a result, these four epitope peptides were capable of inducing phosphorylated peptide-specific HTL cell lines (Fig. 2). The mono-phosphorylated peptide vimentin28-49/phospho-S39 (referred to as p-VimS39) elicited phosphorylated peptide-specific HTL responses from two healthy donors (H7 and T17). These HTLs only reacted to p-VimS39 in a concentration-dependent manner, but not to wt vimentin28-49 (referred to as wt-Vim28-49), indicating that the T cell receptors (TCR) of these HTLs are specific to phosphorylated Ser39. The dual phosphorylated peptide vimentin63-90/phospho-S72 and S83 (referred to as p-VimS72/S83) also elicited phosphorylated peptide-specific HTL responses (K26). This HTL clone reacted to p-VimS72 but not to p-VimS83, demonstrating that TCR of this clone specifically recognize phosphorylated Ser72. Since these responses were inhibited by an anti-HLA-DR mAb (L243), which is specific for HLA-DR, the HTL clones were stimulated in the context of HLA-DR–peptide–TCR molecules (Fig. 2b). Subsequently, a panel of mouse fibroblasts (L-cells) expressing single HLA-DR molecules were used as antigen-presenting cells (APCs) to determine which HLA class II molecules present the phosphorylated vimentin peptides to the HTLs. As shown in Fig. 2c, HTLs H7 and K26 recognized the phosphorylated vimentin peptide in the context of HLA-DR53, while the response of T17 was restricted by HLA-DR14. These results showed that the phosphorylated vimentin peptides were able to bind to several HLA-DR molecules that cover a broad population of cancer patients. In summary, these findings revealed that HTL precursors, which react to p-VimS39 and p-VimS72 and S83, exist in healthy human PBMCs.
Fig. 2.
Induction of CD4 T cell responses using phosphorylated vimentin peptides. a Phosphorylated vimentin-reactive CD4 T cells (H7, T17, and K26) were assessed for their ability to recognize various concentrations of phosphorylated and wild-type (wt) peptides. These cell lines were selected from peptide-reactive CD4 T cells using limiting dilution. Autologous PBMCs were used as antigen-presenting cells (APCs). b MHC restriction analysis of phosphorylated vimentin-reactive CD4 T cells. Peptide-induced T cell responses of phosphorylated vimentin-reactive CD4 T cells (H7, T17, and K26) in the presence of anti-HLA-DR mAb L243 or anti-HLA class I mAb W6/32 (negative control) were evaluated. Autologous PBMCs were used as APCs. c CD4 T cell responses to phosphorylated p53 peptides were measured using L-cells transfected with individual HLA-DR genes as APCs to determine the restricting HLA class II molecules
Direct recognition of phosphorylated vimentin-positive tumor cells by peptide-reactive CD4 T cells
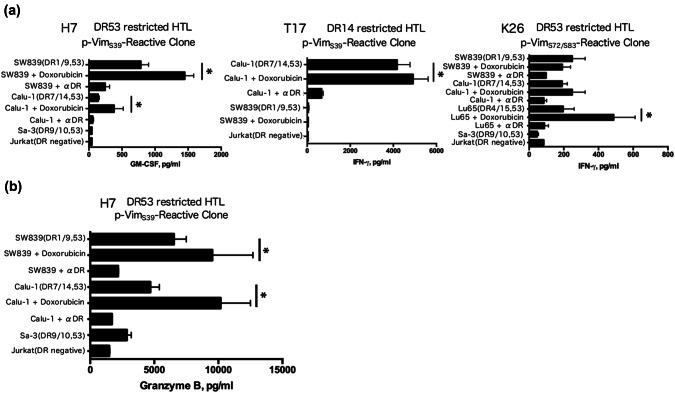
Next, we assessed whether phosphorylated vimentin-reactive HTLs could directly recognize phosphorylated vimentin-expressing tumor cells. Peptide-reactive HTLs and HLA-DR-matched phosphorylated vimentin-positive tumor cells were co-cultured, and IFN-γ levels in the culture supernatant were measured. All phosphorylated vimentin-specific HTLs (H7, T17, and K26) directly recognized HLA-DR-matched phosphorylated vimentin-positive tumor cells, but did not react to HLA-DR-unmatched phosphorylated vimentin-positive tumor cell lines, HLA-DR-matched phosphorylated vimentin-negative tumor cell lines, or a HLA-DR-negative tumor cell line (Fig. 3a). In addition, p-VimS39-reactive HTLs (H7) produced granzyme B against HLA-DR-matched phosphorylated vimentin-positive tumor cells (Fig. 3b), suggesting that these HTLs exhibited cytotoxicity against them. In accordance with the increased expression of phosphorylated vimentin with treatment by doxorubicin (Fig. 1b, c), all phosphorylated vimentin-reactive HTL clones produced more IFN-γ and granzyme B when tumor cells were pretreated with doxorubicin before co-culturing (Fig. 3a, b). These results showed that phosphorylated vimentin-reactive HTLs reacted to tumor cells in an HLA-DR-dependent manner, and the phosphorylation of vimentin was preserved during the antigen-processing phase. Moreover, the chemotherapeutic agent doxorubicin can act as an adjuvant to increase the expression of phosphorylated vimentin.
Fig. 3.
Direct tumor recognition by phosphorylated peptide-specific CD4 T cells. Direct tumor recognition of naturally processed vimentin antigen expressed in tumor cells by p-VimS39-reactive clones (H7 and T17) and the p-VimS72/S83-reactive clone (K26) is shown. a Phosphorylated vimentin-reactive T cells (DR53-restricted-clone H7, K26, and DR14-restricted-clone T17) were evaluated for their capacity to recognize HLA-DR53 positive/p-Vim-positive (SW839, Calu-1, and Lu65), HLA-DR14 positive/p-Vim-positive (Calu-1), DR53-positive/vimentin-negative (Sa-3), and DR-negative (Jurkat) tumor cells. Sa-3 and Jurkat cells were used as negative controls. Tumor cells were treated with 1 µM doxorubicin for 24 h before co-culturing with T cells. b Granzyme B production by the p-VimS39-reactive clone (H7) and the p-VimS72/S83-reactive clone (K26) during co-culturing with tumor cells. Tumor cells were treated with 1 µM doxorubicin for 24 h before co-culturing with T cells. *p < 0.05
Indirect recognition of phosphorylated vimentin-positive colorectal tumor cells by phosphorylated peptide-reactive CD4 T cells
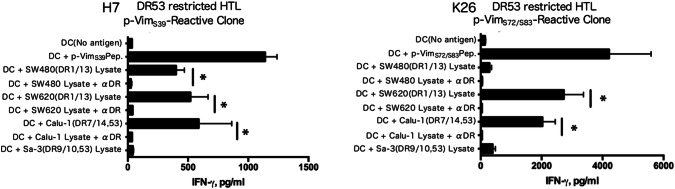
We next evaluated whether phosphorylated vimentin peptide can be processed from tumor cell lysates and successfully presented through MHC class II molecules in autologous DCs. As shown in Fig. 4a, b, p-VimS39-reactive HTLs (H7) and p-VimS72/S83-reactive HTLs (K26) were capable of producing IFN-γ in response to colon cancer cell lysate-loaded DCs, and these reactions were suppressed by an anti-HLA-DR mAb (L243), indicating that phosphorylated vimentin epitopes are preserved during the exogenous antigen-processing pathway.
Fig. 4.
Indirect tumor recognition by phosphorylated peptide-specific CD4 T cells. The ability of p-Vim-reactive CD4 T cells to recognize naturally processed exogenous phosphorylated vimentin was assessed by measuring IFN-γ production. Dendritic cells (DCs) were used as antigen-presenting cells (APCs), and HLA-DR-unmatched CRC tumor cell lysates served as sources of phosphorylated vimentin protein. L243 mAb was used to verify the HLA-DR restriction of these responses. DCs pulsed with p-VimS39 and p-VimS72/S83 were used as positive controls. *p < 0.05
Recognition of phosphorylated vimentin peptides by PBMCs of CRC patients
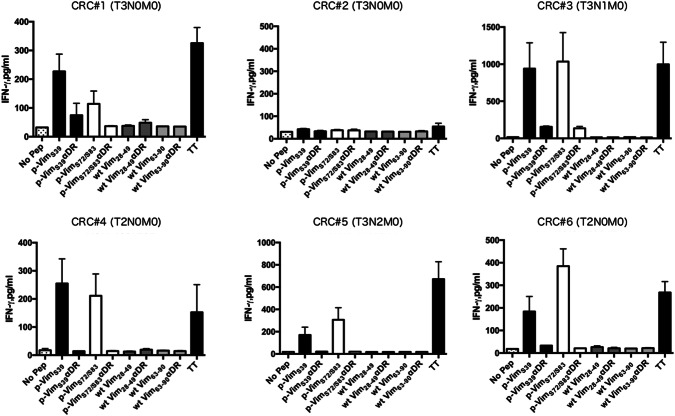
Since it is possible that tumor antigen-specific precursor T cells are diminished in cancer patients, it is important to verify the existence of precursor T cells specific for phosphorylated vimentin peptides in CRC patients to validate the utility of these peptides as a cancer vaccine. To determine the HTL responses to phosphorylated vimentin in CRC patients, we performed short-term culture experiments using peptide-stimulated PBMCs from six CRC patients. Since the volume of blood obtained from these patients was small, it was impossible to induce HTL cell lines for detailed HLA restriction analysis. Since tetanus toxoid (TT830-843) peptide is capable of eliciting strong HTL responses in the vast majority of people regardless of their HLA-DR alleles [20], we used this peptide as a positive control. As shown in Fig. 5, considerable T cell responses to phosphorylated vimentin were observed in five CRC patients. It is intriguing that wild-type vimentin did not induce T cell responses. These responses were inhibited by an anti-HLA-DR mAb, indicating that cytokine production was induced by phosphorylated peptide-reactive HTLs.
Fig. 5.
Evaluation of T cell responses to wild-type and phosphorylated vimentin in CRC patients. PBMCs from six CRC patients (CRC #1-6) were stimulated with peptides, and T cell responses were measured as described in the Materials and methods
Discussion
We showed in the present study that phosphorylated vimentin peptides are likely to be useful as a helper peptide vaccine to treat CRC. Since CTLs are considered to be a direct player that kills tumors in cancer immunotherapy, clinical trials using HTLs have shown promising results [16, 26]. Previously, we showed that peptide-reactive HTLs that were positive for granzyme B could directly kill tumor cells in a killing assay [27]. HTL cell lines that we established in this study produced cytotoxic cytokines, suggesting that HTLs play an important role in direct tumor cell toxicity. Vimentin, a cytoskeletal protein, is one of the clinical parameters of EMT. We showed that more than half of primary CRC and more than 70% of metastatic CRC samples were positive for phosphorylated vimentin (Table 1). Moreover, the precursor lymphocytes that recognize phosphorylated vimentin existed in CRC patients (Fig. 5). Thus, it is feasible to target phosphorylated vimentin as peptide vaccine to treat CRC.
Abnormal phosphorylation of cellular proteins is a hallmark of malignant transformation [10]. Akt, Rho-associated kinase (Rho-kinase), or Polo-like kinase 1 (Plk1) phosphorylate vimentin at Ser39, Ser72, or Ser83 enhance tumor growth, metastasis, and cytokinesis [9, 28, 29]. In oral squamous cell carcinoma, metastatic tumor cells express more vimentin than their non-metastatic counterparts [30]. This datum is consistent with the results of our IHC analysis in CRC patients showing that metastatic tumors tended to express higher levels of phosphorylated vimentin than primary tumors (Table 1). As shown in Supplementary Fig. 1, the expression of phosphorylated vimentin was heterogeneous. Because chemotherapeutic reagents induce phospho-vimentin through the TGF-β and PI3K pathways [31], cells that suffer from cellular stress such as at an invasion front may have higher expression of phospho-vimentin compared to non-stressed cells. Because immune checkpoint blockade with chemotherapy significantly prolongs the survival of patients with lung cancer compared to chemotherapy alone, the combination of immune-suppressive chemotherapy and immunotherapy is a clinically effective approach [32, 33]. It is plausible that chemotherapy-resistant memory T cells survive during chemotherapy [34] and are activated by released tumor antigens from destroyed tumor cells.
Since HTLs have been described to specifically identify post-translationally modified epitopes, including phosphorylated epitopes on MHC II molecules [12, 35, 36], post-translational modification can be a unique target to generate peptide vaccines. To the best of our knowledge, there are no reports focusing on phosphorylated vimentin-specific T cell responses in cancer immunotherapy. The phosphorylated vimentin epitope peptides that we found in this study bound to multiple HLA-DR alleles, including HLA-DR53 (Fig. 2c), indicating that these peptides can be applied to a large population of patients. It should be noted that phosphorylated but not wild-type vimentin induced T cell responses in PBMCs of CRC patients (Fig. 5). This result suggested that the immunogenicity of phosphorylated vimentin is higher than that of wild-type vimentin. Another possibility is that the increased antigen load in CRC patients upregulates the number of precursor T cells against phosphorylated vimentin. Further studies are required to evaluate these possibilities.
In pathology, vimentin is usually used to distinguish mesenchymal tumors from epithelial tumors. Increased expression of vimentin is considered to represent the activation of EMT in malignant tissues. Although it was not significant because of the sample size, metastatic CRC tissues expressed higher levels of phosphorylated vimentin (S72) than primary CRC tissues (Table 1). To improve the efficacy of peptide vaccines, the selection of adjuvants and administration routes is necessary [37]. As shown in this study (Figs. 1, 3), chemotherapeutic reagents such as doxorubicin might be suitable adjuvants to increase the amount of tumor antigen (phosphorylated vimentin) in tumor cells followed by T cell stimulation. Since we proved that phosphorylated peptides were preserved during the exogenous antigen-processing pathway, chemotherapy can also increase the presentation of phosphorylated peptides from dead tumor cells on professional APCs. Because the expression of phosphorylated vimentin in healthy tissues has not been fully investigated, it is important to confirm that phospho-vimentin-reactive HTLs spare healthy tissues in future in vivo studies. As shown in Supplementary Fig. 2, the expression of phosphorylated vimentin was not expressed in healthy colon tissues. The existence of phospho-vimentin-reactive T cell precursors in both healthy donors and colon cancer patients suggests that these cells might not be self-reactive. It is possible that the affinity of these HTLs might be low enough to spare healthy tissues, but is sufficient to react with tumor cells that present high amounts of phosphorylated vimentin-derived peptides.
Conclusions
We discovered novel helper epitopes from phosphorylated vimentin that can elicit antigen-specific HTL reactions. Variants in the amino acid sequences of these peptides have not been reported [38], indicating that these peptides are strongly conserved. Moreover, doxorubicin might work as an adjuvant for peptide-based cancer immunotherapy by upregulating phosphorylated vimentin. Since post-translationally modified epitopes are selectively expressed in malignant tumor cells [12, 13], a peptide vaccine using these phosphorylated peptides could be a suitable therapy for CRC with metastatic lesions, which express a higher level of phosphorylated vimentin.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- APCs
Antigen-presenting cells
- CRC
Colorectal cancer
- CTLs
CD8 cytotoxic T lymphocytes
- DCs
Dendritic cells
- EMT
Epithelial–mesenchymal transition
- HTLs
CD4 helper T lymphocytes
- L-cells
Fibroblast cell lines
- mAb
Monoclonal antibody
- PBMCs
Peripheral blood mononuclear cells
- SCC
Squamous cell carcinoma
- TAA
Tumor-associated antigen
- TCR
T cell receptors
Author contributions
MO, KO, TN, and YH-N contributed to data acquisition. AK, MN, RH, SH, and YY contributed to data acquisition and analysis. TK, TO, and KO contributed to the design and concept and drafting of the manuscript. All authors were involved in data interpretation, preparation, and review of the manuscript draft and approved the final manuscript version for submission.
Funding
This work was supported by the Japanese Society for the Promotion of Science (JSPS) KAKENHI Grant Numbers 17K16884 and 19K07452.
Compliance with ethical standards
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical approval and ethical standards
This study followed the principles of the Helsinki Declaration, and the study protocol was approved by the Asahikawa Medical University Institutional Ethics Committee (approval number #16040-3).
Informed consent
All blood samples from healthy human volunteers and CRC patients (Asahikawa Medical University Hospital, 2016-2018) were obtained after obtaining written informed consent (Institutional Ethics Committee approval number #16040-3). The patients and healthy donors agreed to the use of their specimens and data for research and publication.
Cell line authentication
The CRC cell lines SW480 and SW620 were purchased from KAC Co., Ltd. (Kyoto, Japan). Lung SCC cell lines Calu-1 and Jurkat (T cell lymphoma) were obtained from the American Type Culture Collection (ATCC, Manassas, VA). The gingival SCC cell line Sa-3 and lung large cell carcinoma cell line Lu65 were supplied by the RIKEN Bio-Resource Center (Tsukuba, Japan). The renal cell carcinoma cell line SW839 was obtained from the Cell Resource Center for Biomedical Research Institute of Development (Aging and Cancer, Tohoku University, Sendai, Japan). Cell authentication assays were performed by each company, and the cell passaging was no more than five times.
Footnotes
Mizuho Ohara and Kenzo Ohara have first authorship.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Takumi Kumai, Email: t-kumai@asahikawa-med.ac.jp.
Takayuki Ohkuri, Email: ohkurit@asahikawa-med.ac.jp.
References
- 1.Howlader N, Noone AM, Krapcho M et al. (1975–2016) SEER Cancer Statistics Review. National Cancer Institute. https://seer.cancer.gov/csr/1975_2016/. April 2019
- 2.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687–696. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richardson AM, Havel L, Koyen AE, et al. Vimentin is required for lung adenocarcinoma metastasis via heterotypic tumor cell-cancer-associated fibroblast interactions during collective invasion. Clin Cancer Res. 2017 doi: 10.1158/1078-0432.ccr-17-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toiyama Y, Yasuda H, Saigusa S, Tanaka K, Inoue Y, Goel A, Kusunoki M. Increased expression of Slug and Vimentin as novel predictive biomarkers for lymph node metastasis and poor prognosis in colorectal cancer. Carcinogenesis. 2013;34:2548–2557. doi: 10.1093/carcin/bgt282. [DOI] [PubMed] [Google Scholar]
- 6.Shirahata A, Hibi K. Serum vimentin methylation as a potential marker for colorectal cancer. Anticancer Res. 2014;34:4121–4125. [PubMed] [Google Scholar]
- 7.Liu LG, Yan XB, Xie RT, Jin ZM, Yang Y. Stromal expression of vimentin predicts the clinical outcome of stage II colorectal cancer for high-risk patients. Medical Sci Monit Int Med J Exp Clin Res. 2017;23:2897–2905. doi: 10.12659/MSM.904486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du L, Li J, Lei L, He H, Chen E, Dong J, Yang J. High vimentin expression predicts a poor prognosis and progression in colorectal cancer: a study with meta-analysis and TCGA database. Biomed Res Int. 2018;2018:6387810. doi: 10.1155/2018/6387810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu QS, Rosenblatt K, Huang KL, et al. Vimentin is a novel AKT1 target mediating motility and invasion. Oncogene. 2011;30:457–470. doi: 10.1038/onc.2010.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zarling AL, Polefrone JM, Evans AM, Mikesh LM, Shabanowitz J, Lewis ST, Engelhard VH, Hunt DF. Identification of class I MHC-associated phosphopeptides as targets for cancer immunotherapy. Proc Natl Acad Sci USA. 2006;103:14889–14894. doi: 10.1073/pnas.0604045103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krueger KE, Srivastava S. Posttranslational protein modifications: current implications for cancer detection, prevention, and therapeutics. Mol Cell Proteomics MCP. 2006;5:1799–1810. doi: 10.1074/mcp.R600009-MCP200. [DOI] [PubMed] [Google Scholar]
- 12.Ohara K, Ohkuri T, Kumai T, et al. Targeting phosphorylated p53 to elicit tumor-reactive T helper responses against head and neck squamous cell carcinoma. Oncoimmunology. 2018;7:e1466771. doi: 10.1080/2162402x.2018.1466771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumai T, Ishibashi K, Oikawa K, et al. Induction of tumor-reactive T helper responses by a posttranslational modified epitope from tumor protein p53. Cancer Immunol Immunother. 2014;63:469–478. doi: 10.1007/s00262-014-1533-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumai T, Lee S, Cho HI, Sultan H, Kobayashi H, Harabuchi Y, Celis E. Optimization of peptide vaccines to induce robust antitumor CD4 T-cell responses. Cancer Immunol Res. 2016 doi: 10.1158/2326-6066.cir-16-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumai T, Kobayashi H, Harabuchi Y, Celis E. Peptide vaccines in cancer-old concept revisited. Curr Opin Immunol. 2016;45:1–7. doi: 10.1016/j.coi.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobayashi Y, Sakura T, Miyawaki S, Toga K, Sogo S, Heike Y. A new peptide vaccine OCV-501: in vitro pharmacology and phase 1 study in patients with acute myeloid leukemia. Cancer Immunol Immunother. 2017 doi: 10.1007/s00262-017-1981-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quezada SA, Simpson TR, Peggs KS, et al. Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med. 2010;207:637–650. doi: 10.1084/jem.20091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirata-Nozaki Y, Ohkuri T, Ohara K, et al. PD-L1-specific helper T-cells exhibit effective antitumor responses: new strategy of cancer immunotherapy targeting PD-L1 in head and neck squamous cell carcinoma. J Transl Med. 2019;17:207. doi: 10.1186/s12967-019-1957-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brentville VA, Metheringham RL, Gunn B, Symonds P, Daniels I, Gijon M, Cook K, Xue W, Durrant LG. Citrullinated vimentin presented on MHC-II in tumor cells is a target for CD4+ T-cell-mediated antitumor immunity. Can Res. 2016;76:548–560. doi: 10.1158/0008-5472.can-15-1085. [DOI] [PubMed] [Google Scholar]
- 20.Panina-Bordignon P, Tan A, Termijtelen A, Demotz S, Corradin G, Lanzavecchia A. Universally immunogenic T cell epitopes: promiscuous binding to human MHC class II and promiscuous recognition by T cells. Eur J Immunol. 1989;19:2237–2242. doi: 10.1002/eji.1830191209. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi H, Wood M, Song Y, Appella E, Celis E. Defining promiscuous MHC class II helper T-cell epitopes for the HER2/neu tumor antigen. Can Res. 2000;60:5228–5236. [PubMed] [Google Scholar]
- 22.Kumai T, Matsuda Y, Oikawa K, Aoki N, Kimura S, Harabuchi Y, Celis E, Kobayashi H. EGFR inhibitors augment antitumour helper T-cell responses of HER family-specific immunotherapy. Br J Cancer. 2013;109:2155–2166. doi: 10.1038/bjc.2013.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumai T, Ohkuri T, Nagato T, et al. Targeting HER-3 to elicit antitumor helper T cells against head and neck squamous cell carcinoma. Sci Rep. 2015;5:16280. doi: 10.1038/srep16280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Albert M, Kiefer MV, Sun W, Haller D, Fraker DL, Tuite CM, Stavropoulos SW, Mondschein JI, Soulen MC. Chemoembolization of colorectal liver metastases with cisplatin, doxorubicin, mitomycin C, ethiodol, and polyvinyl alcohol. Cancer. 2011;117:343–352. doi: 10.1002/cncr.25387. [DOI] [PubMed] [Google Scholar]
- 25.Emami F, Banstola A, Vatanara A, Lee S, Kim JO, Jeong JH, Yook S. Doxorubicin and anti-PD-L1 antibody conjugated gold nanoparticles for colorectal cancer photochemotherapy. Mol Pharm. 2019;16:1184–1199. doi: 10.1021/acs.molpharmaceut.8b01157. [DOI] [PubMed] [Google Scholar]
- 26.Obara W, Ohsawa R, Kanehira M, et al. Cancer peptide vaccine therapy developed from oncoantigens identified through genome-wide expression profile analysis for bladder cancer. Jpn J Clin Oncol. 2012;42:591–600. doi: 10.1093/jjco/hys069. [DOI] [PubMed] [Google Scholar]
- 27.Hayashi S, Kumai T, Matsuda Y, et al. Six-transmembrane epithelial antigen of the prostate and enhancer of zeste homolog 2 as immunotherapeutic targets for lung cancer. J Transl Med. 2011;9:191. doi: 10.1186/1479-5876-9-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goto H, Kosako H, Tanabe K, Yanagida M, Sakurai M, Amano M, Kaibuchi K, Inagaki M. Phosphorylation of vimentin by Rho-associated kinase at a unique amino-terminal site that is specifically phosphorylated during cytokinesis. J Biol Chem. 1998;273:11728–11736. doi: 10.1074/jbc.273.19.11728. [DOI] [PubMed] [Google Scholar]
- 29.Yamaguchi T, Goto H, Yokoyama T, et al. Phosphorylation by Cdk1 induces Plk1-mediated vimentin phosphorylation during mitosis. J Cell Biol. 2005;171:431–436. doi: 10.1083/jcb.200504091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu S, Liu L, Ye W, et al. High vimentin expression associated with lymph node metastasis and predicated a poor prognosis in oral squamous cell carcinoma. Sci Rep. 2016;6:38834. doi: 10.1038/srep38834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen WC, Lai YA, Lin YC, Ma JW, Huang LF, Yang NS, Ho CT, Kuo SC, Way TD. Curcumin suppresses doxorubicin-induced epithelial–mesenchymal transition via the inhibition of TGF-beta and PI3K/AKT signaling pathways in triple-negative breast cancer cells. J Agric Food Chem. 2013;61:11817–11824. doi: 10.1021/jf404092f. [DOI] [PubMed] [Google Scholar]
- 32.Horn L, Mansfield AS, Szczesna A, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379:2220–2229. doi: 10.1056/NEJMoa1809064. [DOI] [PubMed] [Google Scholar]
- 33.Harabuchi S, Kosaka A, Yuki Y, et al. Intratumoral STING activations overcome negative impact of cisplatin on antitumor immunity by inflaming tumor microenvironment in squamous cell carcinoma. Biochem Biophys Res Commun. 2019 doi: 10.1016/j.bbrc.2019.11.107. [DOI] [PubMed] [Google Scholar]
- 34.Shibayama Y, Tsukahara T, Emori M, et al. Implication of chemo-resistant memory T cells for immune surveillance in patients with sarcoma receiving chemotherapy. Cancer Sci. 2017;108:1739–1745. doi: 10.1111/cas.13319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Depontieu FR, Qian J, Zarling AL, et al. Identification of tumor-associated, MHC class II-restricted phosphopeptides as targets for immunotherapy. Proc Natl Acad Sci USA. 2009;106:12073–12078. doi: 10.1073/pnas.0903852106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Engelhard VH, Altrich-Vanlith M, Ostankovitch M, Zarling AL. Post-translational modifications of naturally processed MHC-binding epitopes. Curr Opin Immunol. 2006;18:92–97. doi: 10.1016/j.coi.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 37.Kumai T, Fan A, Harabuchi Y, Celis E. Cancer immunotherapy: moving forward with peptide T cell vaccines. Curr Opin Immunol. 2017;47:57–63. doi: 10.1016/j.coi.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.The UniProt Consortium UniProt: the universal protein knowledgebase. Nucl Acids Res. 2017;45:D158-d69. doi: 10.1093/nar/gkw1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.