Abstract
Tumor-related leukocytosis (TRL) is correlated with poor survival in various types of cancers, but the microenvironment of TRL-associated human tumors has not been fully elucidated. Here, we aimed to characterize the immune microenvironment of cancer patients with TRL. The transcriptional signatures of tumor tissues obtained from cervical cancer patients with (TRLpos) and without TRL (TRLneg) were compared. As a surrogate for TRL diagnosis, a leukocytosis signature (LS) score was derived using genes differentially expressed between TRLpos and TRLneg tumors. The immunological profiles of patients in the TCGA database with high (LShigh) or low LS scores were compared. TRLpos tumors were transcriptionally distinct from TRLneg tumors, exhibiting up-regulation of radioresistance and down-regulation of adaptive immune response-related genes. In the TCGA cervical cancer cohort (n = 303), patients with high LS had inferior survival rates compared to those with low LS (P = 0.023). LShigh tumors were enriched in radioresistance, wound healing, and myeloid-derived suppressor cell (MDSC) signatures and had a higher infiltration of M2 macrophages and a lower infiltration of M1 macrophages and lymphocytes. LShigh tumors also expressed higher levels of CXCR2 chemokines, CSF2, and CSF3. In the pan-cancer cohort (n = 9984), LShigh tumors also exhibited poor survival, signatures of a suppressive immune microenvironment, and higher expression of CXCR2 chemokines. Our data provide evidence for a suppressive immune microenvironment in patients with TRL and suggest promising targets, such as the CXCR2 axis, for its therapeutic intervention.
Electronic supplementary material
The online version of this article (10.1007/s00262-020-02545-4) contains supplementary material, which is available to authorized users.
Keywords: Leukocytosis, Tumor microenvironment, Tumor immune evasion, Gene signatures, CXCR2
Introduction
Growing evidence suggests that there is a link between cancer and inflammation [1]. Inflammatory response is considered as one of the hallmarks of cancer and plays central roles at different stages of tumor development and progression [2, 3]. Cancer-related inflammation is known to have tumor-promoting effects by aiding survival of cancer cells, promoting angiogenesis, and suppressing anti-tumor immunity [1]. In 10–20% cancer patients, such inflammatory responses are reflected in peripheral blood as increases in leukocytes referred to as tumor-related leukocytosis (TRL). TRL is associated with a poor response to radiotherapy or chemotherapy and, accordingly, poor survival [4–7]. Despite the dismal prognosis of patients with TRL, there is no effective therapy for such patients.
Myeloid cells, such as myeloid-derived suppressor cells (MDSCs) and tumor-associated macrophages (TAMs), are key components of tumor microenvironment inflammatory responses [8, 9]. MDSCs and TAMs aid tumor development by promoting the cellular stemness of cancer cells, angiogenesis, and epithelial–mesenchymal transition and inhibiting anti-tumor T cell responses [10]. Preclinical studies have shown that increased G-CSF secretion from tumor cells can increase both circulating and intra-tumoral MDSCs [4, 11]. In humans, TRL is associated with a higher frequency of circulating MDSCs and higher G-CSF concentrations [4, 11, 12] and negatively correlated with tumor T-cell infiltration [7, 13]. However, comprehensive analysis of the immune microenvironment of patients with TRL has not been performed.
In the present study, we compared the immune landscapes of tumors from cervical cancer patients with and without TRL using RNA sequencing. Furthermore, we utilized TCGA data to extend our findings to a larger cohort of TRL patients with cervical and non-cervical cancer with the aim of discovering promising TRL therapeutic targets.
Materials and methods
Patients and sample preparation
Patients with pathologically confirmed squamous cell carcinoma of the uterine cervix without distant metastasis who received definitive chemoradiotherapy or radiotherapy were included in the study. TRL was defined as a white blood cell count exceeding 9000/μL at baseline without any evidence of infection [5]. RNA was extracted from formalin-fixed paraffin-embedded (FFPE) tissue sections with an RNeasy FFPE Kit (Qiagen, Hilden, Germany), and only samples that passed the quality control tests were used for RNA sequencing. RNA quality was assessed using an Agilent 2100 bioanalyzer and an RNA 6000 Nano Chip (Agilent Technologies, Palo Alto, CA), and RNA quantification was performed using an ND-2000 Spectrophotometer (Thermo Fisher Scientific, Waltham, MA). This study was approved by the Institutional Review Board (4-2015-0454), and all patients provided informed consent before inclusion in the study.
RNA sequencing and analysis
Four patients with TRL (TRLpos) and four patients without TRL (TRLneg) were eligible for RNA sequencing. Detailed methods of RNA sequencing are provided in Supplementary materials. Gene counts were normalized by library size, and differential gene expression of genes was analyzed using DESeq2 (version 1.24.0). Differentially expressed genes (DEGs) were determined as those with an adjusted P < 0.05 and a log2fold change > 1. Gene set enrichment analysis (GSEA) was performed using Broad Institute software (http://software.broadinstitute.org/gsea/index.jsp). Gene ontology (GO) analysis of the gene expression data obtained from MsigDB (http://www.gsea-msigdb.org/gsea/index.jsp) was performed using GSEA and visualized by the Enrichment Map tool in Cytoscape version 3.7.1 (https://cytoscape.org). The enrichment score for gene sets was calculated using a gene set variation analysis (GSVA) with the GSVA R package (version 1.32.0). Heatmaps were generated using the pheatmap package (version 1.0.12).
The cancer genome atlas (TCGA) data acquisition and definition of subgroups
RNA sequencing data and patient clinical information from the TCGA database were obtained for 9984 patients comprising 32 solid tumors using FireBrowse (Broad Institute). A “leukocytosis signature (LS) score” was calculated for each individual using GSVA and was defined as the enrichment score for the gene set derived from the genes differentially expressed between TRLpos and TRLneg tumors. In detail, the GSVA score for significantly down-regulated genes in the TRLpos tumor was subtracted from the GSVA score of significantly up-regulated genes in TRLpos tumor. Patients within the upper quartile of the LS score were defined as LShigh, with the remaining patients being defined as LSlow.
Immune signature and tumor immune infiltrate analysis
The signature scores for five immune expression signatures, namely the interferon (IFN)-γ response, the transforming growth factor (TGF)-β response, macrophage/monocytes, lymphocyte infiltration, and wound healing, were obtained from a previous paper [14]. These five immune signatures were selected from 160 immune expression signatures that robustly reproduced the co-clustering of the immune signature sets, and signature scores were calculated using GSVA. Data for the fraction of immune cell subsets infiltrating the tissue from the TCGA cohort were calculated using CIBERSORT [15]. The suppressive MDSC gene signature was derived from a previous study that identified the top 100 differentially expressed genes between suppressive MDSCs and monocytes sorted from peripheral blood mononuclear cells [16]. The T cell-inflamed gene expression profile, which is known to predict tumor response to anti-programmed death-1 (PD-1) therapy was calculated by GSVA using a previously reported gene set [17]. The neoantigen load for each patient from the TCGA cohort was obtained from a previous paper [14], which were determined from single-nucleotide variants and indel mutations that were predicted to result in Major histocompatibility complex binding peptides. The T cell receptor (TCR) and B cell receptor (BCR) diversities of patients from the TCGA cohort, which were measured using the Shannon entropy, were obtained from a previous report [14].
Statistical analysis
A Student’s t-test was performed to compare continuous variables between two groups. Survival curves were generated using Kaplan–Meier curves and compared using a log-rank test. A Cox regression was performed to determine the hazard ratio (HR) for the LS score on survival. A Pearson correlation analysis was performed to determine the correlation between two continuous variables. Two-sided P-values < 0.05 were considered significant. All statistical analyses were performed in R version 3.5.1 (http://www.r-project.org) or GraphPad Prism version 6.0 (GraphPad Software Inc., San Diego, CA).
Results
The characteristics of the four TRLpos patients and the four TRLneg patients are summarized in Table 1. TRLpos patients tended to have lower age, larger tumor, and more advanced tumor stage compared to TRLneg patients. In addition, TRLpos patients showed predominance of neutrophils among their leukocytes and a higher neutrophil-to-lymphocyte ratio (NLR) compared to TRLneg patients. All patients had received radiotherapy or chemoradiotherapy. Three of the four TRLpos patients experienced recurrence, while TRLneg patients experienced no recurrence. At last follow-up, two patients were alive among the four TRLpos patients and all TRLneg patients were alive.
Table 1.
Patient characteristics of those with TRL (n = 4) and without TRL (n = 4)
| Patient | Age/sex | Histology | Tumor size (cm) | FIGO stagea | TNM stageb | Leukocyte count (/μL) | ANC (/μL) | ALC (/μL) | NLR (/μL) | Treatment | Recurrence | PFS (m) | OS (m) | Last f/u status |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TRLpos 1 | 37/F | Squamous cell ca., non-keratinizing type | 7.0 | IIB (IIIB) | T2bN1 | 15,770 | 11,380 | 2650 | 4.29 | CCRT | None | 57.8 | 57.8 | Alive |
| TRLpos 2 | 35/F | Squamous cell ca., keratinizing type | 6.8 | IVA (IVA) | T4N1 | 9900 | 8470 | 530 | 15.98 | CCRT | Lung metastasis | 7.8 | 58.4 | Alive |
| TRLpos 3 | 66/F | Squamous cell ca., non-keratinizing type | 6.6 | IVA (IVA) | T4N1 | 11,850 | 9450 | 1660 | 5.69 | RT | Pelvic LN metastasis | 3.5 | 8.3 | Dead |
| TRLpos 4 | 28/F | Squamous cell ca., non-keratinizing type | 7.3 | IIA (IIIB) | T2a2N1 | 12,960 | 8250 | 2200 | 3.75 | CCRT | Lung metastasis | 7.5 | 16.5 | Dead |
| TRLneg 1 | 76/F | Squamous cell ca., non-keratinizing type | 1.9 | IIA1 (IIA1) | T2a1N0 | 6090 | 4200 | 1430 | 2.94 | CCRT | None | 57.8 | 57.8 | Alive |
| TRLneg 2 | 56/F | Squamous cell ca., non-keratinizing type | 5.0 | IB2 (IIIB) | T1b2N1 | 8010 | 3710 | 3340 | 1.11 | CCRT | None | 55.7 | 55.7 | Alive |
| TRLneg 3 | 75/F | Squamous cell ca., non-keratinizing type | 5.6 | IIB (IIIB) | T2bN1 | 8370 | 5300 | 2400 | 2.21 | CCRT | None | 60.8 | 60.8 | Alive |
| TRLneg 4 | 54/F | Squamous cell ca., non-keratinizing type | 3.1 | IIB (IIB) | T2bN0 | 7320 | 2970 | 3890 | 0.76 | CCRT | None | 69.4 | 69.4 | Alive |
TRL, tumor-related leukocytosis; ANC, absolute neutrophil count; ALC, absolute lymphocyte count; NLR, neutrophil-to-lymphocyte ratio; PFS, progression-free survival; OS, overall survival; CCRT, concurrent chemoradiotherapy; RT, radiotherapy; LN, lymph node
a2019 FIGO staging is provided in parenthesis
bAJCC 8th edition
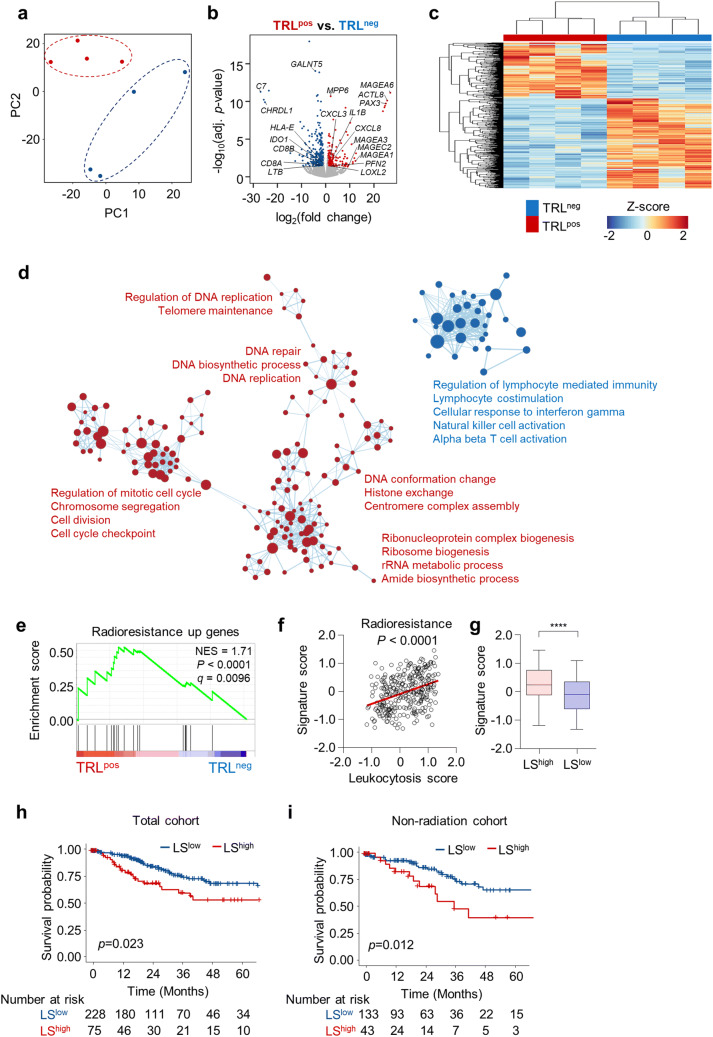
Next, we performed RNA sequencing of tumors obtained from four TRLpos and four TRLneg patients. Principal component analysis of TRLpos and TRLneg tumor gene expression showed distinct transcriptional landscapes (Fig. 1a). There were 591 differentially expressed genes in TRLpos tumors (log2fold change > 1, adjusted P < 0.05), of which 227 genes were significantly up-regulated and 364 genes were significantly down-regulated (Fig. 1b; Supplementary Table 1). Unsupervised hierarchical clustering confirmed that the TRLpos and TRLneg tumor gene signatures were distinct (Fig. 1c). Genes up-regulated in TRLpos tumors included those related to cancer–testis antigens (MAGEA1, MAGEA3, MAGEA6, MAGEC2, ACTL8), neutrophil chemotaxis (CXCL3, CXCL8), inflammatory cytokines (IL1B), and epithelial–mesenchymal transition (LOXL2, PFN2) [18], whereas down-regulated genes included those related to T cells (CD8A, CD8B), T-cell immune response (HLA-E, IDO1), and the complement pathway (C7) (Fig. 1b, Supplementary Table 1). GSEA using GO gene sets showed that TRLneg tumors exhibit enrichment in gene sets related to lymphocyte-mediated immunity, cellular response to IFN-γ, and T cell activation (Fig. 1d), while TRLpos tumors exhibit enrichment in gene sets related to DNA repair, replication, and cell cycle progression, implying the possibility of intrinsic radioresistance in TRLpos tumors (Fig. 1d). Supporting this, TRLpos tumors also showed enrichment in radioresistance-related genes (Fig. 1e) [19]. We further assessed the radioresistance signature in the TCGA cohort of uterine cervical cancer patients. Due to the lack of blood count data in the TCGA cohort and as indicative of TRL diagnosis, we calculated the LS score for each tumor, and defined LShigh and LSlow tumors as TRLpos and TRLneg tumors, respectively. The radioresistance signature score, derived from the radioresistance-related genes [19] by GSVA, showed a significant correlation with the LS (Fig. 1f), such that LShigh tumors exhibited a significantly higher radioresistance signature score than LSlow tumors (Fig. 1g). Patients with LShigh tumors had significantly lower survival rates than patients with LSlow tumors (Fig. 1h). Although TRLpos or LShigh tumors exhibited signatures for radioresistance, a high LS score was still associated with a poor prognosis in patients who did not receive radiotherapy (Fig. 1i), implying that the poor prognosis of patients with TRL may stem not only from the innate radioresistance of tumor cells but also from other factors.
Fig. 1.
TRLpos tumors exhibit a gene expression profile distinct from that of TRLneg tumors. a Principal component analysis of tumors from patients with (TRLpos; n = 4) and without (TRLneg; n = 4) TRL. b Volcano plot demonstrating the differentially expressed genes between the TRLpos and TRLneg tumors. The blue and red dots indicate significantly down- or up-regulated genes in TRLpos tumors, respectively (log2fold change > 1, adjusted P < 0.05). c Unbiased hierarchical clustering analysis of differentially expressed genes in TRLpos and TRLneg tumors. d Expression levels of gene ontology (GO) gene sets in TRLpos and TRLneg tumors. A gene set enrichment analysis (GSEA) was used to identify positive (red) and negative (blue) enrichment of GO gene sets in TRLpos tumors. The size of the nodes represents the number of genes in each pathway, and the links represent genes that are shared by two given pathways. Networks were generated using cytoscape. e GSEA of the radioresistance gene signature in TRLpos (n = 4) and TRLneg (n = 4) tumors. f Correlation plot between the LS score and radioresistance gene signature score in the TCGA uterine cervical cancer cohort (n = 303). Signature scores were calculated using a gene set variation analysis (GSVA). g Radioresistance gene signature scores in LShigh and LSlow tumors. The lines in the boxplot indicate median values, the boxes indicate the IQR values, and the whiskers extend to 1.5 × the IQR values. h, i Overall survival of patients with LSlow tumors and those with LShigh tumors in the whole TCGA cervical cancer cohort (h; n = 303) and patients who did not receive radiotherapy (i; n = 176). Student’s t-test (g), log-rank test (h, i). ****, P < 0.0001
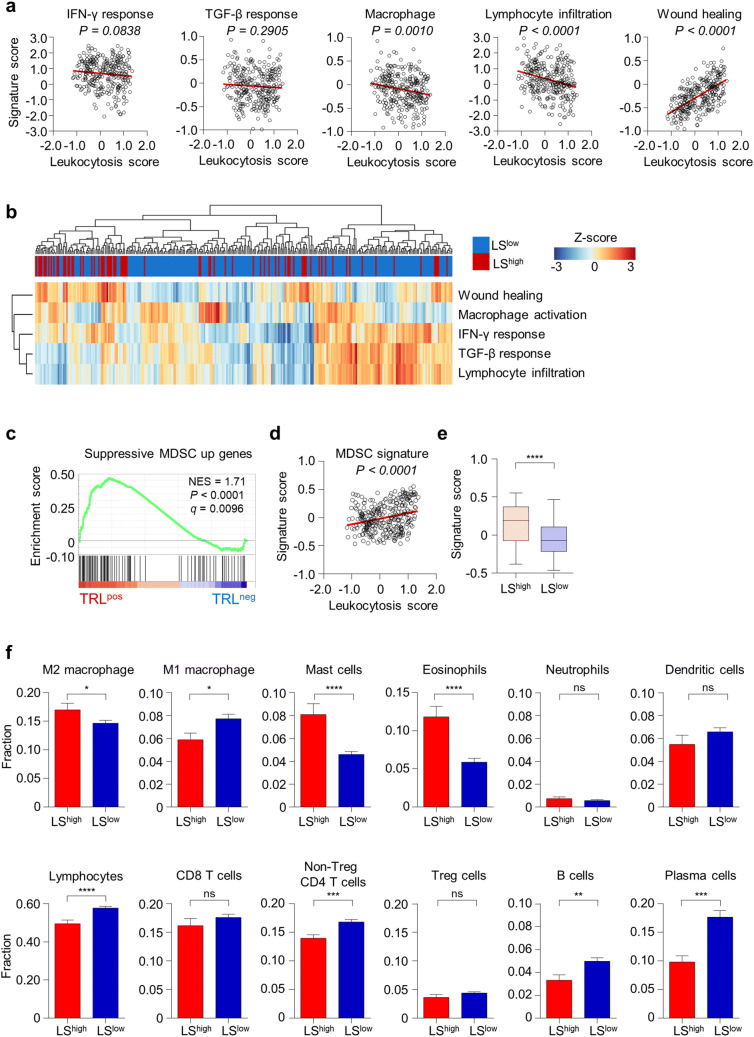
We next investigated the tumor immune microenvironment in uterine cervical cancer patients of the TCGA cohort based on the LS score. The LS score was significantly and negatively correlated with the macrophage/monocyte activation signature and the lymphocyte infiltration signature score and was significantly positively correlated with the wound healing signature score (Fig. 2a). A clustering analysis of LShigh and LSlow tumors also showed that the LShigh tumors were enriched in the cluster with a high wound healing signature score and low macrophage/monocyte activation, IFN-γ response, TGF-β response, and lymphocyte infiltration signature scores (Fig. 2b). However, there were some LShigh tumors that exhibited high IFN-γ response, TGF-β response, and lymphocyte infiltration signature scores, implying heterogeneity in the LShigh tumors (Fig. 2b). We next evaluated the degree of MDSC activation in the TRLpos and TRLneg tumors. The suppressive MDSC gene signature was significantly enriched in TRLpos tumors (Fig. 2c). In the TCGA cohort, the suppressive MDSC gene signature score was significantly correlated with the LS (Fig. 2d), and LShigh tumors exhibited higher suppressive MDSC gene signature scores than LSlow tumors (Fig. 2e). The LShigh tumors had a higher fraction of M2 macrophages, mast cells, and eosinophils and a lower fraction of M1 macrophages and lymphocytes (Fig. 2f). Among lymphocytes, the fractions of non-Treg CD4 T cells, B cells, and plasma cells were significantly higher in TRLneg tumors (Fig. 2f).
Fig. 2.
LShigh tumors have a more suppressive immune microenvironment than LSlow tumors. a Correlation between the LS score and signature scores for the IFN-γ response, the TGF-β response, macrophage, lymphocyte infiltration, and wound healing in the TCGA uterine cervical cancer cohort (n = 303). b Unbiased hierarchical clustering analysis for the signature scores for the IFN-γ response, the TGF-β response, macrophage, lymphocyte infiltration, and wound healing signatures in LShigh (n = 75) and LSlow (n = 228) tumors. c GSEA of the immunosuppressive MDSC gene signature in TRLpos (n = 4) and TRLneg (n = 4) tumors. d Correlation plot between the LS score and the immunosuppressive MDSC gene signature score (n = 303). e Immunosuppressive MDSC gene signature scores in the LShigh (n = 75) and LSlow (n = 228) tumors. The lines in the boxplot indicate median values, the boxes indicate the IQR values, and the whiskers extend to 1.5 × the IQR values. f The fraction of different immune cell subsets in the LShigh (n = 75) and LSlow (n = 228) tumors. The fractions of cells were derived from CIBERSORT. Bar graphs represent mean and s.e.m. Student’s t-test (e, f). ns, not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001
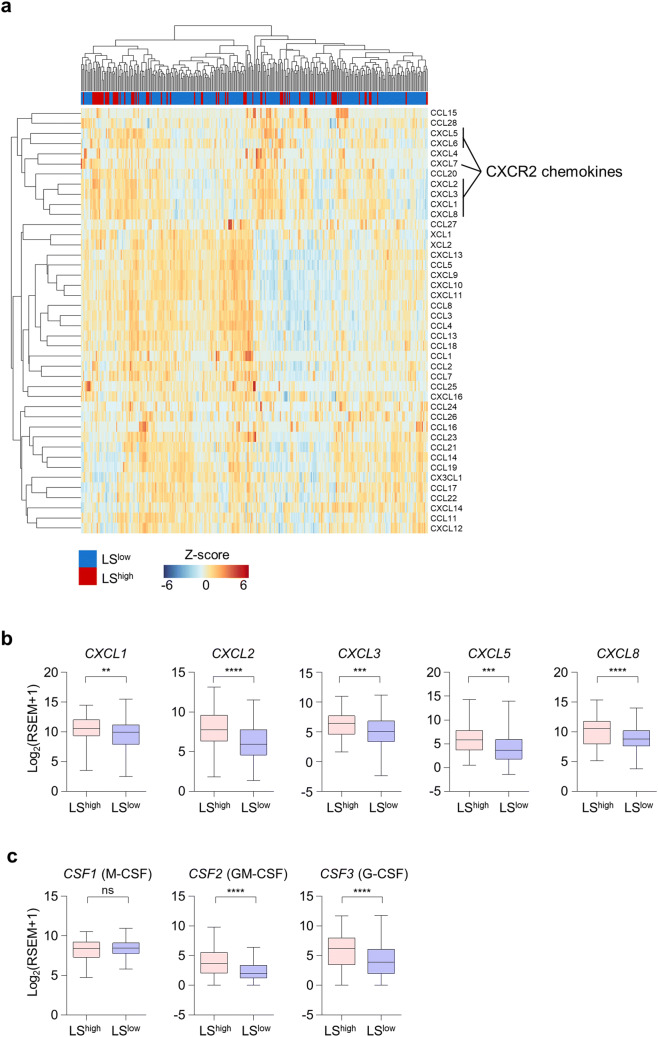
Given the importance of chemotaxis in the recruitment of immune cells to peripheral tissues, we next examined the expression of chemokines. An unsupervised hierarchical clustering of 42 chemokines in 303 uterine cervical tumors demonstrated the clustering of chemokines related to CXCR2, a chemokine receptor that mediates both neutrophil and MDSC trafficking (Fig. 3a). Moreover, the expression of the CXCR2 chemokines CXCL1, CXCL2, CXCL3, CXCL5, and CXCL8 was significantly higher in LShigh tumors (Fig. 3b). In addition to chemokines, we also examined the expression of growth factors for neutrophils or monocytes, such as CSF1 (M-CSF), CSF2 (GM-CSF), and CSF3 (G-CSF), and found that LShigh tumors had a significantly higher expression of CSF2 and CSF3, but not CSF1 compared to LSlow tumors (Fig. 3c). Thus, LShigh tumors showed the potential for enhanced chemotaxis of neutrophils or MDSCs to the tumor site and higher expression of neutrophil- or MDSC-inducing cytokines.
Fig. 3.
Chemokine expression in LShigh and LSlow tumors. a Unbiased hierarchical clustering analysis for gene expression of 42 types of chemokine in the LShigh (n = 75) and LSlow (n = 228) tumors in the TCGA uterine cervical cancer cohort. b Gene expression of selected CXCR2-related chemokines in LShigh (n = 75) and LSlow (n = 228) tumors. c Gene expression of CSF1, CSF2, and CSF3 in LShigh (n = 75) and LSlow (n = 228) tumors. The lines in the boxplot indicate median values, the boxes indicate the IQR values, and the whiskers extend to 1.5 × the IQR values. Student’s t-test (b, c). ns, not significant; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001
Considering the role of MDSCs in disrupting the effectiveness of immune checkpoint blockade, we next investigated the potential of TRLpos and TRLneg or LShigh and LSlow tumors to respond to anti-PD-1 therapy. The T cell-inflamed gene signature, which has been shown to predict responses to anti-PD-1 therapy in multiple types of cancer [17], was found to be significantly enriched in TRLneg tumors (Fig. 4a). The T cell-inflamed gene signature score was also higher in LSlow tumors, with a borderline significance (P = 0.0581; Fig. 4b). The neoantigen number, which is also known to be a predictive marker for anti-PD-1 therapy [20], was similar in the LShigh and LSlow tumors (Fig. 4c). TCR and BCR diversity was significantly lower in the LShigh tumors (Fig. 4d).
Fig. 4.
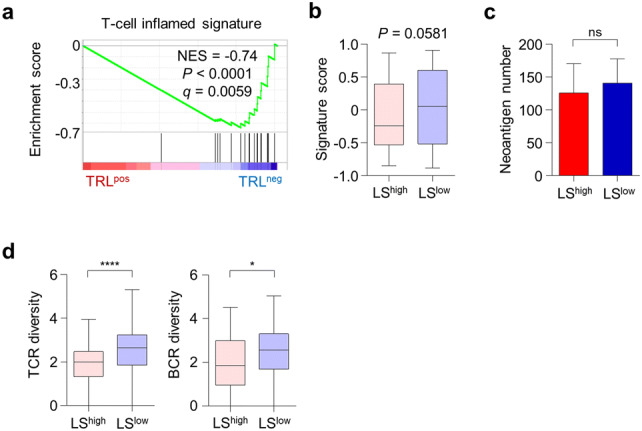
TRLpos and LShigh tumors exhibit features of anti-PD-1 unresponsiveness. a GSEA of the T-cell-inflamed gene signature in TRLpos and TRLneg tumors. b T-cell-inflamed gene signature scores in LShigh (n = 75) and LSlow (n = 228) tumors. c Neoantigen number in LShigh (n = 75) and LSlow (n = 228) tumors. d T-cell receptor (TCR) and B-cell receptor (BCR) diversity in LShigh (n = 75) and LSlow (n = 228) tumors. Diversity indicates the Shannon entropy score. The lines in the boxplot indicate the median values, the boxes indicate the IQR values, and the whiskers extend to 1.5 × the IQR values. Bar graphs represent mean and s.e.m. Student’s t-test (b-d). ns, not significant; *, P < 0.05; ****, P < 0.0001
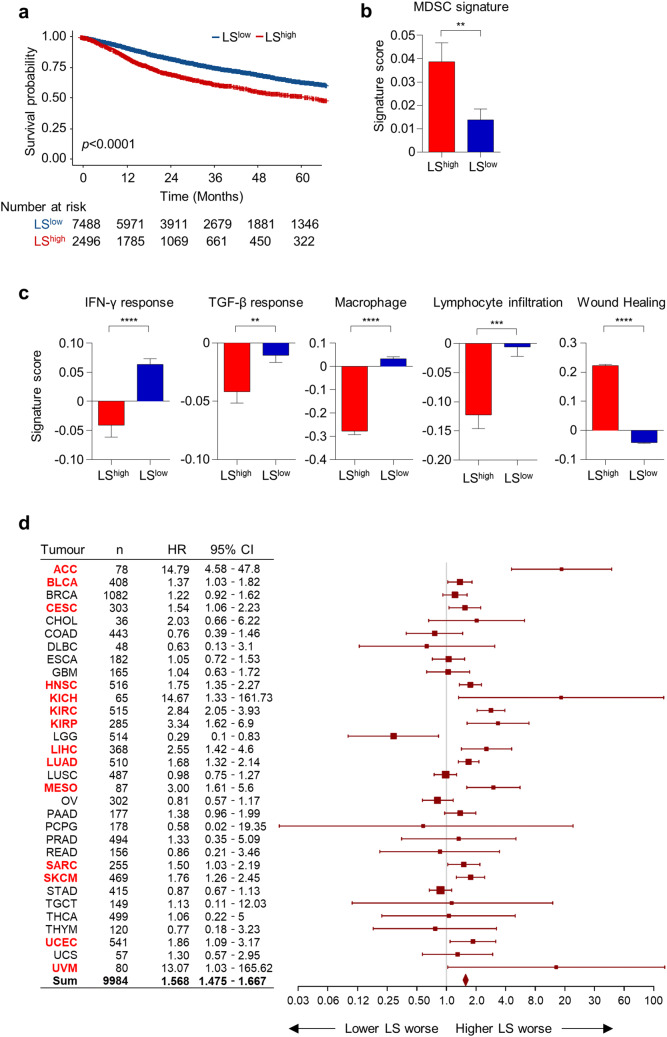
To extend our findings derived from patients with uterine cervical cancer, we compared the survival of patients with LShigh and LSlow tumors and the immune signatures of these tumors in the pan-cancer cohort of the TCGA database comprising 32 types of solid tumors and 9984 patients. Patients with LShigh tumors exhibited a significantly poorer overall survival compared to those with LSlow tumors (HR 1.57, 95% CI 1.48–1.67, P < 0.0001; Fig. 5a). LShigh tumors also had a significantly higher MDSC signature score than LSlow tumors (Fig. 5b). As seen in the TCGA uterine cervical cancer cohort, LShigh tumors exhibited a higher wound healing signature, but lower IFN-γ response, TGF-β response, macrophage/monocyte activation, and lymphocyte infiltration signatures (Fig. 5c). In terms of chemokine expression, LShigh tumors expressed significantly higher levels of CXCL1, CXCL2, CXCL3, CXCL5, and CXCL8 (Supplementary Fig. 1a). In addition, LShigh tumors expressed higher levels of CSF2 and CSF3 (Supplementary Fig. 1b). We next evaluated the prognostic impact of the LS score in the 32 types of solid tumors. A higher LS score was associated with poorer overall survival not only in uterine cervical cancer but also in adrenocortical carcinoma, bladder urothelial carcinoma, head and neck squamous cell carcinoma, kidney chromophobe, kidney renal clear cell carcinoma, kidney renal papillary cell carcinoma, liver hepatocellular carcinoma, lung adenocarcinoma, mesothelioma, sarcoma, skin cutaneous melanoma, uterine corpus endometrial carcinoma, and uveal melanoma (Fig. 5d).
Fig. 5.
Pan-cancer analysis of LShigh and LSlow tumors. a Overall survival of patients with LShigh and LSlow tumors in the TCGA pan-cancer cohort (n = 9984). b Immunosuppressive MDSC gene signature scores in the LShigh (n = 2496) and LSlow (n = 7488) tumors. c Signature scores for the IFN-γ response, the TGF-β response, macrophage, lymphocyte infiltration, and wound healing signatures in LShigh (n = 2496) and LSlow (n = 7488) tumors. d Forest plot demonstrating the hazard ratio and 95% CI of the LS in overall survival among 32 types of solid tumors. Tumor types with a significant association between overall survival and the LS are marked in red. Bar graphs represent mean and s.e.m. Student’s t-test (b, c). Cox regression analysis (d). **, P < 0.01; ***, P < 0.001; ****, P < 0.0001
Discussion
Although a large number of studies have demonstrated the prognostic impact of TRL in various cancer types and treatment settings [4–7], the microenvironment in human TRLpos tumors has not been well studied. Here, we comprehensively characterized the immune microenvironment of TRLpos tumors in patients with cervical cancer, as well as various other types of cancers. TRLpos and TRLneg tumors exhibited distinct transcriptional profiles and TRLpos tumors exhibited increased radioresistant signature, but reduced lymphocyte activation signature. We utilized DEGs from the TRLpos and TRLneg tumors to derive an LS score and defined LShigh and LSlow tumors in the TCGA cohort as representative of TRLpos and TRLneg tumors, respectively. Further analysis revealed that LShigh tumors exhibit a more suppressive immune microenvironment than LSlow tumors, characterized by higher MDSC and wound healing signatures and lower macrophage activation and lymphocyte infiltration signatures. Furthermore, we found that TRLpos or LShigh tumors had higher expression levels of CXCR2 chemokines than TRLneg or LSlow tumors, suggesting the CXCR2 axis may be a promising target for patients with TRL.
TRL in cervical cancer has been suggested as a distinct clinical entity with a lower response rate to radiation and a higher potential for metastasis or locoregional recurrence [5, 11]. As from the previous report [4], we found that patients with TRL tended to have lower age, larger tumor, and more advanced tumor stage than patients without TRL. Importantly, we found that TRLpos and TRLneg tumors have distinct transcriptional profiles. One of the features of TRLpos tumors was enrichment of radioresistant gene signatures. Extending these findings to the TCGA cohort, we found that patients with LShigh tumors also had a higher radioresistant signature and poorer survival than patients with LSlow tumors. However, a high LS score was also associated with poorer survival in patients treated without radiation, which implies that the poor prognosis of LShigh patients may not be solely attributed to radioresistance. As a result, we focused on examining the immune microenvironment.
TRLpos or LShigh tumors exhibited features of suppressive anti-tumor immunity. These tumors were enriched for suppressive MDSC gene signatures, implying that MDSCs are more likely to infiltrate human tumors associated with leukocytosis. In addition, not only MDSCs, but also M2 macrophages, mast cells, and eosinophils were more abundant in LShigh tumors. M2-polarized macrophages in tumors, or TAMs [9], mast cells [21], and eosinophils [22] are known to have pro-tumorigenic effects, which supports the suppressive anti-tumor immunity in patients with TRL. TAMs and mast cells are also key players in the chronic wound healing process [23], and we found that LShigh tumors exhibit an elevated wound healing process signature. The chronic wound healing process has remarkable similarities with tumor growth since during the wound healing process an immunosuppressive environment is established through the action of a diverse array of immune cells and cytokines [24]. LShigh tumors also showed a lower lymphocyte fraction, notably including non-Treg CD4 T cells, B cells, and plasma cells. A higher faction of plasma cells in tumors has been shown to be a predictor of favorable survival, which is associated with tertiary lymphoid structures (TLSs) in the cancer microenvironment [25]. TLSs are ectopic lymphoid organs that develop in non-lymphoid tissues that are composed of a T cell-rich zone and B cell follicle with germinal center characteristics, are surrounded by plasma cells, and represent a privileged site for tumor antigen presentation to T and B cells [26]. The presence of TLSs in tumors has been shown to be associated with favorable clinical outcomes [26].
Although the mechanism behind the formation of TRL in humans is yet to be revealed, preclinical studies have suggested that G-CSF secreted from tumor cells may play a role in inducing the generation of MDSCs from the bone marrow [4, 12]. Here, we also found that the expression of CSF2 (GM-CSF) and CSF3 (G-CSF) is elevated in LShigh tumors. In addition, we also examined the expression of chemokines, since chemotaxis is a crucial process for immune cell recruitment to peripheral tissues. We found that LShigh tumors have a higher expression of CXCR2 chemokines, such as CXCL1, CXCL2, CXCL3, CXCL5, and CXCL8 (IL-8). The CXCR2 chemokine axis has been shown to serve a central role in the recruitment of MDSCs leading to tumor progression, and blocking CXCR2 trafficking leads to enhanced anti-tumor effects of anti-PD-1 therapy in mouse tumor models [27, 28]. In humans, IL-8 has been suggested to be a potent chemotactic factor for MDSCs [29]. Therefore, targeting CXCR2 chemotaxis may be an effective strategy to improve the treatment outcome in patients with TRL. In human subjects, an anti-IL-8 antibody showed a favorable safety profile and reduction in serum IL-8 levels; however, there was no objective tumor response in patients with metastatic or unresectable solid tumors [30]. Although blocking the CXCR2 axis itself did not cause dramatic tumor reductions, it may be promising for combination with other treatments, such as radiotherapy, since local radiotherapy has been shown to enhance the production of IL-8 in tumors [31] and increase the frequency of MDSCs [32].
We found that TRLhigh or LShigh tumors exhibit a significantly lower T-cell-inflamed signature which is a well-established gene signature that predicts the response to anti-PD-1 therapy [17]. In addition, LSlow tumors exhibited a lower TCR and BCR diversity than LShigh tumors. A previous study has also shown that the diversity of peripheral blood T-cell repertoire is negatively correlated with NLR [33]. Furthermore, a higher TCR diversity has been associated with the response to immune checkpoint blockade [34]. Previous studies have also demonstrated that a high absolute neutrophil count or NLR, which is tightly associated with TRL, is associated with a poor response to anti-PD-1 therapy [35–37]. Taken together, tumors associated with leukocytosis are less likely to respond to immune checkpoint blockade and further combinatorial treatments may be needed.
We performed our initial analysis of TRLpos and TRLneg tumors in uterine cervical cancer patients, but found that the findings could be reproduced in multiple types of solid tumors. Cancer types that showed a significant association between a higher LS score and poor overall survival in our study have also been shown to have significant association between TRL or high NLR, with poor prognosis [4–7, 38–40]. However, a higher LS score was significantly associated with poorer survival, but not in all types of cancers. We, thus, suggest that TRL may not have the same prognostic impact in all types of cancers and that the mechanisms for this differential impact in various cancers should be the subject of future investigations.
The current study had some limitations. First, the blood count data lacked in the TCGA cohort, which limited the diagnosis of TRL. As an alternative, we used the DEGs obtained from TRLpos and TRLneg tumors as a surrogate to define TRL in patients in the TCGA cohort. Another limitation is that RNA was extracted from FFPE tissue sections; so as to overcome this limitation, we only used RNA samples that passed quality control tests to assure the accuracy of sequencing data. Moreover, a low number of patients were included for comparison of TRLpos and TRLneg tumors. In addition, TRLpos patients exhibited a heterogeneous clinical response. Although TRLpos patients are suggested to display a distinct clinical entity [4], one of the four patients was recurrence-free for over 4 years, while the others recurred within 1 year post-treatment. Future prospective studies utilizing prospectively collected fresh tissue with blood count data in a larger cohort will better define the immune landscape of tumors associated with leukocytosis.
In conclusion, tumors associated with leukocytosis exhibit a more suppressive immune microenvironment characterized by a higher wound healing signature accompanied by a higher infiltration of MDSCs and TAMs with lower lymphocyte infiltration compared to tumors not associated with leukocytosis. Our findings merit future clinical trials applying novel therapeutics based on the understanding of the distinct immune microenvironment of tumors associated with leukocytosis to improve the treatment outcomes of cancer patients with TRL.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- BCR
B cell receptor
- DEG
Differentially expressed gene
- FFPE
Formalin-fixed paraffin-embedded
- G-CSF
Granulocyte colony-stimulating factor
- GSEA
Gene set enrichment analysis
- GM-CSF
Granulocyte–macrophage colony-stimulating factor
- GO
Gene ontology
- GSVA
Gene set variation analysis
- IFN
Interferon
- LS
Leukocytosis signature
- M-CSF
Macrophage colony-stimulating factor
- MDSC
Myeloid-derived suppressor cells
- NLR
Neutrophil-to-lymphocyte ratio
- PD-1
Programmed death-1
- TAM
Tumor-associated macrophage
- TCGA
The cancer genome atlas
- TCR
T cell receptor
- TGF
Transforming growth factor
- TRL
Tumor-related leukocytosis
Author contributions
KHK and YBK contributed to the study concept and design. JSC and YBK involved in data acquisition. KHK, NSS, JSC, and YBK were involved in data analysis and interpretation. KHK drafted a preliminary version of the paper, and all authors reviewed and critically revised the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional Review Board of Severance Hospital (4-2015-0454) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mabuchi S, Matsumoto Y, Kawano M, Minami K, Seo Y, Sasano T, Takahashi R, Kuroda H, Hisamatsu T, Kakigano A, Hayashi M, Sawada K, Hamasaki T, Morii E, Kurachi H, Matsuura N, Kimura T. Uterine cervical cancer displaying tumor-related leukocytosis: a distinct clinical entity with radioresistant feature. J Natl Cancer Inst. 2014;106(7):dju147. doi: 10.1093/jnci/dju147. [DOI] [PubMed] [Google Scholar]
- 5.Cho Y, Kim KH, Yoon HI, Kim GE, Kim YB. Tumor-related leukocytosis is associated with poor radiation response and clinical outcome in uterine cervical cancer patients. Ann Oncol. 2016;27(11):2067–2074. doi: 10.1093/annonc/mdw308. [DOI] [PubMed] [Google Scholar]
- 6.Connolly GC, Khorana AA, Kuderer NM, Culakova E, Francis CW, Lyman GH. Leukocytosis, thrombosis and early mortality in cancer patients initiating chemotherapy. Thromb Res. 2010;126(2):113–118. doi: 10.1016/j.thromres.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin D, Rodel F, Winkelmann R, Balermpas P, Rodel C, Fokas E. Peripheral Leukocytosis Is Inversely Correlated with Intratumoral CD8 + T-Cell Infiltration and Associated with Worse Outcome after Chemoradiotherapy in Anal Cancer. Front Immunol. 2017;8:1225. doi: 10.3389/fimmu.2017.01225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar V, Patel S, Tcyganov E, Gabrilovich DI. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol. 2016;37(3):208–220. doi: 10.1016/j.it.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pathria P, Louis TL, Varner JA. Targeting tumor-associated macrophages in cancer. Trends Immunol. 2019;40(4):310–327. doi: 10.1016/j.it.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Ugel S, De Sanctis F, Mandruzzato S, Bronte V. Tumor-induced myeloid deviation: when myeloid-derived suppressor cells meet tumor-associated macrophages. J Clin Invest. 2015;125(9):3365–3376. doi: 10.1172/JCI80006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sasano T, Mabuchi S, Kozasa K, Kuroda H, Kawano M, Takahashi R, Komura N, Yokoi E, Matsumoto Y, Hashimoto K, Sawada K, Morii E, Kimura T. The highly metastatic nature of uterine cervical/endometrial cancer displaying tumor-related leukocytosis: clinical and preclinical investigations. Clin Cancer Res. 2018;24(16):4018–4029. doi: 10.1158/1078-0432.CCR-17-2472. [DOI] [PubMed] [Google Scholar]
- 12.Tavakkoli M, Wilkins CR, Mones JV, Mauro MJ. A novel paradigm between leukocytosis, g-csf secretion, neutrophil-to-lymphocyte ratio, myeloid-derived suppressor cells, and prognosis in non-small cell lung cancer. Front Oncol. 2019;9:295. doi: 10.3389/fonc.2019.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu X, Li YQ, Li QG, Ma YL, Peng JJ, Cai SJ. Baseline peripheral blood leukocytosis is negatively correlated with T-cell infiltration predicting worse outcome in colorectal cancers. Front Immunol. 2018;9:2354. doi: 10.3389/fimmu.2018.02354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thorsson V, Gibbs DL, Brown SD, Wolf D, Bortone DS, Ou Yang TH, Porta-Pardo E, Gao GF, Plaisier CL, Eddy JA, Ziv E, Culhane AC, Paull EO, Sivakumar IKA, Gentles AJ, Malhotra R, Farshidfar F, Colaprico A, Parker JS, Mose LE, Vo NS, Liu J, Liu Y, Rader J, Dhankani V, Reynolds SM, Bowlby R, Califano A, Cherniack AD, Anastassiou D, Bedognetti D, Rao A, Chen K, Krasnitz A, Hu H, Malta TM, Noushmehr H, Pedamallu CS, Bullman S, Ojesina AI, Lamb A, Zhou W, Shen H, Choueiri TK, Weinstein JN, Guinney J, Saltz J, Holt RA, Rabkin CE, Lazar AJ, Serody JS, Demicco EG, Disis ML, Vincent BG, Shmulevich L. The Immune Landscape of Cancer. Immunity. 2018;48(4):812–830. doi: 10.1016/j.immuni.2018.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, Hoang CD, Diehn M, Alizadeh AA. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12(5):453–457. doi: 10.1038/nmeth.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yaddanapudi K, Rendon BE, Lamont G, Kim EJ, Al Rayyan N, Richie J, Albeituni S, Waigel S, Wise A, Mitchell RA. MIF is necessary for late-stage melanoma patient MDSC immune suppression and differentiation. Cancer Immunol Res. 2016;4(2):101–112. doi: 10.1158/2326-6066.CIR-15-0070-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ayers M, Lunceford J, Nebozhyn M, Murphy E, Loboda A, Kaufman DR, Albright A, Cheng JD, Kang SP, Shankaran V, Piha-Paul SA, Yearley J, Seiwert TY, Ribas A, McClanahan TK. IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest. 2017;127(8):2930–2940. doi: 10.1172/JCI91190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang YN, Ding WQ, Guo XJ, Yuan XW, Wang DM, Song JG. Epigenetic regulation of Smad2 and Smad3 by profilin-2 promotes lung cancer growth and metastasis. Nat Commun. 2015;6:8230. doi: 10.1038/ncomms9230. [DOI] [PubMed] [Google Scholar]
- 19.Kim HS, Kim SC, Kim SJ, Park CH, Jeung HC, Kim YB, Ahn JB, Chung HC, Rha SY. Identification of a radiosensitivity signature using integrative metaanalysis of published microarray data for NCI-60 cancer cells. BMC Genom. 2012;13:348. doi: 10.1186/1471-2164-13-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348(6230):69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 21.Khazaie K, Blatner NR, Khan MW, Gounari F, Gounaris E, Dennis K, Bonertz A, Tsai FN, Strouch MJ, Cheon E, Phillips JD, Beckhove P, Bentrem DJ. The significant role of mast cells in cancer. Cancer Metastasis Rev. 2011;30(1):45–60. doi: 10.1007/s10555-011-9286-z. [DOI] [PubMed] [Google Scholar]
- 22.Reichman H, Karo-Atar D, Munitz A. Emerging roles for eosinophils in the tumor microenvironment. Trends Cancer. 2016;2(11):664–675. doi: 10.1016/j.trecan.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Schafer M, Werner S. Cancer as an overhealing wound: an old hypothesis revisited. Nat Rev Mol Cell Biol. 2008;9(8):628–638. doi: 10.1038/nrm2455. [DOI] [PubMed] [Google Scholar]
- 24.Galdiero MR, Marone G, Mantovani A. Cancer inflammation and cytokines. Cold Spring Harb Perspect Biol. 2018;10(8):a028662. doi: 10.1101/cshperspect.a028662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kroeger DR, Milne K, Nelson BH. Tumor-infiltrating plasma cells are associated with tertiary lymphoid structures, cytolytic T-cell responses, and superior prognosis in ovarian cancer. Clin Cancer Res. 2016;22(12):3005–3015. doi: 10.1158/1078-0432.CCR-15-2762. [DOI] [PubMed] [Google Scholar]
- 26.Sautes-Fridman C, Petitprez F, Calderaro J, Fridman WH. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat Rev Cancer. 2019;19(6):307–325. doi: 10.1038/s41568-019-0144-6. [DOI] [PubMed] [Google Scholar]
- 27.Steele CW, Karim SA, Leach JDG, Bailey P, Upstill-Goddard R, Rishi L, Foth M, Bryson S, McDaid K, Wilson Z, Eberlein C, Candido JB, Clarke M, Nixon C, Connelly J, Jamieson N, Carter CR, Balkwill F, Chang DK, Evans TRJ, Strathdee D, Biankin AV, Nibbs RJB, Barry ST, Sansom OJ, Morton JP. CXCR2 inhibition profoundly suppresses metastases and augments immunotherapy in pancreatic ductal adenocarcinoma. Cancer Cell. 2016;29(6):832–845. doi: 10.1016/j.ccell.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Highfill SL, Cui Y, Giles AJ, Smith JP, Zhang H, Morse E, Kaplan RN, Mackall CL. Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Sci Transl Med. 2014;6(237):237ra67. doi: 10.1126/scitranslmed.3007974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alfaro C, Teijeira A, Onate C, Perez G, Sanmamed MF, Andueza MP, Alignani D, Labiano S, Azpilikueta A, Rodriguez-Paulete A, Garasa S, Fusco JP, Aznar A, Inoges S, De Pizzol M, Allegretti M, Medina-Echeverz J, Berraondo P, Perez-Gracia JL, Melero I. Tumor-produced interleukin-8 attracts human myeloid-derived suppressor cells and elicits extrusion of neutrophil extracellular traps (NETs) Clin Cancer Res. 2016;22(15):3924–3936. doi: 10.1158/1078-0432.CCR-15-2463. [DOI] [PubMed] [Google Scholar]
- 30.Bilusic M, Heery CR, Collins JM, Donahue RN, Palena C, Madan RA, Karzai F, Marte JL, Strauss J, Gatti-Mays ME, Schlom J, Gulley JL. Phase I trial of HuMax-IL8 (BMS-986253), an anti-IL-8 monoclonal antibody, in patients with metastatic or unresectable solid tumors. J Immunother Cancer. 2019;7(1):240. doi: 10.1186/s40425-019-0706-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin L, Tao H, Karachi A, Long Y, Hou AY, Na M, Dyson KA, Grippin AJ, Deleyrolle LP, Zhang W, Rajon DA, Wang QJ, Yang JC, Kresak JL, Sayour EJ, Rahman M, Bova FJ, Lin Z, Mitchell DA, Huang J. CXCR1- or CXCR2-modified CAR T cells co-opt IL-8 for maximal antitumor efficacy in solid tumors. Nat Commun. 2019;10(1):4016. doi: 10.1038/s41467-019-11869-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parikh F, Duluc D, Imai N, Clark A, Misiukiewicz K, Bonomi M, Gupta V, Patsias A, Parides M, Demicco EG, Zhang DY, Kim-Schulze S, Kao J, Gnjatic S, Oh S, Posner MR, Sikora AG. Chemoradiotherapy-induced upregulation of PD-1 antagonizes immunity to HPV-related oropharyngeal cancer. Cancer Res. 2014;74(24):7205–7216. doi: 10.1158/0008-5472.CAN-14-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu YY, Yang QF, Yang JS, Cao RB, Liang JY, Liu YT, Zeng YL, Chen S, Xia XF, Zhang K, Liu L. Characteristics and prognostic significance of profiling the peripheral blood T-cell receptor repertoire in patients with advanced lung cancer. Int J Cancer. 2019;145(5):1423–1431. doi: 10.1002/ijc.32145. [DOI] [PubMed] [Google Scholar]
- 34.Postow MA, Manuel M, Wong P, Yuan J, Dong Z, Liu C, Perez S, Tanneau I, Noel M, Courtier A, Pasqual N, Wolchok JD. Peripheral T cell receptor diversity is associated with clinical outcomes following ipilimumab treatment in metastatic melanoma. J Immunother Cancer. 2015;3:23. doi: 10.1186/s40425-015-0070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanizaki J, Haratani K, Hayashi H, Chiba Y, Nakamura Y, Yonesaka K, Kudo K, Kaneda H, Hasegawa Y, Tanaka K, Takeda M, Ito A, Nakagawa K. Peripheral blood biomarkers associated with clinical outcome in non-small cell lung cancer patients treated with nivolumab. J Thorac Oncol. 2018;13(1):97–105. doi: 10.1016/j.jtho.2017.10.030. [DOI] [PubMed] [Google Scholar]
- 36.Bagley SJ, Kothari S, Aggarwal C, Bauml JM, Alley EW, Evans TL, Kosteva JA, Ciunci CA, Gabriel PE, Thompson JC, Stonehouse-Lee S, Sherry VE, Gilbert E, Eaby-Sandy B, Mutale F, DiLullo G, Cohen RB, Vachani A, Langer CJ. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer. 2017;106:1–7. doi: 10.1016/j.lungcan.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 37.Ameratunga M, Chenard-Poirier M, Moreno Candilejo I, Pedregal M, Lui A, Dolling D, Aversa C, Ingles Garces A, Ang JE, Banerji U, Kaye S, Gan H, Doger B, Moreno V, de Bono J, Lopez J. Neutrophil-lymphocyte ratio kinetics in patients with advanced solid tumours on phase I trials of PD-1/PD-L1 inhibitors. Eur J Cancer. 2018;89:56–63. doi: 10.1016/j.ejca.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 38.Templeton AJ, McNamara MG, Seruga B, Vera-Badillo FE, Aneja P, Ocana A, Leibowitz-Amit R, Sonpavde G, Knox JJ, Tran B, Tannock IF, Amir E. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6):124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 39.Schernberg A, Huguet F, Moureau-Zabotto L, Chargari C, Rivin Del Campo E, Schlienger M, Escande A, Touboul E, Deutsch E. External validation of leukocytosis and neutrophilia as a prognostic marker in anal carcinoma treated with definitive chemoradiation. Radiother Oncol. 2017;124(1):110–117. doi: 10.1016/j.radonc.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 40.Sebastian N, Wu T, Bazan J, Driscoll E, Willers H, Yegya-Raman N, Bond L, Dwivedi A, Mo X, Tan Y, Xu-Welliver M, Haglund K, Jabbour SK, Keane FK, Williams TM. Pre-treatment neutrophil-lymphocyte ratio is associated with overall mortality in localized non-small cell lung cancer treated with stereotactic body radiotherapy. Radiother Oncol. 2019;134:151–157. doi: 10.1016/j.radonc.2019.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.