Abstract
Prostate cancer is a candidate for immunotherapy because cancer cells express tissue-specific proteins that can be therapeutic targets. However, immune checkpoint inhibitors and active immunization have performed poorly in clinical trials. We developed a novel virus-like particle (VLP) vaccine composed of bovine papillomavirus L1 protein engineered to display surface docking sites. We decorated VLPs with peptides encoding T cell epitopes from two prostate cancer-associated tumor antigens, prostate stem cell antigen (PSCA), and prostatic acid phosphatase (PAP-1 and PAP-2), and a neo-antigen, stimulator of prostatic adenocarcinoma-specific T cells (SPAS-1). The VLP vaccines induced a mean frequency of antigen-specific IFN-γ secreting CD8 + T cells of 2.9% to PSCA, 9.5% to SPAS-1, 0.03% to PAP-1, and 0.03% to PAP-2 in tumor-bearing TRAMP mice. We treated TRAMP mice at 19–20 weeks of age, when mice have advanced stages of carcinogenesis, with either VLP vaccine, anti-PD1 antibody, or combination immunotherapy. The VLP vaccine alone or in combination with anti-PD1 antibody significantly reduced tumor burden, while anti-PD1 antibody had a modest non-significant therapeutic effect. All treatments significantly increased CD3 + and CD8 + T cell infiltration into tumor tissue compared to control mice, and combination therapy resulted in significantly greater CD3 + and CD8 + T cell infiltration than monotherapy. Reduction in tumor burden in vaccine-treated mice was inversely correlated with CD8 + T cell numbers in tumor tissue. No other immunotherapy has shown efficacy in this animal model of advanced prostate cancer, making bovine papillomavirus VLPs an attractive vaccine technology to test in patients with metastatic prostate cancer.
Electronic supplementary material
The online version of this article (10.1007/s00262-020-02493-z) contains supplementary material, which is available to authorized users.
Keywords: Bovine papillomavirus, VLP vaccine, Prostate cancer, Immune checkpoint inhibitor, TRAMP mice, Antigen-specific CD8 + T cells
Introduction
Prostate cancer (PC) is the second most commonly diagnosed cancer in men and a major cause of mortality [2]. Prostate cancer is a candidate for immunotherapy because PC cells express tissue-specific proteins that could act as therapeutic targets [3]. The FDA-approved cell-based prostate cancer vaccine, Sipuleucel-T, has modest benefit but presents feasibility challenges in clinical practice, since it requires ex vivo processing of patient cells [4, 5]. The performance of immune checkpoint inhibitors in prostate cancer patients has been disappointing [6, 7]. Active vaccination with cell-based, viral vector-based, and plasmid DNA vaccines has yielded disappointing results to date [8–14].
As a novel approach to active immunization, we developed a virus-like particle (VLP) vaccine and tested the vaccine in a stringent prostate cancer animal model, late stage carcinogenesis in the TRAMP mouse model of spontaneous prostate cancer. We tested the vaccine alone and in combination with anti-PD-1 antibody, the most commonly used checkpoint inhibitor in clinical oncology.
Material and methods
Production of bovine papillomavirus virus (BPV)-like particles and formulation of prostate cancer antigen BPV VLP vaccine
A BPV L1 capsid protein ORF was constructed, as described previously [15], with insertion of nucleotides encoding a peptide with eight glutamic acids and a cysteine in the HI surface-exposed loop (Supplementary Table S1). A recombinant baculovirus was used to infect High Five insect cells. Cells were resuspended in extraction buffer (20 mM phosphate buffer, pH 6.5, 1 M NaCl, 0.1 mM CaCl2, 50 μm FeCL2) containing protease inhibitors (Roche cOmplete ULTRA, 1 tablet per 10 ml) and subjected to 5 cycles of thawing at 37 °C and freezing in a − 80 °C ethanol bath. The lysate was spun 1 h at 8000 rpm to remove baculovirus particles. The clarified lysate was extracted for 10 min with an equal volume of Vertrel DF (Fisher Scientific). The aqueous layer was loaded onto a 40% sucrose cushion and centrifuged in a SW32Ti rotor at 32,000 rpm for 1.5 h. The sucrose pellet was resuspended in 20 mM phosphate pH 8.0, 0.5 M NaCl, 5 mM MgCl2, and incubated 30 min at 37 °C with 250 U/ml of Salt Active Nuclease (Arcticzymes). After dialysis in 20 mM phosphate pH 6.5, 0.5 M NaCl, the VLP solution was adjusted to 0.01% Tween 80, 0.05% carboxymethyl cellulose, 50 μm FeCL2 and stored at 4 °C Purity was assessed by SDS-PAGE gel analysis and protein concentration was measured by Bradford dye method and uv spectroscopy.
For prostate tumor antigens, we synthesized peptides of 9-amino acids encoding MHC class I-Kb or Db-restricted epitopes from murine prostate stem cell antigen (mPSCA), murine prostatic acid phosphatase (mPAP), or stimulator of prostatic adenocarcinoma-specific T-cells-1 (SPAS-1). Antigens were selected based on functional characterization as tumor antigens and knowledge of the amino acid sequence of T cell epitopes in a C57BL/6 genetic background [16–18]. Each peptide had an N-terminal tag composed of eight arginines flanked by cysteines and followed by two alanines and a tyrosine (AAY) (Supplementary Table 2). The polyarginine-cysteine residues allow docking of peptide to the polyglutamic acid-cysteine site on the surface of the VLP. The AAY sequence is a processing signal for proteases active in MHC class I presentation [19].
For conjugation to the VLP, peptides were solubilized in distilled water at 5 mg/ml (PAP-2 peptide was dissolved at 5 mg/ml in DMSO). Peptides at concentrations between 0.8 and 2.5 mg/ml were reduced with 10 mM Bond-breaker TCEP solution (Thermo Fisher Scientific) for 20 min at 50 °C. After dialysis in 20 mM phosphate buffer, pH 6.5, 0.15 M NaCl, VLP protein (1 mg/ml), and peptide at peptide:L1 protein molar ratios between 4:1 to 8:1 were mixed in the presence of 4 mM glutathione disulfide (GSSG) and 0.8 mM reduced glutathione (GSH), and incubated overnight at 37 °C. To remove unreacted peptide, reactants were dialyzed against 20 mM phosphate pH 6.5, 0.5 M NaCl. The vaccine solutions were adjusted to 0.01% Tween 80, 0.05% carboxymetyl cellulose, 0.5 mM GSSG and 0.05 mM GSH, aliquoted, and stored at − 20 °C. The amount of peptide bound to the VLP was determined by SDS-PAGE analysis and interpolation of sample-peptide band density from a standard curve of known amounts of peptide. Gels were scanned in a BioRad ChemiDocXR imager, and images were analyzed with NIH ImageJ software.
Prostate tumor antigen BPV VLP Vaccination
TRAMP mice 19–20 weeks of age were immunized three times, 1 week apart with 60 ug each of mPSCA, mPAP-1, mPAP-2, and SPAS-1 decorated VLPs, administered in doses of 20 ug by intramuscular, intradermal, and intravenous injections. The intramuscular dose was given into the thigh muscle. The intradermal dose was injected in split doses into the skin of the back of shaved mice. The four vaccines were administered at separate sites. The intravenous dose was administered via the tail vein. As a control, TRAMP mice were immunized with 60 ug of unlabeled VLP protein (empty VLP) administered as described above. TRAMP mice were also treated with three weekly intraperitoneal injections of 200 ug of InVivoMAB anti-mouse PD-1 (CD279), clone RMP1-14 (Bio-X-Cell) starting with the second dose of vaccine, or with three weekly doses of anti-PD-1 antibody alone beginning at 20 weeks of age. Untreated TRAMP mice served as a control for spontaneous tumor burden.
Assessment of tumor burden
At 26 weeks of age, TRAMP mice were sacrificed and the male genitourinary tract was isolated and prostate tissue was separated from bladder, seminal vesicles, and other tissue. Total prostate weight was determined by weighing all prostate lobes and any prostate tumors that were present. The prostate draining lymph nodes and other abdominal organs were inspected for the presence of metastases. The tissue was fixed in 10% neutral buffered formalin.
Immunohistochemistry
Immunohistochemical staining was performed on 5 μm formalin-fixed and paraffin-embedded tissue sections. Slides were deparaffinized and rehydrated and endogenous peroxidases were quenched with BLOXALL (Vector Labs). After blocking with Normal Horse Serum, slides were incubated with primary antibody directed against CD3 or CD8 (Cell Signaling Technology mAb #99940 or #98941). Staining was visualized with secondary antibody ImmPRESS HRP Polymer detection kit and ImmPACT DAB (Vector Labs). Images were taken of four 20X fields, converted into standard TIFF format, and analyzed visually for number of CD3 + or CD8 + T cells. Results are expressed as the average for the four fields.
Intracellular cytokine stain flow cytometry
For immunological assays, mice were sacrificed 10–14 days after the last dose of vaccine. Splenocytes were stimulated with 1 ug/ml of peptide in the presence of brefeldin A (10 ug/ml) overnight at 37 °C in 5% CO2. Cells were stained with Zombie green™ fixable viability dye, treated with Fixation buffer and stored in Cytolast. Cells were permeabilized with Permeabilization buffer and stained with Brilliant Violet BV™ 510-conjugated anti-mouse CD3, clone 17A2, PerCPCy5.5-conjugated anti-mouse CD8α, clone 53–6.7, and PE-conjugated anti-mouse IFNγ, clone XMG1.2. Reagents were purchased from Biolegend. Flow cytometry was performed on an LSR-II flow cytometer and data were analyzed using FACSDiva software. Gating was done on forward and side scatter parameters to select for lymphocytes and singlets. After exclusion of dead cells, CD8 + T lymphocytes were identified on a CD3/CD8 dot plot of gated lymphocytes, and interferon-γ (IFNγ) secreting cells were identified on a CD8/IFNγ dot plot of gated CD8 + T cells. A minimum of 30,000 CD8 + T cells were analyzed.
Tetramer staining
Unstimulated splenocytes were stained with Zombie green™ fixable viability dye, Brilliant Violet BV™ 510-conjugated anti-mouse CD3, and PerCPCy5.5-conjugated anti-mouse CD8α, and MHC class I H-2Db SPAS-1 peptide (STHVNHLHC) tetramer labeled with APC for 1 h on ice in the dark. Cells were fixed and then resuspended in cell staining buffer for analysis by flow cytometry. Gating was done as above and tetramer positive cells were identified on a CD8/tetramer dot plot of gated CD8 + T cells.
Statistical analysis
Prostate weights were compared by the Mann–Whitney rank sum test. Numbers of CD3 and CD8 positive T cells per high-powered field among experimental groups were compared by t test. Frequencies of antigen-specific T cell responses compared to that of unstimulated cultures were analyzed by t test.
Results
Design features of a BPV VLP vaccine
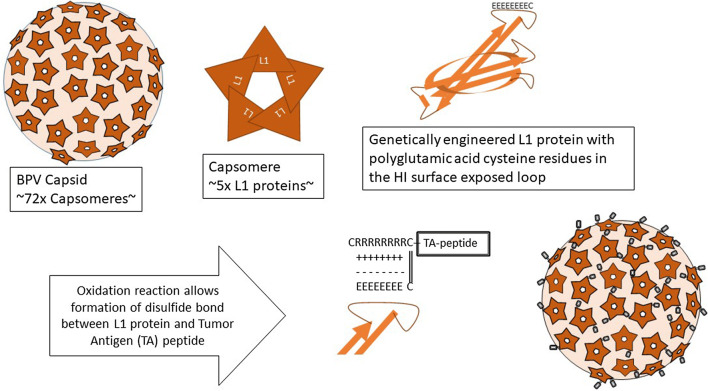
The particles are composed of bovine papillomavirus L1 protein. In order to construct a VLP that can indiscriminately accommodate peptide antigens, we genetically engineered the L1 protein by inserting eight glutamic acid residues and a cysteine residue, replacing nine native amino acids, in a surface-exposed location designated the HI loop [15]. Expression of chimeric L1 protein from a recombinant baculovirus in insect cells results in spontaneous assembly into a VLP displaying the polyglutamic acid motif on the surface. The glutamic acids serve as a docking sites for attachment of protein/peptide antigens with 8N-terminal polyarginines and flanking cysteine residues. Antigens are brought into contact with the particle by electrostatic interactions between the arginine and glutamic acid residues, and the peptide is covalently bound to the VLP by a disulfide bond in an oxidation reaction. A schematic diagram of the formulation of a generic VLP cancer vaccine is shown in Fig. 1.
Fig. 1.
Schematic diagram of formulation of VLP vaccines. Bovine papillomavirus VLPs are 50 nm particles composed of 360 molecules of L1 proteins that form capsomeres of 5 L1 proteins and capsids of 72 capsomeres. The L1 protein was engineered to contain 8 glutamic acids and a cysteine replacing 9 native amino acids at the tip of a surfaced exposed loop, designated the HI loop. Peptides or proteins with an N-terminal tag of 8 arginines flanked by cysteines are brought into contact with the VLP by electrostatic interactions between the glutamic acid and arginine residues and bound to the VLP by disulfide bonds under oxidizing conditions
Peptide content of prostate tumor antigen BPV VLP vaccines
In order to determine the amount of peptide bound to the VLP, serial dilutions of peptide and VLP vaccine were subjected to SDS-PAGE, Coomassie blue staining, and scanning densitometry. SDS-PAGE analysis showed that particle preparations were > 90% pure. Therefore, the amount of particles (in μg of protein) was assumed to represent the amount of L1 protein. L1 has a theoretical MW of 55.7 kDa. The N-terminal tagged PAP-1, PAP-2, PSCA, and SPAS-1 peptides have theoretical MWs of 2.82, 3.04, 2.96, and 2.97 kDa, respectively. Peptide/L1 mass ratios of ~ 19:1 were assumed to represent a 1:1 molar ratio. For the PSCA peptide VLP vaccine, each microgram of VLP had ~ 90 nanograms of conjugated peptide, corresponding to an peptide:L1 mass ratio of 11 or ~ 1.7 peptides per L1 molecule and ~ 600 peptides per VLP particle (Supplementary Figure S1). The number of peptides per VLP particle for PAP-1, PAP-2, and SPAS-1 vaccines was 800, 550, and 120, respectively (Supplementary Figures S2–4). The attachment of more than one peptide per L1 molecule is possible because each peptide antigen contains two cysteine residues allowing for formation of multimeric peptide antigens during the conjugation reaction.
Immunogenicity of prostate tumor antigen BPV VLP vaccine in C57BL/6 mice
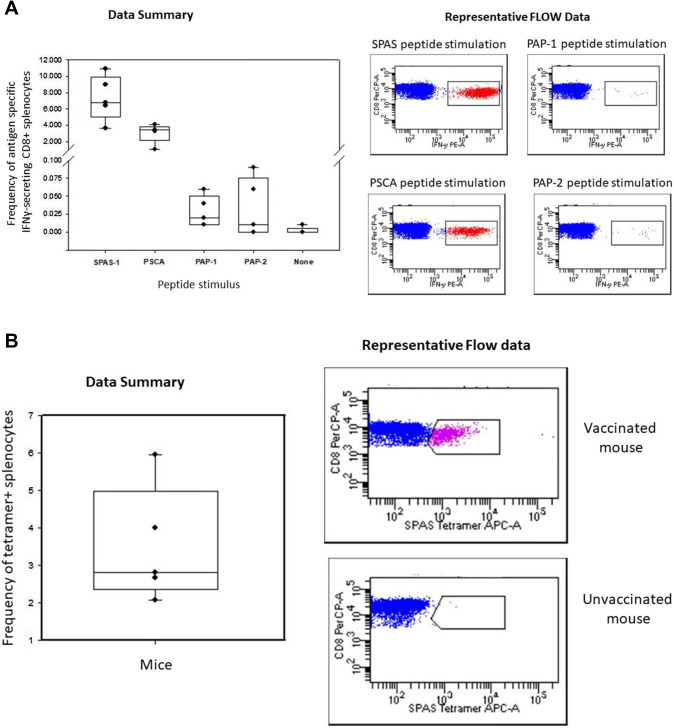
Immunogenicity of the VLP vaccines in wild-type C67BL/6 mice was determined by intracellular IFN-γ cytokine stain flow cytometry of splenocytes harvested 2 weeks post vaccination (Fig. 2a). Mice mounted a robust response to PSCA and SPAS-1, with a mean frequency of IFN-γ-secreting CD8 + T cells of 3.0% and 7.3%, respectively. Responses to PAP-1 and PAP-2 were only slightly above background levels, with a mean frequency of IFN-γ-secreting CD8 + T cells of 0.03% and 0.03%, respectively. As an additional assessment of antigen specificity, unstimulated splenocytes were stained with a SPAS-1 peptide tetramer (Fig. 2b). The mean frequency of tetramer positive cells was 3.5%.
Fig. 2.
Frequency of antigen-specific CD8 + splenocytes 14 days after vaccination of C57BL/6 mice with prostate tumor antigen bovine VLP vaccines. a Splenocytes from vaccinated mice (n = 5 per group) were stimulated overnight with peptide, and the frequency of antigen-specific IFNγ-secreting CD8 + T cells determined by intracellular cytokine flow assay. The left panel shows results presented in a box plot with a superimposed scatter plot of individual data points. The right panel shows representative CD8/IFNγ dot plots generated in DIVA software. With the exception of the response to PAP-2, antigen-specific response were significantly higher than that of background unstimulated cultures (t test, P < 0.05). b Splenocytes from vaccinated mice were surface stained for CD3 and CD8 and with a SPAS-1 peptide MHC complex. The left panel shows results presented in a box plot with a superimposed scatter plot of individual data points. The right panel shows representative CD8/tetramer dot plots of a vaccinated and unvaccinated mouse
Immunogenicity of prostate tumor antigen BPV VLP vaccine in TRAMP mice with advanced stage of carcinogenesis
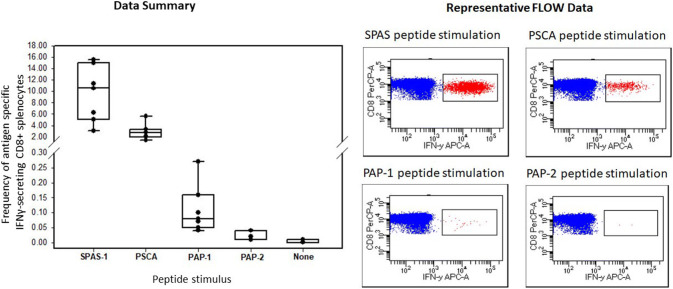
The ability of VLP vaccination to induce antigen-specific CD8 + T cell responses in 14- to 18-week-old tumor-bearing TRAMP mice (n = 7) was also evaluated (Fig. 3). Responses were comparable to that observed in wild-type C57BL/6 mice, with mean frequencies of IFN-γ-secreting PSCA and SPAS-1 antigen-specific CD8 + T cells of 2.9% and 9.5%, respectively. Tumor-bearing TRAMP mice had weak responses to PAP-1 and PAP-2 with mean frequencies of IFN-γ-secreting CD8 + T cells of 0.11% and 0.02%, respectively.
Fig. 3.
Frequency of IFNγ-specific CD8 + splenocytes 14 days after vaccination of 14–18-week-old tumor-bearing TRAMP mice with prostate tumor antigen-VLP vaccines. Splenocytes from vaccinated mice (n = 7) were tested by intracellular cytokine stain, as described in the legend to Fig. 2a. All antigen-specific response were significantly higher than that of background unstimulated cultures (t test, P < 0.05)
Efficacy of prostate tumor antigen BPV VLP vaccination of TRAMP mice with advanced stage of carcinogenesis
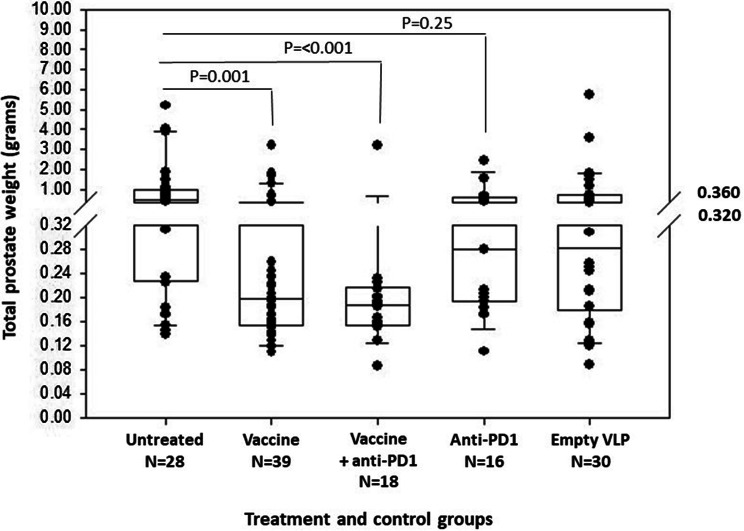
The ability of the prostate tumor antigen BPV VLP vaccines alone or in combination with anti-PD1 checkpoint inhibitor therapy to inhibit tumor growth was evaluated in 20-week-old TRAMP mice. In a pilot study, 21 TRAMP mice were immunized and euthanized at 26 weeks of age. Prostate tissue was dissected and weighed. As compared to unvaccinated control TRAMP mice (n = 28), the VLP vaccines significantly reduced median total prostate weight [vaccine group: median and interquartile range (IQR), 0.187 g (0.155–0.235 g); untreated controls: median (IQR) 0.500 g, (0.228–0.9564 g); Mann–Whitney rank sum test, P = 0.002) (Supplementary Figure S5). Following these results, a study was conducted comparing vaccine alone (n = 18) versus combination immunotherapy with three weekly 200 μg doses of anti-PD1 antibody starting with the second dose of vaccine (n = 18) or three weekly doses of anti-PD1 antibody alone beginning at 20 weeks of age (n = 15). Because the VLPs have endogenous immunostimulatory properties, a control cohort of TRAMP mice (n = 30) was treated with VLPs that were not decorated with prostate tumor antigens (empty VLPs). Because mice came from our breeding colony, they were used for these experiments as they matured to 20 weeks of age. Mice were assigned randomly and alternately to the four experimental groups over the course of 12 months. One batch of vaccine was used for the pilot study and another for the study comparing various treatments. There was no significant difference in vaccine efficacy between mice treated with the two batches of vaccines [initial batch, median (IQR) prostate weight, 0.187 g (0.155–0.255 g) versus second batch, median (IQR) prostate weight, 0.211 g (0.141–0.464 g); Mann–Whitney rank sum test, P = 0.490] (Supplementary Figure S5). Efficacy data from mice treated with the two batches of vaccine, that is, data from the pilot study and the subsequent study comparing vaccine to combination immunotherapy, were combined in the final analysis. The prostate tumor antigen BPV VLP vaccines alone (n = 21 from pilot study and n = 18 from vaccine only in the comparison study, total of n = 39 mice) significantly reduced prostate weight compared to control mice (n = 28). The median (IQR) prostate weight for vaccinated mice was 0.198 g (0.155–0.372 g), as compared to a median (IQR) prostate weight for untreated mice of 0.500 g (0.228–0.954 g) (Mann–Whitney rank sum test, p = 0.001) (Fig. 4). The median (IQR) prostate weight of mice treated with anti-PD1 alone, 0.281 g (0.194–0.608 g), was lower than that of controls, but the difference was not statistically significant (Mann–Whitney rank sum test, P = 0.25) (Fig. 4). The combined treatment with BPV VLP vaccine and anti-PD1 also significantly reduced median (IQR) prostate weight, 0.188 g (0.155–0.217 g), compared to the untreated control group (Mann–Whitney rank sum test, p < 0.001) (Figs. 4, 5). Although the median (IQR) prostate weight was lower for the combination therapy than that of the vaccine alone, the difference was not statistically significant (Mann–Whitney rank sum, P = 0.220). 89% of the mice (16/18) in the combination therapy group had total prostate weights within the normal range for C57BL/6 mice (0.15–0.25 g). Administration of empty VLPs had no statistically significant therapeutic effect: median (IQR) prostate weight, 0.283 g (0.179–0.720 g), as compared to the untreated controls (Mann Whitney rank sum test, P = 0.228). Prostate histology was similar in all groups, with diffuse high-grade PIN replacing normal prostate acini. In some mice, poorly differentiated carcinoma was present that replaced the bulk of the prostate tissue in larger tumors.
Fig. 4.
Prostate tumor antigen bovine VLP vaccine protects 19- to 20-week-old TRAMP mice against tumor growth. 20-week-old TRAMP mice were either vaccinated (Vaccine), treated with anti-PD-1 (Anti-PD-1), or vaccinated and treated with anti-PD-1 (Vaccine + anti-PD-1). Controls consisted of untreated mice (Untreated) or mice vaccinated with VLPs that were not decorated with antigen (Empty VLP). At 26 weeks of age, TRAMP mice were sacrificed, and their prostate dissected and weighed. Results are presented in a box plot with a superimposed scatter plot of individual data points. A break was made in the scale between 0.320 and 0.360 g in order to allow for an expanded depiction of data points below 0.32 g. No data points are excluded. Statistical differences (Mann Whitney rank sum test) between untreated controls and treatment groups are shown
Fig. 5.
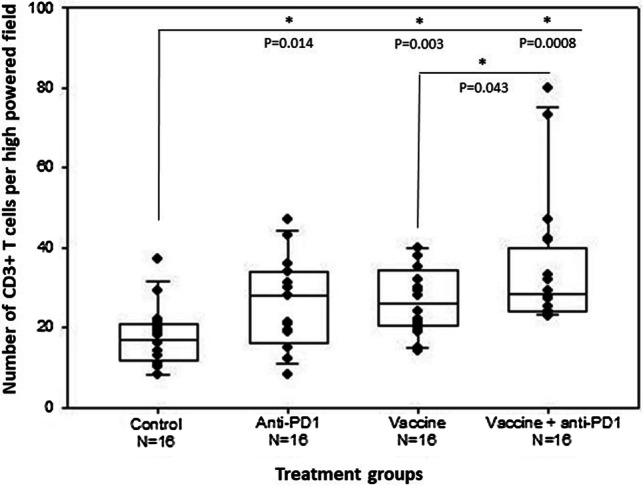
Prostate tumor antigen VLP vaccines and anti-PD1 antibody treatment increase CD3 + T cell infiltration into tumor tissue. 20-week-old TRAMP mice were treated as described in the legend to Fig. 4. At 26 weeks of age. 5 μm tissue sections from formalin-fixed prostate tissue were immunohistochemically stained for CD3 + T cells. The number of CD3 + cells per high-powered field are the average of four images per mouse. Statistically significant differences (t test) between untreated controls and treatment groups are indicated by an asterisk and P value above each group. The statistical difference (one-tailed t test) between vaccine monotherapy and vaccine plus anti-PD1 antibody combination therapy is also shown
T-cell infiltration into the tumor microenvironment induced by prostate tumor antigen BPV VLP vaccination
All treatments significantly increased CD3 + T cell infiltration into tumors compared to untreated mice (P ≤ 0.01). (Fig. 5 and Supplementary Figure S6). The mean CD3 + T cell infiltration was significantly higher in mice treated with the combination therapy compared to vaccine monotherapy (one tailed t test, P = 0.043) (Fig. 5). All treatments also significantly increased CD8 + T cell infiltration into tumors compared to untreated mice (P < 0.001) (Fig. 6 and Supplementary Figure S7). While there was no significant difference in CD8 + T cell infiltration between monotherapies, the combination therapy significantly increased the number of CD8 + T cells compared to vaccine alone (one tailed t test, P = 0.0193) or anti-PD1 alone (one tailed t test, P = 0.0186) (Fig. 6). There was a significant negative correlation between prostate weight and number of CD8 + T cells in tumors from vaccine-treated mice (Pearson correlation coefficient, P = 0.028). There was no significant correlation between CD8 + T cell number and prostate weight for the combination therapy; however, weights were uniformly low in this group.
Fig. 6.
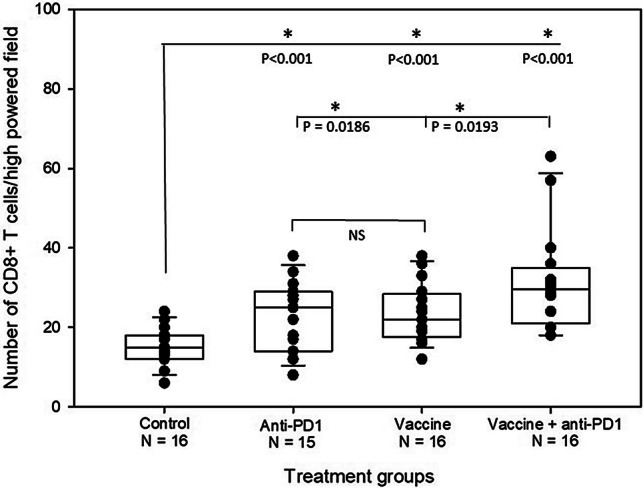
Prostate tumor antigen VLP vaccines and anti-PD1 antibody treatment increase CD8 + T cell infiltration into tumor tissue. 20-week-old TRAMP mice were treated as described in the legend to Fig. 4. At 26 weeks of age, 5 μm tissue sections from formalin-fixed prostate tissue were immunohistochemically stained for CD8 + T cells. The number of CD8 + cells per high-powered field are the average of four images per mouse. Statistically significant differences (t test) between untreated controls and treatment groups are indicated by an asterisk and P value above each group. The statistical difference (one-tailed t test) between combination therapy with vaccine and anti-PD1 versus vaccine or anti-PD1 monotherapy is also shown. There was no statistically significant difference between the monotherapy treatments
Discussion
Active immunization is an attractive approach to cancer immunotherapy, but with the exception of the prostate cancer vaccine, Sipuleucel-T, no cancer vaccines are approved for clinical use. Cancer vaccines have been formulated using plasmid DNA, viral vectors, peptides, or nanoparticles. Limitations to these approaches have hindered clinical efficacy. Plasmid DNA is weakly immunogenic in humans [20, 21]. Viral vectors induce potent immune responses but efficacy is limited by cross-reactive pre-existing immunity due to prior human exposure to genetically related viruses [22]. Additionally, development of anti-vector immunity prevents boosting [23]. Peptides are poorly immunogenic and require an adjuvant; however, few good adjuvants are available for induction of cellular immune responses [24–27]. Nanoparticle vaccines are composed of synthetic materials that are immunologically inert, and how best to enhance their immunogenicity is poorly understood. Additionally, nanoparticles may be rapidly cleared from the body by the reticuloendothelial system or renal filtration [28]. Virus-like particle vaccines are attractive because they harness the power of viral structures to interact with the immune system, but avoid the infectious component. However, most antigens of interest to oncologists are non-viral in origin and do not spontaneously form particles. Chimeric VLPs can be constructed by inserting amino acids encoding tumor antigens into a self-assembling viral protein. Disadvantages of this approach are the limited number of amino acids that can be inserted and the potentially disruptive effect of foreign amino acids on VLP assembly. Furthermore, every tumor antigen presents a unique problem in the design of a self-assembling structure.
Research by others and us have shown that human papillomavirus VLPs have the ability to induce activation and maturation of dendritic cells [29–32]. Of note, VLPs of polyomaviruses, which are closely related structurally to papillomaviruses, have minimal activity [29]. We hypothesized that this unique immunological property of papillomavirus VLPs would substitute for an adjuvant. In order to generate a VLP platform that could accommodate diverse tumor antigens we designed a chimeric bovine papillomavirus capsid protein that presents a surface-exposed docking site allowing promiscuous attachment of antigens to the VLP. We have also shown that chimeric BPV VLPs retain the ability to activate bone-marrow derived dendritic cells and upregulate expression of costimulatory molecules [15].
Herein, we decorated bovine papillomavirus VLPs with peptides encoding well-described MHC class I-restricted epitopes for two prostate tumor antigens, PSCA and PAP, and a neo-antigen, SPAS-1, that spontaneously arises in TRAMP tumors. The VLP vaccines induced robust IFN-γ-secreting PSCA and SPAS-1 antigen-specific CD8 + T cell responses and weaker but detectable responses to PAP epitopes in C57BL/6 mice. Comparison of VLP immunogenicity with that of other vaccine technologies is difficult because different prostate tumor antigens and different methods to assess immunogenicity have been used. A prime boost immunization using a DNA plasmid and Venezuela equine encephalitis viral vector induced a strong ELISPOT response to PSCA [16]. A mouse prostatic acid phosphatase DNA vaccine induced ~ 1.0% frequency of IFNγ-secreting splenocytes when stimulated with PAP-2 peptide [17]. A DNA plasmid vaccine with a PAP-1 Immunobody or the peptide administered in incomplete Freund’s adjuvant induced a ~ 1.7% frequency of PAP-1-specific CD8 + T cells detected using dextramer technology [33]. Combination immunotherapy with TRAMP tumor cells expressing GM-CSF and anti-CTLA-4 antibody induced a 0.4% frequency of SPAS-1-specific IFNγ-secreting splenic CD8 + T cells [18]. The lower response compared to VLP vaccination may be due to higher antigen load achieved with VLP formulation compared to the antigen load provided by immunization with tumor cells. The VLP vaccines were as immunogenic in TRAMP mice, 14–18 weeks of age, as wild-type C57BL/6 mice. Where comparisons have been made, tumor-bearing TRAMP mice have generally had poorer responses to vaccination than wild-type C57BL/6 mice [17, 34, 35]. Because the correlates of protection for vaccines that induce cellular immune responses are unknown, comparison of vaccines based on frequency of induction of CD8 + T cells is of limited value. Empirical tests of vaccine efficacy are more informative.
We tested efficacy in late-stage carcinogenesis in the TRAMP model. In TRAMP mice, simian virus 40 T antigen serves as an oncogene, and transgene expression is under transcriptional control of rat probasin promoter, which directs expression to prostatic epithelium in an androgen-regulated manner, giving rise to tumors at the time of adolescence and restricted to the prostate gland [36]. At puberty, TRAMP mice develop mild hyperplasia, followed by frank neoplasia corresponding to prostatic intraepithelial neoplasia (PIN) in men, and eventually well-differentiated adenocarcinoma (18–20 weeks of age) [37, 38]. One-third of animals develop anaplastic and highly invasive neuroendocrine carcinomas with a propensity for metastasis to lungs, lymph node, and bone (22–26 weeks of age). Most previous vaccine studies treated TRAMP mice at 8–10 weeks of age when the mice exhibit PIN-like lesions [16, 17, 34, 35, 39–41]. In order to mimic more closely application of a prostate cancer vaccine in men, we started treatment at 19–20 weeks of age. The vaccine alone or in combination with anti-PD-1 therapy significantly reduced tumor burden. Of note, in the combination immunotherapy group, only a single mouse had a large tumor (prostate weight ~ 3.0 g), while most mice had prostate weights below the median weight of the control untreated mice (< 0.5 g) and within the normal range for tumor-free C57BL/6 mice (0.150–0.250 g). In contrast to our results, no other prostate tumor vaccine has shown efficacy in late-stage carcinogenesis in the TRAMP model. In fact, the superior protective efficacy of vaccination of TRAMP mice with PIN lesions compared to those with invasive carcinoma has led some to conclude that prostate cancer vaccines should be used in men at early stages of disease [39].
The precise mechanism of BPV VLP vaccine-induced efficacy is unknown but our data strongly support a role for T lymphocytes. The VLP vaccine caused a significantly increased infiltration of CD3 + T cells into the tumor, as reported for other prostate tumor antigen vaccines tested in mice [33, 41, 42]. The vaccine also significantly increased infiltration of CD8 + T cells into the tumor, and there was a significant inverse correlation between tumor weight and number of tumor resident CD8 + T cells in mice treated with vaccine monotherapy. The significantly greater CD3 + and CD8 + T cell infiltration observed in mice treated with combination therapy compared to monotherapy supports the notion that the vaccine and anti-PD1 antibody can act synergistically, as proposed by others [40, 43]. To statistically test for a synergistic or additive effect of VLP vaccine and immune checkpoint inhibitor will require a much larger sample size.
The potential translational value of BPV VLP vaccine for prostate cancer in humans remains to be determined. The TRAMP model may have better predictive value for translatability than other murine models. In fact, the lack of efficacy of immune checkpoint inhibitors in TRAMP mice with advanced disease reflects the disappointing clinical experience with these drugs. Many preclinical animal studies are done in tumor models using tissue culture-adapted cells implanted subcutaneously and are designed to reach short-term endpoints of maximal tumor growth in 30–40 days in untreated controls. In contrast, spontaneous tumor models better mimic the pathophysiology of human tumors since tumors arise from an endogenous source and develop in parallel with host responses. Pathogenesis of neoplasia in TRAMP mice mirrors many features of that in man, with progression from PIN to frank neoplasia to metastatic disease. Similar to human tumors, prostate cancers in TRAMP mice develop without overt signs of local inflammation [44]. To better model clinical applications of immunotherapy, we treated TRAMP mice with advanced stage carcinogenesis and demonstrated efficacy under conditions where other vaccines have had no effect. We have previously shown that the VLP vaccine can be administered repeatedly and maintain CD8 + T cell responses, which could be important for treatment of human cancers [45]. Due to the safety of vaccines compared to immune checkpoint inhibitors, vaccines could be used for patients in active-surveillance or for patients with high-risk, localized prostate cancer. The remarkable efficacy of prostate tumor antigen BPV VLP vaccines in late-stage carcinogenesis in the TRAMP model makes this vaccine an attractive technology to test in patients with metastatic prostate cancer.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the NIH tetramer core facility for provision of dye labeled MHC peptide complexes.
Abbreviations
- AAY
Alanine-alanine–tyrosine
- BPV
Bovine papillomavirus
- DAB
Diaminobenzidine
- CTLA4
Cytotoxic T-lymphocyte-associated protein 4
- GSH
Glutathione
- GSSG
Glutathione disulfide
- IQR
Interquartile range
- ORF
Open reading frame
- PAP
Prostatic acid phosphatase
- PC
Prostate cancer
- PD-1
Programed death-1
- PIN
Prostatic intraepithelial neoplasia
- PSCA
Prostate stem cell antigen
- SPAS
Stimulator of prostatic adenocarcinoma-specific T cells
- TCEP
Tris(2-carboxyethyl)phosphine
- TRAMP
Transgenic adenocarcinoma of the mouse prostate
- VLP
Virus-like particle
Author contributions
BWS contributed to study design and analysis, and supervised immunization of mice, measurement of tumor size and immunohistochemical analysis of tumor tissue. FC produced VLP vaccines and assisted with measurements of tumor size. DTR produced VLP vaccines and performed flow cytometry analysis of the immune response to vaccination. RPV contributed to study design and analysis, and supervised production of VLP vaccines and analysis of vaccine immunogenicity.
Funding
This work was supported by grants from the Allegheny Health Network, Patrick C. Walsh Prostate Cancer Research Fund, and State of Maryland TEDCO program.
Compliance with ethical standards
Conflict of interest
Raphael P Viscidi is the inventor of the bovine VLP vaccine technology and could financially benefit if a product based on the technology is commercialized. All other authors declare that they have no conflicts of interest.
Ethical approval
All animal procedures were performed according to NIH guidelines under protocol MO17M189 approved by the Johns Hopkins University Animal Care and Use Committee.
Animal rights
Breeding pairs of TRAMP mice (C57BL/6-Tg(TRAMP)8247Ng/J)(stock no. 003135) and wild type C57BL/6 J mice (stock no. 000664) were purchased from Jackson Laboratories (Bar Harbor, ME). Tramp mice were bred within our colony on a pure C57BL/6 background.
Cell line authentication
High Five™ Cells (BTI-TN-5B1-4) are a clonal isolate derived from the parental Trichoplusia ni cell line (cabbage looper ovary) and are adapted to serum-free culture (Thermo Fisher Scientific, #B85502). The cells were not further authenticated as they are of insect origin and were used for protein production only.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Miranti CK, Koul HR. Meeting Report of Joint Society of Basic Urologic Research (SBUR) and European Society of Urological Research (ESUR) symposium fall 2017. Am J Clin Exp Urol. 2017;5(1):1–92. doi: 10.11648/j.ajcem.20170501.11. [DOI] [Google Scholar]
- 2.National Cancer Institute (2019) Cancer Stat Facts: Prostate Cancer. https://seer.cancer.gov/statfacts/html/prost.html. Accessed Dec 2019
- 3.Kiessling A, Wehner R, Fussel S, Bachmann M, Wirth MP, Schmitz M. Tumor-associated antigens for specific immunotherapy of prostate cancer. Cancers (Basel) 2012;4:193–217. doi: 10.3390/cancers4010193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 5.Higano CS, Schellhammer PF, Small EJ, Burch PA, Nemunaitis J, Yuh L, Provost N, Frohlich MW. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer. 2009;115:3670–3679. doi: 10.1002/cncr.24429. [DOI] [PubMed] [Google Scholar]
- 6.Beer TM, Kwon ED, Drake CG, Fizazi K, Logothetis C, Gravis G, Ganju V, Polikoff J, Saad F, Humanski P, Piulats JM, Gonzalez MP, Ng SS, Jaeger D, Parnis FX, Franke FA, Puente J, Carvajal R, Sengelov L, McHenry MB, Varma A, van den Eertwegh AJ, Gerritsen W. Randomized, double-blind, phase III trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy-naive castration-resistant prostate cancer. J Clin Oncol. 2017;35:40–47. doi: 10.1200/JCO.2016.69.1584. [DOI] [PubMed] [Google Scholar]
- 7.van den Eertwegh AJ, Versluis J, van den Berg HP, Santegoets SJ, van Moorselaar RJ, van der Sluis TM, Gall HE, Harding TC, Jooss K, Lowy I, Pinedo HM, Scheper RJ, Stam AG, von Blomberg BM, de Gruijl TD, Hege K, Sacks N, Gerritsen WR. Combined immunotherapy with granulocyte-macrophage colony-stimulating factor-transduced allogeneic prostate cancer cells and ipilimumab in patients with metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:509–517. doi: 10.1016/S1470-2045(12)70007-4. [DOI] [PubMed] [Google Scholar]
- 8.Parsons JK, Pinto PA, Pavlovich CP, Uchio E, Kim HL, Nguyen MN, Gulley JL, Jamieson C, Hsu P, Wojtowicz M, Parnes H, Schlom J, Dahut WL, Madan RA, Donahue RN, Chow HS. A randomized, double-blind, phase II trial of PSA-TRICOM (PROSTVAC) in patients with localized prostate cancer: the immunotherapy to prevent progression on active surveillance study. Eur Urol Focus. 2018;4:636–638. doi: 10.1016/j.euf.2018.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kantoff PW, Gulley JL, Pico-Navarro C. Revised overall survival analysis of a phase II, randomized, double-blind, controlled study of PROSTVAC in men with metastatic castration-resistant prostate cancer. J Clin Oncol. 2017;35:124–125. doi: 10.1200/JCO.2016.69.7748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kantoff PW, Schuetz TJ, Blumenstein BA, Glode LM, Bilhartz DL, Wyand M, Manson K, Panicali DL, Laus R, Schlom J, Dahut WL, Arlen PM, Gulley JL, Godfrey WR. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:1099–1105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higano CS, Corman JM, Smith DC, Centeno AS, Steidle CP, Gittleman M, Simons JW, Sacks N, Aimi J, Small EJ. Phase 1/2 dose-escalation study of a GM-CSF-secreting, allogeneic, cellular immunotherapy for metastatic hormone-refractory prostate cancer. Cancer. 2008;113:975–984. doi: 10.1002/cncr.23669. [DOI] [PubMed] [Google Scholar]
- 12.Lubaroff DM. Prostate cancer vaccines in clinical trials. Expert Rev Vaccines. 2012;11:857–868. doi: 10.1586/erv.12.54. [DOI] [PubMed] [Google Scholar]
- 13.McNeel DG, Dunphy EJ, Davies JG, Frye TP, Johnson LE, Staab MJ, Horvath DL, Straus J, Alberti D, Marnocha R, Liu G, Eickhoff JC, Wilding G. Safety and immunological efficacy of a DNA vaccine encoding prostatic acid phosphatase in patients with stage D0 prostate cancer. J Clin Oncol. 2009;27:4047–4054. doi: 10.1200/JCO.2008.19.9968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pavlenko M, Roos AK, Lundqvist A, Palmborg A, Miller AM, Ozenci V, Bergman B, Egevad L, Hellstrom M, Kiessling R, Masucci G, Wersall P, Nilsson S, Pisa P. A phase I trial of DNA vaccination with a plasmid expressing prostate-specific antigen in patients with hormone-refractory prostate cancer. Br J Cancer. 2004;91:688–694. doi: 10.1038/sj.bjc.6602019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pejawar-Gaddy S, Rajawat Y, Hilioti Z, Xue J, Gaddy DF, Finn OJ, Viscidi RP, Bossis I. Generation of a tumor vaccine candidate based on conjugation of a MUC1 peptide to polyionic papillomavirus virus-like particles. Cancer Immunol Immunother. 2010;59:1685–1696. doi: 10.1007/s00262-010-0895-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Hernandez ML, Gray A, Hubby B, Klinger OJ, Kast WM. Prostate stem cell antigen vaccination induces a long-term protective immune response against prostate cancer in the absence of autoimmunity. Cancer Res. 2008;68:861–869. doi: 10.1158/0008-5472.CAN-07-0445. [DOI] [PubMed] [Google Scholar]
- 17.Spies E, Reichardt W, Alvarez G, Groettrup M, Ohlschlager P. An artificial PAP gene breaks self-tolerance and promotes tumor regression in the TRAMP model for prostate carcinoma. Mol Ther. 2012;20:555–564. doi: 10.1038/mt.2011.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fasso M, Waitz R, Hou Y, Rim T, Greenberg NM, Shastri N, Fong L, Allison JP. SPAS-1 (stimulator of prostatic adenocarcinoma-specific T cells)/SH3GLB2: A prostate tumor antigen identified by CTLA-4 blockade. Proc Natl Acad Sci USA. 2008;105:3509–3514. doi: 10.1073/pnas.0712269105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hearn A, York IA, Rock KL. The specificity of trimming of MHC class I-presented peptides in the endoplasmic reticulum. J Immunol. 2009;183:5526–5536. doi: 10.4049/jimmunol.0803663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coban C, Kobiyama K, Aoshi T, Takeshita F, Horii T, Akira S, Ishii KJ. Novel strategies to improve DNA vaccine immunogenicity. Curr Gene Ther. 2011;11:479–484. doi: 10.2174/156652311798192815. [DOI] [PubMed] [Google Scholar]
- 21.Eriksson F, Totterman T, Maltais AK, Pisa P, Yachnin J. DNA vaccine coding for the rhesus prostate specific antigen delivered by intradermal electroporation in patients with relapsed prostate cancer. Vaccine. 2013;31:3843–3848. doi: 10.1016/j.vaccine.2013.06.063. [DOI] [PubMed] [Google Scholar]
- 22.Ura T, Okuda K, Shimada M. Developments in viral vector-based vaccines. Vaccines (Basel) 2014;2:624–641. doi: 10.3390/vaccines2030624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu S. Heterologous prime-boost vaccination. Curr Opin Immunol. 2009;21:346–351. doi: 10.1016/j.coi.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calvo TM, Allard M, Dutoit V, Dietrich PY, Walker PR. Peptides as cancer vaccines. Curr Opin Pharmacol. 2019;47:20–26. doi: 10.1016/j.coph.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 25.Bezu L, Kepp O, Cerrato G, Pol J, Fucikova J, Spisek R, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: Peptide-based vaccines in anticancer therapy. Oncoimmunology. 2018;7:e1511506. doi: 10.1080/2162402X.2018.1511506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Obara W, Sato F, Takeda K, Kato R, Kato Y, Kanehira M, Takata R, Mimata H, Sugai T, Nakamura Y, Fujioka T. Phase I clinical trial of cell division associated 1 (CDCA1) peptide vaccination for castration resistant prostate cancer. Cancer Sci. 2017;108:1452–1457. doi: 10.1111/cas.13278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bastola R, Noh G, Keum T, Bashyal S, Seo JE, Choi J, Oh Y, Cho Y, Lee S. Vaccine adjuvants: smart components to boost the immune system. Arch Pharm Res. 2017;40:1238–1248. doi: 10.1007/s12272-017-0969-z. [DOI] [PubMed] [Google Scholar]
- 28.Wen R, Umeano AC, Kou Y, Xu J, Farooqi AA. Nanoparticle systems for cancer vaccine. Nanomedicine (Lond) 2019;14:627–648. doi: 10.2217/nnm-2018-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lenz P, Day PM, Pang YY, Frye SA, Jensen PN, Lowy DR, Schiller JT. Papillomavirus-like particles induce acute activation of dendritic cells. J Immunol. 2001;166:5346–5355. doi: 10.4049/jimmunol.166.9.5346. [DOI] [PubMed] [Google Scholar]
- 30.Lenz P, Lowy DR, Schiller JT. Papillomavirus virus-like particles induce cytokines characteristic of innate immune responses in plasmacytoid dendritic cells. Eur J Immunol. 2005;35:1548–1556. doi: 10.1002/eji.200425547. [DOI] [PubMed] [Google Scholar]
- 31.Yang R, Murillo FM, Lin KY, Yutzy WH, Uematsu S, Takeda K, Akira S, Viscidi RP, Roden RB. Human papillomavirus type-16 virus-like particles activate complementary defense responses in key dendritic cell subpopulations. J Immunol. 2004;173:2624–2631. doi: 10.4049/jimmunol.173.4.2624. [DOI] [PubMed] [Google Scholar]
- 32.Yang R, Murillo FM, Cui H, Blosser R, Uematsu S, Takeda K, Akira S, Viscidi RP, Roden RB. Papillomavirus-like particles stimulate murine bone marrow-derived dendritic cells to produce alpha interferon and Th1 immune responses via MyD88. J Virol. 2004;78:11152–11160. doi: 10.1128/JVI.78.20.11152-11160.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saif JM, Vadakekolathu J, Rane SS, McDonald D, Ahmad M, Mathieu M, Pockley AG, Durrant L, Metheringham R, Rees RC, McArdle SE. Novel prostate acid phosphatase-based peptide vaccination strategy induces antigen-specific T-cell responses and limits tumour growth in mice. Eur J Immunol. 2014;44:994–1004. doi: 10.1002/eji.201343863. [DOI] [PubMed] [Google Scholar]
- 34.Cappuccini F, Stribbling S, Pollock E, Hill AV, Redchenko I. Immunogenicity and efficacy of the novel cancer vaccine based on simian adenovirus and MVA vectors alone and in combination with PD-1 mAb in a mouse model of prostate cancer. Cancer Immunol Immunother. 2016;65:701–713. doi: 10.1007/s00262-016-1831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mueller M, Reichardt W, Koerner J, Groettrup M. Coencapsulation of tumor lysate and CpG-ODN in PLGA-microspheres enables successful immunotherapy of prostate carcinoma in TRAMP mice. J Control Release. 2012;162:159–166. doi: 10.1016/j.jconrel.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 36.Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO, Cunha GR, Donjacour AA, Matusik RJ, Rosen JM. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci USA. 1995;92:3439–3443. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gingrich JR, Barrios RJ, Foster BA, Greenberg NM. Pathologic progression of autochthonous prostate cancer in the TRAMP model. Prostate Cancer Prostatic Dis. 1999;2:70–75. doi: 10.1038/sj.pcan.4500296. [DOI] [PubMed] [Google Scholar]
- 38.Kido LA, de Almeida LC, Marostica MR, Jr, Cagnon VHA. Transgenic Adenocarcinoma of the Mouse Prostate (TRAMP) model: a good alternative to study PCa progression and chemoprevention approaches. Life Sci. 2019;217:141–147. doi: 10.1016/j.lfs.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 39.Gray A, de la Garcia-Hernandez L, van WM, Kanodia S, Hubby B, Kast WM. Prostate cancer immunotherapy yields superior long-term survival in TRAMP mice when administered at an early stage of carcinogenesis prior to the establishment of tumor-associated immunosuppression at later stages. Vaccine. 2009;27(Suppl 6):G52–G59. doi: 10.1016/j.vaccine.2009.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hurwitz AA, Foster BA, Kwon ED, Truong T, Choi EM, Greenberg NM, Burg MB, Allison JP. Combination immunotherapy of primary prostate cancer in a transgenic mouse model using CTLA-4 blockade. Cancer Res. 2000;60:2444–2448. [PubMed] [Google Scholar]
- 41.Krupa M, Canamero M, Gomez CE, Najera JL, Gil J, Esteban M. Immunization with recombinant DNA and modified vaccinia virus Ankara (MVA) vectors delivering PSCA and STEAP1 antigens inhibits prostate cancer progression. Vaccine. 2011;29:1504–1513. doi: 10.1016/j.vaccine.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 42.Kwilas AR, Ardiani A, Dirmeier U, Wottawah C, Schlom J, Hodge JW. A poxviral-based cancer vaccine the transcription factor twist inhibits primary tumor growth and metastases in a model of metastatic breast cancer and improves survival in a spontaneous prostate cancer model. Oncotarget. 2015;6:28194–28210. doi: 10.18632/oncotarget.4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fu J, Malm IJ, Kadayakkara DK, Levitsky H, Pardoll D, Kim YJ. Preclinical evidence that PD1 blockade cooperates with cancer vaccine TEGVAX to elicit regression of established tumors. Cancer Res. 2014;74:4042–4052. doi: 10.1158/0008-5472.CAN-13-2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shappell SB, Thomas GV, Roberts RL, Herbert R, Ittmann MM, Rubin MA, Humphrey PA, Sundberg JP, Rozengurt N, Barrios R, Ward JM, Cardiff RD. Prostate pathology of genetically engineered mice: definitions and classification. The consensus report from the Bar Harbor meeting of the Mouse Models of Human Cancer Consortium Prostate Pathology Committee. Cancer Res. 2004;64:2270–2305. doi: 10.1158/0008-5472.CAN-03-0946. [DOI] [PubMed] [Google Scholar]
- 45.Beck SE, Queen SE, Viscidi R, Johnson D, Kent SJ, Adams RJ, Tarwater PM, Mankowski JL. Central nervous system-specific consequences of simian immunodeficiency virus Gag escape from major histocompatibility complex class I-mediated control. J Neurovirol. 2016;22:498–507. doi: 10.1007/s13365-015-0420-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.