Abstract
The standard-of-care (SOC) first-line therapy for ovarian cancer (OC) patients is plagued with high relapse rates. Several studies indicated the immune system’s prominent role changing the disease course in OC patients. Chemo-immunotherapy regimens, currently being explored, include oregovomab, which is a monoclonal antibody specific for the OC associated antigen carbohydrate/cancer antigen 125 (CA125) that yielded promising results when administered together with SOC in a previous study. The QPT-ORE-002 multi-site phase II randomized study demonstrated that in patients with advanced OC, oregovomab combined with first-line SOC improved overall and progression-free survival, compared to SOC alone. The study included an Italian cohort in which we demonstrated that adding oregovomab to SOC resulted in increased patient numbers with amplified CA125-specific CD8+T lymphocytes/ml peripheral blood counts, which might explain the improved therapeutic effect of SOC + oregovomab over SOC alone. Predictive for oregovomab efficacy was a less suppressive immune environment at baseline as indicated by low numbers of circulating myeloid-derived suppressor cells, subset type 4, and a low neutrophil-and-monocyte to lymphocyte ratio.
Electronic supplementary material
The online version of this article (10.1007/s00262-019-02456-z) contains supplementary material, which is available to authorized users.
Keywords: Oregovomab, Ovarian cancer, Immune response, Predictive biomarkers, Personalized medicine
Introduction
Cytoreduction followed by chemotherapy with platinum and taxane is the mainstay of ovarian cancer (OC) treatment, although neo-adjuvant chemotherapy is alternatively attempted in an increased number of cases. Despite initial high response rates, 50% of tumors recur within 2 years and no curative relapse therapy exists. Thus, new therapies are urgently needed.
Several studies indicated the immune system’s prominent role changing the disease course in OC patients. In a seminal study, tumor-infiltrating lymphocytes (TILs) were strongly associated with favorable clinical outcomes [1]. This observation was confirmed in subsequent studies, which further identified CD8+T lymphocytes and regulatory CD4+T lymphocytes (Treg) among TILs as crucial anti-tumor immune response effectors and inhibitors, respectively [2–6]. Despite this strong rationale for the immunotherapeutic OC treatment, a recent systematic review reported no successful OC immunotherapeutic approaches established to date [7].
Oregovomab (formerly OvaRex) is an investigational drug designed as an “indirect immunizer” for immunotherapeutic OC treatment. The active component is the murine monoclonal antibody (MAb) B43.13, an IgG1κ immunoglobulin that binds to the carbohydrate/cancer antigen 125 (CA125) with high affinity (1.16 × 1010/M) [8, 9]. CA125 (also known as mucin 16) is a cancer-associated surface glycoprotein expressed in the majority of advanced epithelial OCs [10]. Elevated levels of soluble CA125 are present in the serum of approximately 80% of women with advanced epithelial OC.
Oregovomab binds to CA125 in the blood and local tissues. Immune complex formation with circulating CA125 occurs within 30 min of injection [11]. Antigen processing of the immune complexes enables cross presentation of CA125 peptides and enhances cellular immune responses. A phase III clinical trial of oregovomab maintenance mono-immunotherapy following standard-of-care (SOC) frontline chemotherapy failed to demonstrate clinical efficacy [12]. This may be related to minimal circulating tumor antigen and minimal apoptotic tumor tissue available for interaction with oregovomab in a maintenance setting. This contrasts with frontline combination chemo-immunotherapy. In a randomized, phase II clinical trial of oregovomab combined with SOC as frontline chemo-immunotherapy in advanced OC patients [13], oregovomab was administered in alternative schedules. Patients, infused simultaneously with SOC and oregovomab, demonstrated enhanced CA125-specific cellular immune responses, as well as improved overall clinical results relative to patients infused with oregovomab 1-week-after SOC [13]; the 12-month estimates for progression free survival were 89% and 60% in the simultaneous vs the 1-week-after SOC oregovomab treatment, respectively [13].
Thus, oregovomab requires being administered simultaneously with SOC [13] and in a frontline rather than in maintenance settings [12] to show benefit. Remarkably, oregovomab demonstrated a well-tolerated safety profile in the maintenance and in the frontline settings [12, 13].
The phase II randomized international clinical trial QPT-ORE-002 was conducted to further explore and optimize the frontline oregovomab chemo-immunotherapy of OC relative to an SOC control. While the preliminary clinical results of the trial have been reported elsewhere [14], the present report presents the translational study of immune parameters including CA125-specific cellular immune responses and exploration of immune suppression markers in a subset of patients.
Immunotherapy and vaccine efficacy relies on a functional immune system [15]. We explored two key drivers of immune evasion, the myeloid-derived suppressor cell (MDSC) and Treg pathways, thought to hamper the generation of effective anti-tumor T lymphocyte responses and to correlate with clinical outcomes in cancer immunotherapy studies [15–17]. Both of these cell populations are influenced by several tumor-released factors [18–23] and limit the immune response by promoting immunological tolerance.
Additionally, we explored the baseline neutrophil-and-monocyte-to-lymphocyte ratio (NMLR), because several studies suggested that relative proportions of circulating leukocyte populations may be general immunosuppressive status indicators and are, therefore, associated with poor outcomes in several malignancies [24–26] including OC [27–30].
Materials and methods
Patient eligibility criteria
Patients with documented pre-operative CA125 serum levels greater than 50 U/ml were enrolled at six Italian institutions (Supplementary Table 1) from December 2011 to January 2015. Patients with stage III/IV epithelial adenocarcinoma of ovarian, fallopian tube or peritoneal origin, optimally debulked to RT < 1 cm (40 patients RT = 0 and 41 patients RT < 1 cm) were scheduled to start frontline SOC therapy within 6 weeks after surgery. Additional inclusion/exclusion criteria have been detailed previously [14]. Table 1 provides patient cohort characteristics.
Table 1.
Demographics and baseline characteristics (intention-to-treat population)
| Parameter | Statistics | SOC + oregovomab (N = 39) | SOC alone (N = 42) | Overall (N = 81) |
|---|---|---|---|---|
| Race | ||||
| Caucasian | n (%) | 39 (100.0) | 42 (100.0) | 81 (100.0) |
| Tissue origin | ||||
| Ovaries | n (%) | 35 (89.7) | 38 (90.5) | 73 (90.1) |
| Fallopian tubes | n (%) | 3 (7.7) | 2 (4.8) | 5 (6.2) |
| Peritoneum | n (%) | 0 | 1 (2.4) | 1 (1.2) |
| Missing | n (%) | 1 (2.6) | 1 (2.4) | 2 (2.5) |
| Baseline ECOG performance statusa | ||||
| 0 | n (%) | 33 (84.6) | 41 (97.6) | 74 (91.4) |
| 1 | n (%) | 6 (15.4) | 1 (2.4) | 7 (8.6) |
| 2 | n (%) | 0 | 0 | 0 |
| 3 | n (%) | 0 | 0 | 0 |
| 4 | n (%) | 0 | 0 | 0 |
| FIGO stage at screening | ||||
| III (subclass unknown) | n (%) | 0 | 2 (4.8) | 2 (2.5) |
| IIIA | n (%) | 3 (7.7) | 3 (7.1) | 6 (7.4) |
| IIIB | n (%) | 7 (17.9) | 1 (2.4) | 8 (9.9) |
| IIIC | n (%) | 24 (61.5) | 33 (78.6) | 57 (70.4) |
| IV | n (%) | 5 (12.8) | 3 (7.1) | 8 (9.9) |
| Residual disease status | ||||
| RT = 0 | n (%) | 22 (56.4) | 18 (42.9) | 40 (49.4) |
| RT < 1 | n (%) | 17 (43.6) | 24 (57.1) | 41 (50.6) |
| Age (years)b | Mean (SD) | 56.2 (11.56) | 56.3 (9.68) | 56.3 (10.56) |
| Median | 57.0 | 56.5 | 57.0 | |
| Min, max | 37, 78 | 38, 74 | 37, 78 | |
| Weight (kg) | Mean (SD) | 61.3 (13.69) | 61.6 (13.25) | 61.0 (13.38) |
| Median | 60.0 | 57.0 | 59.0 | |
| Min, max | 39.0, 115.0 | 43.0, 99.0 | 39.0, 115.0 | |
| Height (cm) | Mean (SD) | 160.9 (5.69) | 160.1 (6.51) | 160.5 (6.11) |
| Median | 160.0 | 160.0 | 160.0 | |
| Min, max | 151, 180 | 146, 172 | 146, 180 | |
a0 = Fully active, able to carry on all pre-disease performance without restriction. 1 = Restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature. 2 = Ambulatory and capable of all self-care but unable to carry out any work activities. Up and about more than 50% of waking hours. 3 = Capable of only limited self-care, confined to bed or chair more than 50% of waking hours. 4 = Completely disabled. Cannot carry out any self-care. Totally confined to bed or chair
b Age (years) = (randomization date—date of birth + 1)/365.25
Study design
QPT-ORE-002 is a multi-site phase II randomized study of SOC vs SOC + oregovomab in patients with advanced OC. Briefly, six cycles of SOC were intravenously administered at 3-week intervals. In the chemo-immunotherapy arm, oregovomab (2 mg in 52 ml) was infused over 20 min following sequential paclitaxel and carboplatin infusions at cycles 1, 3, and 5 and as a maintenance dose at cycle 5 + 12 weeks. The primary study endpoint was to evaluate as a surrogate marker of clinical efficacy, a CA125-specific T cell response based on an interferon (IFN)-γ ELISPOT assay. Secondary endpoints included: safety, time to clinical progression (time period from randomization to date of confirmed relapse based on clinical, radiologic, and/or pathologic evaluations), relapse-free survival (RFS; time to clinical progression, death from any cause or censored at last disease assessment), overall survival (OS; observed length of life from randomization to death or censored at the date of last contact), and for this Italian cohort, an analysis of CA125-specific CD8+T lymphocyte induction based on intracellular cytokine staining (ICS) as a potential predictor of efficacy. Not all patients could be studied for all immunological parameters because of a low yield of blood cells following chemotherapy. The treatment extended from randomization to 10 weeks post cycle 6 or to disease relapse if earlier, and a regular patient follow-up took place for up to 3 years from treatment exit to determine RFS and OS.
Cell samples
Peripheral blood (42 ml) was drawn at baseline, cycle 5 and cycle 5 + 13 weeks and sent at room temperature to the central laboratory (Immunology Laboratory at the Università Cattolica del Sacro Cuore, Rome, Italy) within 24 h. Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll–Hypaque gradient centrifugation [31]. Excess (> 15%) PBMC-contaminating granulocytes were eliminated by magnetic sorting using CD15 microbeads (Miltenyi). Monocytes were separated from lymphocytes by magnetic sorting of PBMC using CD14 microbeads (Miltenyi) yielding monocytes (≥ 95% purity) and lymphocytes (≥ 90% purity). Purified monocytes and lymphocytes were cryopreserved in liquid nitrogen for subsequent analyses.
CA125 and MAb-B43.13 for DC pulsing
Pyrogen-free sterile MAb-B43.13 and CA125 were provided by the sponsor (OncoQuest). CA125/MAb-B43.13 immune complex (hereafter referred to as IC) was prepared by incubating 5000 U/ml CA125 and 50 μg/ml MAb-B43.13 at room temperature for 60 min and was immediately used at a final 1:10 dilution.
Intracellular cytokine staining
Monocytes obtained at the three study timepoints were thawed, pooled per patient, and cultured as described previously for 6 days to differentiate them into immature DC (iDC) [32]. The iDC were then pulsed at 37 °C for 4 h with either 500 U/ml CA125, 5 μg/ml MAb-B43.13 or IC. An iDC aliquot was left unprimed. iDC maturation was induced by culturing cells overnight in the presence of 10 ng/ml each of tumor necrosis factor-α (Genzyme), IL-6 (Genzyme) and IL-1β (Genzyme). Pulsed and unprimed mature DC (mDC) were harvested and counted. Corresponding autologous lymphocytes obtained at each time point of the study were thawed and cultured with either pulsed or unprimed mDC (20:1) at 37 °C for 18 h. IFN-γ production by lymphocytes incubated with the staphylococcal enterotoxin B (SEB) in the presence or absence of unprimed autologous mDC served to monitor viability and to set the threshold for IFN-γ detection [31]. Cells were harvested and stained with anti-CD8PE-Cy5 (clone RPA-T8, BD-Biosciences), fixed and permeabilized with the BD-FastImmune intracellular cytokine detection kit (BD-Biosciences) and subsequently stained with anti-CD69FITC (clone FN50, BD-Biosciences), anti-CD3ECD (clone UCHT1, Beckman Coulter) and anti-IFN-γPE (clone B27, BD-Biosciences) for 30 min.
MDSC and Treg
Several studies established the prognostic value of MDSC subsets in a variety of solid malignancies [33–35]. We focused on the MDSC4 subset (CD14+HLA-DRlow/−) as it correlates with efficacy to anti-tumor immunotherapy [36, 37]. MDSC4 were identified using anti-CD14FITC (clone FWKW-1, Exalpha) and anti-HLA-DRPE-CF594 (clone G46-6, BD-Biosciences).
Treg were identified using anti-CD25PE (clone 2A3, BD Bioscience), anti-CD3PerCP (clone UCHT1, BD-Biosciences), anti-CD127FITC (clone ebioRDR5, eBioscience) and anti-CD4ECD (clone SFCI12T4D11, Beckman Coulter) [38, 39].
Flow cytometry
Flow cytometry analysis was performed using a six-parameter EPICS-XL cytometer and Expo 32™ software (Beckman Coulter). The frequency of IFN-γ producing (IFN-γ+) CD8+ T lymphocytes was determined, as depicted in Supplementary Fig. 1. Spontaneous IFN-γ production was assessed following incubation with unprimed mDC and subtracted from patient- and timepoint-matched data. Absolute counts of IFN-γ+ CD8+ T lymphocytes were calculated multiplying the percentage of IFN-γ+ CD8+ T lymphocytes within total lymphocytes by the total lymphocyte count.
The frequency of MDSC4 was determined, as depicted in Supplementary Fig. 2. MDSC4 absolute counts were calculated multiplying the percentage of MDSC4 by the total monocyte count, when available.
Treg were identified as CD3+ CD4+ CD25dim/bright CD127low/neg, as illustrated in Supplementary Fig. 3. Absolute Treg counts were calculated multiplying the Treg frequency within lymphocytes by the total lymphocyte count.
White blood cell counts and differentials were recorded as part of patients’ follow up at each study site.
Neutrophil-and-monocyte-to-lymphocyte ratio
The trial database contains information on total leukocyte and lymphocyte counts for 71 patients (36 patients and 35 patients in the SOC + oregovomab and SOC alone arm, respectively). We derived the baseline (i.e., after primary debulking surgery and just before starting treatment) combined neutrophils-and-monocytes (NM) number, subtracting lymphocyte counts from corresponding total leukocyte counts. The NM-to-lymphocyte ratio (NMLR) was computed as follows: NMLR = NM count/lymphocyte count.
Statistics
Because normality of distribution and homoscedasticity were not verified, the nonparametric Mann–Whitney U test was used (Statistica version 7.1) to verify that baseline immune biomarker values between the two study arms did not differ (Table 2).
Table 2.
Immune biomarkers were evaluated at baseline and compared across the two study groups
| Immune biomarker | SOC + oregovomab | SOC | p valuea | ||||
|---|---|---|---|---|---|---|---|
| N | Median | Min–max | N | Median | Min–max | ||
| Number of CA125-specific IFN-γ+CD8+ T lymphocytes/ml | 17 | 0.00 | 0.00–4682 | 20 | 0.00 | 0.00–1114 | n.s. |
| Number of MDSC4/ml | 23 | 181,407 | 3610–757,390 | 24 | 117,045 | 21,080–300,960 | n.s. |
| Number of Treg/ml | 25 | 56,440 | 11,319–89,041 | 26 | 55,420 | 21,080–130,967 | n.s. |
| NMLR | 36 | 3.046 | 1.338–10.32 | 35 | 2.925 | 1.426–11.375 | n.s. |
p values ≥ 0.05 were considered not significant (n.s.)
aBy Mann–Whitney U test
Differences in changes between timepoints (baseline to cycle 5, cycle 5 to cycle 5 + 13 weeks, and baseline to cycle 5 + 13 weeks) in the two arms were assessed by repeated measure analysis of variance (ANOVA). Only patients with corresponding data for paired timepoints were included in each comparison, leading to varying sample sizes for each comparison. A least-squares estimate of the means from the repeated ANOVA and the corresponding standard error were calculated, using the assumption that variabilities between timepoints were similar. Since this was considered an exploratory dataset, this assumption was not verified. Comparisons were only performed, where ≥ 10 samples were available in paired sample sets (cohorts and timepoints). Due to the many comparisons performed on low sample numbers, for this assay, p values ≤ 0.01 were considered significant.
Cutoff values were calculated by the online application Cutoff Finder (http://molpath.charite.de/cutoff/) [40]. We selected optimal cutoffs as the most significant (log-rank test) splits based on OS data. The optimal cutoff for IFN-γ+CD8+T lymphocytes/ml was determined calculating OS curves for the whole patient population, while optimal cutoffs for Treg/ml, MDSC4/ml and NMLR were determined calculating OS curves for patients in the SOC + oregovomab arm [41].
Chi square test was used to assess significance of differences between proportions of patients in the two study arms categorized according to the defined cutoffs (GraphPad). RFS and OS curves were plotted using Kaplan–Meier method. Survival curves were compared using log-rank test. p values ≤ 0.05 were considered statistically significant.
Results
Patient characteristics
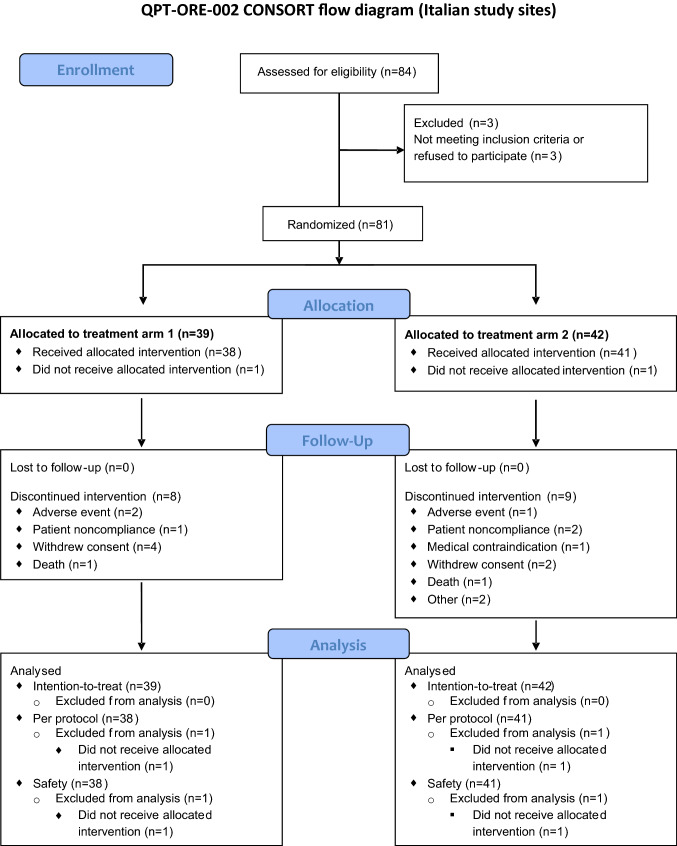
Figure 1 details the treatment schema in a CONSORT flow diagram. Overall, demographics, Eastern Cooperative Oncology Group (ECOG) performance status, medical and cancer-related history were comparable between the two study arms (Table 1). The histopathology was primarily serous adenocarcinoma with grade 3 morphology (88.7%). The present study includes patients from the larger trial cohort for which differential and absolute blood cell counts were available.
Fig. 1.
Study schematic. The CONSORT flow diagram illustrates the different stages of this randomized phase II trial, including patient numbers available at each stage and in each treatment arm (Treatment arm 1 = SOC + oregovomab; Treatment arm 2 = SOC alone)
Treatment efficacy
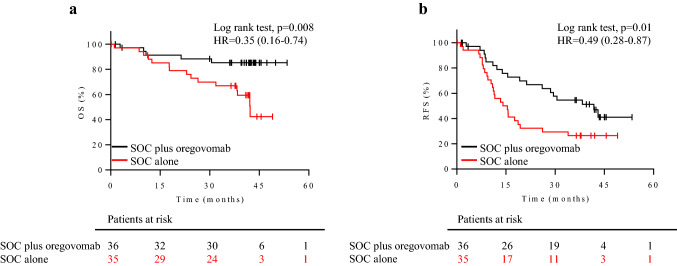
We observed significant differences in OS (p = 0.008, hazard ratio (HR) 0.35, 95% confidence interval (CI) 0.16–0.74) and RFS (p = 0.01, HR 0.49, 95% CI 0.28–0.87) between the SOC + oregovomab and the SOC alone group (Fig. 2a, b) for the cohort at these Italian study sites. Median OS was not achieved in the SOC + oregovomab group. These results are consistent with the overall study data from the full trial cohort (OS p = 0.004, HR 0.35, 95% CI 0.16–0.74, RFS p = 0.003, HR 0.46, 95% CI 0.28–0.77 between the SOC + oregovomab and the SOC alone group, median OS not achieved in the SOC + oregovomab group).
Fig. 2.
Clinical efficacy for the patient cohort included in the present study. a OS and b RFS curves are illustrated for patients stratified by treatment. Black lines: patients on SOC + oregovomab (36 patients). Red lines: patients on SOC alone (35 patients). The corresponding hazard ratio (HR), the associated 95% confidence interval and p values are shown in each graph. Censored patients are indicated on the curves. The number of patients at risk in each group at individual timepoints is also included
CA125-specific CD8+ T lymphocyte response as a surrogate marker for treatment efficacy
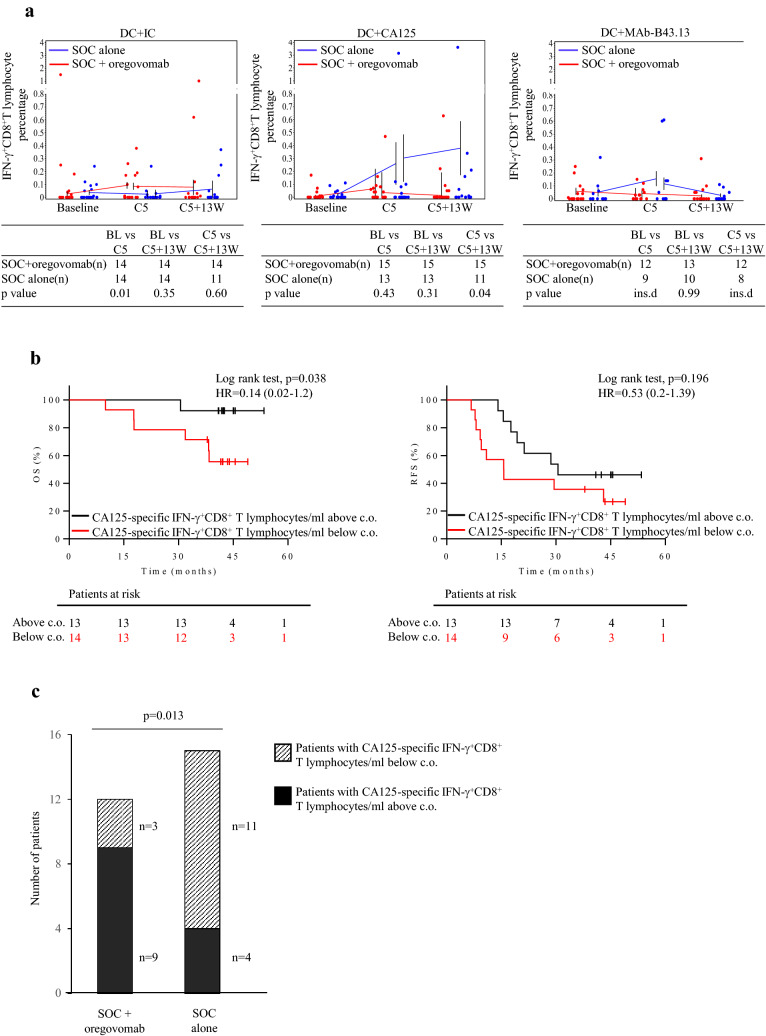
Co-incubation of lymphocytes with IC-pulsed DC revealed that five cycles of SOC with two infusions of oregovomab resulted in an increased frequency of IFN-γ+CD8+T lymphocytes compared to SOC alone (Fig. 3a, left panel and Supplementary Fig. 4a). No such increase was observed following co-incubation with CA125-pulsed DC (Fig. 3a, center panel), indicating less efficient CA125 uptake or presentation [9]. Co-incubation with MAb-B43.13-pulsed DC showed no differences between treatments, although insufficient samples (< 10 in at least one group) were available to assess statistical differences (Fig. 3a, right panel). While the frequency of CA125-specific CD8+ T cells is likely to be more elevated at the tumor site, it is noteworthy that their expansion following SOC + oregovomab treatment can be detected even in peripheral blood.
Fig. 3.
Surrogate markers of clinical efficacy for the cohort of patients included in the present study. a Frequencies of CD8+T lymphocytes producing IFN-γ following an 18 h in vitro stimulation with autologous DC that had been pulsed with either IC (left panel), CA125 (center panel) or MAb-B43.13 (right panel) in patients treated with either SOC + oregovomab (red) or SOC alone (blue) are shown. Dots illustrate individual data points. To generate means, only those patients were taken into account for whom values were available at adjacent timepoints; hence two means and standard errors are provided for cycle 5. The tables below each panel provide patient numbers included in each treatment arm for the respective comparisons being performed, as well as resulting p values. ins.d.: insufficient data. b Kaplan–Meier analysis of OS (left panel) and RFS (right panel) in all patients (i.e., irrespective of treatment). Patients were stratified according to changes in the number of CA125-specific IFN-γ+CD8+ T lymphocytes/ml peripheral blood, the threshold of 6.69 × 103 cells/ml having been defined using Cutoff Finder. Red lines: patients with CA125-specific IFN-γ+CD8+ T lymphocytes/ml below the cutoff. Black lines: patients with CA125-specific IFN-γ+CD8+ T lymphocytes/ml above the cutoff. The corresponding hazard ratio (HR), the associated 95% confidence interval and p values are shown in each graph. Censored patients are indicated on the curves. The number of patients at risk in each group at individual timepoints is also included. c.o.: cutoff. c Number of patients exhibiting a treatment-induced amplification of the CA125- IFN-γ+CD8+T lymphocyte count/ml in the two treatment arms. The relative proportion of patients with changes in the number of CA125-specific IFN-γ+CD8+ T lymphocyte counts above (solid black) or below (hashed) the defined cutoff of 6.69 × 103 lymphocytes/ml in the two study arms significantly differed (p = 0.013)
In keeping with previous observations [13], co-incubation of lymphocytes with IC-pulsed DC showed that 22% and 37% of patients in the SOC + oregovomab and SOC alone groups, respectively (not different by Fisher’s exact test), had circulating CA125-specific CD8+T lymphocytes at baseline (Table 2). Assuming that a defined extent and magnitude of tumor antigen-specific CD8+T lymphocyte expansion is required to attain clinical efficacy, we focused on a treatment’s capacity to generate or stimulate the expansion of CA125-specific CD8+T lymphocytes and normalized data according to individual baseline values in each patient (13 and 14 patients in the SOC + oregovomab and SOC alone arms, respectively).
Treatment-induced changes in the number of CA125-specific IFN-γ+CD8+T lymphocytes/ml (measured after co-incubation with IC-pulsed DC) were calculated as follows: [average CA125-specific IFN-γ+CD8+T lymphocytes/ml at (cycle 5) and (cycle 5 + 13 weeks)]—(CA125-specific IFN-γ+CD8+T lymphocytes/ml at baseline).
We used the web application Cutoff Finder to dichotomize changes in the number of treatment-induced CA125-specific IFN-γ+CD8+T lymphocytes/ml following in vitro stimulation with IC-pulsed DC based on the OS data of the total patient population, i.e., irrespective of treatment. The cutoff that provided the most significant split was an increase of 6.69 × 103 treatment-induced IFN-γ+CD8+T lymphocytes/ml. Patients with CA125-specific CD8+T lymphocytes above this cutoff showed significantly improved OS (p = 0.038, Fig. 3b, left panel). RFS curves also diverged using this same cutoff, though not significant (p = 0.196, Fig. 3b, right panel). Patients with treatment-induced CA125-specific IFN-γ+CD8+T lymphocytes above the cutoff were significantly more frequent in the SOC + oregovomab than in the SOC alone arm (p = 0.013, Fig. 3c; Table 3).
Table 3.
Patient distribution around the cutoffs identified for immune biomarker parameters in the two study arms
| Immune biomarker | Cutoff value | SOC + Oregovomab arm (na/nb, %c) | SOC arm (na/nb, %c) | p valued |
|---|---|---|---|---|
| Change in the number of CA125-specific IFN-γ+CD8+ T lymphocytes/ml | ≥ 6.69x103 | 9/3, 75.0 | 4/11, 26.7 | 0.013 |
| Number of MDSC4/ml | ≥ 137,300 | 10/13, 43.5 | 10/14, 41.7 | n.s. |
| NMLR | ≥ 4.388 | 10/26, 27.8 | 8/27, 22.9 | n.s. |
n.s.: not statistically significant
aNumber of patients with parameter value above cutoff
bNumber of patients with parameter value below cutoff
cPercentage of patients with parameter value above cutoff
dBy Fisher’s exact test
For the international protocol, T cell ELISPOT assessment was a primary endpoint. Technical issues and unavailable optimized reagents precluded per-protocol completion. Therefore, the objective to establish the ELISPOT assessment as a clinical surrogate was not achieved.
Predictive biomarkers of clinical efficacy
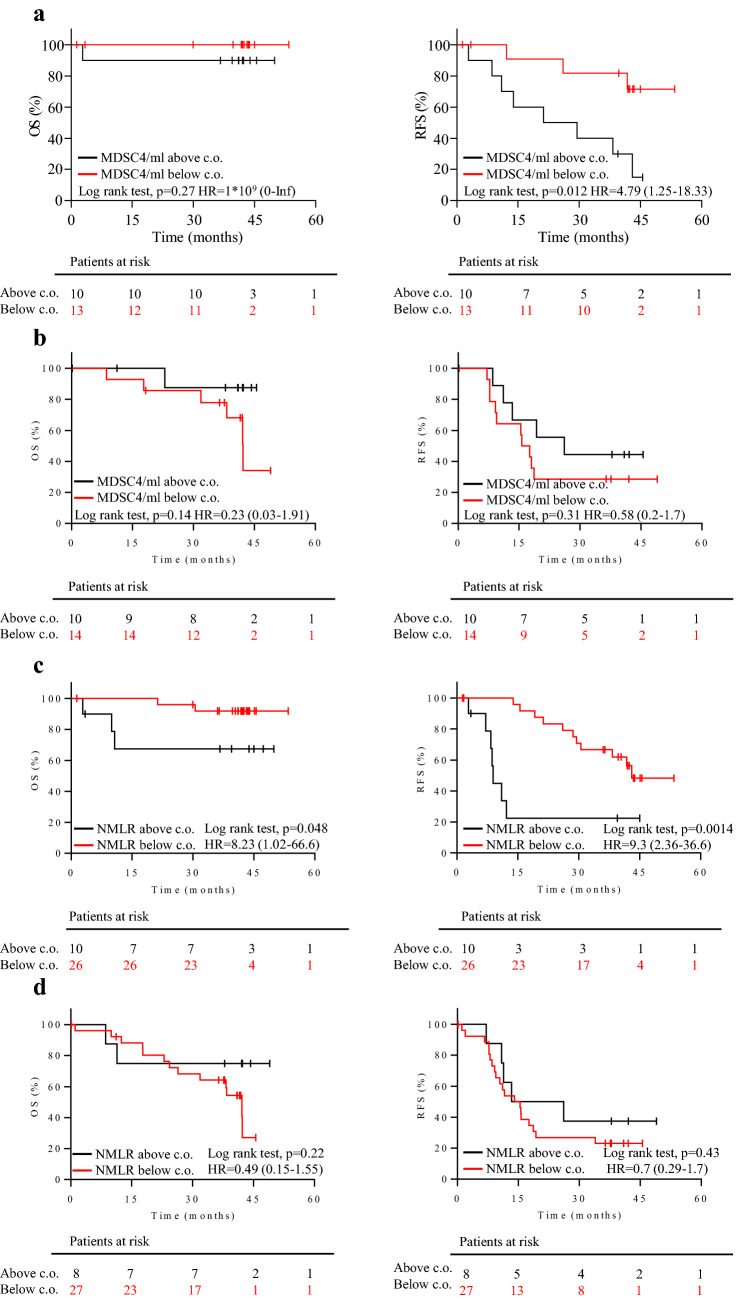
Baseline MDSC4, Treg and NMLR distributions were comparable in the two study groups (Table 2). Based on the OS of SOC + oregovomab patients, the web application Cutoff Finder defined the optimal cutoffs to be 137,300 cells/ml for MDSC4, and 4.388 for NMLR. No meaningful cutoff was obtained for Treg. Patients around the MDSC4 and NMLR cutoffs were similarly distributed across treatment arms (Table 3). The OS and RFS curves of patients stratified according to the MDSC4 and NMLR cutoffs were calculated for each treatment arm. Baseline MDSC4 counts did not correlate with OS (p = 0.27, Fig. 4a, left panel) while being significantly predictive for RFS (p = 0.012, Fig. 4a, right panel) in patients having received SOC + oregovomab. MDSC4 counts did not associate with either OS or RFS in the SOC alone arm (Fig. 4b, left and right panels, respectively). Thus, MDSC4 behaved as a predictive biomarker of relative benefit from oregovomab. Furthermore, patients with MDSC4 counts above the cutoff had similar RFS in the two treatment arms (Fig. 4a, b, right panel, black lines), suggesting that the absence of these suppressor cells allowed oregovomab to confer its therapeutic benefit.
Fig. 4.
Predictive markers of clinical efficacy. a–b Kaplan–Meier survival analysis in patients stratified according to the baseline MDSC4 count. OS and RFS, left and right panel, respectively, in patients in the SOC + oregovomab (a) and SOC alone (b) treatment arms. Patients were stratified according to their distribution around the MDSC4 count cutoff of 137,300 cells/ml. Red lines: patients with MDSC4 count below the cutoff. Black lines: patients with MDSC4 count above the cutoff. c–d Kaplan–Meier survival analysis in patients stratified according to baseline values of NMLR. OS and RFS, left and right panel, respectively, in patients in the SOC + oregovomab (c) and SOC alone (d) treatment arms. Patients were stratified according to their distribution around the NMLR cutoff of 4.388. Red lines: patients with NMLR below the cutoff. Black lines: patients with NMLR above the cutoff. The corresponding hazard ratio (HR), the associated 95% confidence interval and p-values are shown in each graph. In each panel, censored patients are indicated on the curves. The number of patients at risk in each group at individual timepoints is also included. c.o.: cutoff
The NMLR proved to be significantly predictive of both OS and RFS in SOC + oregovomab treated patients (p = 0.048 and p = 0.0014, respectively; Fig. 4c, left and right panels, respectively). Conversely, the NMLR associated with neither OS nor RFS of patients in the SOC alone arm (Fig. 4d, left and right panels, respectively). OS (Fig. 4c, d, left panels, black lines) and RFS (Fig. 4c, d, right panels, black lines) for patients with baseline NMLR > 4.388 were comparable between treatment groups. In the majority of patients (61%, 28/46) NMLR was concordant with MDSC4 count.
Unfortunately, MDSC4 and T lymphocyte response measurements were largely obtained in disparate patient sets, so we were unable to determine whether a correlation between these parameters existed. The same was true for NMLR and T lymphocyte response measurements.
Changes in the number of Treg and MDSC4 following treatment are depicted in the Supplementary Fig. 4b, c, respectively. Notably, Treg but not MDSC4 were transiently affected by SOC treatment.
Discussion
In the present study, we assessed immunological biomarkers of treatment response in advanced OC patients receiving first-line chemotherapy alone or together with oregovomab. Overall, clinical outcomes for this patient cohort were consistent with the larger multicenter trial [Brewer M et al. manuscript submitted]. The present data indicate that oregovomab clinical efficacy is linked to an amplification of a potentially protective CA125-specific cellular immune response when combined with frontline SOC. This confirms and extends a previous study showing that frontline chemo-immunotherapy resulted in a strong immune response to oregovomab using simultaneous oregovomab and chemotherapy infusions [13]. Notably, an antigen-specific cellular immune response has not been reported in a previous mono-immunotherapy protocol in the post frontline maintenance setting [12].
The hypothesis that oregovomab interacts with SOC by amplifying the potentially clinically relevant immune response is in line with preclinical studies showing superior anti-tumor efficacy when combining cancer vaccines with chemotherapy [42, 43]. In the clinical setting, patients suffering from renal cell cancer vaccinated with multiple tumor-associated peptides showed longer OS when pre-treated with a single dose of cyclophosphamide [36]. Mechanisms remain to be defined, which underlie immune interaction of SOC with oregovomab and produce a clinically relevant CA125-specific cellular immune response. Chemotherapy can re-establish immune-surveillance via a cascade of immune activating events, including the induction of immunogenic cancer cell death, cancer antigen spreading, and danger signal generation [44–46]. These same triggering mechanisms may underlie the immuno-stimulatory effects observed after concomitant administration of SOC and oregovomab. Intriguingly, the ability of SOC to facilitate anticancer immune responses may also explain why SOC induced a CA125-specific CD8+ T lymphocyte response in some patients, even in the absence of oregovomab administration. This finding, which has not previously been addressed in the OC setting, warrants further study.
Predictive biomarkers are valuable tools, which enable personalization of treatment regimens. This is of particular importance in cancer settings, where patients often do not have the luxury of time to try different treatment approaches. Because cancer can resist immune destruction through a number of factors contributing to immuno-suppression, we focused on two components of the complex immune suppressive network, which might be detrimental to oregovomab efficacy, namely MDSC4 and Treg. We found that patients with lower baseline MDSC4 counts appeared to benefit more from oregovomab chemo-immunotherapy. Notably, patients in the SOC + oregovomab arm with high baseline MDSC4 counts showed survival curves similar to those of patients treated only with SOC. This observation is relevant for OC patient management, as it implies that the administration of oregovomab to patients with high baseline MDSC4 counts is less likely to show therapeutic benefit. Whether expansion of CA125-specific CD8+ T lymphocytes in circulation is inversely related to baseline MDSC4 cell counts will be addressed in a future study.
The association of baseline MDSC4 with oregovomab efficacy is consistent with the demonstrated negative role of this MDSC subset in the efficacy of immunotherapy in renal cell carcinoma [36] and advanced melanoma patients [37], and concurs with reports that MDSC represent an obstacle for immunotherapies that require an active immune response for clinical efficacy [47–49]. Several strategies are currently being explored to therapeutically target MDSC and improve outcomes of immunotherapies [50–53].
In contrast to MDSC4, the number of baseline circulating Treg, another immuno-suppressive cell type, was devoid of clinical relevance (data not shown). This finding is seemingly out of line with previous studies, where Treg correlated with poor outcome in OC [1–6]. Those studies, however, reported on Treg in the tumor microenvironment, rather than the periphery as in the present study. Moreover, none of the studies addressing the prognostic role of peripheral Treg in cancer included OC [54–56]. It is possible that the immune modulatory effect of SOC is more substantial in the Treg than the myeloid compartment. Supporting this hypothesis, platinum and taxane-based therapies reduced Treg in the tumor microenvironment [57, 58] and had no detectable effect on MDSC [59]. Our results are consistent with those findings, since we observed that SOC treatment induced a transient decrease in peripheral Treg counts, while it did not affect peripheral MDSC4 counts. Thus, we suggest that Treg neutralization by SOC contribute to the observed clinical benefit and the specific immune stimulation ensuing the concomitant SOC and oregovomab administration.
Baseline NMLR had a predictive role in patients undergoing oregovomab vaccination with SOC, where patients with higher baseline NMLR demonstrated a lesser apparent benefit of oregovomab inclusion in their treatment regimen. To our knowledge, this is the first report of baseline NMLR as a predictive biomarker in anti-tumor vaccine therapies, although recently published meta-analyses demonstrated that a high pre-treatment neutrophil-to-lymphocyte ratio (NLR), which is similar to NMLR, served as a negative predictive biomarker of immune-mediated treatment efficacy for immune checkpoint blockade therapies in advanced-stage cancer patients [60, 61].
Several studies indicated that both MDSC and neutrophils can inhibit the anti-tumor cellular immune response in several types of tumors, including OC [62, 63], and most of the factors favoring MDSC generation and activation [19, 49] are pro-inflammatory molecules exerting pressure on the bone marrow to release undifferentiated myeloid cells in addition to mature neutrophils. It is of note that pathologically activated neutrophils can be viewed as MDSC [64] and that immuno-suppressive neutrophils characterized by IL-10 production, a feature of MDSC, have been described to be enriched in melanoma patients and associated with elevated amounts of acute-phase proteins involved in the control of neutrophil plasticity [65]. A recent review discussed the direct detrimental role played by certain cancer-related neutrophil subsets and their possible relationship with MDSC [66]. Since in the present study, the two predictive biomarkers, NMLR and MDSC4 count, overlapped to some extent, we hypothesize that they both reflect an immuno-suppressive environment able to hamper the development of an efficacious anti-tumor immune response following oregovomab chemo-immunotherapy. The planned phase III clinical trial will delineate whether these two factors are associated.
In conclusion, this study demonstrated for the first time that oregovomab treatment in combination with conventional SOC is capable of expanding a potentially clinically efficient CA125-specific cellular immune response in OC patients. Furthermore, high baseline MDSC4 counts and high baseline NMLR represent apparent obstacles to oregovomab chemo-immunotherapy efficacy, highlighting the potential importance of these two predictive biomarkers in anticipating relative responsiveness of newly diagnosed patients and of including agents targeting the myeloid compartment in future therapeutic combinations.
The design of personalized scheduled combinatorial therapies will significantly benefit from a better understanding of the complex interaction between cancer and the innate and adaptive immune systems.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- ANOVA
Analysis of variance
- CA125
Carbohydrate/cancer antigen 125
- CI
Confidence interval
- DC
Dendritic cell
- ECOG
Eastern Cooperative Oncology Group
- HR
Hazard ratio
- IC
Immune complex
- iDC
Immature dendritic cell
- mDC
Mature dendritic cell
- NLR
Neutrophil-to-lymphocyte ratio
- NM
Neutrophil-and-monocyte
- NMLR
Neutrophil-and-monocyte-to-lymphocyte ratio
- OC
Ovarian cancer
- OS
Overall survival
- RFS
Relapse-free survival
- RT
Residual tumor
- SEB
Staphylococcal enterotoxin B
- SOC
Standard of care
- TILs
Tumor-infiltrating lymphocytes
Author contributions
Conception and design: Alessandra Battaglia, Andrea Fattorossi, Madi R. Madiyalakan, Christopher Nicodemus and Giovanni Scambia. Development of methodology: Alessandra Battaglia, Alexia Buzzonetti, Andrea Fattorossi and Marco Fossati. Acquisition and analysis of data: Alexia Buzzonetti and Marco Fossati. Data interpretation: Alessandra Battaglia and Andrea Fattorossi. Statistical analysis: Alessandra Battaglia and Yolanda D. Mahnke with the help of ZellNet (Fort Lee, NJ, USA). Writing and reviewing of manuscript: Alessandra Battaglia, Andrea Fattorossi, Madi R. Madiyalakan, Yolanda D. Mahnke, Christopher Nicodemus and Giovanni Scambia.
Funding
This study was funded by OncoQuest Inc (Edmonton, AB, Canada).
Compliance with ethical standards
Conflict of interest
Yolanda D. Mahnke is an immunology advisor to OncoQuest. Christopher Nicodemus is a consultant to OncoQuest, Inc. and owns shares in Quest PharmaTech, Edmonton Alberta. Madi R. Madiyalakan is an employee of OncoQuest Inc. and Quest PharmaTech and owns shares in OncoQuest Inc. and Quest PharmaTech. The authors declare that there is no other conflict of interest.
Ethical approval
This QPT-ORE-002 study (clinical trial information: NCT01616303) was performed in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. It was approved by the following institutional ethics committees:
| Study site number | Location | Ethics committee |
|---|---|---|
| 001 | Campus Biomedico University, Rome, Italy |
Comitato Etico dell’Università Campus Bio Medico di Roma Via Alvaro del Portillo, 200 00128 Rome |
| 002 | Fondazione Policlinico Universitario A.Gemelli IRCCS, Rome, Italy |
Comitato Etico dell’Università Cattolica del Sacro Cuore e Annesso Policlinico “A. Gemelli” Largo Francesco Vito, 1 00168 Rome |
| 003 | Istituto Nazionale Tumori-IRCCS, Milan, Italy |
Comitato Etico della Fondazione IRCCS “Istituto Nazionale dei Tumori” Via G. Venezian, 1 20133 Milan |
| 005 | Azienda Ospedaliera Cannizzaro, Catania, Italy |
Comitato Etico Catania 1 Via S. Sofia, 78 95123 Catania |
| 006 | Azienda Ospedali Riuniti di Bergamo, Bergamo, Italy |
Comitato Etico della Provincia di Bergamo Piazza OMS, 1 24127 Bergamo |
| 007 | Policlinico Umberto I, Rome, Italy |
Comitato Etico dell’Università Sapienza Viale del Policlinico, 155 00161 Rome |
Informed consent
All patients included in the study provided written informed consent according to the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines. The informed consent, which included consent to the treatment, use of their biological specimen (peripheral blood) and data acquisition and processing, was obtained from patients prior to the initiation of the study.
Footnotes
Some of the results included in this paper were previously published in a poster at the 20th biennial international meeting (November 4–7, 2017, Vienna, Austria) of the European Society of Gynaecological Oncology (ESGO).
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
3/26/2020
The original version of this article unfortunately contained a mistake.
References
- 1.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio MGR, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348(3):203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 2.Mariya T, Hirohashi Y, Torigoe T, Asano T, Kuroda T, Yasuda K, Mizuuchi M, Sonoda T, Saito T, Sato N. Prognostic impact of human leukocyte antigen class I expression and association of platinum resistance with immunologic profiles in epithelial ovarian cancer. Cancer Immunol Res. 2014;2(12):1220–1229. doi: 10.1158/2326-6066.CIR-14-0101. [DOI] [PubMed] [Google Scholar]
- 3.Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N, Honjo T, Fujii S. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8 + T lymphocytes are prognostic factors of human ovarian cancer. Process Natl Acad Sci USA. 2007;104(9):3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10(9):942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 5.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, Kepner J, Odunsi T, Ritter G, Lele S, Chen YT, Ohtani H, Old LJ, Odunsi K. Intraepithelial CD8 + tumor-infiltrating lymphocytes and a high CD8 +/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Process Natl Acad Sci USA. 2005;102(51):18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Preston CC, Maurer MJ, Oberg AL, Visscher DW, Kalli KR, Hartmann LC, Goode EL, Knutson KL. The ratios of CD8 + T cells to CD4 + CD25 + FOXP3 + and FOXP3- T cells correlate with poor clinical outcome in human serous ovarian cancer. PLoS One. 2013;8(11):e80063. doi: 10.1371/journal.pone.0080063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paijens ST, Leffers N, Daemen T, Helfrich W, Boezen HM, Cohlen BJ, Melief CJ, de Bruyn M, Nijman HW. Antigen-specific active immunotherapy for ovarian cancer. Cochrane Database Syst Rev. 2018;9:CD007287. doi: 10.1002/14651858.cd007287.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nustad K, Bast RC, Jr, Brien TJ, Nilsson O, Seguin P, Suresh MR, Saga T, Nozawa S, Bormer OP, de Bruijn HW, Nap M, Vitali A, Gadnell M, Clark J, Shigemasa K, Karlsson B, Kreutz FT, Jette D, Sakahara H, Endo K, Paus E, Warren D, Hammarstrom S, Kenemans P, Hilgers J. Specificity and affinity of 26 monoclonal antibodies against the CA 125 antigen: first report from the ISOBM TD-1 workshop. International Society for Oncodevelopmental Biology and Medicine. Tumour Biol. 1996;17(4):196–219. doi: 10.1159/000217982. [DOI] [PubMed] [Google Scholar]
- 9.Capstick V, Maclean GD, Suresh MR, Bodnar D, Lloyd S, Shepert L, Longenecker BM, Krantz M. Clinical evaluation of a new two-site assay for CA125 antigen. Int J Biol Markers. 1991;6(2):129–135. doi: 10.1177/172460089100600208. [DOI] [PubMed] [Google Scholar]
- 10.Piché A. Pathobiological role of MUC16 mucin (CA125) in ovarian cancer: much more than a tumor biomarker. World J Obstet Gynecol. 2016;5(1):39–49. doi: 10.5317/wjog.v5.i1.39. [DOI] [Google Scholar]
- 11.Noujaim AA, Schultes BC, Baum RP, Madiyalakan R. Induction of CA125-specific B and T cell responses in patients injected with MAb-B43.13–evidence for antibody-mediated antigen-processing and presentation of CA125 in vivo. Cancer Biother Radiopharm. 2001;16(3):187–203. doi: 10.1089/10849780152389384. [DOI] [PubMed] [Google Scholar]
- 12.Berek JS, Taylor PT, Gordon A, Cunningham MJ, Finkler N, Orr J, Jr, Rivkin S, Schultes BC, Whiteside TL, Nicodemus CF. Randomized, placebo-controlled study of oregovomab for consolidation of clinical remission in patients with advanced ovarian cancer. J Clin Oncol. 2004;22(17):3507–3516. doi: 10.1200/jco.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 13.Braly P, Nicodemus CF, Chu C, Collins Y, Edwards R, Gordon A, McGuire W, Schoonmaker C, Whiteside T, Smith LM, Method M. The Immune adjuvant properties of frontline carboplatin-paclitaxel: a randomized phase 2 study of alternative schedules of intravenous oregovomab chemoimmunotherapy in advanced ovarian cancer. J Immunother. 2009;32(1):54–65. doi: 10.1097/CJI.0b013e31818b3dad. [DOI] [PubMed] [Google Scholar]
- 14.Ferrandina G, Braly PS, Terranova C, Salutari V, Ricci C, Raspagliesi F, Lorusso D, Panici PB, Scollo P, Plotti F, Brewer M, Method MW, Holloway RW, Madiyalakan M, Nicodemus CF, Pecorelli SL, Scambia G, Angioli R. A randomized phase II study assessing an optimized schedule of oregovomab (O) anti-CA125 vaccination with carboplatin paclitaxel (CP) relative to CP alone in Frontline treatment of optimally cytoreduced stage III/IV ovarian cancer (EOC) J Clin Oncol. 2017;35(15_suppl):5536. doi: 10.1200/jco.2017.35.15_suppl.5536. [DOI] [Google Scholar]
- 15.Marshall HT, Djamgoz MBA. Immuno-oncology: emerging targets and combination therapies. Front Oncol. 2018;8:315. doi: 10.3389/fonc.2018.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santegoets SJ, Dijkgraaf EM, Battaglia A, Beckhove P, Britten CM, Gallimore A, Godkin A, Gouttefangeas C, de Gruijl TD, Koenen HJ, Scheffold A, Shevach EM, Staats J, Tasken K, Whiteside TL, Kroep JR, Welters MJ, van der Burg SH. Monitoring regulatory T cells in clinical samples: consensus on an essential marker set and gating strategy for regulatory T cell analysis by flow cytometry. Cancer Immunol Immunother. 2015;64(10):1271–1286. doi: 10.1007/s00262-015-1729-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munn DH, Sharma MD, Johnson TS. Treg destabilization and reprogramming: implications for cancer immunotherapy. Cancer Res. 2018;78(18):5191–5199. doi: 10.1158/0008-5472.Can-18-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sinha P, Okoro C, Foell D, Freeze HH, Ostrand-Rosenberg S, Srikrishna G. Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. J Immunol. 2008;181(7):4666–4675. doi: 10.4049/jimmunol.181.7.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tcyganov E, Mastio J, Chen E, Gabrilovich DI. Plasticity of myeloid-derived suppressor cells in cancer. Curr Opin Immunol. 2018;51:76–82. doi: 10.1016/j.coi.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moses K, Brandau S. Human neutrophils: their role in cancer and relation to myeloid-derived suppressor cells. Semin Immunol. 2016;28(2):187–196. doi: 10.1016/j.smim.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 22.Vukmanovic-Stejic M, Zhang Y, Cook JE, Fletcher JM, McQuaid A, Masters JE, Rustin MH, Taams LS, Beverley PC, Macallan DC, Akbar AN. Human CD4 + CD25hi Foxp3 + regulatory T cells are derived by rapid turnover of memory populations in vivo. J Clin Invest. 2006;116(9):2423–2433. doi: 10.1172/jci28941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Facciabene A, Motz GT, Coukos G. T-regulatory cells: key players in tumor immune escape and angiogenesis. Cancer Res. 2012;72(9):2162–2171. doi: 10.1158/0008-5472.Can-11-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X, Zhang W, Yuan X, Fu M, Qian H, Xu W. Neutrophils in cancer development and progression: roles, mechanisms, and implications (Review) Int J Oncol. 2016;49(3):857–867. doi: 10.3892/ijo.2016.3616. [DOI] [PubMed] [Google Scholar]
- 25.Minami S, Ihara S, Komuta K. Pretreatment lymphocyte to monocyte ratio as a prognostic marker for advanced pulmonary squamous cell carcinoma treated with chemotherapy. J Clin Med Res. 2018;10(8):657–664. doi: 10.14740/jocmr3490w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Proctor MJ, McMillan DC, Morrison DS, Fletcher CD, Horgan PG, Clarke SJ. A derived neutrophil to lymphocyte ratio predicts survival in patients with cancer. Br J Cancer. 2012;107(4):695–699. doi: 10.1038/bjc.2012.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim YJ, Lee I, Chung YS, Nam E, Kim S, Kim SW, Kim YT, Lee JY. Pretreatment neutrophil-to-lymphocyte ratio and its dynamic change during neoadjuvant chemotherapy as poor prognostic factors in advanced ovarian cancer. Obstet Gynecol Sci. 2018;61(2):227–234. doi: 10.5468/ogs.2018.61.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baert T, Van Camp J, Vanbrabant L, Busschaert P, Laenen A, Han S, Van Nieuwenhuysen E, Vergote I, Coosemans A. Influence of CA125, platelet count and neutrophil to lymphocyte ratio on the immune system of ovarian cancer patients. Gynecol Oncol. 2018;150(1):31–37. doi: 10.1016/j.ygyno.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 29.Komura N, Mabuchi S, Yokoi E, Kozasa K, Kuroda H, Sasano T, Matsumoto Y, Kimura T. Comparison of clinical utility between neutrophil count and neutrophil-lymphocyte ratio in patients with ovarian cancer: a single institutional experience and a literature review. Int J Clin Oncol. 2018;23(1):104–113. doi: 10.1007/s10147-017-1180-4. [DOI] [PubMed] [Google Scholar]
- 30.Zhou M, Li L, Wang X, Wang C, Wang D. Neutrophil-to-lymphocyte ratio and platelet count predict long-term outcome of stage iiic epithelial ovarian cancer. Cell Physiol Biochem. 2018;46(1):178–186. doi: 10.1159/000488420. [DOI] [PubMed] [Google Scholar]
- 31.Buzzonetti A, Fossati M, Catzola V, Scambia G, Fattorossi A, Battaglia A. Immunological response induced by abagovomab as a maintenance therapy in patients with epithelial ovarian cancer: relationship with survival-a substudy of the MIMOSA trial. Cancer Immunol Immunother. 2014;63(10):1037–1045. doi: 10.1007/s00262-014-1569-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schultes BC, Whiteside TL. Monitoring of immune responses to CA125 with an IFN-g ELISPOT assay. J Immunol Methods. 2003;279:1–15. doi: 10.1016/S0022-1759(03)00253-9. [DOI] [PubMed] [Google Scholar]
- 33.Mandruzzato S, Brandau S, Britten CM, Bronte V, Damuzzo V, Gouttefangeas C, Maurer D, Ottensmeier C, van der Burg SH, Welters MJ, Walter S. Toward harmonized phenotyping of human myeloid-derived suppressor cells by flow cytometry: results from an interim study. Cancer Immunol Immunother. 2016;65(2):161–169. doi: 10.1007/s00262-015-1782-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, Castelli C, Mariani L, Parmiani G, Rivoltini L. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol. 2007;25(18):2546–2553. doi: 10.1200/jco.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- 35.Wang PF, Song SY, Wang TJ, Ji WJ, Li SW, Liu N, Yan CX. Prognostic role of pretreatment circulating MDSCs in patients with solid malignancies: a meta-analysis of 40 studies. Oncoimmunology. 2018;7(10):e1494113. doi: 10.1080/2162402x.2018.1494113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walter S, Weinschenk T, Stenzl A, Zdrojowy R, Pluzanska A, Szczylik C, Staehler M, Brugger W, Dietrich PY, Mendrzyk R, Hilf N, Schoor O, Fritsche J, Mahr A, Maurer D, Vass V, Trautwein C, Lewandrowski P, Flohr C, Pohla H, Stanczak JJ, Bronte V, Mandruzzato S, Biedermann T, Pawelec G, Derhovanessian E, Yamagishi H, Miki T, Hongo F, Takaha N, Hirakawa K, Tanaka H, Stevanovic S, Frisch J, Mayer-Mokler A, Kirner A, Rammensee HG, Reinhardt C, Singh-Jasuja H. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med. 2012;18(8):1254–1261. doi: 10.1038/nm.2883. [DOI] [PubMed] [Google Scholar]
- 37.Martens A, Wistuba-Hamprecht K, Geukes Foppen M, Yuan J, Postow MA, Wong P, Romano E, Khammari A, Dreno B, Capone M, Ascierto PA, Di Giacomo AM, Maio M, Schilling B, Sucker A, Schadendorf D, Hassel JC, Eigentler TK, Martus P, Wolchok JD, Blank C, Pawelec G, Garbe C, Weide B. Baseline peripheral blood biomarkers associated with clinical outcome of advanced melanoma patients treated with ipilimumab. Clin Cancer Res. 2016;22(12):2908–2918. doi: 10.1158/1078-0432.Ccr-15-2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, Solomon M, Selby W, Alexander SI, Nanan R, Kelleher A, de St Fazekas, Groth B. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203(7):1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, de St Fazekas, Groth B, Clayberger C, Soper DM, Ziegler SF, Bluestone JA. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4 + T reg cells. J Exp Med. 2006;203(7):1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Budczies J, Klauschen F, Sinn BV, Gyorffy B, Schmitt WD, Darb-Esfahani S, Denkert C. Cutoff Finder: a comprehensive and straightforward web application enabling rapid biomarker cutoff optimization. PLoS One. 2012;7(12):e51862. doi: 10.1371/journal.pone.0051862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Group F-NBW (2016) BEST (Biomarkers, EndpointS, and other Tools) resource [Internet]. understanding prognostic versus predictive biomarkers. Food and Drug Administration (US). Co-published by National Institutes of Health (US), Bethesda (MD). Silver Spring (MD)
- 42.van der Sluis TC, van Duikeren S, Huppelschoten S, Jordanova ES, Beyranvand Nejad E, Sloots A, Boon L, Smit VT, Welters MJ, Ossendorp F, van de Water B, Arens R, van der Burg SH, Melief CJ. Vaccine-induced tumor necrosis factor-producing T cells synergize with cisplatin to promote tumor cell death. Clin Cancer Res. 2015;21(4):781–794. doi: 10.1158/1078-0432.Ccr-14-2142. [DOI] [PubMed] [Google Scholar]
- 43.Wang X, Liu Y, Diao Y, Gao N, Wan Y, Zhong J, Zheng H, Wang Z, Jin G. Gastric cancer vaccines synthesized using a TLR7 agonist and their synergistic antitumor effects with 5-fluorouracil. J Transl Med. 2018;16(1):120. doi: 10.1186/s12967-018-1501-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell. 2015;28(6):690–714. doi: 10.1016/j.ccell.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 45.Vacchelli E, Ma Y, Baracco EE, Zitvogel L, Kroemer G. Yet another pattern recognition receptor involved in the chemotherapy-induced anticancer immune response: formyl peptide receptor-1. Oncoimmunology. 2016;5(5):e1118600. doi: 10.1080/2162402x.2015.1118600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu J, Waxman DJ. Immunogenic chemotherapy: dose and schedule dependence and combination with immunotherapy. Cancer Lett. 2018;419:210–221. doi: 10.1016/j.canlet.2018.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sade-Feldman M, Kanterman J, Klieger Y, Ish-Shalom E, Olga M, Saragovi A, Shtainberg H, Lotem M, Baniyash M. Clinical significance of circulating CD33 + CD11b + HLA-DR- myeloid cells in patients with stage IV melanoma treated with ipilimumab. Clin Cancer Res. 2016;22(23):5661–5672. doi: 10.1158/1078-0432.Ccr-15-3104. [DOI] [PubMed] [Google Scholar]
- 48.Hansen GL, Gaudernack G, Brunsvig PF, Cvancarova M, Kyte JA. Immunological factors influencing clinical outcome in lung cancer patients after telomerase peptide vaccination. Cancer Immunol Immunother. 2015;64(12):1609–1621. doi: 10.1007/s00262-015-1766-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ostrand-Rosenberg S, Fenselau C. Myeloid-derived suppressor cells: immune-suppressive cells that impair antitumor immunity and are sculpted by their environment. J Immunol. 2018;200(2):422–431. doi: 10.4049/jimmunol.1701019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dominguez GA, Condamine T, Mony S, Hashimoto A, Wang F, Liu Q, Forero A, Bendell J, Witt R, Hockstein N, Kumar P, Gabrilovich DI. Selective targeting of myeloid-derived suppressor cells in cancer patients using DS-8273a, an agonistic TRAIL-R2 antibody. Clin Cancer Res. 2017;23(12):2942–2950. doi: 10.1158/1078-0432.Ccr-16-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu Y, Wei G, Cheng WA, Dong Z, Sun H, Lee VY, Cha SC, Smith DL, Kwak LW, Qin H. Targeting myeloid-derived suppressor cells for cancer immunotherapy. Cancer Immunol Immunother. 2018;67(8):1181–1195. doi: 10.1007/s00262-018-2175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fleming V, Hu X, Weber R, Nagibin V, Groth C, Altevogt P, Utikal J, Umansky V. Targeting myeloid-derived suppressor cells to bypass tumor-induced immunosuppression. Front Immunol. 2018;9:398. doi: 10.3389/fimmu.2018.00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clappaert EJ, Murgaski A, Van Damme H, Kiss M, Laoui D. Diamonds in the rough: harnessing tumor-associated myeloid cells for cancer therapy. Front Immunol. 2018;9:2250. doi: 10.3389/fimmu.2018.02250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kotsakis A, Koinis F, Katsarou A, Gioulbasani M, Aggouraki D, Kentepozidis N, Georgoulias V, Vetsika EK. Prognostic value of circulating regulatory T cell subsets in untreated non-small cell lung cancer patients. Sci Rep. 2016;6:39247. doi: 10.1038/srep39247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu T, Lu J, An H. The relative change in regulatory T cells/T helper lymphocytes ratio as parameter for prediction of therapy efficacy in metastatic colorectal cancer patients. Oncotarget. 2017;8(65):109079–109093. doi: 10.18632/oncotarget.22606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Riemann D, Cwikowski M, Turzer S, Giese T, Grallert M, Schutte W, Seliger B. Blood immune cell biomarkers in lung cancer. Clin Exp Immunol. 2019;195(2):179–189. doi: 10.1111/cei.13219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bohm S, Montfort A, Pearce OMT, Topping J, Chakravarty P, Everitt GLA, Clear A, McDermott JR, Ennis D, Dowe T, Fitzpatrick A, Brockbank EC, Lawrence AC, Jeyarajah A, Faruqi AZ, McNeish IA, Singh N, Lockley M, Bohm FRB. Neoadjuvant chemotherapy modulates the immune microenvironment in metastases of tubo-ovarian high-grade serous carcinoma. Clin Cancer Res. 2016;22(12):3025–3036. doi: 10.1158/1078-0432.CCR-15-2657. [DOI] [PubMed] [Google Scholar]
- 58.Pircher A, Gamerith G, Amann A, Reinold S, Popper H, Gächter A, Palla G, Wöll E, Jamnig H, Gastl G, Wolf AM, Hilbe W, Wolf D. Neoadjuvant chemo-immunotherapy modifies CD4 + CD25 + regulatoryT cells (Treg) in non-small cell lung cancer (NSCLC) patients. Lung Cancer. 2014;85:81–87. doi: 10.1016/j.lungcan.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 59.Shoji SH, Tada K, Kitano S, Nishimura T, Shimada Y, Nagashima K, Aoki K, Hiraoka N, Honma Y, Iwasa S, Takashima A, Kato K, Boku N, Honda K, Yamada T, Heike Y, Hamaguchi T. The peripheral immune status of granulocytic myeloid-derived suppressor cells correlates the survival in advanced gastric cancer patients receiving cisplatin-based chemotherapy. Oncotarget. 2017;8(56):95083–95094. doi: 10.18632/oncotarget.18297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jiang T, Qiao M, Zhao C, Li X, Gao G, Su C, Ren S, Zhou C. Pretreatment neutrophil-to-lymphocyte ratio is associated with outcome of advanced-stage cancer patients treated with immunotherapy: a meta-analysis. Cancer Immunol Immunother. 2018;67(5):713–727. doi: 10.1007/s00262-018-2126-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sacdalan DB, Lucero JA, Sacdalan DL. Prognostic utility of baseline neutrophil-to-lymphocyte ratio in patients receiving immune checkpoint inhibitors: a review and meta-analysis. Onco Targets Ther. 2018;11:955–965. doi: 10.2147/ott.S153290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Walankiewicz M, Grywalska E, Polak G, Kotarski J, Siwicka-Gieroba DJ, Rolinski J. Myeloid-derived suppressor cells in ovarian cancer: friend or foe? Cent Eur J Immunol. 2017;42(4):383–389. doi: 10.5114/ceji.2017.72823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Uribe-Querol E, Rosales C. Neutrophils in cancer: two sides of the same coin. J Immunol Res. 2015;2015:983698. doi: 10.1155/2015/983698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer. 2016;16(7):431–446. doi: 10.1038/nrc.2016.52. [DOI] [PubMed] [Google Scholar]
- 65.De Santo C, Arscott R, Booth S, Karydis I, Jones M, Asher R, Salio M, Middleton M, Cerundolo V. Invariant NKT cells modulate the suppressive activity of IL-10-secreting neutrophils differentiated with serum amyloid A. Nat Immunol. 2010;11(11):1039–1046. doi: 10.1038/ni.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou J, Nefedova Y, Lei A, Gabrilovich D. Neutrophils and PMN-MDSC: their biological role and interaction with stromal cells. Semin Immunol. 2018;35:19–28. doi: 10.1016/j.smim.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.