Abstract
Background
The role of tumor-infiltrating lymphocytes (TILs) in the immune remodeling of tumor microenvironments (TME) in oral squamous cell carcinoma (OSCC) remains controversial. In this study, we pursued a comprehensive characterization of the repertoire of TILs and then analyzed its clinical significance and potential prognostic value.
Methods
Fresh tumor tissue samples and peripheral blood from 83 OSCC patients were collected to comprehensively characterize the phenotypes and frequencies of TILs by flow cytometry. Archived paraffin-embedded tissues derived from 159 OSCC patients were analyzed by immunohistochemistry to further assess the TIL repertoire. The clinical significance of TILs and their potential prognostic value were further analyzed.
Results
A series of unique features of TILs were observed. IL-17 was highly expressed in betel nut chewers, and CD20 was abundantly expressed in patients who did not drink alcohol; high expression of CD138, PD-L1, and Foxp3 was associated with poor prognosis. The Th17/Treg ratio was an independent prognostic factor for patient survival with greater predictive accuracy for overall survival.
Conclusions
Our results suggest an antigen-driven immune response; however, the immune dysfunction within the microenvironment in OSCC and the Th17/Treg balance may play important roles in the modulation of antitumor immunity.
Electronic supplementary material
The online version of this article (10.1007/s00262-020-02479-x) contains supplementary material, which is available to authorized users.
Keywords: Tumor-infiltrating lymphocytes, Immune microenvironment, Oral squamous cell carcinoma, Clinical significance, Prognosis
Introduction
Oral squamous cell carcinoma (OSCC) is the sixth most common cancer worldwide [1]. An estimated 300,400 patients were diagnosed and 145,400 patients died from the disease in 2012 worldwide [1, 2]. Smoking, alcohol drinking, betel nut chewing, and human papillomavirus infection are major risk factors for OSCC [3–5]. Despite the improvement of therapy, the 5-year survival rate of patients with OSCC has remained below 50% over the past three decades [6, 7]. Led by immune checkpoint blockade (ICB) and chimeric antigen receptor (CAR) T-cell therapies, immunotherapy may emerge as a promising option. However, the tumor responses to those immune therapies are not exactly the same and even fail to some tumors, especially solid tumors [8, 9]. Unlike hematological malignancies, solid tumors have more complicated microenvironments that may play intricate roles in the host immune response [10]. Therefore, an improved understanding of the interaction between tumor microenvironments (TME) and host immune systems is needed to optimize the efficacy of current approaches and to develop new strategies for OSCC.
Tumor-infiltrating lymphocytes (TILs) are important features of many solid tumors, and this phenomenon is suggested to be a manifestation of an immune response between tumor cells and immune effector cells [11]. Substantial evidence supports a link between the presence of TILs and the survival of cancer patients. A previous study suggested that high CD8+ T-cell expression among TILs was an independent factor for favorable survival in head and neck squamous cell carcinoma (HNSCC) [12]. High levels of CD4+ TILs combined with low levels of CD8+ TILs were associated with unfavorable prognosis in glioma [13]. The expression of regulatory T cells was not a predictor of patient survival in esophageal squamous cell carcinoma [14].
Although several previous studies have described the obvious infiltrating lymphocytes in OSCC, their role in immune remodeling of the TME remains controversial. However, they are key for understanding the immune responses within the TME and further facilitate treatment stratification in OSCC. In this study, we pursued a comprehensive characterization of the repertoire of TILs and correlated TILs with patient outcomes to elucidate their potential prognostic value in OSCC. We found a series of unique features of TILs in OSCC, demonstrating not only the antigen-driven immune response, but also immune dysfunction within the microenvironment. The clinical significance of TILs was also clarified, revealing that the ratio of T helper 17 (Th17) cells to regulatory T cell (Treg) was a significant independent prognostic factor for OSCC.
Materials and methods
Patients and specimens
Fresh tumor tissue samples and peripheral blood from 83 OSCC patients were collected for flow cytometric analysis to comprehensively characterize the phenotypes and frequencies of TILs. Archived paraffin-embedded tissues derived from 159 OSCC patients were analyzed by immunohistochemistry (IHC) to determine the TIL repertoire and the clinical significance and potential prognostic value of TILs. The patients had no other cancers or diseases, including acute infection or diabetes, and they had not received previous radiotherapy or chemotherapy at the time of the study. The tumor histological grade was determined according to the World Health Organization classification [15]. The clinical staging was assessed according to the eighth edition of the American Joint Committee on Cancer (AJCC) Staging Manual [16]. The patients in the IHC cohort underwent physical examinations or phone interviews once every 3 months until death or the deadline of the study. The mean follow-up time was 48 months. All patients were from the two hospitals (Xiangya Stomatological Hospital and Hunan Cancer Hospital). The demographic information of two cohorts is summarized in Supplemental Tables 1 and 2.
Flow cytometry
Peripheral blood of OSCC patients was collected in EDTA-K2 tubes before surgery and processed immediately. Peripheral blood mononuclear cells (PBMCs) were isolated by using a Ficoll–Hypaque gradient (10771, Sigma, St. Louis, USA). Tumor tissues were obtained during surgery. TILs were obtained by Percoll discontinuous density gradient centrifugation after enzymatic digestion and mechanical dissociation with mesh cell strainers. Flow cytometric analysis was performed with the following fluorescence-tagged anti-human surface Abs according to the manufacturer’s protocol: anti-CD3-PerCP-Cy5.5 (Clone UCHT1, BD Pharmingen, Franklin Lakes, USA), anti-CD4-V450 (Clone RPA-T4, BD Pharmingen), anti-CD8-BV786 (Clone RPA-T8, BD Pharmingen), anti-CD45RA-FITC (Clone HI100, BD Pharmingen), anti-CD45RO-PE-Cy7 (Clone UCHL1, BD Pharmingen), anti-Tim-3-PE (Clone F38–2E2, BioLegend, San Diego, USA), anti-PD-1-APC (Clone MIH4, BD Pharmingen), CD25 (Clone M-A251, BD Pharmingen), anti-IL-17A-PerCP-CY5.5 (Clone N49-653, BD Pharmingen), anti-Foxp3-PE (Clone259D/C7, BD Pharmingen), IOTest Beta Mark TCR-Vβ Repertoire kit (PN IM3497, Beckman Coulter, Marseille, France), anti-CD45-APC-H7 (Clone 2D1, BD Pharmingen), anti-CD19-PerCp-Cy5.5 (Clone HIB19, BD Pharmingen), anti-CD38-BV421(Clone HIT2, BD Horizon), anti-CD27-FITC (Clone m-t271, BD Pharmingen), anti-CD138-PE (Clone MI15, BD Pharmingen), anti-IL10-APC(Clone JES3-19F1, BD Pharmingen), anti-IL4-PE (Clone 8D4-8, BioLegend), anti-INF-γ-FITC (Clone 25723.11, BD Pharmingen), and cell activation cocktail (BioLegend). All flow cytometric analyses were performed on a BD AriaIII instrument (BD Biosciences), and all data analysis was performed using FlowJo v10.0.7 software.
Immunohistochemistry
Paraffin-embedded specimens were cut into 4-mm sections. The slides were stained with the following antibodies: CD4 (Clone 4B12, DAKO, Denmark) at 1:50, CD8 (Clone 144B,DAKO) at 1:100, CD20 (Clone L26, DAKO) at 1:250, CD138 (Clone MI15, DAKO) at 1:100, PD-1 (Clone NAT105, Abcam, Cambridge, USA) at 1:100, PD-L1 (Clone E1L3N, CST, Danvers, USA) at 1:200, IL-0.17(ab9565, Abcam) at 1:100, and Foxp3 (Clone 236A/E7, Abcam) at 1:50. For each slide, five representative fields were selected for initial screening under a Leica light microscope at low power (100 ×). Then, we counted the numbers of each type of immune cell at high power (400 ×). The final density of each section was calculated as the average number of five high-power fields (HPFs). The location of TILs was defined as tumor stroma between tumor nests. All specimens were evaluated by two investigators blinded to the clinical information.
Statistical analysis
The Mann–Whitney U test or Student’s t test was used to compare the expression differences of PBMCs and TILs in OSCCs. The χ2 test was used to analyze the associations between immune cells and clinicopathological parameters. The Kaplan–Meier method was used to estimate overall survival (OS), and survival differences were analyzed by the log-rank test. Univariate and multivariate COX proportional hazard models were used to assess the hazard ratios (HRs) with a confidence interval (CI) of 95% for OS. Receiver operating characteristic (ROC) and area under curve (AUC) analyses were used to estimate the predictive value of immune cells in the survival of OSCC. p < 0.05 was considered statistically significant. All analyses were conducted with SPSS 16.0 and GraphPad Prism 7 software.
Results
Skewing towards memory phenotypes and enrichment of exhausted phenotype in the TIL-Ts of OSCC
Flow cytometric analysis was performed to globally characterize the phenotypes and frequencies of TILs and their matched PBMCs. First, the distribution of helper (CD4+) and cytotoxic (CD8+) T cells within the T-cell population was investigated in TILs and matched PBMCs. Second, naïve and antigen-experienced subpopulations were determined based on the surface markers CD45RA and CD45RO. Our results showed increased CD8+ T cells and decreased CD4+ T cells in TILs (Student’s t test, Fig. 1a); that is, the CD4/CD8 ratio in TILs was reversed compared to that in PBMCs (Student’s t test, Fig. 1b). Among CD4+ and CD8+ T-cell populations, we found a significantly higher frequency of the memory/effector phenotype (CD45RA-CD45RO+) than the naïve phenotype (CD45RA+ CD45RO−) in TILs compared to matched PBMCs (Student’s t test, Fig. 1c, d). The molecules programmed death-1 (PD-1), and T-cell immunoglobulin and mucin-domain containing-3 (Tim-3) were used to evaluate the functional state of T cells. We found increased expression of PD-1 + and Tim-3 + T cells in both CD4+ and CD8+ T cells compared with the matched PBMCs (Mann–Whitney U test, Fig. 1e, f), indicating enrichment of exhausted T cells in tumors. Additionally, when examining these exhausted phenotypes within memory/effector T cells, CD4+ and CD8+ T cells still had a significantly increased expression of PD-1 and Tim-3 in tumors (Mann–Whitney U test, Fig. 1g, h).
Fig. 1.
Flow cytometric analysis for the phenotypes and TCR-Vβ of TILs and matched PBMCs. a Helper (CD4+) and cytotoxic (CD8+) T-cell subsets in TILs and matched PBMCs; b CD4+ /CD8+ T-cell ratio; c, d naïve (CD45RA+ CD45RO−) and antigen-experienced (CD45RA− CD45RO+) population within CD4+ /CD8+ T-cell subsets; e–h T cells displaying exhausted phenotypes (PD-1+ and Tim-3+) within CD4 + /CD8 + subsets and antigen-experienced T cells (CD45RA-CD45RO+); i Th17 (CD4+ IL-17A+), Tc17 (CD8+ IL17A+), CD4+ Tregs (CD4+ CD25+ Foxp3+), CD8+ Tregs (CD8+ CD25+ Foxp3+), and Th17/Treg ratio in CD4+ T cell; j Th17/Th1(IL-17A+ IFN-γ+) and Th17/Th2 population (IL17A+ IL4+) within CD4 + T cell; k antigen-experienced B cells (CD19+ CD27+), plasma cells (CD19+ CD138+), regulatory B cell (CD19+ IL-10+), and plasmablasts (CD19+ CD38+); l, m TCR-Vβ repertoire in CD4+ /CD8+ T cells. All error bars indicate SEM. 0.01 ≤ *p < 0.05, 0.001 ≤ **p < 0.01, ***p < 0.001. p < 0.05 was considered significant; NS not significant
Th17, Tc17, and Tregs are highly expressed in TIL-Ts of OSCC
To analyze the differentiation of the TIL T-cell population, we further investigated the expression of Th17 and Tregs in TILs. The numbers of Th17 (CD4+ IL17A+) and CD4+ Tregs (CD4+ CD25+ Foxp3+) in tumors were significantly greater than in circulating peripheral blood. Next, IL17A+ and CD25+ Foxp3+ T cells were also examined within CD8+ T cells, known as T cytotoxic 17 (Tc17) and CD8+ Tregs. We found that the numbers of Tc17 and CD8+ Tregs were also greater in tumors than in PBMCs. However, the ratios of Th17/Tregs in total CD4+ T lymphocytes showed no significant differences in tumors and PBMCs (Mann–Whitney U test, Fig. 1i).
An additional experiment in a small cohort (six patients) was performed to further investigate the expression of Th17 subtypes within the tumor microenvironment. IFN-γ and IL4 were used to determine the expression of the Th17/Th1 population (CD4+ IL17A+ IFN-γ+) and the Th17/Th2 population (CD4+ IL17A+ IL4+), respectively, by flow cytometry. The results revealed that the expression of Th17/Th1 was relatively higher than Th17/Th2 in tumors; however, in circulating peripheral blood, the expression of Th17/Th1 was significantly higher than Th17/Th2 (Student’s t test, Fig. 1j).
Skewed T-cell receptor β-chain variable region (TCR-Vβ) repertoire among T cells in TILs compared to PBMCs
To assess the clonal expansion of T cells in tumors, we explored the TCR repertoire by TCR-Vβ usage. CD4+ and CD8+ T cells were examined separately. In agreement with other studies, the repertoire of T cell varied substantially among individual patients. Therefore, we focused on the comparison of the TCR repertoire between TILs and matched PBMCs from the same patient. TCR-Vβ repertoires in tumor tissues were quite distinct from those of matched blood in OSCC patients. In CD4+ T cell subpopulations, the TCR Vβ repertoire in tumor tissues was significantly different from that in peripheral blood, with an increased frequency of Vβ7.1, Vβ12, and Vβ11 usage and a decreased frequency of Vβ9 and Vβ5.1 usage (Mann–Whitney U test, Fig. 1l). In CD8+ T cells, overexpression of Vβ12, Vβ23 and Vβ22, and reduced expression of Vβ16 were observed in tumor tissues compared to peripheral blood (Mann–Whitney U test, Fig. 1m). Collectively, the skewed TCR-Vβ repertoire above indicated that T cells clonally expanded in tumors.
Accumulation of antigen-experienced and regulatory phenotypes of TIL-Bs within OSCC
TIL-Bs are the other significant component of lymphocyte infiltrates in tumors; therefore, we analyzed the features of B cells that were infiltrating tumors by flow cytometry. B cells were identified based on the surface expression of CD45 and CD19. Then, CD27, CD38, CD138, and IL-10 were used to distinguish antigen-experienced B cells, plasmablasts, plasma cells and regulatory B cells (Bregs), respectively. Our results indicated that antigen-experienced B cells and Bregs were significantly enriched in tumors; plasmablast and plasma cell numbers were slightly greater in TILs than in corresponding PBMCs (Student’s t test; Fig. 1k).
Immunohistochemistry of TILs within the OSCC microenvironment
As TILs were detected in tumors by flow cytometry, we further assessed the distribution and extent of lymphocytes within tumor microenvironments by IHC. The clinical characterization of TILs was analyzed. We found considerable lymphocyte infiltrates within the microenvironment of OSCC. The majority of immune cells were distributed surrounding the tumor islands as compact clusters, and some were distributed sparsely or infiltrated the tumor islands. The mean numbers of CD4+, CD8+, CD20+, CD138+, PD-1, programmed death ligand 1 (PD-L1), IL-17+ and Foxp3+ were 43,49.5, 39.1,9.2, 9.4, 20, 18.4 and 11 per HPF, respectively (Fig. 3a). These were used as cutoff values to define the high infiltration group and the low infiltration group (Fig. 2a–p). T cells were found in significantly greater numbers than B cells in tumor microenvironment (Student’s t test; Fig. 3b). Among T cells, the number of cells in CD8+ subpopulation was slightly greater than those in CD4+ T cell subpopulations, but the difference was not significant (Student’s t test; Fig. 3a).
Fig. 3.
Correlation between TIL infiltration status and clinicopathologic features. a Mean counts of tumor-infiltrating lymphocytes. The CD8+ subpopulation was larger than the CD4+ subpopulation within infiltrated T cells, but the difference was not significant (Student’s t test). b The infiltrates of T cells were significantly more abundant than those of B cells in tumors (Student’s t test). c IL-17 was highly expressed in betel nut chewers. d CD20 was abundantly expressed in non-alcoholic patients. e IL-17 expression was significantly higher in smaller tumors (T1 + T2). f PD-L1 expression was significantly higher in lager tumors (T3 + T4). g High expression of Foxp3 + was positively associated with clinical stage. h High expression of CD138 was positively associated with clinical stage. i CD138 expression was significantly higher in larger tumors (T3 + T4). All error bars indicate SEM. 0.01 ≤ *p < 0.05, 0.001 ≤ **p < 0.01, ***p < 0.001. p < 0.05 as measured by the Chi-square test was considered significant
Fig. 2.
IHC analysis of immune cell distribution within the microenvironment of OSCC. Considerable infiltration of lymphocytes within the microenvironment of OSCC. The majority of lymphocytes were distributed surrounding the tumor islands as compact clusters. Some were distributed sparsely or infiltrated into tumor islands. a, b Representative images of IHC for high and low CD4 expression; c, d CD8 expression; e, f CD20 expression; g, h PD-L1expression; i, j CD138 expression; k, l IL-17 expression; m, n Foxp3 expression; and o, p PD-1 expression; a, c, e, g, i, k, m, o high expression; b, d, f, h, j, l, n, p low expression; bars: 100 μm
Clinical characterization of TILs by IHC
We explored the clinical association between the various subpopulations of lymphocyte infiltrates and tumor clinical characteristics, including age, sex, smoking status, drinking status, betel nut consumption, tumor size, lymph node metastasis, histological grade, and TNM stage. IL-17 was more highly expressed in betel nut chewers than in nonbetel nut chewers (Chi-square test, Fig. 3c); CD20 was more abundantly expressed in non-alcoholic patients than in alcoholic patients (Chi-square test, Fig. 3d). In addition, the expression of IL-17 was significantly higher in small tumors (T1 and T2) than in large tumors (T3 and T4) (Chi-square test, Fig. 3e). By contrast, the number of PD-L1 + and CD138 + TILs was higher in large tumors than in small tumors (Chi-square test, Fig. 3f, i); higher expression levels of Foxp3+ and CD138+ TIL were positively associated with poor prognosis (Chi-square test, Fig. 3g, h). CD4+ , CD8+ , and PD-1 expression status of TILs and age, sex, smoking/drinking/betel nut consumption history, tumor size, lymph node metastasis, histological grade or TNM stage had no significant relationships. The relationships between the clinicopathological parameters of OSCC and the densities of TILs are summarized in Supplemental Table 3.
A high Th17/Treg ratio was associated with a good prognosis and had a greater predictive accuracy of OS
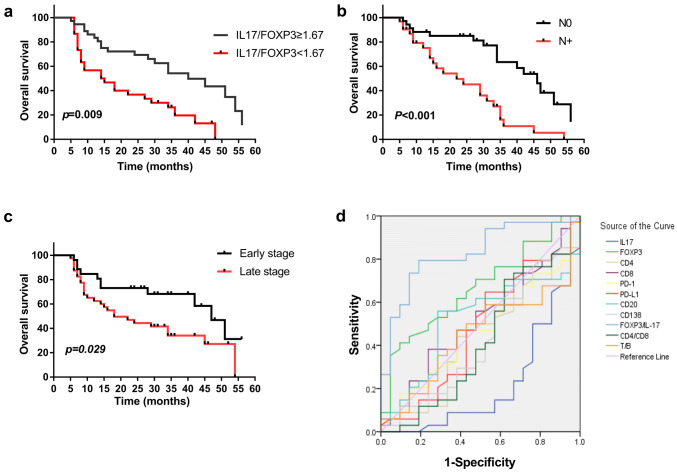
OS was defined as the interval between the date of diagnosis and the date of death or the end of study. The OS rates were significantly higher in patients with high Th17/Treg ratios than in those with low ratios (p = 0.009, Fig. 4a). To determine the independent predictive value of immune cell infiltration in OSCC, we designed Cox proportional hazard models. In the univariate Cox regression model, we found that the following factors had significant positive influences (p < 0.05) on OS: early TNM stages, nonlymphatic metastasis and higher IL17/Foxp3 ratio. However, age, sex, smoking, drinking, betel nut chewing, histological grade, tumor size, presence of CD4+, CD8+, IL-17+, Foxp3+, CD20+, CD138+, PD-1 and PD-L1 had no significant influence on OS (Supplemental Information Table 4). Multivariate Cox regression analysis showed that lymph node metastasis (HR 4.168, p = 0.005), Th17/Treg (HR 0.217, p = 0.002) and CD20 (HR 0.236, p = 0.035) considered independent predictors of prognosis for OSCC (Table 1). However, histological grade, tumor size, presence of CD8+, CD138+, and CD4/CD8 ratio were not considered independent predictors of prognosis for OSCC. To determine the predictive accuracy of immune cells on OS, we performed ROC curve analyses. As shown in Fig. 4D and Table 2, Foxp-3/IL-17 was superior to other immune cells in terms of determining patient prognosis (AUC = 0.829, 95% CI 0.719–0.939). These results suggest that a high Th17/Treg ratio is a useful marker for predicting better prognosis for OSCC.
Fig. 4.
Prognostic significance of tumor-infiltrating immune cells in OSCC. a–c Kaplan–Meier analyses of the prognostic values of IL-17/Foxp3 status, lymph node metastasis status, and clinical stage. d ROC curves indicated the predictive accuracy, sensitivity, and specificity of each potential parameter
Table 1.
Prognostic value of TILs analyzed by a multivariate Cox regression model
| SE | p | HR/RR | 95% CI | ||
|---|---|---|---|---|---|
| Tumor size | T1 + T2 | 0.521 | 0.160 | 0.482 | 0.174–1.336 |
| T3 + T4 | |||||
| N classification | N0 | 0.517 | 0.005 | 4.168 | 1.550–11.781 |
| N1 | |||||
| Pathological types | Type I | 0.911 | 0.116 | 0.238 | 0.040–1.422 |
| Type II–III | |||||
| CD8 | Lo | 0.638 | 0.228 | 2.156 | 0.618–7.521 |
| Hi | |||||
| CD4/CD8 | Lo | 0.492 | 0.421 | 0.673 | 0.257–1.765 |
| Hi | |||||
| IL17/FOXP3 | Lo | 0.492 | 0.002 | 0.217 | 0.083–0.569 |
| Hi | |||||
| CD138 | Lo | 0.536 | 0.242 | 1.872 | 0.655–5.354 |
| HI | |||||
| CD20 | Lo | 0.685 | 0.035 | 0.236 | 0.062–0.906 |
| Hi | |||||
p values showing statistically significance were indicated in bold
Table 2.
Summary of the OS predictive accuracy of immune cells
| Predictive factors | AUC | SE | p | 95% CI | |
|---|---|---|---|---|---|
| IL17 | 0.216 | 0.066 | 0.000 | 0.087 | 0.344 |
| FOXP3 | 0.666 | 0.073 | 0.040 | 0.522 | 0.810 |
| CD4 | 0.447 | 0.081 | 0.510 | 0.289 | 0.605 |
| CD8 | 0.509 | 0.080 | 0.910 | 0.352 | 0.666 |
| PD-1 | 0.457 | 0.078 | 0.591 | 0.304 | 0.609 |
| PD-L1 | 0.473 | 0.083 | 0.742 | 0.311 | 0.636 |
| CD20 | 0.518 | 0.080 | 0.822 | 0.362 | 0.675 |
| CD138 | 0.425 | 0.081 | 0.354 | 0.267 | 0.584 |
| FOXP3/IL17 | 0.829 | 0.056 | 0.000 | 0.719 | 0.939 |
| CD4/CD8 | 0.419 | 0.083 | 0.315 | 0.256 | 0.582 |
| T/B | 0.454 | 0.079 | 0.568 | 0.299 | 0.608 |
p values showing statistically significance were indicated in bold
SE standard error, 95% CI 95% confidence interval
Discussion
The presence of TILs is thought to reflect the host immune response to tumor cells and to be a valuable predictor of patient prognosis [17]. Early observations found that tumor cells express unique antigens that can be specifically recognized by T and B cells and provoke tumor-specific adaptive immunity [18, 19]. Nevertheless, the host immune system fails to control tumor growth, and tumors even exhibit immune privilege, possibly due to immune suppression by the TME [20]. The interaction of infiltrating lymphocytes with tumor cells may play important roles in shaping the immune microenvironment [21]. Although previous studies have shown that lymphocyte infiltration is present in OSCC and may correlate with clinical features, their role in the tumor immune suppression mediated by the TME remains to be thoroughly determined. Therefore, it is urgent to pursue a comprehensive characterization of the TIL repertoire, and then to elucidate its effect on clinical outcome and potential prognostic value in OSCC.
TILs consisted of more T cells than B cells, and CD8+ T cells outnumbered CD4+ T cells in the TME of OSCC, which means the ratio of CD4+ /CD8+ T cells is reversed in the TME compared with the matched peripheral blood. Regarding the effector functions of T cells, our data clearly demonstrate that, from peripheral blood to tumor, the T-cell population of both CD4+ and CD8+ subsets skewed from naïve toward effector/memory. As expected, TCR-Vβ repertoires in the TME that presented a clonal expansion of T cells within tumor tissues were quite distinct from those of matched blood; together these findings, suggest a specific immune response driven by local neoantigens in the TME.
Although memory/effector T cells were enhanced in TIL-Ts, many displayed a phenotype associated with exhaustion (PD-1+, Tim-3+) in tumor tissues, suggesting the inability to mount on effective immune response. The cause for this finding could be that the tumor microenvironment may promote exhaustion due to persistent antigen and/or inflammatory signals. Previous studies reported if T cells are exposed to a persisting antigen for 2–4 weeks, T-cell exhaustion is established [22, 23]. Furthermore, the enrichment of Tregs expressing a negative regulatory marker (Foxp3), which is capable of suppressing the immune response, was displayed in both CD4+ and CD8+ T cells. While T cells were found with antigen-experienced memory/effector phenotypes, the enrichment of exhausted phenotypes and Tregs impaired their antitumor immunity [24]. These findings implied a dysfunction of T cells in the TME, conferring only weak or temporary immune pressure on tumor cells; therefore, this immune response was ultimately ineffective.
Inflammatory responses play a very important role in tumor progression [25]. Therefore, we further examined the IL-17+ and Foxp3+ TILs that play opposite roles during inflammation and immune responses [26]. Our results showed a significantly higher proportion of both Th17 and Tregs in tumor tissues compared to matched peripheral blood, suggesting that the TME itself nourished these cells. Furthermore, Tc17 cells, another type of IL17A-producing cell, were also enriched in tumor tissues. Subsequently, the CD8+ CD25+ Foxp3+ subset of Tregs was found to be more prevalent in the TME than in peripheral blood. In our study, both the TME and peripheral blood showed a skewed balance toward Tregs, but no significant differences in the ratio of Th17 /Treg between TILs and PBMCs. These results imply immune dysfunction in OSCC patients.
B cells are also significant components of lymphocytic infiltrates in a number of solid tumors. In our study, antigen-experienced B cells were in significantly greater numbers in TME than matched PBMC, indicating that specific antigens drive the humoral response in tumors. The term Breg was used for the first time by Mizoguchi and Bhan to designate a subset of B cells with inhibitory properties [27]. Most of the work on Bregs has been focused on IL-10-producing B cells [28, 29], consistent with the findings of our study. Our results revealed Bregs accumulated in the TME; together with T-cell dysfunction, Bregs may contribute to immunosuppression in the TME.
To investigate the role of TILs in tumor biological behavior, a cohort of archived paraffin-embedded tissues from 159 cases of OSCC were analyzed by IHC, focusing on the prognostic value of TILs. The number of CD4+ T cells and CD8+ T cells was not associated with any clinical factors or overall survival in our study. CD8+ T cells exhibit cytotoxic capacities by releasing perforins and granzymes, thereby playing a role in antitumor immunity. Previous studies have reported that CD8+ TILs have favorable effects on the survival of patients with several tumor types [30, 31] including head and neck cancer [32–34]. In agreement with our finding, the numbers of CD4 or CD8 T cells were not associated with overall survival in a small cohort of patients with oral cavity carcinoma [35] or non-small cell lung cancer [36].The different histological types and clinical stages of the studied population, the diverse scoring systems used to calculate TIL expression, and the different techniques of tissue analysis and lymphocyte characterization, could result in the discrepancies of the results of these studies [37]. The role of CD4+ T cells in tumors remains controversial. The paradoxical dualism of CD4+ T cells obligates further differentiation of this subtype into helper and regulatory CD4+ T cells. On the one hand, CD4+ helper T cells perform critical roles in the recruitment, activation and regulation of many facets of the adaptive immune response [38]. On the other hand, CD4+ Tregs can dampen antitumor immunity and promote tumor progression [39]. In the present study, the number of CD4+ TILs had no significant prognostic value, possibly due to the complexity of its components.
In this study, we found that the expression of IL17+ T cells was inversely correlated with tumor size and that the expression of Foxp3+ TILs positively correlated with TNM stage. These results provide comprehensive information regarding the impact of Th17⁄Treg balance on tumor progression in OSCC. Th17 and Tregs accumulated in the tumor microenvironment at early stages of the disease; when tumor cells develop and progress, Th17 infiltrates gradually decrease and Treg infiltrates increase in number as disease progresses. This observation was confirmed by both flow cytometric analysis and IHC staining in our study. IL-17+ T cells were not associated with OS in our study and were not independent prognostic factors for OS. The role of Th17 cells in cancer is still highly controversial. Several lines of evidence suggest that Th17 cells promote a protective antitumor immune response; they mediate their antitumor activity indirectly by facilitating the recruitment of other effector immune cells linking effector T cells\NK cells\dendritic cells and cytotoxic effector cells [40, 41]. The presence of IL-17 positive cells has been positively associated with patient survival in ovarian [42] and prostate cancer [43]. In contrast, Th17 cells show their pro-tumor functions by increasing tumor cell growth, proliferation, and metastasis [44]. The presence of IL-17 positive cells has been reported to be associate with poor survival in HNSCC [45]. A plausible reason for these contradictory results may be the high plasticity of Th17 cells. A previous study showed that Th17 cells can convert into Th1, Treg, type-1 regulatory T (TR1), Th2, or T follicular helper (TFH) cells, which provides them with various and contrary activities and therefore permits them to induce qualitatively discrete responses according to various microenvironments [46]. We found that IL17A /IFNγ and IL17A/ IL4 double-producing CD4+ T cell (Th17/Th1 and Th17/Th2) existed in OSCC. Th17/Th1 was relatively higher than Th17/Th2 in the TME, so Th17 cells induce more Th1-type chemokines, and in turn recruit more Th1-type effector T cells into the tumor microenvironment which can facilitate the antitumor effect of Th17 cells. However, compared with circulating peripheral blood, Th17/Th2 was increased and Th17/Th1 was decreased in the TME because of the immune dysregulation in the microenvironment.
Tregs inhibit antitumor immunity and mediate immune tolerance, favoring tumor growth, and, therefore, might serve as markers of poor prognosis [47]. Increased Treg infiltration in the TME has been associated with worse clinical outcomes in many cancers. Foxp3+ TILs were not associated with OS in our study, suggesting that it was not an independent prognostic factor for overall survival. The small cohort of patients and the relatively short follow-up period in our study might account for this phenomenon. However, when we further evaluated the IL-17/Foxp3 ratio relative to clinical factors, we found that the ratio of IL-17/Foxp3 TIL was positively associated with OS and that a high IL-17/Foxp3 ratios was an independent prognostic factor for OS. Moreover, IL-17/Foxp3 ratios were superior to measurements of other immune cells for determining prognosis.
TIL-Bs play critical roles in the humoral immune system by producing antibodies, priming T cells as antigen-presenting cells (APCs), and secreting cytokines [48]. In the present study, multivariate Cox regression analysis showed that CD20 levels were positively associated with long OS, in line with previous reports on OSCC and several other cancers including non-small cell lung cancer, hepatocellular carcinoma, colorectal cancer, and breast cancer [49–53]. These studies all emphasized that the presence of greater numbers of CD20+ B cells in TME was correlated with favorable prognosis and survival rates. The expression of immune cell-specific CD138 infiltrate was positively correlated with tumor size and TNM stage in our results. We did not find any independent prognostic impact of immune cell-specific CD138 expression in the Kaplan–Meier method or the univariate and multivariate Cox proportional hazard models. In their study, Berntsson et al. also found that immune cell-specific CD138 expression was associated with high-grade tumors, but with smaller tumor sizes and good patient outcomes in univariable but not multivariate analysis. In contrast, tumor cell-specific CD138 expression was an independent predictor of poor patient outcome [52] Previous studies showed that the effects of CD138 expression on tumor prognosis could be positive or neutral or negative. A plausible explanation for this discrepancy may be the different cancer type and cell-type specific CD138 expression. CD138 appeared to be a reliable marker for plasma cells among hematopoietic elements; however, it can also be expressed by nonhematopoietic epithelial and stromal cells in the TME. Similar to many other studies, we also scored immune cell-specific CD138 expression based on cell morphology, but did not indicate the hematopoietic origin of the CD138+ cells with a second marker [54]. Therefore, the biological functions of CD138 in tumors appear to depend on the cellular context and further studies are warranted to validate the prognostical significance of cell-type specific CD138 expression in OSCC.
The expected clinical prognosticators, including lymph node positive/negative and TNM stage, were confirmed in our study. It was unexpected to observe an association of TILs including IL-17 and CD20 with risk factors such as alcohol/betel nut consumption history. However, this was a small cohort, and alcohol use and betel nut consumption were only characterized by current or past users versus never users. Therefore, the association of TILs with risk factors should be evaluated in larger cohorts to obtain definitive indications for potential therapeutic stratification.
Our results demonstrated a comprehensive scenario for lymphocyte infiltrates in OSCC and their relevance to tumor biological behaviors. The repertoire of TILs in OSCC was found to exhibit a series of unique features, demonstrating antigen-driven immune responses, though with an immune dysfunction, within the microenvironment. Furthermore, the clinical significance of TILs was analyzed, revealing that the ratio of Th17/Treg was a significant independent prognostic factor for OSCC and hinting that the balance of Th17 and Treg may play an important role in the modulation of antitumor immunity. In conclusion, these results gave us a deeper understanding of immune microenvironments and their clinical significance in OSCC that will provide opportunities to develop new strategies for immunomodulatory therapy in the future.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- AUC
Area under curve
- Bregs
Regulatory B cells
- CI
Confidence interval
- HNSCC
Head and neck squamous cell carcinoma
- HPFs
High-power fields.
- HRs
Hazard ratios
- OSCC
Oral squamous cell carcinoma
- ROC
Receiver operating characteristic
- Tc17
T cytotoxic 17
- TCR-Vβ
T-cell receptor β chain variable region
- Th17
T helper 17
- Tim-3
T-cell immunoglobulin and mucin-domain containing-3
Author contributions
Conception and design: HZQ, ZYS, and LJF; Data acquisition: ZYS, ZYL, SXL, LY, XDF, BSW, and KL; Evaluation of pathology and IHC: ZYD, YJH, JHH, and ZGY; Data analysis and interpretation: HZQ, ZYS, LJF, ZYL, LY, JJY, KX, and TZT; Writing and review of manuscript: HZQ, ZYS, LJF, and ZGT.
Funding
This work was supported by the National Natural Science Foundation of China (81501110), Hunan Province Natural Science Foundation (2016JJ4093), and China Postdoctoral Science Foundation (2016M592454).
Compliance with ethical standards
Conflict of interest
None of the authors report any conflict of interest.
Ethical approval
This work was approved by the Ethics Committee of Xiangya Stomatological Hospital and School of Stomatology under the Reference Number 2016003 and was conducted in accordance with the principles of the Declaration of Helsinki.
Informed consent
Written informed consent to the use of specimens and clinical data for research purposes was obtained from the patients of the flow cytometry cohort before recruitment into the study. For the IHC cohort, the patient also signed consent to the use of their specimens and clinical data for research purposes before surgical resection.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hongzhi Quan, Email: hongzhi.quan@csu.edu.cn.
Liangjuan Fang, Email: fangliangjuan@163.com.
References
- 1.Sasahira T, Kirita T, Kuniyasu H. Update of molecular pathobiology in oral cancer: a review. Int J Clin Oncol. 2014;19:431–436. doi: 10.1007/s10147-014-0684-4. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Beynon RA, Lang S, Schimansky S, et al. Tobacco smoking and alcohol drinking at diagnosis of head and neck cancer and all-cause mortality: results from head and neck 5000, a prospective observational cohort of people with head and neck cancer. Int J Cancer. 2018;143:1114–1127. doi: 10.1002/ijc.31416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dhull AK, Atri R, Dhankhar R, Chauhan AK, Kaushal V. Major risk factors in head and neck cancer: a retrospective analysis of 12-year experiences. World J Oncol. 2018;9:80–84. doi: 10.14740/wjon1104w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madathil SA, Rousseau MC, Wynant W, Schlecht NF, Netuveli G, Franco EL, Nicolau B. Nonlinear association between betel quid chewing and oral cancer: implications for prevention. Oral Oncol. 2016;60:25–31. doi: 10.1016/j.oraloncology.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 6.Bloebaum M, Poort L, Böckmann R, Kessler P. Survival after curative surgical treatment for primary oral squamous cell carcinoma. J Craniomaxillofac Surg. 2014;42:1572–1576. doi: 10.1016/j.jcms.2014.01.046. [DOI] [PubMed] [Google Scholar]
- 7.Rogers SN, Brown JS, Woolgar JA, Derek L, Patrick M, Shaw RJ, David S, Douglas E, David V. Survival following primary surgery for oral cancer. Oral Oncol. 2009;45:201–211. doi: 10.1016/j.oraloncology.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 8.Cor Hj L, Stefan S, Sabine VS, et al. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management of on-target toxicity. Mol Therap J Am Soc Gene Therap. 2013;21:904–912. doi: 10.1038/mt.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morgan RA, Yang JC, Mio K, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang H, Qiao J, Fu YX. Immunotherapy and tumor microenvironment. Cancer Lett. 2016;370:85–90. doi: 10.1016/j.canlet.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hui L, Chen Y. Tumor microenvironment: Sanctuary of the devil. Cancer Lett. 2015;368:7–13. doi: 10.1016/j.canlet.2015.07.039. [DOI] [PubMed] [Google Scholar]
- 12.Balermpas P, Rödel F, Rödel C, et al. CD8+ tumour-infiltrating lymphocytes in relation to HPV status and clinical outcome in patients with head and neck cancer after postoperative chemoradiotherapy: a multicentre study of the German cancer consortium radiation oncology group (DKTK-ROG) Int J Cancer. 2015;138:171–181. doi: 10.1002/ijc.29683. [DOI] [PubMed] [Google Scholar]
- 13.Han S, Zhang C, Li Q, Dong J, Liu Y, Huang Y, Jiang T, Wu A. Tumour-infiltrating CD4(+) and CD8(+) lymphocytes as predictors of clinical outcome in glioma. Br J Cancer. 2014;110:2560–2568. doi: 10.1038/bjc.2014.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshioka T, Miyamoto M, Cho Y, et al. Infiltrating regulatory T cell numbers is not a factor to predict patient's survival in oesophageal squamous cell carcinoma. Br J Cancer. 2008;98:1258–1263. doi: 10.1038/sj.bjc.6604294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Müller S, et al. Update from the 4th Edition of the World Health Organization of head and neck tumours: tumours of the oral cavity and mobile tongue. Head Neck Pathol. 2017;11:33–40. doi: 10.1038/sj.bjc.6604294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doescher J, Veit JA, Hoffmann TK (2017) The 8th edition of the AJCC cancer staging manual. HNO 1–5 [DOI] [PubMed]
- 17.Sherwood A, Emerson R, Scherer D, et al. Tumor-infiltrating lymphocytes in colorectal tumors display a diversity of T cell receptor sequences that differ from the T cells in adjacent mucosal tissue. Cancer Immunol Immunotherap CII. 2013;62:1453–1461. doi: 10.1007/s00262-013-1446-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gubin MM, Artyomov MN, Mardis ER, Schreiber RD. Tumor neoantigens: building a framework for personalized cancer immunotherapy. J Clin Investig. 2015;125:3413–3421. doi: 10.1172/JCI80008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ward JP, Gubin MM, Schreiber RD. The role of neoantigens in naturally occurring and therapeutically induced immune responses to cancer. Adv Immunol. 2016;130:25–74. doi: 10.1016/bs.ai.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang P, Gu S, Pan D, et al. Signatures of T cell dysfunction and exclusion predict cancer immunotherapy response. Nat Med. 2018;24:1550–1558. doi: 10.1038/s41591-018-0136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science. 2015;348:74. doi: 10.1126/science.aaa6204. [DOI] [PubMed] [Google Scholar]
- 22.Angelosanto JM, Blackburn SD, Crawford A, Wherry EJ. Progressive loss of memory T cell potential and commitment to exhaustion during chronic viral infection. J Virol. 2012;86:8161–8170. doi: 10.1128/JVI.00889-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brooks DG, McGavern DB, Oldstone MBA. Reprogramming of antiviral T cells prevents inactivation and restores T cell activity during persistent viral infection. J Clin Investig. 2006;116:1675. doi: 10.1172/JCI26856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15:486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140:845–858. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 27.Mizoguchi A, Bhan AK. A case for regulatory B cells. J Immunol. 2006;176:705–710. doi: 10.4049/jimmunol.176.2.705. [DOI] [PubMed] [Google Scholar]
- 28.Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16:219–230. doi: 10.1016/S1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- 29.Fillatreau S, Sweenie CH, Mcgeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 30.Chen J, He Q, Liu J, Xiao Y, Xiao C, Chen K, Xie D, Zhang X. CD8+ tumor-infiltrating lymphocytes as a novel prognostic biomarker in lung sarcomatoid carcinoma, a rare subtype of lung cancer. Cancer Manag Res. 2018;10:3505–3511. doi: 10.2147/CMAR.S169074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ye SL, Li XY, Zhao K, Feng T. High expression of CD8 predicts favorable prognosis in patients with lung adenocarcinoma: a cohort study. Medicine. 2017;96:e6472. doi: 10.1097/MD.0000000000006472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balermpas P, Michel Y, Wagenblast J, Seitz O, Weiss C, Rödel F, Rödel C, Fokas E. Tumour-infiltrating lymphocytes predict response to definitive chemoradiotherapy in head and neck cancer. Br J Cancer. 2014;110:501–509. doi: 10.1038/bjc.2013.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fang J, Li X, Ma D, et al. Prognostic significance of tumor infiltrating immune cells in oral squamous cell carcinoma. BMC Cancer. 2017;17:375. doi: 10.1186/s12885-017-3317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cecilia N, Nathalie G, Nikolaos T, et al. CD8+ and CD4+ tumour infiltrating lymphocytes in relation to human papillomavirus status and clinical outcome in tonsillar and base of tongue squamous cell carcinoma. Eur J Cancer. 2013;49:2522–2530. doi: 10.1016/j.ejca.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 35.Wolf GT, Chepeha DB, Bellile E, Nguyen A, Thomas D, McHugh J, University of Michigan H, Neck SP Tumor infiltrating lymphocytes (TIL) and prognosis in oral cavity squamous carcinoma: a preliminary study. Oral Oncol. 2015;51:90–95. doi: 10.1016/j.oraloncology.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jackute J, Zemaitis M, Pranys D, Sitkauskiene B, Miliauskas S, Bajoriunas V, Lavinskiene S, Sakalauskas R. The prognostic influence of tumor infiltrating Foxp3+CD4+, CD4+ and CD8+ T cells in resected non-small cell lung cancer. J Inflamm. 2015;12:63. doi: 10.1186/s12950-015-0108-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu H, Zhang T, Ye J, Li H, Huang J, Li X, Wu B, Huang X, Hou J. Tumor-infiltrating lymphocytes predict response to chemotherapy in patients with advance non-small cell lung cancer. Cancer Immunol Immunotherap CII. 2012;61:1849–1856. doi: 10.1007/s00262-012-1231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zom GG, Khan S, Britten CM, et al. Efficient induction of antitumor immunity by synthetic toll-like receptor ligand-peptide conjugates. Cancer Immunol Res. 2014;2:756–764. doi: 10.1158/2326-6066.CIR-13-0223. [DOI] [PubMed] [Google Scholar]
- 39.Zamarron BF, Chen WJ. Dual roles of immune cells and their factors in cancer development and progression. Int J Biol Sci. 2011;7:651–658. doi: 10.7150/ijbs.7.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sato E, Olson SH, Ahn J, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin-Orozco N, Muranski P, Chung Y, et al. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31:787–798. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kryczek I, Banerjee M, Cheng P, et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114:1141–1149. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sfanos KS, Bruno TC, Maris CH, Xu L, Thoburn CJ, DeMarzo AM, Meeker AK, Isaacs WB, Drake CG. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin Cancer Res. 2008;14:3254–3261. doi: 10.1158/1078-0432.CCR-07-5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Asadzadeh Z, Mohammadi H, Safarzadeh E, Hemmatzadeh M, Mahdian-Shakib A, Jadidi-Niaragh F, Azizi G, Baradaran B. The paradox of Th17 cell functions in tumor immunity. Cell Immunol. 2017;322:15–25. doi: 10.1016/j.cellimm.2017.10.015. [DOI] [PubMed] [Google Scholar]
- 45.Chen X, Wan J, Liu J, Xie W, Diao X, Xu J, Zhu B, Chen Z. Increased IL-17-producing cells correlate with poor survival and lymphangiogenesis in NSCLC patients. Lung Cancer. 2010;69:348–354. doi: 10.1016/j.lungcan.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 46.Guery L, Hugues S. Th17 cell plasticity and functions in cancer immunity. Biomed Res Int. 2015;2015:314620. doi: 10.1155/2015/314620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Di GP, Gerlini G, Caporale R, Sestini S, Brandani P, Urso C, Pimpinelli N, Borgognoni L. T regulatory cells mediate immunosuppresion by adenosine in peripheral blood, sentinel lymph node and TILs from melanoma patients. Cancer Lett. 2018;417:124–130. doi: 10.1016/j.canlet.2017.12.032. [DOI] [PubMed] [Google Scholar]
- 48.Herpen CMLV, Voort RVD, Laak JAVD, et al. Intratumoral rhIL-12 administration in head and neck squamous cell carcinoma patients induces B cell activation. Int J Cancer. 2010;123:2354–2361. doi: 10.1002/ijc.23756. [DOI] [PubMed] [Google Scholar]
- 49.Jie-Yi S, Qiang G, Zhi-Chao W, et al. Margin-infiltrating CD20+ B cells display an atypical memory phenotype and correlate with favorable prognosis in hepatocellular carcinoma. Clin Cancer Res. 2013;19:5994–6005. doi: 10.1158/1078-0432.CCR-12-3497. [DOI] [PubMed] [Google Scholar]
- 50.Marcus S, Daniel BH, Christian VTR, et al. The humoral immune system has a key prognostic impact in node-negative breast cancer. Can Res. 2008;68:5405–5413. doi: 10.1158/0008-5472.CAN-07-5206. [DOI] [PubMed] [Google Scholar]
- 51.Taghavi N, Mohsenifar Z, Baghban AA, Arjomandkhah A. CD20+ Tumor infiltrating B lymphocyte in oral squamous cell carcinoma: correlation with clinicopathologic characteristics and heat shock protein 70 expression. Pathol Res Int. 2018;2018:4810751. doi: 10.1155/2018/4810751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berntsson J, Nodin B, Eberhard J, Micke P, Jirstrom K. Prognostic impact of tumour-infiltrating B cells and plasma cells in colorectal cancer. Int J Cancer. 2016;139:1129–1139. doi: 10.1002/ijc.30138. [DOI] [PubMed] [Google Scholar]
- 53.Al-Shibli KI, Tom D, Samer AS, Magnus P, Bremnes RM, Lill-Tove B. Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clin Cancer Res. 2008;14:5220. doi: 10.1158/1078-0432.CCR-08-0133. [DOI] [PubMed] [Google Scholar]
- 54.Wouters MC, Nelson BH. Prognostic significance of tumor-infiltrating B cells and plasma cells in human cancer. Clin Cancer Res. 2018 doi: 10.1158/1078-0432.CCR-18-1481. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.