Abstract
Purpose
Pre-clinical and early clinical data suggests the microbiome plays an important role in oncogenesis and influences response to immune checkpoint blockade (ICB). The objective of this systematic review and meta-analysis was to determine whether antibiotics affect overall survival (OS) and progression free survival (PFS) in patients with solid malignancies treated with ICB.
Patients and methods
A systematic search of EMBASE, MEDLINE and conference proceedings was conducted for observational studies examining the effect of antibiotics on ICB. A random effects study-level meta-analysis was performed with pooling of the hazards ratio (HR) for OS and PFS. Meta-regression was used to determine the impact of the timing of antibiotic exposure on OS.
Results
766 studies were identified, and 18 studies met the inclusion criteria. Of the 2889 patients included, 826 (28.6%) were exposed to antibiotics. The most common malignancies were lung (59%), renal cell carcinoma (RCC) or urothelial carcinoma (16.3%) and melanoma (18.7%). OS was prolonged in those without antibiotic exposure (pooled HR 1.92, 95% CI 1.37–2.68, p < 0.001). The effect of antibiotics on OS was greater in studies defining antibiotic exposure as 42 days prior to initiation of ICB (HR 3.43, 95% CI 2.29–5.14, p < 0.0001). PFS was also longer in patients who did not receive antibiotics (pooled HR 1.65, 95% CI 1.3–2.1, p < 0.0001).
Conclusion
In patients receiving ICB, OS and PFS are longer in patients who are not exposed to antibiotics. Antibiotic use in the 42 days before starting ICB appears to be most detrimental to outcome.
Electronic supplementary material
The online version of this article (10.1007/s00262-019-02453-2) contains supplementary material, which is available to authorized users.
Keywords: Antibiotics, Immunotherapy, Immune checkpoint blockade, Cancer
Introduction
There is growing interest in the effects of the microbiome on oncogenesis and response to treatment, particularly ICB. Two landmark studies in mice provided the first evidence that the microbiome may directly impact the effectiveness of immunotherapy [1, 2]. More recently, prospective studies have demonstrated that in patients with metastatic melanoma and non-small cell lung cancer (NSCLC) initiating ICB, responses are in part predicted by microbiome diversity and composition [3–5]. If antibiotics disrupt the ecological balance of the microbiome which is essential for immune activation, exposure to antibiotics may compromise the effectiveness of ICB in routine clinical practice.
Following the discovery that antibiotics had a deleterious impact on ICB activity in pre-clinical models, several institution-based retrospective cohort studies have examined the use of antibiotics in the period immediately preceding or following the initiation of immunotherapy and the impact on clinical outcomes. Some have found shorter OS and PFS in those exposed to antibiotics [6–8]. However, the results have not been uniform, with other studies demonstrating either no difference or survival advantage in those receiving antibiotics [5, 9]. Furthermore, the timing of antibiotic exposure varied considerably.
The primary objective of this systematic review and meta-analysis is to examine the impact of antibiotics on the effectiveness of ICB for solid malignancies, as measured by OS and PFS. The secondary objective is to explore the impact of timing of antibiotic exposure on OS and PFS.
Methods
Study eligibility and identification
A systemic search for cohort studies published in English, examining the association between antibiotic use and ICB using MEDLINE and EMBASE was performed on April 26th, 2019. The search strategy is outlined in Supplemental Fig. 1. The following criteria were required for study inclusion: observational cohort studies, studies addressing the impact of antibiotics on the effectiveness of ICB, adult population, solid tumours, and available HR for PFS and/or OS. Studies looking at Chimeric Antigen Receptor T-cell (CAR-T) therapies and bone-marrow transplant were excluded. All identified articles were reviewed by Wilson and Chin for consensus on inclusion. References of included studies were reviewed for any additional publications by manual search. To limit publication bias, unpublished studies were also included. On June 1st, 2019, abstracts from conference proceedings of the American Society of Clinical Oncology and the European Society of Medical Oncology were searched.
Data extraction
For included studies the following data were extracted: the number of patients, type(s) of malignancy, study type (retrospective vs prospective), number of patients exposed to antibiotics, definition of antibiotic exposure, type of ICB agent (anti-PD1/PDL1, anti-CTLA-4, or combination treatment), the proportion of patients with ECOG 0-1, median age and proportion of male patients. Median OS and median PFS, associated HR and 95% Confidence Intervals (CI) for those exposed and not exposed to antibiotics were obtained for each included study. All median OS and PFS times were converted to months (weeks multiplied by 7 divided by 30 to calculate the duration in months). Where the HRs were not available in the presented data, they were derived from the Kaplan-Meir curves using the methodology by Tierney et al. [10]. If the required data was not immediately available from the published abstracts or papers, authors were contacted directly for results.
Statistical analyses
Given the heterogeneity of the studies and patient population, a random effects model was used to pool estimates of effect size for OS and PFS. The χ2 Cochrane Q test was used to detect heterogeneity across the different studies. The definition of antibiotic exposure was categorised into three groups: group 1 publications defined the window of antibiotic exposure as 42 days prior to ICB until initiation at time 0; group 2 publications defined antibiotic exposure as 60 days before and up to 42 days after initiation of ICB; and group 3 publications defined antibiotic exposure as 60 days before and anytime during ICB treatment. The pooled OS and PFS were stratified by antibiotic exposure definition, and we used a test for heterogeneity to determine whether differences between the groups were significant. Meta-regression was also used to examine the effect of antibiotic exposure timing on OS. The HR for OS and PFS were also stratified by type of malignancy to test for heterogeneity. Publication bias was evaluated by examining the funnel plot of the effect size for each observational study against the reciprocal of its standard error. The nominal level of significance was predetermined to be 5% with the exception of publication bias, where significance for the Egger’s test was predetermined to be 10%. All 95% confidence intervals were two-sided.
Results
Studies for inclusion
A total of 751 articles were identified by systematic search, and an additional 15 were identified by manual search of references and conference abstracts. After removal of duplicates and review of the title and/or abstract, 33 studies remained (Fig. 1). A further four abstracts were duplicates of included publications, seven studies were excluded as no univariate HR for OS or PFS were available even after contacting the authors and two were excluded as they were not observational studies [11, 12]. One study [9] was excluded as the population of patients was re-analysed in the subsequent publication by Routy et al. [13]. Another study [14] was excluded as the patients were duplicated in Huemer et al. [5]. The RCC cohort in Routy et al. [13] was excluded as it was updated in the paper by Derosa et al. [7] (Supplemental Table 1). Four studies [5, 7, 13, 15] included separate cohorts and these are presented individually in this meta-analysis. Therefore, a total of 22 cohorts are presented from this point forward for convenience.
Fig. 1.
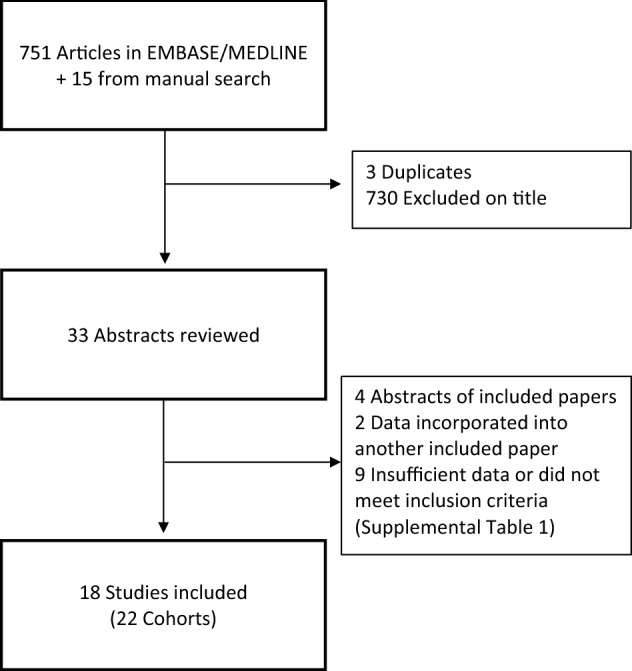
Search strategy
All included studies were published between 2017 and 2019. The studies were conducted predominantly in North America and Europe. One study was prospective [16], while all other studies were retrospective.
Baseline data
A total of 2889 patients were included in this meta-analysis and 826 (28.6%) were exposed to antibiotics. The most common types of malignancy were lung (59%), renal cell carcinoma or urothelial carcinoma (16.3%) and melanoma (18.7%). There were more men included (63.7% of 2408 patients for whom sex was reported). The class of immune-checkpoint blockade was clearly documented for 90% of patients included. The majority of these patients were treated with programmed death 1 (PD-1) or programmed death ligand 1 (PD-L1) inhibitors (92.8%), 5.2% with cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitors and 1.7% combination PD1/PDL1 plus CTLA-4 inhibitor. Functional status was reported in 49.5% of patients, and 84% were ECOG 0-1 (Table 1).
Table 1.
Characteristics of included studies
| Study | Study type | N | Antibiotic exposed N (%) |
Cancer type | Immunotherapy target (N) |
Median age (Abx vs no Abx) or pooled median | ECOG 0–1 or KPS ≥ 90 N (%) |
Male N (%) |
Median PFS Abx vs no Abx (months) | Median OS Abx vs no Abx (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| Group 1 cohorts | ||||||||||
|
Derosa-RCC [7] (France/USA) |
Paper | 121 | 16 (13%) | RCC |
PDL1 (111) PDL1 + CTLA-4 (10) |
61 vs 61 | 118 (49%) | 1.9 vs 7.4 | 17.3 vs 30.6 | |
|
Derosa-NSCLC [7] (France/USA) |
Paper | 239 | 48 (20%) | NSCLC |
PDL1 (205) PDL1 + CTLA-4 (34) |
63 vs 66 | 236 (99%) | 80 (66%) | 1.9 vs 3.8 | 7.9 vs 24.6 |
|
Elkrief [8] (Canada) |
Paper | 74 | 10 (13.5%) | Melanoma |
PD1/PDL1 (54) CTLA-4 (20) |
58 vs 65 | 49 (66%) | 2.4 vs 7.3 | 10.7 vs 18.3 | |
|
Pinato [16] (UK) |
Abstract | 196 | 97 (49%), only 29 in 0–30 days |
NSCLC (119) Melanoma (38) Other (39) |
PD1/PDL1 (189) Not specified (7) |
159 (84%) | 137 (70%) | 2 vs 26 | ||
|
Thompson [22] (USA) |
Abstract | 74 | 18 (24%) | NSCLC | PD1/PDL1 (74) | 66 | 41 (55%) | 2.0 vs 3.8 | 4.0 vs 12.6 | |
|
Sen [19] (USA) |
Paper | 172 | 19 (11%) |
NSCLC 2(1) RCC (25) Melanoma (16) Other (110) |
CTLA-4 (105) PDL1 (67) |
60 | 88 (51%) | 2.5 vs 3.0 | 4.6 vs 8.2 | |
|
Zhao [23] (China) |
Paper | 109 | 20 (18.3%) | NSCLC | PD1 (109) | 57 vs 62 | 107 (98%) | 89 (82%) | 3.7 vs 9.6 | 6.1 vs 21.9 |
| Group 2 Cohorts | ||||||||||
|
Ahmed [6] (USA) |
Paper | 60 | 17 (28%) |
Lung (34) Melanoma (3) RCC (4) Other (19) |
PD1/PDL1 (60) | 52 vs 66 | 38 (63%) | 35 (58%) | 5.0 vs 12.0 | 5.6 vs 20.8 |
|
Hakozaki [41] (Japan) |
Paper | 90 | 13 (14%) | NSCLC | PD1 (90) | 67 vs 68 | 77 (85%) | 57 (63%) | 1.2 vs 4.4 | 8.8 vs NR |
|
Huemer-Salzburg [5] (Austria) |
Paper | 43 | 20 (46.5%) | NSCLC | PD1/PDL1 (43) | 60 | 30 (70%) | 21 (49%) | 7.5 vs 13.6 | |
|
Huemer -Linz [5] (Austria) |
Paper | 53 | 18 (33.9%) | NSCLC | PD1/PDL1 (53) | 66 | 53 (100%) | 32 (60%) | NR vs 10.8 | |
|
(USA) |
Abstract | 146 | 31 (21%) | RCC | PD1/PDL1 (146) | 61 | 104 (71%) | 2.6 vs 8.1 |
65% vs 79% (1-year OS) |
|
|
Mielgo-Rubio [20] (Spain) |
Abstract | 168 | 80 (48%) | NSCLC | PD1/PDL1 (168) | 65 | 121 (72%) | 79.8% | 5.1 vs 7.3 | 8.1 vs 11.9 |
|
Routy-NSCLC [13] (France) |
Paper | 140 | 37 (26%) | NSCLC | PD1/PDL1 (140) | 64 | 129 (92%) | 102 (73%) | 2.8 vs 3.5 | 8.3 vs 15.3 |
|
Routy-Urothelial [13] (France) |
Paper | 42 | 12 (29%) | Urothelial | PD1/PDL1 (42) | 63 | 26 (62%) | 30 (71%) | 1.8 vs 4.3 | 11.5 vs NR |
|
Schett [24] (Switzerland) |
Abstract | 218 | Estimated at 42 (20%) from power calculation | NSCLC | PD1/PDL1 (218) | 1.4 vs 5.8 | 10.6 vs 29.9 | |||
|
Tinsley [25] (UK) |
Paper | 291 | 92 (32%) |
Melanoma (179) NSCLC (64) RCC (48) |
66 | 230 (79%) | 181 (62%) | 3.1 vs 6.3 | 10.7 vs 21.7 | |
| Group 3 Cohorts | ||||||||||
|
Do [17] (USA) |
Abstract | 109 | 87 (80%) | Lung cancer | PD1 (109) | 5.4 vs 17.2 | ||||
|
Hemadri [21] (USA) |
Abstract | 172 | 29 (17%) | Melanoma | PD1/PDL1 (172) | 106 (32%) | 16.6 vs 19.8 | 23.8 vs 35.4 | ||
|
Kulkarni-NSCLC [15] (USA) |
Abstract | 148 | 87 (59%) | NSCLC | PD1/PDL1 (148) | 5 vs 2.5 | 13 vs 8 | |||
|
Kulkarni-RCC [15] (USA) |
Abstract | 55 | 40 (72%) | RCC | PD1/PDL1 (55) | 2.9 vs 5 | ||||
|
Masini [18] (Italy) |
Abstract | 169 | 59 (35%) |
NSCLC (78) Melanoma (57) RCC (29) Other (5) |
PD1/PDL1 (159) CTLA-4 (10) |
|||||
Abx antibiotic, NR not reached, KPS Karnofsky Performance Score
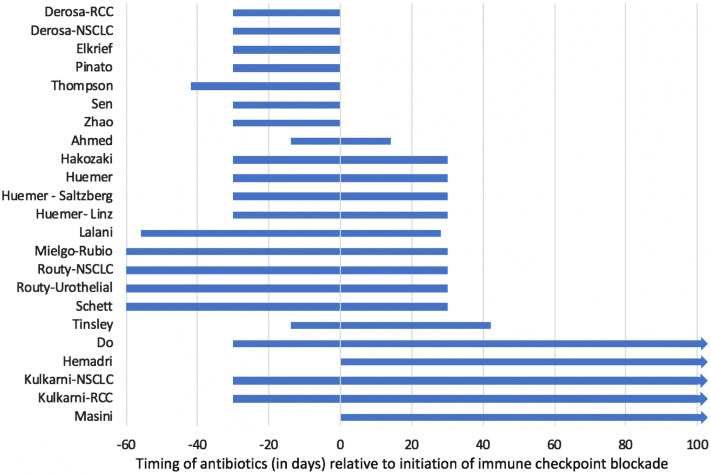
The definition of antibiotic exposure varied considerably across the included cohorts (Fig. 2). Some cohorts defined antibiotic exposure as treatment anytime during ICB [15, 17, 18, 21], while others included narrow definitions such as antibiotic exposure 14 days before or after ICB initiation [6].
Fig. 2.
Definitions of antibiotic exposure by study relative to the initiation of immune checkpoint blockade at time 0. This figure demonstrates the various definitions of antibiotic exposure adopted in each study, relative to the initiation of ICB at time 0. Studies by Do [17], Kulkarni [15], Masini [18] and Hemadri [21] defined antibiotic exposure as any time during ICB, as illustrated with the arrow beyond 100 days
Outcome data
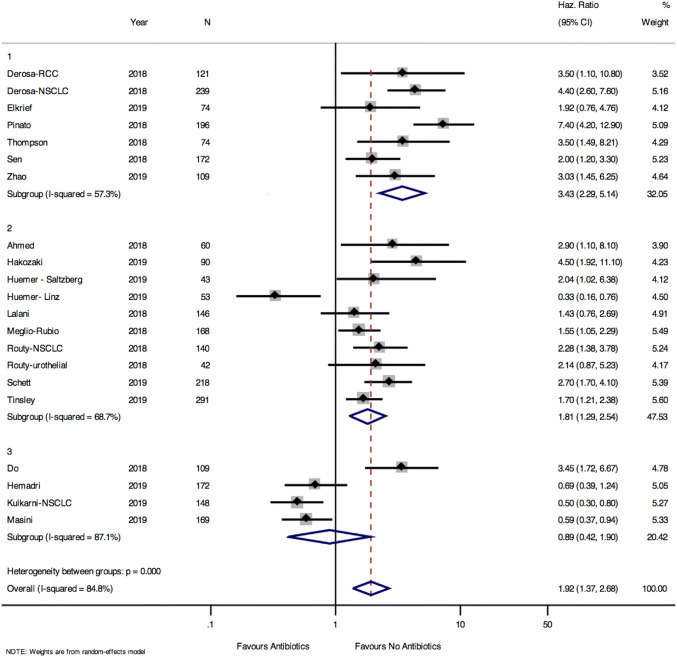
OS data was available for 21 of the 22 cohorts included. Pooled results showed a prolonged OS among those who did not receive antibiotics (pooled HR 1.92, 95% CI 1.37–2.68, p < 0.001) (Fig. 3). There was heterogeneity in the results for OS between studies (Cochrane Q test for heterogeneity p < 0.0001). The funnel plot showed no publication bias for the OS data, with Egger test for small studies p = 0.397 (Supplemental Fig. 2).
Fig. 3.
Pooled hazards ratio for overall survival among those exposed and unexposed to antibiotics stratified by antibiotic exposure definition. Group 1: cohorts defining antibiotic exposure as up to 42 days before initiation of ICB. Group 2: cohorts defining antibiotic exposure as 60 days before and 42 days after initiation of ICB. Group 3: cohorts defining antibiotic exposure as 60 days before and anytime during ICB
The HR for OS were stratified by the cohort definition of antibiotic exposure (Fig. 3). Among group 1 cohorts (antibiotic exposure within 42 days before initiation of ICB), OS was prolonged in those who were not exposed to antibiotics (HR 3.43, 95% CI 2.29–5.14, p < 0.0001). For group 2 cohorts (antibiotic use within 60 days before or 42 days after initiation of ICB) those unexposed to antibiotics still had prolonged OS, but the effect was less pronounced (HR 1.81, 95% CI 1.29–2.54, p = 0.001). However, for group 3 cohorts (antibiotic exposure 60 days before and anytime during ICB) there was no difference in OS between those exposed and unexposed to antibiotics (HR 0.89, 95% CI 0.42–1.90, p = 0.76). Meta-regression demonstrated a strong association between the antibiotic window and the effect of antibiotics on OS (p = 0.002, Supplemental Fig. 3). Overall, these results suggest that the impact of antibiotics on OS is greatest in the period immediately prior to initiation of immunotherapy. When the HRs for OS were stratified by tumour type, we also found differences in the effects of antibiotics on outcome (NSCLC HR 2.00 95% CI 1.23–3.25, RCC HR 1.86 95% CI 1.16–2.98, melanoma HR 1.08 95% CI 0.4–2.92, mixed tumour types HR 2.07 95% CI 0.94–4.57, test for heterogeneity between subgroups p = 0.06) (Supplemental Fig. 4).
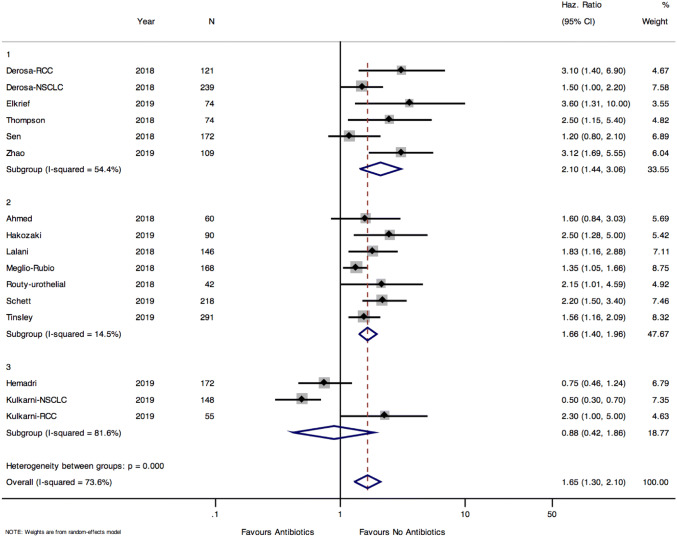
PFS data was available for 16 of the 22 cohorts included. Pooled PFS was longer in patients who did not receive antibiotics compared to those who were treated with antibiotics (pooled HR 1.65, 95% CI 1.3–2.1, p < 0.0001) (Fig. 4). There was significant heterogeneity between cohorts, and between the groups of cohorts when stratified by antibiotic exposure window (test for heterogeneity between groups p < 0.001). The effect of antibiotics on PFS was greatest in group 1 cohorts (HR 2.1 95% CI 1.44–3.06) followed by group 2 cohorts (HR 1.66 95% CI 1.4–1.96). There was no effect of antibiotics on PFS in group 3 cohorts (HR 0.88 95% CI 0.42–1.86). There was no evidence of publication bias in the funnel plot, confirmed by the Egger test for small study effect (p = 0.12) (Supplemental Fig. 5). PFS was longer in patients who did not receive antibiotics in cohorts of NSCLC patients (HR 1.64 95% CI 1.07–2.52) and RCC (HR 2.13 95% CI 1.54–2.93) and mixed tumour types (HR 1.47 95% CI 1.16–1.86), but not for melanoma (HR 1.54 95% CI 0.33–7.12) (Supplemental Fig. 6).
Fig. 4.
Pooled hazards ratio for progression free survival among those exposed and unexposed to antibiotics stratified by antibiotic exposure definition. Group 1: cohorts defining antibiotic exposure as 42 days before initiation of ICB. Group 2: cohorts defining antibiotic exposure as 60 days before and 42 days after initiation of ICB. Group 3: cohorts defining antibiotic exposure as 60 days before and anytime during ICB
Of the included cohorts, 7 [5, 17–21] did not present any multivariate analysis (Supplemental Table 2). Of the 12 cohorts that reported a multivariate analysis for OS, 8 [6, 7, 13, 16, 22–25] remained significant after adjusting for other variables. Of the ten cohorts that presented a multivariate analysis for PFS, 9 [7, 8, 13, 15, 22, 23, 25, 26] remained significant after adjusting for other variables. The types of variables included in the multivariate models differed by cohort as listed in Supplemental Table 2. These results show that even after adjusting for baseline confounders, antibiotics remained associated with worse OS in 66.7% of cohorts and worse PFS in 90% of cohorts for which adjusted analysis was performed.
Discussion
Our pooled results demonstrate that OS was almost two times longer and PFS was 1.65 times longer in patients who did not receive antibiotics either before or during treatment with ICB.
This is the first meta-analysis to characterise how timing of antibiotic exposure might impact on responsiveness to ICB. OS was 3.4 times longer in patients who did not receive any antibiotics in the 42 days prior to ICB (group 1 cohorts). In contrast, pooled results from studies defining antibiotic exposure as 60 days prior or any time during ICB found no differences in OS (group 3 cohorts). These pooled findings are also supported by stratified analyses within studies. Sen et al. [19] presented a stratified analysis by timing of antibiotic onset, and found no significant difference in overall survival in patients receiving antibiotics during ICB or in the 30–60 days prior to ICB initiation, as opposed to the significant difference in OS for antibiotic use 0–30 days prior to IO initiation. Pinato et al. [27] also stratified antibiotic exposure by timing, and found that the effects of antibiotics on survival were much greater in those exposed 0–30 days before initiation of immunotherapy (HR 7.4, 95% CI 4.3–12.8), compared to those exposed during immunotherapy (HR 0.9, 95% CI 0.5–1.4). In a recent study [28] of 12 healthy men treated with a 4-day course of meropenem, vancomycin and gentamicin, the gut microbiota composition had recovered to near baseline by 42 days. Another study treated three patients with ciprofloxacin and found that most species had recovered to a pre-antibiotic state by 4 weeks [29]. These recovery times support our findings that antibiotic exposure in the 42-day period prior to start of immunotherapy is particularly harmful to the microbiome, and most likely to negatively impact on the effectiveness of ICB.
While there is evidence to support the critical window prior to initiation of ICB, our understanding of the critical window after initiation of ICB is limited. Pre-clinical models to explore how long the microbiome takes to prime and activate the immune system after exposure to ICB are needed.
The antibiotic exposure windows defined in our study are broad and overlapping. For example, the exposure window in group 3 cohorts (60 days prior and any time during ICB) also includes patients exposed to antibiotics within the definition of group 1 cohorts (42 days prior to ICB initiation). This may explain some of the heterogeneity seen within the results for group 2 and 3 cohorts, as opposed to group 1 cohorts, where the results are more homogenous. Moreover, we cannot be certain that a patient exposed to antibiotics within 42 days of initiation of ICB, was not also later exposed to antibiotics during treatment with ICB. Unfortunately, detailed data regarding the timing of exposure for each patient was not available in the setting of this study-level meta-analysis, and therefore, this result should be considered hypothesis generating. A patient-level analysis to more precisely explore the timing of antibiotic exposure is needed.
When the pooled HR for OS were stratified by tumour type, overall survival was prolonged in patients with NSCLC and RCC/urothelial cancers who were not exposed to antibiotics. While we recognise that RCC and urothelial cancers have different biology and responses to immunotherapy, the effect of antibiotics on outcome in the RCC and urothelial cancer studies was similar, as demonstrated by the similar HR for PFS and OS between these studies. Antibiotic exposure did not affect OS in the studies of melanoma patients. This likely reflects the small number of melanoma studies included in the meta-analysis (only 2 cohorts). This result may also be confounded by the broad definition of antibiotic use adopted in one [21] of these two studies, or by other baseline differences between the populations.
Metagenomic research is ongoing to further clarify which bacterial species predict response to ICB. Several studies have demonstrated differences in the stool microbial composition in responders and non-responders to ICB for lung, renal [13] and melanoma [3, 4, 30, 31] patients. However, the bacterial signatures correlating with response to ICB have not yet been validated in a prospective trial. The bacteria that predicted response to therapy differed between studies. Frankel et al. found that the presence of Bacteroides, among others, predicted ICB response, while Chaput found that Faecalibacterium was predictive of response. Matson et al. identified Bifidobacterium longum as a marker of ICB response, while Gopalakrishnan et al. identified Ruminococcaceae. Finally, Routy et al. found a correlation between the abundance of Akkermansia muciniphilia in stools and clinical response to ICB. Different microbial species have diverse immunomodulatory effects independent of microbial phylogeny [32], supporting the hypothesis that imbalances in the gut flora might alter the immune systems’ ability to respond to ICB for malignancy, rather than the presence or absence of any one particular species. Additionally, pre-clinical models have shown that poor response to ICB may be reversed by faecal compensation with bacteria [13, 30], raising the possibility of improving responsiveness to ICB through therapeutic manipulation of the microbiome. A recent randomised study of oral supplementation of Akkermansia muciniphilia improved several metabolic parameters in obese patients, providing a proof of concept that oral manipulation of the microbiome could be used to alter disease outcomes [33], and similar studies in cancer are needed. The mechanisms by which the microbiome primes or activate the immune system’s response to ICB remains an area of active research.
To date, no circulating markers of gut health have been prospectively validated to predict response to immunotherapy. However, in a recent study, patients responding to nivolumab for NSCLC were found to have higher plasma citrulline levels compared to non-responders [34]. Citrulline is an amino acid produced almost exclusively by enterocytes. It has been validated as a marker for chemotherapy induced mucosal barrier injury in paediatric patients [35]. Its role in predicting gut health in the setting of antibiotic use and response to immunotherapy should be further explored. Moreover, this same study [34] examined the blood microbiome and found several signatures that were predictive of clinical response to ICB. Whether or not antibiotics impact on the blood microbiome and negatively affect PFS and OS remains to be seen.
The microbiome may also play a role in modulating immune related toxicities. Vetizou et al. [1] demonstrated that oral inoculation with Bacteroides in combination with Burkholderia reduced immune related colitis in mice. Therefore, antibiotic use could affect the severity and frequency of immune related toxicities in patients treated with ICB. In one retrospective study [14], there was no difference in the grade of immune related toxicities in patients exposed or unexposed to antibiotics. However, further research is warranted.
There are several important limitations of this study. This systematic review and meta-analysis only included observational studies. However, our search identified two studies [11, 12] analysing prospective randomised controlled trial data. In a pooled analysis of the OAK [36] and POPLAR [37] trials for NSCLC, median OS was shorter among those exposed to antibiotics (8.54 vs 14.06 months, HR 1.32, 95% CI 1.06–1.63), and this association remained significant after adjusting for potential confounders [12]. On the other hand, Weinstock et al. [11] pooled results from seven clinical trials in urothelial cancer and found no difference in OS (9.23 vs 9.86 months) or PFS (105 vs 101 days). For the latter study, antibiotic exposure was defined as any time during ICB, further supporting our results that the timing of antibiotic exposure is important. Therefore, while these studies are not included in the pooled results of this meta-analysis, they provide further support for the findings presented in this paper. Similar analyses for other large randomised studies of immunotherapy in solid malignancies would be helpful, with attention paid to the antibiotic exposure window.
Secondly, this study does not account for differences in the types of antibiotics, route of administration or duration of use. Longer treatment durations and broader spectrum antibiotics may have more detrimental effects on gut microbiome, thereby having a greater impact on OS and PFS. Ahmed et al. [6] found PFS was longer in patients receiving narrow vs broad spectrum antibiotics; however, the sample size was small (HR 1.8, 95% CI 0.86–3.89). Tinsley et al. [25, 38] found that longer durations and multiple courses of antibiotics had more significant effects on OS. Galli et al. [39] also found that patients with longer antibiotic exposures had shorter PFS and OS. Mielgo-Rubio found that patients who received intravenous antibiotics has shorter OS and PFS compared to those who received oral antibiotics (OS 2.9 vs 14.2 months, p = 0.0001, PFS 2.2 vs 5.9 months, p = 0.001) [20]. Unfortunately, there was insufficient data to examine the effect of antibiotic type, duration and route on clinical outcomes in this study and further research is warranted. Furthermore, our study was not able to determine whether the detrimental effects seen on OS and PFS from antibiotic exposure are due to the antibiotics themselves, or whether having an infection alone might negatively influence the response to immunotherapy. Further studies examining the PFS and OS stratified by antibiotic indication (prophylactic antibiotic use vs treatment of infection) could help to clarify this important point.
Thirdly, this study-level meta-analysis cannot adjust for patient-level confounders. Differences in baseline performance status, age and comorbidities among those treated with antibiotics could drive poorer survival outcomes and cannot be accounted for in the present study. Among the cohorts that did present a multivariate analysis, antibiotics remained associated with worse OS in 66.7% and worse PFS in 90%. Further research adjusting for potential confounders is needed.
Finally, while this study focused on the impact of antibiotic exposure on OS and PFS, there is growing literature that proton-pump inhibitors, corticosteroids and vaccines may also influence the outcomes and safety of ICB [40]. Further exploration of how these drugs might impact the microbiome thereby indirectly impacting response to ICB should be undertaken.
Conclusions
In this meta-analysis of 18 observational studies including 22 distinct cohorts, pooled HR for OS and PFS were longer in patients who were not exposed to antibiotics. The timing of antibiotic exposure is a significant effect modifier in the association between antibiotics and response to ICB, with antibiotic exposure immediately prior to initiation of ICB having the greatest impact on OS. Without undermining the role of antibiotics in patients with infections, careful consideration of the use of antibiotics and the subsequent timing of ICB initiation is warranted.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- CAR-T
Chimeric antigen receptor T cell
- CTLA-4
Cytotoxic T lymphocyte associated protein 4
- ECOG
Eastern Co-operative Oncology Group
- HR
Hazard ratio
- ICB
Immune checkpoint blockade
- NSCLC
Non-small cell lung cancer
- OS
Overall survival
- PD-1
Programmed cell death protein—1
- PD-L1
Programmed death—ligand 1
- PFS
Progression free survival
- RCC
Renal cell carcinoma
Author contributions
BEW: project design, data extraction, data analysis, manuscript writing. BR: manuscript revision and advice. AN: manuscript revision and advice. VC: project design, data extraction, data interpretation and manuscript revision
Funding
No relevant funding.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
As this study was based on published data, no ethics approval was sought for the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vétizou M, Pitt JM, Daillère R, Lepage P, Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong CP. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350(6264):1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Lei YM, Jabri B, Alegre M-L. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti–PD-L1 efficacy. Science. 2015;350(6264):1084–1089. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gopalakrishnan V, Spencer C, Nezi L, Reuben A, Andrews M, Karpinets T, Prieto P, Vicente D, Hoffman K, Wei S. Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients. Science. 2018;359(6371):97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaput N, Lepage P, Coutzac C, Soularue E, Le Roux K, Monot C, Boselli L, Routier E, Cassard L, Collins M. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol. 2017;28(6):1368–1379. doi: 10.1093/annonc/mdx108. [DOI] [PubMed] [Google Scholar]
- 5.Huemer F, Rinnerthaler G, Lang D, Hackl H, Lamprecht B, Greil R. Association between antibiotics use and outcome in patients with NSCLC treated with immunotherapeutics. Ann Oncol. 2019;30(4):652–653. doi: 10.1093/annonc/mdz021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmed J, Kumar A, Parikh K, Anwar A, Knoll BM, Puccio C, Chun H, Fanucchi M, Lim SH. Use of broad-spectrum antibiotics impacts outcome in patients treated with immune checkpoint inhibitors. OncoImmunology. 2018;7(11):e1507670. doi: 10.1080/2162402X.2018.1507670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Derosa L, Hellmann M, Spaziano M, Halpenny D, Fidelle M, Rizvi H, Long N, Plodkowski A, Arbour K, Chaft J. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann Oncol. 2018;29(6):1437–1444. doi: 10.1093/annonc/mdy103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elkrief A, El Raichani L, Richard C, Messaoudene M, Belkaid W, Malo J, Belanger K, Miller W, Jamal R, Letarte N. Antibiotics are associated with decreased progression-free survival of advanced melanoma patients treated with immune checkpoint inhibitors. OncoImmunology. 2019;8:1–6. doi: 10.1080/2162402X.2019.1568812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaderbhai C, Richard C, Fumet JD, Aarnink A, Foucher P, Coudert B, Favier L, Lagrange A, Limagne E, Boidot R. Antibiotic use does not appear to influence response to Nivolumab. Anticancer Res. 2017;37(6):3195–3200. doi: 10.21873/anticanres.11680. [DOI] [PubMed] [Google Scholar]
- 10.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8(1):16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weinstock CMV, Fernandes LL, Tang S, Agrawal S, Brave MH, Ning YM, Singh H, Suzman DL, Xu J, Goldberg KB, Srihara R, Ibrahim A, Theoret MR, Beaver JA, Pazdur R. Impact of antibiotic use on clinical outcomes in patents with urothelial cancer receiving a programmed death protein 1 or programmed death ligant (and-PD1/L1) antibody. J Clin Oncol. 2019;37:4557. doi: 10.1200/JCO.2019.37.15_suppl.4557. [DOI] [PubMed] [Google Scholar]
- 12.Chalabi M, Cardona A, Nagarkar D, Scala AD, Albert M, Kok M, Powles T, Herrera F. 50O: Effects of antibiotics and proton pump inhibitors in NSCLC patients treated with atezolizumab and docetaxel: pooled analysis of the OAK and POPLAR trials. Ann Oncol. 2018;29(supplemental 10):486.001. doi: 10.1016/j.annonc.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Routy B, Le Chatelier E, Derosa L, Duong CP, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP. Gut microbiome influences efficacy of PD-1—based immunotherapy against epithelial tumors. Science. 2018;359(6371):91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 14.Huemer F, Rinnerthaler G, Westphal T, Hackl H, Hutarew G, Gampenrieder SP, Weiss L, Greil R. Impact of antibiotic treatment on immune-checkpoint blockade efficacy in advanced non-squamous non-small cell lung cancer. Oncotarget. 2018;9(23):16512–16520. doi: 10.18632/oncotarget.24751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kulkarni AKM, Pease DF, Want Y, deFor TE, Patel M. Impact of antibiotics and proton pump inhibitors on clinical outcomes of immune check point blockers in advanced non-small cell lung cancers and metastatic renal cell cancers. J Clin Oncol. 2019;37:e20520. doi: 10.1200/JCO.2019.37.15_suppl.e20520. [DOI] [Google Scholar]
- 16.Pinato DJHS, Ottaviani D, Urus H, Patel A, et al. Antibiotic treatment prior to immune checkpoint inhibitor therapy as a tumor-agnostic predictive correlate of response in routine clinical practice. J Clin Oncol. 2019;37(supplemental 8):147. doi: 10.1200/JCO.2019.37.8_suppl.147. [DOI] [Google Scholar]
- 17.Do TP, Hegde AM, Cherry CR, Stroud CRG, Sharma N, Cherukuri SD, Bowling M, Walker PR. Antibiotic use and overall survival in lung cancer patients receiving nivolumab. J Clin Oncol. 2019;36(supplement 15):e15109. [Google Scholar]
- 18.Masini CBA, Romagnani A, Bonelli C, Fantinel E, Pagano M, Banzi M, Prati G, Gasparini E, Moretti G, Gervasi E, Gnoni R, Stridi G, Pinto C. Results of an Italian CORE-IMMUNO study: safety and clinical-related biomarkers as predictors of immunotherapy (IT) benefit in real-world treatment of various advanced tumors (ATs) J Clin Oncol. 2019;37:e14156. doi: 10.1200/JCO.2019.37.15_suppl.e14156. [DOI] [Google Scholar]
- 19.Sen S, Carmagnani Pestana R, Hess K, Viola G, Subbiah V. Impact of antibiotic use on survival in patients with advanced cancers treated on immune checkpoint inhibitor phase I clinical trials. Ann Oncol. 2018;29(12):2396–2398. doi: 10.1093/annonc/mdy453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mielgo-Rubio X, Chara L, Sotelo-Lezama M, Castro RL, Rubio-Martínez J, Velastegui A, Olier-Garate C, Falagan S, Gómez-Barreda I, Bautista-Sanz P. Antibiotic use and PD-1 inhibitors: shorter survival in lung cancer, especially when given intravenously. Type of infection also matters. J Thorac Oncol. 2018;13(10):S389. doi: 10.1016/j.jtho.2018.08.395. [DOI] [Google Scholar]
- 21.Hemadri ALH, Lin Y, Rose A, Sander C, Najjar Y, Zarour HM, Kirkwood JM, Davar D. Association of medication and antibiotic use with response and survival in advanced melanoma receiving PD-1 inhibtors. J Clin Oncol. 2019;37(supplemental 15):9572. doi: 10.1200/JCO.2019.37.15_suppl.9572. [DOI] [Google Scholar]
- 22.Thompson J, Szabo A, Arce-Lara C, Menon S. Microbiome and immunotherapy: antibiotic use is associated with inferior survival for lung cancer patients receiving PD-1 inhibitors. J Thorac Oncol. 2017;12(11 Supplement 2):S1998. doi: 10.1016/j.jtho.2017.09.926. [DOI] [Google Scholar]
- 23.Zhao S, Gao G, Li W, Li X, Zhao C, Jiang T, Jia Y, He Y, Li A, Su C. Antibiotics are associated with attenuated efficacy of anti-PD-1/PD-L1 therapies in Chinese patients with advanced non-small cell lung cancer. Lung Cancer. 2019;130:10–17. doi: 10.1016/j.lungcan.2019.01.017. [DOI] [PubMed] [Google Scholar]
- 24.Schett ARS, Mauti LA, Schmid S, Appenzeller C, Curioni-Fontecedro A, Frueh M, Joerger M. Progsnotic impact of the use of antibiotics in patients with advanced non-small cell lung cancer receiving PD-(L)1 targeting monoclonal antibodies. Ann Oncol. 2019;30(Supplemental 2):157. [Google Scholar]
- 25.Tinsley N, Zhou C, Tan G, Rack S, Lorigan P, Blackhall F, Krebs M, Carter L, Thistlethwaite F, Graham D. Cumulative Antibiotic Use Significantly Decreases Efficacy of Checkpoint Inhibitors in Patients with Advanced Cancer. Oncologist. 2019;24:1–9. doi: 10.1634/theoncologist.2017-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lalani A-KA, Xie W, Lin X, Steinharter JA, Martini DJ, Duquette A, Bosse D, McKay RR, Simantov R, Wei XX. Antibiotic use and outcomes with systemic therapy in metastatic renal cell carcinoma (mRCC) J Clin Oncol 36(supplemental. 2018;6):607. doi: 10.1200/JCO.2018.36.6_suppl.607. [DOI] [Google Scholar]
- 27.Pinato DJ, Howlett S, Ottaviani D, Urus H, Patel A, Mineo T, Brock C, Power D, Hatcher O, Falconer A. Association of prior antibiotic treatment with survival and response to immune checkpoint inhibitor therapy in patients with cancer. JAMA Oncol. 2019;5(12):1774–1778. doi: 10.1001/jamaoncol.2019.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palleja A, Mikkelsen KH, Forslund SK, Kashani A, Allin KH, Nielsen T, Hansen TH, Liang S, Feng Q, Zhang C, et al. Recovery of gut microbiota of healthy adults following antibiotic exposure. Nat Microbiol. 2018;3(11):1255–1265. doi: 10.1038/s41564-018-0257-9. [DOI] [PubMed] [Google Scholar]
- 29.Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008;6(11):e280. doi: 10.1371/journal.pbio.0060280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre M-L, Luke JJ, Gajewski TF. The commensal microbiome is associated with anti–PD-1 efficacy in metastatic melanoma patients. Science. 2018;359(6371):104–108. doi: 10.1126/science.aao3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frankel AE, Coughlin LA, Kim J, Froehlich TW, Xie Y, Frenkel EP, Koh AY. Metagenomic shotgun sequencing and unbiased metabolomic profiling identify specific human gut microbiota and metabolites associated with immune checkpoint therapy efficacy in melanoma patients. Neoplasia. 2017;19(10):848–855. doi: 10.1016/j.neo.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geva-Zatorsky N, Sefik E, Kua L, Pasman L, Tan TG, Ortiz-Lopez A, Yanortsang TB, Yang L, Jupp R, Mathis D. Mining the human gut microbiota for immunomodulatory organisms. Cell. 2017;168(5):928–943. doi: 10.1016/j.cell.2017.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Depommier C, Everard A, Druart C, Plovier H, Van Hul M, Vieira-Silva S, Falony G, Raes J, Maiter D, Delzenne NM. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med. 2019;25(7):1096. doi: 10.1038/s41591-019-0495-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krief JO, de Tauriers PH, Dumenil C, Neveux N, Dumoulin J, Giraud V, Labrune S, Tisserand J, Julie C, Emile J-F. Role of antibiotic use, plasma citrulline and blood microbiome in advanced non-small cell lung cancer patients treated with nivolumab. J Immunother Cancer. 2019;7(1):176. doi: 10.1186/s40425-019-0658-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Vliet MJ, Tissing WJ, Rings EH, Koetse HA, Stellaard F, Kamps WA, de Bont ES. Citrulline as a marker for chemotherapy induced mucosal barrier injury in pediatric patients. Pediatr Blood Cancer. 2009;53(7):1188–1194. doi: 10.1002/pbc.22210. [DOI] [PubMed] [Google Scholar]
- 36.Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, Von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols MC. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D, Artal-Cortes A, Lewanski C. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837–1846. doi: 10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
- 38.Tinsley NZC, Villa SS, Tan G, Lorigan P, Blackhall FH, Elliott T, Krebs M, Carter L, Thistlethwaite F, Hughes A, Cook N. Cumulative antibiotic use and efficacy of immune checkpoint inhibitors in patients with advanced cancer. J Clin Oncol 36(supplemental. 2018;15):3010. doi: 10.1200/JCO.2018.36.15_suppl.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galli G, Poggi M, Fuca G, Imbimbo M, Proto C, Signorelli D, Vitali M, Zilembo N, Ganzinelli M, De Braud F, et al. Impact of antibiotics on outcome of metastatic non small cell lung cancer patients treated with immunotherapy. J Thorac Oncol. 2018;13(Supplement 10):S389. doi: 10.1016/j.jtho.2018.08.396. [DOI] [Google Scholar]
- 40.Rossi G, Pezzuto A, Sini C, Tuzi A, Citarella F, McCusker MG, Nigro O, Tanda E, Russo A. Concomitant medications during immune checkpoint blockage in cancer patients: novel insights in this emerging clinical scenario. Crit Rev Oncol Hematol. 2019;142:26–34. doi: 10.1016/j.critrevonc.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 41.Hakozaki T, Okuma Y, Omori M, Hosomi Y. Impact of prior antibiotic use on the efficacy of nivolumab for non-small cell lung cancer. Oncol Lett. 2019;17(3):2946–2952. doi: 10.3892/ol.2019.9899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lalani AKA (2019) Effect of antibiotic use on outcomes with systemic therapies in metastatic renal cell carcinoma. In. Personal communication regarding unpublished data
- 43.Khan U, Peña C, Brouwer J, Hoffman K, Choudhury AR, Zhang C, Thakkar P, Betel D, Sarkar S, Sonnenberg G. Impact of antibiotic use on response to treatment with immune checkpoint inhibitors. J Clin Oncol 37(supplemental. 2019;4):143. doi: 10.1200/JCO.2019.37.4_suppl.143. [DOI] [Google Scholar]
- 44.Agarwal A, Pond GR, Curran C, Nassar A, Nuzzo PV, Kumar V, McGregor BA, Wei XX, Harshman LC, Choueiri TK, et al. Impact of concurrent medications on outcomes with PD1/PDL1 inhibitors for metastatic urothelial carcinoma. J Clin Oncol. 2019;37(Supplement 7):435. doi: 10.1200/JCO.2019.37.7_suppl.435. [DOI] [Google Scholar]
- 45.Metges Jean-Philippe, Michaud Emmanuelle, Deniel Lagadec Delphine, Marhuenda Fanny, Chaslerie Anicet, Grude Francoise. Impact of anti-infectious and corticosteroids on immunotherapy: Nivolumab and pembrozilumab follow-up in a French study. Journal of Clinical Oncology. 2018;36(15_suppl):e15157–e15157. doi: 10.1200/JCO.2018.36.15_suppl.e15157. [DOI] [Google Scholar]
- 46.Spakowicz DHM, Tinoco G, Patel SH, Murkart JT, Verscharegen SF, Ekndra KL, Hoffman S, Philippon J, Quiroga DM, Otterson GA, Owen DH. Effect of concomittant medications on overall survival in patients with cancer undergoing immunotherapy. J Clin Oncol 37(supplemental. 2019;8):94. doi: 10.1200/JCO.2019.37.8_suppl.94. [DOI] [Google Scholar]
- 47.Wang YWD, Helmink BA, Gopalakrishnan V, Choi K, Dupont HL, et al. Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nat Rev. 2018;24(12):1804–1808. doi: 10.1038/s41591-018-0238-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kapoor VRJ, Boyce T, Pankratz VS, Rixe O. Effect of antibiotic exposure in patients with metastatic melanoma treated with PD-1 inhibitor or CTLA-4 inhibitor or a combination of both. Am Soc Clin Oncol. 2019;37(supplemental 15):e14141. doi: 10.1200/JCO.2019.37.15_suppl.e14141. [DOI] [Google Scholar]
- 49.Derosa L, Routy B, Enot D, Baciarello G, Massard C, Loriot Y, Fizazi K, Escudier BJ, Zitvogel L, Albiges L. Impact of antibiotics on outcome in patients with metastatic renal cell carcinoma treated with immune checkpoint inhibitors. J Clin Oncol. 2017;35(6 Supplement 1):462. doi: 10.1200/JCO.2017.35.6_suppl.462. [DOI] [Google Scholar]
- 50.Derosa L, Routy B, Enot D, Fidelle M, Gubet AG, Goldwasser F, Zitvogel L, Loriot Y, Albiges L, Escudier B. Impact of antibiotics on outcome in patients with metastatic renal cell carcinoma treated with immune checkpoint inhibitors. Kidney Cancer. 2018;2(Supplement 1):S24–S25. [Google Scholar]
- 51.Derosa L, Routy B, Mezquita L, Naltet C, Enot D, Fidelle M, Goubet AG, Soria JC, Massard C, Goldwasser F, et al. Antibiotics prescription to decrease progression-free survival (PFS) and overall survival (OS) in patients with advanced cancers treated with PD1/PDL1 immune checkpoint inhibitors. J Clin Oncol. 2017;35(15 Supplement 1):3015. doi: 10.1200/JCO.2017.35.15_suppl.3015. [DOI] [Google Scholar]
- 52.Zhao S, Jia Y, Jiang T, Li X, Li W, Gao G, Zhao C, He Y, Chen X, Su C, et al. Antibiotics attenuate the clinical benefit of anti-PD-(L)1 immunotherapies in Chinese patients with advanced non-small cell lung cancer. J Thorac Oncol. 2018;13(10 Supplement):S930. doi: 10.1016/j.jtho.2018.08.1728. [DOI] [Google Scholar]
- 53.Wilson BE, Routy B, Nagrial A, Chin V. The effect of antibiotics on clinical outcomes in immune-checkpoint blockade: a systematic review and meta-analysis of observational studies. Asia-Pacific J Clin Oncol. 2019;15(supplement 9):359. doi: 10.1007/s00262-019-02453-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.