Abstract
CD160 is an Ig-like glycoprotein expressed by the majority of circulating natural killer cells and γδ T cells. Whether CD160 could regulate CD8+ T-cell functions remains unknown. In this study, we investigated the effects of CD160 on CD8+ T cells in pancreatic cancer. First, we found that the frequency of PD-1+ cells was comparable between CD160+ and CD160−CD8+ T cells, with the former presenting significantly higher PD-1 expression level. In contrast, the frequency of TIM-3+ cells was higher among CD160+ cells but the expression level was comparable between CD160+ and CD160−CD8+ T cells. The IFN-γ and IL-2-expressing CD8+ T cells, directly ex vivo, were highly enriched in the CD160+ subset. However, when CD160+ and CD160−CD8+ T cells were stimulated, the proliferation levels of CD160+ and CD160− cells were initially comparable, but were significantly lower in CD160+CD8+ T cells than in CD160−CD8+ T cells later on. The IFN-γ and IL-2 transcription levels were initially higher in CD160+CD8+ T cells, but eventually reduced in CD160+CD8+ T cells compared to CD160−CD8+ T cells. Also, CD160+CD8+ T cells presented lower cytotoxic capacity than CD160−CD8+ T cells. Interestingly, we observed that tumor-infiltrating CD8+ T cells were significantly enriched with the CD160+ subset in pancreatic cancer patients. In addition, patients with higher frequencies of tumor CD160+CD8+ T cells presented lower survival. Overall, these data demonstrated that tumor-infiltrating CD8+ T cells were enriched with the CD160+ subset in pancreatic cancer, with active effector responses directly ex vivo but limited potential for further activation.
Electronic supplementary material
The online version of this article (10.1007/s00262-020-02500-3) contains supplementary material, which is available to authorized users.
Keywords: CD160, CD8+ T cells, Effector responses, Pancreatic cancer
Introduction
CD160 is an Ig-like glycoprotein that acts as an activating receptor on the surface of several lymphocyte subsets [1]. It is expressed by all of the small intestinal intraepithelial T cells and the majority of circulating natural killer (NK) cells and circulating γδ T cells [2]. A minor population of circulating CD4+ and CD8+ T cells also express CD160 [2, 3]. CD160 has not been found on normal B cells but is found on malignant B-cell chronic lymphocytic leukemia [4, 5].
CD160 binds broadly but weakly to MHC class I molecules and strongly to the regulatory molecule herpes virus entry mediator (HVEM) [3, 6–8]. Engagement of CD160 with HLA-C is thought to mediate NK cytotoxicity and induce a unique cytokine expression profile with the production of IFN-γ, TNF-α, and IL-6 [6, 9]. However, a later study using CD160−/− mice has demonstrated that CD160-deficient NK cells present regular cytotoxicity but severely reduced IFN-γ expression [10]. CD160 may also act as a co-receptor in T cells lacking CD28 expression and participate in TCR signal transduction to promote proliferation [11]. Interestingly, CD160 is slightly upregulated in CD4+ T cells upon anti-CD3/CD28 stimulation, and the engagement of CD160 with HVEM strongly inhibits CD4+ T-cell proliferation and cytokine expression in a dose-dependent manner [3]. Similarly, blockade of CD160 can restore cell proliferation in CD160+ CD8+ T cells, but unlike the situation in CD4+ T cells, anti-CD3/CD28 stimulation does not upregulate CD160 in CD8+ T cells [12]. In contrast, CD56dim NK cells, which represent over 90% of all circulating NK cells, highly express CD160, and upon engagement with HVEM, CD160 induces strong phosphorylation of ERK1/2 and AKT and enhances NK-mediated lysis of target cells [13]. Together, it is clear that CD160 performs critical functions in the regulation of immune responses, and the specific direction of CD160-mediated regulation appears to be dependent on many factors, including the interacting ligand, the cell type, and the activation status.
Recently in hepatocellular carcinoma, patients with lower density of intratumoral CD160+ cells presented worse diseases and higher recurrence rates [14]. The intrahepatic NK cells were distinguished into CD160+ and CD160− subsets, in which CD160+ NK cells presented higher levels of activating molecules, such as NKp30, NKp44, NKp46, NKG2D, CD244, and IFN-γ, and lower inhibitory receptor B- and T-lymphocyte attenuator (BTLA) and CTLA-4, than CD160− NK cells. However, the intratumoral CD160+ NK cells were more exhausted than their peritumoral CD160+ NK cell counterparts and presented lower IFN-γ expression, which could be restored by TGF-β inhibition. In CD160−/− mice, adoptive transfer of CD160+ NK cells resulted in stronger control of early tumor [10]. These studies indicated that CD160+ NK cells were likely beneficial to cancer patients.
However, given that CD160 is best characterized with inhibition in T cells, the role of CD160-expressing T cells in cancer patients remains unclear and should be investigated independently. In this study, the characteristics of CD160+ CD8+ T cells were examined in patients with pancreatic cancer.
Methods
Study participants
Fifteen patients with resectable pancreatic cancer were recruited in this study. These patients consisted of 10 men and 5 women between 41 and 72 years of age. All patients were HLA-A2 positive. Diagnosis of pancreatic cancer was performed via imaging, ultrasound, and blood tests and confirmed using biopsy. All patients presented resectable tumor, and both the peripheral blood and tumor samples were collected. Familial pancreatic cancer was not observed in this cohort of participants. In addition, potential participants with autoimmune diseases, chronic virus infections, alcoholic liver diseases, cirrhosis, and rare inherited conditions were excluded.
Sample collection
Peripheral blood mononuclear cells (PBMCs) were collected using standard centrifugation method across the Ficoll gradient. Tumor-infiltrating lymphocytes (TILs) were collected using enzyme digestion according to a previous published protocol [15]. Briefly, the resected tumor was rinsed in 10 × penicillin–streptomycin (Thermo Fisher), and then minced into small fragments. The tumor was then incubated in a shaking water bath at 37 ˚C for 2 h in the presence of 1 mg/mL collagenase IV, 30 U/mL DNase, and 100 µg/mL hyaluronidase (Sigma). The resulting suspension was then strained across a 70-µm cell strainer to obtain TILs. To collect pure CD8+ T cells, PBMCs and TILs were processed using the Human CD8 T cell Enrichment Kit (Stemcell) according to the manufacturer’s procedures. This negative magnetic isolation process yielded a purity of above 97%, as verified in flow cytometry.
Flow cytometry
For the expression of inhibitory molecules, CD8+ T cells from PBMCs and TILs were incubated with anti-human CD3, CD8, CD160, PD-1, and TIM-3 (BioLegend) and LIVE/DEAD Violet stain (Invitrogen) for 30 min in dark. For the expression of intracellular cytokines, CD8+ T cells were incubated with 3 µg/mL each of GolgiPlug and GolgiStop (BD Biosciences) for 5 h and then incubated with anti-human CD8 and CD160 and Violet stain for 30 min in dark. After washing, CD8+ T cells were incubated for 20 min in CytoFix/CytoPerm buffer (BD Biosciences) and then incubated with anti-human IFN-γ and IL-2 for 30 min in dark. The cells were washed to remove excess antibodies and fixed in 2% formaldehyde. Samples were acquired in FACSCanto cytometer and investigated in FowJo Software (version 10).
CD8+ T-cell stimulation
CD8+ T cells were incubated with phycoerythrin (PE)-anti-CD160 and then separated into CD160− and CD160+ subsets using PE-Positive Selection kit (Stemcell). Each subset was then plated at 104 cells per well per 100 µL complete medium in a 96-well round bottom plate, and incubated with 1 bead per cell Human T-Activator CD3/CD28 (Thermo Fisher) and 20 U/mL recombinant human (rh)IL-2 (R&D systems). The cells were harvested at 12 h, 24 h, 48 h, and 96 h post-stimulation, and separated into two portions. One portion was incubated for 5 h with 0.5 µCi/mL radioactive thymidine (Amersham) and then harvested for reading in a direct β-counter. The other portion was treated using the RNeasy Mini kit (Qiagen) and the SuperScript IV Reverse Transcriptase kit (Invitrogen) to obtain cDNA. The expression of IFNG and IL2 transcripts was then evaluated using commercial TaqMan assays (Hs00989291_m1 for IFNG and Hs00174114_m1 for IL2). Relative levels to the reference gene GAPDH were presented in the figures.
Cytotoxicity assay
CD8+ T cells were separated into CD160+ and CD160− subsets and stimulated by anti-CD3/CD28 and rhIL-2 as described above. After 48 h, stimulated CD8+ T cells were co-incubated with Capan-2 cells (ATCC) at various ratios. The amount of cell lysis was then measured using the LDH Cytotoxicity Assay kit (Invitrogen), according to the manufacturer’s procedures. Maximum lysis used pure water in place of culture media, and minimum lysis did not contain CD8+ T cells. Specific lysis was calculated as (experiment − minimum)/(maximum − minimum) × 100.
Statistical analysis
Results between CD160+ and CD160− cells were compared using Student’s t-test. Correlation between tumor and blood was examined using Pearson correlation test. Differences in specific lysis between CD160+ and CD160− cells were examined using ordinary 2-way ANOVA. A p value smaller than 0.05 was considered statistically significant.
Results
CD160+ CD8+ T-cell characteristics in cancer patients
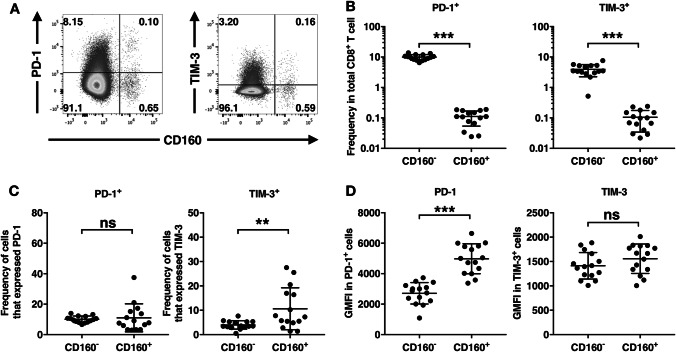
First, fresh circulating CD8+ T cells from 15 pancreatic cancer patients were isolated from total PBMCs using negative magnetic sorting (Supplementary Figure). To examine the ex vivo expression of inhibitory receptors PD-1 and TIM-3, surface staining was performed on CD8+ T cells immediately following isolation (Fig. 1a). The vast majority of PD-1+ and TIM-3+ cells was found in the CD160− population (Fig. 1b). However, the percentage of CD160+ CD8+ T cells that expressed PD-1 was comparable to the percentage of CD160− CD8+ T cells that expressed PD-1; while, the percentage of CD160+ CD8+ T cells that expressed TIM-3 was significantly higher than the percentage of CD160− CD8+ T cells that expressed TIM-3 (Fig. 1c). Interestingly, the level of PD-1 expression by PD-1-expressing CD160+ CD8+ T cells was higher than the level of PD-1 expression by PD-1-expressing CD160− CD8+ T cells; while, the level of TIM-3 expression was comparable between TIM-3-expressing CD160+ CD8+ T cells and TIM-3-expressing CD160− CD8+ T cells (Fig. 1d).
Fig. 1.
Expression of PD-1 and TIM-3 by CD160+ vs. CD160− CD8+ T cells. Fresh circulating CD8+ T cells were stained directly ex vivo. a Representative gating of CD160 vs. PD-1 and CD160 vs. TIM-3 in one representative individual, pre-gated on CD8+ T cells. b Frequencies of PD-1+CD160− and PD-1+CD160+ cells in total CD8+ T cells, and TIM-3+CD160− and TIM-3+CD160+ cells in total CD8+ T cells. c Percent of CD160− or CD160+ CD8+ T cells that expressed PD-1 and TIM-3. d Geometric mead fluorescence intensity (GMFI) of PD-1 and TIM-3 in PD-1+ and TIM-3+ cells, respectively, from CD160− or CD160+ CD8+ T cells. ns not significant. ***p < 0.001
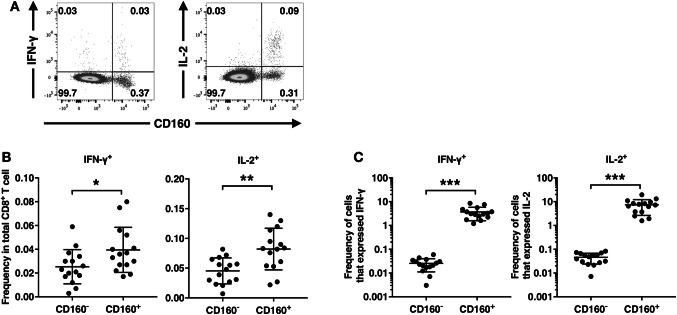
To retain ex vivo cytokine expression intracellularly, freshly isolated CD8+ T cells were incubated for 5 h with Golgi inhibitors in the absence of any other stimulating agents. The expression of cytokines IFN-γ and IL-2 by CD160+ and CD160− CD8+ T cells was then examined using intracellular staining (Fig. 2a). More IFN-γ+ and IL-2+ cells belonged to the CD160+ CD8+ T-cell subset than the CD160− CD8+ T-cell subset (Fig. 2b). Subsequently, the percentages of CD160+ cells and CD160− CD8+ T cells that expressed IFN-γ and IL-2 were calculated. The frequency of IFN-γ+ and IL-2+ cells was significantly higher in CD160+ CD8+ T cells than in CD160− CD8+ T cells (Fig. 2c). In these patients, no significant association was observed between the levels of PD-1 and TIM-3 expression and the levels of IFN-γ and IL-2 expression (data not shown).
Fig. 2.
Expression of intracellular cytokines by CD160+ vs. CD160− CD8+ T cells. Fresh circulating CD8+ T cells were incubated with Golgi inhibitors for 5 h, and the expression of cytokines was examined via intracellular flow cytometry. a Representative gating of CD160 vs. IFN-γ and CD160 vs. IL-2 in one representative individual, pre-gated on CD8+ T cells. b Percent of IFN-γ+ and IL-2+ cells found in the CD160− or CD160+ subset. c Percent of CD160− or CD160+ CD8+ T cells that expressed IFN-γ and IL-2. *p < 0.05. **p < 0.01. ***p < 0.001
Overall, the above results demonstrated that under direct ex vivo condition, CD160+ CD8+ T cells presented higher inhibitory receptor expression and higher cytokine expression than CD160− CD8+ T cells.
CD160+ CD8+ T cells presented limited proliferation potential and higher cell death following stimulation
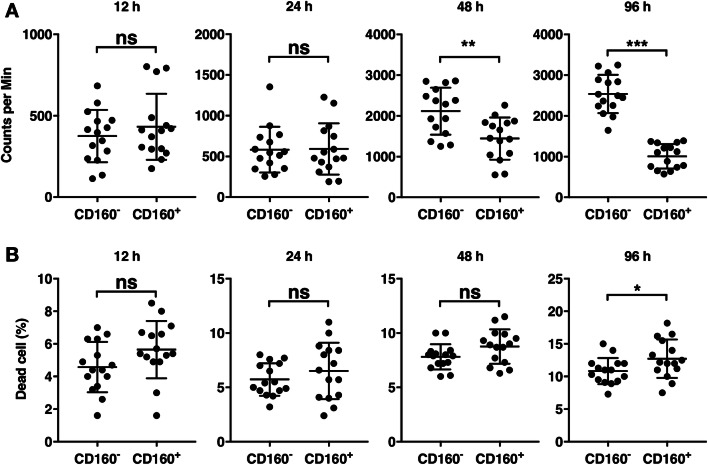
To examine the effector responses of CD160+ and CD160− CD8+ T cells upon activation, we separated CD160+ and CD160− CD8+ T cells and stimulated each population using pan-T-cell activating anti-CD3/CD28 beads and the T-cell growth factor IL-2. The proliferation of each population was examined using radioactive thymidine incorporation assay. At 12 h and 24 h, CD160− and CD160+ CD8+ T cells presented comparable levels of thymidine incorporation (Fig. 3a). However, at 48 h and 96 h, CD160+ CD8+ T cells demonstrated significantly lower proliferation than CD160− CD8+ T cells (Fig. 3a). To examine the viability of CD160+ and CD160− CD8+ T cells, the numbers of live and dead cells were counted using Trypan Blue dye at each time point before pulsing with radioactive thymidine. The frequencies of dead cells were compared between CD160+ and CD160− CD8+ T cells (Fig. 3b). Between 12 and 48 h, no significant difference was observed between CD160+ and CD160− CD8+ T cells (Fig. 3b). At 96 h, however, CD160+ CD8+ T cells presented slightly, but significantly, higher levels of dead cells than CD160− CD8+ T cells (Fig. 3b).
Fig. 3.
Proliferation of stimulated CD160+ and CD160− CD8+ T cells. Circulating CD8+ T cells were separated into CD160+ and CD160− subsets, each stimulated by anti-CD3/CD28 beads and IL-2. The cells were then pulsed with radioactive thymidine at 12 h, 24 h, 48 h, and 96 h after stimulation. a The level of thymidine incorporation at each time point. b The frequency of dead cells at each time point. ns not significant. **p < 0.01. ***p < 0.001
CD160+ CD8+ T cells presented lower cytokine expression following stimulation
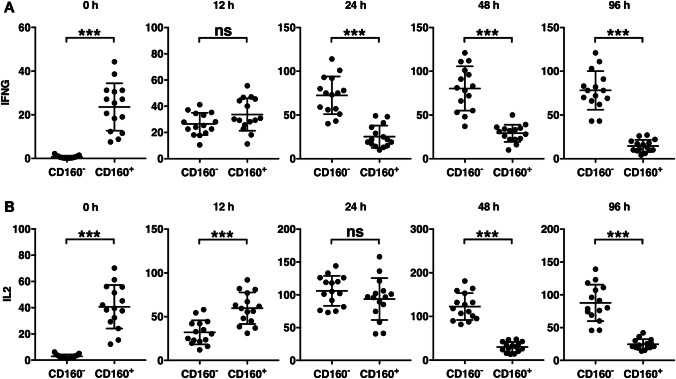
To investigate the cytokine expression of CD160− and CD160+ CD8+ T cells in response to activation, each population was stimulated with anti-CD3/CD28 and IL-2, and a portion of the cells was lysed at various time points for transcript analysis. Prior to stimulation (time 0), the IFN-γ transcription in CD160+ CD8+ T cells was significantly higher than the IFN-γ transcription in CD160− CD8+ T cells (Fig. 4a), which was consistent with the intracellular staining data (Fig. 2). However, 12 h into the stimulation, no significant difference between CD160− CD8+ T cells and CD160+ CD8+ T cells was observed (Fig. 4a), and at 24 h onward, the IFN-γ transcription in CD160− CD8+ T cells was significantly higher than that in CD160+ CD8+ T cells. The IL-2 transcription level was significantly higher in CD160+ CD8+ T cells than in CD160− CD8+ T cells before stimulation and at 12 h after stimulation (Fig. 4b). However, at 24 h post-stimulation, no difference between CD160− CD8+ T cells and CD160+ CD8+ T cells was seen, and from 48 h onward, CD160− CD8+ T cells presented significantly higher IL-2 expression than CD160+ CD8+ T cells.
Fig. 4.
Expression of cytokines by stimulated CD160+ and CD160− CD8+ T cells. Circulating CD8+ T cells were separated into CD160+ and CD160− subsets, each stimulated by anti-CD3/CD28 antibodies and IL-2. The (a) IFN-γ and (b) IL-2 transcript levels were examined before stimulation (time 0) and at 12 h, 24 h, 48 h, and 96 h after stimulation. ns not significant. ***p < 0.001
CD160+ CD8+ T cells presented reduced cytotoxicity compared to CD160− CD8+ T cells
Subsequently, we measured the cytotoxicity by CD160+ CD8+ T cells and CD160− CD8+ T cells form 15 pancreatic cancer patients. CD160+ CD8+ T cells and CD160− CD8+ T cells were activated using anti-CD3/CD28 beads and IL-2 for 48 h. Activated CD8+ T cells (effector cells) were then co-incubated with Capan-2, a human pancreatic adenocarcinoma cell line, acting as target cells. The Capan-2 cell line was chosen because both Capan-2 and all of our patients were HLA-A2 positive. At every effector/target ratio investigated in this study, the cytotoxicity mediated by CD160+ CD8+ T cells was significantly lower than the cytotoxicity mediated by CD160− CD8+ T cells (Fig. 5a). The expression of granzyme B and perforin was then examined in activated CD160+ CD8+ T cells and CD160− CD8+ T cells. CD160+ CD8+ T cells presented significantly lower granzyme B and lower perforin than CD160− CD8+ T cells (Fig. 5b).
Fig. 5.
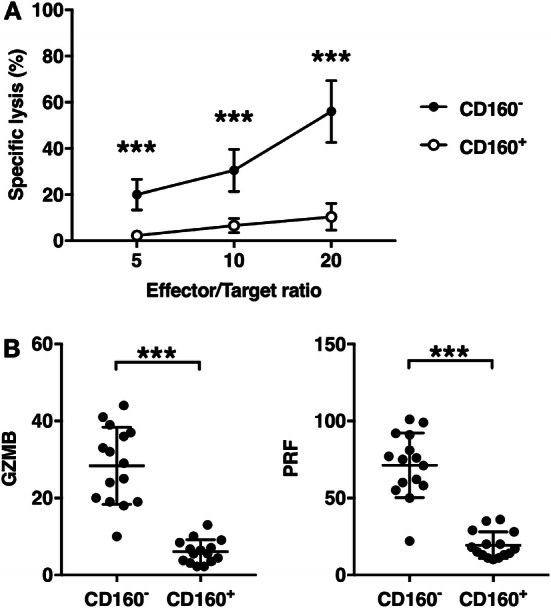
Cytotoxicity of CD160+ and CD160− CD8+ T cells. Circulating CD8+ T cells were separated into CD160+ and CD160− subsets, each stimulated by anti-CD3/CD28 antibodies and IL-2 for 48 h. a Stimulated CD8+ T cells were co-incubated with target Capan-2 cells at ratios indicated above for 12 h. The specific lysis was then measured using lactate dehydrogenase release assay. Mean ± SD of 15 pancreatic patients was shown. b Stimulated CD8+ T cells were lysed and the granzyme B (GZMB) and perforin (PRF) transcript levels were examined. ns not significant. ***p < 0.001
The CD160+ subset was enriched in tumor-infiltrating CD8+ T cells
Finally, the characteristics of CD160+ CD8+ T cells in resected tumor were investigated. Compared to the frequencies of CD160+ CD8+ T cells in the blood of the patients, the frequencies of CD160+ CD8+ T cells in the tumors were significantly elevated (Fig. 6a). No correlation between the frequency of CD160+ CD8+ T cells in blood and the frequency of CD160+ CD8+ T cells in tumor was observed (Fig. 6b).
Fig. 6.
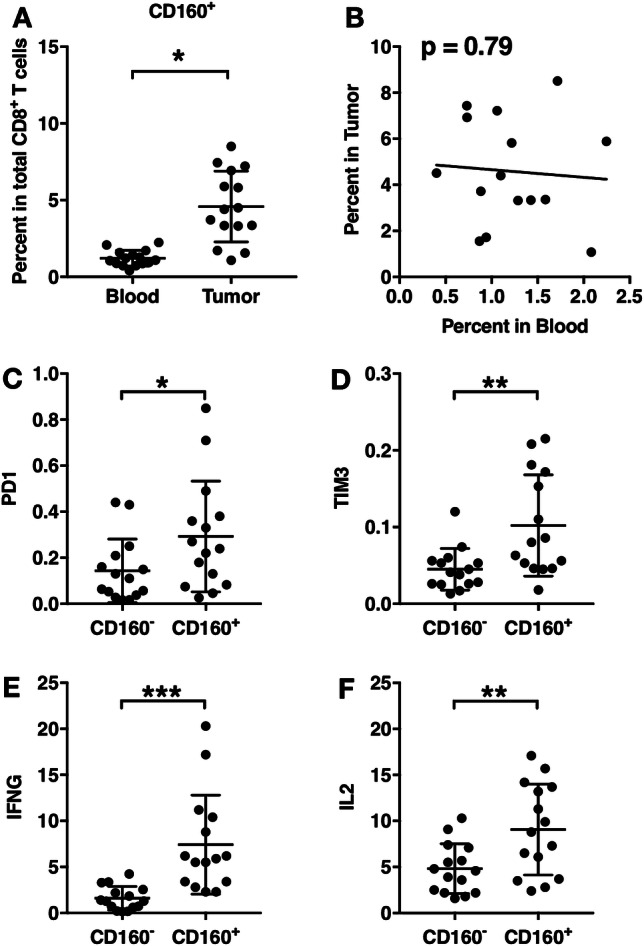
Characteristics of CD160+ tumor-infiltrating CD8+ T cells. a CD160+ cell frequencies in tumor-infiltrating CD8+ T cells, compared with that in circulating CD8+ T cells from the same patients. b The correlation between the percent of CD160+ CD8+ T cells in tumor vs. that in blood. c–f CD160+ and CD160− tumor-infiltrating CD8+ T cells were sorted and the transcript levels of PD-1, TIM-3, IFN-γ, and IL-2 were examined using PCR. *p < 0.05. **p < 0.01. ***p < 0.001
The mRNA transcript levels of cytokines and inhibitory molecules were then examined in CD160+ and CD160− tumor-infiltrating CD8+ T cells, immediately after isolation and sorting. Tumor-infiltrating CD160+ CD8+ T cells presented significantly higher PD-1 than tumor-infiltrating CD160− CD8+ T cells (Fig. 6c). The expression levels of TIM-3, IFN-γ, and IL-2 were also significantly higher in tumor-infiltrating CD160+ CD8+ T cells than in tumor-infiltrating CD160− CD8+ T cells (Fig. 6d, f). These trends in tumors were similar to those in the peripheral blood.
Patients with higher frequencies of tumor CD160+ CD8+ T cells presented lower survival
The survival of pancreatic cancer patients after tumor resection was recorded, and we examined whether the length of survival was associated with the frequency of CD160+ cells in CD8+ T cells. No association between the blood frequencies of CD160+ CD8+ T cells and the survival of the patients was observed (Fig. 7a). In contrast, patients with higher frequencies of CD160+ cells in tumor-infiltrating CD8+ T cells presented shorter survival time (Fig. 7b).
Fig. 7.
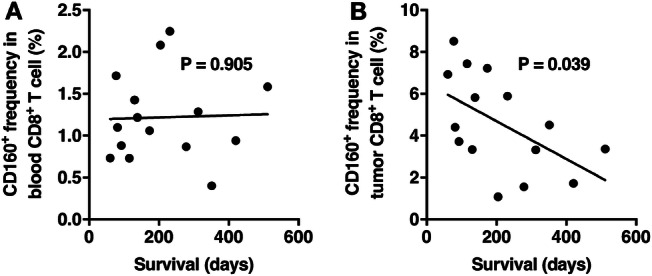
The association between the frequencies of CD160+ CD8+ T cells and the length of survival after tumor resection. a The association between the duration of patient survival and the frequencies of CD160+ CD8+ T cells in the peripheral blood. b The association between the duration of patient survival and the frequencies of CD160+ CD8+ T cells in the resected tumor. Pearson correlation test
Discussion
In this study, we examined the characteristics of CD160+ CD8+ T cells in comparison with CD160− CD8+ T cells. The CD160+ CD8+ T cells directly ex vivo presented comparable PD-1 expression and significantly higher TIM-3 expression than CD160− CD8+ T cells. TIM-3 was first found on IFN-γ-expressing CD4+ and CD8+ T cells [16]. Later studies demonstrated that TIM-3 was associated with exhausted T cells in chronic virus infections and TILs, and engagement with TIM-3 ligand could trigger T-cell apoptosis [17, 18]. Interestingly, by staining for intracellular IFN-γ and IL-2 expression directly ex vivo, we found that the frequencies of IFN-γ+ and IL-2+ cells were significantly higher in CD160+ CD8+ T cells than in CD160− CD8+ T cells. These data combined seemed to suggest that the CD160+ CD8+ T cells represented an activated CD8+ T-cell subset directly ex vivo.
To investigate the function of CD160+ CD8+ T cells upon activation, we separated CD160+ CD8+ T cells and CD160− CD8+ T cells prior to stimulation. This was done to prevent alterations in CD160 expression in stimulated cells. CD160+ CD8+ T cells and CD160− CD8+ T cells were then activated by anti-CD3/CD28 and IL-2 in separate cell cultures. Upon CD3/CD28/IL-2-mediated stimulation, the proliferation capacity of CD160+ and CD160− CD8+ T cells was comparable at 12 and 24 h post-stimulation, but was significantly lower in CD160+ CD8+ T cells than in CD160− CD8+ T cells at 48 h and 96 h post-stimulation. Also, the cell death following activation was comparable between 12 and 48 h post-stimulation, but was slightly higher in CD160+ CD8+ T cells than in CD160− CD8+ T cells at 96 h post-stimulation. However, the difference in cell death occurred at 96 h, while the difference in proliferation occurred much earlier at 48 h, suggesting that the higher death in CD160+ CD8+ T cells was only a secondary contributor to lower proliferation. The underlying cause of elevated cell death in CD160+ CD8+ T cells has not been investigated in this study. Activation-induced cell death may have acted in both CD160− and CD160+ CD8+ T cells [19]. Also, CD160+ CD8+ T cells may have been subjected to additional suppression due to the interactions between CD160 and its ligand HVEM [3, 8, 20]. These possibilities should be investigated in future studies.
Besides proliferation, we also compared cytokine production and cytotoxicity between CD160+ CD8+ T cells than in CD160− CD8+ T cells. The IFN-γ and IL-2 transcription levels were initially higher in CD160+ CD8+ T cells but eventually reduced in CD160+ CD8+ T cells compared to CD160− CD8+ T cells. Also, we observed that CD160+ CD8+ T cells presented lower cytotoxic capacity than CD160− CD8+ T cells. This was different from some previous studies, which suggested that CD160 could be used as a marker that selected NK cells and cytotoxic T cells with high cytotoxic capacity [6, 7, 21]. One possible explanation was that the CD160− CD8+ T cells might become CD160+ cells upon anti-CD3/CD28 and IL-2 stimulation, and it was those CD160-upregulated cells that mediated cytotoxicity. However, Vigano et al. demonstrated that unlike PD-1, which could be elevated following stimulation and T-cell activation, CD160 did not present the same expression dynamic [12]. In anti-CD3/CD28-stimulated CD8+ T cells, the expression of CD160 was in fact downregulated with long duration of stimulation, while the expression of PD-1 was markedly upregulated. Hence, the reason for the discrepancies between our current study and previous investigations could not be explained by CD160 expression in stimulated CD8+ T cells.
A major limitation of this study is that the specific signaling cascade downstream of CD160 has not been addressed in these CD160+ CD8+ T cells. Hence, it is unclear whether stimulated CD160+ CD8+ T cells intrinsically presented lower activation potential, or required interaction with ligands. Two ligands have been identified to interact with CD160, including the MHC class I molecules and HVEM. Interestingly, HVEM is prevalently expressed by pancreatic tumor cells [22]. Its interaction with CD160+ CD8+ T cells should be further examined, potentially using HVEM-Fc fusion proteins. In addition, whether HVEM and MHC class I molecules send similar or different signals to CD160+ CD8+ T cells remains to be investigated.
Another possibility is that the function of CD160+ CD8+ T cells could be inhibited via other inhibitory receptors that were co-expressed with CD160, such as TIM-3 in blood CD160+ CD8+ T cells and PD-1 and TIM-3 in tumor CD160+ CD8+ T cells. Indeed, we found that the levels of CD160+ CD8+ T cells were inversely associated with patient survival, which appeared paradoxical since tumor CD160+ CD8+ T cells had significantly higher IFNG and IL2 expression than CD160− CD8+ T cells. To what extent is this caused by the associated PD-1 and TIM-3 is unclear. Both PD-1 and TIM-3 can serve as markers of activated/effector cells in addition to their role in suppressing further activation and label exhausted T cells [23, 24]. The available data seem to suggest that CD160+ CD8+ T cells have basal cytokine production directly ex vivo due to chronic activation but are unable to respond to further activation due to exhaustion. In the current study, the frequencies of CD160+ CD8+ T cells and those that expressed PD-1 and TIM-3 were low, which prevented further functional analyses. In the future, diseases that are characterized by chronic activation and/or exhaustion, such as autoimmune diseases and infections, might be investigated for the frequency and characteristics of CD160+ CD8+ T cells. CD8+ T cells that co-express CD160 and PD-1 and/or TIM-3 may be sorted for the examination of proliferation, cytokine expression, granzyme B and perforin expression, and cytotoxicity. In addition, whether PD-1 and TIM-3 blockade, in combination with CD160 blockade could elevate the effector response of CD160+ CD8+ T cells should be examined.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- BTLA
B and T lymphocyte attenuator
- GMFI
Geometric mead fluorescence intensity
- GZMB
Granzyme B
- HVEM
Herpes virus entry mediator
- PRF
Perforin
- Rh
Recombinant human
Author contributions
SL, WZ, KL, and YW conceived and designed the study; SL and WZ conducted experiments; SL, WZ, and YW analyzed data; SL, WZ, and KL drafted the manuscript; YW supervised the study and reviewed the manuscript. All authors read and approved the final manuscript.
Funding
No relevant funding.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments. All protocols were reviewed and approved by the Ethics Committee of the First Hospital of Jilin University (160116).
Informed consent
All patients provided written informed consent at the time recruitment. All patients gave the consent to the use of data and materials for research and publication or consent to this particular study.
Cell line authentication
Capan-2 cells were purchased from the American Type Culture Collection.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Maïza H, Leca G, Mansur IG, Schiavon V, Boumsell L, Bensussan A. A novel 80-kD cell surface structure identifies human circulating lymphocytes with natural killer activity. J Exp Med. 1993;178(3):1121–1126. doi: 10.1084/jem.178.3.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anumanthan A, Boumsell L, Christ AD, Blumberg RS, Voss SD, Patel AT, Robertson MJ, Nadler LM, Freeman GJ. Cloning of BY55, a novel Ig superfamily cloning of BY55, a novel ig superfamily member expressed on NK Cells, CTL, and intestinal intraepithelial lymphocytes. J Immunol. 1998;161(6):2780–2790. [PubMed] [Google Scholar]
- 3.Cai G, Anumanthan A, Brown JA, Greenfield EA, Zhu B, Freeman GJ. CD160 inhibits activation of human CD4+ T cells through interaction with herpesvirus entry mediator. Nat Immunol. 2008;9(2):176–185. doi: 10.1038/ni1554. [DOI] [PubMed] [Google Scholar]
- 4.Farren TW, Giustiniani J, Liu FT, Tsitsikas DA, Macey MG, Cavenagh JD, Oakervee HE, Taussig D, Newland AC, Calaminici M, Bensussan A, Jenner M, Gribben JG, Agrawal SG. Differential and tumor-specific expression of CD160 in B-cell malignancies. Blood. 2011;118(8):2174–2183. doi: 10.1182/blood-2011-02-334326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu FT, Giustiniani J, Farren T, Jia L, Bensussan A, Gribben JG, Agrawal SG. CD160 signaling mediates PI3K-dependent survival and growth signals in chronic lymphocytic leukemia. Blood. 2010;115(15):3079–3088. doi: 10.1182/blood-2009-08-239483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barakonyi A, Rabot M, Marie-Cardine A, Aguerre-Girr M, Polgar B, Schiavon V, Bensussan A, Le Bouteiller P. Cutting edge: engagement of CD160 by its HLA-C physiological ligand triggers a unique cytokine profile secretion in the cytotoxic peripheral blood NK cell subset. J Immunol. 2004;173(9):5349–5354. doi: 10.4049/jimmunol.173.9.5349. [DOI] [PubMed] [Google Scholar]
- 7.Maeda M, Carpenito C, Russell RC, Dasanjh J, Veinotte LL, Ohta H. Murine CD160, Ig-like receptor on NK cells and NKT cells, recognizes classical and nonclassical MHC class I and regulates NK cell activation. J Immunol. 2005;175(7):4426–4432. doi: 10.4049/jimmunol.175.7.4426. [DOI] [PubMed] [Google Scholar]
- 8.Muscate F, Stetter N, Schramm C, Schulze zur Wiesch J, Bosurgi L, Jacobs T. HVEM and CD160: regulators of immunopathology during malaria blood-stage. Front Immunol. 2018;9:2611. doi: 10.3389/fimmu.2018.02611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le Bouteiller P, Barakonyi A, Giustiniani J, Lenfant F, Marie-Cardine A, Aguerre-Girr M, Rabot M, Hilgert I, Mami-Chouaib F, Tabiasco J, Boumsell L, Bensussan A. Engagement of CD160 receptor by HLA-C is a triggering mechanism used by circulating natural killer cells to mediate cytotoxicity. Proc Natl Acad Sci. 2002;99(26):16963–16968. doi: 10.1073/pnas.012681099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tu TC, Brown NK, Kim T-J, Wroblewska J, Yang X, Guo X. CD160 is essential for NK-mediated IFN-γ production. J Exp Med. 2015;212(3):415–429. doi: 10.1084/jem.20131601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nikolova M, Marie-Cardine A, Boumsell L, Bensussan A. BY55/CD160 acts as a co-receptor in TCR signal transduction of a human circulating cytotoxic effector T lymphocyte subset lacking CD28 expression. Int Immunol. 2002;14(5):445–451. doi: 10.1093/intimm/14.5.445. [DOI] [PubMed] [Google Scholar]
- 12.Viganò S, Banga R, Bellanger F, Pellaton C, Farina A, Comte D. CD160-associated CD8 T-cell functional impairment is independent of PD-1 expression. PLoS Pathog. 2014;10(9):e1004380. doi: 10.1371/journal.ppat.1004380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sedy JR, Bjordahl RL, Bekiaris V, Macauley MG, Ware BC, Norris PS, Lurain NS, Benedict CA, Ware CF. CD160 Activation by herpesvirus entry mediator augments inflammatory cytokine production and cytolytic function by NK cells. J Immunol. 2013;191(2):828–836. doi: 10.4049/jimmunol.1300894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun H, Xu J, Huang Q, Huang M, Li K, Qu K. Reduced CD160 expression contributes to impaired NK cell function and poor clinical outcomes in patients with HCC. Cancer Res. 2018;79(7):1714–1721. doi: 10.1158/0008-5472.CAN-19-0630. [DOI] [PubMed] [Google Scholar]
- 15.Hall ML, Liu H, Malafa M, Centeno B, Hodul PJ, Pimiento J. Expansion of tumor-infiltrating lymphocytes (TIL) from human pancreatic tumors. J Immunother Cancer. 2016;4:61. doi: 10.1186/s40425-016-0164-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, Manning S, Greenfield EA, Coyle AJ, Sobel RA, Freeman GJ, Kuchroo VK. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415:536–541. doi: 10.1038/415536a. [DOI] [PubMed] [Google Scholar]
- 17.Jones RB, Ndhlovu LC, Barbour JD, Sheth PM, Jha AR, Long BR. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med. 2008;205:2763–2779. doi: 10.1084/jem.20081398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Green DR, Droin N, Pinkoski M. Activation-induced cell death in T cells. Immunol Rev. 2003;193:70–81. doi: 10.1034/j.1600-065X.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- 20.Cai G, Freeman GJ. The CD160, BTLA, LIGHT/HVEM pathway: a bidirectional switch regulating T-cell activation. Immunol Rev. 2009;229(1):244–258. doi: 10.1111/j.1600-065X.2009.00783.x. [DOI] [PubMed] [Google Scholar]
- 21.Tsujimura K, Obata Y, Matsudaira Y, Nishida K, Akatsuka Y, Ito Y, Demachi-Okamura A, Kuzushima K, Takahashi T. Characterization of murine CD160+ CD8+ T lymphocytes. Immunol Lett. 2006;106(1):48–56. doi: 10.1016/j.imlet.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Sideras K, Biermann K, Yap K, Mancham S, Boor PPC, Hansen BE, Stoop HJA, Peppelenbosch MP, van Eijck CH, Sleijfer S, Kwekkeboom J, Bruno MJ. Tumor cell expression of immune inhibitory molecules and tumor-infiltrating lymphocyte count predict cancer-specific survival in pancreatic and ampullary cancer. Int J Cancer. 2017;141(3):572–582. doi: 10.1002/ijc.30760. [DOI] [PubMed] [Google Scholar]
- 23.Han G, Chen G, Shen B, Li Y. Tim-3: an activation marker and activation limiter of innate immune cells. Front Immunol. 2013;4:449. doi: 10.3389/fimmu.2013.00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simon S, Labarriere N. PD-1 expression on tumor-specific T cells: friend or foe for immunotherapy? Oncoimmunology. 2017;7(1):e1364828. doi: 10.1080/2162402X.2017.1364828. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.