Abstract
High grade ovarian serous cancer (HGSC) is a malignant disease with high mortality. Glycosylation plays important roles in tumor invasion and immune evasion, but its effect on the immune microenvironment of HGSC remains unclear. This study examined the association of glycosyltransferase expression with HGSC prognosis and explored the underlying mechanism using clinical specimens and integrated bioinformatic analyses. We identified a cluster of 15 glycogenes associated with reduced overall survival, and GALNT10 was found to be an independent predictor of HGSC prognosis. The high GALNT10 expression was associated with increased regulatory CD4+ T cells infiltration and decreased granzyme B expression in CD8+ T cells. The expression of GALNT10 and its product, Tn antigen, in HGSC specimens was associated with the increased infiltration of M2 macrophages and neutrophils, and the decreased infiltration of CD3+ T cells, NK cells, and B cells. Taken collectively, high GALNT10 expression confers with immunosuppressive microenvironment to promote tumor progression and predicts poor clinical outcomes in HGSC patients.
Electronic supplementary material
The online version of this article (10.1007/s00262-019-02454-1) contains supplementary material, which is available to authorized users.
Keywords: High grade serous ovarian cancer, Glycosylation, GALNT10, M2 macrophage, Neutrophil, Immunosuppression
Introduction
Despite various screening and treatment approaches, ovarian cancer is one of the most malignant types of cancer in females worldwide [1]. High-grade serous cancer, the most common histological subtype of ovarian cancer, is associated with high recurrence rates and resistance to chemotherapy, and it accounts for approximately 80% of ovarian cancer deaths [2]. Cytoreductive surgery and modified chemotherapy are the main treatment approaches, however, platinum-based chemotherapy and drug resistance are challenges in the treatment of HGSC [3]. Recently, genomic analyses of HGSC have identified several genetic alterations in TP53, BRCA1, BRCA2, and PTEN, as well as other genes, but these genes play insignificant roles in the treatment of this disease. Therefore, there is an urgent need to classify the HGSCS subtypes and to obtain more information on metastatic rates and survival outcomes [4].
Glycosyltransferase, also known as glycogene, is a type of enzyme that mediates protein and lipid modifications. It plays crucial roles in normal cell development and other physiological processes. It is also an important biomarker, as it can define specific targets for therapeutic intervention [5]. For example, the abnormal glycosylation on the surface of tumor cells can markedly alter the tumor microenvironment and immune function, as well as promote metastasis, which can greatly impact tumor progression, and even introduce new diagnostic and prognostic features [5, 6]. For ovarian cancer, CA125 has heavily O-glycosylated epitopes that are encoded by MUC16, whereas Tn antigen (GalNAc-α-O-Ser/Thr) and sialyl Tn (sTn) have been reported to associate with the growth and metastasis of breast and gastric tumors [7].
In this study, we used unsupervised classification clustering to analyze the expression of glycogenes in order to obtain a new subtype and to relate the overall survival (OS) of HGSC patients with the expression of GALNT10 by LASSO Cox regression analysis. GALNT10 is a member of the O-glycosyltransferase family and a prognostic biomarker of HGSC patients. Here, we report that GALNT10 expression was associated with tumor progression and immunosuppression, indicating that GALNT10 plays a role in HGSC.
Materials and methods
Study population
All microarray data were publicly downloaded from the Cancer Genome Atlas (TCGA) and Gene Expression Omnibus (GEO), as shown in Table S1. We used standardized expression values from existing clinical data, which included the overall survival, and selected patients with HGSC. If a gene was represented by multiple probes, the maximum expression value was selected. The tumor invasion database was download from the Tumor Ovarian Serous Cystadenocarcinoma-TCGA of the R2 Tool (https://hgserver1.amc.nl/cgi-bin/r2/main.cgi). Lymphatic invasion indicated that tumors invaded the lymphatic system, and venous invasion indicated that tumors invaded the vascular system.
Cohort 1: A retrospective series of 83 formalin-fixed, paraffin-embedded specimens were obtained from patients with HGSC who underwent primary surgery in the absence of neoadjuvant chemotherapy between March 2013 and November 2015 from the tissue bank of the Obstetrics and Gynecology Hospital, Fudan University. The characteristics of these patients are summarized in Table S2. Pathological staging was performed according to the FIGO (International Federation of Gynecology and Obstetrics) Classification (2018), and histological types were determined according to the current WHO (World Health Organization) Classification [8]. Data on the clinical outcomes were obtained retrospectively by questioning families. The last follow-up was in August 2017.
Cohort 2: A retrospective series of 117 formalin-fixed, paraffin-embedded specimens from patients with HGSC was collected between May 2013 and August 2018 at Suzhou Municipal Hospital. The characteristics of these patients are summarized in Table S3.
Cohort 3: An additional series of 33 specimens were collected for flow cytometry from patients with HGSC at the Obstetrics & Gynecology Hospital, Fudan University between June 2018 and April 2019. The characteristics of these patients are summarized in Table S4.
Immunohistochemistry and immunohistochemical analysis
Immunohistochemistry (IHC) was performed using tissue microarrays prepared with 4-μm tissue sections from formalin-fixed, paraffin-embedded specimens. To reduce the selection bias, we selected two different tissue sections from each specimen. After the tissue sections were deparaffinized and rehydrated, antigen retrieval was performed. The sections were blocked and incubated with primary antibodies, namely anti-GALNT10 (diluted 1/100), anti-CD3 (diluted 1/600), anti-CD8 (diluted 1/900), anti-CD20 (diluted 1/800), anti-CD56 (diluted 1/800), anti-CD68 + (diluted 1/400), anti-C163 (diluted 1/400), and anti-CD66b (diluted 1/400). The Tn antigen was measured by the avidin–biotin–peroxidase (ABP) method with Vicia villosa agglutinin (VVA) lectin (VVA lectin, diluted 1/1000) (Table S5). The isotype antibody served as negative control. The immunoreactivity of GALNT10, CD3, CD8, CD20, CD56, CD68, CD163, CD66, and Tn antigen ranged from yellow to brown, and it was analyzed using an algorithm developed for the ImageJ Software at a magnification of 200 × . The variation coefficients of the two specimens from each specimen were < 15%.
Confocal immunofluorescent microscopy
Frozen sections were stained with the anti-GALNT10 primary antibody, followed by incubation with an Alexa Fluor 488-conjugated donkey anti-rabbit secondary antibody and FITC (fluorescein isothiocyanate)-conjugated VVA (Table S5). The sections were subsequently washed and counterstained with 4′,6-diamidine-2-phenylindole (DAPI). Images were taken at a magnification of 400 × with a Leica SP8 confocal microscope running under the Leica Application Suite X Software.
Flow cytometry analysis
Single-cell suspensions were prepared from 33 fresh tumors of cohort 3 with the Neural Tissue Dissociation Kit (Miltenyi Biotec). The cells were subsequently treated with FcR-blocking reagent and stained with fluorochrome-conjugated antibodies against cell surface markers for 30 min at 4 °C in the dark. For intracellular staining, surface-stained cells were permeabilized and fixed with the Fixation/Permeabilization Solution Kit (BD Biosciences) for 20 min, and then incubated with intracellular fluorochrome-conjugated antibodies for 30 min. Flow cytometry analysis was performed with a Beckman CytoFLEX System running under FlowJo 10.0 Software (TreeStar). The compensation was dependent on the BD CompBeads Set (BD Biosciences), which was automatically adjusted by the flow cytometer. The antibodies that were used in this experiment are summarized in Table S5.
Statistical analysis
Hierarchical clustering was performed with the Mev 4.9.0 Software using mean-centered expression values of the glycogenes. One hundred and sixty-six glycogenes were selected from 190 glycogenes in the GlycoGeneDataBase (http://acgg.asia/ggdb2/). Differential gene expression analysis and meta-analysis were accomplished by the “Desq2” and “metafor” packages in R, respectively. LASSO Cox regression analysis was used to obtain the correlation coefficients for the 15 glycogenes with the OS, and the variable selection was achieved by the “glmnet” package in R. Gene Set Enrichment Analysis (GSEA) and ToppFun GO analyses (https://toppgene.cchmc.org/enrichment.jsp) were used to identify the functions of GALNT10. The degree of immune cell infiltration was calculated with the CIBERSORT Tool (https://cibersort.stanford.edu/). Statistical analyses were performed with Medcalc 18.0, SPSS Statistics 21.0, and R 3.5.1 (http://www.rproject.org/).
Results
Transcriptional analysis of glycogenes in two subgroups of patients with HGSC with different OS
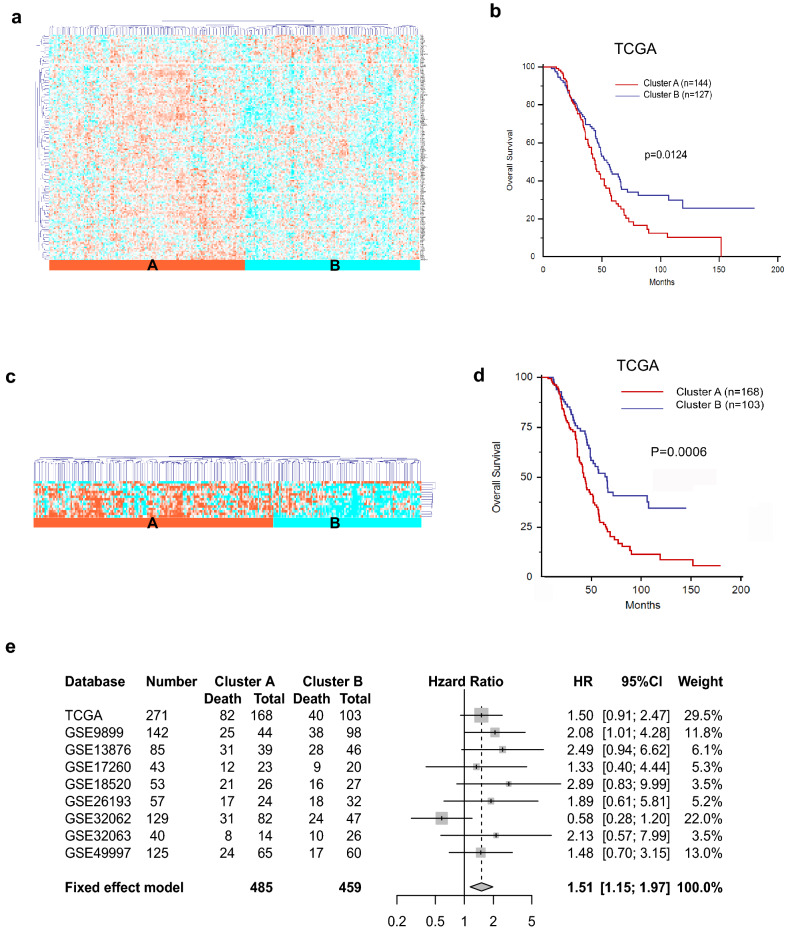
The TCGA cohort of 271 patients was divided into two subgroups, namely cluster A and cluster B, based on the unsupervised hierarchical clustering analysis of 166 glycogenes (Fig. 1a, Table S6). Cluster A showed a relatively higher expression of glycogenes than that of cluster B. Kaplan–Meier survival analysis showed that cluster A was significantly associated with shorter OS (Fig. 1b, p = 0.0124).
Fig. 1.
Identification of glycosyltransferase gene (glycogene)-derived subtypes of high-grade ovarian cancer (HGSC). a Unsupervised hierarchical clustering based on 166 glycogenes in 271 patients with HGSC from the TCGA cohort, demonstrating two major clusters, cluster A (n = 144) and B (n = 127). b Cluster A was significantly associated with a shorter OS (median survival time, 44.05 months; 95% CI 38.01–51.87) compared with cluster B (median survival time, 54.07 months; 95% CI 45.96–65.47; p = 0.0124). c Cluster analysis was performed using 15 glycogenes (designated as the 15-glycogene signature) in 271 patients with HGSC from the TCGA cohort, and the 15-glycogene subtype, which included clusters A (n = 168) and B (n = 103), was established. d Cluster A was significantly associated with a shorter OS (p = 0.0006). e Meta-analysis of the 15-glycogene signature in nine HGSC cohorts, which included the TCGA cohort and eight GEO databases (GSE9899, GSE13876, GSE17260, GSE18520, GSE26193, GSE32063, GSE32062, and GSE49997) (HR 1.51; 95% CI 1.15–1.97)
The analysis of differentially expressed genes in clusters A and B yielded 128 genes, which was further reduced to 15 genes (B3GALNT2, MGAT5, NDST1, GALNT2, POFUT1, DPM3, ST3GAL2, HS6ST1, CHSY1, MGAT3, NDST2, EXTL3, B4GALT5, MGAT2, and GALNT10) (Tables S7 and 8) to comprise the glycogene signature.
Prognostic characteristics of the 15-glycogene signature
Unsupervised hierarchical clustering analysis of the 15 glycogenes in the TCGA cohort (Fig. 1c) revealed that the expression levels of the 15 glycogenes in cluster A were higher than those of cluster B, whereas the OS was shorter in cluster A than that in cluster B (Fig. 1d, p = 0.0006). We also analyzed another eight groups (GSE9899, GSE13876, GSE17260, GSE18520, GSE26193, GSE32062, GSE32063, and GSE49997) of HGSC patients from the GEO Database to confirm the correlation between the expression of the 15 glycogenes in cluster A and the OS (Fig. S1). Meta-analysis of the TCGA cohort and the eight groups supported the initial characterization of the 15 glycogenes, as well as the correlation between the expression of the glycogenes in cluster A with a worse prognosis (Fig. 1e, hazard ratio (HR), 1.51; 95% confidence interval (CI), 1.15–1.97).
Elevated GALNT10 expression is related to tumor invasion and the prognosis
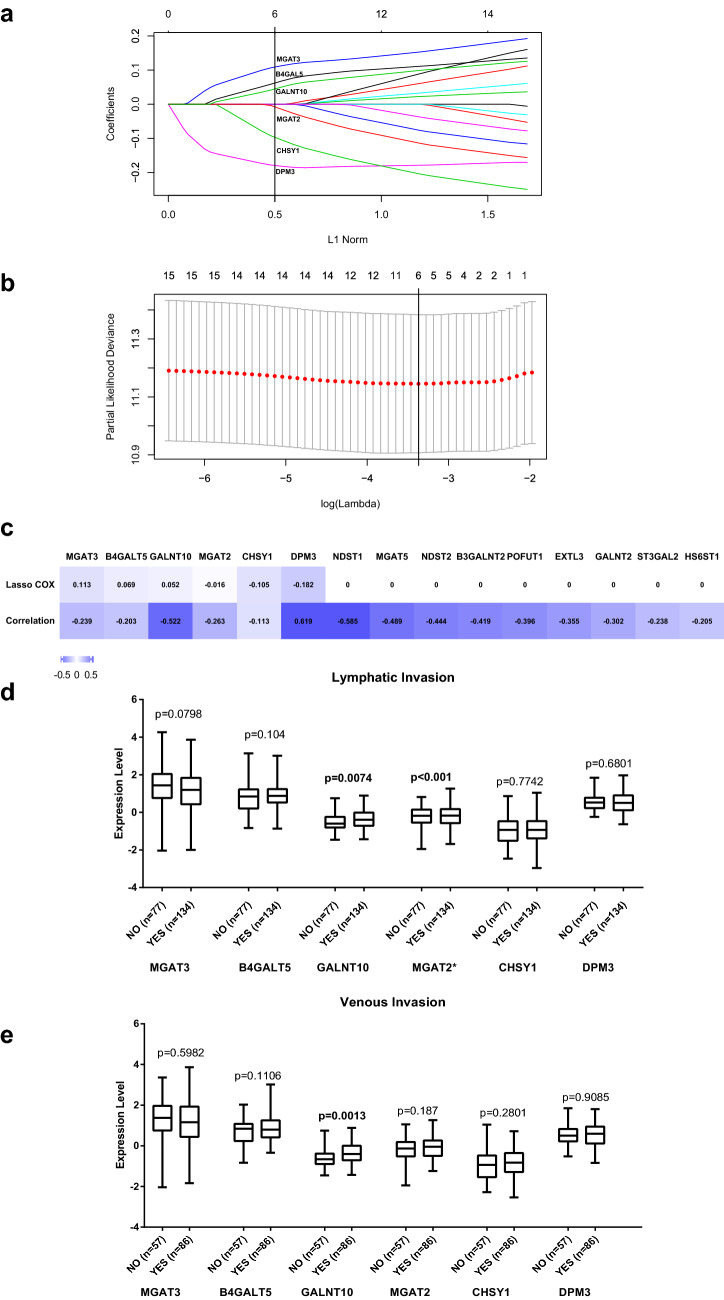
LASSO Cox regression analysis was used to obtain correlation coefficients for the 15 glycogenes to select the best regression effect (Fig. 2a, γ = 0.5, λ = 0.0378) and to cross-validate the results (Fig. 2b). We compared the LASSO Cox regression and correlation coefficients after clustering the 15 glycogenes and then selected six genes with the highest coefficients (Fig. 2c). Only GALNT10 was positively correlated with lymphatic (p = 0.0074) and venous invasion (p = 0.0013) with high significance (Fig. 2d, e).
Fig. 2.
Correlations of the 15 glycogenes with prognosis and tumor invasion in the TCGA cohort. a LASSO Cox regression coefficient profiles of the 15 glycogenes with the OS from the TCGA cohort. A dashed vertical line indicates the value (γ = 0.5) chosen by the fivefold cross-validation of data. b Partial likelihood deviance for the LASSO Cox regression coefficient profiles. A light dashed vertical line indicates the minimum partial likelihood deviance. A bold dashed vertical line indicates the partial likelihood deviance at the value (λ = 0.0378). c LASSO Cox regression coefficients and relative coefficients of the 15-gylcogene signature. Both coefficients were not zero for MGAT3, B4GAT5, GALNT10, MGAT2, CHSY1, and DPM3. Only the p value of CHSY1 was > 0.05. d Association of lymphatic and venous invasion with the expression of MGAT3, B4GAT5, GALNT10, MGAT2, CHSY1, and DPM3. Only GALNT10 was significantly associated with lymphatic and venous invasion (p = 0.0074, p = 0.0013)
Elevated GALNT10 expression is associated with decreased survival
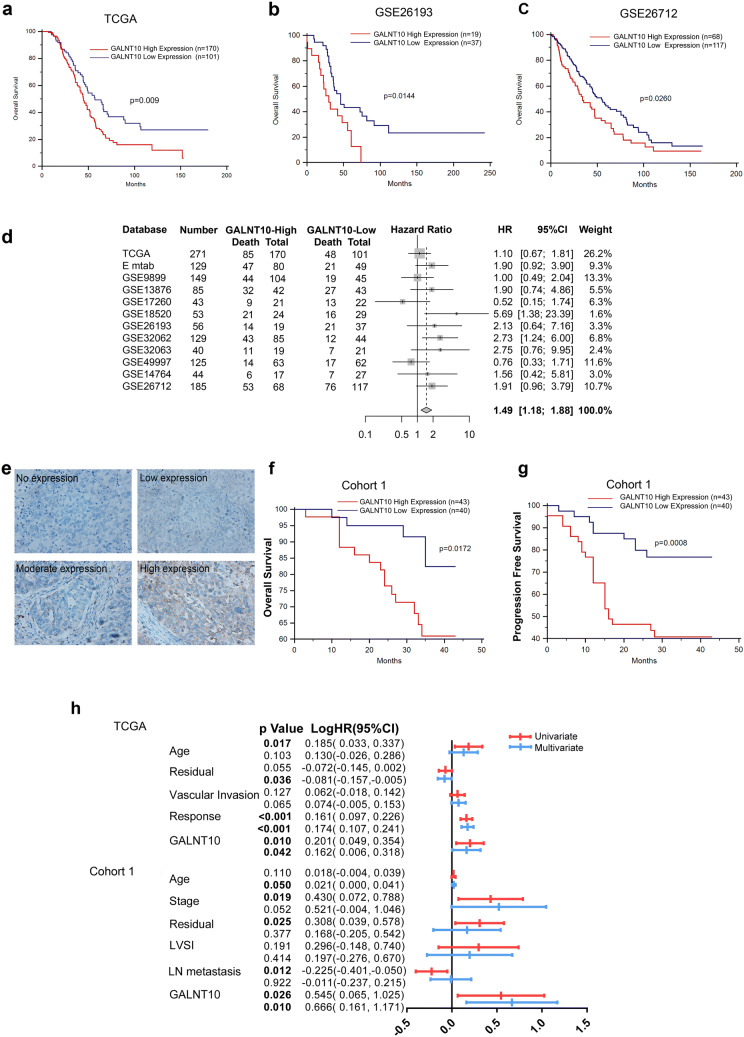
Kaplan–Meier survival analysis revealed that patients in the TCGA cohort with high GALNT10 expression had the shortest OS (Fig. 3a, p = 0.009, Table S9). Similar results were obtained for patients from the GSE26193 and GSE26712 groups (Fig. 3b, c, p = 0.0144, p = 0.026). Meta-analysis of GALNT10 expression in patients of the TCGA cohort and the 11 GEO databases showed that high GALNT10 expression was associated with poor prognosis (Fig. 3d; HR, 1.49; 95% CI, 1.18–1.88; Fig. S2).
Fig. 3.
GALNT10 expression is a predictor of poor survival of patients with HGSC. a GALNT10 expression was significantly correlated with the OS of HGSC patients from the TCGA cohort (p = 0.009). b GALNT10 expression was significantly correlated with the OS of HGSC patients from the GSE26193 database (p = 0.0144). c GALNT10 expression was significantly correlated with the OS of HGSC patients from the GSE26712 database (p = 0.0260). d Meta-analysis of high GALNT10 expression in HGSC patients from 12 databases (HR 1.49; 95% CI 1.18–1.88). e Representative image of GALNT10 staining in an HGSC specimen (40 ×). f GALNT10 expression was significantly correlated with the OS of HGSC patients from cohort 1 (p = 0.0172). g GALNT10 expression was significantly correlated with the PFS of HGSC patients from cohort 1 (p = 0.0008). h Univariate and multivariate COX regression analyses of clinical variables associated with the OS of HGSC patients from the TCGA cohort and cohort 1
Compared with normal tissues, GALNT10 staining was most obvious in the cytoplasm of tumor cells (Figs. 3e and S3). Kaplan–Meier analysis revealed that elevated GALNT10 expression was correlated with a shorter OS and progression-free survival (PFS) in cohort 1 (Fig. 3f, g, p = 0.0172, p = 0.0008, Table S10). Furthermore, multivariate Cox regression analysis indicated that GALNT10 expression was an independent factor of the OS in the TCGA cohort (p = 0.042; logHR, 0.162; 95% CI 0.016–0.318) and cohort 1 (p = 0.010; logHR, 0.666; 95% CI 0.161–1.171) (Fig. 3h, Table S11).
GALNT10 expression is associated with the immunosuppressive tumor microenvironment
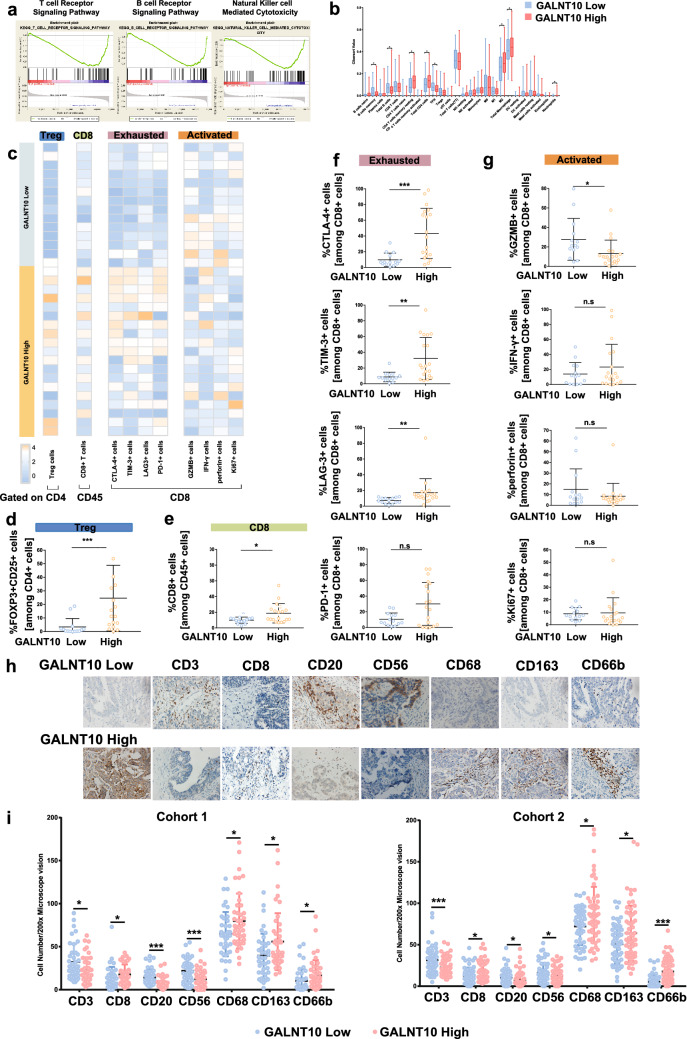
We used GSEA to identify enriched or depleted gene sets in tumor specimens with elevated GALNT10 expression. The results of GSEA of the c2 dataset revealed that the levels of genes associated with cell adhesion and metabolism were significantly upregulated in tumor specimens with elevated GALNT10 expression. By contrast, the levels of genes associated with adaptive immunity were significantly downregulated in tumor specimens with elevated GALNT10 expression. For example, the expression of B-cell-specific genes, such as those encoding Igα and Igβ of the B cell antigen receptor complex and CD3E, CD3D, CD3G, and CD247 of the T cell receptor (TCR) complex, was decreased with enrichment scores (ES) of -0.55 and -0.58 (Fig. 4a). The expression of genes associated with NK cell-mediated cytotoxic activity was also significantly reduced in tumor specimens with high GALNT10 expression with an ES of − 0.53 (Fig. 4a). The results of GSEA of the c7 dataset revealed that macrophages, neutrophils, NK cells, and B cells played more significant roles in the HGSC with high GALNT10 expression (Fig. S4). In addition, CD4+ T cell, macrophage, M2 macrophage, and neutrophil counts were increased in the high expression group, whereas B cell and T follicular helper cell counts were decreased according to the CIBERSORT analysis (Fig. 4b).
Fig. 4.
GALNT10 expression associates with the immunosuppressive tumor microenvironment. a GSEA KEGG analysis of high and low GALNT10 expression, as follows: T cell receptor signaling pathway, ES = − 0.58, FDR < 105; B cell receptor signaling pathway, ES = − 0.55, FDR = 0.006; NK cell-mediated cytotoxicity, ES = − 0.53, FDR < 105. b The results of CIBERSORT analysis of high and low GALNT10 expression. c Heatmap showing results of the flow cytometric analysis of Tregs, CD8+ T cells, as well as immune exhaustion makers (CTLA-4, TIM-3, LAG3, and PD1) and immune activation makers (GZMB, IFN-α, perforin, and Ki67) in CD8+ T cells of HGSC specimens with low and high GALNT10 expression. d The proportion of FOXP3+ CD25+ cells in CD4+ T cells of HGSC specimens with low and high GALNT10 expression. e The proportion of CD8+ T cells in CD45+ cells of HGSC specimens with low and high GALNT10 expression. f The proportion of immune exhaustion markers (CTLA-4, TIM-3, LAG3, and PD1) in CD8+ T cells of HGSC specimens with low and high GALNT10 expression. g The proportion of immune activation makers (GZMB, IFN-α, perforin, and Ki67) in CD8+ T cells of HGSC specimens with low and high GALNT10 expression. h Representative images of GALNT10, CD3, CD8, CD20, CD56, CD68, CD163, and CD66b staining in HGSC specimens (40 ×). i Comparison of CD3, CD8, CD20, CD56, CD68, CD163, and CD66b staining in high and low GALNT groups from cohort 1 and 2 (*p < 0.05; **p < 0.01; ***p < 0.001; n.s., not significant)
To validate the correlations between GALNT10 expression and the infiltration of Tregs and CD8+ T cells, flow cytometry was performed on 33 freshly resected HSGCs from cohort 3 whose GALNT10 status had been previously assessed by IHC (Fig. 4c). We observed significant infiltration of Tregs and CD8+ T cells in tumor specimens with high GALNT10 expression (Fig. 4d). The expression of several activations (Granzyme B, IFN-α, perforin, and Ki-67) and exhaustion (CTLA-4, TIM-3, LAG3, and PD-1) biomarkers in CD8+ T cells was also examined. The proportion of CD8+ T cells expressing CTLA-4, TIM-3, and LAG3 was significantly increased, whereas the proportion of CD8+ T cells expressing Granzyme B (GZMB) was significantly decreased in tumor specimens with elevated GALNT10 expression (Fig. 4e–g). Furthermore, the infiltration of CD3+ T cells, B cells, and NK cells was decreased, whereas the infiltration of CD8+ T cells, macrophages, M2 macrophages, and neutrophils was increased in tumor specimens with high GALNT10 expression (Fig. 4h, i).
Tn antigen correlates with the immunosuppressive tumor microenvironment
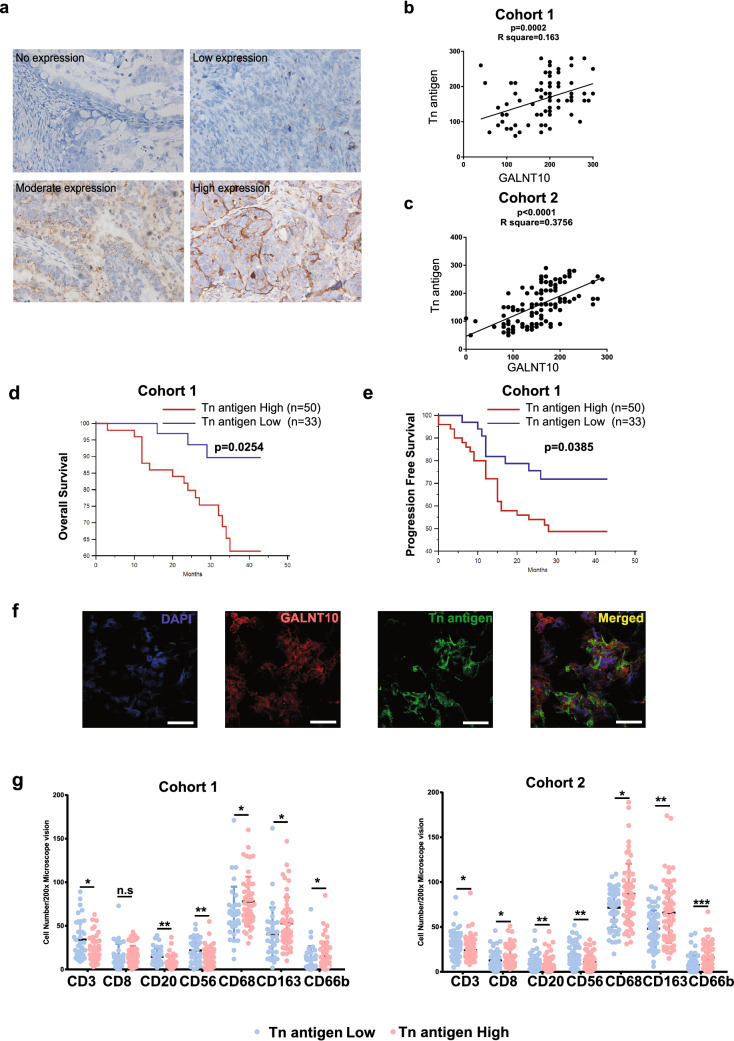
The Tn antigen (GalNAc-α-O-Ser/Thr), as a representative product of GALNT10, was specifically recognized by VVA lectin (Fig. 5a). Tn antigen expression was positively correlated with GALNT10 expression in both cohorts (Fig. 5b, c; p = 0.0002, R2 = 0.163; p < 0.0001, R2 = 0.3756), as well as a shorter OS (p = 0.0254) and a shorter PFS (p = 0.0385) in cohort 1 (Fig. 5d, e). Tn antigen expression in HGSC specimens was associated with the expression and localization of GALNT10 (Fig. 5f). Except for CD8+ T cells in cohort 1, the infiltration of macrophages, M2 macrophages, and neutrophils was significantly increased in the group with high Tn antigen expression, whereas the infiltration of CD3+ T cells, B cells, and NK cells was significantly decreased (Fig. 5g).
Fig. 5.
Tn antigen expression associates with the immunosuppressive tumor microenvironment. a Representative image of Tn antigen staining in an HGSC specimen (40 ×). b Linear regression analysis of GALNT10 and Tn antigen in cohort 1 (p = 0.0002; R2 = 0.163). c Linear regression analysis of GALNT10 and Tn antigen in cohort 2 (p < 0.0001; R2 = 0.3756). d Tn antigen was significantly correlated with the OS of HGSC patients from cohort 1 (p = 0.0254). e Tn antigen was significantly correlated with the PFS of HGSC patients from cohort 1 (p = 0.0385). f Identical views of the same microscopic field of an HGSC specimen stained with DAPI (blue, i), GALNT10 (red, ii), and Tn antigen (green, iii). The images were merged (iv) to show colocalization of GALNT10 and Tn antigen. Scale bars, 40 μm. g Comparison of CD3, CD8, CD20, CD56, CD68, CD163, and CD66b staining with that of Tn antigen in high and low GALNT10 expression groups from cohort 1 and 2 (*p < 0.05; **p < 0.01, ***p < 0.001; n.s., not significant)
Discussion
This study established a novel signature of 15 glycogenes related to the poor OS of HGSC. Analysis of LASSO Cox regression and tumor invasion data identified GALNT10 from the glycogene signature as a predictor of poor prognosis. The results of GSEA and flow cytometry data showed that GALNT10 expression was high in the immunosuppressive tumor microenvironment. The results of CIBERSORT and IHC indicated the difference of immune cell infiltration associated with GALNT10 expression. Furthermore, Tn antigen was associated with the expression and localization of GALNT10, and Tn antigen showed the same trend as GALNT10 in regard to the infiltration of immune cells.
O-glycosylation plays important roles in the survival, migration, and invasion of cancer cells [9–11]. An abnormal form of O-glycan has been reported in different solid tumors, including those of the ovary, bladder, breast, cervix, colon, and lung [12–14]. GalNAc O-glycosylation, a post-translational modification of proteins, is mediated by a family of approximately 20 homologous genes that encode UDP-GalNAc, namely polypeptide GalNAc-transferases (GALNTs) [15]. Interestingly, the expression of various GALNT genes is highly restricted to cells and tissues during development and differentiation [16]. For example, GALNT10 expression was reported in the small intestine, stomach, pancreas, ovary, thyroid gland, and spleen [17]. In other studies, GALNT10 expression was associated with the OS of patients with ovarian cancer [18], whereas elevated GALNT10 expression was a negative prognostic indicator of renal cell carcinoma [19]. In addition, GALNT genes have been reported to associate with the recurrence and metastasis of HGSC, as well as other cancers [20].
Here, we found that GALNT10 promoted tumor progression by affecting cell adhesion and immunity. The results of GSEA revealed changes in cell adhesion, adaptive immunity, and immune cell infiltration. A modulation of GALNT expression can increase tumor invasion and immune suppression [21]. For example, GALNT10 can alter the invasion and metastasis of tumor cells by regulating cell–cell/cell–extracellular matrix adhesion, cell junction dynamics, cell signaling, cell migration, and the epithelial-mesenchymal-transition [5, 9]. The NK cell-mediated downregulation of B cells and T cells and the concomitant cytotoxicity suggested increased immunosuppression in HGSC specimens, which was consistent with the infiltration of immune cells, especially Tregs, as well as the increase in abnormal CD8+ T cells expressing CTLA-4, TIM-3, and LAG3 [22, 23]. It was previously reported that increased GALNT7 expression could promote the differentiation of T cells into Tregs to enhance invasion and immunosuppression [24, 25]. Moreover, the TGF-β level was upregulated in GALNT14-overexpressing cells, which could promote the differentiation of T cells into Tregs [26, 27]. Tumor infiltrating-Tregs are considered to function as anti-tumor immune suppressors, and they can reduce anti-tumor immune responses [28]. The heterogeneity of the immune infiltration in HGSC suggested a different immune state. The infiltration of macrophage, M2-like macrophage, and neutrophil was increased in HGSC with GALNT10 high expression. Several studies have reported that tumor-associated macrophages (TAMs), especially M2-like TAMs and tumor-associated neutrophils (TANs), could facilitate immunosuppression and tumor progression [29–33], suggesting that GALNT10 may induce immunosuppression by regulating Tregs and other immune cells.
Tn antigen, as a representative product, is expressed in 10–90% of bladder, cervical, ovarian, colon, lung, stomach, and prostate tumors; however, it is weakly/not expressed in normal adult tissues [34]. GALNT-induced O-glycosylation may also lead to changes in the biosynthesis of several other cancer-associated glycans such as sialyl-Tn and Sialyl-T [16]. Cancer-associated O-glycans are highly sialylated by sialyltransferase upon Tn (GalNAc-) and T (Galβ1-3GalNAc-) antigens [6]. An increase in STn and Sialyl-T (ST) antigens can promote tumor cell metastasis and invasion, as well as evasion of immune surveillance [6, 35]. Both MUC16 and STn-glycoforming MUC16, which are highly expressed in ovarian cancers, are currently being used as diagnostic markers and predictors of clinical outcomes [36, 37].
A previous study reported that Tn glycosylation of mucin can affect B cell and T cell immunogenicity, thereby promoting the immune escape of tumor cells [38]. In bladder cells, heavily O-glycosylated MUC1 can interact with Gal-3 to retard the interaction between tumor cells and NK cells, thereby promoting tumor cell survival and metastasis [39]. Myeloid tumor cells express STn antigen, which binds Siglec-15 in macrophages, thereby resulting in TGF-β secretion [40]. Furthermore, STn could cause dysfunctional dendritic cell and reduce the secretion of Th1 cytokines, such as IL-12 and TNF-α, in bladder cancer [41]. Similarly, sialylation of T antigen in MUC1 binds to Singlec-9 on TAMs to initiate inhibitory immune signaling in breast cancer [42]. The tumor cells expressing abnormal O-glycans may disrupt antigen-processing and the immune response by activating the MGL receptor on dendritic cells [43].
There were several limitations in this study. First, the cohort size in this retrospective study was small, and the histological subtypes were limited. Second, this study only analyzed the association between GALNT10 expression and immune cell infiltration.
In conclusion, we established a 15-glycogene signature, and GALNT10 was found to be an independent predictor of HGSC prognosis. Our findings indicate that GALNT10 may have induced tumor immunosuppression, which depended on the association between Tn antigen expression and immune cell infiltration.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- CI
Confidence interval
- ES
Enrichment scores
- GEO
Gene expression Omnibus
- GSEA
Gene set enrichment analysis
- GZMB
Granzyme B
- HGSC
High grade serous ovarian cancer
- HR
Hazard ratio
- MUC
Mucin
- ST
Sialyl-T
- sTn
Sialyl Tn
- TAM
Tumor-associated macrophages
- TCGA
The cancer genome atlas
- VVA
Vicia villosa agglutinin
Author contribution
HL designed and executed experiments and contributed to the revision of the manuscript. CX contributed to the design of the experiments and prepared the manuscript. GZ and MY performed IHC, IF, and flow cytometry experiments and analyzed the bioinformatics data. JL contributed to the analysis of the data and manuscript preparation. YW contributed to the analysis of the immune cell infiltration data.
Funding
This work was supported by funding from the National Key R&D Program of China (2016YFC1303100), National Natural Science Foundation of China (31570803, 81773090), and Research Program of Shanghai Municipal Commission of Health and Family Planning (20154Y0049).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Obstetrics and Gynecology Hospital, Fudan University (Kyy2016-49, for cohort 1; Kyy2017-27, for cohort 3) and the Ethics Committee of Suzhou Municipal Hospital (2018-0715, for cohort 2).
Informed consent
All participants included in this study provided written informed consent, which allowed us to use their specimens and data for publication. In this study, patients in cohort 1 and 2 provided informed consent prior to surgery for the use of their paraffin-embedded tissue blocks after a definitive diagnosis, and patients in cohort 3 provided informed consent for use of fresh tissues before surgery.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guodong Zhang, Jiaqi Lu, and Moran Yang have contributed equally to this article.
Contributor Information
Haiou Liu, Email: liuhaiou@fudan.edu.cn.
Congjian Xu, Email: xucongjian@fudan.edu.cn.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Trabert B, DeSantis CE, Miller KD, Samimi G, Runowicz CD, Gaudet MM, Jemal A, Siegel RL. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68(4):284–296. doi: 10.3322/caac.21456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Labidi-Galy SI, Papp E, Hallberg D, Niknafs N, Adleff V, Noe M, Bhattacharya R, Novak M, Jones S, Phallen J, Hruban CA, Hirsch MS, Lin DI, Schwartz L, Maire CL, Tille JC, Bowden M, Ayhan A, Wood LD, Scharpf RB, Kurman R, Wang TL, Shih IM, Karchin R, Drapkin R, Velculescu VE. High grade serous ovarian carcinomas originate in the fallopian tube. Nat Commun. 2017;8(1):1093. doi: 10.1038/s41467-017-00962-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cancer Genome Atlas Research N Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474(7353):609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oliveira-Ferrer L, Legler K, Milde-Langosch K. Role of protein glycosylation in cancer metastasis. Semin Cancer Biol. 2017;44:141–152. doi: 10.1016/j.semcancer.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Pinho SS, Reis CA. Glycosylation in cancer: mechanisms and clinical implications. Nat Rev Cancer. 2015;15(9):540–555. doi: 10.1038/nrc3982. [DOI] [PubMed] [Google Scholar]
- 7.Rao TD, Fernandez-Tejada A, Axelrod A, Rosales N, Yan X, Thapi S, Wang A, Park KJ, Nemieboka B, Xiang J, Lewis JS, Olvera N, Levine DA, Danishefsky SJ, Spriggs DR. Antibodies against specific MUC16 glycosylation sites inhibit ovarian cancer growth. ACS Chem Biol. 2017;12(8):2085–2096. doi: 10.1021/acschembio.7b00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Javadi S, Ganeshan DM, Qayyum A, Iyer RB, Bhosale P. Ovarian cancer, the revised FIGO staging system, and the role of imaging. AJR Am J Roentgenol. 2016;206(6):1351–1360. doi: 10.2214/ajr.15.15199. [DOI] [PubMed] [Google Scholar]
- 9.Rodrigues JG, Balmana M, Macedo JA, Pocas J, Fernandes A, de Freitas JCM, Pinho SS, Gomes J, Magalhaes A, Gomes C, Mereiter S, Reis CA. Glycosylation in cancer: selected roles in tumour progression, immune modulation and metastasis. Cell Immunol. 2018 doi: 10.1016/j.cellimm.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Hanson Ryan, Hollingsworth Michael. Functional Consequences of Differential O-glycosylation of MUC1, MUC4, and MUC16 (Downstream Effects on Signaling) Biomolecules. 2016;6(3):34. doi: 10.3390/biom6030034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brockhausen I. Mucin-type O-glycans in human colon and breast cancer: glycodynamics and functions. EMBO Rep. 2006;7(6):599–604. doi: 10.1038/sj.embor.7400705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohyama C. Glycosylation in bladder cancer. Int J Clin Oncol. 2008;13(4):308–313. doi: 10.1007/s10147-008-0809-8. [DOI] [PubMed] [Google Scholar]
- 13.Langbecker D, Janda M. Systematic review of interventions to improve the provision of information for adults with primary brain tumors and their caregivers. Front Oncol. 2015;5:1. doi: 10.3389/fonc.2015.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stowell SR, Ju T, Cummings RD. Protein glycosylation in cancer. Annu Rev Pathol. 2015;10:473–510. doi: 10.1146/annurev-pathol-012414-040438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steentoft C, Vakhrushev SY, Joshi HJ, Kong Y, Vester-Christensen MB, Schjoldager KT, Lavrsen K, Dabelsteen S, Pedersen NB, Marcos-Silva L, Gupta R, Bennett EP, Mandel U, Brunak S, Wandall HH, Levery SB, Clausen H. Precision mapping of the human O-GalNAc glycoproteome through SimpleCell technology. EMBO J. 2013;32(10):1478–1488. doi: 10.1038/emboj.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gomes J, Mereiter S, Magalhaes A, Reis CA. Early GalNAc O-glycosylation: pushing the tumor boundaries. Cancer Cell. 2017;32(5):544–545. doi: 10.1016/j.ccell.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 17.Cheng L, Tachibana K, Zhang Y, Guo J, Kahori Tachibana K, Kameyama A, Wang H, Hiruma T, Iwasaki H, Togayachi A, Kudo T, Narimatsu H. Characterization of a novel human UDP-GalNAc transferase, pp-GalNAc-T10. FEBS Lett. 2002;531(2):115–121. doi: 10.1016/S0014-5793(02)03399-9. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Q, Burdette JE, Wang JP. Integrative network analysis of TCGA data for ovarian cancer. BMC Syst Biol. 2014;8:1338. doi: 10.1186/s12918-014-0136-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu Q, Yang L, Liu H, Zhang W, Le X, Xu J. Elevated expression of N-acetylgalactosaminyltransferase 10 predicts poor survival and early recurrence of patients with clear-cell renal cell carcinoma. Ann Surg Oncol. 2015;22(7):2446–2453. doi: 10.1245/s10434-014-4236-y. [DOI] [PubMed] [Google Scholar]
- 20.Sheta R, Bachvarova M, Plante M, Gregoire J, Renaud MC, Sebastianelli A, Popa I, Bachvarov D. Altered expression of different GalNActransferases is associated with disease progression and poor prognosis in women with high-grade serous ovarian cancer. Int J Oncol. 2017;51(6):1887–1897. doi: 10.3892/ijo.2017.4147. [DOI] [PubMed] [Google Scholar]
- 21.Gaziel-Sovran A, Hernando E. miRNA-mediated GALNT modulation of invasion and immune suppression. OncoImmunology. 2014;1(5):746–748. doi: 10.4161/onci.19535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fucikova J, Rakova J, Hensler M, Kasikova L, Belicova L, Hladikova K, Truxova I, Skapa P, Laco J, Pecen L, Praznovec I, Halaska MJ, Brtnicky T, Kodet R, Fialova A, Pineau J, Gey A, Tartour E, Ryska A, Galluzzi L, Spisek R. TIM-3 dictates functional orientation of the immune infiltrate in ovarian cancer. Clin Cancer Res. 2019 doi: 10.1158/1078-0432.Ccr-18-4175. [DOI] [PubMed] [Google Scholar]
- 23.Catakovic K, Klieser E, Neureiter D, Geisberger R. T cell exhaustion: from pathophysiological basics to tumor immunotherapy. Cell Commun Signal CCS. 2017;15(1):1. doi: 10.1186/s12964-016-0160-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaziel-Sovran A, Segura MF, Di Micco R, Collins MK, Hanniford D, Vega-Saenz de Miera E, Rakus JF, Dankert JF, Shang S, Kerbel RS, Bhardwaj N, Shao Y, Darvishian F, Zavadil J, Erlebacher A, Mahal LK, Osman I, Hernando E. miR-30b/30d regulation of GalNAc transferases enhances invasion and immunosuppression during metastasis. Cancer Cell. 2011;20(1):104–118. doi: 10.1016/j.ccr.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu H, Chen J, Li D, Liu X, Li L, Wang K. MicroRNA-30e functions as a tumor suppressor in cervical carcinoma cells through targeting GALNT7. Transl Oncol. 2017;10(6):876–885. doi: 10.1016/j.tranon.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huanna T, Tao Z, Xiangfei W, Longfei A, Yuanyuan X, Jianhua W, Cuifang Z, Manjing J, Wenjing C, Shaochuan Q, Feifei X, Naikang L, Jinchao Z, Chen W. GALNT14 mediates tumor invasion and migration in breast cancer cell MCF-7. Mol Carcinog. 2015;54(10):1159–1171. doi: 10.1002/mc.22186. [DOI] [PubMed] [Google Scholar]
- 27.Dahmani Amina, Delisle Jean-Sébastien. TGF-β in T Cell Biology: Implications for Cancer Immunotherapy. Cancers. 2018;10(6):194. doi: 10.3390/cancers10060194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whiteside TL. FOXP3 + Treg as a therapeutic target for promoting anti-tumor immunity. Expert Opin Therap Targets. 2018;22(4):353–363. doi: 10.1080/14728222.2018.1451514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41(1):49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tariq M, Zhang J, Liang G, Ding L, He Q, Yang B. Macrophage polarization: anti-cancer strategies to target tumor-associated macrophage in breast cancer. J Cell Biochem. 2017;118(9):2484–2501. doi: 10.1002/jcb.25895. [DOI] [PubMed] [Google Scholar]
- 32.Hurt B, Schulick R, Edil B, El Kasmi KC, Barnett C., Jr Cancer-promoting mechanisms of tumor-associated neutrophils. Am J Surg. 2017;214(5):938–944. doi: 10.1016/j.amjsurg.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 33.Sionov RV, Fridlender ZG, Granot Z. The multifaceted roles neutrophils play in the tumor microenvironment. Cancer Microenviron. 2015;8(3):125–158. doi: 10.1007/s12307-014-0147-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fu C, Zhao H, Wang Y, Cai H, Xiao Y, Zeng Y, Chen H. Tumor-associated antigens: Tn antigen, sTn antigen, and T antigen. HLA. 2016;88(6):275–286. doi: 10.1111/tan.12900. [DOI] [PubMed] [Google Scholar]
- 35.Schultz MJ, Swindall AF, Bellis SL. Regulation of the metastatic cell phenotype by sialylated glycans. Cancer Metastasis Rev. 2012;31(3–4):501–518. doi: 10.1007/s10555-012-9359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghattass K, El-Sitt S, Zibara K, Rayes S, Haddadin MJ, El-Sabban M, Gali-Muhtasib H. The quinoxaline di-N-oxide DCQ blocks breast cancer metastasis in vitro and in vivo by targeting the hypoxia inducible factor-1 pathway. Molecular Cancer. 2014;13:12. doi: 10.1186/1476-4598-13-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vasudev NS, Trigonis I, Cairns DA, Hall GD, Jackson DP, Broadhead T, Buxton J, Hutson R, Nugent D, Perren TJ. The prognostic and predictive value of CA-125 regression during neoadjuvant chemotherapy for advanced ovarian or primary peritoneal carcinoma. Arch Gynecol Obst. 2011;284(1):221–227. doi: 10.1007/s00404-010-1655-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Freire T, Lo-Man R, Bay S, Leclerc C. Tn glycosylation of the MUC6 protein modulates its immunogenicity and promotes the induction of Th17-biased T cell responses. J Biol Chem. 2011;286(10):7797–7811. doi: 10.1074/jbc.M110.209742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suzuki Y, Sutoh M, Hatakeyama S, Mori K, Yamamoto H, Koie T, Saitoh H, Yamaya K, Funyu T, Habuchi T, Arai Y, Fukuda M, Ohyama C, Tsuboi S. MUC1 carrying core 2 O-glycans functions as a molecular shield against NK cell attack, promoting bladder tumor metastasis. Int J Oncol. 2012;40(6):1831–1838. doi: 10.3892/ijo.2012.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takamiya R, Ohtsubo K, Takamatsu S, Taniguchi N, Angata T. The interaction between Siglec-15 and tumor-associated sialyl-Tn antigen enhances TGF-beta secretion from monocytes/macrophages through the DAP12-Syk pathway. Glycobiology. 2013;23(2):178–187. doi: 10.1093/glycob/cws139. [DOI] [PubMed] [Google Scholar]
- 41.Carrascal MA, Severino PF, Guadalupe Cabral M, Silva M, Ferreira JA, Calais F, Quinto H, Pen C, Ligeiro D, Santos LL, Dall’Olio F, Videira PA. Sialyl Tn-expressing bladder cancer cells induce a tolerogenic phenotype in innate and adaptive immune cells. Molecular Oncol. 2014;8(3):753–765. doi: 10.1016/j.molonc.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beatson R, Tajadura-Ortega V, Achkova D, Picco G, Tsourouktsoglou TD, Klausing S, Hillier M, Maher J, Noll T, Crocker PR, Taylor-Papadimitriou J, Burchell JM. The mucin MUC1 modulates the tumor immunological microenvironment through engagement of the lectin Siglec-9. Nat Immunol. 2016;17(11):1273–1281. doi: 10.1038/ni.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Madsen CB, Petersen C, Lavrsen K, Harndahl M, Buus S, Clausen H, Pedersen AE, Wandall HH. Cancer associated aberrant protein O-glycosylation can modify antigen processing and immune response. PLoS One. 2012;7(11):e50139. doi: 10.1371/journal.pone.0050139. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.