Abstract
Cancer is one of the main causes of mortality worldwide and a major public health concern. Among various strategies, therapeutic vaccines have been developed to stimulate anti-tumoral immune responses. However, in spite of extensive studies, this approach suffers from a lack of efficacy. Recently, we designed the MAG-Tn3 vaccine, aiming to induce antibody responses against Tn, a tumor-associated carbohydrate antigen. The Tn antigen is of interest because it is expressed by several adenocarcinomas, but not normal cells. The fully synthetic glycopeptide vaccine MAG-Tn3 is composed of four arms built on three adjacent Tn moieties associated with the tetanus toxin-derived peptide TT830–844 CD4+ T-cell epitope. This promiscuous CD4+ T-cell epitope can bind to a wide range of HLA–DRB molecules and is thus expected to activate CD4+ T-cell responses in a large part of the human population. The MAG-Tn3 vaccine was formulated with the GSK-proprietary immunostimulant AS15, composed of CpG7909, MPL, and QS21, which has been shown to stimulate both innate and humoral responses, in addition to being well tolerated. Here, seven patients with localized breast cancer with a high-risk of relapse were immunized with the MAG-Tn3 vaccine formulated with AS15. The first results of phase I clinical trial demonstrated that all vaccinated patients developed high levels of Tn-specific antibodies. Moreover, these antibodies specifically recognized Tn-expressing human tumor cells and killed them through a complement-dependent cytotoxicity mechanism. Overall, this study establishes, for the first time, the capacity of a fully synthetic glycopeptide cancer vaccine to induce specific immune responses in humans.
Electronic supplementary material
The online version of this article (10.1007/s00262-020-02503-0) contains supplementary material, which is available to authorized users.
Keywords: Tn antigen, Cancer vaccine, Antibodies, Breast cancer
Introduction
Prophylactic vaccines are one of the greatest triumphs of modern medicine. Originally intended to protect against infectious diseases, new approaches have been developed to design therapeutic vaccines against cancer, based on the stimulation of the host immune system. Autologous dendritic-cell (DC) vaccines are among the most widely studied strategies and consist of priming the host’s DCs ex vivo and injecting them into the patients [1]. With 289 clinical trials ongoing in 2014, this strategy has shown some success and has resulted in the approval of the Sipuleucel-T vaccine by the FDA (American Food and Drug Administration) [2]. This DC-based vaccine, composed of the prostate acid phosphatase antigen associated with granulocyte-macrophage colony-stimulating factor (GM-CSF), demonstrated a relative significant 22% reduction in the risk of death in patients with metastatic castration-resistant prostate cancer in phase III clinical trial [3]. More recently, immune checkpoint inhibitors have emerged as an essential player in the therapeutic arsenal against cancer but have failed to provide a clinical benefit to a majority of patients. One explanation for such low efficacy could be the absence of tumor-specific T cells in non-responder patients, a deficit that could be corrected by immunization with a cancer vaccine [4]. It has thus been proposed to combine immune checkpoint inhibitors with vaccines [5]. Several studies have indeed shown improved clinical outcomes when combining cancer vaccines with anti-CTLA4 [6] or anti-PD1 [7]. Better knowledge of tumor-specific antigens (TSAs), including neoantigens arising from somatic cancer mutations [8], have also contributed to rekindling the interest in cancer vaccines.
Thus, studies focusing on TAAs (tumor-associated antigens) aberrantly expressed on tumor cells are still pertinent [9]. Among them, TACAs (tumor-associated carbohydrate antigens) are promising targets in cancer immunotherapy. Their expression is due to impairment of the glycosylation process that occurs in malignant cells. One of the most prevalent TACAs is the Thomsen-nouveau (Tn) antigen, a carbohydrate composed of a serine or threonine residue O-linked to an alpha-N-acetylgalactosamine [10]. The National Cancer Institute (NCI) has declared the Tn antigen to be of interest for the development of cancer vaccines [11]. A key feature of this antigen is its selective expression by adenocarcinomas, but not normal cells. The expression of the Tn antigen is 90% in breast cancer [12] and 70–90% in colon, bladder, lung, bladder, cervix, ovary, and stomach cancer [13–15]. This antigen, along with other O-glycans, has been shown to be associated with a bad prognosis, notably playing a role in cell growth and invasion [16].
As a result, investigations have been carried on to design antibodies targeting the Tn antigen. However, although several mAbs have been developed and promising results were obtained in pre-clinical studies, their specificity remains to be precisely defined as well as their potential therapeutic effect before their testing in clinical trials [17]. In addition, unlike HER2, no cut-off of the Tn-expression on the tumor that could be associated with Tn treatment efficacy has been established, as Tn+ tumors are rather homogeneously stained [12].
Clinical trials have also been performed with the Tn antigen. A phase I clinical trial using Tn trimers coupled to KLH, in association with the QS21 immunostimulant, demonstrated good immunogenicity and safety in patients with prostate cancer [18]. More recently, a DC-based vaccine, called Tn-MUC1, has been designed. Tn-MUC1 loaded onto autologous DCs was found to be safe and induce Tn-MUC1-specific CD4+ and/or CD8+ T-cell responses in five of seven patients with non-metastatic castration-resistant prostate cancer [19].
Here, we designed a synthetic vaccine, MAG-Tn3, composed of four arms of three Tn antigens linked to the tetanus toxoid-derived TT830–844 peptide. As a fully synthetic compound, it presents several key features, such as allowing accurate and reliable quality control of the vaccine [20]. The challenge represented by the synthesis of MAG-Tn3 has been recently circumvented, allowing multigram preparation of a highly-pure clinical batch [21].
The MAG-Tn3 vaccine was designed to induce an anti-Tn antibody response in all vaccinated subjects. The carbohydrate Tn antigen is not sufficient alone to mount an antigen-specific antibody response and must be linked to CD4+ T-cell epitopes [22]. Thus, the tetanus toxin-derived epitope TT830–844 was included in the vaccine design as a source of T-cell help. Indeed, this promiscuous epitope has been shown to bind to a wide set of HLA–DRB1 molecules largely expressed in the population [23].
This vaccine was associated with AS15, an immunostimulant composed of CpG7909 (TLR9 agonist), monophosphoryl lipid A (MPL, a TLR4 agonist), and QS21. This combination has been found to be highly immunogenic in large-scale clinical trials while being well tolerated [24]. We previously demonstrated that the MAG-Tn3 vaccine combined with alum efficiently reduced the tumor burden in the TA3/Ha mouse model [25]. Moreover, in association with AS15, MAG-Tn3 has been recently found to be well tolerated in non-human primates and induced robust Tn-specific IgM and IgG responses [23].
Given the promising preclinical results of the MAG-Tn3 vaccine, we designed a phase I clinical trial (ClinicalTrials.gov identifier: NCT02364492) to treat patients with localized breast cancer, in adjuvant setting, with a high-risk of relapse. For this study, the MAG-Tn3 vaccine formulated with the AS15 immunostimulant (MAG-Tn3/AS15) was administered intramuscularly to the patients. Immunization with MAG-Tn3/AS15 induced marked levels of Tn-specific IgM and IgG responses in all patients. These Tn-specific antibodies recognized human Tn+ but not Tn− tumor cell lines. In addition, these antibodies were extremely potent in killing Tn+ Jurkat cells through a complement-dependent cytotoxicity mechanism. Overall, we demonstrate, for the first time, the immunogenicity of a fully synthetic glycopeptidic cancer vaccine in humans.
Materials and methods
Patient inclusion and clinical trial design
The MAGTRIVACSEIN phase I clinical trial (https://clinicaltrials.gov/ct2/show/NCT02364492) consists of an open-label study of the use of the fully synthetic vaccine MAG-Tn3 (Supplementary Fig. S1), formulated with the immunostimulant AS15, as a therapeutic vaccine candidate in patients with localized breast cancer at high-risk of relapse. The study protocol, all amendments and informed consents were approved by the French “Agence Nationale de la Sécurité du Médicament” (ANSM, EudraCT 2013-004970-90) and by one of the French ethical research committees “Comités de Protection des Personnes (CPP)”. Information about patient enrolment, main inclusion and exclusion criteria and the MAG-Tn3/AS15 administration and blood sampling are detailed in the Supplementary Fig. S2. The patient characteristics, treatment, and tumor status are presented in Table 1. Informed consent was obtained from each patient after the nature and possible consequence of the study have been explained.
Table 1.
Clinical characteristics of patients with breast cancer included in the study
| Patient 01 | Patient 02 | Patient 03 | Patient 04 | Patient 05 | Patient 06 | Patient 07 | |
|---|---|---|---|---|---|---|---|
| Age (years) | 44 | 61 | 65 | 55 | 46 | 66 | 57 |
| Sex | Female | Female | Female | Female | Female | Female | Female |
| Estrogen receptor (%) | 70 | 100 | Unknown | 90 | 0 | 90 | 0 |
| Progesterone receptor (%) | 2 | 0 | Unknown | 70 | 0 | 20 | 0 |
| HER2 receptor | Not expressed/not amplified | Not expressed/not amplified | Not expressed/not amplified | Not expressed/not amplified | Not expressed/not amplified | Not expressed/not amplified | Not expressed/not amplified |
| TNM classification | T2, N3, M0 | T1, N1, M0 | T3, N1, M0 | T2, N3, M0 | T3, N0, M0 | T1, N2, M0 | T1, N1, M0 |
| Chemotherapy | Adjuvant | Adjuvant | Neo-adjuvant | Adjuvant | Neo-adjuvant | Adjuvant | Adjuvant |
| Chemotherapy | High dose ECa and 3–4b taxotere | 3–4 FECc and 3–4 docetaxel | 6 cycles 75 mg/m2 EC + carboplatin + taxol | 3–4 EC and 3–4 docetaxel | High dose EC and 3–4 taxol | 3–4 FEC and 3–4 docetaxel | 3–4 EC and 3–4 docetaxel |
| Surgery | Tumorectomy and axillary curettage | Mastectomy and axillary curettage | Mastectomy and axillary curettage | Mastectomy and axillary curettage | Mastectomy and axillary curettage | Mastectomy and axillary curettage | Mastectomy and axillary curettage |
| Radiotherapy | Breast, ganglionic areas, chest wall | Breast, ganglionic areas | Breast, ganglionic areas | Chest wall | Ganglionic areas, chest wall | Breast, ganglionic areas, chest wall | Ganglionic areas, chest wall |
| Hormonotherapy | Exemestane and enantone | Aromatase inhibitor | No | Aromatase inhibitor | No | Aromatase inhibitor | No |
| MAG-Tn3 dose (µg) | 30 | 30 | 30 | 100 | 100 | 100 | 100 |
| Serious adverse effect after MAG-Tn3 immunization | No | No | No | No | No | No | No |
General information, tumor status, and previous treatment of the patients included in the clinical trial
aEC: epirubicin/cyclophosphamide
b3–4: 3–4 cycles
cFEC: fluorouracil/epirubicin/cyclophosphamide
Preparation of the MAG-Tn3/AS15 vaccine
The fully synthetic multiple antigenic glycopeptide is assembled onto a tetravalent lysine core. It displays four arms, consisting of three Tn glycotopes (MAG-Tn3) linked to the N-ter of the tetanus toxoid-derived peptide TT830–844. The MAG-Tn3 vaccine was produced by Lonza under GMP conditions as previously described [26]. The tetanus toxoid TT830–844 peptide (QYIKANSKFIGITEL) was obtained from Polypeptide. To enhance immune response, the MAG-Tn3 vaccine was formulated with AS15, an immunostimulant containing 3-O-desacyl-4′-monophosphoryl lipid A (MPL) (50 µg, produced by GSK), Quillaja saponaria Molina, fraction 21 (QS-21) (50 µg, licensed by GSK from Antigenics LLC, a wholly owned subsidiary of Agenus Inc., a Delaware, USA corporation), CpG 7909 synthetic oligodeoxynucleotides (ODNs) containing unmethylated CpG motifs [380 µg, in-licensed from Pfizer (Coley)] and liposome. AS01B is a combination of QS-21 and MPL. The MAG-Tn3/AS15 formulation consisted of lyophilized MAG-Tn3 combined with CpG 7909 and reconstituted with the AS01B adjuvant (500 μL), extemporaneously.
Cell culture
Jurkat, MCF7, SHIN3, LS174T, LS180, SKBR3, and MDA231 human cell lines (ATCC) were cultured in RPMI 1640 medium (Fischer Scientific), supplemented with 10% fetal bovine serum (Fischer Scientific) and antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin, Fischer Scientific).
Monoclonal antibodies and reagents
The murine monoclonal anti-Tn monoclonal antibodies 83D4 [12], 6E11 [12], and 8D4 [23] were produced by BioXcell. Trastuzumab anti-HER2 humanized antibody was provided by Enzo Life Sciences, Inc. The purified immunoglobulin from non-human primates used as a positive control for the CDC assay was obtained in a preclinical study using MAG-Tn3/AS15 vaccination [23]. The proapoptotic molecule staurosporin, used as positive control for the cytotoxicity, assay was provided by Fischer Scientific.
HLA genotyping
The high resolution typing for HLA-A, -B, -C, -DRB1, -DQB1 and DPB1 was performed by sequence-based typing Sanger sequencing using AlleleSEQR (Abbot). HLA-DPB1 typing was performed using PCR SSO (single specific oligonucleotide) reverse (OneLambda, Inc.)
Measurement of T cell responses by ELISPOT
Multi-well polyvinylidene fluoride membrane plates were coated with anti-IFNγ or anti-IL-2 capture antibody. Briefly, 25.104 PBMCs per well were incubated with complete medium alone or containing 5 µM of the tetanus toxoid TT830–844 peptide (QYIKANSKFIGITEL) for 18 h. Then, biotinylated anti-cytokine detection antibody was added to each well. ELISPOT plates were incubated with avidin alkaline phosphatase and freshly prepared NBT (nitro-blue tetrazolium chloride) and the BCIP (5-bromo-4-chloro-3′-indolyphosphate p-toluidine salt) substrate then added for spot formation. Plates were washed with distilled water to arrest spot development. Spots were counted using an automated ELISPOT plate reader and the results expressed as spot counts per million cells. All samples were tested in triplicate wells.
Analysis of Tn-specific antibody responses by ELISA
ELISA was performed as previously described [23]. Sera from patients were incubated in neutravidin pre-coated plates (Fischer Scientific) coated with in-house synthetic biotinylated Tn3-G6K(Biot)G glycopeptide [25]. Anti-human IgM-HRP and IgG-HRP detection antibodies (Sigma-Aldrich) were used at dilutions of 1:12,500 and 1:1000, respectively. For anti-Tn IgG1, IgG2, IgG3, and IgG4 titer determination, twofold dilutions of sera of patients, ranging from 1:50 to 1:1600, were tested. The anti-human IgG1, IgG2, IgG3, and IgG4 detection antibodies coupled with alkaline phosphatase (Southern Biotech) were used at a 1:500 dilution. Optical densities were recorded at 450 nm for HRP and 405 nm for alkaline phosphatase using a Multiskan spectrum (Fischer Scientific) plate reader. Antibody titers were calculated as previously described [23]. Briefly, ELISA antibody titers were determined by linear regression analysis, plotting the dilution versus optical density. The titers were calculated as the dilution giving twice the optical density of serum of the same patient before immunization. Titers are given as the arithmetic mean ± SD of the log10 titers.
Flow-cytometry analysis of the capacity of sera from patients to recognize Tn+ human cell lines
Sera from patients were tested for their ability to bind to Tn-expressing human cell lines at 1:50, 1:200, 1:800, and 1:3200 dilutions, as previously described [23]. Briefly, the sera were incubated with human Jurkat, MCF-7, SHIN3, LS174T, LS180, SKBR3, or MDA231 cells for 15 min at 4 °C in PBS containing 2% FCS (Fischer Scientific). After washing, the cells were stained with goat anti-human polyclonal IgM-FITC and IgG-PE (Southern Biotech) at dilutions of 1:250 or 1:10, respectively. Cells were fixed using 100 µL BD cytofix (BD) and data acquired using an LSR Fortessa cytometer. Data were analyzed using FlowJo software.
Direct and complement-dependent cytotoxicity assays
Direct and complement-dependent cytotoxicity (CDC) were assayed using purified immunoglobulin isolated from sera, or with monoclonal antibody 8D4. Salts were removed from the sera using ZebaSpin Desalting Columns (Fischer scientific) and the immunoglobulins (Ig) purified using the Melon Gel IgG Purification Kit (Fischer scientific), following the manufacturer’s procedures. The immunoglobulin concentration was determined by the Bradford protein assay. Jurkat, MCF-7 or MDA231 cells were incubated at 37 °C with various concentrations of purified immunoglobulins in the presence or absence of 1% rabbit complement-MA (Cedarlane). Dead cells were stained by adding 40 µL Celltiter-Blue (Promega) to each well. The fluorescence emission was recorded using a Glomax discoverer plate reader (Promega).
The percentage of direct cytotoxicity was calculated as 100 × ((Fluorescence of sample) − (Fluorescence of sample without Ig))/(Fluorescence of sample without Ig) and that of CDC as 100 × ((Fluorescence of sample with complement) − (Fluorescence of sample without Ig))/(Fluorescence of sample without Ig).
Statistical analysis
Statistical analyses were performed using two-tailed paired or unpaired parametric Student’s t tests (GraphPad Software). p values < 0.05 were considered statistically significant.
Results
Immunization of breast-cancer patients with the MAG-Tn3 vaccine formulated with AS15 does not alter immune parameters
We evaluated the immune responses induced by the MAG-Tn3/AS15 vaccine (Supplementary Fig. S1) in seven patients with breast cancer with a high risk of relapse because the Tn antigen is widely expressed in breast cancer [12], supporting a vaccine strategy targeting this carbohydrate tumor antigen. The patients included in this phase I clinical trial (MAGTRIVACSEIN) had non-metastatic breast cancer that did not express the HER2 receptor (Table 1). These patients received at least six cycles of chemotherapy, in addition to surgery and radiotherapy, before being eligible for inclusion in the trial (Supplementary Fig. S2A). The immunization protocol consisted of an intramuscular injection of the MAG-Tn3/AS15 vaccine every 3 weeks for a total of six immunizations (Supplementary Fig. S2B). The main aim of this phase I clinical trial was to assess the safety and immunogenicity of the MAG-Tn3/AS15 vaccine. In the present study, we analyzed the immune responses of the first seven patients who completed the protocol.
MAG-Tn3/AS15 vaccination did not significantly modify the total concentration of circulating IgG1, IgG2, IgG3, or IgG4 immunoglobulins in most of the patients (Supplementary Fig. S3). We also observed no major changes in blood leukocyte populations (Supplementary Table S1) or the myeloid and lymphoid populations present in the peripheral blood mononuclear cells (PBMCs) of these patients (Supplementary Fig. S4).
Immunization of breast cancer patients with the MAG-Tn3 vaccine induces TT-specific T-cell responses in all patients
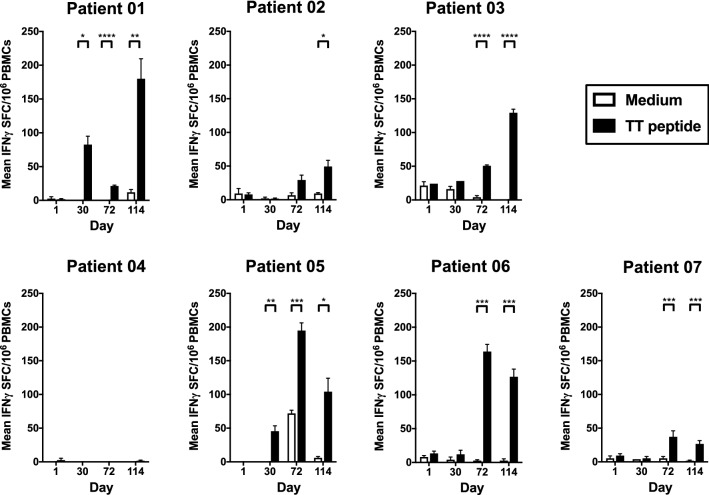
We used the promiscuous tetanus toxin-derived TT830–844 epitope [27] as a T-helper epitope to induce anti-Tn antibody responses in all patients, as this peptide can bind to a large set of HLA–DRB molecules [23]. We analyzed the HLA genotype profile of the seven patients immunized with the MAG-Tn3/AS15 vaccine by biallelic sequencing (Table 2). Six of the seven patients displayed at least one allele described to efficiently bind the TT830–844 peptide (patient 01: alleles 04:05 and 11:01; patient 2: allele 07:01; patient 04: allele 04:05; patient 05: allele 04:01and patients 06 and 7: allele 11:01) [23]. Only patient 03 did not possess an HLA–DRB1 allele known to bind the TT830–844 peptide. The PBMCs of the patients were stimulated in vitro with the TT830–844 peptide and the production of IFNγ measured by ELISPOT to analyze their TT-specific T-cell responses. Following immunization with 30 μg of the MAG-Tn3/AS15 vaccine, a significant TT-specific T-cell responses was detected in all 3 patients and following the 100 μg dose administration in 3 out of the 4 patients (Fig. 1). We measured the production of IL-2 by PBMCs stimulated with the TT830–844 peptide by ELISPOT to confirm these results (Supplementary Fig. S5). A production of IL-2 was observed for all patients, including patient 04, who developed a weak but detectable IL-2 response after in vitro stimulation with the TT peptide. We observed a high level of IL-2 production with the PBMCs of patient 05 incubated with medium alone following vaccination, which may be linked to previous exposure to TT.
Table 2.
HLA genotype of patients
| Patient | Allele | HLA-A | HLA-B | HLA-C | HLA-DRB1 | HLA-DQB1 | HLA-DPB1 |
|---|---|---|---|---|---|---|---|
| Patient 01 | Allele 1 | 02:05 | 08:01 | 07a | 04:05 | 03:01 | 01a |
| Allele 2 | 30:02 | 15:16 | 14:02 | 11:01 | 03:02 | 03a | |
| Patient 02 | Allele 1 | 01:01 | 08:01 | 06:02 | 03:01 | 02:01 | 04:01 |
| Allele 2 | 24:02 | 57:01 | 07a | 07a | 03:03 | 04a | |
| Patient 03 | Allele 1 | 01:01 | 35:02 | 04:01 | 04:02 | 03:01 | 04:01 |
| Allele 2 | 24:02 | 57:01 | 06:02 | 11:04 | 03:02 | 05a | |
| Patient 04 | Allele 1 | 03:01 | 35:01 | 06:02 | 04:05 | 03:02 | 02:01 |
| Allele 2 | 29:02 | 45:01 | 16:04 | 15:01 | 06:02 | 02a | |
| Patient 05 | Allele 1 | 03:01 | 15:01 | 03:04 | 04:01 | 03:02 | 04a |
| Allele 2 | 31:01 | 18:01 | 07:01 | 14:54 | 05:03 | 04a | |
| Patient 06 | Allele 1 | 02:01 | 15:01 | 03a | 04:04 | 03:01 | 02a |
| Allele 2 | 23a | 49:01 | 07a | 11a | 03:02 | 580a | |
| Patient 07 | Allele 1 | 03:01 | 15:01 | 05:01 | 11:01 | 03a | 04a |
| Allele 2 | 32:01 | 44:02 | 07:04 | 11:04 | 03a | 05a |
Biallelic sequencing for loci of class I HLA-A, HLA-B, and HLA-C and class II HLA-DRB1, HLA-DQB1, and HLA-DPB1
aSpecific HLA allele could not be unambiguously defined
Fig. 1.
Immunization with the MAG-Tn3 vaccine induces activation of a specific anti-TT T-cell response. Patients were immunized on days 0, 21, 42, 63, 84, and 105 with the MAG-Tn3 vaccine formulated with the immunostimulant AS15. Patients 01, 02, and 03 received intramuscular injections of 30 µg MAG-Tn3, whereas patients 04, 05, 06, and 07 received 100 µg MAG-Tn3. PMBCs were harvested at various times after immunization and stimulated for 18 h with either medium or the tetanus toxin (TT830–844) peptide. IFNγ production was evaluated by ELISPOT and is expressed as the mean of spot forming colonies (SFC) per 106 cells ± SEM of quadruplicates from two independent experiments. The statistical significance of the differences was determined by the unpaired Student’s t test, comparing the SFC obtained with medium or TT peptide for each timepoint. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
Immunization of breast cancer patients with the MAG-Tn3 vaccine induces Tn-specific antibody responses in all patients
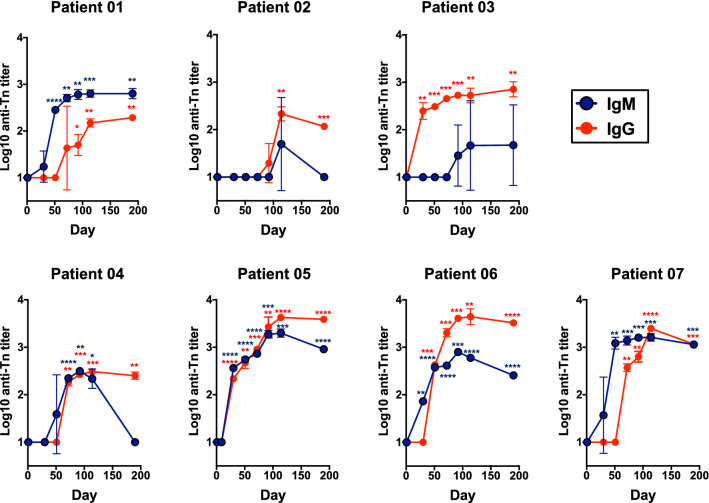
We assessed the capacity of the MAG-Tn3/AS15 vaccine to induce antibody responses in patients by evaluating the IgM- and IgG-specific anti-Tn titers by an ELISA using a Tn3 antigen bearing an irrelevant peptide. None of the patients showed detectable anti-Tn antibodies before immunization (Fig. 2). In contrast, anti-Tn antibody responses were induced in the seven patients who received either 30 or 100 μg of the MAG-Tn3/AS15 vaccine. The level and kinetics of these responses differed between patients. We observed low anti-Tn IgM titers for patients 02 and 03, whereas the anti-Tn IgM response of patient 04 was higher but decreased rapidly. The IgM response for patients 01, 05, 06, and 07 reached 2.5–3 log10 titers after three or four injections and remained stable. All patients showed good anti-Tn IgG titers, although those immunized with 100 μg MAG-Tn3 yielded a more robust and sustained anti-Tn response, with the exception of patient 04. The peak of the anti-Tn responses was reached between days 92 and 114 after the first immunization, i.e. after five to six immunizations with the MAG-Tn3 vaccine. These anti-Tn IgG responses persisted for at least 3 months after the last MAG-Tn3/AS15 immunization.
Fig. 2.
Immunization with the MAG-Tn3 vaccine induces a specific anti-Tn antibody response in all patients. Patients were immunized on days 0, 21, 42, 63, 84, and 105 with the MAG-Tn3 vaccine formulated with the immunostimulant AS15. Patients 01, 02, and 03 received intramuscular injections of 30 µg MAG-Tn3, whereas patients 04, 05, 06, and 07 received 100 µg MAG-Tn3. Sera were collected at various times after immunization and tested for anti-Tn specific IgM and IgG responses by ELISA. Antibody titers are expressed as the mean ± SD of log10 titer of quadruplicates from two independent experiments. The statistical significance of the differences was determined by the unpaired Student’s t test relative to baseline. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
We then characterized the IgG subclass profile of these anti-Tn responses by ELISA (Supplementary Fig. S6). No IgG subclass was detectable for patients 01, 02, or 04, probably due to their lower level of anti-Tn IgG antibodies. In contrast, patients 03, 05, 06, and 07 showed a Tn-specific IgG3-oriented profile. In addition, the anti-Tn IgG2 and IgG1 titers increased significantly in patients 05 and 06, respectively.
The antibodies induced by the MAG-Tn3 vaccine recognize Tn+ human tumor cells
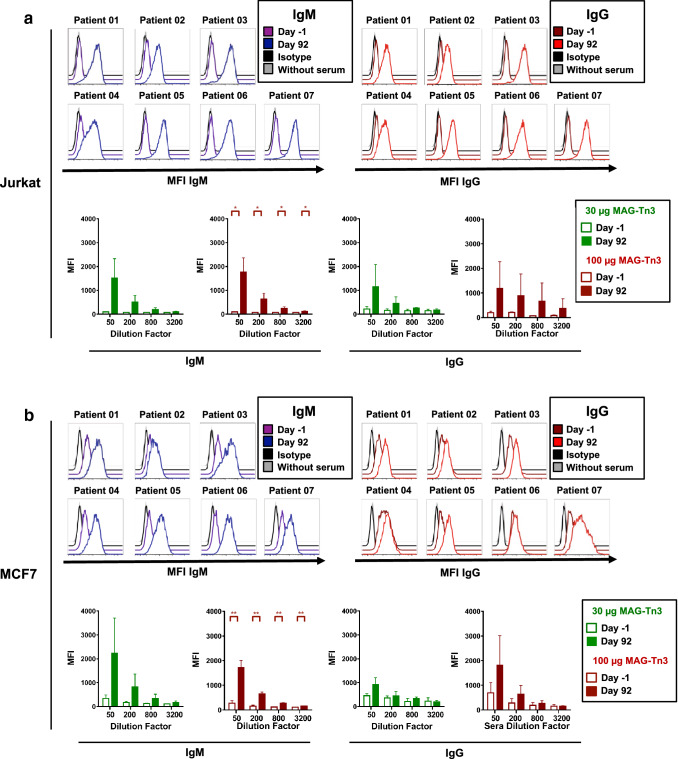
To determine whether the anti-Tn antibodies induced in patients by the MAG-Tn3 vaccine are able to recognize Tn+ tumor cells, we first evaluated the expression of the Tn antigen by several relevant cell lines using the 83D4 anti-Tn murine monoclonal antibody [12]. We observed a particularly high Tn-expression on Jurkat and MCF7 cell lines, whereas SHIN3, LS180, LS174T cell lines did express a more heterogeneous level of Tn (Supplementary Fig. S7). SKBR3 and MDA231 showed no or very low Tn expression. Based on these results, we selected for our analysis the Jurkat and MCF7 cell lines as Tn+ cell lines and MDA231 and SKBR3 cell lines as a Tn− control.
We then analyzed the capacity of sera from immunized patients, harvested either before immunization or at day 92, to recognize these cell lines by flow cytometry. All patients developed IgM and IgG antibodies capable of recognizing Jurkat (Fig. 3a) and MCF7 Tn+ cells (Fig. 3b) following immunization. In contrast, these sera did not recognize the two Tn− cell lines, SKBR3 and MDA231 (Supplementary Fig. S8). We did not observe significant differences between the capacity of the sera from patients immunized with either 30 or 100 µg of the MAG-Tn3 vaccine to recognize Tn+ cells due to the heterogeneity of the responses and the limited number of patients.
Fig. 3.
Anti-Tn antibodies induced in cancer patients by the MAG-Tn3 vaccine recognize human Tn+ tumor cells. Patients were immunized on days 0, 21, 42, 63, 84, and 105 with the MAG-Tn3 vaccine formulated with the immunostimulant AS15. Patients 01, 02, and 03 received intramuscular injections of 30 µg MAG-Tn3, whereas patients 04, 05, 06, and 07 received 100 µg MAG-Tn3. Pre- and post-immunization sera were analyzed for their ability to recognize Jurkat (a) and MCF7 (b) Tn+ human cell lines. Cells were incubated with 1/50, 1/200, 1/800, or 1/3,200 diluted sera and then stained with anti-human IgM-FITC or IgG-PE antibodies and analyzed by flow cytometry. In a and b, the upper panels show histograms of one representative experiment, showing the mean fluorescence intensity (MFI) for IgM and IgG expression at a 1/50 serum dilution for each patient. The lower panels show the mean MFI ± SD obtained in two independent experiments with sera from patients immunized either with 30 or 100 µg of MAG-Tn3. The statistical significance of the differences was determined by the paired Student’s t test comparing the results obtained with sera before and after immunization. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
The antibodies induced in patients immunized with the MAG-Tn3 vaccine can kill Tn+ Jurkat tumor cells in the presence of complement
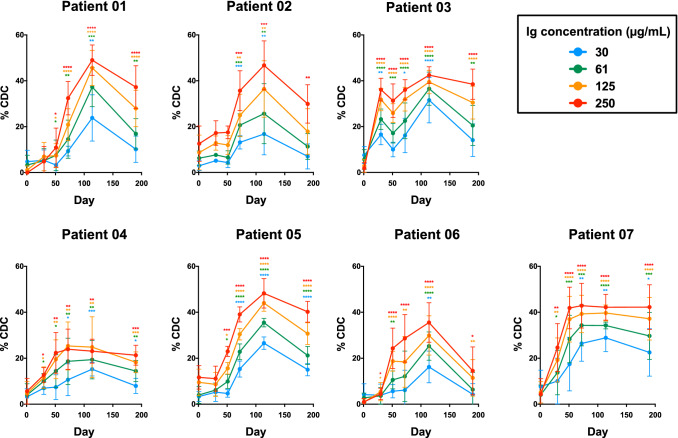
We then assessed the potential anti-tumoral effect of the antibody responses induced in MAG-Tn3-immunized patients by analyzing the capacity of their sera to kill Tn+ tumor cells. Tn+ Jurkat and MCF7 cells were incubated with immunoglobulins purified from the patients for 5 h and their viability determined by their capacity to convert a redox dye (resazurin) into a fluorescent end product (resorufin). While only a very low percentage of cytotoxicity was observed after the incubation of Tn+ Jurkat and MCF7 cells with the purified immunoglobulins (Supplementary Fig. S9), when Tn+ Jurkat cells were incubated with purified immunoglobulins in the presence of complement, a high and sustained cytotoxic effect was observed with the sera harvested after immunization with the MAG-Tn3 vaccine (Fig. 4). This cytotoxic effect was dependent upon the dose of purified immunoglobulins used, was detectable in most of the patients very early after immunization, peaked around day 114, and was still detectable at the last timepoint studied, i.e. 3 months after the last MAG-Tn3/AS15 immunization. These results demonstrate the strong capacity of the anti-Tn antibodies to kill Tn+ Jurkat tumor cells through complement-dependent cytotoxicity (CDC), even at low concentrations. However, we observed no significant CDC with the Tn+ MCF7 cells or, as expected, the Tn− MDA231 cells (Supplementary Fig. S10). The absence of CDC with the MCF7 cells could be related to the level of Tn at the surface of those cells, which has been found to be lower than on Jurkat cells (Supplementary Fig. S7). A second explanation could be linked to the proteinic backbone which carries the Tn antigen. Indeed, in Jurkat cell line, Tn is expressed on leukosialin [28] whereas it is expressed on mucin for MCF7 [29]. This difference could play a role in the exposure of the Tn antigen at the cell surface and thus could affect the ability of anti-Tn antibody to bind its target. Finally, the physiological properties of those cell lines may also differ (cell adherence, cell membrane structure, resistance to apoptosis…) and could impact the results.
Fig. 4.
Anti-Tn antibodies induced in cancer patients by the MAG-Tn3 vaccine efficiently kill Tn+ Jurkat cells through complement-dependent cytotoxicity. Patients were immunized on days 0, 21, 42, 63, 84, and 105 with the MAG-Tn3 vaccine formulated with the immunostimulant AS15. Patients 01, 02, and 03 received intramuscular injections of 30 µg MAG-Tn3, whereas patients 04, 05, 06, and 07 received 100 µg MAG-Tn3. Pre- and post-immunization sera were analyzed for their ability to kill the Jurkat Tn+ human cell line. Tn+ Jurkat cells were incubated with immunoglobulins purified from the sera of patients at four concentrations (250, 125, 61, and 30 µg/mL) and complement. After 5 h, cell viability was measured using the Celltiter-Blue kit® (Promega). The percentage of CDC was calculated as described in Materials and Methods and the results are expressed as the mean ± SD of four to six replicates from two to three independent experiments. The statistical significance of the differences was determined relative to baseline by the unpaired Student’s t test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
In addition, we also evaluated the direct and complement-dependent cytotoxicity of the 8D4 anti-Tn monoclonal antibody [23, 30] on Jurkat, MCF7 and MDA231 cells. As shown in the Supplementary Fig. S11, in the presence of complement, 8D4 killed Jurkat cells, but not MCF7 and MDA231 cells. Interestingly, the level of CDC obtained with this monoclonal antibody was lower than the cytotoxicity obtained with similar concentration of total immunoglobulins purified from MAG-Tn3 vaccinated patients, suggesting a strong cytotoxic property of the anti-Tn antibodies induced in these patients. We also analyzed the purified immunoglobulins for their capacity to kill tumor cells by antibody-dependent cell cytotoxicity (ADCC). This assay was based on flow cytometry to discriminate effector from target cells and determine the percentage of live and dead cells (Supplementary Fig. S12A). Two types of positive controls were used to establish the assay. First, we used the humanized anti-HER2 trastuzumab monoclonal antibody, which was incubated with SKBR3 target HER2+ cells, and human primary CD56+ NK cells as effector cells (Supplementary Fig. S12B). These results confirmed the ability of CD56+ NK cells to efficiently kill SKBR3 cells in the presence of trastuzumab. Then, the anti-Tn murine monoclonal antibody 6E11 was incubated with Tn+ Jurkat cells or Tn− MDA231 cells (Supplementary Fig. S12C). This experiment clearly demonstrated that the monoclonal anti-Tn antibody can kill Tn+ Jurkat cells in the presence of NK cells, but not Tn− MDA231 cells. Finally, we tested the capacity of purified immunoglobulins from MAG-Tn3/AS15-immunized patients to kill Tn+ Jurkat cells in the presence of NK cells and observed no significant ADCC activity (Supplementary Fig. S12D).
Discussion
This study represents the first proof of concept of the immunogenicity of a fully synthetic glycopeptide vaccine in humans. With its unique design, associating four arms carrying three Tn moieties and the promiscuous TT830–844 CD4+ T-helper epitope, the MAG-Tn3 vaccine induced high levels of Tn-specific antibodies in all patients. In addition, the anti-Tn antibodies induced by the MAG-Tn3 vaccine recognized Tn-expressing human tumor cells and killed them through CDC.
In this study, seven patients with localized breast cancer with a high-risk of relapse were immunized with the MAG-Tn3/AS15 vaccine. The first results of this phase I clinical trial show that vaccinated patients developed high levels of IgM and IgG Tn-specific antibodies. The Tn antibody responses appeared to higher than those obtained in previous clinical trials involving the Tn antigen. Here, the anti-Tn titers were determined by ELISA and calculated using linear regression analysis from an appropriate array of serum dilutions. Immunization of patients with a Tn-KLH conjugate was previously shown to induce only moderate and heterogeneous levels of anti-Tn IgM and IgG titers, ranging from approximately 102 to 103 after immunization versus the titers of 102 to 104 obtained in our study [18]. The titers found in the other study were also obtained by ELISA, but using a different calculation method (highest dilution yielding an optical density above 0.1), which could minimize the titer differences obtained between the two studies [18]. These results, obtained in humans, corroborate our previous findings in non-human primates, demonstrating that the Tn-KLH conjugate induces much lower IgG titers than the synthetic MAG-Tn3 [22]. Moreover, the only synthetic Tn vaccine reported in clinical trials, a palmitoyl-Tn conjugate, failed to induce anti-Tn responses [18]. A KLH-based vaccine, combining six self-antigens, including Tn, as well as GM2, Globo H, Lewisy, glycosylated MUC-1-32mer, and TF, has also been developed [31]. Unlike the monovalent version, the hexavalent vaccine failed to induce anti-Tn IgG responses.
The strong antibody response obtained in patients immunized with the MAG-Tn3 vaccine, relative to that to the KLH-Tn conjugate, can be explained by the vaccine design. Indeed, this fully synthetic vaccine was specifically designed to obtain a high density of the Tn carbohydrate antigen, increasing the antibody response directed against this tumor antigen [26]. In addition, the Tn cluster of MAG-Tn3 is different from that of KLH-Tn, as it is based on a Ser-Thr-Thr instead of a Ser-Ser-Ser sequence. This aglyconic part of the Tn structure (Ser vs Thr) has been shown to play a key role in anti-Tn specificity for breast cancer detection by monoclonal antibodies [12] and may have important implications for the immunogenicity of Tn-based vaccines.
In addition, we have previously shown that MAG-Tn3 binds to the macrophage galactose-type lectin (MGL) receptor, which is expressed by dermal DCs and is thus captured very efficiently by these antigen-presenting cells, leading to the induction of anti-Tn antibody responses, even in the absence of adjuvant [32]. Finally, this high antibody response could also be explained by the formulation of the MAG-Tn3 vaccine with the AS15 immunostimulant. Initially, alum was used to demonstrate the efficacy of the MAG-Tn3 vaccine to reduce the tumor burden in the TA3/Ha mouse model [25]. However, further studies in non-human primates demonstrated that formulation with AS15 improved the immunogenicity of the MAG-Tn3 vaccine [23]. In addition to strongly enhancing innate and adaptive responses in experimental models, AS15 has been safely used in large-scale clinical trials [33].
Another key factor leading to the induction of a strong anti-Tn antibody response is the activation of CD4+ helper T cells, which has been found to be required for inducing the anti-Tn IgG response [22, 23]. The promiscuous nature of the tetanus toxin-derived peptide was demonstrated 30 years ago [27] and this peptide has been used with success in mice as a carrier protein in various contexts [34] and has been shown to enhance the cytotoxic activity of antibodies against tumor cells [35]. We have also previously demonstrated that this TT peptide induced the activation of CD4+ T cells and the development of anti-Tn IgG responses in various models, including inbred DR1*A2 and C3H/HeN mice, outbred NMRI and CD-1 mice, and non-human primates [23].
Here, we observed significant IFNγ production by the PBMCs from six of seven patients following in vitro stimulation by the TT830–844 peptide. Similar results were obtained for the production of IL-2, with the exception of patient 05, for whom a very high background was observed at all timepoints. A memory response against the TT830–844 peptide could explain such spontaneous IFNγ production, as vaccination against tetanus is mandatory in France. This property could increase the immunogenicity of the vaccine. Surprisingly, we observed no CD4+ T cell activation in patient 04, in spite of the expression of MHC-II alleles capable of binding this TT epitope. Conversely, patient 03 did not express MHC-II alleles capable of binding to the TT peptide but developed a CD4+ T-cell response, shown by the production of IFNγ. These discrepancies can be explained by the method used to determine the capacity of the TT peptide to bind to HLA-DRB molecules. In our previous study, we used the MHC-class II ProImmune REVEAL assay, which relies on detection of the native conformation of the MHC-peptide complex by a labeled antibody, allowing the detection of low-affinity HLA-peptide binding [23]. Another approach, tetramer-guided epitope mapping, consisting of CD4+ T cell stimulation with peptides followed by staining with the corresponding tetramers, has been found to be more stringent and, in this case, the TT830–844 peptide failed to recognize low-affinity HLA alleles, such as DRB1*04:01 [36]. In addition, James et al. [36] reported heterogeneity among patients with identical HLA haplotypes, suggesting that other parameters may interfere with the presentation of the HLA-DRB molecule. Finally, we cannot exclude that TT830–844 specific CD4+ T cells were activated in patient 04 but did not produce detectable amounts of IFNγ or IL-2. Activated polyfunctional CD4+ T cell populations can indeed harbor a very diverse profile, and are thus able to produce a very large set of cytokines (TNFα, T-bet, etc.) [37].
Here, we also demonstrate that the Tn-specific antibodies induced by immunization with the MAG-Tn3/AS15 vaccine efficiently kill Tn+ Jurkat cells in the presence of complement. The ability of Tn-specific antibodies to induce CDC in vitro is an interesting feature that has also been observed in preclinical studies and humans following immunization with the Tn-KLH vaccine [18, 23]. This is in agreement with the fact that MAG-Tn3/AS15 induced mainly IgG3 anti-Tn antibodies, an immunoglobulin subclass known to strongly activate the classical complement pathway [38]. IgG3 antibodies can also strongly bind to the FcγRIIIa receptor, making it an important ADCC activator [39, 40]. However, we failed to detect ADCC activity of the anti-Tn antibodies induced in the patients. Several studies using vaccines targeting Tn or sTn carbohydrates have reported an ADCC response in vaccinees [22, 41]. Technical limitations, such as insufficient sensitivity of the method or insufficient amounts of anti-Tn immunoglobulins, may explain the lack of ADCC observed in our study.
Our results suggest that the anti-Tn antibodies induced by MAG-Tn3 may exert a cytotoxic effect against tumor cells in the patients that is mainly by CDC. First, complement proteins have been demonstrated to be present inside the tumor for various cancers, including breast cancer [42]. In addition, the anti-CD20 monoclonal antibody rituximab has been reported to induce an anti-tumoral effect by CDC, rather than ADCC. Indeed, the protective effect of rituximab against EL4, a lymphoma cell line, was shown to be abolished in a knockout mouse lacking C1q, but not mice lacking NK cells or neutrophils [43]. Other monoclonal antibodies, such as ofatumumab can also mediate CDC [44]. Conversely, other studies have demonstrated that the depletion of C3, a central effector of the complement cascade, can enhance ADCC activity induced by rituximab and increase its efficacy [45].
As the MAG-Tn3 vaccine was designed to trigger antibody responses, other approaches aiming to induce cytotoxic CD8+ T cells could be complementary. Indeed, CAR (chimeric antigen receptor) T cells targeting the Tn antigen have been recently found to efficiently eliminate Tn-MUC1-expressing tumors in a mouse model [46]. However, the efficiency of such strategies strongly depends on the tumor micro-environment, which affects the capacity of T cells to kill tumor cells [47]. Although CD8+ T cells can potentially exert a cytotoxic effect in highly inflamed or “hot” tumors, they fail to penetrate non-inflamed or “cold” tumors. [48]. Cancer vaccines that can turn cold into hot tumors could play a very valuable role in this context [49]. Thus, synergistic approaches that combine cancer vaccines with molecules such as anti-PD1 or anti-CTLA4, which are inefficient in cold tumors, have recently emerged [5]. The MAG-Tn3 vaccine, as an inducer of potent and tumor-specific antibodies, may be particularly well suited to complete a multifaceted strategy combining a tumor-specific T-cell inducer and immune-checkpoint inhibitor, aiming to induce both humoral and cellular compartments of the immune response.
Overall, we have shown that a fully characterized and synthetic glycopeptide vaccine can elicit strong antibody responses in humans against the Tn antigen, which is expressed by several adenocarcinomas, but not normal cells. This vaccine can be synthesized at a large scale using good manufacturing procedures. This breakthrough represents an important steppingstone in the development of multimeric synthetic vaccines involving glycopeptide antigens.
Data and materials availability
All the data for this study are present in the main text or in the Supplementary Materials.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Jamila Louahed (GSK Vaccines) for her continuous support, helpful discussions and critical review of the publication and the clinical protocol. We thank the CB UTechS platform and the molecular biophysics facility (especially Sylviane Hoos) for their support and help with the various devices and Luisa Nardini, Natalia Zmarlak, Ferdinand Nanfack Minkeu, and Inge Holm for sharing equipment. We thank Eleonore De Guillebon from the Hôpital Européen Georges Pompidou and Thomas Bachelot from the Centre Hospitalier Léon Bérard for their valuable support in the organization of this clinical trial. We thank the hospital staff for their daily work on the project and the patients for their agreement to participate in the trial.
Author contributions
CL conceived and supervised the study, discussed the data, and wrote the manuscript. PR designed and performed the experiments analyzed the results and wrote the manuscript. CA coordinated the clinical trial, participated in the collection of the clinical data, and provided input for the manuscript. SB developed the process of vaccine synthesis and provided input for the manuscript. CG prepared the Tn3 antigen used in the ELISA. MC (investigator coordinator), SD, JM, CG, DL, FP, M-PS, OT, and AV designed and performed oversight of the clinical trial.
Funding
The work was funded by the donors of the MAG-Tn3 program. GlaxoSmithKline Biological SA provided AS15 and preparing the final formulation of the MAG/AS15 vaccine.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Garg AD, Coulie PG, Van den Eynde BJ, Agostinis P. Integrating next-generation dendritic cell vaccines into the current cancer immunotherapy landscape. Trends Immunol. 2017;38:577–593. doi: 10.1016/j.it.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed MS, Bae YS. Dendritic cell-based therapeutic cancer vaccines: past, present and future. Clin Exp Vaccine Res. 2014;3:113–116. doi: 10.7774/cevr.2014.3.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 4.Vermaelen K. Vaccine strategies to improve anti-cancer cellular immune responses. Front Immunol. 2019;10:8. doi: 10.3389/fimmu.2019.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dillman RO. Is there a role for therapeutic cancer vaccines in the age of checkpoint inhibitors? Hum Vaccines Immunother. 2017;13:528–532. doi: 10.1080/21645515.2016.1244149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilgenhof S, Corthals J, Heirman C, van Baren N, Lucas S, Kvistborg P, Thielemans K, Neyns B. Phase II study of autologous monocyte-derived mRNA electroporated dendritic cells (TriMixDC-MEL) plus ipilimumab in patients with pretreated advanced melanoma. J Clin Oncol. 2016;34:1330–1338. doi: 10.1200/JCO.2015.63.4121. [DOI] [PubMed] [Google Scholar]
- 7.Gibney GT, Kudchadkar RR, DeConti RC, et al. Safety, correlative markers, and clinical results of adjuvant nivolumab in combination with vaccine in resected high-risk metastatic melanoma. Clin Cancer Res. 2015;21:712–720. doi: 10.1158/1078-0432.CCR-14-2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ott PA, Wu CJ. Cancer vaccines: steering T cells down the right path to eradicate tumors. Cancer Discov. 2019 doi: 10.1158/2159-8290.cd-18-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hollingsworth RE, Jansen K. Turning the corner on therapeutic cancer vaccines. NPJ Vaccines. 2019;4:7. doi: 10.1038/s41541-019-0103-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu C, Zhao H, Wang Y, Cai H, Xiao Y, Zeng Y, Chen H. Tumor-associated antigens: Tn antigen, sTn antigen, and T antigen. HLA. 2016;88:275–286. doi: 10.1111/tan.12900. [DOI] [PubMed] [Google Scholar]
- 11.Cheever MA, Allison JP, Ferris AS, et al. The prioritization of cancer antigens: a National Cancer Institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15:5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mazal D, Lo-Man R, Bay S, et al. Monoclonal antibodies toward different Tn-amino acid backbones display distinct recognition patterns on human cancer cells. Implications for effective immuno-targeting of cancer. Cancer Immunol Immunother. 2013;62:1107–1122. doi: 10.1007/s00262-013-1425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desai PR. Immunoreactive T and Tn antigens in malignancy: role in carcinoma diagnosis, prognosis, and immunotherapy. Transfus Med Rev. 2000;14:312–325. doi: 10.1053/tmrv.2000.16229. [DOI] [PubMed] [Google Scholar]
- 14.Springer GF. T and Tn, general carcinoma autoantigens. Science. 1984;224:1198–1206. doi: 10.1126/science.6729450. [DOI] [PubMed] [Google Scholar]
- 15.Springer GF. Immunoreactive T and Tn epitopes in cancer diagnosis, prognosis, and immunotherapy. J Mol Med. 1997;75:594–602. doi: 10.1007/s001090050144. [DOI] [PubMed] [Google Scholar]
- 16.Radhakrishnan P, Dabelsteen S, Madsen FB, et al. Immature truncated O-glycophenotype of cancer directly induces oncogenic features. Proc Natl Acad Sci USA. 2014;111:E4066–E4075. doi: 10.1073/pnas.1406619111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loureiro LR, Carrascal MA, Barbas A, Ramalho JS, Novo C, Delannoy P, Videira PA. Challenges in antibody development against Tn and sialyl-Tn antigens. Biomolecules. 2015;5:1783–1809. doi: 10.3390/biom5031783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slovin SF, Ragupathi G, Musselli C, et al. Fully synthetic carbohydrate-based vaccines in biochemically relapsed prostate cancer: clinical trial results with alpha-N-acetylgalactosamine-O-serine/threonine conjugate vaccine. J Clin Oncol. 2003;21:2489–4292. doi: 10.1200/JCO.2003.04.112. [DOI] [PubMed] [Google Scholar]
- 19.Scheid E, Major P, Bergeron A, et al. Tn-MUC1 DC vaccination of rhesus macaques and a phase I/II trial in patients with nonmetastatic castrate-resistant prostate cancer. Cancer Immunol Res. 2016;4:881–892. doi: 10.1158/2326-6066.CIR-15-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skwarczynski M, Toth I. Peptide-based synthetic vaccines. Chem Sci. 2016;7:842–854. doi: 10.1039/c5sc03892h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ganneau C, Simenel C, Emptas E, et al. Large-scale synthesis and structural analysis of a synthetic glycopeptide dendrimer as an anti-cancer vaccine candidate. Org Biomol Chem. 2017 doi: 10.1039/c6ob01931e. [DOI] [PubMed] [Google Scholar]
- 22.Lo-Man R, Vichier-Guerre S, Perraut R, et al. A fully synthetic therapeutic vaccine candidate targeting carcinoma-associated Tn carbohydrate antigen induces tumor-specific antibodies in nonhuman primates. Cancer Res. 2004;64:4987–4994. doi: 10.1158/0008-5472.CAN-04-0252. [DOI] [PubMed] [Google Scholar]
- 23.Laubreton D, Bay S, Sedlik C, et al. The fully synthetic MAG-Tn3 therapeutic vaccine containing the tetanus toxoid-derived TT830–844 universal epitope provides anti-tumor immunity. Cancer Immunol Immunother. 2016;65:315–325. doi: 10.1007/s00262-016-1802-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dreno B, Thompson JF, Smithers BM, et al. MAGE-A3 immunotherapeutic as adjuvant therapy for patients with resected, MAGE-A3-positive, stage III melanoma (DERMA): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2018;19:916–929. doi: 10.1016/s1470-2045(18)30254-7. [DOI] [PubMed] [Google Scholar]
- 25.Lo-Man R, Vichier-Guerre S, Bay S, Dériaud E, Cantacuzène D, Leclerc C. Anti-tumor immunity provided by a synthetic multiple antigenic glycopeptide displaying a tri-Tn glycotope. J Immunol. 2001;166:2849–2854. doi: 10.4049/jimmunol.166.4.2849. [DOI] [PubMed] [Google Scholar]
- 26.Bay S, Lo-Man R, Osinaga E, Nakada H, Leclerc C, Cantacuzène D. Preparation of a multiple antigen glycopeptide (MAG) carrying the Tn antigen. A possible approach to a synthetic carbohydrate vaccine. J Pept Res. 1997;49:620–625. doi: 10.1111/j.1399-3011.1997.tb01171.x. [DOI] [PubMed] [Google Scholar]
- 27.Panina-Bordignon P, Tan A, Termijtelen A, Demotz S, Corradin G, Lanzavecchia A. Universally immunogenic T cell epitopes: promiscuous binding to human MHC class II and promiscuous recognition by T cells. Eur J Immunol. 1989;19:2237–2242. doi: 10.1002/eji.1830191209. [DOI] [PubMed] [Google Scholar]
- 28.Nakada H, Inoue M, Tanaka N, Numata Y, Kitagawa H, Fukui S, Yamashina I. Expression of the Tn antigen on T-lymphoid cell line Jurkat. Biochem Biophys Res Commun. 1991;179:762–767. doi: 10.1016/0006-291X(91)91882-D. [DOI] [PubMed] [Google Scholar]
- 29.Tarp MA, Clausen H. Mucin-type O-glycosylation and its potential use in drug and vaccine development. Biochim Biophys Acta. 2008;1780:546–563. doi: 10.1016/j.bbagen.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 30.Coelho H, Matsushita T, Artigas G, et al. The quest for anticancer vaccines: deciphering the fine-epitope specificity of cancer-related monoclonal antibodies by combining microarray screening and saturation transfer difference NMR. J Am Chem Soc. 2015;137:12438–12441. doi: 10.1021/jacs.5b06787. [DOI] [PubMed] [Google Scholar]
- 31.Slovin SF, Ragupathi G, Fernandez C, et al. A polyvalent vaccine for high-risk prostate patients: “are more antigens better?”. Cancer Immunol Immunother. 2007;56:1921–1930. doi: 10.1007/s00262-007-0335-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freire T, Zhang X, Dériaud E, et al. Glycosidic Tn-based vaccines targeting dermal dendritic cells favor germinal center B-cell development and potent antibody response in the absence of adjuvant. Blood. 2010;116:3526–3536. doi: 10.1182/blood-2010-04-279133. [DOI] [PubMed] [Google Scholar]
- 33.Kruit WH, Suciu S, Dreno B, et al. Selection of immunostimulant AS15 for active immunization with MAGE-A3 protein: results of a randomized phase II study of the European Organisation for Research and Treatment of Cancer Melanoma Group in Metastatic Melanoma. J Clin Oncol. 2013;31:2413–2420. doi: 10.1200/JCO.2012.43.7111. [DOI] [PubMed] [Google Scholar]
- 34.Bouche FB, Steinmetz A, Yanagi Y, Muller CP. Induction of broadly neutralizing antibodies against measles virus mutants using a polyepitope vaccine strategy. Vaccine. 2005;23:2074–2077. doi: 10.1016/j.vaccine.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 35.Yu Z, Healy F, Valmori D, Escobar P, Corradin G, Mach JP. Peptide-antibody conjugates for tumour therapy: a MHC-class-II-restricted tetanus toxin peptide coupled to an anti-Ig light chain antibody can induce cytotoxic lysis of a human B-cell lymphoma by specific CD4 T cells. Int J Cancer. 1994;56:244–248. doi: 10.1002/ijc.2910560217. [DOI] [PubMed] [Google Scholar]
- 36.James EA, Bui J, Berger D, Huston L, Roti M, Kwok WW. Tetramer-guided epitope mapping reveals broad, individualized repertoires of tetanus toxin-specific CD4+ T cells and suggests HLA-based differences in epitope recognition. Int Immunol. 2007;19:1291–1301. doi: 10.1093/intimm/dxm099. [DOI] [PubMed] [Google Scholar]
- 37.Deng N, Weaver JM, Mosmann TR. Cytokine diversity in the Th1-dominated human anti-influenza response caused by variable cytokine expression by Th1 cells, and a minor population of uncommitted IL-2+IFNgamma-Thpp cells. PLoS ONE. 2014;9:e95986. doi: 10.1371/journal.pone.0095986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mamidi S, Höne S, Kirschfink M. The complement system in cancer: ambivalence between tumour destruction and promotion. Immunobiology. 2017;222:45–54. doi: 10.1016/j.imbio.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 39.Irani V, Guy AJ, Andrew D, Beeson JG, Ramsland PA, Richards JS. Molecular properties of human IgG subclasses and their implications for designing therapeutic monoclonal antibodies against infectious diseases. Mol Immunol. 2015;67:171–182. doi: 10.1016/j.molimm.2015.03.255. [DOI] [PubMed] [Google Scholar]
- 40.Bruhns P, Iannascoli B, England P, Mancardi DA, Fernandez N, Jorieux S, Daeron M. Specificity and affinity of human Fc receptors and their polymorphic variants for human IgG subclasses. Blood. 2009;113:3716–3725. doi: 10.1182/blood-2008-09-179754. [DOI] [PubMed] [Google Scholar]
- 41.Holmberg LA, Sandmaier BM. Vaccination with Theratope (STn-KLH) as treatment for breast cancer. Expert Rev Vaccines. 2004;3:655–663. doi: 10.1586/14760584.3.6.655. [DOI] [PubMed] [Google Scholar]
- 42.Afshar-Kharghan V. The role of the complement system in cancer. J Clin Investig. 2017;127:780–789. doi: 10.1172/JCI90962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Di Gaetano N, Cittera E, Nota R, Vecchi A, Grieco V, Scanziani E, Botto M, Introna M, Golay J. Complement activation determines the therapeutic activity of rituximab in vivo. J Immunol. 2014;171:1581–1587. doi: 10.4049/jimmunol.171.3.1581. [DOI] [PubMed] [Google Scholar]
- 44.Bologna L, Gotti E, Da Roit F, Intermesoli T, Rambaldi A, Introna M, Golay J. Ofatumumab is more efficient than rituximab in lysing B chronic lymphocytic leukemia cells in whole blood and in combination with chemotherapy. J Immunol. 2012;190:231–239. doi: 10.4049/jimmunol.1202645. [DOI] [PubMed] [Google Scholar]
- 45.Wang SY, Veeramani S, Racila E, Cagley J, Fritzinger DC, Vogel CW, St John W, Weiner GJ. Depletion of the C3 component of complement enhances the ability of rituximab-coated target cells to activate human NK cells and improves the efficacy of monoclonal antibody therapy in an in vivo model. Blood. 2009;114:5322–5330. doi: 10.1182/blood-2009-01-200469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Posey AD, Schwab RD, Boesteanu AC, et al. Engineered CAR T cells targeting the cancer-associated Tn-glycoform of the membrane mucin MUC1 control adenocarcinoma. Immunity. 2016;44:1444–1454. doi: 10.1016/j.immuni.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science. 2015;348:74–80. doi: 10.1126/science.aaa6204. [DOI] [PubMed] [Google Scholar]
- 48.van der Woude LL, Gorris MAJ, Halilovic A, Figdor CG, de Vries IJM. Migrating into the tumor: a roadmap for T cells. Trends Cancer. 2017;3:797–808. doi: 10.1016/j.trecan.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 49.Karyampudi L, Lamichhane P, Scheid AD, Kalli KR, Shreeder B, Krempski JW, Behrens MD, Knutson KL. Accumulation of memory precursor CD8 T cells in regressing tumors following combination therapy with vaccine and anti-PD-1 antibody. Cancer Res. 2014;74:2974–2985. doi: 10.1158/0008-5472.CAN-13-2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.