Abstract
Immune checkpoint inhibitors (ICIs) represent a major breakthrough for cancer treatment. However, evidence regarding the use of ICIs in pancreatic cancer (PC) remained scarce. To assess the efficacy and safety of ICIs plus chemotherapy, patients with advanced PC were retrospectively recruited and were treated with either chemotherapy alone or chemotherapy plus ICIs. Patients previously treated with any agents targeting T-cell co-stimulation or checkpoint pathways were excluded. The primary outcome was overall survival (OS). The secondary outcomes were progression-free survival (PFS), overall response rate (ORR) and safety. In total, 58 patients were included (combination, n = 22; chemotherapy, n = 36). The combination group showed a significantly longer OS than the chemotherapy group [median, 18.1 vs 6.1 months, hazard ratio (HR) 0.46 (0.23–0.90), P = 0.021]. The median PFSs were 3.2 months in the combination group and 2.0 months in the chemotherapy group [HR 0.57 (0.32–0.99), P = 0.041]. The combination group and the chemotherapy group had similar ORRs (18.2% vs 19.4%, P = 0.906). All patients who achieved a partial response received a doublet chemotherapy regimen regardless of co-treatment with ICIs. Grade 3 or higher adverse events occurred in 31.8% of the patients in the combination group and in 16.9% of those receiving chemotherapy. Although the incidence of serious treatment-related adverse events was higher in the combination group than in the chemotherapy group, the difference was not significant (P = 0.183). Our findings suggest that the combination of ICIs with chemotherapy is both effective and tolerable for advanced PC. ICIs combined with a doublet chemotherapy regimen might be a preferable choice.
Electronic supplementary material
The online version of this article (10.1007/s00262-019-02452-3) contains supplementary material, which is available to authorized users.
Keywords: Pancreatic cancer, Immune checkpoint inhibitors, Combination therapy, Efficacy, Safety
Introduction
Pancreatic cancer (PC), a highly lethal disease as reflected by its high incidence and mortality, is usually asymptomatic and most patients are diagnosed with advanced disease [2–4]. Surgery provides the only potential possibility for a cure, however, only less than 20% of patients are eligible for resection, and most patients still eventually recur [3]. For advanced PC, gemcitabine or two combination therapies [gemcitabine with nab-paclitaxel and fluorouracil/leucovorin plus irinotecan plus oxaliplatin (FOLFIRINOX)] have been widely acknowledged as the standard systemic therapies since 2010 [5–7]. However, published data indicated that the clinical benefit of chemotherapy was still far from ideal. For example, among patients with advanced-stage PC, the 5-year survival rate after standard chemotherapy is only 2% [2]. Given the lack of effective treatment, considerable efforts have been devoted to improving the outcomes of patients with advanced PC.
Immune checkpoint inhibitors (ICIs) targeting programmed cell death protein 1 (PD-1) or PD-1 ligand (PD-L1) have emerged as promising treatment strategies in cancer care that lead to durable antitumor activities and improved survival in various malignancies [8–10]. However, there is limited evidence on the efficacy of ICIs in PC. The PD-1 inhibitor pembrolizumab appeared to be efficacious in a subset of PC patients who were microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) [11]. However, the prevalence of dMMR/MSI-H is low in PC, as shown by genetic profiling of 385 PC cases where a hypermutated profile (all related to dMMR) was found in only less than 2% (4/385) of the cases [12]. In addition, two phase I trials and one phase II trial demonstrated that anti-PD-1/PD-L1 monotherapy failed to elicit any response in unselected advanced PC patients [13–15]. Hence, overall, except for MSI-H tumors, PCs are considered resistant to single-agent immunotherapy. Based on published research, there are three major hurdles to overcome before immunotherapy could be widely applied in PC treatment: a low level of mutational load [16], a largely immunosuppressive microenvironment [17], and few infiltrating T cells [18]. Thus, the incorporation of additional therapies that can convert the “cold” microenvironment to a “hot” one becomes a key strategy to enhance the clinical activity of immunotherapy.
Chemotherapy may promote the release of tumor neoantigens by inducing tumor cell death, which in turn triggers an anticancer immune response [19]. Immunotherapy administered alongside chemotherapy is predicted to synergistically enhance the antitumor effects of either therapy alone [20]. For advanced PC, ICIs combined with chemotherapy have been investigated. In a phase II study, pembrolizumab plus doublet chemotherapy in metastatic PC achieved an overall response rate (ORR) of 20.0% (3/15) and a disease control rate (DCR) of 86.7% (13/15) [21]. Another study presented at the 2018 American Society of Clinical Oncology-gastrointestinal cancer (ASCO-GI) meeting showed that nivolumab combined with three chemotherapeutic drugs produced a DCR of 100% and an ORR of 80% [22]. However, these two studies were both single armed. A prospective cohort study of two ICIs (PD-1 inhibitor and CTLA-4 inhibitor) plus chemotherapy compared with chemotherapy alone showed that combination therapy was both effective and tolerable [23]. Although this strategy significantly improved the therapeutic effect, it was unclear which drug played a major role since two different ICIs were utilized. Moreover, such a complex treatment regimen might also increase cost and toxicity.
Herein, we evaluated the efficacy and safety of ICIs plus chemotherapy compared with chemotherapy alone for advanced PC. This study may improve our understanding of the effects of immunotherapy combined with chemotherapy on PC.
Patients and methods
Participants and study design
Patients with unresectable advanced PC having been treated with at least one cycle of anti-PD-1/PD-L1 combination therapy or chemotherapy between June 2015 and May 2018 at the People’s Liberation Army General Hospital (Beijing) were retrospectively included. To ensure data quality, the protocol, case report form (CRF), and standard operating procedure (SOP) of data collection were prospectively designed before the launch of this study.
Patients were identified via electronic medical records based on the following eligibility criteria: (1) biopsy confirmed metastatic PC and (2) received at least one cycle of ICIs plus chemotherapy or chemotherapy. Patients who had been previously exposed to any agent targeting T-cell co-stimulation or immune checkpoints were excluded from this study.
Data collection and study objectives
Two investigators independently extracted and verified information on the clinico-pathological characteristics and treatment histories of the patients. All imaging data were independently assessed by two physicians. Any inconsistent evaluation results were further determined by the director of the imaging center. The data were last edited on Oct 30, 2018.
Overall survival (OS) was the primary outcome and was defined as the time from the treatment initiation to death for any reason. The secondary outcomes included progression-free survival (PFS), ORR, DCR and safety (treatment-related adverse events). PFS was defined as the time from the treatment initiation to disease progression or death by any cause. ORR [the percentage of patients with a confirmed complete/partial response (CR/PR)] and DCR [the proportion of patients with CR, PR, or stable disease (SD)] were assessed according to the RECIST criteria [24]. Data on adverse events were collected according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0 [25]. Patients who were alive and did not experience any of these events were censored on the date of the last follow-up. The study was reported according to the Transparent Reporting of Evaluations with Nonrandomized Designs (TREND) [26].
Statistical analysis
Patients who did not complete the first cycle of treatment were replaced in the final study outputs. The baseline characteristics and response data between the two groups were compared using the Chi-square test or Fisher’s exact test for categorical variables and the Mann–Whitney U test for continuous or ordinal variables. OS and PFS were analyzed using the Kaplan–Meier method with a P value determined by the log-rank test. Hazard ratios (HR) were estimated using Cox proportional hazards regression. Two-sided P values were evaluated and P < 0.05 was considered statistically significant. All statistical analyses were performed with SPSS statistical software (version 20.0, IBM Corporation, USA) and GraphPad Prism (version 6, GraphPad Software Inc., USA).
Results
Patient and tumor characteristics
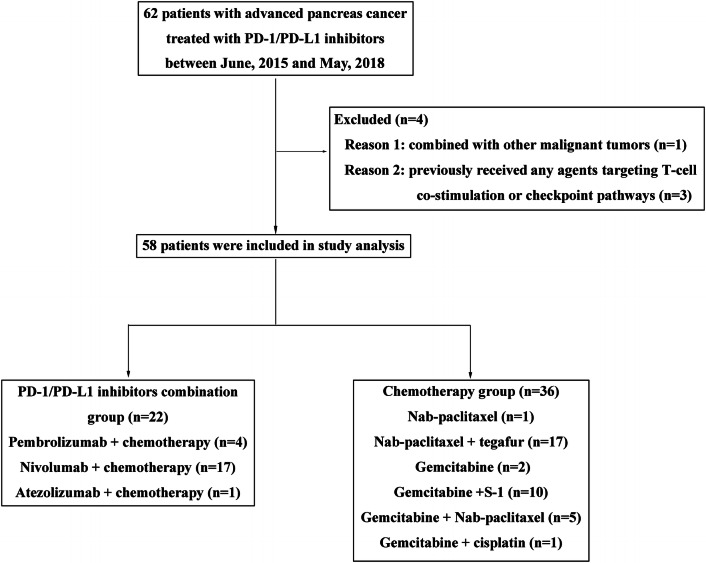
A total of 58 patients with metastatic PC were enrolled in this study, 36 patients treated with chemotherapy (chemotherapy group) and 22 patients treated with a combination of ICIs and chemotherapy (combination group) (Fig. 1). In the chemotherapy group, the most common regimen was nab-paclitaxel plus tegafur (47.2%, 17/36), and only three patients received a single chemotherapeutic drug. In the combination group, 17 patients were treated with nivolumab plus chemotherapy, 4 with pembrolizumab plus chemotherapy, and 1 with atezolizumab plus chemotherapy. The treatment strategy for each individual is shown in Supplementary Table S1. The patients’ baseline characteristics are shown in Table 1, and most demographics and disease characteristics were in general well balanced between the two groups. Both groups had a higher percentage of males than females. The majority of patients never smoked or consumed alcohol, and most of the patients had an ECOG performance status (PS) of 0–2. The liver and lymph nodes were the most common sites of metastases. Approximately one-third of the patients in both groups had undergone surgery. In addition, treatment-naive patients were more common in the chemotherapy group than in the combination group (97.7% vs 72.3%, P = 0.016).
Fig. 1.
Flow diagram of the study
Table 1.
Baseline characteristics
| Characteristics | Combination group (n = 22) | Chemotherapy group (n = 36) | P value |
|---|---|---|---|
| Median age, years (range) | 56.0 (34–73) | 54.0 (30–77) | 0.573 |
| Sex, n (%) | 0.955 | ||
| Male | 13 (59.1%) | 21 (58.3%) | |
| Female | 9 (40.9%) | 15 (41.7%) | |
| Alcohol history, n (%) | 0.967 | ||
| Former or current | 6 (27.3%) | 10 (27.8%) | |
| Never or unknown | 16 (72.7%) | 26 (72.2%) | |
| Pancreatic tumor location, n (%) | 0.484 | ||
| Head | 12 (54.5%) | 23 (63.0%) | |
| Body or tail | 10 (45.5%) | 13 (36.1%) | |
| Smoking history, n (%) | 0.673 | ||
| Former or current | 5 (22.7%) | 10 (27.8%) | |
| Never or unknown | 17 (77.3%) | 26 (72.2%) | |
| ECOG performance status, n (%) | 0.261 | ||
| 0–2 | 21 (95.5%) | 31 (86.1%) | |
| > 2 | 1 (4.5%) | 5 (13.9%) | |
| Previous surgery, n (%) | 0.092 | ||
| Yes | 8 (36.4%) | 6 (16.7%) | |
| No | 14 (63.6%) | 30 (83.3%) | |
| Number of prior lines of treatment for metastatic disease, n (%) | 0.016 | ||
| 0 | 17 (72.3%) | 35 (97.2%) | |
| ≥ 1 | 5 (22.7%) | 1 (2.8%) | |
| Site of metastases, n (%) | |||
| Liver | 20 (90.9%) | 29 (80.6%) | 0.295 |
| Lymph node | 20 (90.9%) | 31 (86.1%) | 0.590 |
| Lung | 5 (22.7%) | 4 (11.1%) | 0.240 |
| Peritoneal | 4 (18.2%) | 6 (16.7%) | 0.883 |
| Other | 5 (22.7%) | 8 (22.2%) | 0.965 |
| Number of metastases | 0.484 | ||
| 0–2 | 12 (54.5%) | 23 (63.9%) | |
| ≥ 3 | 10 (45.5%) | 13 (36.1%) |
ECOG Eastern Cooperative Oncology Group
Efficacy
The median follow-up for all patients after the commencement of study treatment was 6.9 months (range 2.0–29.1 months). In total, 56 (96.6%) patients experienced disease progression and 48 (82.6%) patients died. The median OS (mOS) was 18.1 months (95% CI 4.0–32.2) in the combination group and 6.1 months (95% CI 5.0–7.3) in the chemotherapy group (HR 0.46, 95% CI 0.23–0.90, P = 0.021, Fig. 2a). The median PFS (mPFS) was 3.2 months (95% CI 2.0–4.5) in the combination group compared with 2.0 months (95% CI 1.9–2.0) in the chemotherapy group with an HR of 0.57 (95% CI 0.32–0.99) (P = 0.041, Fig. 2b). OS and PFS analyses of subgroups stratified according to baseline demographics and disease characteristics showed that most subgroups obtained greater clinical benefits from ICIs plus chemotherapy than from chemotherapy alone (Supplementary Figure S1). In particular, patients with no prior surgical treatment or fewer metastases presented with a significantly longer mOS with combination treatment, and patients with liver metastases also had a significantly longer mPFS when treated with ICIs plus chemotherapy rather than chemotherapy alone.
Fig. 2.
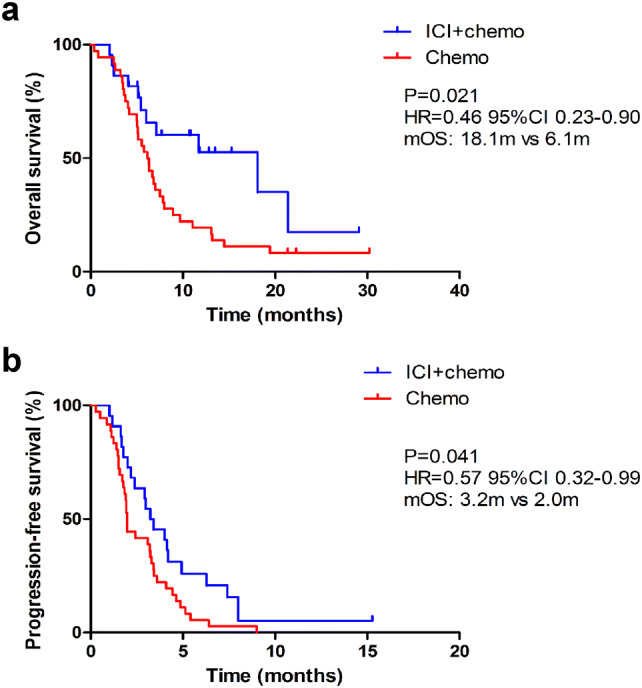
Kaplan–Meier estimates of overall survival (a) and progression-free survival (b) comparing chemotherapy alone and chemotherapy combined with immune checkpoint inhibitors (ICIs). mOS median overall survival, HR hazard ratio, ICI + chemo the group of patients receiving immune checkpoint inhibitors and chemotherapy, Chemo the group of patients receiving chemotherapy
As of data cut-off, the ORRs were similar between the combination group and the chemotherapy group (18.2% vs 19.4%), and the difference was not statistically significant (P = 0.906, Table 2 and Fig. 3). In addition, 59.1% (13/22) of the patients who received ICIs plus chemotherapy achieved disease control while the DCR for the chemotherapy group was 58.3% (21/36) (P = 0.955). The median change from baseline was 10% (range − 86 to 68%) for the combination immunotherapy group and 13% (range − 83 to 70%) for the chemotherapy group.
Table 2.
Tumor response to treatment for the overall population
| Combination group (n = 22) | Chemotherapy group (n = 36) | P value | |
|---|---|---|---|
| Objective response, n (%; 95% CI) | 4 (18.2%; 6.5–36.9) | 7 (19.4%; 9.5–33.5) | 0.906 |
| Disease control rate, n (%; 95% CI) | 13 (59.1%; 39.5–76.7) | 21 (58.3%; 67.0–88.9) | 0.955 |
| Best overall response, n (%) | |||
| Complete response | 0 | 0 | |
| Partial response | 4 (18.2) | 7 (19.4%) | |
| Stable disease | 9 (40.9%) | 14 (38.9%) | |
| Progressive disease | 9 (40.9%) | 15 (41.7%) |
Fig. 3.
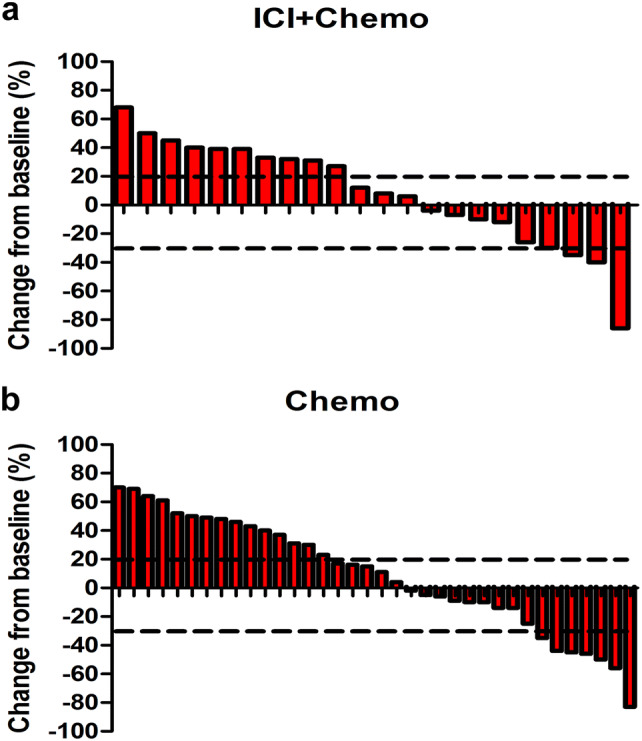
Waterfall plots of the best percentage change. a The best percentage change in tumor size from baseline for individual patients in the combination immunotherapy group. b The best percentage change in tumor size from baseline for individual patients in the chemotherapy group. ICI + chemo the group of patients receiving immune checkpoint inhibitors and chemotherapy, Chemo the group of patients receiving chemotherapy
Safety
All the treatment-related adverse events (TRAEs) are shown in Table 3. Most TRAEs were grades 1–2 with a predominance of neutropenia (6 out of 13 patients on ICIs plus chemotherapy and 19 out of 36 patients on chemotherapy). The incidence of grade 3–4 TRAEs was higher in the combination group (31.8%) than in the chemotherapy group (16.7%), but the difference was not statistically significant (P = 0.183). In the combination group, the most common serious TRAEs were neutropenia (3/22, 13.6%), thrombocytopenia (2/22, 9.1%), and nausea (1/22, 4.5%) and the chemotherapy group followed a similar pattern with 8.3% (3/36) neutropenia, 5.6% (2/36) thrombocytopenia, and 2.8% (1/36) nausea. No autoimmune events or drug-related deaths occurred in either group.
Table 3.
Treatment-related adverse events
| Combination group (n = 22) | Chemotherapy group (n = 36) | |||
|---|---|---|---|---|
| Grade 1–4 | Grade 3–4 | Grade 1–4 | Grade 3–4 | |
| Any terma | 19 (86.4%) | 7 (31.8%) | 30 (83.3%) | 6 (16.7%) |
| Nausea | 6 (27.3%) | 1 (4.5%) | 15 (41.7%) | 1 (2.8%) |
| Diarrhea | 1 (4.5%) | 0 | 0 | 0 |
| Fever | 1 (4.5%) | 0 | 0 | 0 |
| Fatigue | 1 (4.5%) | 0 | 0 | 0 |
| Anemia | 1 (4.5%) | 0 | 4 (11.1%) | 0 |
| Creatinine | 0 | 0 | 2 (5.6%) | 0 |
| Skin rash | 0 | 0 | 1 (2.7%) | 0 |
| Neurotoxicity | 0 | 0 | 8 (22.2%) | 1 (2.8%) |
| Pulmonitis | 1 (4.5%) | 0 | 0 | 0 |
| AST elevation | 2 (4.5%) | 0 | 0 | 0 |
| ALT elevation | 0 | 0 | 0 | 0 |
| Thrombocytopenia | 5 (22.7%) | 2 (9.1%) | 3 (8.3%) | 2 (5.6%) |
| Leukopenia | 1 (9.1%) | 0 | 0 | 0 |
| Neutropenia | 9 (40.9%) | 3 (13.6%) | 22 (61.1%) | 3 (8.3%) |
AST aspartate aminotransferase, ALT alanine aminotransferase
aListed are all adverse events that occurred during the whole treatment process regardless of attribution to any treatment regimens
Discussion
This retrospective study evaluated the efficacy and safety of chemotherapy alone or in combination with ICIs in PC patients who were treatment-naive or had progressed on prior chemotherapy. Our study demonstrated in a cohort of 58 such patients that adding ICIs to conventional chemotherapy resulted in an approximately 50% reduction in the risk of death. Although ICIs combined with chemotherapy was not associated with a higher ORR or DCR, the mOS and mPFS were significantly prolonged. The trend of greater survival benefits associated with the ICI combination was also observed in all subgroups. Significantly improved survival was observed in the subgroups with no previous surgical treatment or fewer metastatic sites. In addition, ICIs plus chemotherapy was tolerable without unexpected toxicity. Collectively, this retrospective study showed that introducing immunotherapy into conventional chemotherapy may have a favorable effect on patients’ outcomes.
In both groups, the patients who achieved PR were all treated with a doublet chemotherapy regimen rather than single-agent chemotherapy regimen, whether combined with ICIs or not. In previous research, gemcitabine-based chemotherapy or targeted therapy has generally not demonstrated a significant survival benefit over gemcitabine monotherapy [27]. It was not until a decade ago that accumulating evidence started to show that combination chemotherapy regimens, e.g., nab-paclitaxel plus gemcitabine [6] or FOLFIRINOX [7], were associated with a survival advantage. We acknowledge the fact that the chemotherapy regimens adopted in our study were diverse, e.g., the proportion of tegafur or S-1 was relatively higher in the chemotherapy group than in the combination group. S-1 is an oral drug composed of tegafur, gimestat, and oteracil potassium [28], and S-1 monotherapy has been shown to be less toxic and well tolerable in PC [29]. The GEST study indicated that S-1 was non-inferior to gemcitabine and that adding S-1 did not significantly improve the survival time compared to gemcitabine alone in PC patients [29]. A phase II trial of adjuvant chemotherapy revealed that tegafur/uracil and gemcitabine provided similar efficacy to gemcitabine alone in resected PC patients [30]. A meta-analysis evaluating 12 different chemotherapeutic regimens revealed that except for FOLFIRINOX or gemcitabine monotherapy, the rest of the chemotherapy regimens, such as gemcitabine combined with S-1 (tegafur) or nab-paclitaxel, have similar efficacies [31]. Thus, it is unlikely that the heterogeneous chemotherapy regimens have significantly affected patients’ outcomes.
Currently, some oncologists believe that chemotherapy induces an immunosuppressive microenvironment. Although there is no direct evidence showing immunosuppression by chemotherapy, a previous publication indicated that the immune system could be reset by re-obtaining various immune cell subsets [32]. In addition, several observations have indicated that despite their immunosuppressive effects, some conventional chemotherapeutic agents rely on cancer cell-extrinsic molecular and cellular cascades to stimulate antitumour immunity [33]. For example, gemcitabine is one of the standard chemotherapy regimens for PC, and previous research has proven that it not only promotes the apoptosis of tumor cells to increase antigen presentation [34] but also elicits naive T-cell activation to reverse the immunosuppressive microenvironment [35]. Paclitaxel may be a particularly strong immunostimulant, because it is able to both activate CD8+ T cells and suppress immunosuppressive cells, such as regulatory T cells [36]. As the mechanisms of chemotherapeutic drugs are diverse, combining chemotherapies of different classes might maximize the clinical benefits. Thus, immunotherapy plus a doublet chemotherapy may be a preferable treatment regimen for PC.
The safety profile of ICIs combined with chemotherapy observed in this study was consistent with that seen previously for the treatment of other tumor types and advanced PC. The Keynote-189 trial showed that the incidence rates of grade 3 or higher TRAEs were similar between the pembrolizumab plus chemotherapy group and the placebo plus chemotherapy group (67.2% vs 65.8%) [37]. The results from a clinical study on pembrolizumab, gemcitabine, and nab-paclitaxel in metastatic PC indicated that grade 3 or higher events occurred in 53% of the patients and the most common adverse events were neutropenia (46.7%), thrombocytopenia (20%), hyponatremia (13.3%), AST elevation (6.7%), and ALT elevation (6.7%) [21]. In the current study, although the overall safety profile appeared to be worse with ICIs plus chemotherapy than with chemotherapy, the difference was not statistically significant and most immune-related adverse events were mild and controllable without any treatment interventions.
An important limitation of this study was the lack of patients receiving ICI monotherapy. Future studies should explore whether the addition of ICIs to chemotherapy also has greater efficacy than ICI monotherapy in these patients. In addition, although we prospectively designed the study, it was retrospective in nature. The small sample size might also have introduced selection bias and recall bias. Although these factors may have compromised the validity and reliability of the conclusions, the real-world data will still shed some light on the performance of ICIs plus chemotherapy in advanced PC and these results are worth further investigation in a prospective manner.
In conclusion, ICIs combined with chemotherapy are both efficacious and tolerable for patients with advanced PC. A doublet chemotherapy in conjunction with ICIs may be a preferable choice for those who are refractory to conventional therapy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the patients and their families.
Abbreviations
- ASCO
American Society of Clinical Oncology
- ASCO-GI
American Society of Clinical Oncology-gastrointestinal cancer
- CR
Complete response
- DCR
Disease control rate
- ECOG
Eastern Cooperative Oncology Group
- FOLFIRINOX
Fluorouracil/leucovorin plus irinotecan plus oxaliplatin
- GEST
Gemcitabine and S-1 Trial
- HR
Hazard ratio
- ICI
Immune checkpoint inhibitor
- dMMR
Mismatch repair deficient
- mOS
Median overall survival
- mPFS
Median progression-free survival
- MSI-H
Microsatellite instability-high
- ORR
Overall response rate
- PC
Pancreatic cancer
- PR
Partial response
- PS
Performance status
- SD
Stable disease
- TRAE
Treatment-related adverse event
Author contributions
Conception and design: JXM, DYS, SLC and YH. Protocol writing: JXM, DYS, JS, SLC and YH. Data collection: CH, JLW, YYQ, GYC, XYL, PFC, JZ, WSD, SXC, ZZW, XZ and ZCY. Data analysis: JS, JXM and CG. Manuscript writing: JS, JXM, DYS and JLW. Manuscript revisions: CG, YH and SLC. All authors contributed to writing and reviewing the manuscript and approved the final version of the manuscript.
Funding
This work was funded by the National Natural Science Foundation of China [81402552 to Yi Hu, 81672996 to Yi Hu].
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval and ethical standards
The study was approved by the institutional review board of the People’s Liberation Army General Hospital, Beijing, China (approval number: S2018-144-02). This clinical study was conducted in accordance with the Helsinki declaration and its later amendments.
Informed consent
Because of the retrospective nature of the study, informed consent was waived by the People’s Liberation Army General Hospital, Beijing. This paper does not contain any individual person’s data in any form.
Footnotes
Parts of the data have been published as an abstract at the 2019 American Society of Clinical Oncology (ASCO) Annual Meeting which was held during May 31–June 4, in Chicago, IL, USA [1].
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Junxun Ma and Danyang Sun are co-first authors.
Contributor Information
Shangli Cai, Email: shangli.cai@3dmedcare.com.
Yi Hu, Email: huyi_0912@126.com.
References
- 1.Sun DY, Ma JX, Wang JL, Han C, Qian YY, Chen GY, et al. Anti-PD-1 therapy combined with chemotherapy in patients with advanced pancreas cancer in a real-world clinical setting. J Clin Oncol. 2019;37(15_suppl):e14103. doi: 10.1200/JCO.2019.37.15_suppl.e14103. [DOI] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 3.Gillen S, Schuster T, Meyer Zum Büschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7:e1000267. doi: 10.1371/journal.pmed.1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Roessel S, Kasumova GG, Verheij J, Najarian RM, Maggino L, de Pastena M, International validation of the eighth edition of the American Joint Committee on Cancer (AJCC) et al. TNM staging system in patients with resected pancreatic cancer. JAMA Surg. 2018;153:183617. doi: 10.1001/jamasurg.2018.3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burris HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 6.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 8.Hammers H, Plimack ER, Infante JR, Ernstoff MS, Rini BI, McDermott DF, et al. Phase I study of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma. Ann Oncol. 2014;25(suppl):iv361–iv362. doi: 10.1093/annonc/mdu342.3. [DOI] [Google Scholar]
- 9.Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387:1909–1920. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heery CR, O’Sullivan-Coyne G, Madan RA, Cordes L, Rajan A, Rauckhorst M, et al. Avelumab for metastatic or locally advanced previously treated solid tumors (JAVELIN Solid Tumor): a phase 1a, multicohort, dose-escalation trial. Lancet Oncol. 2017;18:587–598. doi: 10.1016/S1470-2045(17)30239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Humphris JL, Patch AM, Nones K, Bailey PJ, Johns AL, McKay S, et al. Hypermutation in pancreatic cancer. Gastroenterology. 2017;152:68–74. doi: 10.1053/j.gastro.2016.09.060. [DOI] [PubMed] [Google Scholar]
- 13.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patnaik A, Kang SP, Rasco D, Papadopoulos KP, Elassaiss-Schaap J, Beeram M, et al. Phase I study of pembrolizumab (MK-3475; Anti-PD-1 Monoclonal Antibody) in patients with advanced solid tumors. Clin Cancer Res. 2015;21:4286–4293. doi: 10.1158/1078-0432.CCR-14-2607. [DOI] [PubMed] [Google Scholar]
- 15.Royal RE, Levy C, Turner K, Mathur A, Hughes M, Kammula US, et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;33:828–833. doi: 10.1097/CJI.0b013e3181eec14c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–442. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vonderheide RH, Bayne LJ. Inflammatory networks and immune surveillance of pancreatic carcinoma. Curr Opin Immunol. 2013;25:200–205. doi: 10.1016/j.coi.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winograd R, Byrne KT, Evans RA, Odorizzi PM, Meyer AR, Bajor DL, et al. Induction of T-cell immunity overcomes complete resistance to PD-1 and CTLA-4 blockade and improves survival in pancreatic carcinoma. Cancer Immunol Res. 2015;3:399–411. doi: 10.1158/2326-6066.CIR-14-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity. 2013;39:74–88. doi: 10.1016/j.immuni.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 20.Smyth MJ, Ngiow SF, Ribas A, Teng MW. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat Rev Clin Oncol. 2016;13:143–158. doi: 10.1038/nrclinonc.2015.209. [DOI] [PubMed] [Google Scholar]
- 21.Weiss GJ, Blaydorn L, Beck J, Bornemann-Kolatzki K, Urnovitz H, Schütz E, et al. Phase Ib/II study of gemcitabine, nab-paclitaxel, and pembrolizumab in metastatic pancreatic adenocarcinoma. Investig New Drugs. 2018;36:96–102. doi: 10.1007/s10637-017-0525-1. [DOI] [PubMed] [Google Scholar]
- 22.Borazanci EH, Jameson GS, Board MJ, Ramanathan RK, Korn RL, Caldwell L, et al. A phase II pilot trial of nivolumab (N) + albumin bound paclitaxel (AP) + paricalcitol (P) + cisplatin (C) + gemcitabine (G) (NAPPCG) in patients with previously untreated metastatic pancreatic ductal adenocarcinoma (PDAC) J Clin Oncol. 2018;36(4suppl):abstract358. doi: 10.1200/JCO.2018.36.4_suppl.358. [DOI] [Google Scholar]
- 23.Renouf DJ, Dhani NC, Kavan P, Jonker DJ, Wei ACC, Hsu T, et al. The Canadian Cancer Trials Group PA.7 trial: results from the safety run in of a randomized phase II study of gemcitabine (GEM) and nab-paclitaxel (Nab-P) versus GEM, nab-P, durvalumab (D), and tremelimumab (T) as first-line therapy in metastatic pancreatic ductal adenocarcinoma (mPDAC) J Clin Oncol. 2018;36(4suppl):abstract349. doi: 10.1200/JCO.2018.36.4_suppl.349. [DOI] [Google Scholar]
- 24.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 25.Chen AP, Setser A, Anadkat MJ, Cotliar J, Olsen EA, Garden BC, et al. Grading dermatologic adverse events of cancer treatments: The Common Terminology Criteria for Adverse Events Version 4.0. J AM Acad Dermatol. 2012;67:1025–1039. doi: 10.1016/j.jaad.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 26.Des Jarlais DC, Lyles C, Crepaz N, TREND Group Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: the TREND statement. Am J Public Health. 2005;94:361–366. doi: 10.2105/AJPH.94.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Marco M, Di Cicilia R, Macchini M, Nobili E, Vecchiarelli S, Brandi G, et al. Metastatic pancreatic cancer: is gemcitabine still the best standard treatment? (Review) Oncol Rep. 2010;23:1183–1192. doi: 10.3892/or_00000749. [DOI] [PubMed] [Google Scholar]
- 28.Shirasaka T, Shimamoto Y, Ohshimo H, Yamaguchi M, Kato T, Yonekura K, et al. Development of a novel form of an oral 5-fluorouracil derivative (S-1) directed to the potentiation of the tumor selective cytotoxicity of 5-fluorouracil by two biochemical modulators. Anticancer Drugs. 1996;7:548–557. doi: 10.1097/00001813-199607000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Ueno H, Ioka T, Ikeda M, Ohkawa S, Yanagimoto H, Boku N, et al. Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol. 2013;31:1640–1648. doi: 10.1200/JCO.2012.43.3680. [DOI] [PubMed] [Google Scholar]
- 30.Yoshitomi H, Togawa A, Kimura F, Ito H, Shimizu H, Yoshidome H, et al. A randomized phase II trial of adjuvant chemotherapy with uracil/tegafur and gemcitabine versus gemcitabine alone in patients with resected pancreatic cancer. Cancer. 2008;113:2448–2456. doi: 10.1002/cncr.23863. [DOI] [PubMed] [Google Scholar]
- 31.Zhang SH, Liu GF, Li XF, Liu L, Yu SN. Efficacy of different chemotherapy regimens in treatment of advanced or metastatic pancreatic cancer: a network meta-analysis. J Cell Physiol. 2018;233:3352–3374. doi: 10.1002/jcp.26183. [DOI] [PubMed] [Google Scholar]
- 32.Bracci L, Schiavoni G, Sistigu A, Belardelli F. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ. 2014;21:15–25. doi: 10.1038/cdd.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galluzzi L, Senovilla L, Zitvogel L, Kroemer G. The secret ally: immunostimulation by anticancer drugs. Nat Rev Drug Discov. 2012;11:215–233. doi: 10.1038/nrd3626. [DOI] [PubMed] [Google Scholar]
- 34.Nowak AK, Lake RA, Marzo AL, Scott B, Heath WR, Collins EJ, et al. Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor-specific CD8 T cells. J Immunol. 2003;170:4013–4905. doi: 10.4049/jimmunol.170.10.4905. [DOI] [PubMed] [Google Scholar]
- 35.Plate JM, Plate AE, Shott S, Bograd S, Harris JE. Effect of gemcitabine on immune cells in subjects with adenocarcinoma of the pancreas. Cancer Immunol Immunother. 2005;54:915–925. doi: 10.1007/s00262-004-0638-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soliman HH. Nab-Paclitaxel as a potential partner with checkpoint inhibitors in solid tumors. Onco Targets Ther. 2016;10:101–112. doi: 10.2147/OTT.S122974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.