Abstract
Cathepsins are lysosomal peptidases involved in intracellular protein catabolism as well as in various other physiological and pathological processes. Several members of the family, most notably cathepsins B, S, K and L, are frequently overexpressed in cancer and have been associated with remodeling of the proteins of the extracellular matrix, a process leading to tumor cell migration, invasion and metastasis. In addition, lysosomal cathepsins play a role in innate and adaptive immunity, regulation of antigen presentation, Toll-like receptor signaling, cytokine secretion, apoptosis, autophagy, differentiation, migration and cytotoxicity. In cancer, the cells of innate immunity, such as myeloid cells, are often subverted to the regulatory immunosuppressive phenotype. Most studies indicate that lysosomal cathepsins reinforce the pro-tumoral activity of myeloid-derived suppressor cells and tumor-associated macrophages as well as of neutrophils. On the other hand, in cytotoxic natural killer cells, tumor cells suppress lysosomal peptidases in their activation of perforin and granzymes, thus diminishing their killing ability. With multifaceted actions, lysosomal peptidases constitute an important regulatory mechanism for fine-tuning the anti-tumor immune response.
Keywords: Lysosomal peptidases, Cathepsins, Cancer immunity, Innate immune response, Myeloid suppressor cells, CITIM 2019
Introduction
Peptidases are proteolytic enzymes present in all living organisms. In broad terms, they are distinguished as being either extracellular or intracellular, the latter being targeted to cytoplasm, nucleus and endosomal/lysosomal vesicles. On the basis of structural similarity, peptidases are assigned to different families, which are further grouped into clans. A clan comprises peptidases with the same catalytic mechanism and is named according to the active site amino acid that is the donor of a group for a nucleophilic attack on the peptide bond, i.e., aspartic, cysteine, glutamic, metallo, serine and threonine. There are also peptidases where these site residues are unknown or are mixed in addition to the self-cleaving asparagine peptide lyases [1].
Cysteine peptidases are the most abundant lysosomal peptidases. They comprise 11 papain-like cathepsins in humans (cathepsins B, C, F, H, L, K, O, S, V, X and W) and a caspase-like asparaginyl endopeptidase (AEP), also called legumain. Several other peptidases are present in lysosomes, including cathepsins D and E and aspartic acid peptidases related to pepsin, while lysosomal serine peptidases include cathepsin A, neutrophil elastase, cathepsin G and granzymes. The majority of lysosomal peptidases are endopeptidases, cleaving peptide bonds in the middle of polypeptide chains, but some, such as carboxy exopeptidases, cathepsins B and X and amino exopeptidases cathepsins C and H [1, 2], trim their substrates at the C- or N-terminus. Lysosomes are not merely waste disposal compartments for protein breakdown, but act as complex sensory organelles, responding actively to changes in cell physiology. They regulate cell survival, for example by releasing cathepsins to trigger apoptosis in response to cell stress. Indeed, the term lysosomal can be misleading, since these peptidases have also been detected in nuclei, cytosol, secretory vesicles, at the cell membrane and in the extracellular space [1]. In immune cells, the site- and substrate-specific activity of cysteine lysosomal peptidases regulates important biological processes, such as antigen presentation, cytokine secretion, cell differentiation and migration. Hence, their proteolytic activities require rigorous control that is achieved by transcriptional regulation, expression as zymogens, posttranslational processing, compartmentalization and ultimately endogenous peptidase inhibitors. Elevated protein levels/activities of cysteine lysosomal peptidases, detected in patients with neurodegenerative and autoimmune disorders and in cancer, have been correlated in most cases with poor patient prognosis [1, 2].
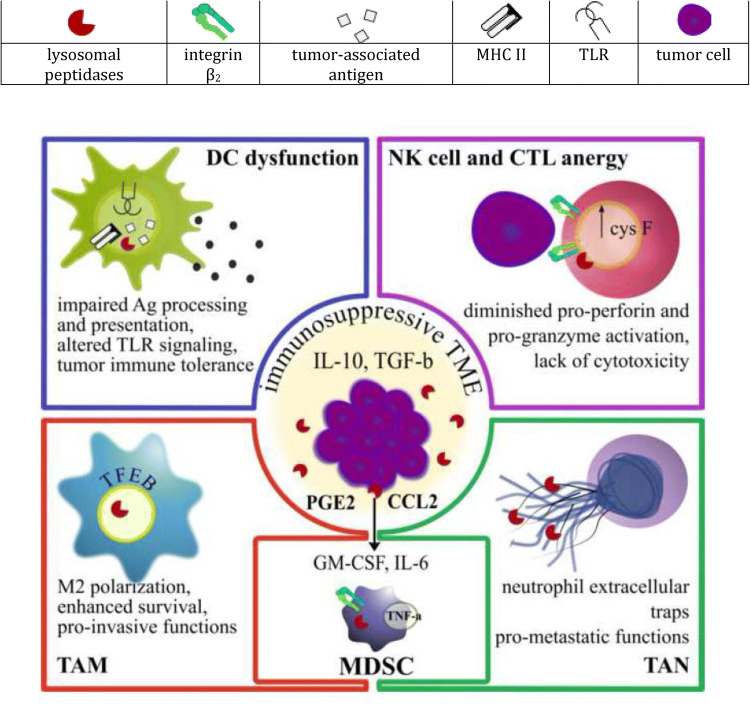
Innate immune cells play a key role in cancer immune surveillance and are necessary for stimulating the effector arm of the immune system to favor tumor rejection. However, established tumors evade immune destruction by converting myeloid cells to potent immunosuppressive regulatory cells that abundantly express lysosomal peptidases. The following review will discuss the impact of lysosomal peptidases on the anti-tumor immune response and pro-tumorigenic functions of myeloid-derived suppressor cells (MDSC), tumor-associated macrophages (TAM) and tumor-associated neutrophils (as summarized in Fig. 1).
Fig. 1.
Lysosomal peptidases, as regulators of immune response in cancer, subvert myeloid cells to promote immunosuppression and cytotoxic cell dysfunction
Lysosomal peptidases in dendritic cells
Dendritic cells (DC) are considered the most important antigen-presenting cells, connecting innate and adaptive immunity through the activation of naïve T cells. They are characterized by a number of unique features that enable their eminent function in antigen sampling and presentation, facilitated by high expression of major histocompatibility complex class II (MHC class II) molecules. Intracellular trafficking and cell surface expression of MHC class II molecules is a tightly regulated process and is, to a large extent, determined by the fate of the class II chaperone, the invariant chain. Degradation of the latter is regulated by the lysosomal peptidases [3]. On the other hand, DCs also express Toll-like receptors (TLRs) that offer several sensitive mediators to trigger an adaptive immune response. Nevertheless, lysosomal peptidases also interfere with TLR activation, which is necessary for DC maturation and further efficient T cell stimulation [4].
The role of cathepsins S and L in antigen processing and presentation
Successful elimination of cancer cells depends greatly on the ability of the immune system to activate cytotoxic T lymphocytes (CTL). This activation requires DC cross-presentation of the tumor-associated antigens to CTL via the MHC I pathway. Another pathway of antigen presentation, mediated by MHC II molecules, is no less important, since MHC II bound peptides prime helper T cells that either enhance or weaken CTL-mediated tumor cell killing.
Gene editing experiments in mice have revealed non-redundant roles for lysosomal cysteine cathepsins L and S in MHC II antigen presentation. Both cathepsins help to release the chaperon invariant chain fragment p10 in order to facilitate antigenic peptide binding. Cathepsin L, and its human homolog cathepsin V, cleave invariant chain in cortical thymic epithelial cells, where positive selection of helper T cells takes place, whereas cathepsin S cleaves invariant chain in peripheral antigen-presenting cells, such as DC, macrophages and B cells [3]. Accordingly, numerous cathepsin S inhibitors have been evaluated in preclinical as well as clinical studies for their efficacy in decreasing the hyper-responsive immune reaction in rheumatoid arthritis, multiple sclerosis, bronchial asthma and myasthenia gravis [2]. A role of cathepsin S in TAP-independent cross-priming has also been confirmed in vivo [5]. Regardless of the cell type and antigen presentation pathway, formation or destruction of an antigenic epitope depends on the combined action of lysosomal peptidases, although a specific peptidase may be dominant in unlocking certain epitopes, as is the case of cathepsin S in processing of myelin basic protein and proinsulin [6]. Activity of lysosomal peptidases in the endo-phagosomal system is strictly regulated by lysosomal pH and redox state, which are controlled by lysosomal proton pump V-ATPase and by NADPH oxidase 2, NOX2 [7], respectively. Cathepsin S activity and stability are also influenced by γ-interferon-inducible thiol reductase, GILT [8].
Functional plasticity, a common characteristic of myeloid cells, endows them with the potential to respond in a variety of ways, according to external and internal stimuli. Therefore in cancer, myeloid cells play a regulatory role and gain pro-tumor functions. Constitutive activation of STAT3 signaling, fueled by tumor-derived cytokines such as IL-10 and IL-6, mediates the switch from immunostimulatory to tolerogenic myeloid cell phenotype. Indeed, IL-10 and IL-6 have been shown to induce mouse bone-marrow-derived DC tolerance by reducing MHC II (both cytokines) as well as invariant chain and human leukocyte antigen DM (HLA-DM) protein levels (IL-6 only). The effect was attributable to increased cathepsin S activity, presumably due to the loss of its endogenous inhibitor cystatin C [9]. While Zavašnik et al. did not observe co-localization between cathepsin S and cystatin C in human DC differentiated from monocyte precursors [10], Martino et al. showed that cystatin C interaction with cathepsin S is relevant for human hematopoietic stem cells during commitment to DC. Cathepsin S could form a tight but reversible interaction with cystatin C specifically in hematopoietic stem cells and could be released from its inhibitor by mature cathepsin D. Importantly, the appearance of mature cathepsins S and D was delayed during the process of differentiation toward immunogenic DC in comparison with tolerogenic DC. Disturbance in the balance of cathepsins D, S and cystatin C expression at the time of cell commitment could steer cells from an immunogenic toward a tolerogenic phenotype [11]. These findings not only highlight important functional differences between DC from disparate origins, but also imply that myeloid cells are extremely sensitive to temporally regulated changes in lysosomal peptidase activities during differentiation.
Multiple lysosomal peptidases act on Toll-like receptor signaling
Pattern recognition receptors comprise an important evolutionarily conserved system for regulating inflammation by innate immune cells. Among them, TLRs are the best known and can be activated either by pathogen- or danger-associated molecular patterns. TLR engagement in cancer or stromal cells either boosts carcinogenesis, by promoting immunotolerance, production of tumor-promoting cytokines (IL-1β, TNF-α, IL-6), stimulation of proliferation, and prevention of apoptosis, or it can suppress tumor progression and enhance anti-tumor immunity [12]. In any case, TLR activation is a prerequisite for DC maturation that is required for effective T cell stimulation. Lysosomal peptidases contribute to the activation of nucleic acid-sensing endosomal TLR-3, TLR-7 and TLR-9. In a two-step cleaving process, a large portion of receptor loop is removed by multiple cysteine cathepsins or AEP, followed by cysteine cathepsin-specific trimming of the remaining N-terminal part [4]. Furthermore, lysosomal peptidases have been shown to interact even with cell surface residing TLRs, most importantly TLR-4. Cathepsin V has been co-immunoprecipitated with TLR-4 and its downstream signaling component IRAK-4 from human monocyte-derived macrophages. Silencing or pharmacological inhibition of cathepsin V attenuated TNF-α release from HMGB1-stimulated macrophages, holding a promise for treating chronic TLR-4-driven inflammation [13]. Intriguingly, lysosomal peptidases could bypass the need for TLR engagement for DC activation and stimulation of antigen-specific responses. It has been shown that cathepsin B release from lysosomes is critical for DC-stimulatory activity of vaccine adjuvant QS-21, a non-toxic saponin found in the bark of Quillaja saaponaria tree. When mice deficient in cathepsin B were vaccinated with a combination of antigen and adjuvant, their DC failed to elicit antigen-specific T cell responses as shown by diminished IL-2, TNF-α and IFN-γ levels [14].
Lysosomal peptidases in macrophages and monocytic MDSC
The tumor microenvironment attracts inflammatory monocytes and provides cues for differentiation to TAM. Depending on environmental cues, TAM were initially classified as anti-tumoral (M1) or immunosuppressive (M2) polarized macrophages. Such a distinction applies well for in vitro differentiated macrophages, but, in cancer patients, macrophages adopt diverse phenotypes according to the tumor type, stage of progression and location within the tumor. In contrast to terminally differentiated macrophages, MDSC are immature myeloid cells that have been arrested in their differentiation toward DC, neutrophils or macrophages. They are usually subclassified as monocytic or polymorphonuclear MDSC. Their expansion is driven by tumor-derived growth factors and cytokines, through induction of emergency myelopoiesis. TAM and monocytic MDSC share mechanisms of immunosuppression and the latter readily differentiate to TAM at the tumor site, so distinguishing between them is not easy. Nevertheless, both cell types are associated with advanced stage and poor prognosis and reduced survival of cancer patients. Hence, depletion or reprogramming of myeloid cells toward a tumor-rejecting phenotype is becoming increasingly important strategy in cancer immunotherapy [15].
Lysosomal cathepsins are involved in macrophage cell death, polarization and macrophage-assisted cancer cell invasion
Lysosomal cathepsins are necessary for the survival of macrophages as they regulate closely related processes of autophagy and apoptosis. Impaired autophagy triggers apoptosis due to mitochondrial damage and to the subsequent generation of reactive oxygen species [16]. Interestingly, lysosomes self-regulate their biogenesis and autophagic flux by controlling the activity of transcription factor EB through lysosomal cysteine peptidase cathepsin B [17]. Moreover, blocking autophagy by a broad-spectrum pharmacological inhibitor of cathepsins B, L and S, GB111-NH2, specifically eliminated TAM, which resulted in reduced tumor burden in an in vivo model of mammary cancer [16]. Lysosomal peptidases that leak from destabilized lysosomes can trigger cell death by cleaving pro-apoptotic Bid. On the other hand, they have been shown to suppress another type of programmed cell death, called necroptosis. Necroptosis in macrophages is initiated through protein kinase Rip-1 and prevented by both caspase-8 and cysteine cathepsins (cathepsins B and S). Since cysteine cathepsins are overexpressed in myeloid cells at the site of inflammation, inhibiting necroptosis could be an important mechanism for enhancing macrophage survival in cancer [18].
Besides macrophage survival, evidence exists that lysosomal cathepsins exacerbate an immunosuppressive phenotype of macrophages in either autocrine or paracrine manner. It has been shown that macrophage intrinsic cathepsin S promotes macrophage M2 polarization during tumor development [19]. Moreover, in a mouse model of colon cancer, gut microbiota bolstered tumor cells to secrete cathepsin K, which in turn polarized macrophages toward an M2 phenotype by activating their TLR-4 [20]. Intriguingly, TLR-4 is also important for M2-macrophage-tumor cell cross talk in that it enhances the epithelial to mesenchymal transition of cancer cells [21].
Cancer cells often up-regulate the surface receptor CD47 that binds to signal regulatory protein α (SIRP α) on macrophages in order to avoid phagocytosis. Chemotherapy-resistant hepatocellular carcinoma stem cells have been found to overexpress CD47 along with cathepsin S, in contrast to more differentiated tumor cells. Cathepsin S, secreted by CD47 + cells, could enhance tumor cell stemness and invasive properties by activating protease-activated receptor 2 (PAR2) [22]. Since PAR2 receptor also regulates functions of myeloid cells, cathepsin S could be important for modulation of the innate immune response. Furthermore, cathepsin S has been found expressed differentially in subsets of tumor-infiltrating macrophages in a transgenic TRAMP mouse model and in prostate cancer patients. The density of cathepsin S-positive macrophages was markedly increased in poorly differentiated or high-grade, castration-resistant tumors, relative to differentiated tumors [23]. Recently, cathepsin B has also been acknowledged in the macrophage-assisted pro-metastatic process called perineural invasion, whereby small nerves in the vicinity of a tumor serve as a conduit for cancer cell spread. Cathepsin B was abundant in macrophages that were attracted to the invaded nerves and was associated with local cancer recurrence in pancreatic ductal adenocarcinoma cancer patients. Importantly, reduced nerve invasion and improved nerve function were observed in cathepsin B knockout mice [24].
Exosomes of tumor and MDSC origin contain certain lysosomal peptidases with specific activity
Most cells, including tumor cells, discharge nano-sized vesicles called exosomes that constitute functional RNA, DNA and proteins. Exosomes are potent mediators of long-distance cell communication, and several studies have demonstrated that tumor-derived exosomes promote MDSC expansion. Lysosomal AEP is enriched in exosomes isolated from pancreatic ductal adenocarcinoma patients and contributes significantly to cancer cell invasion in vitro. In addition, exosomal AEP plays a role in signal transduction, as shown by the fact that AEP-overexpressing exosomes activated the pro-survival PI3K/Akt pathway in cancer cells [25]. Similarly, cathepsin B in microvesicles shed by ovarian cancer cells promoted endothelial cell invasiveness under low pH conditions [26]. MDSC also shed their own exosomes, driving polarization of macrophages toward the tumor-promoting M2 phenotype. Importantly, cathepsins B and G were detected among the biologically active molecules in these exosomes [27]. The specific functions of exosome-derived lysosomal peptidases deserve further consideration, since they could exert systemic impact on cancer immunity.
The prominent role of cysteine cathepsins in MDSC biology has also been proven in complex in vivo models. For example, MDSC expansion in a mouse model of hereditary polyposis is dependent on cathepsin B. MDSC failed to accumulate in mice deficient in cathepsin B, probably due to reduced TNF-α secretion [28] since cathepsin B is known to regulate the trafficking of TNF-α-containing vesicles [29]. In another model of prostate cancer bone metastasis, cathepsin K has been shown to contribute to macrophage infiltration and to overexpression of cathepsin B, CCL2 and cyclooxygenase 2 (COX2) [30], thus favoring conditions for expansion and recruitment of MDSC. In contrast to cathepsin K, cathepsins B, L and X appear to oppose bone metastasis progression, since they block MDSC differentiation to osteoclasts [31].
In MDSC, some lysosomal peptidases have the specific activity of modifying the mediators of inflammation. Chemokine CCL2 is one of the most potent cytokines for attracting MDSC and TAM to the tumor site. Interestingly, CCL2 production is coupled to cathepsin S-mediated cleavage of MHC II chaperone invariant chain (CD74). The liberated intracellular domain fragment of invariant chain is then translocated to the nucleus, where it activates NF-κB and CCL2 transcription [32]. After mobilization to the tumor site, myeloid cells encounter a highly immunosuppressive environment, promoting transcription of genes for attenuating an anti-tumor immune response. In particular, increased COX2 activity in the tumor microenvironment has been associated with the induction of MDSC. Notably, broad-spectrum inhibitors of calpain and cathepsins B, H and L suppressed maturation of COX2, thus reducing the production of the potent immunosuppressive agent prostaglandin E2 [33].
Lysosomal peptidases in neutrophils and polymorphonuclear MDSC
Like macrophages, neutrophils shift between N1 and N2 polarization states. As short-lived cells, they are often neglected in studies that explore immune infiltrates in cancer. Immunosuppressive neutrophil-like cells are often characterized in mouse tumor models as polymorphonuclear MDSC. However, in humans, distinction between MDSC and tumor-associated neutrophils is debatable [34]. In neutrophils, lysosomal enzymes are sequestered into specialized secretory vesicles, azurophilic granules that contain a distinct set of serine peptidases. Most important for the function of neutrophils are cathepsin G, neutrophil elastase and proteinase-3, which have to be converted to the active form by cysteine peptidase cathepsin C. Unsurprisingly, cathepsin C deficiency in humans, known as Papillon–Lefèvre syndrome, is marked by failed neutrophil function [35]. Similarly to that in macrophages, cell survival in neutrophils is regulated by lysosomal peptidases. Aspartic peptidase cathepsin D and cysteine peptidase cathepsin B can initiate apoptosis by cleaving caspase-8 or Bid, respectively. Reactive oxygen species trigger leakage of either cathepsin in the cytosol, but the molecular mechanism for selecting which of the cathepsins will prevail in executing neutrophil cell death remains to be elucidated [36].
Neutrophil extracellular traps-associated peptidases
Activated neutrophils can extrude neutrophil extracellular traps (NET) that comprise web-like de-condensed chromatin structures, decorated with antimicrobial peptides and neutrophil peptidases. In cancer patients, NET are believed to facilitate cancer cell dissemination, on the one hand entrapping them in a pro-tumorigenic environment and, on the other, carrying molecules that activate other leukocytes and endothelial cells [34]. Neutrophil elastase, for example, can stimulate TLR-4 signaling in macrophages to induce NF-κB activation and increase cathepsin B expression. In addition, neutrophil elastase processes cathepsin B to its active form [37]. NET-incorporated cathepsin G also contributes to cancer patient morbidity by promoting thrombosis. Cathepsin G can activate vascular endothelium to increase the expression of adhesive molecules and thrombogenic tissue factor by converting pro-IL-1α in its active form [38]. In addition, cathepsin G has been shown to promote spheroidal homotypic aggregation of breast cancer cells, supporting a metastasis-promoting role for cathepsin G [39].
Involvement of integrins in polymorphonuclear MDSC recruitment
Myeloid cells utilize leukocyte-specific β2 integrin transmembrane adhesion receptors for regulation of adhesion, migration and signaling. The ability of cathepsin X to modulate signal transduction via integrins macrophage-1 antigen (Mac-1) and lymphocyte function-associated antigen 1 (LFA-1) has been studied extensively [40]. It was demonstrated that cathepsin X cleaves β2 chain cytoplasmic domain sequentially which, in turn, modulates the binding of adaptor proteins talin and α-actinin. These adaptor proteins link integrin receptors to the actin cytoskeleton, thereby enabling the formation of signaling complexes that transmit information in and out of the cell [40]. Deficiency of integrin Mac-1 (also CD11b/CD18) in a mouse model of colorectal cancer was found to abrogate trafficking of polymorphonuclear MDSC to the tumor site. Further, lack of CD11b+ cell infiltration also correlated with decreased TNF-α levels and prevented proliferative Wnt/β-catenin signaling in cancer cells [41]. Since cathepsin X is frequently up-regulated in cancer, it could contribute to constitutive Mac-1 activation in myeloid cells. On the other hand, lack of Mac-1 on human neutrophils reduces antibody-dependent cell cytotoxicity due to impaired formation of immunological synapses [42]. Outcomes of Mac-1 receptor activation and signaling therefore appear to depend on context and on neutrophil phenotype.
Lysosomal peptidases in natural killer cells
Natural killer (NK) cells are innate lymphoid MHC I non-restricted cytotoxic cells that are as equally important for killing cancer cells as CTL, but without the need for prior sensitization. However, NK cells preferentially target poorly differentiated tumors and cancer stem-like cells with lower levels of MHC I [43].
NK cells kill their targets in a manner similar to that by CTL, either through the death receptor pathway or by releasing cytotoxic granules containing perforin and granzymes. As their name implies, perforin molecules imbed in the target cell membrane, thereby forming openings for the entry of granzymes that induce cell death through caspase activation. Both types of effector molecules are stored as inactive precursors that need to be cleaved by lysosomal peptidases. An important, although non-redundant, role in cleaving pro-perforin has been reported for cathepsin L [44]. Similarly, pro-granzymes A and B are converted to their active forms by cathepsins C and H [45]. The activities of lysosomal peptidases in cytotoxic cells are regulated primarily by their leukocyte-specific endogenous inhibitor, cystatin F. Notably, cystatin F is synthesized as an inactive dimer that has first to be converted to a monomer, possibly by another lysosomal peptidase, cathepsin V, to become a potent inhibitor of cathepsins C and H. Studies on CTL and NK cells by our group revealed a novel mechanism of tumor cell immune evasion, whereby excessive inhibition of cysteine cathepsins by cystatin F affects immune cell cytotoxicity. Indeed, cystatin F has been up-regulated after induction of functional anergy in NK cells [46] and in CTL [47], resulting in decreased activities of cathepsins C, H and L and impaired activation of granzymes A and B that were translated to reduced cytotoxicity. Additionally, exogenously supplied cystatin F has been shown to significantly reduce the cytotoxicity of otherwise functional NK cells. Tumor cells are known to secrete inactive dimeric cystatin F that could be internalized into endolysosomal compartments and converted to active monomer [48]. To summarize, cystatin F may act as a regulatory switch to prevent cytotoxic molecule activation and dampen cytotoxic activity of NK cells and CTL in cancer.
In cell–cell interactions, integrin LFA-1, expressed on NK cells, is essential for establishing firm contact with the target cell. When LFA-1 binds its ligand ICAM-1 on target cells, talin accumulates at the site of contact and brings together WASP and Arp2/3 complex to promote actin polymerization, eventually resulting in polarization of cytotoxic granules toward target cells [49]. Interestingly, LFA-1 is more prone to activation in mature NK cell subsets with the highest cytotoxic potential [50]. Cathepsin X has been shown to modulate the adhesiveness of LFA-1 by facilitating a transition from a moderate- to a high-affinity conformation [40]. Cathepsin X may thus contribute importantly to NK cell immunological synapse stability and LFA-1 signaling.
Conclusion
Knowledge concerning the mechanisms underlying the spread of cancer at the cellular and molecular levels is expanding rapidly. Lysosomal peptidases have been recognized as important factors triggering the initiation, growth and proliferation of tumor cells, angiogenesis, invasion and metastasis. However, the central key players governing the initiation and progression of tumors remain poorly established. More attention has lately been paid to the role of immune cells in cancer immune surveillance, where the fundamental principles of innate and adaptive immunity may explain how immune cells generate a specific response to tumor tissue. One of the reasons is that tumor cells escape the immune response by converting myeloid cells to potent immunosuppressive regulatory cells. These cells express abundantly lysosomal peptidases such as cathepsin B, L, S and K. Lysosomal peptidases have been shown to be involved in maintaining the immunosuppressive program in MDSC, TAM and TAN. On the other hand, lysosomal peptidases, mainly cathepsins S and L, interfere with DC cross-presentation of the tumor-associated antigen to the CTL, leading to an inadequate anti-tumor immune response. Importantly, in a highly immunosuppressive tumor microenvironment, CTL and NK cells becomes anergic and lose the ability to eliminate tumor cells due to inactivation of lysosomal peptidases. Indeed, regulatory axes that control expression and activity of lysosomal peptidases conform according to the functional state of the immune cells. Thus, the diverse outcomes of lysosomal peptidase functions reflect the plasticity of the immune system and can be either immunostimulatory or immunosuppressive (Table 1). Finally, elucidating the role of lysosomal peptidases in innate immune cells with regard to cancer immunity could aid in identifying and developing novel therapeutic strategies.
Table 1.
Cathepsin-dependent mechanisms for regulation of immune cell functions in health and in cancer
| Normal | Pathologic |
|---|---|
| Cell fate | |
| (a) Death or survival | |
| Lysosomal integrity is necessary for prevention of apoptosis [16, 18] | Cat (B, D, L, S) promote autophagy and survival of tumor-associated myeloid cells [16, [36] |
| CatB is the main regulator of lysosomal biogenesis and autophagic flux [17] | CatB and catS inhibit macrophage necroptosis [18] |
| (b) Differentiation and polarization | |
| Myeloid cell functions depend on interplay between catD and catS and their endogenous inhibitors (cystatin C) during cell differentiation and maturation [9, 11] | CatK and catS drive myeloid cell polarization toward regulatory and immunosuppressive phenotype [19, 20] |
| AEP, catB and catG are present in cancer exosomes [25, 27] | |
| Immune system activation | |
| (a) Innate immunity | |
| Multiple cat and AEP can trigger TLR signaling, therefore controlling innate immune cell activation and inflammation [13, 20] | Cat contribute to enrichment of tumor-promoting inflammatory mediators such as CCL2 (catS), prostaglandin E2 (cat B, H, L) and TNF-α (catB) [ [29, 32, 33] |
| (b) Adaptive immunity | |
| CatL/V and catS degrade invariant chain, facilitating MHC II antigen presentation and CD4 + (catS also CD8+) T cell activation [3, 5] | Impaired antigen presentation and processing of antigenic peptides by catL/V and catS lead to defective immune response [2] |
| Cell motility and mobilization | |
| CatX mediates affinity switch of leukocyte main adhesion molecules LFA-1 and Mac-1 [40] | TAM-derived cat (B, K, L, S) enable tumor cell invasion through extracellular matrix and adjacent nerves [23, 24] |
| NET-incorporated catG activates vascular endothelium by processing pro-IL-1α [38] | |
| Immune cell cytotoxicity | |
| Tumor elimination | Tumor escape |
| Cat (C, H, L) in secretory granules of CD8 + and NK cells are main activators of perforin and granzymes [44, 45] | By secreting endogenous cat inhibitor, cystatin F, tumor cells impair perforin and granzyme activation [ [46–48] |
Cathepsins contribute to immune system homeostasis at different levels. Tight regulation of their activity is lost in cancer, where cathepsins promote survival, accumulation and pro-tumorigenic functions of innate immune cells (TAM, MDSC and TAN). At the same time, they interfere with the activation and tumor killing ability of adaptive immune cells
Cat cathepsin
Acknowledgements
We sincerely thank Professor Roger H. Pain for critical reading of the manuscript.
Abbreviations
- AEP
Asparaginyl endopeptidase
- Arp 2/3
Actin-related proteins 2/3
- COX2
Cyclooxygenase 2
- CTL
Cytotoxic T lymphocytes
- DC
Dendritic cells
- HLA-DM
Human leukocyte antigen DM
- HMGB1
High-mobility group box 1
- GILT
Γ-interferon-inducible thiol reductase
- ICAM-1
Intercellular adhesion molecule 1
- IRAK4
IL-1 receptor-associated kinase 4
- LFA-1
Lymphocyte function-associated antigen 1
- Mac-1
Macrophage-1 antigen
- MDSC
Myeloid-derived suppressor cells
- MHC II
Major histocompatibility complex class II
- NET
Neutrophil extracellular traps
- NK
Natural killer
- NOX2
NADPH oxidase 2
- PAR2
Protease-activated receptor 2
- SIRP α
Signal regulatory protein α
- STAT3
Signal transducer and activator of transcription 3
- TAM
Tumor-associated macrophages
- TLR
Toll-like receptors
- WASP
Wiskott–Aldrich syndrome protein
Author contributions
TJ drafted the manuscript and designed the figure. UPF, AP and JK finalized and complemented the manuscript.
Funding
This work was supported by the Grants P4-0127 and J4-8227 from the Research Agency of the Republic of Slovenia (to Janko Kos).
Compliance with ethical standards
Conflict of interest
The authors declare no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pišlar A, Perišić Nanut M, Kos J. Lysosomal cysteine peptidases—molecules signaling tumor cell death and survival. Semin Cancer Biol. 2015;35:168–179. doi: 10.1016/j.semcancer.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Kramer L, Turk D, Turk B. The future of cysteine cathepsins in disease management. Trends Pharmacol Sci. 2017;38:873–898. doi: 10.1016/j.tips.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Honey K, Rudensky AY. Lysosomal cysteine proteases regulate antigen presentation. Nat Rev Immunol. 2003;3:472–482. doi: 10.1038/nri1110. [DOI] [PubMed] [Google Scholar]
- 4.Ewald SE, Engel A, Lee J, Wang M, Bogyo M, Barton GM. Nucleic acid recognition by Toll-like receptors is coupled to stepwise processing by cathepsins and asparagine endopeptidase. J Exp Med. 2011;208:643–651. doi: 10.1084/jem.20100682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen L, Sigal LJ, Boes M, Rock KL. Important role of cathepsin S in generating peptides for TAP-independent MHC class I cross presentation in vivo. Immunity. 2004;21:155–165. doi: 10.1016/j.immuni.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Stoeckle C, Quecke P, Rückrich T, Burster T, Reich M, Weber E, et al. Cathepsin S dominates autoantigen processing in human thymic dendritic cells. J Autoimmun. 2012;38:332–343. doi: 10.1016/j.jaut.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Savina A, Jancic C, Hugues S, Guermonprez P, Vargas P, Moura IC, et al. NOX2 controls phagosomal pH to regulate antigen processing during cross presentation by dendritic cells. Cell. 2006;126:205–218. doi: 10.1016/j.cell.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 8.Balce DR, Allan ERO, McKenna N, Yates RM. γ-interferon-inducible lysosomal thiol reductase (GILT) maintains phagosomal proteolysis in alternatively activated macrophages. J Biol Chem. 2014;289:31891–31904. doi: 10.1074/jbc.M114.584391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitamura H, Kamon H, Sawa S, Park S-J, Katunuma N, Ishihara K, et al. IL-6-STAT3 controls intracellular MHC Class II αβ dimer level through cathepsin S activity in dendritic cells. Immunity. 2005;23:491–502. doi: 10.1016/j.immuni.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Zavasnik-Bergant T. Differentiation- and maturation-dependent content, localization, and secretion of cystatin C in human dendritic cells. J Leukoc Biol. 2005;78:122–134. doi: 10.1189/jlb.0804451. [DOI] [PubMed] [Google Scholar]
- 11.Martino S, Tiribuzi R, Ciraci E, Makrypidi G, D’Angelo F, di Girolamo I, et al. Coordinated involvement of cathepsins S, D and cystatin C in the commitment of hematopoietic stem cells to dendritic cells. Int J Biochem Cell Biol. 2011;43:775–783. doi: 10.1016/j.biocel.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Pradere J-P, Dapito DH, Schwabe RF. The Yin and Yang of Toll-like receptors in cancer. Oncogene. 2014;33:3485–3495. doi: 10.1038/onc.2013.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pribis JP, Al-Abed Y, Yang H, Gero D, Xu H, Montenegro MF, et al. The HIV Protease Inhibitor Saquinavir Inhibits HMGB1-Driven Inflammation by Targeting the Interaction of Cathepsin V with TLR4/MyD88. Mol Med. 2015;21:749–757. doi: 10.2119/molmed.2015.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Welsby I, Detienne S, N’Kuli F, Thomas S, Wouters S, Bechtold V, et al. Lysosome-dependent activation of human dendritic cells by the vaccine adjuvant QS-21. Front Immunol. 2016;7:663. doi: 10.3389/fimmu.2016.00663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ugel S, De Sanctis F, Mandruzzato S, Bronte V. Tumor-induced myeloid deviation: when myeloid-derived suppressor cells meet tumor-associated macrophages. J Clin Invest. 2015;125:3365–3376. doi: 10.1172/JCI80006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salpeter SJ, Pozniak Y, Merquiol E, Ben-Nun Y, Geiger T, Blum G. A novel cysteine cathepsin inhibitor yields macrophage cell death and mammary tumor regression. Oncogene. 2015;34:6066–6078. doi: 10.1038/onc.2015.51. [DOI] [PubMed] [Google Scholar]
- 17.Qi X, Man SM, Malireddi RKS, Karki R, Lupfer C, Gurung P, et al. Cathepsin B modulates lysosomal biogenesis and host defense against Francisella novicida infection. J Exp Med. 2016;213:2081–2097. doi: 10.1084/jem.20151938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McComb S, Shutinoski B, Thurston S, Cessford E, Kumar K, Sad S. Cathepsins limit macrophage necroptosis through cleavage of Rip1 kinase. J Immunol. 2014;192:5671–5678. doi: 10.4049/jimmunol.1303380. [DOI] [PubMed] [Google Scholar]
- 19.Yang M, Liu J, Shao J, Qin Y, Ji Q, Zhang X, et al. Cathepsin S-mediated autophagic flux in tumor-associated macrophages accelerate tumor development by promoting M2 polarization. Mol Cancer. 2014;13:43. doi: 10.1186/1476-4598-13-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li R, Zhou R, Wang H, Li W, Pan M, Yao X, et al. Gut microbiota-stimulated cathepsin K secretion mediates TLR4-dependent M2 macrophage polarization and promotes tumor metastasis in colorectal cancer. Cell Death Differ. 2019 doi: 10.1038/s41418-019-0312-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yao R-R, Li J-H, Zhang R, Chen R-X, Wang Y-H. M2-polarized tumor-associated macrophages facilitated migration and epithelial-mesenchymal transition of HCC cells via the TLR4/STAT3 signaling pathway. World J Surg Oncol. 2018;16:9. doi: 10.1186/s12957-018-1312-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee TK-W, Cheung VC-H, Lu P, Lau EYT, Ma S, Tang KH, et al. Blockade of CD47-mediated cathepsin S/protease-activated receptor 2 signaling provides a therapeutic target for hepatocellular carcinoma. Hepatology. 2014;60:179–191. doi: 10.1002/hep.27070. [DOI] [PubMed] [Google Scholar]
- 23.Lindahl C, Simonsson M, Bergh A, Thysell E, Antti H, Sund M, et al. Increased levels of macrophage-secreted cathepsin S during prostate cancer progression in TRAMP mice and patients. Cancer Genom Proteomics. 2009;6:149–59. http://cgp.iiarjournals.org/content/6/3/149.abstract [PubMed]
- 24.Bakst RL, Xiong H, Chen C-H, Deborde S, Lyubchik A, Zhou Y, et al. Inflammatory monocytes promote perineural invasion via CCL2-mediated recruitment and cathepsin B expression. Cancer Res. 2017;77:6400–6414. doi: 10.1158/0008-5472.CAN-17-1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan Q, Yuan W-B, Sun X, Zhang M-J, Cen F, Zhou S-Y, et al. Asparaginyl endopeptidase enhances pancreatic ductal adenocarcinoma cell invasion in an exosome-dependent manner and correlates with poor prognosis. Int J Oncol. 2018 doi: 10.3892/ijo.2018.4318. [DOI] [PubMed] [Google Scholar]
- 26.Giusti I, D’Ascenzo S, Millimaggi D, Taraboletti G, Carta G, Franceschini N, et al. Cathepsin B mediates the pH-dependent proinvasive activity of tumor-shed microvesicles. Neoplasia. 2008;10:481–488. doi: 10.1593/neo.08178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burke M, Choksawangkarn W, Edwards N, Ostrand-Rosenberg S, Fenselau C. Exosomes from myeloid-derived suppressor cells carry biologically active proteins. J Proteome Res. 2014;13:836–843. doi: 10.1021/pr400879c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gounaris E, Tung CH, Restaino C, Maehr R, Kohler R, Joyce JA, et al. Live imaging of cysteine-cathepsin activity reveals dynamics of focal inflammation, angiogenesis, and polyp growth. PLoS One. 2008;3:e2916. doi: 10.1371/journal.pone.0002916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ha S-D, Martins A, Khazaie K, Han J, Chan BMC, Kim SO. Cathepsin B is involved in the trafficking of TNF- -containing vesicles to the plasma membrane in macrophages. J Immunol. 2008;181:690–697. doi: 10.4049/jimmunol.181.1.690. [DOI] [PubMed] [Google Scholar]
- 30.Herroon MK, Rajagurubandara E, Rudy DL, Chalasani A, Hardaway AL, Podgorski I. Macrophage cathepsin K promotes prostate tumor progression in bone. Oncogene. 2013;32:1580–1593. doi: 10.1038/onc.2012.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edgington-Mitchell LE, Rautela J, Duivenvoorden HM, Jayatilleke KM, van der Linden WA, Verdoes M, et al. Cysteine cathepsin activity suppresses osteoclastogenesis of myeloid-derived suppressor cells in breast cancer. Oncotarget. 2015;6:8–10. doi: 10.18632/oncotarget.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilkinson RDA, Magorrian SM, Williams R, Young A, Small DM, Scott CJ, et al. CCL2 is transcriptionally controlled by the lysosomal protease cathepsin S in a CD74-dependent manner. Oncotarget. 2015;6:29725–29739. doi: 10.18632/oncotarget.5065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mancini A, Jovanovic DV, He QW, Di Battista JA. Site-specific proteolysis of cyclooxygenase-2: a putative step in inflammatory prostaglandin E2 biosynthesis. J Cell Biochem. 2007;101:425–441. doi: 10.1002/jcb.21191. [DOI] [PubMed] [Google Scholar]
- 34.Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer. 2016;16:431. doi: 10.1038/nrc.2016.52. [DOI] [PubMed] [Google Scholar]
- 35.Pham CTN, Ivanovich JL, Raptis SZ, Zehnbauer B, Ley TJ. Papillon-Lefèvre Syndrome: correlating the molecular, cellular, and clinical consequences of cathepsin C/dipeptidyl peptidase i deficiency in humans. J Immunol. 2004;173:7277. doi: 10.4049/jimmunol.173.12.7277. [DOI] [PubMed] [Google Scholar]
- 36.Conus S, Perozzo R, Reinheckel T, Peters C, Scapozza L, Yousefi S, et al. Caspase-8 is activated by cathepsin D initiating neutrophil apoptosis during the resolution of inflammation. J Exp Med. 2008;205:685–698. doi: 10.1084/jem.20072152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geraghty P, Rogan MP, Greene CM, Boxio RMM, Poiriert T, O’Mahony M, et al. Neutrophil elastase up-regulates cathepsin B and matrix metalloprotease-2 expression. J Immunol. 2007;178:5871–5878. doi: 10.4049/jimmunol.178.9.5871. [DOI] [PubMed] [Google Scholar]
- 38.Folco EJ, Mawson TL, Vromman A, Bernardes-Souza B, Franck G, Persson O, et al. Neutrophil extracellular traps induce endothelial cell activation and tissue factor production through interleukin-1alpha and cathepsin G. Arterioscler Thromb Vasc Biol. 2018;38:1901–1912. doi: 10.1161/ATVBAHA.118.311150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yui S, Osawa Y, Ichisugi T, Morimoto-Kamata R. Neutrophil cathepsin G, but not elastase, induces aggregation of MCF-7 mammary carcinoma cells by a protease activity-dependent cell-oriented mechanism. Mediators Inflamm. 2014;2014:971409. doi: 10.1155/2014/971409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jevnikar Z, Obermajer N, Kos J. LFA-1 fine-tuning by cathepsin X. IUBMB Life. 2011 doi: 10.1002/iub.505. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Q-Q, Hu X-W, Liu Y-L, Ye Z-J, Gui Y-H, Zhou D-L, et al. CD11b deficiency suppresses intestinal tumor growth by reducing myeloid cell recruitment. Sci Rep. 2015;5:15948. doi: 10.1038/srep15948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Spriel AB, Leusen JH, van Egmond M, Dijkman HB, Assmann KJ, Mayadas TN, et al. Mac-1 (CD11b/CD18) is essential for Fc receptor-mediated neutrophil cytotoxicity and immunologic synapse formation. Blood. 2001;97:2478–2486. doi: 10.1182/blood.V97.8.2478. [DOI] [PubMed] [Google Scholar]
- 43.Kaur K, Nanut MP, Ko M-W, Safaie T, Kos J, Jewett A. Natural killer cells target and differentiate cancer stem-like cells/undifferentiated tumors: strategies to optimize their growth and expansion for effective cancer immunotherapy. Curr Opin Immunol. 2018;51:170–180. doi: 10.1016/j.coi.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 44.Konjar Š, Sutton VR, Hoves S, Repnik U, Yagita H, Reinheckel T, et al. Human and mouse perforin are processed in part through cleavage by the lysosomal cysteine proteinase cathepsin L. Immunology. 2010;131:257–267. doi: 10.1111/j.1365-2567.2010.03299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.D’Angelo ME, Bird PI, Peters C, Reinheckel T, Trapani JA, Sutton VR. Cathepsin H is an additional convertase of pro-granzyme B. J Biol Chem. 2010;285:20514–20519. doi: 10.1074/jbc.M109.094573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Magister S, Tseng H-C, Bui VT, Kos J, Jewett A. Regulation of split anergy in natural killer cells by inhibition of cathepsins C and H and cystatin F. Oncotarget. 2015;6:22310–22327. doi: 10.18632/oncotarget.4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prunk M, Nanut MP, Sabotic J, Svajger U, Kos J. Increased cystatin F levels correlate with decreased cytotoxicity of cytotoxic T cells. Radiol Oncol. 2019;53:57–68. doi: 10.2478/raon-2019-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perišić Nanut M, Sabotič J, Švajger U, Jewett A, Kos J. Cystatin F affects natural killer cell cytotoxicity. Front Immunol. 2017;8:1459. doi: 10.3389/fimmu.2017.01459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mace EM, Zhang J, Siminovitch KA, Takei F. Elucidation of the integrin LFA-1-mediated signaling pathway of actin polarization in natural killer cells. Blood. 2010;116:1272–1279. doi: 10.1182/blood-2009-12-261487. [DOI] [PubMed] [Google Scholar]
- 50.Urlaub D, Höfer K, Müller M-L, Watzl C. LFA-1 activation in NK cells and their subsets: influence of receptors, maturation, and cytokine stimulation. J Immunol. 2017;198:1944–1951. doi: 10.4049/jimmunol.1601004. [DOI] [PubMed] [Google Scholar]