Abstract
From a metabolic perspective, cancer may be considered as a metabolic disease characterized by reprogrammed glycolytic metabolism. The aim of the present study was to investigate CD147-mediated glucose metabolic regulation in hepatocellular carcinoma (HCC) and its contribution to altered immune responses in the tumor microenvironment. Several HCC cell lines and corresponding nude mice xenografts models differing in CD147 expressions were established to directly investigate the role of CD147 in the reprogramming of glucose metabolism, and to determine the underlying molecular mechanisms. Immunohistochemistry (IHC) analyses and flow cytometry were used to identify the relationship between reprogrammed glycolysis and immunosuppression in HCC. Upregulated CD147 expressions were found to be associated with enhanced expressions of GLUT1, MCT1 in HCC tumorous tissues. CD147 promoted the glycolytic metabolism in HCC cell lines in vitro via the PI3K/Akt/mTOR signaling pathway. A positive correlation existed between a profile of immunosuppressive lymphocytes infiltration and CD147 expression in HCC tissues. Accumulation of FOXP3-expressing regulatory T cells was induced under a stimulation with lactate in vitro. In conclusion, CD147 promoted glycolytic metabolism in HCC via the PI3K/Akt/mTOR signaling pathway, and was related to immunosuppression in HCC.
Electronic supplementary material
The online version of this article (10.1007/s00262-019-02457-y) contains supplementary material, which is available to authorized users.
Keywords: Glucose metabolism, CD147, Immunosuppression, Hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignancies worldwide, ranking fifth in morbidity and third in mortality [1]. Immunotherapy, such as immune checkpoint blockade with anti-cytotoxic T lymphocytes-associated protein-4 (CTLA-4) and anti-programmed cell death-1 (PD-1) antibodies, has attracted increasing attention [2, 3], and has been considered as a promising therapeutic approach for HCC with advanced stages. However, the benefit of immunotherapy in HCC is currently compromised by an immunosuppressive tumor microenvironment [4]. A combined and thorough analysis of different immunosuppressive components in local tumor microenvironment has been performed by various investigators in previous studies to determine the underlying mechanisms employed by tumor cells to circumvent the host’s immune surveillance [5]. The mechanisms responsible for an impaired anti-tumor immunity or induced immunosuppression in the tumor micro-milieu are complicated [6], and recently there has been an increasing momentum to focus on cellular metabolisms [7].
From a metabolic perspective, tumor may be considered as a system-level disease with dysregulated metabolisms [8]. There is growing awareness that transformed cells undergo a metabolic reprogramming characterized by a shift of their energy metabolism into aerobic glycolysis to fulfill an increasing biosynthetic demand of tumors cells, even under aerobic conditions which could physiologically support the mitochondrial oxidative respiration, a phenomenon now known as “Warburg effect” [9]. In fact, apparently distinct tumor immune escape and tumor metabolic switch are substantially linked to each other [10]. An increased dependence on aerobic glycolysis by tumor cells inevitably leads to a fierce nutrient competition between tumor cells and the surrounding immune cells in the tumor microenvironment [11], as immune cells also rely on increased aerobic glycolysis to support extensive proliferation, and effector T cells (Teff) differentiation and function upon activation [12, 13]. This nutrient deficiency imposed on immune cells by the tumor may result in attenuated anti-tumor immunity [14]. Apart from the resource restriction imposed by tumor cells, metabolites themselves, such as an abundance of lactate in the tumor micro-milieu due to an increased dependence on aerobic glycolysis by tumor cells, have also been demonstrated to exert inhibitory effects on various immune cells [15, 16]. Thus, the metabolic imbalance brought about by tumor cells contributes to impaired immune cell function and consequent immune cell anergy and tumor immune escape [11, 17].
CD147, also known as extracellular matrix metalloproteinase inducer (EMMPRIN), has been reported to be upregulated on the surfaces of various types of malignant tumors [18]. Especially, it has been becoming increasingly accepted that upregulation of CD147 in HCC significantly contributes to tumor progression and may be used as an early diagnostic biomarker, and is a significantly unfavorable prognostic factor which functions through multiple molecular mechanisms [19]. Intriguingly, CD147 has also been described as a chaperone of monocarboxylate transporter 1 (MCT1) and MCT4 which catalyze the transport of monocarboxylates such as lactate across the plasma membrane [20], suggesting the involvement of CD147 in the metabolic regulation of tumor cells [21]. In addition, recent identification of a previously unrecognized glucose metabolic mechanism responsible for the tumorigenic capacity of CD147 in HCC [22] has warranted further exploration of the potential immunosuppressive role of CD147 in HCC.
In the present study, correlation analyses between CD147 and MCT1, MCT4 and glucose transporter 1 (GLUT1) in tumorous tissues of HCC were performed. Next, several HCC cell lines and corresponding nude mice xenograft models with different status of CD147 expression were established to determine the involvement of CD147 in the promotion of glucose metabolism in HCC. Subsequently, the molecular mechanism by which CD147 enhanced the glucose metabolism in HCC was determined. Finally, correlation between CD147 expression levels and the status of local anti-tumor immunity in HCC was determined, and a potentially immunoregulatory role of lactate in regulatory T cell accumulation in local tumor microenvironment was identified. The present study demonstrated a glucose-metabolism-enhancing role of CD147 in HCC, and uncovered a correlation between immune evasion of HCC and CD147 expression. The present investigation may contribute to the development of CD147-associated therapeutic strategies and an improvement in tumor immunotherapies for HCC.
Materials and methods
Patients and tissue samples
A total of 43 patients with HCC seen at the Center of Hepatobiliary Surgery, Peking University People’s Hospital (Beijing, China) were enrolled in the present study. Clinical characteristics of the included patients are summarized in Table 1. Tumor and non-tumor (> 5 cm from the tumor margin) tissues were collected at the time of surgery from the included patients for flow cytometric analyses. In addition, formalin-fixed and paraffin-embedded slides from the tumorous tissues of the recruited patients were also collected for IHC staining. HCC was diagnosed according to the diagnostic guidelines of the European association for the study of the liver.
Table 1.
Clinical characteristics and CD147 expression of 43 HCC patients included in this study
| Variables | Total | CD147 expression | p value | ||
|---|---|---|---|---|---|
| n | ∓ (%) | ++ (%) | +++ (%) | ||
| Age | |||||
| < 57 | 21 | 5 | 6 | 10 | 0.772 |
| ≥ 57 | 22 | 4 | 7 | 11 | |
| Sex | |||||
| Female | 10 | 2 | 3 | 5 | 0.925 |
| Male | 33 | 7 | 10 | 16 | |
| α-fetoprotein (ng/ml) | |||||
| < 405 | 21 | 4 | 5 | 12 | 0.369 |
| ≥ 405 | 22 | 5 | 8 | 9 | |
| HBs Ag | |||||
| – | 15 | 2 | 7 | 6 | 0.782 |
| + | 28 | 7 | 6 | 15 | |
| HCV Ab | |||||
| – | 40 | 9 | 11 | 20 | 0.959 |
| + | 3 | 0 | 2 | 1 | |
| TNM stage | |||||
| I | 7 | 3 | 3 | 1 | 0.191 |
| II | 16 | 3 | 3 | 10 | |
| III | 15 | 2 | 5 | 8 | |
| IV | 5 | 1 | 2 | 2 | |
| Differentiation | |||||
| High | 23 | 3 | 5 | 15 | 0.076 |
| Medium | 7 | 3 | 2 | 2 | |
| Low | 13 | 3 | 6 | 4 | |
| GLUT1 expression | |||||
| ∓ | 6 | 4 | 1 | 1 | 0.004 |
| ++ | 15 | 3 | 8 | 4 | |
| +++ | 22 | 2 | 4 | 16 | |
| MCT1 expression | |||||
| ∓ | 11 | 6 | 3 | 2 | 0.009 |
| ++ | 19 | 2 | 7 | 10 | |
| +++ | 13 | 1 | 3 | 9 | |
| MCT4 expression | |||||
| ∓ | 12 | 2 | 4 | 6 | 0.106 |
| ++ | 17 | 2 | 4 | 11 | |
| +++ | 14 | 5 | 5 | 4 | |
HBs Ag hepatitis B surface antigen, HCV Ab hepatitis C virus antibody, TNM Tumor Node Metastasis
Immunohistochemical analyses
Streptavidin–biotin–peroxidase IHC staining method was used in the present study. Briefly, formalin-fixed and paraffin-embedded sections from tumorous tissues of patients with HCC were deparaffinized by sequential washing with xylene, rehydration in a descending series of graded ethanol solutions and washing with phosphate buffer solution (PBS) to remove residual graded ethanol. Antigen retrieval was performed in citrate buffer (0.01 M, pH 6.0) and tissues were microwaved for 3 min. Endogenous peroxidases were blocked with 3% hydrogen peroxide for 20 min at room temperature, after which, slides were incubated overnight at 4 °C with one of the following primary antibodies: anti-CD147, anti-GLUT1, anti-MCT1 and anti-MCT4, (1:100, Cell Signaling Technology, Beverly, MA, USA), and followed by incubations with appropriate secondary horseradish peroxidase (HRP)-conjugated secondary antibody (1:2000; Santa Cruz Biotechnology, Inc., Dallas, TX) for 1 h at room temperature to stain the sections. Signals were visualized using 3,3-diaminobenzidine buffer (DAB). Slides were counterstained with haematoxylin, followed by dehydration and mounting. PBS was used instead of primary antibodies for the negative control. CD147, GLUT1, MCT1 and MCT4 expression levels were determined by examining the percentages of positively stained cells and the intensities of staining of the cytoplasm and cell membrane. The percentage of immune-reactivity was graded on a scale from 0 to 3 (0, ≤ 5% positive cells; 1, 5–25% positive cells; 2, 25–50% positive cells; 3, > 50% positive cells). The staining intensity was stratified into the following three categories (0, no staining or stained pale yellow; 1, weak staining intensity with stained brown; 2, strong staining intensity with stained tan). The two scores were multiplied to obtain a composite expression score. The final expression level was classified as follows: 0, weakly positive/negative (±); 1–3, moderate positive (++); or 4–6, strongly positive (+++).
Antibodies and flow cytometric analyses
Fluorescence-labeled monoclonal antibodies specific to CD3, CD4, CD8, CD56 and appropriate isotype-matched controls were purchased from BD Pharmingen (San Diego, CA, USA). Anti-FOXP3 was obtained from eBioscience (San Diego, CA, USA). For flow cytometric analyses, single cell suspensions of mononuclear cells (MNC) from freshly dissected tumor and non-tumor tissues were prepared by mechanical dissociation, collagenase treatment and centrifugation using FICOLL density gradients to separate tumor-infiltrating lymphocytes (TILs) and non-tumor-infiltrating lymphocytes (NILs) as described previously [5]. To stain for the cell surface markers, TILs and NILs were incubated with a series of fluorescence-labeled monoclonal antibodies for 30 min on ice. For FOXP3 staining, cells for were first fixed and permeabilized using Cytofix/Cytoperm (eBioscience) for 1 h after an appropriate surface staining according to the manufacturer’s instructions. Data were acquired using a Fluorescence Activated Cell Sorting (FACS) Caliber (BD Biosciences), and analyzed using CellQuest software (BD Biosciences, San Diego, CA, USA).
Lentiviral packaging and transduction to modulate CD147 expression in hepatoma cells
Before lentiviral packaging and transduction, a small hairpin RNA shRNA targeting human CD147 mRNA sequence and full-length CD147 cDNA were cloned into pLL3.7 and pLVX-IRES-Zsgreen lentiviral vectors (Youbao Biotechnology, Changsha, China), respectively. The sequence of the shRNA used was 5′-GTACAAGATCACTGACTCT-3′. Briefly, the sequence encoding CD147 was amplified from SMMC-7721 cDNA using specific primers containing endonuclease restriction sites at both ends (Forward, EcoRI; Reverse, BamHI) and the fragment was cloned into pLVX-IRES-Zsgreen lentiviral vector which had been digested using EcoRI and BamHI. The shRNA sequences targeting CD147 were synthesized at Suzhou Genepharma Gene technologies (Suzhoui, China) (Forward, HpaI; Reverse, XhoI). A control shRNA sequence was also cloned into pLL3.7 lentiviral vector as a silencing negative control. The primer sequences for amplification of CD147 were: Forward, 5′-GCAGCGGTTGGAGGTTGT-3′, and reverse, 5′-AGCCACGGATGCCCAGGAAGG-3′. Lentiviral particles were packaged using transient co-transfection of HEK 293T cells with pSPAX2, pMD2.G combined with either pLVX-IRES-Zsgreen-CD147, pLL3.7-CD147-shRNA, or negative control lentiviral vector. The lentiviral supernatants were collected 48 h afterwards, and the viral titers were determined through a transduction of HEK 293T cells by limiting dilutions. For the lentiviral transduction of SMMC-7721 cells and HepG2 cells, one single round of transduction with lentiviral supernatant was performed using a MOI (multiplicity of transduction) of 10 in the presence of 5 μg/ml PolyBrene (Sigma-Aldrich, Oakville, Canada). Following lentiviral transduction, cells were continually cultured in the complete medium for 8 days. Zsgreen+ and enhanced green fluorescent protein (EGFP)+ hepatoma cells were sorted using aBD FACS Aria (BD Biosciences, San Diego, CA) to a purity of 95–98%. The levels of CD147 expression in these purified transduced cells were assessed using western blotting assays prior to phenotypic and functional assays.
Western blotting
To reveal the molecular mechanism by which CD147 enhanced the glucose mechanism in HCC, the activation status of the phosphatidylinositol 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) signaling pathway was evaluated using western blotting. Briefly, lentivirally transduced SMMC-7721 and HepG2 cells were lysed using radio-immunoprecipitation assay (RIPA) buffer supplemented with a protease/phosphatase inhibitor cocktail (Cell Signaling Technology, Beverly, MA, USA), and equal amounts of proteins were loaded and separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). As described previously, the proteins separated in gels were then transferred to polyvinylidene difluoride (PVDF) membranes and blocked with 5% skimmed milk powder for 1 h. Subsequently, the membranes were incubated overnight at 4 °C with one of the following primary antibodies: anti-p-mTOR/mTOR, anti-p-Akt/Akt, anti-p-p70S6K/p70S6K, anti-p-4E-BP1/4E-BP1, anti-CD147 and anti-β-actin (1:1000, Cell Signaling Technology). After incubation, membranes were washed using tris-buffered saline (TBS) containing 0.1% Tween 20; the membranes were finally incubated with goat anti-rabbit horseradish peroxidase (HRP) conjugated secondary antibody (1:2000; Santa Cruz Biotechnology, Inc., Dallas, TX). Signals on the membranes were visualized using enhanced chemiluminescence (ECL) reagents (Millipore, Bedford, MA).
Measurement of in vitro glycolytic capacity of lentivirally transduced hepatoma cells with different levels of CD147 expression
Lentivirally transduced SMMC-7721 cells and HepG2 cells were cultured in complete dulbecco’s modified eagle medium (DMEM) supplemented with 10% fetal calf serum (FCS) at 37 °C in the 5% CO2 cell incubator. After 6 h, cells were added with 1 ml of medium containing 300 μCi 18F-fluorodeoxyglucose (18F-FDG, synthesized by our laboratory) to each culture well (6-well culture plate) and cells were further cultured for 30, 60, and 120 min. After incubation, rinsing and digestion, single cell suspensions were prepared in PBS to detect the 18F-FDG uptakes by lentivirally transduced cells with different levels of CD147 expression. The radioactivity was counted using a γ-radioimmunoassay counter (Cobra Quantum; Packard) after cells were counted, and the results were normalized to the radioactivity of 1 × 104 cells. Lactate production by lentivirally transduced hepatoma cells was measured using a lactate assay kit (BioVision, Mountain View, CA) according to the manufacturer’s instructions, and lactate dehydrogenase (LDH) activity was measured using lactate dehydrogenase activity assay kit (BioVision). Extracellular pH was measured with a pH meter (PBS-3E, Leici; Shanghai), and all pH measurements were taken within 2 min of samples collection.
18F-FDG positron emission tomography/computed tomography (PET/CT) imaging of nude mice xenograft models with different levels of CD147 expression
Approximately six-week-old female BALB/c nude mice with an average body weight of 18–22 g were maintained in specific pathogen-free condition for use in the present study. Lentivirally transduced SMMC-7721 cells in the logarithmic phase were harvested and resuspended in PBS at a density of 1 × 107 cells/ml. Xenografts were initiated by subcutaneous injections of 2 × 106 lentivirally transduced SMMC-7721 cells with CD147 overexpression or with an endogenous level of CD147 in a final volume of 0.2 ml into the right axillary fossa; whereas 2 × 106 lentivirally transduced SMMC-7721 cells with a decreased expression of CD147 were injected into the left axillary fossa. Xenografts size was regularly monitored, and time–tumor volume curves were plotted to assess xenograft growth. Tumor volumes were calculated using the following formula: V = 1/2 × (L × W2). 18F-FDG PET/CT (GE HealthCare, Waukesha, WI, USA) imaging was performed following intravenous injection of 18F-FDG at a concentration of 10 μCi/g. Briefly, CT was performed followed by PET scanning, and the acquired and reconstructed PET/CT images using attenuation correction (PET VCAR; GE Healthcare, USA) were transferred to a workstation (GE Advantage Workstation 4.6) for image fusion. Maximal standard uptake values (SUVmax) of the xenografts were calculated as the primary outcome measurement.
Lactate stimulation assay and transwell co-culture system
Peripheral blood mononuclear cells (PBMC) were isolated from healthy donors (Tianjin Blood Center) by centrifugations of peripheral blood samples on FICOLL density gradients. Freshly obtained PBMCs were then sorted using BD FACS Aria (BD Biosciences, San Diego, CA) to a purity of 95–98%. Purified CD3+CD4+ T cells were seeded into 24-well plates at a density of 5 × 105 cells/well and activated in 10% FCS Roswell Park Memorial Institute (RPMI) 1640 mediums supplemented with anti-CD3, anti-CD28 (1 μg/ml, Biolegend, San Diego, CA) plus interleukin 2 (IL-2, 10 ng/ml, Biolegend, San Diego, CA) in the presence or absence of transforming growth factor β1 (TGF-β1, 10 ng/ml, Biolegend, San Diego, CA). For the lactate stimulation assay, 20 mM sodium l-lactate (Sigma-Aldrich, Saint Louis, MO) was added to the stimulating culture medium mentioned above. For the co-culture system, purified CD3+CD4+ T cells were seeded into the upper chambers of a transwell co-culture system with or without 2-Deoxy-d-glucose (2-DG, 5 mmol/L, ApexBio Tech LCC), a glycolysis inhibitor; whereas established lentivirally transduced SMMC-7721 cells differing in CD147 expression were seeded into the lower chambers. At Day 4, lymphocytes were harvested, and examined for the FOXP3 expression using flow cytometry.
Statistical analyses
Data were presented as the mean ± standard error of the mean for percentages. Student’s unpaired t test and one-way ANOVA were performed to determine the means of two or more groups, respectively, using GraphPad Prism software (Graphpad, La Jolla, CA, USA). Paired t test was used for comparisons between the means of tumor tissues and the matching non-tumor tissues. Correlations between CD147 expressions and MCT1, GLUT1, MCT4 expressions, and correlations between CD147 expressions and the glycolytic capacities of hepatoma cells and the percentages of lymphoid populations in TILs were determined by Spearman’s rank correlation analyses using the SPSS 21.0 statistical software package (SPSS, Chicago, IL, USA). p < 0.05 was considered to indicate statistically significant differences.
Results
Correlation between CD147 and MCT1, GLUT1, and MCT4 in tumorous tissues of HCC patients
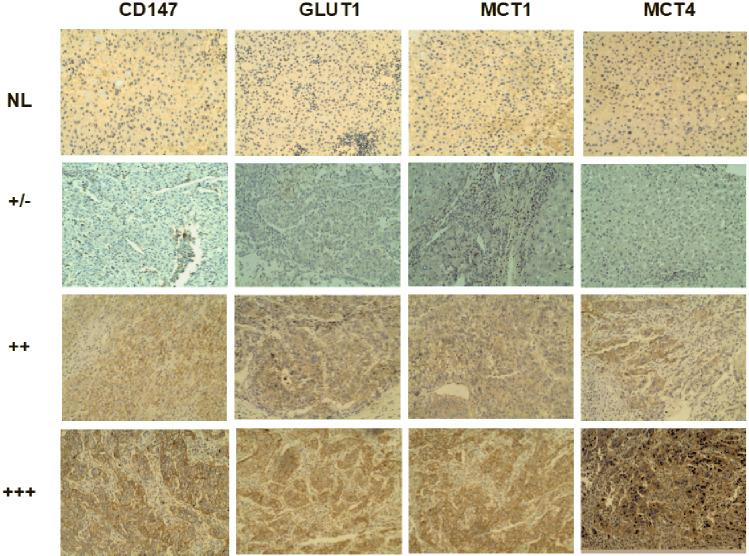
IHC staining of paraffin-embedded sections from normal livers and tumorous tissues of patients with HCC were performed to determine the status of CD147 expression and the correlations between CD147 and GLUT1, MCT1 and MCT4 (Fig. 1). Based on the levels of CD147 expression, samples were divided into three groups (∓, ++, +++). As presented in Table 1, GLUT1 and MCT1 expressions differed significantly between all three groups, and there was no significant difference in MCT4 expression between the three groups. Spearman’s rank correlation analyses demonstrated that the expression levels of CD147 positively correlated with the expression of MCT1 and GLUT1 (Table S1, S2); whereas a significant correlation between expression of CD147 and MCT4 was not suggested (Table S3). Thus, it was hypothesized that CD147 may be involved in the glucose metabolic reprogramming of HCC, at least partially through interactions with MCT1 and GLUT1.
Fig. 1.
CD147 expression in tumorous tissues obtained from patients with HCC is positively correlated with MCT1 and GLUT1 expression. Expression levels of CD147, MCT1, MCT4 and GLUT1 in HCC tissues and normal livers were detected using IHC staining. An IHC profile was shown from one representative normal liver (NL) and three representative HCC patients with weakly positive/negative (± , lower), positive (++, middle) and strongly positive (+++, upper) staining for each marker, Magnification, 200×. NL normal liver
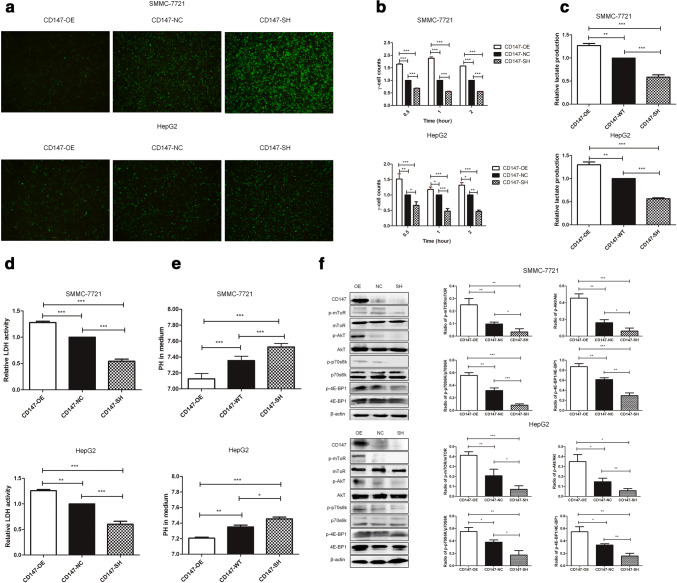
CD147 enhances the reprogramming of glucose metabolism in HCC cells via the PI3K/Akt/mTOR signaling pathway
To systematically examine the role of CD147 in the reprogramming of glucose metabolism in HCC, stable SMMC-7721 and HepG2 cell lines with different levels of CD147 expression were established: CD147-OE cells were lentivirally transduced to overexpress CD147; CD147-NC cells were with negative control lentiviral transduction to express endogenous levels of CD147; CD147-SH cells were with lentiviral transduction to knock down the endogenous levels of CD147 (Fig. 2a). To detect 18F-FDG uptakes by these lentivirally transduced cell lines in vitro, the stable cell lines were incubated with newly synthesized 18F-FDG for 0.5 h, 1 h and 2 h. The results of a γ-radioimmunoassay illustrated that the stable CD147-OE cells exhibited a significantly higher uptake of 18F-FDG compared with CD147-NC or CD147-SH cells (Fig. 2b). Consistently, lentivirally transduced hepatoma cells with CD147 overexpression were characterized by an increased lactate production (Fig. 2c) and an enhanced LDH activity (Fig. 2d). In addition, a lower pH value was also observed in the culture medium of the stable CD147-OE cells compared to that of the stable CD147-NC and CD147-SH cells (Fig. 2e). Spearman’s rank correlation analyses revealed positive correlations (p < 0.001) between the levels of CD147 expression and the 18F-FDG uptake, the lactate production, the LDH activity and the extracellular pH values. To summarize, CD147 significantly contributed to the enhanced glycolysis in HCC.
Fig. 2.
CD147 enhances the reprogramming of glucose metabolism in HCC in vitro via the PI3K/Akt/mTOR signaling pathway. a Efficiencies for lentiviral transduction of SMMG-7721 (upper) and HepG2 (lower) cells were shown under fluorescence microscope. b18F-FDG uptakes, c lactate productions, d LDH activities and e extracellular pH in the medium of lentivirally transduced SMMC-7721 cells (upper) and HepG2 cells (lower) were measured. Data were presented as relative values normalized to the respective negative control group. f Expression of members of the PI3K/Akt/mTOR signaling pathway in lentivirally transduced SMMC-7721 cells (left) and HepG2 cells (right) were examined by western blotting assay, and the ratio of phosphorylated proteins to total proteins was used to evaluate the degree of activation. *p < 0.05, **p < 0.01, ***p < 0.001
The possible molecular signaling pathways involved in the CD147-mediated regulation of glucose metabolism in HCC were next assessed. The PI3K/Akt/mTOR signaling pathway has been found to be highly activated and contributes to the reprogramming of glycolytic metabolism in tumor [22]. To investigate the effect of PI3K/Akt/mTOR pathway in the regulation of glucose metabolism induced by CD147, the expression levels of p-Akt, p-mTOR, p-p70S6K and p-4EBP1 and the corresponding total proteins were measured in lentivirally transduced SMMC-7721 cells and HepG2 cells (Fig. 2f). As expected, overexpression of CD147 enhanced the activation of PI3K/Akt/mTOR signaling in SMMC-7721 cells and HepG2 cells. Conversely, the protein levels of p-Akt, p-mTOR, p-p70S6K and p-4EBP1 were decreased in CD147-SH lentivirally transduced SMCC-7721 cells and HepG2 cells compared with that in CD147-NC lentivirally transduced counterparts. There results suggested that the PI3K/Akt/mTOR signaling may be an important mediator in the regulation of glucose metabolism mediated by CD147 in HCC.
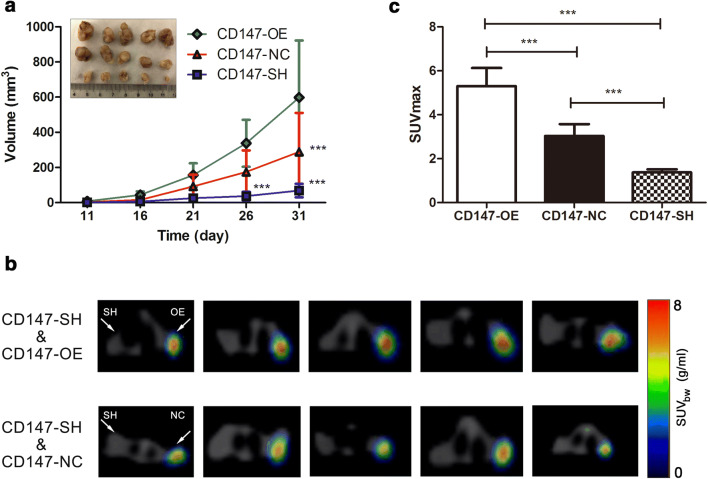
18F-FDG PET-CT imaging of nude mice SMMC-7721 xenograft models with different levels of CD147 expression
Base on the results in vitro, the influence of CD147 overexpression and CD147 loss on the tumor growth was assessed by establishing nude mice SMMC-7721 xenograft models with different levels of CD147 expression. As illustrated in the time–tumor volume curves (Fig. 3a), xenografts developed from CD147-OE cells exhibited increased tumor growths in comparison with that developed from CD147-NC cells. Conversely, xenografts developed from CD147-SH cells exhibited dramatically attenuated tumor growths compared to that developed from CD147-NC cells and CD147-OE cells. The uptakes of glucose in vivo by xenografts were evaluated by 18F-FDG PET-CT imaging (Fig. 3b), and SUVmax was used to semi-quantitatively analyze the radioactive uptake of 18F-FDG. In accordance with the results regarding the glucose uptakes by lentivirally transduced SMMC-7721 cells in vitro, the radioactivity accumulated in xenograft from CD147-OE was substantially higher than that in xenografts from CD147-SH and CD147-NC (Fig. 3c). On the opposite, a significant decrease in the 18F-FDG uptake was found in xenograft from CD147-SH in contrast with that from CD147-NC (Fig. 3c), suggesting that glucose uptakes by xenografts in vivo were positively associated with CD147 expression.
Fig. 3.
Overexpression of CD147 may promote proliferation and glucose uptake in HCC cells in vivo. Nude mice SMMC-7721 xenograft models with different levels of CD147 expression were established, and then 18F-FDG PET-CT imaging was performed to determine the effect of CD147 on glucose uptakes in vivo. a Time–volume curves were plotted to monitor the developments of xenografts, and the xenografts were extracted from the nude mice and photographed. b Axial view of 18F-FDG PET-CT imaging of the nude mice SMMC-7721 cell xenograft models. A total of 5 representative PET/CT fused images were shown for each group, including CD147-SH and CD147-OE (upper) and CD147-SH and CD147-NC (lower). c SUVmax was used to semi-quantitatively evaluate the glucose uptakes in the xenografts in vivo. Histograms were drawn to identify the differences existed in SUVmax for xenografts developed from lentiviral transduced SMMC-7721 cells with different levels of CD147 expression. ***p < 0.001
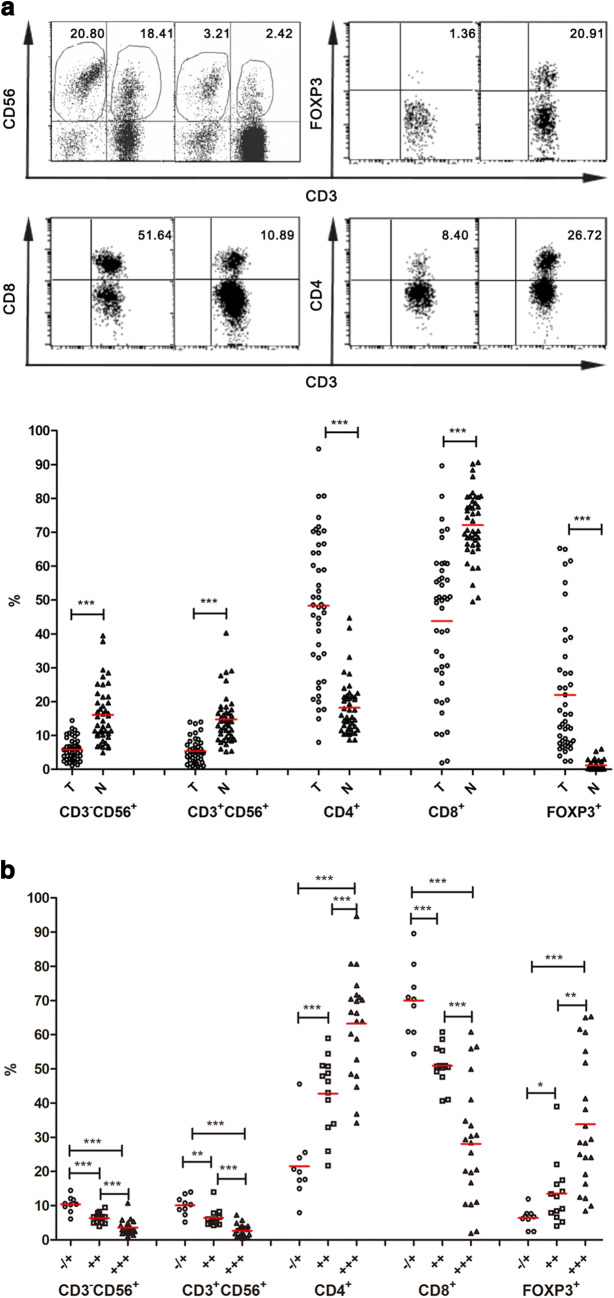
Correlation between the lymphocyte composition in TILs and the expression of CD147 in tumor tissues of HCC
To reveal potential changes in the composition of TILs compared with NILs, flow cytometric analyses were performed on the infiltrating lymphocytes isolated from a total of 43 paired tumor samples and adjacent non-tumor samples. In the CD3+ population, the percentage of CD8+ cells was found to be markedly reduced in TILs compared with that in NILs (48.7 ± 3.3 vs. 72.1 ± 1.5%, p < 0.001). Concurrently, there was a significant increase in the percentage of CD4+ cells in TILs compared with that in NILs (48.3 ± 3.2 vs. 18.2 ± 1.2%, p < 0.001) (Fig. 4a). As to CD3+CD56− natural killer (NK) cells and CD3+CD56+ natural T (NT) cells, remarkable reductions for both of them were observed in TILs in comparison to that in NILs (NK, 5.9 ± 0.5 vs. 16.1 ± 1.3%; NT, 5.4 ± 0.6 vs. 14.8 ± 1.1%, p < 0.001). The percentage of FOXP3-expressing cells was significantly higher in the CD3+ population of TILs than that in NILs (22.0 ± 2.8 vs. 1.21 ± 0.2%, p < 0.001). Taken together, these results suggested a remarkable change in the composition of TILs compared to that of NILs for patients with HCC. The identification of a glycolytic mechanism responsible for the tumorigenic capacity of CD147 in HCC prompted an investigation into whether the levels of CD147 expression in HCC could be used to predict any differences in the status of tumor-infiltrating lymphocytes. Thus, a relationship between the lymphocyte composition in TILs and the CD147 expression in HCC was assessed. Samples were divided into three groups based on CD147 expression status in tumorous tissues of HCC, respective comparisons of the percentages of CD3+CD56− NK cells and CD3+CD56+ NT cells in TILs and the proportions of CD4+, CD8+ and FOXP3+ cells in the CD3+ population in TILs were performed between groups (Fig. 4b). The results from a one-way ANOVA analysis indicated statistically significant differences (p < 0.001) between the group means of all the lymphocytes mentioned above. Spearman’s rank correlation analyses were performed to further evaluate the association between the lymphocyte composition in TILs and the expression level of CD147 in tumorous tissues of HCC. A positive correlation (p < 0.001) was observed between the expression level of CD147 and the percentage of CD4+ and FOXP3+ cells in the CD3+ population in TILs; whereas the expression level of CD147 was negatively correlated (p < 0.001) with the percentage of CD3+CD56− NK cells, CD3+CD56+ NT cells in TILs, and the proportions of CD8+ cells in the CD3+ population in TILs.
Fig. 4.
Comparative analyses of the TIL repertoires in HCC tissues with different levels of CD147 expressions. a Infiltrating lymphocyte compositions in tumor tissues and adjacent matching normal tissues obtained from patients with HCC, including the percentages of NT (CD3+CD56+) and NK (CD3−CD56+), CD4+, CD8+ and FOXP3+ subsets in CD3+ lymphocytes were analyzed using flow cytometry. A representative profile of scatter plots was shown (upper). b Based on the levels of CD147 expression (∓, ++, +++) in tumorous tissues of HCC, TILs were divided into three groups. A comparative analysis was performed between those three groups for each lymphocyte subsets mentioned above. *p < 0.05, **p < 0.01, ***p < 0.001
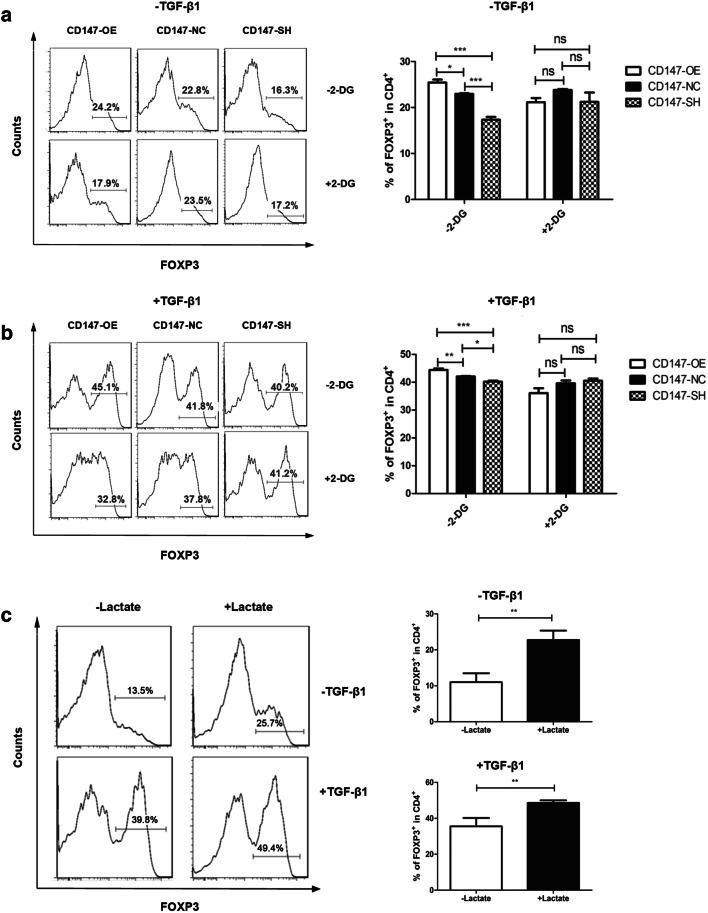
An accumulation of FOXP3-expressing regulatory T cells was induced in a transwell co-culture system and by a stimulation with lactate
To confirm the relationship between enhanced glycolysis mediated by CD147 in HCC and an accumulation of FOXP3-expressing regulatory T cells in vitro, a transwell co-culture system was used. As shown in Fig. 5a, under T cell stimulation without TGF-β1, CD3+CD4+ T cells co-cultured with CD147-OE or CD147-NC lentivirally transduced SMMC-7721 cells were found to be with higher percentages of FOXP3-expressing regulatory T cells in comparison with that co-cultured with CD147-SH lentivirally transduced SMMC-7721 cells. Under polarizing condition to form induced regulatory T cells, TGF-β1 was shown to induce FOXP3 expression in CD3+CD4+ T cells (Fig. 5b). As expected, an addition of 2-DG, a glycolysis inhibitor, to the transwell co-culture system resulted in a remarkable decrease of FOXP3-expressing regulatory cells in CD3+CD4+ T cells co-cultured with lentivirally transduced SMMC-7721 cells with CD147 overexpression (Fig. 5a, b); whereas no significant differences in the percentages of FOXP3-expressing regulatory T cells were observed in CD3+CD4+ T cells co-cultured with CD147-NC or CD147-SH lentivirally transduced SMMC-7721 cells treated with or without 2-DG (Fig. 5a, b). As described above, enhanced glycolysis mediated by CD147 in hepatoma cells resulted in a significant increase in lactate production in the medium. Lactate had been previously reported to contribute to tumor immunosuppression [15, 16]. Based on the results of the transwell co-culture system, the role of lactate in FOXP3-expressing regulatory T cell inductions in HCC was assessed. As shown in Fig. 5c, an addition of sodium L-lactate to activated CD3+CD4+ T cells in the presence or absence of TGF-β1 resulted in a notable induced increase in FOXP3-expressing regulatory T cells. These results suggested that a high concentration of lactate in the tumor microenvironment produced by glycolytic tumor cells may induce an immunosuppressive network due, in part, to an accumulation of regulatory T cells.
Fig. 5.
Co-culture of T cells with hepatoma cells and a stimulation of T cells with exogenous lactate promote an accumulation of FOXP3-expressing regulatory T cells. CD3+CD4+ T cells were sorted and purified from PBMCs using FACS, then activated in stimulating culture medium in the presence or absence of TGF-β1. At Day 4, lymphocytes were harvested, and FOXP3 expression was examined using flow cytometry. For the co-culture system, purified CD3+CD4+ T cells were seeded into the upper chambers of a transwell co-culture system a without or b with a glycolysis inhibitor, 2-DG, and the established lentiviral transduced SMMC-7721 cells were seeded into the lower chambers. c For the lactate stimulation assay, 20-mM sodium L-lactate was added to the stimulating culture medium mentioned above. A representative flow cytometry histogram profile from one repeat was shown for each group (left). Quantitative analyses of three independent experiments using PBMCs from three different healthy donors were summarized (right). *p < 0.05, **p < 0.01, ***p < 0.001
Discussion
Increasing evidence has demonstrated that tumor cells prefer to uptake and utilize glucose via glycolysis even in the presence of sufficient oxygen, a phenomenon popularly referred to as “Warburg effect” [9]. Thus, aerobic glycolysis, which endows tumor cells with a proliferative advantage in the tumor microenvironment, has been suggested as a vital metabolic hallmark of tumor [7]. As a well-known tumor-associated antigen, CD147 has been verified to promote the tumorigenesis and tumor progression via multiple molecular mechanisms [23, 24], particularly in HCC. However, the correlation between CD147 and metabolic reprogramming in HCC has been rarely studied [22]. In the present study, an important involvement of CD147 in metabolic regulation of glucose in HCC had been confirmed. As shown, upregulation of CD147 increased the uptake of 18F-FDG in hepatoma cells both in vitro and in vivo; whereas decreased expression of CD147 led to a dramatic reduction in the uptake of 18F-FDG. Consistently, the lactate productions in the culture medium and the LDH activities of these lentivirally transduced hepatoma cells with upregulated expression of CD147 were markedly increased compared with the cells with lower levels of CD147 expression. Additionally, the pH of the culture medium of cells overexpressing CD147 was dramatically decreased probably due to an increased accumulation of lactate production by tumor cells. Furthermore, a positive correlation between CD147 and MCT1 and between CD147 and GLUT1 expression in HCC tissues were uncovered in the present study, confirming an essential role of CD147 in glucose uptake and lactate flux across the plasma membrane as a chaperon to MCT1.
Several intracellular signaling pathway molecules in the metabolic reprogramming of tumor cells had been identified [25]. Among these, activation of the PI3K/Akt/mTOR signaling pathway had been found to contribute to the metabolic transformation of tumor cells [26]. Several recent studies had shown that CD147 enhanced the activity of PI3K/Akt/mTOR pathway in HCC cells [27, 28]. As an ancillary protein required for the expression and function of MCTs, CD147 was reported to initiate the activation of PI3K/Akt pathway by chaperoning MCT1 to effectively export redundant lactate in HCC cells due to enhanced tumor glycolysis [22]. Consistently, in the current study, a positive correlation was observed between the degree of activation of PI3K/Akt/mTOR pathway-associated molecules and the expression level of CD147 using several established HCC cell lines with different levels of CD147 expression.
Immune evasion is another hallmark of cancer. Numerous studies had demonstrated the presence of an immunosuppressive network in the tumor micro-milieu [6]. Although tumor glycolysis and evasion of immune surveillance are often viewed and studied as distinct processes, numerous reports are increasingly suggesting that they are fundamentally intimately linked [10, 11, 29, 30]. The results from the present study demonstrated that the percentages of tumor infiltrating NK (CD3−CD56+), NT (CD3+CD56+) and CD8+ T cells were negatively correlated with CD147 expression in tumorous tissues of HCC. Concurrently, the prevalence of tumor-infiltrating CD4+ T cells and FOXP3-expressing regulatory T cells were positively correlated with CD147 expression in tumorous tissues of HCC.
Besides tumor cells themselves in the tumor microenvironment, various neighboring activated immune cells also depend on increased glycolysis to maintain proper cell expansion, cell lineage differentiation and immunological function exertion [12]. An overlapping dependence on enhanced glycolysis by tumor cells and surrounding immune cells inevitably results in a metabolic competition in the tumor microenvironment [11]. The tumor-imposed glucose limitation may directly lead to a dampened glucose uptake and thus metabolic reprogramming of immune cells which ultimately causes impaired immunological functions [11, 13, 14]. As reported, glucose deprivation or blockage of glycolysis remarkably inhibited multiple key gene expression events and effector functions in CD8+ effector T cells (Teff) [31]. Consistently, the transwell co-culture system used in the present study demonstrated that an increase of FOXP3-expressing regulatory T cells was induced when CD3+CD4+ T cells were co-cultured with CD147-OE lentivirally transduced SMMC-7721 cells, and this was significantly reversed by 2-DG, an inhibitor of glycolysis. Apart from the tumor-imposed nutrient restriction on immune cells, several tumor-derived immunomodulatory metabolic intermediates, such as lactate, also significantly engage in the metabolic interplay between tumor cells and immune cells [32–34]. The concentration of lactate in the tumor microenvironment may be as high as 40 mM, and this high lactate concentration certainly exerts a deleterious effect on a variety of tumor-infiltrating immune cells [35]. Tumor cell-derived extracellular lactate has been demonstrated to block the differentiation from monocytes to dendritic cells (DCs) and inhibit the cytokine release from differentiated DCs [36]. The cytotoxic activity of NK cells is also significantly decreased when cultured in the presence of lactate [15, 16, 37, 38]. Furthermore, the proliferation and cytokine production of cytotoxic T lymphocytes (CTLs) in the adaptive arm of the immune system may be suppressed up to 95%, and their cytotoxic activity was inhibited by 50% [39]. In the present study, addition of lactate into the culture medium of CD3+CD4+ T cells under activation with or without TGF-β1 resulted in a significant increase in FOXP3-expressing T cells. Intriguingly, the transcription factor, FOXP3, had been reported to participate in a metabolic adaptation to induce oxidative phosphorylation but decrease glycolysis, which consequently allowed regulatory T cells to thrive and function under a metabolically challenging local tumor environment with low glucose and high lactate [40].
Despite valuable results achieved in the current investigation, several limitations should also be noticed. Firstly, as glucose is the primary energy source for the majority of cells, a focus was placed on altered glucose metabolism in HCC in the present study; the potential involvement of amino acid, lipid and nucleotide metabolism with respect to CD147 was not examined. Secondly, uptake of 18F-FDG in vitro and in vivo, lactate production and LDH activity were used to reflect the accelerated aerobic glycolysis induced by CD147 overexpression. However, the rate of oxygen consumption and the status of mitochondrial oxidative phosphorylation should also be considered to strengthen the conclusion. A direct correlational analysis between enhanced glycolysis mediated by CD147 in HCC and the local immunosuppression in animal experiments was not performed due to some experimental setting limitations. In the end, the association between metabolic reprogramming of immune cells and impaired anti-tumor immune responses should be considered in future studies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Wenwen Yu at Tianjin Medical University Cancer Institute and Hospital (Tianjin, China) for her assistance in FACS analyses.
Abbreviations
- CTL
Cytotoxic T lymphocyte
- CTLA-4
Cytotoxic T lymphocytes associated protein-4
- DAB
Diaminobenzidine buffer
- DC
Dendritic cell
- 2-DG
2-Deoxy-d-glucose
- DMEM
Dulbecco’s modified eagle medium
- ECL
Enhanced chemiluminescence
- EGFP
Enhanced green fluorescent protein
- EMMPRIN
Extracellular matrix metalloproteinase inducer
- FACS
Fluorescence Activated Cell Sorting
- FCS
Fetal calf serum
- 18F-FDG
18F-fluorodeoxyglucose
- FOXP3
Forkhead box protein 3
- GLUT1
Glucose transporter 1
- HBs Ag
Hepatitis B surface antigen
- HCC
Hepatocellular carcinoma
- HCV Ab
Hepatitis C virus antibody
- HRP
Horseradish peroxidase
- IHC
Immunohistochemistry
- IL-2
Interleukin 2
- LDH
Lactate dehydrogenase
- MCT1/4
Monocarboxylate transporters 1 and 4
- MNC
Mononuclear cell
- MOI
Multiplicity of transduction
- mTOR
Mammalian target of rapamycin
- NIL
Non-tumor-infiltrating lymphocytes
- NK
Natural killer
- NT
Natural T
- OCR
Oxygen consumption
- PBMC
Peripheral blood mononuclear cell
- PBS
Phosphate buffer solution
- PD-1
Programmed cell death-1
- PET/CT
Positron Emission Tomography/Computed Tomography
- PI3K
Phosphatidylinositol 3-kinase
- PVDF
Polyvinylidene difluoride membranes
- RIPA
Radio-Immunoprecipitation Assay
- RPMI
Roswell Park Memorial Institute
- SDS-PAGE
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- ShRNA
Small hairpin RNA
- SPSS
Statistical software package
- SUVmax
Maximal standard uptake value
- TBS
Tris buffer saline
- Teff
Effector T cell
- TGF-β1
Transforming growth factor
- TIL
Tumor-infiltrating lymphocytes
- TNM
Tumor Node Metastasis
Author contributions
XFL and YFZ contributed to the experimental design, the operation of the experiments, data analyses and interpretation of the results in this study. They both contributed to drafting the manuscript. WCM performed the statistical analyses and drafted the manuscript. QF and JJL performed the cellular and immunological experiments. GTY and PHC established the nude mice xenograft models and performed the imaging tests in vivo. DD and WC designed the study and interpreted the results. LSQ and XZY designed the study and reviewed the draft of the manuscript. WGX was responsible for data interpretation and assessing the final content of the manuscript. All authors approved the final version of the manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Grant Nos. 81601377, 81501984 and 2018ZX09201015), the Tianjin Natural Science Fund (Grant Nos. 16JCZDJC35200, 17JCYBJC25100, 18PTZWHZ00100 and H2018206600), the Science and Technology Development Fund of Tianjin Education Commission for Higher Education (Grant Nos. 2018KJ057 and 2018KJ061), the Tianjin Medical University Cancer Institute and Hospital Fund (Grant Nos. Y1601, B1605, B1719, Y1805 and Y1810) and the Incubation Project of the National Clinical Research Center for Cancer (Grant No. N14B09).
Compliance with ethical standards
Conflict of interest
The authors declare no potential conflicts of interest.
Ethical approval and ethical standards
All procedures were performed in accordance with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The present study was approved by Hospital Ethic Review Committee of Peking University People’s Hospital (Beijing, China) on June 6, 2017. All protocols involving animals were in strict accordance with institutional guidelines for the care and use of experimental animals and were approved by the animal ethical review committee of Tianjin Medical University and Cancer Institute (Tianjin, China) on August 18, 2018.
Informed consent
All patients provided written informed consent before surgery for use of their specimens for research. Oral informed consent was obtained from the healthy donors at the Tianjin Blood Center to the use of their blood for research purposes.
Animal source
Female BALB/c nude mice (Beijing Vital River Laboratory Animal Technology Co., Ltd) with an average body weight of 18–22 g were purchased and maintained in specific pathogen-free condition for use.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiaofeng Li, Yufan Zhang and Wenchao Ma contributed equally to this work.
Contributor Information
Lisha Qi, Email: qli01@tmu.edu.cn.
Xiaozhou Yu, Email: xyu@tmu.edu.cn.
Wengui Xu, Email: wenguixy@yeah.net, Email: wxu06@tmu.edu.cn.
References
- 1.Lafaro KJ, Demirjian AN, Pawlik TM. Epidemiology of hepatocellular carcinoma. Surg Oncol Clin N Am. 2015;24:1–17. doi: 10.1016/j.soc.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Sangro B, Gomez-Martin C, de la Mata M, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59:81–88. doi: 10.1016/j.jhep.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 3.Liu X, Qin S. Immune checkpoint inhibitors in hepatocellular carcinoma: opportunities and challenges. Oncologist. 2019;24:S3–S10. doi: 10.1634/theoncologist.2019-IO-S1-s01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Floudas CS, Brar G, Greten TF. Immunotherapy: current status and future perspectives. Dig Dis Sci. 2019;64:1030–1040. doi: 10.1007/s10620-019-05516-7. [DOI] [PubMed] [Google Scholar]
- 5.Li X, Peng J, Pang Y, et al. Identification of a FOXP3(+)CD3(+)CD56(+) population with immunosuppressive function in cancer tissues of human hepatocellular carcinoma. Sci Rep. 2015;5:14757. doi: 10.1038/srep14757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Speiser DE, Ho PC, Verdeil G. Regulatory circuits of T cell function in cancer. Nat Rev Immunol. 2016;16:599–611. doi: 10.1038/nri.2016.80. [DOI] [PubMed] [Google Scholar]
- 7.Renner K, Singer K, Koehl GE, et al. Metabolic Hallmarks of tumor and immune cells in the tumor microenvironment. Front Immunol. 2017;8:248. doi: 10.3389/fimmu.2017.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell. 2008;13:472–482. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Hsu PP, Sabatini DM. Cancer cell metabolism: warburg and beyond. Cell. 2008;134:703–707. doi: 10.1016/j.cell.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 10.Villalba M, Rathore MG, Lopez-Royuela N, Krzywinska E, Garaude J, Allende-Vega N. From tumor cell metabolism to tumor immune escape. Int J Biochem Cell Biol. 2013;45:106–113. doi: 10.1016/j.biocel.2012.04.024. [DOI] [PubMed] [Google Scholar]
- 11.Chang CH, Qiu J, O’Sullivan D, et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015;162:1229–1241. doi: 10.1016/j.cell.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacIver NJ, Michalek RD, Rathmell JC. Metabolic regulation of T lymphocytes. Annu Rev Immunol. 2013;31:259–283. doi: 10.1146/annurev-immunol-032712-095956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siska PJ, Rathmell JC. T Cell metabolic fitness in anti-tumor immunity. Trends Immunol. 2015;36:257–264. doi: 10.1016/j.it.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Domblides C, Lartigue L, Faustin B. Control of the antitumor immune response by cancer metabolism. Cells. 2019;8(pii):E104. doi: 10.3390/cells8020104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brand A, Singer K, Koehl GE, et al. LDHA-associated lactic acid production blunts tumor immunosurveillance by T and NK Cells. Cell Metab. 2016;24:657–671. doi: 10.1016/j.cmet.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Scott KE, Cleveland JL. Lactate wreaks havoc on tumor-infiltrating T and NK cells. Cell Metab. 2016;24:649–650. doi: 10.1016/j.cmet.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 17.Zhao E, Maj T, Kryczek I, et al. Cancer mediates effector T cell dysfunction by targeting micro RNAs and EZH2 via glycolysis restriction. Nat Immunol. 2016;17:95–103. doi: 10.1038/ni.3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xin X, Zeng X, Gu H, et al. CD147/EMMPRIN overexpression and prognosis in cancer: a systematic review and meta-analysis. Sci Rep. 2016;6:32804. doi: 10.1038/srep32804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng F, Li H, You Q, et al. CD147 as a novel prognostic biomarker for hepatocellular carcinoma: a meta-analysis. Biomed Res Int. 2017;2017:5019367. doi: 10.1155/2017/5019367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X, Yu X, Dai D, Song X, Xu W. The altered glucose metabolism in tumor and a tumor acidic microenvironment associated with extracellular matrix metalloproteinase inducer and monocarboxylate transporters. Oncotarget. 2016;7:23141–23155. doi: 10.18632/oncotarget.8153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ke X, Fei F, Chen Y, et al. Hypoxia upregulates CD147 through a combined effect of HIF-1α and Sp1 to promote glycolysis and tumor progression in epithelial solid tumors. Carcinogenesis. 2012;33:1598–1607. doi: 10.1093/carcin/bgs196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang Q, Li J, Xing J, et al. CD147 promotes reprogramming of glucose metabolism and cell proliferation in HCC cells by inhibiting the p53-dependent signaling pathway. J Hepatol. 2014;61:859–866. doi: 10.1016/j.jhep.2014.04.035. [DOI] [PubMed] [Google Scholar]
- 23.Dai JY, Dou KF, Wang CH, et al. The interaction of HAb18G/CD147 with integrin alpha6beta1 and its implications for the invasion potential of human hepatoma cells. BMC Cancer. 2009;9:337. doi: 10.1186/1471-2407-9-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao P, Zhang W, Tang J, et al. Annexin II promotes invasion and migration of human hepatocellular carcinoma cells in vitro via its interaction with HAb18G/CD147. Cancer Sci. 2010;101:387–395. doi: 10.1111/j.1349-7006.2009.01420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeung SJ, Pan J, Lee MH. Roles of p53, MYC and HIF-1 in regulating glycolysis—the seventh hallmark of cancer. Cell Mol Life Sci. 2008;65:3981–3999. doi: 10.1007/s00018-008-8224-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng YL, Li L, Jia YX, et al. LINC01554-mediated glucose metabolism reprogramming suppresses tumorigenicity in hepatocellular carcinoma via downregulating PKM2 expression and inhibiting Akt/mTOR signaling pathway. Theranostics. 2019;9:796–810. doi: 10.7150/thno.28992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X, Fu Q, Zhu Y, et al. CD147-mediated glucose metabolic regulation contributes to the predictive role of 18 F-FDG PET/CT imaging for EGFR-TKI treatment sensitivity in NSCLC. Mol Carcinog. 2019;58:247–257. doi: 10.1002/mc.22923. [DOI] [PubMed] [Google Scholar]
- 28.Gou X, Tang X, Kong DK, et al. CD147 is increased in HCC cells under starvation and reduces cell death through upregulating p-mTOR in vitro. Apoptosis. 2016;21:110–119. doi: 10.1007/s10495-015-1189-y. [DOI] [PubMed] [Google Scholar]
- 29.Ganapathy-Kanniappan S. Linking tumor glycolysis and immune evasion in cancer: emerging concepts and therapeutic opportunities. Biochim Biophys Acta Rev Cancer. 2017;1868:212–220. doi: 10.1016/j.bbcan.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 30.Ganapathy-Kanniappan S. Taming tumor glycolysis and potential implications for immunotherapy. Front Oncol. 2017;7:36. doi: 10.3389/fonc.2017.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cham CM, Driessens G, O’Keefe JP, Gajewski TF. Glucose deprivation inhibits multiple key gene expression events and effector functions in CD8+ T cells. Eur J Immunol. 2008;38:2438–2450. doi: 10.1002/eji.200838289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.San-Millán I, Brooks GA. Reexamining cancer metabolism: lactate production for carcinogenesis could be the purpose and explanation of the Warburg Effect. Carcinogenesis. 2017;38:119–133. doi: 10.1093/carcin/bgw127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirschhaeuser F, Sattler UG, Mueller-Klieser W. Lactate: a metabolic key player in cancer. Cancer Res. 2011;71:6921–6925. doi: 10.1158/0008-5472.CAN-11-1457. [DOI] [PubMed] [Google Scholar]
- 34.Romero-Garcia S, Moreno-Altamirano MM, Prado-Garcia H, Sánchez-García FJ. Lactate contribution to the tumor microenvironment: mechanisms, effects on immune cells and therapeutic relevance. Front Immunol. 2016;7:52. doi: 10.3389/fimmu.2016.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feng J, Yang H, Zhang Y, et al. Tumor cell-derived lactate induces TAZ dependent upregulation of PD-L1 through GPR81 in human lung cancer cells. Oncogene. 2017;36:5829–5839. doi: 10.1038/onc.2017.188. [DOI] [PubMed] [Google Scholar]
- 36.Caronni N, Simoncello F, Stafetta F, et al. Downregulation of membrane trafficking proteins and lactate conditioning determine loss of dendritic cell function in lung cancer. Cancer Res. 2018;78:1685–1699. doi: 10.1158/0008-5472.CAN-17-1307. [DOI] [PubMed] [Google Scholar]
- 37.Harmon C, Robinson MW, Hand F, et al. Lactate-mediated acidification of tumor microenvironment induces apoptosis of liver-resident NK cells in colorectal liver metastasis. Cancer Immunol Res. 2019;7:335–346. doi: 10.1158/2326-6066.CIR-18-0481. [DOI] [PubMed] [Google Scholar]
- 38.Long Y, Gao Z, Hu X, et al. Downregulation of MCT4 for lactate exchange promotes the cytotoxicity of NK cells in breast carcinoma. Cancer Med. 2018;7:4690–4700. doi: 10.1002/cam4.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fischer K, Hoffmann P, Voelkl S, et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. 2007;109:3812–3819. doi: 10.1182/blood-2006-07-035972. [DOI] [PubMed] [Google Scholar]
- 40.Angelin A, Gil-de-Gómez L, Dahiya S, et al. FOXP3 reprograms T cell metabolism to function in low-glucose, high-lactate environments. Cell Metab. 2017;25:1282–1293. doi: 10.1016/j.cmet.2016.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.